Abstract
cAMP response element binding protein (CREB) is a key transcriptional regulator that regulates the transcription of genes related with neuronal differentiation, synaptic plasticity, learning and memory. Brain derived neurotrophic factor (BDNF), is a CREB dependent gene which plays a pivotal role in the pathogenesis of epilepsy and central comorbid condi-tions associated with epilepsy. However, the beneficial or detrimental consequences of CREB-BDNF activation on the in-duction and/or progression of seizures depend specifically on the region of brain involved and the time of activation. The bi-oactive molecules that alter the activity of CREB in a way to have specialized effects in different brain regions and neural cir-cuits involved could potentially be utilized for therapeutic purposes. Flavonoids are the polyphenolic compounds which lead to phosphorylation of CREB in the hippocampus, followed by increase in extracellular signal regulated kinase (ERK) and BDNF. Several members of flavonoid family have also showed suppression of epileptic seizures via interaction with CREB/BDNF pathway. Moreover, epilepsy is often accompanied by a number of behavioural and psychological comorbid conditions that further gets aggravated by the use of conventional antiepileptic drug therapy. Multiple studies have also sup-ported the beneficial effects of flavonoids in cognitive and memory impairments by upregulation of CREB-BDNF pathway. The current review is an attempt to collate the available preclinical and clinical studies to establish the therapeutic potential of various dietary flavonoids in comprehensive management of epilepsy with relation to CREB-BDNF pathway.
Keywords: cAMP response element binding protein, bioavailability, epilepsy comorbidities, neuroinflammation, neurotrophins, cognitive deficit
1. INTRODUCTION
Epilepsy is a chronic brain disorder having multifaceted underlying causes. Abnormal electric discharge usually occurs as a result of different perinatal and postnatal injuries of neuronal circuits. According to the World Health Organisation (WHO), approximately 2.4 million people are diagnosed with epilepsy each year and it affects around 65 million people worldwide [1, 2]. The International League Against Epilepsy (ILAE) has approved a revised classification of epilepsy at three different levels. The classification is based on seizure type, epilepsy type and syndrome. Different epileptic groups are categorised as focal epilepsy, generalized epilepsy, combined (generalized, and focal) epilepsy, and an unknown epilepsy. Epilepsy syndrome refers to a higher level where a specific syndrome-based diagnosis is made [3]. Epilepsy can occur at any age but is highly prevalent in children and elderly population. In general population the prevalence of epilepsy is estimated to be 6.4 per 1,000 whereas the lifetime or cumulative incidence rate is 7.6 per 1,000 [4].
Several antiepileptic drugs (AEDs) are available in the market, but still 30% of patients do not respond to these agents and develop a condition called as refractory or drug resistant epilepsy [5]. Till date, surgery is considered to be the most reliable way to treat drug resistant epilepsy, however at several instances it results in postoperative recurrence of seizures [6]. A number of new medicines have been identified that can be used along with the conventional AED therapy for the management of drug resistant epilepsy like, oxcarbazepine, levetiracetam, lacosamide, stiripentol, retigabine, eslicarbazepine, brivaracetam and ganaxolone, etc. Unfortunately, their efficacy cannot be considered superior to the first-generation AEDs [7]. Ethosuximide and valproate are the drugs of choice in absence seizures. On the other hand, perampanel and lacosamide have proven to be the most efficacious in controlling tonic-clonic seizures in generalized epilepsy [8]. Despite all these, a number of side effects are associated with the use of AEDs that fairly affects the cognitive and psychological health of patients. The management of these comorbid conditions in epileptic patients is equally important as they affect the overall quality of life. The major central comorbid conditions include learning impairments, intellectual disability, sleep deprivation, mood swings and psychiatric issues such as depression, anxiety, psychosis, autism, spectrum disorders, etc. [9]. Previous reports have depicted that the use of AEDs further worsen the comorbid conditions associated with epilepsy. Therefore, there is an utmost need to look forward to novel therapeutic approaches for the comprehensive management of epilepsy with minimum possible side effects. Earlier the emphasis was on therapeutic agents acting through modulation of GABA (gamma amino butyric acid)/glutamate/ion channel functions. Recent research has led to the emergence of newer antiepileptic agents that act via novel mechanisms apart from direct modulation of neural excito-inhibitory functions. Hence, the interest of researchers has been shifted towards novel therapies that act via diverse mechanisms. Several food-based therapies have come into light as an efficient non-pharmacological approach for the management of epilepsy [10].
