Abstract
Identification and development of newer and better antimicrobials from natural products represent ongoing research efforts by many investigators. Curcumin is a polyphenol commonly found in the plant Curcuma longa (better known as turmeric). It has been reported to possess several bioactivities including antioxidant, anti-cancer, anti-inflammatory, anti-diabetic, anti-fibrotic, and antimicrobial properties. However, little is known about the antimicrobial mode of action of curcumin, thus undermining its prospects as an alternative antimicrobial agent. In this study, we investigated the mechanism of antimicrobial action by curcumin. The mechanism of inhibition was evaluated in representatives of Gram negative (Escherichia coli) and Gram positive (Staphylococcus aureus) bacteria isolates, treated with either curcumin singly or in combination with ascorbic acid (1000 μg/mL). Results showed that curcumin has broad antimicrobial capacity. In addition, curcumin only and/or co-treatment with ascorbic acid caused lipid peroxidation in S. aureus and E. coli, and by extension led to DNA damage, indicative of oxidative stress. It is plausible that the oxidative might be related to the activation of the kynurenine pathway in S. aureus but not in E. coli. Furthermore, curcumin exposure led to elevated total antioxidant capacity (TAC) and level of total thiol, but decreased nitric oxide level in the bacteria isolates. Together, the findings suggest that oxidative stress and DNA damage might be partly responsible for the antimicrobial action of curcumin.
Keywords: Biochemistry, Microbiology, Toxicology, Natural product chemistry, Organic chemistry, Antimicrobials, Medicinal biochemistry, Microbial infection, Natural products, Polyphenols
Biochemistry; Microbiology; Toxicology; Natural product chemistry; Organic chemistry; Antimicrobials; Medicinal biochemistry; Microbial infection; Natural products; Polyphenols
1. Introduction
Plant polyphenols are among the prospective compounds being investigated as potential alternative antimicrobial agents [1]. They are often effective in the treatment of infectious diseases while reducing many of the side effects usually associated with the use of synthetic antimicrobial agents. Considerable resources have been directed into isolating and purifying these natural chemical substances to ascertain their medical effectiveness and also to understand their bioactivities [2]. Polyphenols are secondary plant metabolites that protect the plant against infection by pathogens, the invasion of herbivores, and the attraction of pollinators; they also execute structural functions and defend the plant against ultraviolet radiation [3]. They may contribute to the astringency, bitterness or even color of foods. Polyphenols are naturally occurring compounds found largely in fruits, vegetables, nuts, seeds, and flowers, bark of trees, cereals, and beverages such as chocolate, wine, and tea [3, 4]. Investigations have shown that polyphenols possess a number of bioactive and medicinal properties, including antioxidant, anti-carcinogenic, anti-allergic, anti-inflammatory, antibacterial, antiviral, anti-fungal, vasodilatory motion, anti-hypertensive, antithrombotic, anti-aging, and hepatoprotective [5].
Curcumin is a polyphenol found mainly in the plant Curcuma longa, a rhizome that is popularly known as turmeric. A member of the Zingiberaceae family, Curcuma longa is a spice used in cooking various dishes around the world, especially Asian dishes. Historically, this plant has also been used to treat various human diseases [6]. In recent years, curcumin has attracted considerable interest due to its promising medicinal value; it is a potent immune modulatory agent possessing antioxidant, anti-fibrotic, anti-viral and anti-infective effects [7]. Indeed, curcumin has continued to draw attention because of such bioactivities as anti-inflammatory, anti-tumor, anti-diabetic, anti-cancer and antimicrobial properties [8]. Curcumin also mitigates stress-induced activation of IDO-kynurenine pathway. In particular, curcumin has been used for a range of antimicrobial purposes, because of its well-established bioactive properties and few negative side effects [9]. Studies have shown the inhibitory potency of curcumin against a wide range of bacteria, viruses, and fungi as well as parasites [9, 10, 11]. Additionally, curcumin has been recommended as an adjuvant therapy to enhance the antimicrobial properties of available antibiotics [6].
However, in spite of the promising antimicrobial potencies of curcumin, little is known about its mechanisms of action; only a few studies have reported the mechanistic antimicrobial action by curcumin [10, 11]. This gap in knowledge impedes curcumin's prospects as an alternative antimicrobial agent. For this reason, the present work investigated the mechanisms of antimicrobial action of curcumin by means of the determination of biochemical indices. The biochemical assays performed in this study included the evaluation of redox status, DNA damage and activation of the kynurenine pathway. Studies have demonstrated that curcumin was not only capable of causing oxidative stress, but DNA damage as well as the activation of kynurenine pathway [12, 13, 14].
2. Materials and methods
2.1. Chemical and reagents
The curcumin, kynurenine standard, gallic acid, tannic acid, and Ehrlich reagent were products of Sigma Chemicals Co. (St. Louis, Missouri, USA). The agar and broth came from HiMedia (Mumbai, India). The chemical reagents were of analytical grade and were used as supplied. Every compound used in this work was dissolved using Dimethyl sulfoxide (DMSO).
