Abstract
Background and aims
B cells involvement in animal models of atherosclerosis has been unequivocally established. However, the role of these cells in patients with atherosclerosis is almost unknown. Besides the production of antibodies, B cells can also exhibit regulatory functions mainly through IL-10. Here, we characterized human B cell subsets, their production of IL-10 in patients with atherosclerosis and their potential association with inflammation.
Methods
Patients with confirmed atherosclerotic events and controls with low cardiovascular risk were included. B cells subsets were determined in mononuclear cells (PBMC) using flow cytometry. PBMC were cultured ex vivo (5 h) and in vitro (48 h) to determine IL-10+ B cells and in some cases TNF-α+ and IFN-γ+ CD4+ T cells. The inflammatory state of the participants was determined through high sensitivity C reactive protein levels.
Results
Increase in percentage and number of plasmablasts was observed in patients with atherosclerosis compared with controls. A decreased frequency of IL-10+ B cells was observed in patients, both in ex vivo and in vitro cultures. This decrease was detected in transitional, memory, and plasmablast subsets. Interestingly, the reduction of IL-10+ B cells negatively and significantly correlated with the inflammatory condition of the studied subjects and associated with an increased frequency of TNF-α+ and IFN-γ+ CD4+ T cells. The blockade of IL-10R did not show further effect in T cells activation.
Conclusions
There is an association between the inflammatory state and a reduction of IL-10+ B cells that could contribute to the development of atherosclerosis.
Keywords: Immunology, Inflammation, Health sciences, Cardiology, Cardiovascular system, Immune system, Immune disorder, Atherosclerosis, B cells, Regulatory B cell, IL-10, B cell subset, C reactive protein, Cholesterol
Immunology; Inflammation; Health sciences; Cardiology; Cardiovascular system; Immune system; Immune disorder; Atherosclerosis, B cells, Regulatory B cell, IL-10, B cell subset, C reactive protein, Cholesterol.
1. Introduction
Atherosclerosis is considered a systemic inflammatory disease, and is also considered as the main cause of cardiovascular diseases, in which both innate and adaptive immunity play important roles [1]. It has been suggested a potential contribution of autoimmune responses in atherogenesis and progression of atherosclerotic plaques [2, 3]. This concept is partially based on the presence of different autoantibodies against heat-shock protein 60/65 and oxidized low density lipoprotein (oxLDL) in peripheral blood in these patients and in animal models [4, 5, 6, 7]. Also, there is association between the titles of these autoantibodies and the severity of coronary heart disease and myocardial infarction [7, 8, 9, 10]. This suggests that humoral immune responses and B cells seem to contribute to atherosclerosis development.
Actually, different evidence in animal models have unequivocally revealed the involvement of B cells in atherosclerosis. For example, depletion of mature B cell with anti-CD20 or anti-B cell activating factor-receptor (BAFFR) monoclonal antibodies, reduces the size of atherosclerotic plaques in hyperlipidemic apolipoprotein E-deficient (apoE−/−) mice [11, 12]. However, only a few B cells are located within the atheroma, and the vast majority are found in the adventitial layer forming tertiary lymphoid organs [13]. In these structures, the presence of B2 B cells together with T follicular helper cells, plasma cells and germinal center reactions have been described [14]. On the other hand, circulating B2 cells subset appear to have proatherogenic effect, while the innate and non-circulating B1 cells seem to exert a protective role [15, 16]. The deleterious effect of B2 cells have been related to the production of IgG and IgE antibodies, whereas the protective effect of B1 cells has been associated with IgM antibodies which recognize oxidized specific epitopes [16, 17]. Nevertheless, there is some contradictory information regarding the function of B2 cells in atherosclerosis [18].
The evidence about the role of B cells in atherosclerosis is scarce in humans, but it has been proven that there is both dysregulation and activation of B cells in atherosclerotic coronary heart disease, as was seen through whole blood gene expression profiles and enrichment analysis [19]. The immunostaining of peri-renal aortic specimens from 114 samples confirmed the extensive and progressive presence of cellular components of adaptive immunity, including B cells, in this atherosclerotic tissue [20]. B cells mainly infiltrate the adventitia, developing small lymph follicles, as observed in human samples of coronary atherosclerotic lesions evaluated by immunohistochemistry [21]. Carotid tissue from 19 patients undergoing carotid endarterectomy showed that the adventitial resident B2 lymphocytes were mostly mature oligoclonal cells that expressed an activated phenotype and positive stain for IL-6 and TNF-α. Interestingly, the immunoglobulin repertoires of the clones isolated from these samples were different between adventitia and the draining lymph nodes, suggesting that those cells were expanded in situ or that they came from different sources [22].
B cells have been described as cells with regulatory capabilities, mainly through IL-10 production, both in mice and in humans. Different B cell subsets seem to be capable to produce IL-10 and to negatively modulate T cell responses and therefore these cells are considered as regulatory B cells (Breg) [23, 24, 25, 26]. IL-10 is an anti-inflammatory cytokine and a key element in the dysregulation of the immune response in patients with atherosclerosis, with well-known anti-atherogenic properties [27]. However, the involvement of Breg has only been studied in murine models of atherosclerosis with conflicting results [28, 29]. This could be related with the fact that different B cell subsets produce IL-10 and can regulate the production of IFN-γ and TNF-α in hyperlipidemic apoE−/− mice [30]. However, the evidence regarding the distribution of B cell subsets and their IL-10 production by human patients with atherosclerosis is even scarcer. The mRNA and protein levels of IL-10 have been studied in total B cells from atherosclerotic patients by RT-PCR and western blot, showing that they were significantly lower compared with healthy controls [22, 31].
