Abstract
In this study, antibacterial, antioxidant, and anticancer effects of Cu nanoparticles (CuNPs) fixed on cellulosic walnut shell material were investigated. Firstly, three types of walnut shell-supported copper nanoparticles with various sizes (CuNP-WS1 15–22 nm, CuNP-WS2 60–80 nm and, CuNP-WS3 aggregated of metallic nanoparticles) were synthesized. Antibacterial properties of CuNPs were studied on three strains of bacteria; Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes. DPPH (1, 1-Diphenyl-2-picrylhydrazyl) method was used to examining antioxidant properties. Cytotoxic effects of the synthesized nanoparticles on the cancer cell line were studied. Antimicrobial properties of CuNPs showed that these nanomaterials affect both Gram-positive and Gram-negative bacteria. The antioxidant properties of CuNPs increased significantly by increasing the concentration to 10%. CuNPs appeared to have a dose-dependent cytotoxic effect on K562 cells. However, the IC50 of the synthesized nanoparticles against the K562 (25.24 ± 5 μg/mL) cancer cells was lower significantly (P < 0.01) of the IC50 of these compounds against PBMCs (42.54 ± 6.2 μg/mL).
Keywords: Microbiology, Immunology, Material science of foods, Nanomaterials, Natural product synthesis, Antibacterial, Anticancer, Antioxidant, Cu nanoparticles, Walnut shell
Microbiology; Immunology; Material science of foods; Nanomaterials; Natural product synthesis; Antibacterial; Anticancer; Antioxidant; Cu nanoparticles; Walnut shell.
1. Introduction
Nanotechnology, known as manipulation science, is used to design and organize materials on a nanoscale. The control of nanoscale synthesis stages allows for the creation of new materials with unique features such as quantum size and high surface-to-volume ratio, flexibility, ductility, high yield strength, rigidity and macro quantum tunneling effect (Stoimenov et al., 2002; Din et al., 2017). These features make nanotechnology widely used in various industrial fields such as pharmaceutical and drug delivery, food and feed technology, cosmetics, biomedical, environmental health, electronics and energy, mechanics and space industries (Saranyaadevi et al., 2014). On the other hand, researchers have recently investigated the efficacy of nanoparticles against antibiotic-resistant microorganisms and the treatment of cancers. The antibacterial effect of the metal nanoparticles has been attributed to the small size and the high surface to particle size ratio. The presence of such properties allows particles to be able to interact more effectively, in direct contact with germs and bacteria, and consequently, gradually release metal ions along with the membrane (Liu et al., 2009). Studies showed that some of the oxidative nanoparticles act selectively so that they exert a very toxic and destructive effect when exposed to bacterial cells, although they do not have such an effect on human cells (Nowack et al., 2011).
Copper is one of the useful and necessary materials in the structure of the human body, and it causes the balance and stability of the tissues. Copper and its compounds are introduced by the American Environmental Protection Agency (EPA) as a potent antibacterial agent. Copper nanoparticles (CuNPs) quickly accumulate in colloid environments. The other advantage of CuNPs is the rapid oxidation and formation of a form of copper oxide that can be easily mixed with polymers or macromolecules and, over time, maintains some of its physical and chemical properties (Kim et al., 2007; Usman et al., 2012). Copper exhibits intense antibacterial activity against a wide range of microorganisms, so that, E. coli and B. subtilis bacteria are more susceptible to acceptance of the antibacterial effect of copper nanoparticles among other bacteria and hence, their percentage viability decreases with increasing nanoparticle concentrations (Schrand et al., 2010). Also, the Gram-negative bacteria react against the CuNPs in a shorter period time and kill faster than the other species (Theron et al., 2008). The antibacterial characteristics of silver nanoparticles have been widely studied and several possible mechanisms of possible action have been proposed. Based on one of these, silver nanoparticles penetrated inside the cell in contact with the cellar, resulting in the death of the cell (Sondi and Salopek-Sondi, 2004). The researchers also believe that the mechanism of action of CuNPs is similar to that of silver nanoparticles (Gogoi et al., 2006).
Many cancers initially respond to chemotherapy, but after a while, they develop their resistance to chemotherapy. Besides, chemotherapy drugs cause adverse reactions. Therefore developing an effective, inexpensive and environmentally friendly way to treat cancers is essential. On the other hand, nanoparticles have a specific capacity for delivering the drug and have the ability to photoluminescence efficiently (Sajja et al., 2009; Akhtar-Zaidi et al., 2012). Also, their very tiny size makes them useful for delivering targeted nanomedicines in organs such as the brain that is protected by a blood-brain barrier (Faraji and Wipf, 2009). Of course, these nanoparticles can also be used to treat cancers (Yang et al., 2010).