2.2. Bacterial isolates and growth media
The bacterial isolates used in this study included Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Klebsiella pneumoniae. These microbial isolates were obtained from the laboratory of the Department of Microbiology, Landmark University, Nigeria. Isolates were inoculated into nutrient broth, stored frozen under sterile conditions, and subcultured for further studies.
2.3. Antibacterial determinations
2.3.1. Determination of the zone of inhibition
Determination of the zone of inhibition was performed using the pour plate method. Nutrient agar (OXOID) was prepared in 50 mL quantities in 100 mL-capacity conical flasks, following the manufacturer's instruction and sterilized in an autoclave for 15 min. After sterilization, the flaks containing the sterile agar were cooled (45 °C ± 2 °C), before inoculating with 1 mL of broth culture of the respective bacteria and mixed thoroughly. Following mixing, the inoculated agar was dispensed in 20 mL quantity into Petri dishes and allowed to solidify. Following solidification of the bacteria growth medium, a sterile cork borer was used to make holes in the solidified agar. The holes made were then filled with the respective test compounds (curcumin, gallotannin, gallic acid, ascorbic acid) that were previously dissolved in DMSO (1 mg/mL w/v). The respective compounds allowed to diffuse in the agar before incubation (37 °C ± 2 °C) for 24 h. An antibiotic (amoxicillin) was included as reference to validate the assay. The diameter in mm of the zone of inhibition around each hole was measured by placing a metric ruler on the bottom of the plate. The whole procedure was carried out in an inoculating chamber.
2.3.2. Minimum inhibitory concentration (MIC) determination
Determination of minimum inhibitory concentration (MIC) was performed by using the standard tube dilution method. MIC was only determined for the curcumin. In this test, test tubes containing 10 mL of sterile nutrient broth were inoculated with 0.5 mL of the respective bacterial suspensions. Each of the inoculated test tubes were then added known concentration of the curcumin (0.1–1 mg/mL). Cell suspension without any curcumin concentration was used as the control. After inoculation and addition of curcumin, the tubes were incubated at 45 °C ± 2 °C) for 24 h for observation for growth. The lowest dilution of curcumin that showed no turbidity (indicative of growth) was recorded as the MIC.
2.4. Treatments of cells for biochemical assays
To accomplish the biochemical experiment, the Gram-positive S. aureus and the Gram-negative E. coli were selected as representative.
2.4.1. Curcumin only
-
○
The S. aureus was treated with curcumin at concentrations of 600 μg/mL (MIC), 1200 μg/mL (2x MIC), and 1800 μg/mL (3x MIC).
-
○
For E. coli, curcumin treatments were at concentrations of 400 μg/mL (MIC), 800 μg/mL (2x MIC), and 1200 μg/mL (3x MIC).
2.4.2. Curcumin and co-treatment with ascorbic acid
-
○
The treatments were as described above except for the simultaneous treatment with ascorbic acid (AA) at 1 mg/mL. Ascorbic acid was included as an antioxidant to protect against presumed oxidative stress caused by curcumin treatment. From our preliminary studies, ascorbic acid at 1000 μg/mL did not inhibit the growth of the bacteria isolates.
2.5. Harvesting of cells for biochemical assays
Harvesting of cells for biochemical assays was as reported previously [15]. Briefly, the bacteria isolates, namely E. coli and S. aureus, were treated in nutrient broth with curcumin at specified concentrations; control, MIC, 2x MIC, and 3x MIC values or in combination with ascorbic acid (1 mg/mL). After a 24-h incubation at 37 °C, the cells were harvested by centrifugation at 5,000 g for 10 min (model C5, LW Scientific, USA). Aliquot of the supernatant was taken for kynurenine assay. The pelleted cells were washed with normal saline three times, resuspended, and thereafter homogenized and stored frozen until required for analysis.
2.6. Biochemical assays
Where applicable, biochemical parameters were determined by spectrophotometry (Jenway, Staffordshire, United Kingdom). Determination of kynurenine in bacteria culture suspension was performed according to a procedure described previously [16]. Nitric oxide concentration was measured as the nitrite level according to a method described by Adeyemi and Sulaiman [17]. Total antioxidant capacity (TAC) of cell lysates was determined as described by Adeyemi et al. [18]. Total protein concentration was estimated according to a method described by Gornall et al. [19] with slight modification; potassium iodide was added to the biuret reagent to prevent precipitation of Cu2+ ions. Total thiol level was assayed according to a method described by Beutler et al. [20]. Lipid peroxidation was estimated as malondialdehyde (MDA) using the method described by Varshney and Kale [21]. DNA fragmentation was determined using the diphenylamine (DPA) assay as described elsewhere [22].
2.7. Statistical analysis
Results were analyzed by using a one-way analysis of variance (ANOVA) (GraphPad Software Inc., California, US). Data are expressed as the average of two replicates ± standard error of the mean (SEM). Comparison of mean values among groups was achieved by using Tukey's post-hoc test; p < 0.05 was considered to indicate a significant difference. Experiments were performed independently two times.