Hence, the characterization of human B cell subsets and their production of IL-10 would help to better understand the involvement of these cells in human atherosclerosis, and to clarify which of these subsets truly have a pro or anti-atherogenic role. In this study, we evaluated the frequency of circulating B2 cell subsets (Memory, Mature and Transitional) and their IL-10 production in patients with atherosclerosis.
2. Materials and methods
2.1. Patients and controls
Patients with confirmed previous atherosclerotic events (myocardial infarction, stroke or acute limb ischemic event) from the cardiovascular unit at Hospital Universitario San Vicente Fundación (HUSVF, Medellin, Colombia), were included in this study; as well as controls with low cardiovascular risk (LCVR) according to Framingham score [32], defined as healthy donors with a calculated risk lower than normal risk from general population. This score was calculated using Cardiovascular Disease tool for 10-year risk (available at www.framinghamheartstudy.org). The main demographic and clinical data from patients and LCVR are shown in Table 1. Atherosclerotic patients were under different treatments with captopril, metoprolol, warfarin, acetylsalicylic acid and statins. Patients and controls were paired by gender and age range. Only controls with a Framingham score lower than 9% were included for the analysis of B cells; therefore, there is smaller number of controls than patients in those results. All patients and controls signed an informed consent previously approved by the ethics committee from the Instituto de Investigaciones Médicas (Facultad de Medicina, Universidad de Antioquia, Medellin, Colombia) and HUSVF with file number 014–2011.
Table 1.
Main demographic and clinical data from patients and LCVR.
| LCVRa | Patientsa | |
|---|---|---|
| Age (years old) | 55 (45–67) | 57 (47–67) |
| n | 19 | 19 |
| Sex (Men/Women) | 12/7 | 8/11 |
| Framingham score | 8.9 (2.7–19.5) | 30.7 (5.3–86) **** |
| Body Mass Index (kg/m2) | 22.4 (18.13–27.68) | 25.1 (21.1–40) ** |
| Blood pressure (mmHg) | 120 (100–125) | 140 (115–180) **** |
| HDLb levels (mg/dL) | 57.48 (31.48–87.81) | 35 (17–53) **** |
| Cholesterol (mg/dL) | 201.95 (150.7–260) | 206 (132–296) |
| hsCRPc (μg/mL) | 1.3 (0.8–5.6) | 7.5 (4.6–85.3) *** |
Comparisons between patients and LCVR were performed using Mann−Whitney test, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Median and range.
HDL (High density lipoproteins).
hsCRP (high sensitivity C reactive protein).
2.2. Isolation of mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood from healthy individuals and patients by centrifugation on Histopaque (Sigma-Aldrich, MO) at 900 xg, 30 min. Cell suspensions were washed twice with phosphate-buffered saline (PBS, Gibco, NY) at 250 xg, 10 min and room temperature. Total cell number was calculated in Neubauer chamber and viability by trypan blue exclusion (Sigma-Aldrich), and Live/Dead staining (viability >98%, data not shown).
2.3. Detection of IL-10 producing B cells
All cultures were performed in RPMI 1640 Glutamax medium (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS, Gibco), 4 mM L-glutamine (Gibco) and 1% penicillin/streptomycin (Gibco). PBMC were cultured at a concentration of 2 × 106 cells/mL in the media described in two types of culture: 1) Ex vivo stimulation with 10 μg/mL lipopolysaccharide (LPS from Escherichia coli, serotype 0111:B4, Sigma-Aldrich) or 10 μg/mL CpG (ODN2006, Invivogen, CA), combined with 50 ng/mL PMA (phorbol 12-myristate 13-acetate, Sigma-Aldrich) and 1 μg/mL Ionomycin (Sigma-Aldrich), in the presence of 5 μg/mL Brefeldin A (Sigma-Aldrich) (PIB) for 5 h. 2) In vitro stimulation with an anti-CD40 agonist (Clone HM40-3, Becton Dickinson (BD), CA) for 48 h, with re-stimulation at the last 5 h with LPS + PIB or CpG + PIB as explained for ex vivo culture. As control, cells were cultured without LPS, CpG, PMA and Ionomycin, in the presence of Brefeldin A in the last 5 h. Subsequently, flow cytometry was performed to detect IL-10+ B cells as it is explained forward.
Also, IL-10R blocking antibody (CDw210a, clone 3F9 from BD Biosciences) was used in some cultures of total PBMC from controls and patients with in vitro stimulation.
2.4. Multiparametric flow cytometry
Cell suspensions were washed with PBS at 600 ×g for 5 min at 4 °C. Cells were incubated with Live/Dead Fixable Aqua Dead Cell Stain Kit (Invitrogen, CA) for 15 min and washed twice with PBS. Cell pellets were incubated with blocking buffer (10% FBS, 0.1% bovine serum albumin (Sigma- Aldrich), and 0.01% sodium azide (Sigma-Aldrich) in PBS, pH = 7.35) for 15 min at 4 °C. Specific monoclonal antibodies were added in 100 μL of PBS and incubated for 25 min at 4 °C in the dark. Anti-human CD1d-PE (51.1), CD4-Pacific Blue (OKT4), CD19-Pacific Blue (HIB19), CD24-FITC (ML5), CD27-PE-Cy7 (O323), CD38-PerCP (HIT2), CD40-APC (5C3), CD80-APC (2D10), CD86-PE-Cy5 (IT2.2), IL-10-APC (JES3-19F1), IFN-γ-APC (4SB3), and TNF-α-PE-Cy7 (MAb11) monoclonal antibodies were purchased from Biolegend (CA). Cells suspensions were washed with PBS and then fixed with Foxp3/Transcription Factor Staining Buffer Set Kit (eBiosciences, CA) for 1 h. Then, cells were washed with permeabilization buffer from the same kit and centrifuged at 600 ×g for 5 min at 4 °C. Antibodies against IL-10, TNF-α or IFN-γ were incubated for 30 min in 100 μL of permeabilization buffer from the same kit. Finally, cells were washed with permeabilization buffer and PBS. Cells were acquired with the flow cytometer FACS Canto-II (BD). Data were analyzed using FlowJo software vX (Tree Star, CA) and reported as percentage of viable cells (live/dead negative). B cell subsets studied were: Memory (CD19+CD24hiCD38−), Mature (CD19+CD24low/−CD38low/−), Transitional (CD19+CD24hiCD38hi), and Plasmablast (CD19+CD24−CD38hi).