Nevertheless, to use CuNPs in various fields, it is essential to prepare it with low cost, easy availability, environment-friendly and green chemistry procedures. The green synthesis method is compatible with food and medical applications and the potential to enhance the antimicrobial and antioxidant effects. For this reason, biosynthesis of nanoparticles contains different biological bodies such as plants, has been considered by researchers (Din et al., 2017).
According to the principles of green chemistry, walnut shell-supported CuNPs were synthesized and investigated in previous studies (Zamani et al., 2018). The present study was set out to investigate the antibacterial and antioxidant effects. Furthermore, the cytotoxic effects of the synthesized nanoparticles on the cancer cell line were also studied.
2. Material and methods
2.1. Copper (II) sulfate anhydrous (for analysis) and sodium borohydride (for analysis) were purchased from Merck and used without further purification
2.1.1. Preparation of metal nanoparticles fixed on walnut shell
Three types of walnut shell-supported copper nanoparticles with various sizes (15–80 nm) were synthesized using our previously described procedure. Briefly, the walnut shells (WS) were washed in the shade and room temperature. Then crushed using a high-speed mill up to 40 mesh sizes. Ten g of WS and 100 mL of CuSO4 aqueous solution (0.03 M, 0.07 M and 0.14 M) were mixed in 50 °C for two hours. In the next step, water in a rotary evaporator was removed. The reduction of Cu2+ was carried out by the drop-wise addition of 0.25 M ethanol (Merck Millipore) solution of NaBH4 (30 mL, 70 mL, 140 mL) to the ethanol mixture of obtained solid and intensive stirring was continued for three hours. In the final stage Cu loading on WS was 1.35% (CuNP-WS1), 3.03% (CuNP-WS2) and 8.14% (CuNP-WS3), respectively, based on Atomic Absorption Spectroscopy (AAS) analysis (Zamani et al., 2018).
2.2. Antimicrobial effects of nanoparticles
2.2.1. Determination of MIC and MBC
The broth macro-dilution method was used for the determination of minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) (Alizadeh et al., 2014). Standard strains of Staphylococcus aureus (ATCC29923), Listeria monocytogenes (ATCC19115) and Escherichia coli (PTCC1533) bacteria were obtained from Laboratory of Department of Food Hygiene and Quality Control, Faculty of Veterinary Medicine, Urmia University, Iran. After that, the compounds were dissolved in dimethyl sulfoxide (DMSO, Merck Millipore) and then twofold serial dilutions were made to achieve concentration range from 4000 to 62.5 ppm in sterile test tubes containing BHI broth (Merck Millipore). Each tube contained 105 CFU/mL bacterial inoculum. For every experiment, two growth controls consisting of BHI broth without compounds and BHI broth containing DMSO inoculated with the diluted medium culture was selected. The contents in the tubes were mixed thoroughly and incubated at 37 °C for 24 h. The lowest concentration of each agent showing visually no growth was taken as its minimal inhibitory concentration (MIC). This confirmed by plating 100 mL samples from clear wells onto BHI agar medium. Furthermore, the lowest concentration of nano compounds that kills >99.9% of the initial bacterial population, considered as MBC.
2.2.2. Agar well diffusion method
The antimicrobial activity of nanoparticles was evaluated by the agar well diffusion method. Briefly, the nutrient broth medium (Merck Millipore) was used to prepare a bacterial suspension with a standard concentration of 0.5 McFarland. 0.1mLof the inoculums of test organism was spread uniformly on Muller Hinton agar medium (Merck Millipore) and then wells were prepared using a sterile cork borer. Cups were filled with 10 mg of each antimicrobial sample. Plates were incubated at 37 °C for 24 h. The zone of inhibition of bacterial growth around the well was measured with a caliper (Muraleedaran and Mujeeb, 2015).
2.3. Antioxidant effects of nanoparticles
For this purpose, the free radical scavenging ability on the 2-diphenyl-2-picrylhydrazyl (DPPH) (Merck, Darmstadt, Germany) method was used. In this method, different concentrations of nanoparticles, 10, 8, 6, 4, 2, and 1 mg/mL were prepared and 1.7 mL of 1 mM DPPH solution was added to each concentration. For the control sample, the walnut shell was used. All stages have done except the fixation of CuNPs on it. Samples were allowed to stand in a dark environment for 30 min). The supernatant was collected at 11,963g before measuring the absorbance at 517 nm using a spectrophotometer (S2100; Unico Dayton, USA). Then percentage free radical scavenging was calculated by applying the following formula:
Where,
Ac, is the absorbance of the control (containing all reagents except the test compound) and As, is the absorbance of the sample (Karunakaran et al., 2013).