3. Results
3.1. Antibacterial determinations
Among the test compounds, only curcumin was active against all bacterial isolates used in this study. Gallic and tannic acids were active against only a few of the bacteria isolates (Table 1). Thus, curcumin was selected for further experiments. Furthermore, to determine the MIC, bacteria isolates were treated for 24 h with curcumin. Results revealed MIC values ranging between 300 and 1500 μg/mL (Table 2).
Table 1.
The zone of inhibition (in mm) after 24 h of treatment.
| Organism | Curcumin (1000 μg/mL) | Gallic acid (1000 μg/mL) | Tannic acid (1000 μg/mL) | DMSO | Ascorbic acid (1000 μg/mL) | Amoxillin (1000 μg/mL) |
|---|---|---|---|---|---|---|
| S. aureus | 18.5 ± 0.5 | - | 18.0 ± 0.2 | - | - | 24.5 ± 5.5 |
| E. coli | 23.0 ± 2.0 | 15.0 ± 0.6 | 17.0 ± 0.4 | - | - | 25.5 ± 3.5 |
| P. aeruginosa | 20.5 ± 0.5 | - | - | - | - | 33.5 ± 1.5 |
| K. pneumoniae | 20.5 ± 1.5 | - | - | - | - | - |
| B. subtilis | 22.5 ± 2.5 | 12.5 ± 0.5 | - | - | - | 16.0 ± 1.0 |
(-) means no inhibition was observed.
Table 2.
The minimum inhibitory concentration (MIC) of curcumin after 24 h of treatment.
| Organism | MIC value (μg/mL) |
|---|---|
| S. aureus | 600 ± 50.21 |
| E. coli | 400 ± 63.05 |
| P. aeruginosa | 300 ± 38.52 |
| B. subtilis | 700 ± 54.05 |
| K. pneumonia | 1500 ± 73.25 |
3.2. Biochemical indices
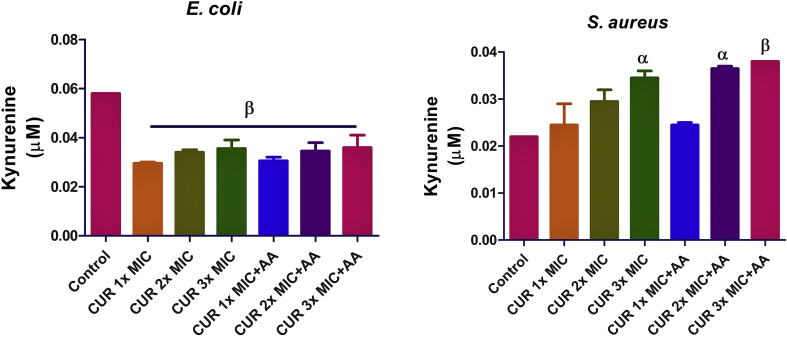
In E. coli, the kynurenine level was decreased (p < 0.01) compared to the untreated group following treatment with curcumin only. Co-treatment with ascorbic acid also reduced the kynurenine level, though not significantly but more than when cells were treated with curcumin only (Figure 1). In S. aureus, at 2x MIC of curcumin only, there was a significant increase (p < 0.05) in the kynurenine level. The same effect was observed for co-treatment with ascorbic acid, but again the difference was not significant when compared with curcumin only.
Figure 1.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on kynurenine level in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05 and β at p < 0.01 versus the control.
3.2.1. Effect of curcumin on nitric oxide level
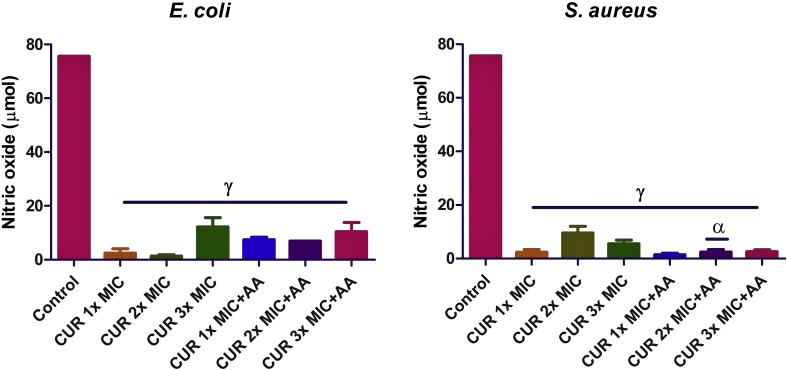
In E. coli (Figure 2), treatment with curcumin only caused a drastic decrease (p < 0.0001) in the nitric oxide level compared with the control (untreated group). Meanwhile, co-treatment with ascorbic acid did not cause any significant change when compared to the cells treated with curcumin only. In S. aureus, curcumin only also caused a drastic decrease (p < 0.0001) in the nitric oxide level relative to the control (untreated group). Likewise, co-treatment at 2x MIC of curcumin with ascorbic acid caused a decrease in nitric oxide compared with 2x MIC curcumin only.
