Abstract
Background
Limited data exist on the use of bedaquiline and delamanid in adolescents with rifampicin-resistant tuberculosis (RR-TB). We describe RR-TB treatment of adolescents (10–19 years) with injectable-free regimens containing these drugs in Khayelitsha, South Africa.
Methods
This retrospective study included adolescents initiating injectable-free RR-TB treatment regimens containing bedaquiline and/or delamanid from February 2015 to June 2018. We report adverse events (AEs) of interest, sputum culture conversion (SCC), and final end-of-treatment outcomes.
Findings
Twenty-two patients were included; median age at treatment initiation was 17 years (interquartile range [IQR] 15-18), and six (27%) were HIV-positive (median CD4 count 191 cells/mm3 [IQR 157-204]). Eight (36%) patients had RR-TB with fluoroquinolone resistance; ten (45%), eight (36%), and four (18%) patients received regimens containing bedaquiline, delamanid, or the combination of bedaquiline and delamanid, respectively. The median durations of exposure to bedaquiline and delamanid were 5·6 (IQR 5·5-8·4) and 9·4 (IQR 5·9-14·4) months, respectively. There were 49 AEs of interest which occurred in 17 (77%) patients. Fourteen (64%) patients had pulmonary TB with positive sputum cultures at bedaquiline and/or delamanid initiation; among these SCC at month 6 was 79%. Final end-of-treatment outcomes for the 22 adolescent were: 17 (77%) successfully treated, two (9%) lost-to-follow-up, two (9%) treatment failed, and one (5%) died.
Interpretation
This study found that injectable-free regimens containing bedaquiline and/or delamanid in a programmatic setting were effective and well tolerated in adolescents and should be routinely provided for RR-TB treatment in this age group as recommended by the World Health Organisation.
Panel: Research in Context.
Evidence before this study
We searched: PubMED, Google Scholar, OVID, and the Cochrane Database of Systematic Reviews and trial registries (clinicaltrials.gov and the South African National Clinical Trials Register) for studies published before May 10, 2019 to identify studies where bedaquiline and/or delamanid were given to adolescents (defined as persons 10–19 years of age) for treatment of rifampicin-resistant tuberculosis (RR-TB); abstracts of the International Union Against Tuberculosis and Lung Disease's Union World Conference on Lung Health from 2015 to 2018, the Conference on Retroviruses and Opportunistic Infections from 2016–2018, and the International Conference on Antimicrobial Agents and Chemotherapy from 2015 to 2019; and evidence from unpublished studies from January 1, 2013 through May 10, 2019, on short-term safety and dosing of delamanid and bedaquiline in children ages 3 years and above (delamanid) and 6 years and above (bedaquiline).
Some cohort studies on bedaquiline in adults included a small number of adolescents aged 12 years and above but detailed outcomes and safety in this population specifically were not assessed; additionally a 2016 retrospective review of 16 older children and adolescents with RR-TB who received delamanid through Otsuka's compassionate use programme was published as a letter to the editor and reported early safety and effectiveness but only culture status was assessed at the time of the analysis. Multiple clinical trials were identified assessing dosing, efficacy and safety of bedaquiline in children and adolescents (Janssen C211, NCT02354041; P1108, NCT 02906007; EndTB, NCT02754765) and delamanid in children and adolescents (Otsuka 232, NCT01856634; Otsuka 233, NCT01859223; IMPAACT 2005, NCT03141060; EndTB, NCT02754765). The endTB trial (multiple arms containing bedaquiline and/or delamanid) includes patients aged 15 years and older, and IMPAACT 2020 assessing injectable-free therapy in adolescents aged 10–19 years is being planned. Finally, a systematic review and individual patient data meta-analysis of 975 children and adolescents treated for RR-TB did not include any patients who received bedaquiline or delamanid.
Added value of this study
Our data from this programmatic setting in Khayelitsha, South Africa—a major epidemic hotspot for RR-TB and HIV and one with a growing adolescent population—found that end-of-treatment effectiveness and safety outcomes were excellent among this population treated with injectable-free regimens containing bedaquiline and/or delamanid for RR-TB, including those living with HIV. There were few instances of Grade 3 and 4 adverse events of interest during bedaquiline and/or delamanid exposure and over three fourths of adolescents were successfully treated. Additionally, there were no cases of cardiotoxicity or QTcF prolongation >500 ms.
Implications of all the available evidence
Although the new World Health Organization recommendations stress the importance of injectable-free regimens for the treatment of RR-TB and advise that injectables should be avoided in paediatric populations, many countries are only providing bedaquiline and delamanid to people over the age of 18 years, thus excluding vulnerable adolescents. Our study has shown that denying access to these medications to persons under the age of 18 is no longer justified. Furthermore, because most countries are rolling out injectable-free regimens in a phased approach or “pilot” fashion, our data support prioritizing adolescents, including those living with HIV, to receive injectable-free regimens for RR-TB.
Alt-text: Unlabelled box
1. Introduction
Adolescents are a vulnerable population when it comes to rifampicin-resistant tuberculosis (RR-TB), a form of TB that was estimated to cost the global economy 3 billion United States Dollars in 2018 and which will be responsible for one-third of deaths from antimicrobial resistance if radical action is not taken [1], [2], [3]. Studies have shown that adolescents may have worse RR-TB outcomes compared with both younger and older populations, especially if they are co-infected with HIV [4]. Given that adolescents are experiencing significant transitional periods in their lives [5], they may also be more likely to suffer from the impact of the serious and well-described adverse events (AEs) associated with commonly-used second-line anti-tuberculosis medications, especially the permanent hearing loss associated with injectable-agents [6].