2.5. Mononuclear cell culture
PBMC from controls and patients were cultured with 0.5 μg/mL anti-CD3ε (BD, clone HIT3a) and 1 μg/mL CD40L for 48 h. Cells were re-stimulated with PIB at the last 5 h and immediately labeled and acquired in a flow cytometer as previously described. Cultures were performed in the presence or absence of 10 μg/mL anti-CDw210a (anti-IL-10Ra, clone 3F9, BD). Results were reported as percentage of TNF-α+ and IFN-γ+ viable CD4+ T cells.
2.6. Determination of cholesterol levels
Serum lipid profile (cholesterol, LDL and HDL) was determined on Dimension RxL Max clinical chemistry system (DADE Behring, IL) in IPS Universitaria (Universidad de Antioquia).
2.7. Human serum C reactive protein (CRP) levels
Serum CRP levels were measured by Human hsCRP ELISA (Biovendor, Czech Republic) following manufacturer's instructions and using an ELx800 Absorbance Microplate Reader (Biotek Instruments, VT) at 450 nm OD.
2.8. Statistical analysis
To compare the frequency of IL-10+ B cells between patients and controls, a Mann−Whitney test was performed. Correlations were performed with the Spearman correlation coefficient. Data was analyzed in Prism 6.0 software (GraphPad, CA), and p values < 0.05 were considered statistically significant. Data from cultures were evaluated using Two-way ANOVA with multiple comparisons test of Šidák. All comparisons were performed among the study groups; however, only those that were statistically significant were highlighted in the figures.
3. Results
3.1. Increased frequency and number of plasmablasts in patients with atherosclerosis
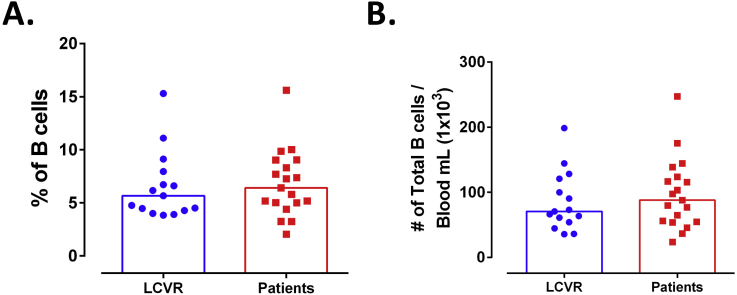
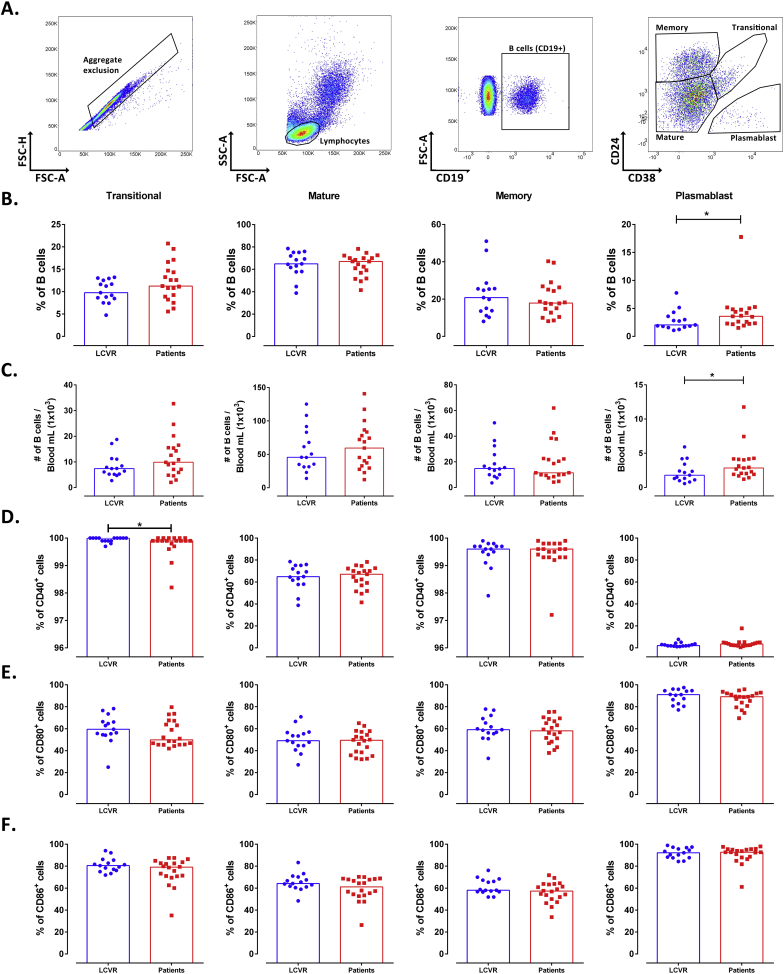
There were not differences in the frequency and number of total B cells between patients with atherosclerosis and LCVR controls (Figure 1). Total PBMC were analyzed according to the expression of CD19, CD24 and CD38 by flow cytometry in Transitional, Mature, Memory, and Plasmablast subsets (Figure 2A). As expected, most of B cells correspond to the mature phenotype (Figure 2B). A significant increase in the frequency and number of plasmablasts in patients was observed compared with LCVR controls (Figure 2B, C). The percentage of CD40+ B cells was lower in Transitional subset of patients compared with LCVR controls (Figure 2D). There were no differences in the frequency of the evaluated B cell subsets expressing CD80 and CD86 among patients and controls (Figures 2E, F). Nor were any significant differences in the expression of these molecules (mean fluorescence intensity, MFI) in the B cell subsets of atherosclerotic patients compared with controls (Data not shown).