2.4. K562 cells culture
The K562 erythroleukemia cell line was prepared from the Pasteur Institute of Iran. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Merck, Darmstadt, Germany), supplemented with 10% fetal calf serum (FCS) and penicillin plus streptomycin (100 IU/mL and 100 μg/mL, respectively) (Sigma Aldrich Chemical Co., Steinheim, Germany). The K562 cells were cultured in a 5% CO2 humidified atmosphere at 37 °C to reach 80% confluency.
2.5. PBMC isolation
Heparinized blood samples (20 mL) were gathered from the five volunteers. Samples were centrifuged at 400 g at 4 °C for 10 min to isolate a buffy-coat layer. The collected cells were diluted 1: 2 in PBS (Merck Millipore) and centrifuged over Histopaque 1083 for 30 min at 800 g in 18 °C. The cells were re-suspended and hypotonic lysis was used to remove contaminant erythrocytes. PBMCs were washed three times and then re-suspended in DMEM, which contained 10% fetal calf serum.
2.6. Cell treatment
The K562 cells or PBMCs were plated in 96-well flat-bottomed plates (1×105 cells/100 μl/well) and incubated for 24h serial dilution of Green CuO NPs (0, 50, 100, 200 and 400 μM).
After incubation, cultures were pulsed with 20 μl of a 5 mg/mL solution of MTT (Merck Millipore) in PBS at 37 °C for 4h. Then, the medium was removed and 150 mL DMSO was added. The plate was shaken vigorously to dissolve Formosan crystal and the optical density (OD) was measured using a microplate reader (Dynatech, Denkendorf, Germany) at 550 nm. The experiments were performed in triplicate and the results were reported as the survivability present according to the ratio of (OD550 of treated cells to OD550 of non-treated cells) × 100. The Inhibitory 50% concentration (IC 50) was determined by linear regression (Abtahi Froushani et al., 2014).
2.7. Statistical analysis
Data were analyzed using IBM SPSS software version 24. The IC50 was assessed by linear regression analysis using Minitab software (version 18, State College PA 16801-3210, USA).
3. Results
Three sizes of supported CuNPs were prepared via the mixing of walnut shell and Cu (II) aqueous solution followed by evaporation of water and reduction of copper ions by an ethanolic solution of NaBH4. The formation of copper nanoparticles was observed by transmission electron microscopy (TEM) and energy dispersive X-ray spectroscope (EDX) The structure stability of CuNPs was investigated by the thermogravimetric (TGA) under air (supplementary material) (Zamani et al., 2018). TEM image give 15–22 and 60–80 nm particle size for CuNP-WS1, CuNP-WS2 respectively and clear aggregation of nanoparticles and non-supported ones were detected in the case of CuNP-WS3 (supplementary material, Figure 1). The EDX patterns of CuNP-WS1 confirms that the nanoparticles are Cu. The carbon and oxygen peaks obtain from the walnut shell (supplementary material, Figure 2). The TGA results showed that CuNP-WS sample is stable up to 200 °C (supplementary material, Figure 3). From the FT-IR spectrum of CuNP-WS3 it is observed that the peak at 1622 cm−1 is assigned to carboxylate ions (-COO-) responsible for stabilization of copper (I) oxide NPs and the peak at 622 cm−1 is due to Cu–O vibration of Cu2O nanoparticles (supplementary material, Figure 4). Also based on the XPS spectra Cu2p3/2 and Cu2p1/2 signals centered at 932.7 and 952 respectively were consistent with Cu0 or Cu+ (supplementary material, Figure 5) (Zamani et al., 2018).
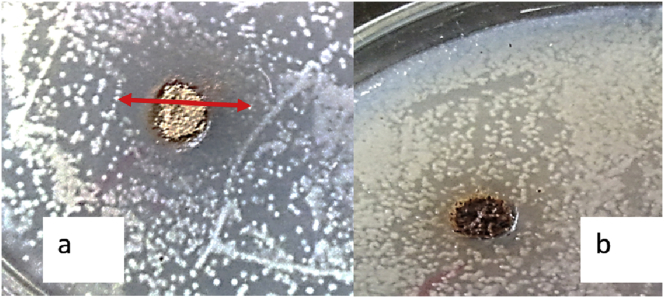
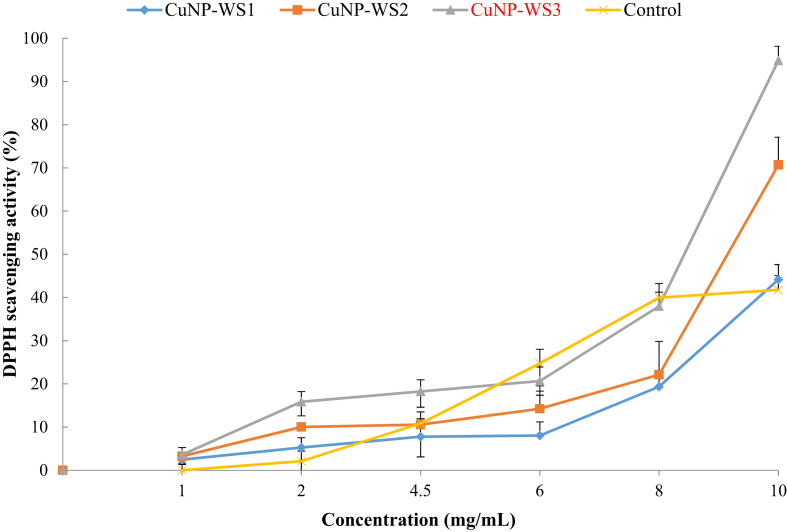
The results of the antimicrobial activity of the nanoparticles prepared by the diffusion method in the well are shown in Table and Figure 1. According to the results of Table 1, the highest antimicrobial effect was observed on L. monocytogenes as for CuNP-WS3 (15.0 ± 1.1 mm) and lowest as for CuNP-WS1 on S. aureus (6.3 ± 1.1 mm). Results of MIC and MBC of copper nanoparticles (Cu NPs) (Table 2) also confirm this difference. Percentage of DPPH free radical scavenging in different concentrations of CuNPs (%) presented in Figure 2. All samples showed antioxidant effect but the highest effect was observed in the CuNP-WS3 sample so that there was a significant difference (p ≤ 0.05) in concentrations of 2, 4.5 and 10 mg compared to the other samples.