The World Health Organization (WHO) has now recommended all-oral treatment regimens for the majority of people with RR-TB; however, these guidelines reveal the limited experience of all-oral regimens in adolescents [7]. Many countries have restricted the use of newer medications including bedaquiline and delamanid to people over the age of 18 years. While there is growing experience using these drugs in older children and adolescents, most published data on bedaquiline and delamanid in this population provide only interim efficacy and safety outcomes, and pragmatic data that describe safety, effectiveness, and outcomes at the end-of-treatment are urgently needed. A retrospective multi-site cohort review of 27 adolescents receiving bedaquiline found the drug was safe, and 23 participants had negative sputum cultures at the time of analysis. No end-of-treatment outcomes were reported, however, and none of the adolescents had HIV [8]. In a multi-site retrospective record review of 16 children and adolescents who received delamanid under compassionate use for the treatment of RR-TB (all but two children had fluoroquinolone-resistant strains), 81% had negative sputum cultures at the time of the analysis, but only six children had completed 24 weeks of treatment with delamanid. Excellent safety parameters were also reported, but only three of the adolescents were living with HIV [9]. A large retrospective review of persons receiving bedaquiline for the treatment of RR-TB in South Africa—which demonstrated both significantly improved treatment outcomes and lower mortality among persons who received bedaquiline compared with those who did not—included 39 adolescents 15–18 years of age but no detailed exploration of the adolescent population was presented [10]. A systematic review and individual patient data meta-analysis of 975 children and adolescents treated for RR-TB did not include any patients who received bedaquiline or delamanid [11].
Robust data on effectiveness and safety at the end-of-treatment among adolescents who received injectable-free regimens containing bedaquiline, delamanid or both could support the WHO recommendation that “the avoidance of an injectable-containing regimen is particularly desirable in children” [12]. recognizing that adolescents with RR-TB may have worse treatment outcomes and suffer disproportionately from the serious AEs associated with injectable medications, Médecins Sans Frontières (MSF) partnered with the Province of the Western Cape and the City of Cape Town to provide injectable-free regimens containing bedaquiline, delamanid, or both, for all adolescents diagnosed with RR-TB in the peri-urban township of Khayelitsha, South Africa. The objective of this retrospective analysis was to describe early and end-of-treatment safety and effectiveness of injectable-free regimens containing bedaquiline and/or delamanid for the treatment of RR-TB in this population.
2. Methods
2.1. Study design and population
This was a retrospective cohort study of all consecutive adolescents aged 10–19 years, initiating injectable-free RR-TB treatment regimens containing bedaquiline and/or delamanid from February 2015 to June 2018 in Khayelitsha, South Africa.
2.2. Inclusion criteria and eligibility for bedaquiline and/or delamanid
Adolescents were included if they received at least 1 week of bedaquiline and/or delamanid for the treatment of RR-TB. Bedaquiline and/or delamanid were offered to adolescents aged 10–19 years within an anti-tuberculosis regimen containing at least four other effective drugs for the treatment of RR-TB. Some of these patients had exposure to injectable agents prior to the initiation of bedaquiline and/or delamanid. Patients were eligible to receive regimens containing both bedaquiline and delamanid if they met the following indications: (1) limited options to design a regimen with at least five effective drugs; (2) floroquinolone resistant; and (3) patients whose RR-TB treatment previously failed. Patients were eligible to receive bedaquiline or delamanid if they had drug intolerance requiring substitution of specific second-line drugs in the treatment regimen. Patients were prioritized for treatment with delamanid if they were on fixed dose combination anti-retroviral therapy and/or for the adolescents less than 15 years of age.
2.3. Procedures
Adolescents with RR-TB were managed free of charge in primary and secondary public healthcare facilities within the decentralised RR-TB programme in Khayelitsha [13]. Some children and adolescents in this setting are treated for RR-TB in the absence of bacteriological confirmation if they have clinically diagnosed RR-TB. MSF began supporting the roll-out of bedaquiline and delamanid for adolescents in Khayelitsha in 2015. Bedaquiline was available on a case-by-case basis for children and adolescents <18 years of age through the expanded National Bedaquiline Clinical Access Programme [14] and delamanid was available for adolescents through MSF and later through the national Delamanid Clinical Access Programme.
The dose of bedaquiline was 400 mg daily for 2-weeks, followed by 200 mg three times a week for those >30 kg; 100 mg of delamanid was administered twice daily, with the exception of the 10 year old patient who received 50 mg twice daily. Bedaquiline and delamanid were administered for a period of 6-months but could be extended beyond 6-months if there was a clinical indication. Monitoring of patients on bedaquiline and/or delamanid for safety occurred monthly; this included clinical assessments and serial laboratory tests, including albumin, haemoglobin, electrolyte measurements, and renal and liver function tests.
Electrocardiogram monitoring was carried out monthly for patients receiving either bedaquiline or delamanid, in addition to other potentially QT prolonging drugs such as moxifloxacin and/or clofazimine. Patients receiving both bedaquiline and delamanid had twice monthly electrocardiograms for the first 8 weeks then monthly thereafter. QTc intervals were corrected for heart rate using the Fridericia formula (QTcF) [15]. If the QTcF interval was prolonged (i.e. QTcF >450 ms), the electrocardiogram was repeated and treatment decisions were based on the average QTcF measurement. To eliminate potential calculation errors, the study team independently and retrospectively calculated the QTcF intervals using the QT and heart rate values from the electrocardiograms obtained from clinical files. In cases of clinically significant cardiotoxicity, e.g. QTcF prolongation > 500 ms, patients were managed according to WHO guidelines [16], local provincial guidance, and clinician discretion.