Figure 1.
No changes in frequency and number of total B cells in patients with atherosclerosis. A. Frequency and B. number of B cells (CD19+) in mononuclear cells. Median and data from 15 LCVR and 20 patients are shown. Mann-Whitney test.
Figure 2.
Increased frequency and number of Plasmablasts in patients with atherosclerosis. A. Strategy of analysis of PBMC: Aggregate exclusion (Left), selection of lymphocytes (Center-left), selection of B cells (CD19+) (Center-right), and B cell subsets (Right) according to CD24 and CD38 expression. Transitional (CD19+CD24hiCD38hi), Mature (CD19+CD24low/−CD38low/-), Memory (CD19+CD24hiCD38 low/-) and Plasmablast (CD19+CD24-CD38hi). B. Frequency and C. number of B cell subsets. D-F. Frequency of D. CD40+, E. CD80+ and F. CD86+ cells on Transitional, Mature, Memory and Plasmablast subsets. Median and data from 15 LCVR and 20 patients are shown. Mann−Whitney test. *p < 0.05.
3.2. Low frequency of IL-10+ B cells in atherosclerotic patients
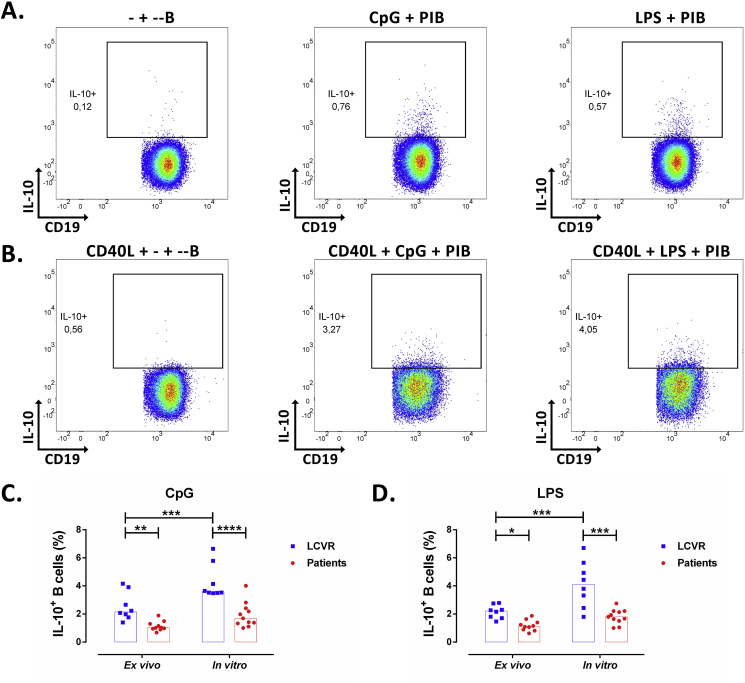
Two different types of cultures were performed to evaluate IL-10 production by B cells, ex vivo and in vitro, as it was explained in detail in Materials and Methods. Total B cells were selected from the region of viable cells (Live/Dead negative) according to the expression of CD19 in a similar way as mentioned for Figure 2A. In all cases, the selection of IL-10+ cells in total B cells stimulated with CpG and LPS was based on the detection of this cytokine in cells cultured only with Brefeldin A (Figures 3A, B). As expected, in vitro culture allows the detection of a higher percentage of IL-10+ B cells, with both LPS and CpG treatments, compared with ex vivo culture. Also, no differences were observed in the frequency of IL-10 positive B cells, between CpG and LPS stimuli (Figure 3). Decreased frequency of IL-10+ B cells was observed in PBMC of patients compared with LCVR controls, after ex vivo and in vitro cultures and with both classes of stimuli (Figures 3C, D).
Figure 3.
Decreased frequency of IL-10+ B cells in patients with atherosclerosis. A-B. Representative pseudocolor plots of IL-10+ B cells according to IL-10 and CD19 expression under A. ex vivo and B. in vitro cultures of mononuclear cells from a control. IL-10+ B cells in cultures in absence (Left) or with re-stimulation with CpG (Center) or LPS (Right) plus PIB in the last 5h in both type of cultures. Frequency of IL-10+ B cells under ex vivo and in vitro stimulations and re-stimulation with C. CpG plus PIB and D. LPS plus PIB as showed in A and B. Median and data from 8 LCVR and 10–11 patients. Two-way ANOVA with Šidák post-test. ****p < 0.0001 ***p < 0.001, **p < 0.01, and *p < 0.05.
