Key Points
Question
Is terbinafine use in pregnancy associated with an increased risk of major malformations and spontaneous abortion?
Findings
This Danish nationwide cohort study included all pregnancies with use of oral and topical terbinafine in pregnancy during the study period 1997-2016 (n = 4065) as well as 40 650 unexposed pregnancies. In propensity score–matched comparisons, no significant differences in the risk of major malformations or spontaneous abortion between oral terbinafine-exposed, topical terbinafine-exposed, and unexposed pregnancies were identified.
Meaning
Oral or topical terbinafine use in pregnancy does not appear to be associated with an increased risk of major malformations or spontaneous abortion.
Abstract
Importance
Terbinafine is a commonly used antifungal agent, but safety data of its use in pregnancy are limited.
Objective
To examine the association between oral and topical terbinafine exposure in pregnancy and the risk of major malformations and spontaneous abortion.
Design, Setting, and Participants
A nationwide, registry-based cohort study was conducted in Denmark from January 1, 1997, to December 31, 2016, in a cohort of 1 650 649 pregnancies. Data analysis was performed from July 11 to October 20, 2019. Pregnancies were matched on propensity scores comparing oral terbinafine exposed vs unexposed (1:10 ratio), topical terbinafine exposed vs unexposed (1:10), and oral vs topical terbinafine exposed (1:1).
Exposures
Filled prescriptions for oral or topical terbinafine.
Main Outcomes and Measures
Logistic regression was used to compute prevalence odds ratios for the primary outcome of major malformations and Cox proportional hazards regression was used to compute hazard ratios for the secondary outcome of spontaneous abortion.
Results
Based on a cohort of 1 650 649 pregnancies, oral terbinafine-exposed (n = 891 pregnancies; mean [SD] age, 30.4 [6] years) and topical terbinafine-exposed (n = 3174; mean [SD] age, 29.5 [5.4] years) pregnancies were identified; up to a total of 40 650 unexposed pregnancies were included for the matched outcome analyses. In propensity-matched comparisons of the risk of major malformations, the prevalence odds ratios were 1.01 (95% CI, 0.63-1.62) for oral terbinafine-exposed vs unexposed pregnancies (absolute risk difference [ARD], 0.04%; 95% CI, −1.69% to 1.76%), 1.08 (95% CI, 0.81-1.44) for topical terbinafine-exposed vs unexposed pregnancies (ARD, 0.26%; 95% CI, −0.73% to 1.26%), and 1.18 (95% CI, 0.61-2.29) for oral vs topical terbinafine-exposed pregnancies (ARD, 0.59%; 95% CI, −1.71% to 2.88%). For the risk of spontaneous abortion, the hazard ratios were 1.06 (95% CI, 0.86-1.32) for oral terbinafine-exposed vs unexposed pregnancies (ARD, 0.13%; 95% CI, −1.97% to 2.24%), 1.04 (95% CI, 0.88-1.21) for topical terbinafine-exposed vs unexposed pregnancies (ARD, 0.17%; 95% CI, −0.64% to 0.98%), and 1.19 (95% CI, 0.84-1.70) for oral vs topical terbinafine-exposed (ARD, 1.13%; 95% CI, −2.23% to 4.50%) pregnancies.
Conclusions and Relevance
Among pregnancies exposed to oral or topical terbinafine, no increased risk of major malformations or spontaneous abortion was identified.
This cohort study examines the association between use of oral or topical terbinafine and malformations and spontaneous abortions in women who receive terbinafine during pregnancy.
Introduction
Terbinafine is an allylamine antifungal agent, available in both topical and systemic formulations and indicated for treatment of common infections, such as dermatophytic skin infections, onychomycosis, and other cutaneous dermatophytosis; off-label use includes treatment of sporotrichosis.1,2,3 Terbinafine works primarily as a fungicidal agent, as opposed to the fungistatic azole agents, by interfering with fungal ergosterol biosynthesis by inhibiting squalene epoxidase in the fungal cell membrane. This mechanism of action leads to a deficiency of ergosterol and an intracellular accumulation of squalene, thus disrupting fungal membrane functioning and cell wall synthesis, resulting in fungal cell death.4,5,6
While terbinafine is a commonly used effective antifungal agent and generally well tolerated in the nonpregnant population, limited data exist on the fetal safety of use in pregnancy.2,7,8,9 Data from animal studies do not suggest a fetal risk of terbinafine exposure in pregnancy,10 but findings may not be applicable to the human response. To our knowledge, only 1 preliminary report has previously provided data concerning the fetal safety of terbinafine use in pregnancy.11 The study included 54 exposed pregnancies, of whom 26 received oral formulations, and, despite the lack of statistical testing, the data presented were not suggestive of a potential fetal risk. Furthermore, terbinafine is selective for fungal enzymes and has only minimal effects on mammalian cholesterol synthesis12; in addition, when terbinafine is applied topically, less than 5% has been reported to be absorbed systemically.6,13,14 The limited human data do not allow a sufficient evaluation of the potential fetal risk with terbinafine use in pregnant women. Consequently, until more data become available, caution is advised and use of terbinafine preferably should be avoided during pregnancy.1,8,15
Given the wide use of terbinafine to treat common fungal infections, the lack of fetal safety data on terbinafine exposure in pregnancy emerges as an issue of considerable relevance for routine clinical care. In a nationwide, registry-based cohort study from 1997 to 2016, we investigated the associated risk of the primary outcome major malformations and the secondary outcome spontaneous abortion with oral and topical terbinafine exposure in pregnancy with use of a propensity score–matched design.