The National Health Laboratory Service (NHLS) conducted the monthly bacteriological monitoring (smear microscopy, and TB culture liquid media) as per the local guidelines [17]. When cultures were positive for Mycobacterium tuberculosis, additional drug susceptibility testing (DST) was conducted for first- and second-line anti-tuberculosis medications at diagnosis or upon request.
2.4. Outcomes
Safety was measured by the occurrence of treatment-emergent AEs of interest; these were limited to laboratory-monitored parameters (increased alanine aminotransferase, decreased white blood cell count, anaemia, and thrombocytopaenia), and clinically assessed QTcF prolongation. These measures were graded according to the EndTB Severity Grading Scale [18] and the NHLS normal reference ranges for adolescents based on age and sex were used to determine the lower and upper limits of normal for the investigated laboratory parameters [19].
Effectiveness was assessed using sputum culture conversion (SCC) and final treatment outcomes. SCC was measured 6-months after initiation of bedaquiline and/or delamanid. SCC was defined as two consecutive negative cultures taken at least 30 days apart in a patient with a positive specimen at bedaquiline and/or delamanid initiation [20]. Sputum reversion to positive was defined as two consecutive positive cultures at least 30 days apart occurring after SCC. Standard WHO definitions were used for RR-TB treatment outcomes [20]; favourable and unfavourable outcomes were defined as cure and treatment completion and loss to follow-up (LTFU), death, and treatment failure, respectively.
2.5. Data sources and statistical analysis
Data were obtained from the South African Electronic RR-TB Register (EDR.web), paper RR-TB registers, patients’ medical records (including electrocardiogram readings), and the National Health Laboratory Service Database (TrakCare). Data entered in EDR.web were validated using information in patient medical records and RR-TB registers. These systems are used for prospective, routine programmatic monitoring.
Clinical and demographic characteristics were described using proportions for categorical variables and using medians and interquartile range (IQR) for continuous variables. Chi square or Fisher tests and Wilcoxon rank-sum tests were used to determine differences in clinical and demographic characteristics based on HIV-status. Factors were considered statistically significant if there was a two-tailed P-value of <0·05. Box plots were used to show the distribution of the QTcF values from baseline to month 6 (median, interquartile range, and range). Time to culture conversion was displayed using a Kaplan Meier curve and a life table was used to report on culture status up until month 6. Stata version 14·1 (College Station, Texas, USA) package was utilised for statistical analysis.
2.6. Ethics
Ethical approval was obtained from the University of Cape Town Human Research Ethics Committee (HREC 499/2011) for this study. Additionally this research fulfilled the exemption criteria set by the MSF Ethics Review Board for a posteriori analyses of routinely collected clinical data.
2.7. Role of the funding source
The funder had no role in this study design, data collection, data analysis, data interpretation, or the manuscript writing. The corresponding author had full access to all the data included in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Patient and treatment characteristics
From February 2015 to June 2018, 22 adolescents were initiated on injectable-free regimens containing bedaquiline and/or delamanid. Clinical and demographic characteristics of this cohort, stratified by HIV status, are described in Table 1; there were no significant differences based on HIV status.
Table 1.
Clinical and demographic characteristics of adolescents who initiated injectable-free regimens containing bedaquiline and/or delamanid between February 2015 and June 2018 in Khayelitsha, South Africa.
| Total Patients N (%) or Median (IQR) |
HIV-Positive N (%) or Median (IQR) |
HIV-Negative N (%) or Median (IQR) |
P-value | |
|---|---|---|---|---|
| Number of Patients | 22 (100·0) | 6 (100·0) | 16 (100·0) | ·· |
| Age at treatment initiation, (y) | 17·0 (15·0–18·0) | 16·5 (15·0–18·0) | 17·0 (14·5–19·0) | 0·68 |
|
Sex Male |
13 (59·1) |
2 (33·3) |
11 (68·7) |
0·16 |
| Weight (kg) | 49·5 (40·0–56·0) | 45·0 (39·0–51·0) | 50·5 (44·5–58·0) | 0·40 |
|
Body mass index (kg/m2) (n = 19) |
18·8 (17·0–20·6) * | 19·3 (18·5–20·1) | 18·7 (16·6–20·6) * | 0·38 |
| CD4 count at treatment initiation (cells/mm3) | ·· | 190.5 (157·0–204·0) | ·· | ·· |
| On anti-retroviral therapy at Bedaquiline and/or Delamanid Initiation | ·· | 4 (66·7) | ·· | ·· |
| Median weeks to anti-retroviral therapy following Bedaquiline and/or Delamanid Initiation (n = 2) | ·· | 5·8 (2·1–9·4) | ·· | ·· |
|
NNRTI/PI used in anti-retroviral Therapy Efavirenz Lopinavir/Ritonavir Nevirapine |
·· |
2 (33·3) 1 (16·7) 3 (50·0) |
·· | ·· |
|
RR-TB disease classification Clinically diagnosed RR-TB GeneXpert rifampicin-resistance without further confirmation Rifampicin-mono resistant TB Multi-drug resistant TB RR-TB with fluoroquinolone resistance |
2 (9·1) 1 (4·5) 3 (13·6) 8 (36·4) 8 (36·4) |
0 (0·0) 0 (0·0) 0 (0·0) 4 (66·7) 2 (33·3) |
2 (12·5) 1 (6·3) 3 (18·7) 4 (25·0) 6 (37·5) |
0·55 |
|
Previous TB treatment history None First-line TB treatment Second-line TB treatment |
15 (68·2) 4 (18·2) 3 (13·6) |
4 (66·7) 1 (16·7) 1 (16·7) |
11 (68·7) 3 (18·8) 2 (12·5) |
1·0 |
|
Disease Site Pulmonary TB Extra-pulmonary TB |
20 (90·9) 2 (9·1)* |
6 (100·0) 0 (0·0) |
14 (87·5) 2 (12·5)* |
1·0 |
|
Culture Status at Bedaquiline and/or Delamanid Initiation Negative Positive Not Done |
5 (25·0) 15 (68·2) 2 (9·1) |
0 (0·0) 6 (100·0) 0 (0·0) |
5 (31·3) 9 (56·2) 2 (12·5) |
0·23 |
|
Median QTcF interval at Bedaquiline and/or Delamanid Initiation (ms) (n = 20) |
399·0 (388·0–416·0)+ | 399·0 (390·0–413·0) | 403·5 (386·0–423·0)+ | 0·74 |
Abbreviations: IQR, Interquartile range; RR-TB, Rifampicin-resistant Tuberculosis; TB, Tuberculosis; QTcF, QT corrected using the Frederica formula. *n = 1 Extra-pulmonary TB of the spine (bone tissue), n = 1 Extra-pulmonary TB of the lymph nodes. *n = 3 missing baseline body mass index. +n = 2 missing baseline QTcF. The comparisons underlying the p-values presented in this table relate to the HIV status of the included participants.