Figure 2.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on nitric oxide level in E. coli and S. aureus. Nitric oxide (NO) concentration was measured as nitrate/nitrite level. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05 versus CUR 2x MIC, and γ at p < 0.0001 versus the control.
3.2.2. Effect of curcumin on total antioxidant capacity
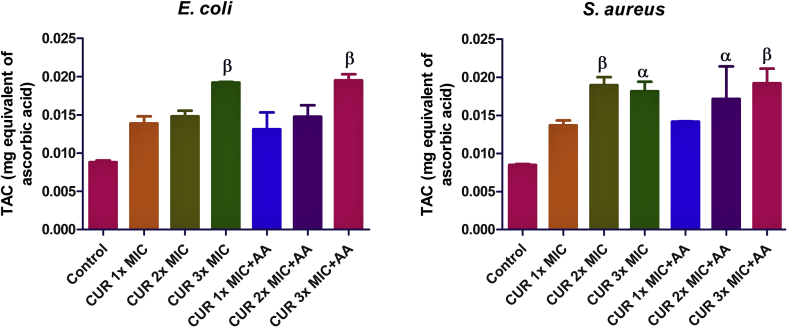
In E. coli there was a dose-dependent increase in TAC, but the sole instance in which it was significant (p < 0.01) relative to the control was at 2x MIC of curcumin only (Figure 3). Meanwhile, co-treatment with ascorbic acid did not cause any significant change compared to curcumin only. The same trend was also observed for S. aureus, but the increase was significant at both 2x MIC (p < 0.01) and 3x MIC (p < 0.05) of curcumin relative to the control.
Figure 3.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on total antioxidant capacity in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05 and β at p < 0.01 versus the control.
3.2.3. Effect of curcumin on total protein level
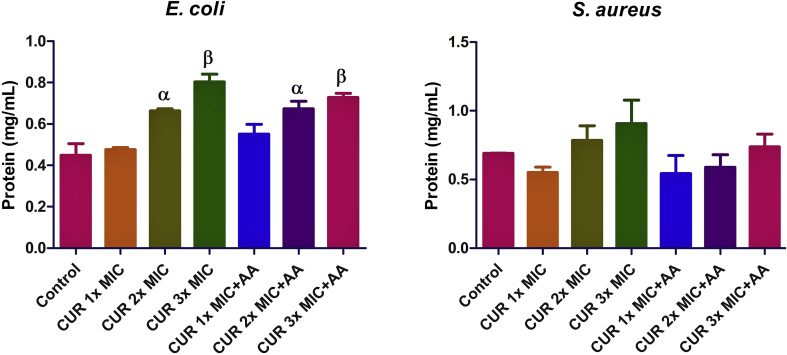
In E. coli, the increase in the total protein level following treatment with curcumin only was dose-dependent. It was significant only at 2x MIC (p < 0.05) and 3x MIC (p < 0.01) of curcumin (Figure 4). Co-treatment with ascorbic acid did not cause any significant change compared to curcumin only. In S. aureus, there was no significant change compared to the control.
Figure 4.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on total protein level in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05 and β at p < 0.01 versus the control.
3.2.4. Effect of curcumin on total thiol level
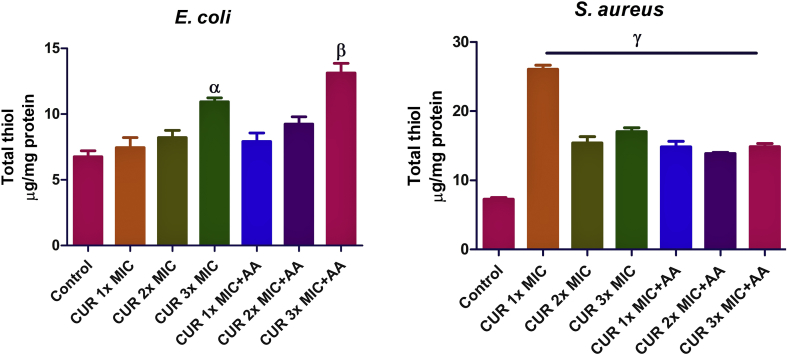
In E. coli, there was a dose-dependent increase in the total thiol level, but it was statistically significant relative to the control (p < 0.05) only at 3x MIC of curcumin (Figure 5). Also, co-treatment of cells at 3x MIC of curcumin along with ascorbic acid elevated the total thiol level significantly more (p < 0.01) than treatment with 2x MIC curcumin only. Meanwhile, in S. aureus, treatment with 1x MIC of curcumin significantly (p < 0.05) increased the total thiol level when compared to the control; in contrast, co-treatment with ascorbic acid significantly decreased the total thiol level at 1x MIC of curcumin.
Figure 5.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on total thiol level in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05, β at p < 0.01, and γ at p < 0.0001 versus the control.