Methods
Data Sources and Study Design
We conducted a historical registry-based cohort study from January 1, 1997, until December 31, 2016. Data analysis was performed from July 11 to October 20, 2019. With use of the unique personal identification number that is assigned to all inhabitants of Denmark, we linked individual-level data from different nationwide registries. The source population was established on the basis of all pregnancies (ie, both pregnancies ending in live birth and fetal death) registered in the Medical Birth Registry and the National Patient Registry during the study period.16,17 The Medical Birth Registry contains information of all live births and stillbirths since 1978, including birth-related and maternal-related variables. The National Patient Registry contains information on pregnancies ending in spontaneous or induced abortive outcomes, as well as hospital care use and diagnoses assigned by the treating physician according to the International Statistical Classification of Diseases, Tenth Revision (ICD-10). The gestational age registered at the date of birth or abortive outcome allowed us to follow up the cohort from pregnancy onset. The gestational age is estimated based on maternal report of the first day of the last menstrual period and subsequently ultrasonographically confirmed at the antenatal screening. Multiple pregnancy records with overlapping dates and records with implausible or missing information on gestational age were excluded. The study cohorts for the analyses of major malformation were derived from pregnancies resulting in live births, and the study cohorts for the analyses of spontaneous abortion were derived from all pregnancies. Demographic and socioeconomic information were obtained from the Danish Civil Registration System and Statistics Denmark.18 With use of the Registry of Medicinal Product Statistics, we obtained information on all filled prescriptions, including Anatomical Therapeutic Chemical (ATC) code and date of filled prescriptions, for terbinafine as well as for other drugs from all pharmacies in Denmark.19
The study was approved by the Danish Data Protection Agency. Ethical approval and informed consent are not required for registry-based research in Denmark.
The study included 3 comparison groups for the analyses of the risk of major malformations and spontaneous abortion. These groups included oral terbinafine-exposed pregnancies, topical terbinafine-exposed pregnancies, and unexposed pregnancies. With use of separate propensity score matching, we compared oral terbinafine-exposed vs unexposed pregnancies, topical terbinafine-exposed vs unexposed pregnancies, and oral terbinafine- vs topical terbinafine-exposed pregnancies in distinct cohorts.
Exposure was defined as at least 1 filled prescription for oral terbinafine (ATC: D01BA02) or topical terbinafine (ATC: D01AE15) identified via the Registry of Medicinal Product Statistics. The first day of exposure was defined by the date of first filled prescription (index date). We defined the exposure time windows according to the specific outcome analyses: the first trimester for the analyses of major malformations and before 22 completed gestational weeks for spontaneous abortion. Women with a filled prescription for oral terbinafine during the 14 days prior to pregnancy onset were allowed inclusion in the oral terbinafine-exposed pregnancy group. For the topical terbinafine-exposed pregnancy group, we excluded pregnancies in case of filled prescriptions for oral terbinafine within 6 months prior to pregnancy onset. The unexposed comparison group consisted of pregnant women with no filled prescriptions for oral or topical terbinafine or any other topical or systemic antifungal drugs (ATC: D01A, D01BA, J02AB, and J02AC) in pregnancy as well as in 1 year prior to pregnancy onset.
For a preplanned sensitivity analysis of the association between oral terbinafine and major malformations, we defined an additional comparative group of pregnant women with filled prescriptions for oral terbinafine in the time interval from 1 year until 3 months before conception but with no filled prescription in pregnancy or in the 3 months prior to pregnancy onset.
With use of the National Patient Registry, we identified outcome cases diagnosed via inpatient or outpatient care. The primary outcome was major malformations, defined as an infant who, within the first year of life, was diagnosed with a major malformation in accordance with the European Surveillance of Congenital Anomalies classification system of subgroups of major congenital anomalies.20 We excluded major malformations with known causes (eg, Down syndrome and fetal alcohol syndrome) and minor defects according to the European Surveillance of Congenital Anomalies exclusion list (eMethods in the Supplement).20,21 The secondary outcome was spontaneous abortion, defined as a pregnancy ending in fetal death before 22 completed gestational weeks (ICD-10 code O021 or O03).22 Spontaneous abortions occurring earlier than gestational week 6 were not included owing to the risk of misclassification of these very early abortions in the registry.
We used propensity score matching to take into account baseline characteristics that could be associated with the risk of the outcomes. For each outcome analysis, individual propensity score estimations and matchings were performed.23 The propensity scores were estimated using a logistic regression model and included all variables reported in eTable 1 in the Supplement. Based on the propensity scores, we created distinct matched cohorts of oral terbinafine-exposed vs unexposed pregnancies (matched in a 1:10 ratio), topical terbinafine-exposed vs unexposed pregnancies (1:10), and oral terbinafine-exposed vs topical terbinafine-exposed pregnancies (1:1). Matching was performed using the greedy nearest neighbor matching algorithm (caliper width 0.02 on the propensity-score scale).24,25
Statistical Analysis
Three comparative study cohorts were constructed for both the analyses of major malformations and spontaneous abortion. We imputed missing values (0%-4.1% missing; eTable 2 in the Supplement) using the mode value. The quality of matching was assessed by standardized differences. A covariate was considered well balanced between matched groups if the standardized difference was less than 10%.