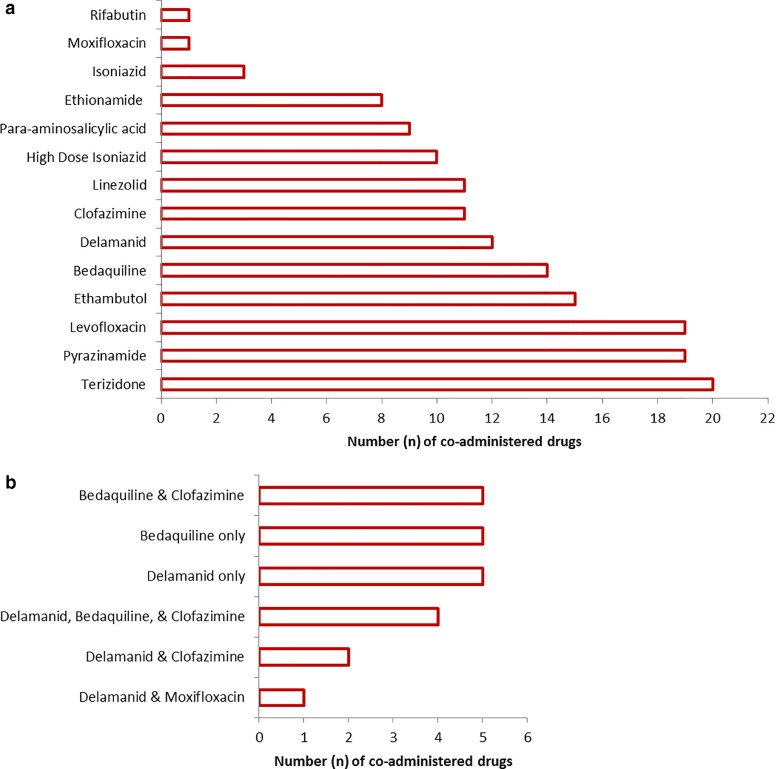
Overall, ten (45%) patients received regimens containing bedaquiline without delamanid and eight (36%) received regimens containing delamanid without bedaquiline. Four (18%) patients received bedaquiline and delamanid together (RR-TB treatment was failing in one patient and the other three had RR-TB with fluoroquinolone resistance). Ten (46%) adolescents received bedaquiline and/or delamanid as a substitute for the injectable agent, ten (46%) received one or both drugs due to limited therapeutic options as a result of second-line drug resistance, and two (8%) patients received bedaquiline and/or delamanid following previous RR-TB treatment failure. Overall, only seven (32%) patients started bedaquiline and/or delamanid at RR-TB treatment initiation; four on bedaquiline, two on delamanid, and one on both drugs together. The median time from RR-TB treatment initiation to receiving bedaquiline and delamanid was 5·6 (IQR 0·0-7·9) and 4·7 (IQR 1·1-9·1) weeks, respectively, while the median duration of exposure to these drugs was 24·5 (IQR 24·0-36·4) and 40·9 (IQR 25·4-62·8) weeks, respectively. Eleven (50%) patients received a regimen containing linezolid (600 mg daily aside from one patient who received 300 mg daily). The co-administered TB drugs as well as the combinations of QT prolonging drugs can be seen in Fig. 1.
Fig. 1.
Number of (a) all co-administered tuberculosis medications (b) combinations of QT-prolonging tuberculosis medications co-administered among adolescents who initiated injectable-free regimens containing bedaquiline and/or delamanid between February 2015 and June 2018 in Khayelitsha, South Africa.
3.2. Treatment emergent adverse events of interest
Overall, there were 49 treatment emergent AEs of interest which occurred in 17 (77%) patients (13·8 events per 100 person-months). In total there were five severe AEs, three grade 3 and two grade 4, reported among three (14%) patients (Table 2). All five severe AEs were haematological with a median time to the onset of 3·0 (IQR 1·8-15·1) months; only two of these three patients were receiving linezolid.
Table 2.