3.2.5. Effect of curcumin on lipid peroxidation
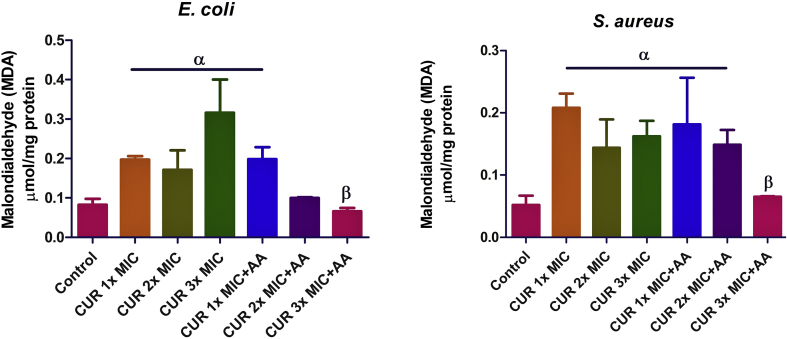
In E. coli, the concentration of MDA in cells treated with 3x MIC curcumin was elevated relative to the control (Figure 6). However, there was a significant decrease (p < 0.05) in the concentration of MDA in cells treated with 3x MIC of curcumin and ascorbic acid, compared with those treated with 3x MIC of curcumin only. In S. aureus, curcumin only and/or co-treatment with ascorbic acid caused a dose-dependent increase in MDA levels, but the increase was not statistically significant relative to the control.
Figure 6.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on malondialdehyde (MDA) level in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM). α is significant at p < 0.05 versus the control and β at p < 0.01 versus CUR 3x MIC.
3.2.6. Effect of curcumin on DNA fragmentation
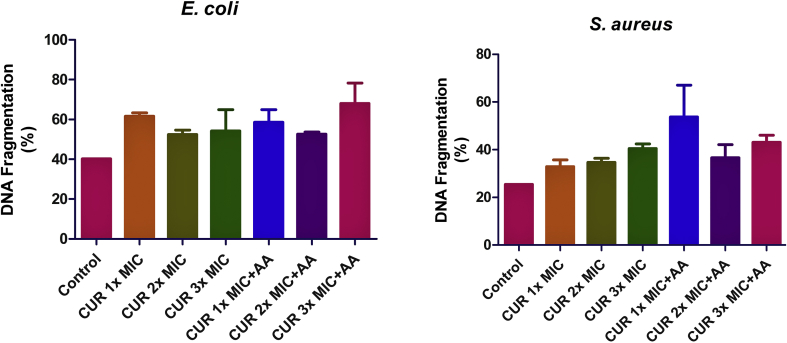
In both E. coli and S. aureus (Figure 7), curcumin treatments caused DNA fragmentation relative to the control. Co-treatment with ascorbic acid failed to abate the DNA fragmentation caused by curcumin treatment at all dose levels.
Figure 7.
Effect of curcumin (CUR) treatment and/or co-treatment with ascorbic acid (AA) on DNA fragmentation in E. coli and S. aureus. Data are presented as the mean of two replicates ± standard error of the mean (SEM).
4. Discussion
Microbial infections remain a global public health challenge due to the rising number of cases of drug resistance [23, 24]. Therefore, there is a pressing need to seek new classes of antimicrobials [25, 26]. Polyphenols are a class of prospective antimicrobials, but their mechanisms of action is not well defined. Therefore, in this study, the mechanism of antimicrobial action of curcumin was investigated. Curcumin treatment restricted growth of both the Gram negative and positive bacteria isolates, indicating a broad antimicrobial capacity. This finding is consistent with existing studies [11, 12, 27]. In a previous study, it was reported that the MIC value of curcumin was 187.5–500 mg/mL against S. aureus and also between 93.8 and 250 mg/mL against E. coli [27]. In the present study, MIC was 600 and 400 μg/mL respectively against S. aureus and E. coli.
To assess whether curcumin could be affecting L-tryptophan metabolism as part of its antimicrobial action, we measured the kynurenine level in the culture medium. Kynurenine is a major degradation product of L-tryptophan. Our results showed that treatment only with curcumin activated the kynurenine pathway in S. aureus, but not in E. coli. For S. aureus, the activation of the kynurenine pathway by curcumin might have caused a decreasing local concentration of L-tryptophan available to support bacteria growth, thereby starving bacterial cells of an essential nutrient. Additionally, in this study, curcumin caused oxidative stress in the bacterial cells, and this result might be connected with the activation of the kynurenine pathway in S. aureus. This possibility gains credence when we consider that studies have linked oxidative stress with the activation of the kynurenine pathway both in vivo and in vitro [17]. On the other hand, the decrease in the E. coli kynurenine level may indicate that curcumin or its metabolites are inhibiting the activity of indoleamine 2,3-dioxygenase (IDO-1, the rate-limiting enzyme responsible for the formation of kynurenine from L-tryptophan) [17]. This line of thought would be consistent with prior investigations that showed that curcumin suppressed the induction of IDO-1 [13].