Figure 1.
Pictures showing inhibition zones of Listeria monocytogenes exposed to CuNP-WS2 (a); Control (b).
Table 1.
Antimicrobial effect of Cu NPs by agar well diffusion method by diameter of the inhibition zone (mm).
| Concentration (mg/mL) | Staphylococcus aureus | Escherichia coli | Listeria monocytogenes | |
|---|---|---|---|---|
| CuNP-WS1 | 5 | 6.3 ± 1.1aA∗ | 6.41 ± 2.27aA | 9.8 ± 2.0aAB |
| CuNP-WS2 | 5 | 8.2 ± 2.5aA | 9.29 ± 0.5bA | 12.4 ± 0.9aB |
| CuNP-WS3 | 5 | 11.6 ± 1.4bA | 10.2 ± 1.2bA | 15.0 ± 1.1bB |
| ∗∗Control | - | 0cA | 0cA | 0cA |
∗Values are given as mean ± SD. Same row and the same column with different letters indicates significantly different (P < 0.05).
∗∗Control: Walnut shell powder.
Table 2.
MIC and MBC of Cu NPs (ppm).
| Bacteria |
Staphylococcus aureus |
Escherichia coli |
Listeria monocytogenes |
|||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| CuNP-WS1 | 1000 | ≥1000 | ≥1000 | ≥1000 | 500 | 1000 |
| CuNP-WS2 | 250 | 500 | 500 | 1000 | 125 | 250 |
| CuNP-WS3 | 125 | 250 | 250 | 500 | 62.5 | 125 |
| Control | 1000 | ≥1000 | 0 | 0 | 0 | 0 |
Figure 2.
Percentage of DPPH free radical scavenging in different concentration of CuNPs (%).
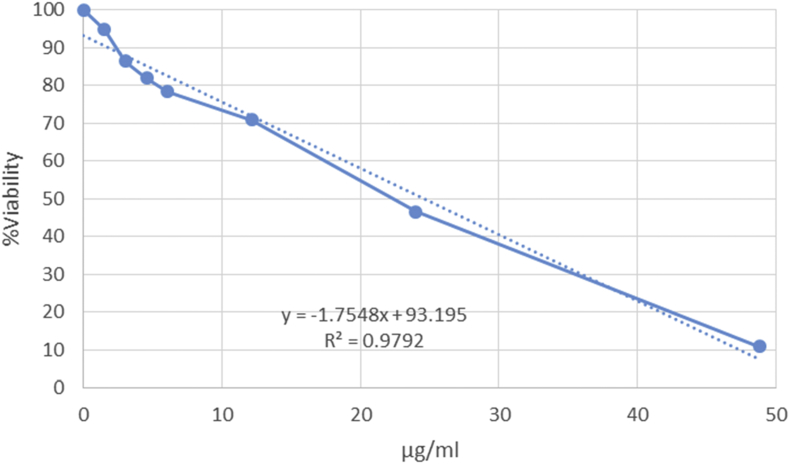
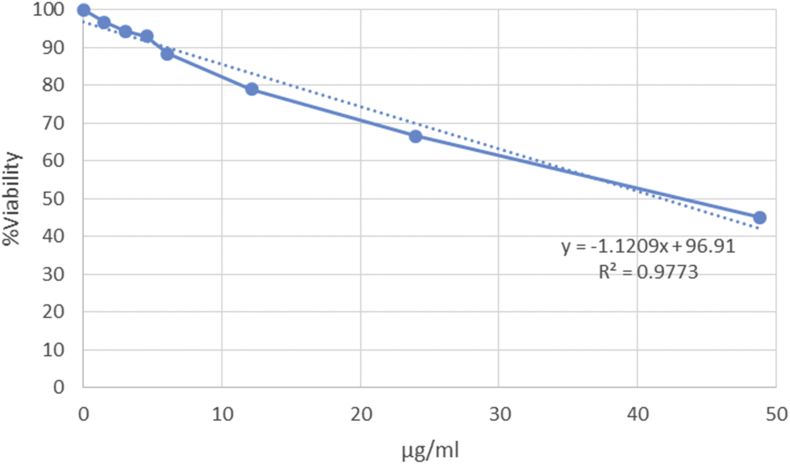
Statistical analysis of the correlation coefficient (R2) showed a linear relationship between the percent of viability and CuNP or PBMCs concentration. IC50 indexes were calculated based on the equation expressed in Figure 3 or 4. As shown in Figure 3, CuNP appears to have a dose-dependent cytotoxic effect on K562 cells. However, the IC50 of the synthesized nanoparticles against the K562 (25.24 ± 5 μg/mL) cancer cells was lower significantly (P < 0.01) of the IC50 of these compounds against PBMCs (42.54 ± 6.2 μg/mL) (Figures 3 and 4). Given that the effects of synthesized nanoparticles at much lower concentrations are capable of destroying 50% of the cancerous blood cells (K562) compared to healthy blood cells (PBMCs), this is partly specific.
Figure 3.
Evaluation of CuNPs effects on K562 cancer cells. Statistical analysis of correlation coefficient (R2) showed a linear relationship between the percent of viability and CuNP concentration. IC50 was calculated based on this equation: (50 (50% viability) = -1.7548x+93.195).
Figure 4.
Evaluation of CuNPs effects on healthy blood cells (PBMCs). Statistical analysis of correlation coefficient (R2) showed a linear relationship between the percent of viability and PBMC concentration. IC50 was calculated based on this equation: (50 (50% viability) = -1.2309x+96.91).
4. Discussion
Although chemical methods are available for the synthesis of CuNPs, there is a trend in the scientific literature on green synthesis of CuNPs. Cellulose is one of the most common organic polymers. In this study, a hard walnut shell was selected as the nanoparticle substrate. This substrate can to stabilize particles at the appropriate size due to its numerous phenolic groups. In the present study, Cu nanoparticles showed good antimicrobial effect against L. monocytogenese in compare to the other tested microorganisms. Our findings are in contrast with other reports that indicate higher activity of copper