Number of treatment emergent adverse events of interest based on grade among adolescents who initiated injectable-free regimens containing bedaquiline and/or delamanid between February 2015 and June 2018 in Khayelitsha, South Africa.
| Adverse Events of Interest | Median (IQR) time to onset (months) | Grade 1 Instances/Patient N/N (%) | Grade 2 Instances/Patient N/N (%) |
Grade 3 Instances/Patient N/N (%) |
Grade 4 Instances/Patient N/N (%) |
Total Instances/Patient N/N (%) |
|---|---|---|---|---|---|---|
| Alanine aminotransferase increased | 2·2 (1·5–3·5) | 8 n = 4 (18%) |
2 n = 2 (9%) |
0 | 0 | 10 n = 4 (18%) |
| White blood cell decreased | 6·4 (1·80–19·0) | 14 n = 6 (27%) |
0 | 0 | 0 | 14 n = 6 (27%) |
| Anaemia | 2·4 (1·8–4·6) | 8 n = 5 (23%) |
8 n = 6 (27%) |
3 n = 2 (9%) |
1 n = 1 (5%) |
20 n = 7 (32%) |
| Thrombocytopaenia | 1·3 | 0 | 0 | 0 | 1 n = 1 (5%) |
1 n = 1 (5%) |
| QTcF prolongation | 3·0 (2·0–4·5) | 4 n = 4 (18%) |
0 | 0 | 0 | 4 n = 4 (18%) |
| Total of each grade | 3·0 (1·8–6·5) | 34 n= 16 (73%) |
10 n = 7 (32%) |
3 n = 2 (9%) |
2 n = 2 (9%) |
49 n = 17 (77%) |
Abbreviations: IQR, interquartile range; QTcF, QT corrected using the Frederica formula.
Twelve (55%) patients experienced haematological AEs and half (n = 6) of them had anaemia. Only seven (58%) of these 12 patients were on a regimen containing linezolid; all seven received linezolid doses of 600 mg daily. Three of the five (60%) patients not on linezolid had Grade 1 hematological AEs at treatment initiation; three patients (n = 2 HIV-positive and n = 1 HIV-negative) had Grade 1 decreased white blood cell counts and one HIV-positive patient had existing grade 1 anaemia. The remaining two patients not on linezolid (n = 1 HIV-positive and n = 1 HIV-negative) did not have any existing hematological AEs at treatment initiation. Although insignificant, there was a trend towards a greater frequency of hematological AEs among those on linezolid containing regimens (64% and 45% linezolid containing and non-linezolid containing regimens, respectively, p = 0·67). The median time to the onset of these hematological AEs was 3·0 (IQR 1·8-15·1) months.
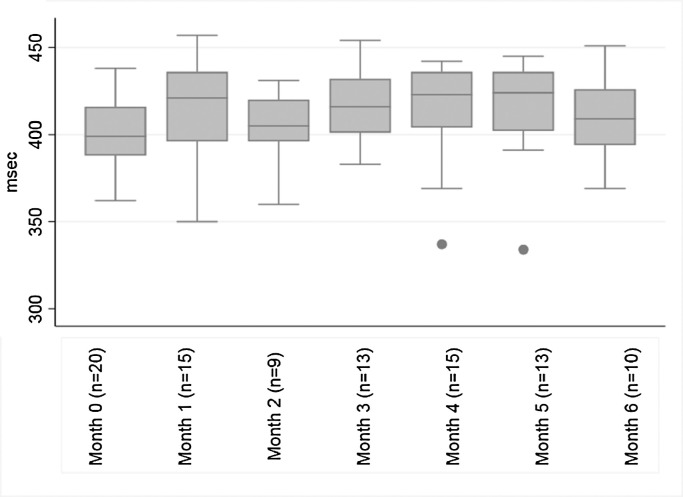
No patient had a baseline QTcF value >=450 ms. There were four instances of grade 1 QTcF prolongation (QTcF 450–480 ms) which occurred in four (18%) patients at months 1 (n = 1), 3 (n = 2), and 6 (n = 1, Table 2, Fig. 2). While there were many missing electrocardiogram results over the 6-month follow-up period, there were no instances of QTcF> 500 ms, cardiac arrhythmia, or unexplained sudden death reported in this cohort.
Fig. 2.
Distribution of median QTcF measurements over the first 6-months of treatment among adolescents who initiated injectable-free regimens containing bedaquiline and/or delamanid between February 2015 and June 2018 in Khayelitsha, South Africa.
There were no deaths as a result of any of the AEs reported, nor were there any cases in which bedaquiline and/or delamanid were permanently discontinued as a result of the AEs reported.
3.3. Effectiveness
Twenty (91%) patients had pulmonary TB, five (26%) of whom had negative sputum cultures when bedaquiline and/or delamanid was initiated. Four (80%) of these patients had persistently negative cultures until the end of treatment and were assigned successful treatment outcomes. The fifth patient was LTFU 8·2 months after delamanid initiation. Another patient (n = 1, 5%) did not have a culture completed when bedaquiline and/or delamanid was initiated, nor did this patient have cultures taken during treatment; this patient was assigned a successful end-of-treatment outcome
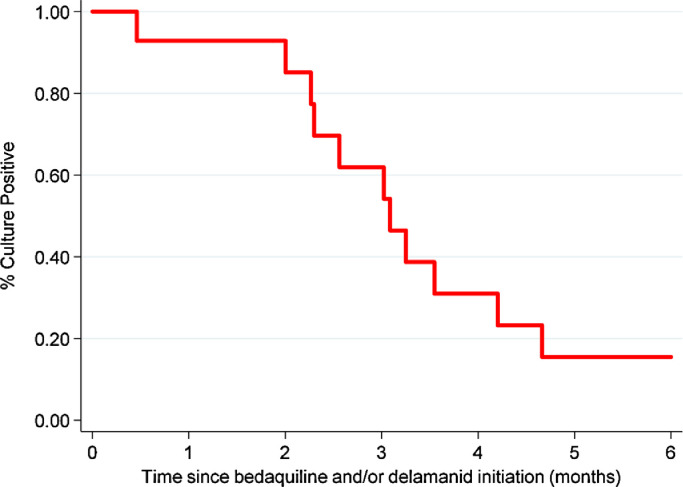
Fourteen (70%) of the 20 patients with pulmonary TB had positive sputum cultures at bedaquiline and/or delamanid initiation; among these, SCC at month 6 of RR-TB treatment was 79% (Fig. 3). Among the 11 patients who had SCC by month 6, the median time to SCC was 2·3 (IQR 0·8-2·7) months. There was no significant difference in the rate of SCC by HIV status (p = 0·54).