A logistic regression model was used to compute prevalence odds ratios (ORs) for the associated risk of major malformations. A Cox proportional hazards regression model was used to compute hazard ratios (HRs) for the associated risk of spontaneous abortion, with gestational age (days) as the underlying time scale. We used a Wald test to assess the interaction between time scale and exposure. Pregnancies were censored if abortive outcome occurred on that date other than spontaneous abortion. For the exposed vs unexposed pregnancies comparison analyses of spontaneous abortion, the gestational day on the first day of terbinafine exposure (index date) was added as an additional matching criterion, that is, unexposed pregnancies were eligible as matches if the pregnancy had lasted until the index date. Follow-up for both the terbinafine-exposed and unexposed pregnancies started at the respective index date. For the oral vs topical terbinafine comparison analysis of spontaneous abortion, we added the gestational week of the first day of exposure as a matching criterion, and pregnancies were followed up from their respective index date.
Preplanned sensitivity analyses of the association between oral terbinafine exposure and major malformations included comparing pregnancies with previous oral terbinafine use before pregnancy onset (propensity score matched in a 1:2 ratio), a subgroup analysis of pregnancies with filled prescriptions in gestational weeks 4 to 10 (a time during which a teratogenic effect in general is considered to be most pronounced, if present), exposures occurring at any time in pregnancy, pregnancies exposed only in the second and third trimesters, subgroup analyses of singleton pregnancies, and exclusion of pregnancies with filled prescriptions in the 14 days prior to pregnancy onset. Furthermore, we assessed the association between oral terbinafine use and risk of spontaneous abortion compared with unexposed by subgroup analyses of pregnancies with filled prescriptions later than gestational week 5 and excluding pregnancies in which the prescriptions were filled within the 14 days prior to pregnancy onset.
All measures of associations are reported with the corresponding 95% CIs. All statistical tests were 2-sided; we considered CIs not overlapping 1.0 to indicate statistical significance. SAS software, version 9.4 (SAS Institute Inc), was used for all analyses.
Results
Study Cohorts
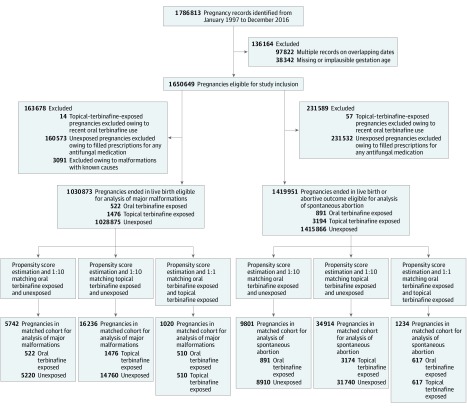
The Figure displays a flowchart of the cohort selection and construction of the study cohorts. The source cohort consisted of a total of 1 650 649 pregnancies. Subsequent to the application of the respective exclusion criteria and exposure definitions, 1 030 873 pregnancies were eligible for inclusion for the analyses of major malformations and 1 419 951 pregnancies were eligible for inclusion for the analyses of spontaneous abortion; eTable 3 and eTable 4 in the Supplement report the unmatched baseline characteristics. Of these pregnancies, oral terbinafine- (n = 891 pregnancies; mean [SD] age, 30.4 [6] years) and topical terbinafine- (n = 3174; mean [SD] age, 29.5 [5.4] years) exposed pregnancies were identified. Up to a total of 40 650 unexposed pregnancies were included for the matched outcome analyses. Following the propensity score estimation and matching, the cohorts used in the analyses of major malformations included 5742 pregnancies for the comparison of oral terbinafine exposed vs unexposed (matched in a 1:10 ratio), 16 236 pregnancies for topical terbinafine exposed vs unexposed (1:10 ratio), and 1020 pregnancies for oral vs topical terbinafine exposed (1:1 ratio). The matched cohorts for the analyses of spontaneous abortion included 9801 pregnancies for oral terbinafine exposed vs unexposed, 34 914 pregnancies for topical terbinafine exposed vs unexposed, and 1234 pregnancies for oral vs topical terbinafine exposed Table 1 and eTable 5 in the Supplement report the baseline characteristics of the matched cohorts for the primary outcome major malformations and secondary outcome spontaneous abortion, respectively. Across all matched cohorts, baseline characteristics were well balanced between groups with standardized differences less than 10% (eTable 6 and eTable 7 in the Supplement).
Figure. Flowchart of Study Cohort.
Cohort selection process for the 3-group comparison of oral terbinafine-exposed, topical terbinafine-exposed, and unexposed pregnancies for the risk of major malformations and spontaneous abortion.