nanoparticles against Gram-negative microorganisms (Usman et al., 2013). Metal oxide nanoparticles are antimicrobial substances that are prepared with a high contact surface and specific crystalline structure. These nanoparticles have many active sites on their edges and corners (Klasen, 2000). The antibacterial effects of Cu nanoparticles may be associated with characteristics of certain bacterial species. There are differences in membrane structure between gram-positive and gram-negative bacteria (Das et al., 2010). CuNPs have been shown to generate reactive oxygen species (ROS) and to ultimately result in cell death (Yadav et al., 2017). In one study, copper nanoparticles were shown to act as an efflux inhibitor, and its antibacterial effect is due to the effects of particles itself, as well as the effects of ions (Christena et al., 2015). Also, CuNPs can cause changes in the structure of the membrane by binding to the cell wall of the bacteria and reacting with the membrane proteins, which ultimately leads to the release of intracellular materials and the death of the bacteria (Mallick et al., 2012).
The antimicrobial activity of copper nanoparticles has been demonstrated in several studies. Due to the variety of preparation methods, as well as the different antimicrobial protocols, it is difficult to compare their results. In one study, linoleic acid capped copper nanoparticles (150-100 nm) have been prepared through the reduction of cupric ions by ethanol and it was found to be much effective on S. aureus, E. coli and S. bacillus respectively (Das et al., 2010). In another study, the nanoparticles fixed on the surface of the cloth showed a decrease in the growth of Aspergillus niger, S. aureus and E. coli after 48 h of incubation (Duran et al., 2010). In the study of Miranda et al., the antibacterial and rheological properties of copper nanoparticles/PVC composites were studied. It has been shown that copper nanoparticles/PVC composites have a significant inhibitory effect on the growth of E. coli. The results of the study found that copper nanoparticles/PVC composite has excellent potential for use in medical applications (Miranda et al., 2018). In another study, the antibacterial activity of Cu Nanoparticles against some bacteria (E. coli, Staphylococcus aureus and Pseudomonas aeruginosa) were studied. CuNPs antibacterial activity was evaluated using well diffusion method. The most significant effect on bacteria was on E. coli and the least effect on Pseudomonas aeruginosa (Yadav et al., 2017). Lv et al. synthesized copper nanoparticles using Shewanella loihica PV-4 and studied its antibacterial properties. In this study, it has been shown that the synthesized copper nanoparticles, using the effect on the bacterial cell membrane and cytoplasmic compounds, cause the damage and death of E. coli Lv et al. 2018. Result of antimicrobial effects of nanoparticles in both methods showed that CuNP-WS3 had the most effective that indicating a direct relationship between copper concentration and antimicrobial activity. This is in complete agreement with the study that synthesized copper nanoparticle using Capparis Zeylanica leaf Extract, was investigated (Saranyaadevi et al., 2014).