Moreover, our study revealed that curcumin at all dose levels caused lipid peroxidation, as reflected in elevated MDA levels when compared with the control. Lipid peroxidation is a degradation process that primarily affects the tissues, resulting in the formation of free radicals and unsaturated fatty acid saturation [28]. Lipid peroxidation is a principal marker for oxidative damage due to high production of reactive oxygen species (ROS); determination of the MDA level is used for this assessment. In E. coli, the MDA level increased with increasing concentrations of curcumin, but when the same concentrations were co-administered with ascorbic acid, the MDA level decreased dose-dependently. This result implies that ascorbic acid remarkably reversed the oxidative effect of curcumin treatment on E. coli. In S. aureus, there was a general increase in the MDA level across all treatments. Elevated MDA levels can contribute to the excess production of hydroxyl radicals [25].
Many studies have demonstrated that an excessive generation of intracellular ROS may directly cause oxidative stress, which causes damage to components of the cell, including nucleic acids, proteins, and lipids, thereby leading to apoptosis [14]. The lipid peroxidation caused by curcumin treatment and/or co-treatment with ascorbic acids might have led to DNA fragmentation in these bacteria cells. In our work, the percentage of DNA fragmentation was elevated in cells treated with curcumin only, as well as in those co-treated with ascorbic acid, when compared with the untreated control group for both E. coli and S. aureus. This may not be surprising, since studies on the mechanism of curcumin as an anti-cancer agent have shown that production of ROS effectively causes DNA fragmentation, which eventually leads to cellular death [14]. In addition, curcumin has been shown to cause oxidative stress and promotion of ROS as part of its antimicrobial action [12]. Therefore, curcumin treatment might have caused oxidative stress and (by extension) DNA damage as part of the biochemical mechanisms leading to its antimicrobial action against E. coli and S. aureus.
On the other hand, following curcumin treatment, both E. coli and S. aureus showed elevated levels of total thiol. It is possible that the bacterial isolates increased the synthesis of thiol groups as a form of adaptive mechanism in response to the oxidative stress imposed by curcumin exposure. Moreover, curcumin may form adducts with cellular proteins; in particular, thiol reactivity of curcumin has been reported [29], and in response bacterial species such as E. coli and S. aureus might increase their capacity for synthesis of thiol groups [30, 31, 32, 33]. Increased capacity for thiol synthesis has been linked to drug resistance in E. coli [30].
In addition, subtle variance in antibacterial effects of curcumin on E. coli and S. aureus, as observed in this study, might also be connected with the difference in bacterial cell wall morphology. S. aureus, which lacks a lipopolysaccharide outer cell layer, seems to be more sensitive to curcumin-induced oxidative stress than E. coli. In the meantime, investigations have shown that the antimicrobial activities of polyphenols may include diverse modes of action, such as permeabilization and disruption of the plasma membrane, as well as the inhibition of enzymes by oxidized products, perhaps by means of reactions with sulfhydryl groups or by interactions that are nonspecific with proteins. For example, one mechanism could be the generation of reactive quinones that react with proteins and amino acids. Alternatively, the inhibition of nucleic acid formation in Gram-positive and Gram-negative bacteria could also be a mechanism [25]. Previous studies have reported that curcumin caused perturbation of the lipid bilayer in cells [34]. Also, it has been noted that curcumin at higher concentrations can have antibacterial activities against Gram-negative and Gram-positive bacteria through membrane permeabilization [11]. In another study, curcumin blocked bacterial cell wall formation [10].
Considering that both curcumin and ascorbic acid possess antioxidant properties [35, 36], we determined the antioxidant status of treated cells. The ability to fight against oxidative stress can be evaluated by measuring the cellular antioxidant capacity. The TAC is an important index that measures the operational status of the antioxidant defense system and can indicate the state of antioxidant enzyme systems in living organisms [15]. In the present study, there was a general increase in TAC levels among the cells treated with curcumin only, as well as those co-treated with ascorbic acid, in a dose-dependent manner. The increased TAC levels might be due to the antioxidant capacity of curcumin and ascorbic acid, which both substances are known to possess [35, 36]. Additionally, treatments with curcumin only and co-treatments with ascorbic acid at all doses drastically reduced nitric oxide levels in both S. aureus and E. coli. This reduction might be connected with the radical scavenging capacity of both curcumin and ascorbic acid and is compatible with their antioxidant character. It is plausible that both curcumin and ascorbic acid possess scavenging activity against nitric oxide, thereby making it unavailable for detection.