Flavonoids, also known as Vitamin P are the polyphenolic compounds naturally present in most of the plants. They are the secondary metabolites that provide ultraviolet protection and colour to the plants. As they are widely distributed in nature, flavonoids constitute a part of diet which is consumed on regular basis with minimal adverse effects reported. Major dietary sources of flavonoids include vegetables, fruits, tea, coffee, juices and red wine [11]. They are unique in displaying a wide range of pharmacological activities such as antioxidant, anti-inflammatory, metal ions chelating, vasoprotective, anti-infective, hepatoprotective and anticancer. Flavonoids are also neurologically active as they have shown protection against neuroinflammation and neurotoxins. They have also been reported to improve cognitive functions [12, 13].
Various preclinical, as well as clinical studies have discovered that inflammatory mediators like, cytokines, prostaglandins and others released from the neurons, as well as peripheral tissues play an important role in seizure induction and epileptogenesis [14]. Flavonoids exert a strong anti-inflammatory action by exhibiting free-radical scavenging activity in the brain or by directly altering the neuroinflammatory cascades [15]. Apart from their antioxidant and anti-inflammatory potential, flavonoids also act on a number of targets involved in the pathogenesis of epilepsy including, GABA receptors, opioid receptors, N-methyl-D-aspartate (NMDA) receptors, calcium and sodium ion channels [16].
2. FLAVONOIDS AND EPILEPSY
In traditional system of medicine, several plants containing polyphenolic compounds have been used for the management of neurological disorders since time immemorial. These molecules modulate the neuronal chemical equilibrium by influencing the function of different neurotransmitters. Pharmacological effects of flavonoids can be accredited to their antioxidant, anti-inflammatory properties or their potential to influence the signalling molecules in various cellular cascades. Flavonoids can interact directly with cellular receptors and proteins (kinases and enzymes), resulting in physiological responses and gene expression alterations that can lead to neuroprotection [17]. In epilepsy, following seizures the antioxidant defence mechanisms are diminished with the generation of free radicals in the brain. Free radical generation further contributes to DNA and protein damage, tissue injury, inflammation and apoptosis [18]. It has been found that there exists a direct relationship between neural inflammation and seizures. Seizure activity leads to the production of numerous inflammatory molecules such as IL-1β, TNF-α, IL-6, prostaglandin E2. These changes further prompt the oxidative stress and neuronal damage in epileptic patients which later ends up in worsening of seizures [19]. Many of the flavonoids and their metabolites are reported to have antioxidant and anti-inflammatory potential. Therefore, this class of secondary metabolites holds a substantial potential in the management of epilepsy and associated behavioural complications.
Studies formerly conducted have demonstrated the anticonvulsive effect of flavonoids or flavonoid-rich fractions isolated from innumerable plant extracts (Table 1). The effect of luteolin isolated by Eclipta alba leaves (5, 10 and 20 mg/kg; i.p.) was studied in pentylenetetrazole (PTZ)-induced acute and chronic models of epilepsy in mouse. Luteolin treatment inhibited kindling in a dose-dependent manner. It also caused a marked reduction in malondialdehyde (MDA) level and restored glutathione (GSH) in kindled mice [20]. Citraro et al. [21] tested flavonoid-rich extract from orange juice (Citrus sinensis var. Tarocco) in genetically audiogenic seizures (AGS)-susceptible DBA/2 mouse and PTZ-induced seizures in ICR-CD1 mice. Administration of orange juice (40 mg/kg; i.p.), 30 min prior to PTZ treatment or auditory stimulation exhibited anticonvulsant effects, and also prevented PTZ-induced tonic seizures. The juice consisted of a mixture of phytochemicals with hesperidin and narirutin being the major flavonoids. Inhibition of NMDA receptors at the glycine binding site and agonistic activity on GABAA receptors can be considered as the possible mechanism of action for the anticonvulsant effect of orange juice [21]. In another study performed by Chang et al. [22], citrus flavonoid hesperidin was found to reduce kainic acid-induced neuronal damage and glutamate excitotoxicity in the rat hippocampus. In the same study, it was proposed that hesperidin attenuates 4-Aminopyridine (4-AP) mediated elevation in cytosolic Ca 2+ and presynaptically inhibits glutamate release in vitro in the synaptosomes (hippocampal nerve terminals) [22].