Fig. 3.
Effect of bedaquiline and/or delamanid treatment on sputum cultures positive for tuberculosis among adolescents who initiated injectable-free regimens containing bedaquiline and/or delamanid between February 2015 and June 2018 in Khayelitsha, South Africa.
Details of the three patients whose sputum cultures did not convert by month-6 are as follows. One patient who received both bedaquiline and delamanid was assigned an outcome of treatment failure 13·1 months after bedaquiline initiation; this was a HIV-positive male with fluoroquinolone-resistant RR-TB and no previous TB treatment history. At the end of the study period, this patient was still alive and receiving a salvage regimen. Another patient died 1·9 months after initiating both bedaquiline and delamanid while still on treatment with both of these medications; this was a 17 year old, HIV-positive female (CD4 count, 198·0 cells/mm3) with extensively drug-resistant tuberculosis and no previous TB treatment history. She did not experience any Grade 3 or 4 AEs of interest. The third patient was LTFU 5·2 months after delamanid initiation; this patient was a HIV-negative female with rifampicin-mono-resistant TB and no previous TB treatment history.
End-of-treatment outcomes for the 22 adolescents were as follows: 17 (77%) successfully treated, two (9%) LTFU, two (9%) were failed by treatment, and one (5%) died. Among the four patients on the combination of bedaquiline and delamanid, two (50%) were successfully treated, one (25%) was failed by treatment, and one died (25%). The median times to LTFU and treatment failure, after initiation of bedaquiline and/or delamanid, were 6·7 (IQR 5·2-8·2) and 16·7 (IQR 13·1-20·2) months, respectively. There was no significant difference in the frequency of successful outcomes based on HIV status (p = 0·59). The remaining patient who was failed by treatment, that included bedaquiline but not delamanid, was a HIV-negative female with fluoroquinolone resistant RR-TB; SCC occurred within 4·2 months of bedaquiline initiation however, cultures reconverted to positive 13·8 months after initial SCC when she was no longer on bedaquiline. Nineteen months after the initiation of bedaquiline she was assigned an outcome of failed by treatment.
Both individuals in whom treatment had failed had experienced adherence challenges, as noted in clinical records; however, we did not have sufficient data to report on number of missed doses, nor on the extent of disease and severity throughout treatment. Both individuals are still alive and receiving salvage regimens tailored according to extended DST results, and one is due to be discharged with a successful outcome after a year of monthly negative sputum TB cultures.
4. Discussion
We report on end-of-treatment outcomes for adolescents, including those living with HIV, who received injectable-free regimens containing bedaquiline and/or delamanid for the treatment of RR-TB in a routine programmatic setting. Nearly half of these patients received regimens containing bedaquiline and/or delamanid as part of a strategy to prioritise injectable-free regimens. Although we report on a small cohort of adolescents from one programmatic setting, our findings are important in that, despite the high rates of HIV and fluoroquinolone-resistant RR-TB, they show good safety and excellent effectiveness. Our findings support the notion that there is no evidence based reason to restrict adolescents from receiving injectable-free regimens containing bedaquiline and delamanid, and thus they should be prioritised for broader access to such regimens as recommended in the updated WHO treatment guidelines [12].
Historically, adolescents have been denied new anti-tuberculosis medications largely due to safety concerns. In this cohort, although AEs were frequent, we report a very low incidence of severe treatment emergent AEs of interest (1·4 events per 100 person-months). Although we were not able to assess the causal anti-tuberculosis drug/s associated with these Grade 3 and 4 AEs, neither anaemia nor thrombocytopaenia are commonly associated with exposure to bedaquiline and/or delamanid. The majority of AEs experienced in this cohort were haematologic (71%); although non-significant, there was a trend towards a greater frequency of haematological AEs among those on regimens containing linezolid, a drug known to significantly impact this laboratory monitored parameter [21]. Hematological abnormalities in person's not receiving linezolid points to other causes of bone marrow suppression in this population, including TB itself or HIV; however, the small sample size limits the ability to draw any strong conclusions. There were no cases of cardiac arrhythmia or QTcF >500 ms, despite the fact that some patients received the combination of bedaquiline and delamanid. These data are supported by findings from recently published adult cohorts which also showed low frequencies of QTcF prolongation among patients on bedaquiline, and sometimes in combination with delamanid [22,23]. Additionally, based on the evidence from ACTG5343, it has been demonstrated that the use of bedaquiline and delamanid in combination is no more than additive [24]. In these studies with more rigorous monitoring, none of the instances of QTcF prolongation were associated with cardiac arrhythmia or death. Based on our experience, patient safety needs to be balanced with logistics around frequent clinic visits for ECG's; this should be taken into account when deciding on context-relevant monitoring schedules. For patients on bedaquiline, delamanid, and other QT prolonging drugs, monthly ECG monitoring seems reasonable. Of note, patients included in this cohort were on a median of seven anti-tuberculosis medications, many of which could have attributed to the AEs observed. Finally, there were no deaths or instances of permanent discontinuation of bedaquiline and/or delamanid as a result of the AEs reported.