3.3. Low frequency of IL-10+ cells in almost all B cell subsets of atherosclerotic patients
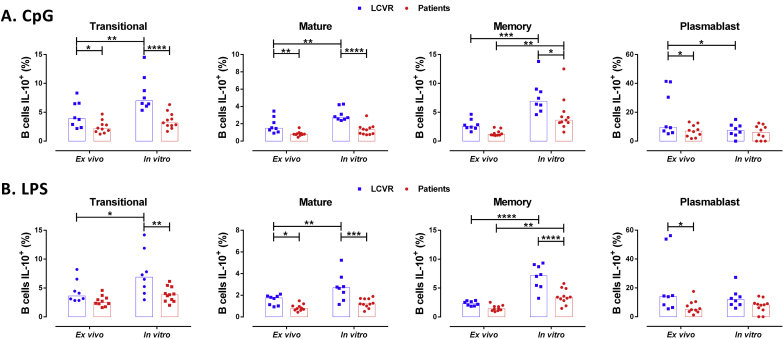
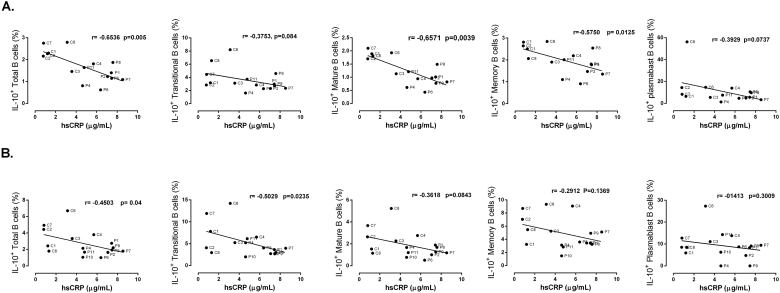
To verify which B cell subset was associated with the changes described in patients, the frequency of IL-10+ cells in each B cell subset was evaluated (Figure 4). In all B cell subsets, the decreased production of IL-10 was detected in patients with atherosclerosis; however, Transitional, Memory, and Plasmablast presented the lowest percentage of IL-10+ B cells, compared with controls. Under CpG stimulation, decreased frequency of IL-10+ B cells was observed in Transitional, Mature, and Memory subsets, but not in Plasmablast (Figure 4A); whereas under LPS treatment, this reduction was detected in all B cell subsets (Figure 4B). Interestingly, there was a negative correlation between the frequency of IL-10+ B cells and the levels of hsCRP in serum, which was observed in total cells and in the different B subsets and were more significant with the ex vivo culture data compared with in vitro culture after LPS stimulus (Figure 5), and was not detected with CpG data (Data not shown).
Figure 4.
Decrease in the frequency of IL-10+ B cell subsets in patients with atherosclerosis. A-B. Frequency of IL-10+ B cell subsets under ex vivo and in vitro stimulation and re-stimulation with A. CpG or B. LPS plus PIB in the last 5 h as described in Materials and Methods. Median and data from 8 LCVR and 10–11 patients. Two-way ANOVA with Šidák post-test. ****p < 0.0001, ***p < 0.001, **p < 0.01 and *p < 0.05.
Figure 5.
Correlations between the frequency of IL-10+ B cells and hsCRP levels in serum. Spearman correlation between serum hsCRP levels and the percentage of IL-10+ in total B cells and each B cell subset after A. ex vivo and B. in vitro cultures with LPS. Data from 7 controls (C) and 9–10 patients (P) are shown.
On the other hand, similar results were obtained after a further analysis of B cell subsets including the evaluation of Immature cells (CD19+CD10+) (Data not shown).
3.4. Altered regulation of CD4+TNF-α+ and CD4+IFN-γ+ cells by B cells in atherosclerotic patients
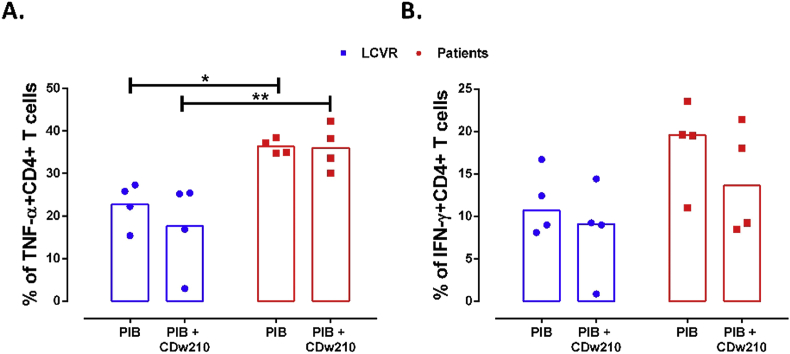
To prove whether the decreased frequency of IL-10+ B cells could have any functional consequence in the activation of CD4+ T cells, total PBMC from patients and controls were cultured with T (anti-CD3ε) and B stimuli (CD40L). The frequency of CD4+ TNF-α+ and IFN-γ+ T cells was increased in atherosclerotic patients compared with LCVR controls. This difference was statistically significant only for TNF-α+ cells (Figure 6). However, the addition of IL-10R blocking did not have any impact in the frequency of TNF-α+ and IFN-γ+ CD4+ T cells (Figure 6). These results suggested that CD4+ T cells from patients had an increased inflammatory response compared with controls, which seem to be related with less regulatory function of B cells; however, the frequency of TNF-α+ and IFN-γ+ CD4+ T cells did not seem to be regulated through IL-10, since its blockade had no effect on the cultures from patients and controls.
Figure 6.