Table 1. Studies showing antiepileptic/ anticonvulsive effect of major dietary flavonoids.
| S. No. | Tested Flavonoid | Dose/ Source | Model | Species | Proposed Mechanism | Refs. | |
|---|---|---|---|---|---|---|---|
| 1. | Apigenin | 25,50 mg/kg; i.p. (Methanol extract of flower bud of Carduus crispus) |
Kainic acid induced seizures | Male ICR mice and SD rats |
Quenching of ROS and inhibition of glutathione depletion | Han et al. [118] | |
| 2. | Baicalein | 100 mg/kg; i.p. (Purified from Scutellaria baicalensis) |
Pilocarpine induced SE |
Male SD rats | Antioxidant activity and reduction in caspase-3 expression | Liu et al. [119] | |
| 3. | Gossypin | 5,10,20 mg/kg; p.o. (Sigma) |
PTZ, Strychnine and Maximal Electroshock induced seizures | Swiss albino mice | GABAergic mediation, glycine inhibition and protection from electrical kindling effect | Rasilingam et al. [120] | |
| 4. | Hesperidin/ Hesperitin | 5 to 60 µM (In vitro), 500 mg /kg; p.o (In vivo) (Sigma) | Electrical stimulation and Treatment with TEA, PTZ, 4-AP and Bicuculline | Male SD rats | Modulation of calcium dependent potassium channels | Dimpfel [121] | |
| Hesperidin | 100,200 mg/kg; p.o | PTZ induced convulsions | Male LACA mice | Modulation of GABAA-benzodiazepine receptor complex | Kumar et al. [122] | ||
| 5. | Kaempferol | 12.5, 25, 50, 75 mg/kg; p.o. (Leaf extract of Crinum jagus L.) |
PTZ induced kindling | Male Swiss albino mice | Potentiation of GABAergic neurotransmission, suppression of glutamate, elevation in glutathione and SOD | Taiwe et al. [123] | |
| 6. | Licochalcone | 15 mg/kg; i.p. | Kainic acid induced seizures |
C57BL6/J (Wild type) |
Inhibition of JNK1 | Busquets et al. [124] | |
| 7. | Luteolin | 5,10,20 mg/kg; i.p. (Methanol extract of Eclipta alba leaves) |
Acute PTZ model and PTZ-induced kindling |
Swiss albino mice | Facilitation of GABA mediated chloride channel opening and upsurge in seizure threshold | Tambe et al. [20] | |
| Luteolin | 50, 100 mg/kg; p.o. (Nanjing Zelang Biotechnology) |
PTZ induced kindling | Male SD rats | Activation of PKA/CREB/BDNF pathway | Zhen et al. [125] | ||
| 8. | Naringin | 80 mg/kg; i.p. | Kainic acid induced seizures | Male C57BL/6 mice | Suppresses KA-induced mTORC1 activation | Jang et al. [126] | |
| 9. | Naringenin | 20, 40 mg/kg; p.o. (Sigma) |
Pilocarpine induced SE | Swiss albino mice | Reduction in oxidative stress (Increase in SOD and CAT activity, Reduced MDA) | Shakeel et al. [127] | |
| Naringenin 4′,7- dimethyl ether |
100 µL; i.p. (Chemically synthetized) |
PTZ acute seizure model, psychomotor seizures (6-Hz) | Zebrafish (AB strain), Male C57Bl/6 and NMRI mice |
Action on GABAA-receptor, attenuation of IL-1β, TLR4/ NF-κB | Copmans et al. [128] | ||
| Naringenin 7-O-methyl ether | 100 µL; i.p. (Sigma) |
PTZ induced seizures | Zebrafish (AB strain) |
Modulation of GABAA dependent chloride channels, downregulation of TNF-α, IL-6, and IL-1β |
Copmans et al. [128] | ||
| 10. | Proanthocyanidin | 100, 200 mg/kg p.o | PTZ induced kindling | Male SD rats | Reduction in oxidative stress | Zhen et al. [129] | |
| S. No. | Tested Flavonoid | Dose/ Source | Model | Species | Proposed Mechanism | Refs. | |
| 11. | Quercetin | 5, 10, 20, 40 mg/kg; i.p. (Sigma) |
PTZ and PTX induced seizures | Male Wistar rats | Modulation of GABAA receptors | Sefil et al. [130] | |
| 12. | Rutin | 10, 50, 100 mg/kg; i.p. (Sigma) |
PTZ induced kindling | Male Wistar rats | ERK1-CREB, PI3K-mTOR upregulation, modulation of GABAergic and opioid receptors | Nassiri-Asl et al. [131] | |
| 13. | Vitexin | μM; 50, 100, 200 i.c.v. (Fluka) |
PTZ induced seizures | Male Wistar rats | Modulation of GABAA-benzodiazepine receptor complex | Abbasi et al. [23] | |
Abbrevations: 4AP: 4 aminopyridine; BDNF: Brain derived neurotropic growth factor; CAT: Catalase; CREB: Cyclic-AMP response element binding; ERK: Extracellular signal-regulated kinase; GABA: Gaba aminobutyric acid; GR: Glutathione reductase; ICR: Institute of Cancer Research; i.c.v.: Intracerebroventricular; IL 1 β: Interleukin 1 beta; JNK: c-Jun N-terminal kinase; KA: Kainic acid; LACA: Laboratory Animal Centre A-strain; MDA: Malondialdehyde; mTOR: Mammalian target of rapamycin; NF-Κb: Nuclear factor kappa-light-chain-enhancer of activated B cells; NMRI: Naval Medical Research Institute; PI3K: Phosphoinositide 3-kinases; PKA: Protein kinase A; PTX: Picrotoxin; PTZ: Pentylenetetrazole; ROS: Reactive oxygen species; SD: Sprague Dawley; SOD: Superoxide dismutase; SE: Status epilepticus; TEA: Tetraethylammonium; TLR4: Toll-like receptor 4; TNF: Tumor necrosis factor.