Table 1. Baseline Characteristics of Matched Pregnancy Cohorts on Propensity Scores for Analyses of Major Malformations.
| Characteristic | Oral Terbinafine vs Unexposed, No. (%) | Topical Terbinafine vs Unexposed, No. (%) | Oral vs Topical Terbinafine Exposed, No. (%) | |||
|---|---|---|---|---|---|---|
| Oral Terbinafine (n = 522) | Unexposed (n = 5220) | Topical Terbinafine (n = 1476) | Unexposed (n = 14 760) | Oral Terbinafine (n = 510) | Topical Terbinafine (n = 510) | |
| Age at pregnancy onset, y | ||||||
| ≤19 | 7 (1.3) | 104 (2.0) | 23 (1.6) | 273 (1.8) | 7 (1.4) | 5 (1.0) |
| 20-24 | 50 (9.6) | 497 (9.5) | 215 (14.6) | 2188 (14.8) | 50 (9.8) | 54 (10.6) |
| 25-29 | 162 (31.0) | 1591 (30.5) | 506 (34.3) | 5143 (34.8) | 157 (30.8) | 160 (31.4) |
| 30-34 | 201 (38.5) | 2094 (40.1) | 503 (34.1) | 5107 (34.6) | 198 (38.8) | 199 (39.0) |
| ≥35 | 102 (19.5) | 934 (17.9) | 229 (15.5) | 2049 (13.9) | 98 (19.2) | 92 (18.0) |
| Married or living with partner | 445 (85.2) | 4482 (85.9) | 1283 (86.9) | 12 738 (86.3) | 437 (85.7) | 432 (84.7) |
| Place of birth | ||||||
| Denmark | 423 (81.0) | 4285 (82.1) | 1256 (85.1) | 12 766 (86.5) | 417 (81.8) | 424 (83.1) |
| Europe | 32 (6.1) | 246 (4.7) | 76 (5.1) | 704 (4.8) | 31 (6.1) | 29 (5.7) |
| Outside of Europe | 67 (12.8) | 689 (13.2) | 144 (9.8) | 1290 (8.7) | 62 (12.2) | 57 (11.2) |
| Region of residence | ||||||
| Capital Denmark | 149 (28.5) | 1544 (29.6) | 432 (29.3) | 4300 (29.1) | 144 (28.2) | 150 (29.4) |
| Zealand Denmark | 58 (11.1) | 563 (10.8) | 205 (13.9) | 2092 (14.2) | 58 (11.4) | 54 (10.6) |
| Southern Denmark | 109 (20.9) | 1103 (21.1) | 293 (19.9) | 2900 (19.6) | 109 (21.4) | 105 (20.6) |
| Central Denmark | 149 (28.5) | 1488 (28.5) | 387 (26.2) | 3886 (26.3) | 144 (28.2) | 145 (28.4) |
| North Denmark | 57 (10.9) | 522 (10.0) | 159 (10.8) | 1582 (10.7) | 55 (10.8) | 56 (11.0) |
| Gross household income, quartile | ||||||
| 1 | 129 (24.7) | 1236 (23.7) | 405 (27.4) | 4083 (27.7) | 118 (23.1) | 133 (26.1) |
| 2 | 124 (23.8) | 1176 (22.5) | 448 (30.4) | 4352 (29.5) | 113 (22.2) | 107 (21.0) |
| 3 | 134 (25.7) | 1401 (26.8) | 376 (25.5) | 3835 (26.0) | 114 (22.4) | 106 (20.8) |
| 4 | 135 (25.9) | 1407 (27.0) | 247 (16.7) | 2490 (16.9) | 165 (32.4) | 164 (32.2) |
| Educational level, y | ||||||
| <12 | 124 (23.8) | 1266 (24.3) | 383 (25.9) | 3937 (26.7) | 122 (23.9) | 129 (25.3) |
| 12-13 | 93 (17.8) | 956 (18.3) | 232 (15.7) | 2249 (15.2) | 90 (17.6) | 88 (17.3) |
| 14-15 | 132 (25.3) | 1290 (24.7) | 402 (27.2) | 4010 (27.2) | 128 (25.1) | 128 (25.1) |
| >15 | 173 (33.1) | 1708 (32.7) | 459 (31.1) | 4564 (30.9) | 170 (33.3) | 165 (32.4) |
| Year of pregnancy onset | ||||||
| 1997-2000 | 69 (13.2) | 724 (13.9) | 523 (35.4) | 5322 (36.1) | 69 (13.5) | 72 (14.1) |
| 2001-2004 | 104 (19.9) | 1051 (20.1) | 352 (23.8) | 3501 (23.7) | 104 (20.4) | 99 (19.4) |
| 2005-2008 | 134 (25.7) | 1425 (27.3) | 268 (18.2) | 2616 (17.7) | 131 (25.7) | 139 (27.3) |
| 2009-2012 | 122 (23.4) | 1227 (23.5) | 194 (13.1) | 1977 (13.4) | 118 (23.1) | 112 (22.0) |
| 2013-2016 | 93 (17.8) | 793 (15.2) | 139 (9.4) | 1344 (9.1) | 88 (17.3) | 88 (17.3) |
| Parity | ||||||
| 1 | 228 (43.7) | 2341 (44.8) | 699 (47.4) | 7144 (48.4) | 225 (44.1) | 225 (44.1) |
| 2 | 177 (33.9) | 1672 (32.0) | 529 (35.8) | 5289 (35.8) | 174 (34.1) | 181 (35.5) |
| ≥3 | 117 (22.4) | 1207 (23.1) | 248 (16.8) | 2327 (15.8) | 111 (21.8) | 104 (20.4) |