In this cohort of adolescents, among whom 27% were HIV co-infected and 36% had RR-TB with fluoroquinolone resistance, we report a treatment success rate of 77%. This is a notable finding, as it challenges the literature suggesting that adolescents treated for RR-TB have poor outcomes [4,25]. It is possible that the patients included in the previous cohorts were treated with ineffective regimens containing suboptimal, toxic drugs, thus explaining the higher rates of unsuccessful outcomes [26], [27], [28]. Additionally, the treatment success rate for the adolescents included in this cohort is higher than that reported for adult populations globally [29]. However, it is similar to the success rate of 76% reported in a Peruvian cohort of adolescents, despite the fact that new anti-tuberculosis medications were not used. This might be explained by the fact that none of the Peruvian adolescents were living with HIV [30]. As this study included a small cohort of adolescents, this might have allowed for more focused social support in the form of a peer support group with a dedicated counselor to check up on the adolescents regularly. Additionally, these adolescent were provided with tailored support through mobile phones and social media platforms; this may have contributed to the good outcomes observed. Our data suggest that even in this difficult to treat population, for whom treatment outcomes have historically been poor, high rates of treatment success can be achieved with injectable-free regimens that include novel agents.
The practice of denying this vulnerable population access to new anti-tuberculosis medications, including injectable-free regimens, is detrimental considering the impact that injectable agents have on the cognitive development of adolescents as a result of hearing loss [31]. Although South Africa has recommended injectable-free regimens for the treatment of RR-TB, the limited safety and effectiveness data from programmatic settings has left clinicians feeling hesitant in the implementation of these new guidelines. Additionally, the majority of adolescents globally are being denied programmatic access to such regimens despite the WHO recommendations. Our data should reassure clinicians and policy makers, that the use of injectable-free regimens containing bedaquiline and/or delamanid in adolescents is both safe and effective. Deployment of these regimens along with supplemental capacity building on the holistic and decentralised management of RR-TB in pediatric and adolescent populations should be the norm.
This study was subject to several limitations. First this was a retrospective study of routinely collected programmatic data with a small study population, conducted at a single site. Thus, these factors might limit the generalisability of our findings. Second, there was no contemporary control group which limits the comparability of these results. Third, QTcF prolongation might have been underestimated in this study due to infrequency of electrocardiogram monitoring; these missing data are a reflection of the reality of RR-TB treatment administration and patient monitoring under routine programmatic conditions. These data suggest that frequent electrocardiogram monitoring might play a less substantial role in clinical decision making. Fourth, as AEs were not systematically documented in clinical files we were only able to report on AEs of interest limited to laboratory-monitored parameters and clinically assessed QTcF prolongation. Thus, this study does not give a comprehensive overview of all the AEs faced by adolescents on RR-TB treatment. Fifth, as a result of the small sample size, it would be difficult to draw any firm conclusions on factors associated with poor treatment outcomes among this cohort. This is an important area for future research. Nonetheless our study provides real-world data on a population for which there is a paucity of data in the literature.
Adolescents are systematically denied access to the novel RR-TB drugs that have been associated with lower mortality and improved outcomes in adults. We found that injectable-free RR-TB treatment regimens containing bedaquiline and/or delamanid were well tolerated and achieved excellent outcomes in this cohort of adolescents in the programmatic setting of Khayelitsha, South Africa. There is an urgent need to expand access to novel RR-TB medications, such as bedaquiline and delamanid, in injectable-free regimens in order to ensure the best chance of cure among this vulnerable group. In the past, adolescents have been overlooked in many aspects of the RR-TB care cascade: it is time for the tide to shift and for National TB Programs to prioritize adolescents in the uptake and roll-out of new treatment guidelines.
Declaration of competing interest
We declare no competing interests.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the Provincial Government of the Western Cape and Cape Town City Health for supporting and managing the RR-TB programme. Importantly, the authors would like to acknowledge MSF staff involved in implementation activities and people living with RR-TB in Khayelitsha.
Funding
Médecins Sans Frontières (MSF).