A majority of the members of flavonoid family have demonstrated selective affinity for benzodiazepine binding site of GABAA receptors, leading to allosteric modulation of chloride flux. Abbasi et al. [23] illustrated that vitexin (100 and 200 μm; i.c.v) increased the seizure onset time in minimal clonic seizures and generalized tonic-clonic seizures induced by PTZ (90 mg/kg, i.p.) in rats. Anticonvulsive effect of vitexin was reversed by flumazenil indicating that vitexin might act through modulation of GABAA receptor
complex [23]. Flavonoid-rich ethyl acetate extract of the leaves of Anisomeles malabarica was tested by Choudhary et al. [24] in PTZ and Maximal electroshock (MES) seizure model of Wistar rats. Pretreatment with the extract (25 and 50 mg/kg; i.p.) displayed anticonvulsive effect, but also exhibited neurotoxicity, indicated by decreased locomotor activity and motor activity performance. However, chronic treatment (1 week) at lower doses (6.25 and 12.5 mg/kg; i.p,) inhibited epileptic seizures, without causing neurotoxic effects [24].
Flavonoids have also proved themselves as effective anti-inflammatory and antioxidant agents. Quercetin attenuated neuroinflammation in astrocytes and microglia via inhibition of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), cytokine production, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and reactive oxygen species (ROS) generation [25]. In a similar manner, naringenin inhibited nitric oxide (NO), iNOS, COX-2 and proinflammatory mediators in cultured murine microglial cells [26]. Furthermore, flavonoids have also shown beneficial effects against neurological conditions that are often associated with epilepsy. Flavonoids have demonstrated antidepressant action by affecting serotonergic, noradrenergic and dopaminergic systems in hypophysis cerebri and brainstem. They also cause a reduction in the bioamines reuptake by synaptosomes and show inhibitory action on monoamine oxidase type A and B [27].
Mitochondrial dysfunction is another early event involved in the pathogenesis of various neurological disorders including epilepsy. Impaired mitochondrial function is directly involved in the alteration of apoptotic cascade, increased production of ROS and generation of seizure foci in temporal lobe epilepsy. As electron transport chain is associated with the generation of ATP and cellular Ca 2+ homeostasis, alteration in mitochondrial function affects neuronal excitability, synaptic transmission and triggers neuronal apoptosis that is the hallmark of drug resistant epilepsy [28, 29]. Flavonoids modulate mitochondrial functions by enzyme inhibition, scavenging ROS or by influencing the activity of various transcription factors involved in oxidative pathways Fig. (1). Quercetin, resveratrol and curcumin initiate the release of cytochrome C from mitochondria and upregulates the expression of proapoptotic protein Bcl-2 [30]. Lagoa et al. [31] showed that kaempferol, apigenin, curcumin and quercetin decrease the activity of Complex-I in mitochondrial respiratory chain, which is competitively inhibited in the presence of coenzyme Q1. Also, quercetin, kaempferol and epicatechin were able to inhibit stoichiometrically purified cytochrome c. Gao et al. [32] demonstrated swollen and disrupted mitochondria in the hippocampus of pilocarpine treated rats having prolonged seizures, along with a decrease in the expression of mitochondria-encoded COX subunit III (COXIII). These studies indicate that flavonoids can provide an alternate therapy in the management of refractory epilepsy by counteracting mitochondrial dysfunction.
Fig. (1).