| Multiple-birth pregnancy | 11 (2.1) | 123 (2.4) | 33 (2.2) | 305 (2.1) | 11 (2.2) | 12 (2.4) |
| Smoking during pregnancy | 82 (15.7) | 813 (15.6) | 272 (18.4) | 2612 (17.7) | 80 (15.7) | 83 (16.3) |
| Previous pregnancy with major malformations outcome | 13 (2.5) | 121 (2.3) | 16 (1.1) | 143 (1.0) | 10 (2.0) | 10 (2.0) |
| Antidiabetic drugs used in past year | 5 (1.0) | 29 (0.6) | 21 (1.4) | 161 (1.1) | 5 (1.0) | 6 (1.2) |
| Drugs used for IVF in past 3 mo | 16 (3.1) | 176 (3.4) | 87 (5.9) | 781 (5.3) | 16 (3.1) | 15 (2.9) |
| No. of drugs used in past year | ||||||
| 1-2 | 170 (32.6) | 1733 (33.2) | 487 (33.0) | 4876 (33.0) | 159 (31.2) | 170 (33.3) |
| 3-4 | 133 (25.5) | 1250 (23.9) | 400 (27.1) | 4167 (28.2) | 135 (26.5) | 134 (26.3) |
| ≥5 | 173 (33.1) | 1780 (34.1) | 381 (25.8) | 3653 (24.7) | 174 (34.1) | 176 (34.5) |
| No. of hospitalizations in past year | ||||||
| 1 | 74 (14.2) | 634 (12.1) | 226 (15.3) | 2046 (13.9) | 74 (14.5) | 65 (12.7) |
| 2 | 9 (1.7) | 95 (1.8) | 27 (1.8) | 199 (1.3) | 9 (1.8) | 11 (2.2) |
| ≥3 | 5 (1.0) | 35 (0.7) | 15 (1.0) | 129 (0.9) | 5 (1.0) | 5 (1.0) |
| No. of outpatient contacts in past year | ||||||
| 1 | 82 (15.7) | 704 (13.5) | 207 (14.0) | 2100 (14.2) | 79 (15.5) | 73 (14.3) |
| 2 | 27 (5.2) | 258 (4.9) | 84 (5.7) | 681 (4.6) | 27 (5.3) | 21 (4.1) |
| ≥3 | 11 (2.1) | 96 (1.8) | 36 (2.4) | 284 (1.9) | 11 (2.2) | 12 (2.4) |
Abbreviation: IVF, in vitro fertilization.
Major Malformations
Table 2 reports the matched analyses of the primary outcome major malformations for the 3 comparative study cohorts. For the comparison of pregnancies exposed to oral terbinafine vs unexposed, infants diagnosed with a major malformation occurred in 20 pregnancies (3.8%) exposed to oral terbinafine compared with 198 unexposed pregnancies (3.8%) (prevalence OR, 1.01; 95% CI, 0.63-1.62), corresponding to an absolute risk difference (ARD) of 0.04% (95% CI, −1.69% to 1.76%). For the topical terbinafine-exposed vs unexposed pregnancies comparison, a major malformation occurred in 53 pregnancies (3.6%) exposed to topical terbinafine compared with 419 unexposed pregnancies (3.3%) (prevalence OR, 1.08; 95% CI, 0.81-1.44), with an ARD of 0.26% (95% CI, −0.73% to 1.26%). In the comparison of oral terbinafine- vs topical terbinafine-exposed pregnancies, a major malformation occurred in 20 pregnancies (3.9%) exposed to oral terbinafine vs 17 pregnancies (3.3%) exposed to topical terbinafine (prevalence OR, 1.18; 95% CI, 0.61-2.29), with an ARD of 0.59% (95% CI, −1.71% to 2.88%).
Table 2. Association Between Oral and Topical Terbinafine Use in Pregnancy and Risk of the Primary Outcome Major Malformations in Matched Cohorts on Propensity Scores.
| Major Malformations | Total No. of Pregnancies | Malformations, No. (%) | Prevalence OR (95% CI) |
|---|---|---|---|
| Oral terbinafine exposed vs unexposed matched pregnancies | |||
| Oral terbinafine | 522 | 20 (3.8) | 1.01 (0.63-1.62) |
| Unexposed | 5220 | 198 (3.8) | 1 [Reference] |
| Topical terbinafine exposed vs unexposed matched pregnancies | |||
| Topical terbinafine | 1476 | 53 (3.6) | 1.08 (0.81-1.44) |
| Unexposed | 14 760 | 491 (3.3) | 1 [Reference] |
| Oral vs topical terbinafine-exposed matched pregnancies | |||
| Oral terbinafine | 510 | 20 (3.9) | 1.18 (0.61-2.29) |
| Topical terbinafine | 510 | 17 (3.3) | 1 [Reference] |
Abbreviation: OR, odds ratio.