References
- 1.Furin J., Tommasi M., Garcia-Prats A. Drug-resistant tuberculosis : will grand promises fail children and adolescents? Lancet Child Adolesc Heal. 2018;2:237–238. doi: 10.1016/S2352-4642(18)30068-3. [DOI] [PubMed] [Google Scholar]
- 2.The Economist Intelligence Unit It's time to end drug-resistant tuberculosis. Case Action. 2019 http://www.eiu.com/graphics/marketing/pdf/its-time-to-end-drug-resistant-tuberculosis-full-report.pdf Accessed 10 May 2019. [Google Scholar]
- 3.London: Wellcome Trust and HM Government Tackling drug-resistant infections globally: final report and recommendations. Rev Antimicrob Resist. 2016 https://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf (Accessed 10 May 2019) [Google Scholar]
- 4.Moyo S., Furin J., Hughes J. Outcomes in adolescents undergoing treatment for drug-resistant tuberculosis in Cape Town, South Africa, 2008–2013. Arch Pediatr Infect Dis. 2014;3:e17934. [Google Scholar]
- 5.Patton G.C., Sawyer S.M., Santelli J.S. Our future : a lancet commission on adolescent health and wellbeing. Lancet Comm. 2016;387:2423–2478. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon J., Godfrey-Faussett P., Jacobs K., Ebrahim A., Hesseling A., Schaaf H. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J. 2012;40:1277–1286. doi: 10.1183/09031936.00044812. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019. https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?sequence=1&isAllowed=y (Accessed 10 May 2019). [PubMed]
- 8.Achar J., Hewison C., Cavalheiro A.P. Off-Label use of bedaquiline in children and adolescents with multidrug-resistant tuberculosis. Emerg Infect Dis. 2017;23:1711–1713. doi: 10.3201/eid2310.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tadolini M., Garcia-Prats A.J., D'Ambrosio L. Compassionate use of new drugs in children and adolescents with multidrug-resistant and extensively drug-resistant tuberculosis: early experiences and challenges. Eur Respir J. 2016;48:938–943. doi: 10.1183/13993003.00705-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnippel K., Ndjeka N., Maartens G. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir. 2018;2600:1–8. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 11.Harausz E.P., Garcia-prats A.J., Law S. Treatment and outcomes in children with multidrug-resistant tuberculosis : a systematic review and individual patient data meta- analysis. PLOS Med. 2018;15 doi: 10.1371/journal.pmed.1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Rapid communication: key changes to treatment of Multidrug- and Rifampicin-Resistant Tuberculosis (MDR/RR-TB). 2018. https://www.who.int/tb/publications/2018/WHO_RapidCommunicationMDRTB.pdf?ua=1 (Accessed 10 May 2019).
- 13.Cox H., Daniels J.F., Muller O. Impact of decentralized care and the xpert mtb/rif test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infect Dis. 2015;2:ofv014. doi: 10.1093/ofid/ofv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariem R., Cox V., de Azevedo V. The experience of bedaquiline implementation at a decentralized clinic in South Africa. Public Heal Action. 2016;6:190–192. doi: 10.5588/pha.16.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenberk B., Vandael E., Robyns T. Which QT correction formulae to use for QT monitoring. J Am Heart Assoc. 2016;5:1–10. doi: 10.1161/JAHA.116.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Active tuberculosis drug-safety monitoring and management (aDSM): framework for implementation. 2015. https://apps.who.int/iris/bitstream/handle/10665/204465/WHO_HTM_TB_2015.28_eng.pdf?sequence=1 (Accessed 10 May 2019).
- 17.Department of Health: Republic of South Africa. Interim clinical guidance for the implementation of injectable-free regimens for rifampicin-resistant tuberculosis in adults, adolescents and children. 2018. http://www.tbonline.info/media/uploads/documents/dr_tb_clinical_guidelines_for_rsa_september_2018.pdf (Accessed 10 May 2019).
- 18.EndTB. EndTB clinical and programmatic guide for patient management with new TB drugs. 2018. https://samumsf.org/sites/default/files/2018-06/EndTBGuide for New TB Drugs Version 4.0.pdf (Accessed 10 May 2019).
- 19.National Health Labratory Service . 2016. NHLS handbook: standard operating procedure.http://www.pathology.uct.ac.za/sites/default/files/image_tool/images/231/documents/NHLS_Handbook_2015.pdf Accessed 10 May 2019. [Google Scholar]
- 20.World Health Organization. Definitions and reporting framework for tuberculosis–2013 revision. 2013. http://apps.who.int/iris/handle/10665/79199 (Accessed 10 May 2019).
- 21.Cox H., Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis : a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2012;16:447–454. doi: 10.5588/ijtld.11.0451. [DOI] [PubMed] [Google Scholar]
- 22.Guglielmetti L., Le Du D., Jachym M. Compassionate use of Bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis. 2015;60:188–194. doi: 10.1093/cid/ciu786. [DOI] [PubMed] [Google Scholar]
- 23.Ferlazzo G., Mohr E., Laxmeshwar C. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of drug-resistant tuberculosis patients in Armenia, India and South Africa: a retrospective cohort study. Lancet Infect Dis. 2018;18:536–544. doi: 10.1016/S1473-3099(18)30100-2. [DOI] [PubMed] [Google Scholar]
- 24.Dooley K.E., Rosenkranz S.L., Conradie F. QT effects of bedaquiline, delamanid or both in MDR-TB patients: the deliberate trial. CROI. 2019 [Google Scholar]
- 25.Isaakidis P., Paryani R., Khan S. Poor outcomes in a cohort of HIV-Infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLoS One. 2013;8:e68869. doi: 10.1371/journal.pone.0068869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N., Ahuja S., Akkerman O.W. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis : an individual patient data. Lancet. 2018;392:821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson K.R., Tierney D.B., Jeon C.Y., Mitnick C.D., Murray M.B. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S., Zhang Y., Sun F. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther. 2016;23:e521–e530. doi: 10.1097/01.mjt.0000433951.09030.5a. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Global tuberculosis report 2018. https://www.who.int/tb/publications/global_report/en/ (Accessed May 10, 2019).
- 30.Tierney D.B., Milstein M.B., Manjourides J., Furin J.J., Mitnick C.D. Treatment outcomes for adolescents with multidrug-resistant tuberculosis in Lima, Peru. Glob Pediatr Heal. 2016;3:1–5. doi: 10.1177/2333794X16674382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddon J.A., Thee S., Jacobs K., Ebrahim A., Hesseling A.C., Schaaf H.S. Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect. 2013;66:320–329. doi: 10.1016/j.jinf.2012.09.002. [DOI] [PubMed] [Google Scholar]