In addition, the elevated level of total thiol could be a result of increased synthesis by the bacterial isolates in response to the effect of treatment with curcumin (with or without ascorbic acid). Also, ascorbic acid is required as a cofactor for replenishing total thiol stores. Moreover, previous research has revealed curcumin's capacity to cause elevation of total thiol levels [37]. Total thiols function by scavenging radicals intracellularly. They protect the cell against ROS, mutagens, toxins, and drugs. Total thiols are also essential for the cellular metabolism of a variety of amino acids, enzymes, and hormones. In the present study, curcumin caused oxidative stress, thus leading to elevated total thiol and TAC levels in the bacteria isolates. This result was not unexpected, as bacterial species including E. coli and S. aureus have cell redox systems to cope with oxidative damage [30, 32]. On the other hand, the reduced nitric oxide level may indicate the scavenging capacity of curcumin, compatible with its antioxidant character, but contrasts with its potential to cause oxidative stress and DNA damage in arresting microbial growth. Nonetheless, these seemingly opposite impacts of curcumin are plausible. Studies have shown that some phenolic compounds with antioxidant properties such as curcumin and gallotannin are capable of causing ROS production and facilitating oxidative stress as part of their cytotoxic action [14, 29, 38].
In conclusion, this study showed that curcumin treatment caused lipid peroxidation and, by extension, led to DNA fragmentation in E. coli and S. aureus. However, co-treatment with ascorbic acid, rather than abating the cellular oxidative stress and DNA fragmentation, apparently potentiated these oxidative processes in an additive manner. This finding further reinforces our opinion that oxidative stress might be responsible for the antimicrobial action of curcumin. Moreover, curcumin apparently activated the kynurenine pathway in S. aureus, and this process might be connected with the ensuing oxidative stress caused by the treatment. The evidence of oxidative stress is further strengthened by the elevated levels of total thiol groups and TAC in the bacterial isolates. In contrast, the reduced level of nitric oxide might be a result of the radical scavenging capacity of curcumin and/or ascorbic acid, but would be compatible with these substances’ potential to cause oxidative cellular damage in their toxic action. Overall, the data support the conclusion that curcumin might have caused oxidative stress and DNA damage, leading to the death and/or arrested growth of E. coli and S. aureus.
Declarations
Author contribution statement
Oluyomi Stephen ADEYEMI, Benjamin Oghenerobor AKPOR: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Joy Ihuoma Obeme-Imom, Damilare ROTIMI: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gaber El-saber BATIHA: Performed the experiments; Wrote the paper.
Akinyomade OWOLABI: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Appreciate the Ton Duc Thang University, Ho Chi Minh City, Vietnam. The authors also wish to thank the laboratory staff in the Landmark University Departments of Biochemistry and Microbiology for their technical support. Bruce Barron and Carey are appreciated for review and editorial assistance.
References
- 1.Gupta A.K., Mishra R., Lal R.K. Genetic resources, diversity, characterization and utilization of agronomical traits in turmeric (Curcuma longa L.) Ind. Crop. Prod. 2015;77:708–712. [Google Scholar]
- 2.Su Y., Ma L., Wen Y., Wang H., Zhang S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules. 2014;19(8):12630–12639. doi: 10.3390/molecules190812630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutrim C.S., Cortez M.A.S. A review on polyphenols: classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018;71(3):564–578. [Google Scholar]
- 4.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Sin-Yeang T., Kitson L., Syed A.A., Alan S.K., Suat-Cheng P. Antibacterial action of curcumin against Staphylococcus aureus: a brief review. J. Trop. Med. 2016:10. doi: 10.1155/2016/2853045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima G.P.P., Vianello F., Corrêa C.R., Campos R.A.D.S., Borguini M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014:1065–1082. [Google Scholar]
- 8.Zhang W., Guob Y., Hanb W., Yangb M., Wenc L., Wang K., Jian P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharm. 2019;67:138–144. doi: 10.1016/j.intimp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Moghadamtousi S., Abdul Kadir H., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014 doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai D., Singh J.K., Roy N., Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem. J. 2008;410(1):147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi P., Singh M., Kumari H., Kumari A., Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS One. 2015;10(3) doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlar I., Droby S., Choudhary R., Rodov V. The mode of antimicrobial action of curcumin depends on the delivery system: monolithic nanoparticles vs. Supramolecular inclusion complex. RSC Adv. 2017;7:42559–42569. [Google Scholar]