DPPH is a stable compound that accepts hydrogen or electrons from silver nanoparticles. The results obtained in the DPPH assay showed effective free radical inhibition by CuNP-WSs. Similar findings with enhanced DPPH scavenging activity by platinum, selenium, silver nanoparticles (Watanabe et al., 2009; Huang et al., 2003; Reddy et al., 2014) have been reported. CuO nanoparticles exhibited free radical scavenging activity, which is relatively higher in comparison with other metal oxide nanoparticles (Das et al., 2013). Few studies have been conducted on the antioxidant effects of copper nanoparticles. In one study by Ghosh et al. (2015) antioxidant effect of copper nanoparticles synthesized by the medicinal plant (Dioscorea bulbifera) was studied. CuNPs showed 40.81 ± 1.44% scavenging activity against the DPPH radicals. In another study, the mean percentage inhibition values of synthesized CuNPs and powdered leaves of E. prostrata were 32, 34, 41, 46 and 53%; and 29, 32, 37, 43 and 48%, respectively (Chung et al., 2017). According to the results of DPPH test of CuNP-WS3 (Figure 1), by increasing the concentration from 1 to 10 mg/mL, percent of scavenging increase from 3.61 to 94.82, respectively. Our experiments confirm that DPPH activity of the nanoparticles increasees in a dose-dependent manner (Reddy et al., 2014). These results have some similarities with Dipankar and Murugan's (2012) findings. So that the I. herbstii using AgNPs exhibited more inhibition with more scavenging activity of the DPPH than I. herbstii leaf ethanolic extract (Dipankar and Murugan, 2012).
Wang et al. studied the toxic effects of Zn nanoparticles (Zn NPs) on the oral cancer cell line. The results of this study showed that Zn NPs treatment reduced the viability of cancer cells (Wang et al., 2018). In another study by Wei et al., it was shown that silica nanoparticles induce and enhance the human cellular cancer cell line autophagy. The results of both studies are consistent with the results obtained by our study. Also, in other studies, the effects of gold and silver nanoparticles were investigated on the hepatocellular carcinoma cell line, healthy alveolar cells and human lung cancer cell lines, respectively. In the case of nanoparticles and their use in the treatment of diseases and cancers, in addition to the effect of nanoparticles on cancer cells, their toxicity should also be evaluated on healthy cells of the body (Foldbjerg et al., 2011; Paino et al., 2012).
Traditional anticancer agents have many sides’ effects and caused a dramatic death in the normal cell such as hematopoietic and immune cells in addition to the cancer cell (Anand et al., 2008). Here, we used PBMCs as a normal cell for evaluation of unwanted side effects. It is clear that an agent with minimal cytotoxic effect against healthy cells and maximal cytotoxic effect against tumor cells is more favorable. The dose-dependent cytotoxicity of CuO NPs in contrast to cancer cell lines, as was described beforehand (Shafagh et al., 2015). Former studies advocated that CuO NPs caused cytotoxicity in mammalian cells through oxidative stress in a dose-dependent manner (Jose et al., 2011; Anand et al., 2008). The DNA damage is due to oxidative stress but, there is little information practically the genotoxicity of Green CuO NPs exposure to human cells (Ahamed et al., 2010). It suggested that the Cu NPs by inducing apoptosis have cytotoxic effects against Hela cells of human histiocytic lymphoma, human cervical cancer origins and U937, respectively (Jose et al., 2011). Here, we showed that Green CuO NPs had cytotoxic effects against K562 cell line. Of note, the IC50 value of the green CuO NPs against K562 cell line was higher than the IC50 value of the green CuO NPs against PBMCs. The results of the present study showed that CuNP was able to degrade 50% of blood cancer cells (K562) at significantly lower concentrations compared with healthy blood cells (PBMCs). Therefore, it seems reasonable to use this compound for in vivo studies, and it is expected that these nanoparticles will have a broad therapeutic index in possible in vivo studies.
5. Conclusion
The CuNPs were successfully synthesized by fixing on cellulosic walnut shell material. The data has already been published (Zamani et al., 2018). Antimicrobial properties of CuNPs showed that these nanomaterials affect both Gram-positive and Gram-negative bacteria. Similarly, it exhibited excellent antioxidant properties. CuNPs appeared to have a dose-dependent cytotoxic effect on K562 cells. Finally, the NPs evaluated in this work may be effectively utilized for many pharmaceutical and food industry applications.