Increase in the frequency of TNF-α+ CD4+ T cells in PBMC from patients with atherosclerosis. Culture of total PBMC from controls and patients with anti-CD3ε plus CD40L during 48 h with re-stimulation with PIB in the last 5 h, in the presence or absence of IL-10R blocking antibody (CDw210a). Frequency of A. TNF-α+ and B. IFN-γ+ CD4+ T cells. Median and data from 4 LCVR and 4 patients are shown. Two-way ANOVA with Šidák post-test. **p < 0.01 and *p < 0.05.
4. Discussion
Several studies have shown that white blood cells count associates with acute infarction mortality [33] and is a predictor factor of recurrent ischemic events [34]. Although we did not find differences in B cells count between patients and controls, our results showed a significant increase in the frequency and number of plasmablasts in patients compared with LCVR controls, which could be relevant due to their role as antibodies and cytokines producing cells. Even though there is not consensus about the function of plasmablasts as cytokine producer cells in humans, this function has been probed in mouse models [35]. There is scarce information of the role of B cells in human atherosclerosis, some studies have shown that high levels of B-cell specific cytokines such as CCL7 and BAFF predict increased risk of death and recurrent myocardial infarction [36]. This agrees with the main detection of mature B2 (conventional) plasmablasts in arterial adventitia from atherosclerotic patients [22]. These and our results suggest that B cells, and specifically plasmablasts may play important roles in human atherosclerosis.
Some molecules such as CD80, CD86 and CD40 have been reported to be necessary for regulation by B cells [25, 37]. CD40 molecule has been described as relevant for stimulating B cells regulatory capacity, through interaction with activated T cells expressing CD40L (CD154) [38]. Contrary to previous results from our group in apoE−/− mice with atherosclerosis [39], herein we showed a decreased percentage of transitional CD40+ B cells in patients compared with LCVR controls; which could suggest that signaling through CD40 in this B cell subset [40] could be altered in atherosclerotic patients. In accordance with this, it was observed that patients with high percentages of CD40+ B cell, exhibit a lower risk of stroke [41]. Therefore, it is possible to speculate that the low frequency of CD40+ cells observed in Transitional subset of patients with atherosclerosis could contribute partially to the less percentage of IL-10+ B cells.
In addition, previous results from our group have shown that exposure of mice B cells to hyperlipemic conditions is sufficient to promote lipid accumulation in these cells and to down regulate CD19 surface expression [42]. However, CD19 expression was not different between B cells from patients and controls. This difference between mouse model and human atherosclerosis could suggest differences in the effects of circulating lipids on B cells between species or could be related with the drug treatment that those patients receive.
IL-10 has been described as the main molecule involved in the regulation executed by B cells [23, 24, 38]. As expected [25, 43], in vitro culture allows the detection of a higher percentage of IL-10+ B cells with both LPS and CpG treatments compared with ex vivo culture; which is explained because B cells are capacitated under in vitro culture, whereas in ex vivo cultures we are detecting the IL-10 of in vivo capacitated cells. Stimulation with CpG and LPS has been used in order to trigger the regulatory capacity of Bregs [38, 44]. Decreased frequency of IL-10+ B cells was observed in PBMC of patients compared with LCVR controls, after ex vivo and in vitro cultures and with both classes of stimuli. These results showed that all B cell subsets of atherosclerotic patients have a wide alteration in IL-10 production, which has been previously described as protective in this disease [45].
Similar results have been reported in patients with systemic lupus erythematosus [25], other chronic inflammatory disease in which atherosclerosis development is accelerated [46]. Our group has reported in apoE−/− atherosclerosis mouse model an increase in IL-10+ B cells compared with control mice, showing that most of the evaluated IL-10+ B cell subsets presented an increased frequency in atherosclerotic mice [30], contrary to the observation here in atherosclerotic patients. Interestingly, lower capacity of one type or regulatory B cells (Tim1+) to up-regulate IL-10 production after anti-BCR plus CpG stimulation was previously reported in patients [47]. Although it is no clear what is the function of IL-10 produced by B cells in atherosclerosis and the mechanism involved in this defect, the reduction of IL-10+ B cells in humans could have an important impact in the pathophysiology of this disease because IL-10 have a clear atheroprotective role [48].
In the same line of evidence, we also observed significant increased frequency of TNF-α+ CD4+ T cells in PBMC from patients with atherosclerosis, compared with controls. Also, even though not statistically significant, we could observe a trend towards an increase in IFN-γ+ CD4+ T cells. This could be explained by an altered regulatory capacity of B cell subsets in atherosclerosis. However, the blockade of IL-10 apparently did not induce any difference in the frequency of TNF-α-producing CD4+ T cells, which suggests that the regulation executed by B cells in atherosclerotic patients does not depend directly on this cytokine; so other regulatory mechanisms of B cells could be altered in these patients that need to be explored in further studies. However, it worth mentioning that we cannot discard the effect of other cells than B cells in the evaluated CD4 responses, since the experiments were performed in PBMC and that this blocking antibody could have other biological effects that could interfere in the observed results.
The hsCRP protein has been described as an inflammatory marker related to atherosclerosis development. Levels of hsCRP in serum ≥ 3 μg/mL are used in the clinical setting as unspecific marker of inflammation, infection, and tissue injury, associated with an acute-phase response and is actually considered as a predictor factor of future cardiovascular events [49]. As expected, significantly increased hsCRP levels were observed in the serum of patients compared with controls (Table 1), suggesting that under atherosclerosis there is an inflammatory state. Since we used LCVR with similar age ranges to the atherosclerotic patients, we could also suggest that control subjects selected based on Framingham score agreed with the results of hsCRP, suggesting lower inflammatory state in these subjects. We observed a negative and significant correlation between the percentage of total IL-10+ B cells and hsCRP levels in ex vivo and in vitro cultures. This correlation was also detected in mature and memory B cells in ex vivo cultures, showing that at higher levels of hsCRP there is lower frequency of IL-10-producing B cells.