Spontaneous Abortion
Table 3 reports the matched analyses of the secondary outcome spontaneous abortion for the 3 comparative study cohorts. The proportional hazards assumption was fulfilled for all analyses on spontaneous abortion. For the comparison of pregnancies exposed to oral terbinafine vs unexposed (median index date of gestational day 11; interquartile range [IQR], −2 to 28), spontaneous abortion occurred in 93 pregnancies (10.4%) exposed to oral terbinafine compared with 918 unexposed pregnancies (10.3%) (HR, 1.06; 95% CI, 0.86-1.32), corresponding to an ARD of 0.13% (95% CI, −1.97% to 2.24%). For the topical terbinafine-exposed vs unexposed pregnancies comparison (median index date, day 66; IQR, 31-111), spontaneous abortion occurred in 166 pregnancies (5.2%) exposed to topical terbinafine compared with 1607 unexposed pregnancies (5.1%) (HR, 1.04; 95% CI, 0.88-1.21), with an ARD of 0.17% (95% CI, −0.64% to 0.98%). In the comparison of oral terbinafine- vs topical terbinafine-exposed pregnancies (median index date, oral: day 20; IQR, 8-36; topical, day 20; IQR, 7-37), spontaneous abortion occurred in 66 pregnancies with oral terbinafine exposure (10.7%) vs 59 pregnancies (9.6%) with topical exposure (HR, 1.19; 95% CI, 0.84-1.70), with an ARD of 1.13% (95% CI −2.23% to 4.50%).
Table 3. Association Between Oral and Topical Terbinafine Use in Pregnancy and Risk of the Secondary Outcome Spontaneous Abortions in Matched Cohorts on Propensity Scores.
| Spontaneous Abortion | Total No. of Pregnancies | Spontaneous Abortion, No. (%) | Hazard Ratio (95% CI) |
|---|---|---|---|
| Oral terbinafine-exposed vs unexposed matched pregnancies | |||
| Oral terbinafine | 891 | 93 (10.4) | 1.06 (0.86-1.32) |
| Unexposed | 8910 | 918 (10.3) | 1 [Reference] |
| Topical terbinafine-exposed vs unexposed matched pregnancies | |||
| Topical terbinafine | 3174 | 166 (5.2) | 1.04 (0.88-1.21) |
| Unexposed | 31 740 | 1607 (5.1) | 1 [Reference] |
| Oral vs topical terbinafine-exposed matched pregnancies | |||
| Oral terbinafine | 617 | 66 (10.7) | 1.19 (0.84-1.70) |
| Topical terbinafine | 617 | 59 (9.6) | 1 [Reference] |
Sensitivity Analyses
Table 4 reports the results of the sensitivity analyses of the association between oral terbinafine exposure in pregnancy and risk of major malformations. No statistically significant associations were identified in any of these sensitivity analyses. Sensitivity analyses of the association between oral terbinafine use and risk of spontaneous abortion did not provide any statistically significant results (eTable 8 in the Supplement).
Table 4. Sensitivity Analyses of the Association Between Oral Terbinafine Use in Pregnancy and Risk of Major Malformations.
| Sensitivity Analyses | Oral Terbinafine Exposed | Comparison Group | Prevalence OR (95% CI) | ||
|---|---|---|---|---|---|
| Total No. of Pregnancies | Malformations, No. (%) | Total No. of Pregnancies | Malformations, No. (%) | ||
| Compared with unexposed pregnancies with recent oral terbinafine usea | 491 | 18 (3.7) | 971 | 31 (3.2) | 1.15 (0.64-2.08) |
| Oral terbinafine-exposed pregnancies compared with unexposed pregnancies | |||||
| Filled prescription in gestational weeks 4-10 | 130 | 5 (3.8) | 5220 | 198 (3.8) | 1.02 (0.41-2.51) |
| Exposure anytime during entire pregnancy | 613 | 23 (3.8) | 6130 | 227 (3.7) | 1.01 (0.66-1.57) |
| Exposure in 2nd and 3rd trimester only | 91 | 3 (3.3) | 6130 | 227 (3.7) | 0.89 (0.28-2.82) |
| Accumulative terbinafine dose >7000 mgb | 220 | 11 (5.0) | 5220 | 198 (3.8) | 1.08 (0.55-2.14) |
| Singleton pregnancies only | 511 | 19 (3.7) | 5097 | 193 (3.8) | 0.98 (0.61-1.59) |
| Filled prescription in gestational day 0-84 | 363 | 13 (3.6) | 5220 | 198 (3.8) | 0.94 (0.53-1.67) |
Abbreviation: OR, odds ratio.
Oral terbinafine-exposed pregnancies compared to pregnancies with oral terbinafine use in the last year prior to pregnancy only, matched in 1:2 ratio.
The median of the accumulative dose of oral terbinafine within the first trimester.
Discussion
In this nationwide cohort study, we found no statistically significant differences in the risk of major malformations or spontaneous abortion comparing oral terbinafine-exposed, topical terbinafine-exposed, and unexposed pregnancies in propensity score–matched analyses.
Previous data have not allowed for a proper assessment of the fetal risk of terbinafine use among pregnant women. Consequently, use of systemic terbinafine during pregnancy is currently not advised, and the US Food and Drug Administration recommends that terbinafine therapy should not be initiated during pregnancy.8,15,26 Among the 54 pregnant women exposed to terbinafine enrolled in 1 study, 1 major malformation and 3 spontaneous abortions were observed.11 Although no reports of statistical testing were presented in the published abstract, the authors concluded that the preliminary results were suggestive of no increased fetal risk. As such, the results of our study support this notion as well as the preclinical safety data, while expanding the body of available epidemiologic fetal safety data.
Oral terbinafine exposure in pregnancy was not associated with an increased risk of major malformations compared with unexposed pregnancies as well as compared with pregnancies exposed to topical terbinafine. Several sensitivity analyses were carried out, none of which provided evidence of a significant association. Given the relative rarity of the individual malformations, the power to address the risk of specific malformations in cohort studies is limited.21,27 Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation.