Declarations
Author contribution statement
Tooraj Mehdizadeh, Asghar Zamani, Seyyed Meysam Abtahi Froushani: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Urmia University, Urmia, Iran.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supporting Information
References
- Abtahi Froushani S.M., Delirezh N., Hobbenaghi R., Mosayebi G. Synergistic effects of atorvastatin and all-trans retinoic acid in ameliorating animal model of multiple sclerosis. Immunol. Invest. 2014 Sep 24;43(1):54–68. doi: 10.3109/08820139.2013.825269. [DOI] [PubMed] [Google Scholar]
- Ahamed M., Siddiqui M.A., Akhtar M.J., Ahmad I., Pant A.B., Alhadlaq H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010 May 28;396(2):578–583. doi: 10.1016/j.bbrc.2010.04.156. [DOI] [PubMed] [Google Scholar]
- Akhtar-Zaidi B., Cowper-Sal-lari R., Corradin O., Saiakhova A., Bartels C.F., Balasubramanian D., Myeroff L., Lutterbaugh J., Jarrar A., Kalady M.F., Willis J., Moore J.H., Tesar P.J., Laframboise T., Markowitz S., Lupien M., Scacheri P.C. May 11 Epigenomic enhancer profiling defines a signature of colon cancer. Science. 2012;336(6082):736–739. doi: 10.1126/science.1217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh H., Salouti M., Shapouri R. Mar. Bactericidal effect of silver nanoparticles on intramacrophage Brucella abortus 544. Jundishapur J. Microbiol. 2014;7(3) doi: 10.5812/jjm.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B. Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. (N. Y.) 2008 Sep;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christena L.R., Mangalagowri V., Pradheeba P., Ahmed K.B., Shalini B.I., Vidyalakshmi M. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015;5:12899–12909. [Google Scholar]
- Chung I.M., Abdul Rahuman A., Marimuthu S., Vishnu Kirthi A., Anbarasan K., Padmini P., Rajakumar G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Therapeut. Med. 2017 Now;14(1):18–24. doi: 10.3892/etm.2017.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Gang S., Nath S.S., Bhattacharjee R. Linoleic acid capped copper nanoparticles for antibacterial activity. J. Bionanoscience. 2010;4:82–86. [Google Scholar]
- Das D., Nath B.C., Phukon P., Dolui S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces. 2013;101:430–433. doi: 10.1016/j.colsurfb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Din M.I., Arshad F., Hussain Z., Mukhtar M. Green adeptness in the synthesis and stabilization of copper nanoparticles: catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017;12(1):638. doi: 10.1186/s11671-017-2399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipankar C., Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces. 2012;98:112–119. doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Duran N., Marcato P.D., De Conti R., Alves O.L., Costa F.T.M., Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity, and possible mechanisms of action. Braz. Chem. Soc. 2010;21(6):949–959. [Google Scholar]
- Faraji A.H., Wipf P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009 Apr;17(8):2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- Foldbjerg R., Dang D.A., Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011 Jul;85(7):743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- Ghosh S., More P., Nitnavare R., Jagtap S., Chippalkatti R., Derle A., Kitture R., Asok A., Kale S., Singh S., Shaikh M.L. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J. Nanomed. Nanotechnol. 2015 Sep;(S6):1. [Google Scholar]
- Gogoi S.K., Gopinath P., Paul A., Ramesh A., Ghosh S.S., Chattopadhyay A. Green fluorescent protein-expressing Escherichia coli as amodel system for investigating the antimicrobial activities of silver nanoparticles. Langmuir. 2006 Oct;22(22):9322–9328. doi: 10.1021/la060661v. [DOI] [PubMed] [Google Scholar]
- Huang B., Zhang J., Hou J., Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003;35(7):805–813. doi: 10.1016/s0891-5849(03)00428-3. [DOI] [PubMed] [Google Scholar]
- Jose G.P., Santra S., Mandal S.K., Sengupta T.K. Mar. Singlet oxygen mediated DNA degradation by copper nanoparticles: potential towards cytotoxic effect on cancer cells. J. Nanobiotechnol. 2011;9(1):1–8. doi: 10.1186/1477-3155-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran G., Suriyaprabha R., Manivasakan P., Yuvakkumar R., Rajendran V., Kannan N. Screening of in vitro cytotoxicity, antioxidant potential and bioactivity of nano-and micro-ZrO2 and-TiO2 particles Ecotoxicol. Environ. Saf. 2013 Jul;1(93):191–197. doi: 10.1016/j.ecoenv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwan g C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Mar. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Klasen H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000 Mar;26(2):117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Liu Y., He L., Mustapha A., Li H., Hu Z.Q., Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009 Oct;107(4):1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- Lv Q., Zhang B., Xing X., Zhao Y., Cai R., Wang W., Gu Q. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. J. Hazard Mater. 2018 Apr;347:141–149. doi: 10.1016/j.jhazmat.2017.12.070. [DOI] [PubMed] [Google Scholar]