- 13.Yamamoto R., Yamamoto Y., Imai S., Fukutomi R., Ozawa Y., Abe M., Matuo Y., Saito K. Effects of various phytochemicals on indoleamine 2,3-dioxygenase 1 activity: galanal is a novel, competitive inhibitor of the enzyme. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A., Kasinathan A., Ganesan R., Balasubramanian A., Bhaskaran J., Suresh S., Sivalingam .N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species–independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018;51:67–81. doi: 10.1016/j.nutres.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Meng D., Wang Z., Guo H., Wang Y. Oxidative stress response in two representative bacteria exposed to atrazine. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2012;334(2):95–101. doi: 10.1111/j.1574-6968.2012.02625.x. [DOI] [PubMed] [Google Scholar]
- 16.Adeyemi O.S., Murata Y., Sugi T., Kato K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017;12:1647. doi: 10.2147/IJN.S122178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeyemi O.S., Sulaiman F.A. Co-administration of iron sulphate and nitroglycerin promoted oxidative stress and mild tissue damage in Wistar rats. Comp. Clin. Pathol. 2014;23(5):1525–1533. [Google Scholar]
- 18.Adeyemi O.S., Atolani O., Banerjee P., Arolasafe G., Preissner R., Etukudoh P., Ibraheem O. Computational and experimental validation of antioxidant properties of synthesized bioactive ferulic acid derivatives. Int. J. Food Prop. 2018;21(1):101–113. [Google Scholar]
- 19.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 20.Beutler O., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 21.Varshney R., Kale R.K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 22.Adeyemi O.S., Uloko R.A., Awakan O.J., Adeyanju A.A., Otohinoyi D.A. The oral administration of silver nanoparticles activates the kynurenine pathway in rat brain independently of oxidative stress. Chem. Biol. Interact. 2019;302:22–27. doi: 10.1016/j.cbi.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal J., Siddiqui R., Kazmi S.U., Khan N.A. A simple assay to screen antimicrobial compounds potentiating the activity of current antibiotics. BioMed Res. Int. 2013:4. doi: 10.1155/2013/927323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent I.S., Ehmann D.E., Mills S.D., Perros M., Barrett M.P. Untargeted metabolomics to ascertain antibiotic modes of action. Antimicrob. Agents Chemother. 2016;60(4):2281–2291. doi: 10.1128/AAC.02109-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esmaeilnejad B., Samiei A., Mirzaei Y., Farhang-Pajuh F. Assessment of oxidative/nitrosative stress biomarkers and DNA damage in Haemonchus contortus, following exposure to zinc oxide nanoparticles. Acta Parasitol. 2018;63(3):563–571. doi: 10.1515/ap-2018-0065. [DOI] [PubMed] [Google Scholar]
- 26.Borges A., Ferreira C., Saavedra M.J., Simoes M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 27.Gunes H., Gulen D., Mutlu R., Gumus A., Tas T., Topkaya A.E. Antibacterial effects of curcumin: an in vitro minimum inhibitory concentration study. Toxicol. Ind. Health. 2016;32(2):246–250. doi: 10.1177/0748233713498458. [DOI] [PubMed] [Google Scholar]
- 28.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longevity. 2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. Curcumin may (not) defy science. ACS Med. Chem. Lett. 2017;8(5):467–470. doi: 10.1021/acsmedchemlett.7b00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kominkova M., Michalek P., Cihalova K., Guran R., Cernei N., Nejdl L., Smerkova K., Dostalova S., Chudobova D., Heger Z., Vesely R., Gumulec J., Kynicky J., Xhaxhiu K., Zitka O., Adam V., Kizek R. Study of linkage between glutathione pathway and the antibiotic resistance of Escherichia coli from patients’ swabs. Int. J. Mol. Sci. 2015 Mar 31;16(4):7210–7229. doi: 10.3390/ijms16047210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linzner N., Loi V.V., Fritsch V.N., Tung Q.N., Stenzel S., Wirtz M., Hell R., Hamilton C.J., Tedin K., Fulde M., Antelmann H. Staphylococcus aureus uses the bacilliredoxin (BrxAB)/Bacillithiol disulfide reductase (YpdA) redox pathway to defend against oxidative stress under infections. Front. Microbiol. 2019;10:1355. doi: 10.3389/fmicb.2019.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loi V.V., Huyen N.T.T., Busche T., Tung Q.N., Gruhlke M.C.H., Kalinowski J., Bernhardt J., Slusarenko A.J., Antelmann H. Staphylococcus aureus responds to allicin by global S-thioallylation - role of the Brx/BSH/YpdA pathway and the disulfide reductase MerA to overcome allicin stress. Free Radic. Biol. Med. 2019;139:55–69. doi: 10.1016/j.freeradbiomed.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Perera V.R., Newton G.L., Pogliano K. Bacillithiol: a key protective thiol in Staphylococcus aureus. Expert Rev. Anti-infect. Ther. 2015;13(9):1089–1107. doi: 10.1586/14787210.2015.1064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingólfsson H.I., Thakur P., Herold K.F., Hobart E.A., Ramsey N.B., Periole X., de Jong D.H., Zwama M., Yilmaz D., Hall K. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014;9:1788–1798. doi: 10.1021/cb500086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Košťálová D., Bezáková L., Račkovác L., Mošovská S., Šturdík E. Therapeutic potential of curcumin in medicinal chemistry. Acta Chim. Slovaca. 2013;6(1):89–99. [Google Scholar]
- 36.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syng-ai C., Kumari A.L., Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol. Canc. Therapeut. 2004;3(9):1101–1108. [PubMed] [Google Scholar]
- 38.Adeyemi O.S. Gallotannin promoted cellular death through alteration of redox status. Comp. Clin. Pathol. 2018;27(5):1269–1272. [Google Scholar]