According to current guidelines, topical terbinafine can be safely used during pregnancy because of limited systemic absorption.1,28 We are not aware of any previous studies investigating the specific fetal risk of topical terbinafine use in pregnant women. Thus, to our knowledge, this study provides the first data from real-world routine clinical practice and reports no increased risk of major malformation and spontaneous abortion with topical terbinafine use in pregnancy compared with unexposed pregnancies. Although this study may help inform clinicians, patients, and drug regulatory authorities regarding the fetal safety of terbinafine use in pregnancy when clinically indicated, the results should be confirmed in other populations.
Strengths and Limitations
The study has several strengths and limitations. The data were derived from a cohort of all pregnancies in Denmark from 1997 through 2016 and included all filled prescriptions for terbinafine in this period, making the results generalizable to similar populations. The nationwide registries facilitated an independent and detailed assessment of exposures, outcomes, and covariates throughout the study period. The registrations of major malformations and spontaneous abortions in the National Patient Registry have high validity with positive predictive values of 88% and 97%, respectively.29,30 Use of oral and topical terbinafine was based on filled prescriptions. If the pregnant women did not adhere to the dispensed drug, the association with the outcome of the exposed pregnancies would be biased toward the unexposed pregnancies. In addition, although oral terbinafine is a prescription-only drug, topical terbinafine is available as an over-the-counter preparation (29.2% of the supply was personally identifiable during the study period31); this availability could also introduce misclassification of exposure, which would bias the results toward the association with topical terbinafine exposure. To minimize the risk of this potential misclassification, we excluded pregnancies with use of any antifungal preparations in pregnancy as well as prior to pregnancy onset.
To increase the probability of isolating any differential associations to the outcomes, we used propensity score matching to take into account a wide range of characteristics that might have influenced the associations. Covariates were well balanced across all matched cohorts. However, residual confounding cannot be fully excluded, particularly to the extent that potential confounding factors not adjusted for (eg, other drug use or body mass index) were differently distributed between matched groups and not accounted for through adjustment for correlated variables (ie, proxies) included in the propensity score. Given the propensity-matched comparison design, results from the 3 study cohorts should primarily be interpreted separately. A main concern of observational cohort studies reporting null findings is inherited unadjusted factors that could mask a true association. Given our large study size, including all pregnancies in Denmark, and that such factors would need to be common or strongly associated with the exposure and inversely associated with the outcome, we expect such residual confounding to be limited or unlikely.
Another potential confounding issue would be if the indications for treatment with terbinafine were associated with the outcomes. The analyses of oral vs topical terbinafine-exposed pregnancies did not support this notion. Still, confounding by severity of the fungal infections cannot be ruled out, since oral terbinafine use may likely be associated with more-pronounced infections. Information on the indication for a prescribed drug is unattainable in the Danish registries. Under the assumption that the fungal infections were present also during the first trimester, and thus potentially teratogenic in relation to the risk of congenital malformations, the sensitivity analysis of pregnancies exposed to oral terbinafine later in pregnancy was not suggestive of this type of confounding.
Conclusions
This nationwide cohort study found no increased risk of major malformations or spontaneous abortion among pregnancies exposed to oral or topical terbinafine. These results suggest that terbinafine can be used relatively safely in both oral and topical formulations during pregnancy.
eMethods. Definition of the Major Malformations
eTable 1. Definition of Covariates Included in Propensity Score With Definitions and Data Sources
eTable 2. Number of Missing Values (Percentages) for the Unmatched Study Cohorts
eTable 3. Baseline Characteristics of Unmatched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies for Analyses of the Primary Outcome
eTable 4. Baseline Characteristics of Unmatched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies for Analyses of the Secondary Outcome
eTable 5. Baseline Characteristics of Matched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies on Propensity Scores for Analyses of the Secondary Outcome
eTable 6. Standardized Differences Comparison of Oral and Topical Terbinafine-Exposed and Unexposed Pregnancies Before Propensity Score Matching
eTable 7. Standardized Differences for Comparison of Oral and Topical Terbinafine-Exposed and Unexposed Pregnancies After Propensity Score Matching
eTable 8. Sensitivity Analyses for the Association Between Oral Terbinafine Use and Spontaneous Abortion as Compared With Unexposed Pregnancies Where Restricting According to Different Exposure Time Window Definitions
eReferences
References
- 1.Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother. 2015;70(1):14-22. doi: 10.1093/jac/dku355 [DOI] [PubMed] [Google Scholar]
- 2.Fuller LC, Barton RC, Mohd Mustapa MF, Proudfoot LE, Punjabi SP, Higgins EM. British Association of Dermatologists’ guidelines for the management of tinea capitis 2014. Br J Dermatol. 2014;171(3):454-463. doi: 10.1111/bjd.13196 [DOI] [PubMed] [Google Scholar]
- 3.Kauffman CA, Bustamante B, Chapman SW, Pappas PG; Infectious Diseases Society of America . Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(10):1255-1265. doi: 10.1086/522765 [DOI] [PubMed] [Google Scholar]
- 4.Ryder NS. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 1992;126(suppl 39):2-7. doi: 10.1111/j.1365-2133.1992.tb00001.x [DOI] [PubMed] [Google Scholar]
- 5.Debruyne D, Coquerel A. Pharmacokinetics of antifungal agents in onychomycoses. Clin Pharmacokinet. 2001;40(6):441-472. doi: 10.2165/00003088-200140060-00005 [DOI] [PubMed] [Google Scholar]
- 6.McClellan KJ, Wiseman LR, Markham A. Terbinafine: an update of its use in superficial mycoses. Drugs. 1999;58(1):179-202. doi: 10.2165/00003495-199958010-00018 [DOI] [PubMed] [Google Scholar]
- 7.Patel VM, Schwartz RA, Lambert WC. Topical antiviral and antifungal medications in pregnancy: a review of safety profiles. J Eur Acad Dermatol Venereol. 2017;31(9):1440-1446. doi: 10.1111/jdv.14297 [DOI] [PubMed] [Google Scholar]
- 8.Sobel JD. Use of antifungal drugs in pregnancy: a focus on safety. Drug Saf. 2000;23(1):77-85. doi: 10.2165/00002018-200023010-00005 [DOI] [PubMed] [Google Scholar]
- 9.Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80(4):853-867. doi: 10.1016/j.jaad.2018.05.1260 [DOI] [PubMed] [Google Scholar]
- 10.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 11.Sarkar MS, Rowland K, Koren G Pregnancy outcome following gestational exposure to terbinafine: a prospective comparative study [abstract]. https://onlinelibrary.wiley.com/doi/pdf/10.1002/bdrb.10026. Accessed August 14, 2019.