- Mallick S., Sharma S., Banerjee M., Ghosh S.S., Chattopadhyay A., Paul A. Feb. Iodine-stabilized Cu nanoparticle chitosan composite for antibacterial applications. ACS Appl. Mater. Interfaces. 2012;4(3):1313–1323. doi: 10.1021/am201586w. [DOI] [PubMed] [Google Scholar]
- Miranda C., Rodríguez-Llamazares S., Castaño J., Mondaca M.A. Cu nanoparticles/PVC composites: thermal, rheological, and antibacterial properties. Adv. Polym. Technol. 2018 Apr;37(3):937–942. [Google Scholar]
- Muraleedaran K., Mujeeb V.A. Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015 Jun;1(77):266–272. doi: 10.1016/j.ijbiomac.2015.03.058. [DOI] [PubMed] [Google Scholar]
- Nowack B., Krug H.F., Height M. Feb 15. 120 years of nanosilver history: implications for policy makers. Environ. Sci. Technol. 2011;45(4):1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- Paino I.M., Marangoni V.S., de Oliveira Rde C., Antunes L.M., Zucolotto V. Cyto and genotoxicity of gold nanoparticles in human hepatocellular carcinoma and peripheral blood mononuclear cells. Toxicol. Lett. 2012 Nov;215(2):119–125. doi: 10.1016/j.toxlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Reddy N.J., Vali D.N., Rani M., Rani S.S. Aug. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C. 2014;34:115–122. doi: 10.1016/j.msec.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Sajja H.K., East M.P., Mao H., Wang Y.A., Nie S., Yang L. Mar. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr. Drug Discov. Technol. 2009;6(1):43–51. doi: 10.2174/157016309787581066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranyaadevi K., Subha V., Ravindran R.E., Renganathan S. Sep. Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int. J. Chem. Tech. Res. 2014;6(10):4433–4454. [Google Scholar]
- Schrand A.M., Rahman M.F., Hussain S.M., Schlager J.J., Smith D.A., Syed A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010 Sep;2(5):554–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- Shafagh M., Rahmani F., Delirezh N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran J. Basic Med. Sci. 2015 Oct;18(10):993–1000. [PMC free article] [PubMed] [Google Scholar]
- Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004 Jul;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002 Jul;18(17):6679–6686. [Google Scholar]
- Theron J., Walker J.A., Cloete T.E. Nanotechnology and watertreatment: applications and emerging opportunities. Crit. Rev. Microbiol. 2008;34(1):43–69. doi: 10.1080/10408410701710442. [DOI] [PubMed] [Google Scholar]
- Usman M.S., Ibrahim N.A., Shameli K., Zainuddin N., Junus W.M. Copper nanoparticles mediated by chitosan: synthesis and characterization via chemical methods. Molecules. 2012 Dec;17(12):14928–14936. doi: 10.3390/molecules171214928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M.S., El Zowalaty M.E., Shameli K., Zainuddin N., Salama M., Ibrahim N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013;8:4467. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gao S., Wang S., Xu Z., Wei L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018 Jun;13:3441–3450. doi: 10.2147/IJN.S165699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Kajita M., Kim J., Kanayama A., Takahashi K., Mashino T., Miyamoto Y. Now. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology. 2009;20(45):455105. doi: 10.1088/0957-4484/20/45/455105. [DOI] [PubMed] [Google Scholar]
- Wei F., Wang Y., Luo Z., Li Y., Duan Y. New findings of silica nanoparticles induced ER autophagy in human colon cancer cell. Sci. Rep. 2017 Feb;14(7):1–11. doi: 10.1038/srep42591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav L., Tripathi R.M., Prasad R., Pudake R.N., Mittal J. Antibacterial activity of Cu nanoparticles against E. coli, Staphylococcus aureus and Pseudomonas aeruginosa. Nano Biomed. Eng. 2017;9(1):9–14. [Google Scholar]
- Yang T.P., Beazley C., Montgomery S.B., Dimas A.S., Gutierrez-Arcelus M. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics. 2010 Oct;26(19):2474–2476. doi: 10.1093/bioinformatics/btq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani A., Poursattar Marjani A., Nikoo A., Heidarpour M., Dehghan A. Synthesis and characterization of copper nanoparticles on walnut shell for catalytic reduction and C-C coupling reaction. Inorg. Nano-Met. Chem. 2018;48(3):176–181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information