These results show a potential association of IL-10+ B cells with the inflammatory state of patients with atherosclerosis, as well as reveal some differences in B cell phenotype between murine model and atherosclerotic patients. In concordance with our results a reduction in mRNA and protein levels of IL-10 was previously reported in atherosclerotic patients compared with healthy controls [22, 31]. However, more research should be performed in order to confirm this apparent anti-inflammatory role of Breg cells in human atherosclerosis and its usage as possible targets of immunotherapy.
5. Conclusions
Patients with atherosclerosis present an increased number of plasmablasts in peripheral blood as well as a decreased frequency of IL-10+ B cells, both in ex vivo and in vitro cultures and in different B subsets; this suggests the presence of a general deficiency in innate and adaptive regulatory B cells of atherosclerotic patients; however, the regulatory functions of these cells seem to be executed by other mechanisms different than IL-10 production.
The low frequency of IL-10+ B cells correlates directly with the high inflammation status and increase cardiovascular risk (hsCRP) in patients with atherosclerosis. This altered regulatory function could explain partially the predominant inflammatory responses described in atherosclerotic patients.
Declarations
Author contribution statement
Héctor Rincón-Arévalo: Performed the experiments; Wrote the paper.
Julio C. Quintero: Performed the experiments.
Fernando Fortich: Contributed reagents, materials, analysis tools or data.
Mauricio Rojas, Gloria Vásquez: Analyzed and interpreted the data.
Diana Castaño, Lina M. Yassin: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by financial support by COLCIENCIAS (111554431390) and Universidad de Antioquia (Programa de Sostenibilidad) grants and the program Jóvenes Investigadores e Innovadores de Colciencias.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Diana Castaño, Email: diana.castano@udea.edu.co.
Lina M. Yassin, Email: yascatorce@yahoo.com.
References
- 1.Ketelhuth D.F., Hansson G.K. Adaptive response of T and B cells in atherosclerosis. Circ. Res. 2016;118(4):668–678. doi: 10.1161/CIRCRESAHA.115.306427. [DOI] [PubMed] [Google Scholar]
- 2.Wick G., Xu Q. Atherosclerosis--an autoimmune disease. Exp. Gerontol. 1999;34(4):559–566. [PubMed] [Google Scholar]
- 3.Wick G., Perschinka H., Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22(12):665–669. doi: 10.1016/s1471-4906(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 4.Mandal K., Jahangiri M., Xu Q. Autoimmunity to heat shock proteins in atherosclerosis. Autoimmun. Rev. 2004;3(2):31–37. doi: 10.1016/S1568-9972(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 5.Bartolini Gritti B., Binder C.J. Oxidation-specific epitopes are major targets of innate immunity in atherothrombosis. Hämostaseologie. 2016;36(2):89–96. doi: 10.5482/HAMO-14-11-0069. [DOI] [PubMed] [Google Scholar]
- 6.Chou M.Y., Fogelstrand L., Hartvigsen K., Hansen L.F., Woelkers D. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perschinka H., Mayr M., Millonig G., Mayerl C., van der Zee R. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23(6):1060–1065. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 8.Puurunen M., Manttari M., Manninen V., Tenkanen L., Alfthan G. Antibody against oxidized low-density lipoprotein predicting myocardial infarction. Arch. Intern. Med. 1994;154(22):2605–2609. [PubMed] [Google Scholar]
- 9.Kovanen P.T., Manttari M., Palosuo T., Manninen V., Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch. Intern. Med. 1998;158(13):1434–1439. doi: 10.1001/archinte.158.13.1434. [DOI] [PubMed] [Google Scholar]
- 10.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Immune-inflammatory responses in atherosclerosis: role of an adaptive immunity mainly driven by T and B cells. Immunobiology. 2016;221(9):1014–1033. doi: 10.1016/j.imbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Ait-Oufella H., Herbin O., Bouaziz J.D., Binder C.J., Uyttenhove C. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010;207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyaw T., Cui P., Tay C., Kanellakis P., Hosseini H. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(-/-) mice. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanta S.K., Yin C., Peng L., Srikakulapu P., Bontha V. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ. Res. 2014;114(11):1772–1787. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 14.Yin C., Mohanta S.K., Srikakulapu P., Weber C., Habenicht A.J. Artery tertiary lymphoid organs: powerhouses of atherosclerosis immunity. Front. Immunol. 2016;7:387. doi: 10.3389/fimmu.2016.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyaw T., Tay C., Hosseini H., Kanellakis P., Gadowski T. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PloS One. 2012;7(1) doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld S.M., Perry H.M., Gonen A., Prohaska T.A., Srikakulapu P. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ. Res. 2015;117(3):e28–39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsiantoulas D., Diehl C.J., Witztum J.L., Binder C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014;114(11):1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doran A.C., Lipinski M.J., Oldham S.N., Garmey J.C., Campbell K.A. B-cell aortic homing and atheroprotection depend on Id3. Circ. Res. 2012;110(1):e1–12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huan T., Zhang B., Wang Z., Joehanes R., Zhu J. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijk R.A., Duinisveld A.J., Schaapherder A.F., Mulder-Stapel A., Hamming J.F. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J. Am. Heart Assoc. 2015;4(4) doi: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M., Sangawa A., Sasaki Y., Yamashita M., Tanaka-Shintani M. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J. Atherosclerosis Thromb. 2007;14(6):325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 22.Hamze M., Desmetz C., Berthe M.L., Roger P., Boulle N. Characterization of resident B cells of vascular walls in human atherosclerotic patients. J. Immunol. 2013;191(6):3006–3016. doi: 10.4049/jimmunol.1202870. [DOI] [PubMed] [Google Scholar]