- 12.Birnbaum JE. Pharmacology of the allylamines. J Am Acad Dermatol. 1990;23(4, pt 2):782-785. doi: 10.1016/0190-9622(90)70288-S [DOI] [PubMed] [Google Scholar]
- 13.Schaefer C, Peters PW, Miller RK. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. Cambridge, MA: Academic Press; 2014. [Google Scholar]
- 14.Kyle AA, Dahl MV. Topical therapy for fungal infections. Am J Clin Dermatol. 2004;5(6):443-451. doi: 10.2165/00128071-200405060-00009 [DOI] [PubMed] [Google Scholar]
- 15.Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I—pregnancy. J Am Acad Dermatol. 2014;70(3):401.e1-401.e14. doi: 10.1016/j.jaad.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 16.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320-323. [PubMed] [Google Scholar]
- 17.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 suppl):30-33. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22-25. [DOI] [PubMed] [Google Scholar]
- 19.Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 suppl):38-41. [DOI] [PubMed] [Google Scholar]
- 20.European Surveillance of Congenital Anomalies. Eurocat guide 1.4 and reference documents. https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf. Updated December 28, 2018. Accessed May 16, 2019.
- 21.Huybrechts KF, Bateman BT, Hernández-Díaz S. Use of real-world evidence from healthcare utilization data to evaluate drug safety during pregnancy. Pharmacoepidemiol Drug Saf. 2019;28(7):906-922. doi: 10.1002/pds.4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohse SR, Farkas DK, Lohse N, et al. Validation of spontaneous abortion diagnoses in the Danish National Registry of Patients. Clin Epidemiol. 2010;2:247-250. doi: 10.2147/CLEP.S13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604-611. doi: 10.1161/CIRCOUTCOMES.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171-184. doi: 10.1002/bimj.200810488 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. doi: 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamisil [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020539s029,022071s013lbl.pdf. Accessed October 21, 2019.
- 27.Mitchell AA. Studies of drug-induced birth defects In: Strom BL, Kimmel SE, Hennessy S, eds. Pharmacoepidemiology. Fifth Edition. Indianapolis: John Wiley & Sons, Ltd; 2012:487-504. doi: 10.1002/9781119959946.ch28 [DOI] [Google Scholar]
- 28.Tyler KH. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58(1):112-118. doi: 10.1097/GRF.0000000000000089 [DOI] [PubMed] [Google Scholar]
- 29.Larsen H, Nielsen GL, Bendsen J, Flint C, Olsen J, Sørensen HT. Predictive value and completeness of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12-16. doi: 10.1080/14034940210134194 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Danish Health Data Authority The Register of Medicinal Product Statistics. medstat.dk. Accessed January 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Definition of the Major Malformations
eTable 1. Definition of Covariates Included in Propensity Score With Definitions and Data Sources
eTable 2. Number of Missing Values (Percentages) for the Unmatched Study Cohorts
eTable 3. Baseline Characteristics of Unmatched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies for Analyses of the Primary Outcome
eTable 4. Baseline Characteristics of Unmatched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies for Analyses of the Secondary Outcome
eTable 5. Baseline Characteristics of Matched Cohorts of Oral and Topical Terbinafine Exposed and Unexposed Pregnancies on Propensity Scores for Analyses of the Secondary Outcome
eTable 6. Standardized Differences Comparison of Oral and Topical Terbinafine-Exposed and Unexposed Pregnancies Before Propensity Score Matching
eTable 7. Standardized Differences for Comparison of Oral and Topical Terbinafine-Exposed and Unexposed Pregnancies After Propensity Score Matching
eTable 8. Sensitivity Analyses for the Association Between Oral Terbinafine Use and Spontaneous Abortion as Compared With Unexposed Pregnancies Where Restricting According to Different Exposure Time Window Definitions
eReferences