- 23.Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 24.Mauri C., Gray D., Mushtaq N., Londei M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair P.A., Norena L.Y., Flores-Borja F., Rawlings D.J., Isenberg D.A. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Iwata Y., Matsushita T., Horikawa M., Dilillo D.J., Yanaba K. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potteaux S., Esposito B., van Oostrom O., Brun V., Ardouin P. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24(8):1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 28.Strom A.C., Cross A.J., Cole J.E., Blair P.A., Leib C. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via IL-10. Thromb. Haemostasis. 2015;114(4):835–847. doi: 10.1160/TH14-12-1084. [DOI] [PubMed] [Google Scholar]
- 29.Sage A.P., Nus M., Baker L.L., Finigan A.J., Masters L.M. Regulatory B cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler. Thromb. Vasc. Biol. 2015;35(8):1770–1773. doi: 10.1161/ATVBAHA.115.305568. [DOI] [PubMed] [Google Scholar]
- 30.Rincon-Arevalo H., Villa-Pulgarin J., Tabares J., Rojas M., Vasquez G. Interleukin-10 production and T cell-suppressive capacity in B cell subsets from atherosclerotic apoE (-/-) mice. Immunol. Res. 2017;65(5):995–1008. doi: 10.1007/s12026-017-8939-6. [DOI] [PubMed] [Google Scholar]
- 31.Ren Z.Q., Liu N., Zhao K. Micro RNA-19a suppresses IL-10 in peripheral B cells from patients with atherosclerosis. Cytokine. 2016;86:86–91. doi: 10.1016/j.cyto.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Tsao C.W., Vasan R.S. Cohort profile: the Framingham heart study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 2015;44(6):1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barron H.V., Harr S.D., Radford M.J., Wang Y., Krumholz H.M. The association between white blood cell count and acute myocardial infarction mortality in patients > or =65 years of age: findings from the cooperative cardiovascular project. J. Am. Coll. Cardiol. 2001;38(6):1654–1661. doi: 10.1016/s0735-1097(01)01613-8. [DOI] [PubMed] [Google Scholar]
- 34.Grau A.J., Boddy A.W., Dukovic D.A., Buggle F., Lichy C. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35(5):1147–1152. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto M., Baba A., Yokota T., Nishikawa H., Ohkawa Y. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41(6):1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Zouggari Y., Ait-Oufella H., Bonnin P., Simon T., Sage A.P. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013;19(10):1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores-Borja F., Bosma A., Ng D., Reddy V., Ehrenstein M.R. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013;5(173) doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 38.Baba Y., Matsumoto M., Kurosaki T. Signals controlling the development and activity of regulatory B-lineage cells. Int. Immunol. 2015;27(10):487–493. doi: 10.1093/intimm/dxv027. [DOI] [PubMed] [Google Scholar]
- 39.Rincon-Arevalo H., Castano D., Villa-Pulgarin J., Rojas M., Vasquez G. Dyslipidemia-associated alterations in B cell subpopulation frequency and phenotype during experimental atherosclerosis. Atherosclerosis. 2016;247:118–126. doi: 10.1016/j.atherosclerosis.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Blair P.A., Chavez-Rueda K.A., Evans J.G., Shlomchik M.J., Eddaoudi A. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J. Immunol. 2009;182(6):3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantani P.T., Ljungcrantz I., Andersson L., Alm R., Hedblad B. Circulating CD40+ and CD86+ B cell subsets demonstrate opposing associations with risk of stroke. Arterioscler. Thromb. Vasc. Biol. 2014;34(1):211–218. doi: 10.1161/ATVBAHA.113.302667. [DOI] [PubMed] [Google Scholar]
- 42.Rincon-Arevalo H., Sanchez-Parra C.C., Castano D., Yassin L., Vasquez G. Regulatory B cells and mechanisms. Int. Rev. Immunol. 2016;35(2):156–176. doi: 10.3109/08830185.2015.1015719. [DOI] [PubMed] [Google Scholar]
- 43.Heinemann K., Wilde B., Hoerning A., Tebbe B., Kribben A. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand. J. Rheumatol. 2016;45(4):312–316. doi: 10.3109/03009742.2015.1126346. [DOI] [PubMed] [Google Scholar]
- 44.Lampropoulou V., Calderon-Gomez E., Roch T., Neves P., Shen P. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll-like receptors in immunity. Immunol. Rev. 2010;233(1):146–161. doi: 10.1111/j.0105-2896.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 45.Mallat Z., Besnard S., Duriez M., Deleuze V., Emmanuel F. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999;85(8):e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 46.Hahn B.H., Grossman J., Chen W., McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J. Autoimmun. 2007;28(2-3):69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Gu X.L., He H., Lin L., Luo G.X., Wen Y.F. Tim-1(+) B cells suppress T cell interferon-gamma production and promote Foxp3 expression, but have impaired regulatory function in coronary artery disease. APMIS. 2017;125(10):872–879. doi: 10.1111/apm.12729. [DOI] [PubMed] [Google Scholar]
- 48.Ait-Oufella H., Taleb S., Mallat Z., Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 49.Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int. J. Cardiol. 2013;168(6):5126–5134. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]