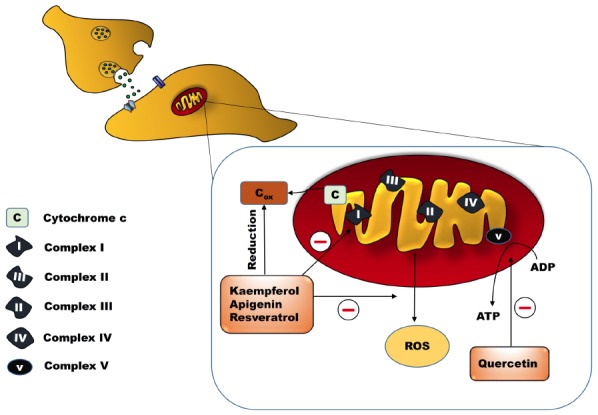
Modulation of mitochondrial electron transport chain by dietary flavonoids. Flavonoids such as apigenin, quercetin, kaempferol and resveratrol inhibit the activity of complex I in mitochondrial electron transport chain. Therefore, control the excessive ROS generation and ATP production causing diminution of neuronal excitability. ADP: Adenosine diphosphate; ATP: Adenosine triphosphate; Cox: Oxidised cytochrome C and ROS: Reactive oxygen species. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Nevertheless, there are studies revealing ambiguous behaviour of few flavonoids in epileptic seizure models. In a study performed by Avallone et al. [33] apigenin isolated from methanolic extract of dried flowers of Matricaria chamomilla reduced the seizure latency (0.5 to 50 mg/kg; i.p) in picrotoxin-induced seizures in rats. It also diminished GABA initiated Cl- currents dose-dependently in cultured cerebellar granule cells, which was blocked by co-application of a benzodiazepine receptor antagonist (Ro 15-1788) [33]. In an electrophysiological model on recombinant GABAA receptors expressed in Xenopus laevis oocytes, chemically synthesised hispidulin (4′,5,7-trihydroxy-6-methoxyflavone) at a concentration from 50 nM to 10 µM showed a positive allosteric modulatory effect on α1β2γ2 subunit of GABAA receptors and stimulated GABA‐induced Cl- influx. In the same study, apigenin acted as a negative allosteric modulator of GABAA [34]. Therefore, the role of apigenin in seizures remains exceedingly unclear. Interestingly, apigenin treatment showed a marked reversal of cognitive deficit and behavioural impairments in PTZ kindled mice. Apigenin treatment at a dose of 10 and 20 mg/kg; p.o. for 20 days showed protection against kindling induced anxiety, depression, learning and memory impairments, without affecting seizure activity [35]. Ethanolic extract of Citrus macroptera fruit peel, rich in flavonoids (333.00 mg/g equivalent of quercetin) was also tested in MES and PTZ induced seizure model at a concentration of 250 mg/kg and 500 mg/kg in mice. It showed a dose-dependent anxiolytic and antidepressant effect. However, increased duration of tonic hind limb extension and prolonged duration of convulsions was observed in MES and PTZ models, respectively in extract treated groups [36].
3. BIOAVAILABILITY AND DRUG INTERACTIONS
Bioavailability of flavonoids has always been a great concern as it varies drastically among compounds belonging to separate groups, and sometimes between the members of the same group. Interestingly, in vivo concentration of flavonoids or their metabolites is not always proportional to their abundance in diet [37]. Despite the health claims of flavonoids, it is a known fact that flavonoids generally exhibit a low bioavailability. Flavonoids with large molecular weights and having complex structures can have even lower bioavailability [38]. Among all dietary flavonoids, isoflavones are considered to be greatest absorbed, flavanols, flavanones and flavonol are the intermediate ones, while anthocyanins, proanthocyanidins and flavanol gallates are the least absorbed [39]. After ingestion from oral route, flavonoids get absorbed through the intestine. Inside the enterocytes, there are two plasma membrane barriers for solutes to overcome apical (luminal) and the basolateral (vascular). Selective passage through these barriers determines the amount that reaches the systemic circulation. It can be assumed that bioavailability of flavonoids is dependent on cell membrane penetrability, protein binding and presystemic metabolism, which further depends upon their chemical structure. Generally, most of the flavonoids undergo conjugation with glucuronic acid, sulfate or O-methylation in the liver and the small intestine. Conjugated metabolites can be found in plasma after flavonoid ingestion. Flavonoids that are not absorbed from the small intestine, undergo metabolism in the colon by microorganisms, which breakdown the ring structure. The microflora causes fission of the A-ring and the heterocyclic ring resulting in the formation of phenolic acids and hydroxylated aromatic compounds, C ring is generally degraded. Various other reactions such as dihydroxylation, demethylation and decarboxylation also take place. Metabolites include phenylpropionic acid, hydroxyphenylpropionic acid, phenylvaleric acid, hydroxylated phenylvalerolactones, phenylacetic, benzoic acids and their derivatives [40, 41]. Enterohepatic circulation and microbial action play an important role in the metabolism of flavonoids yet, absorption from small intestine is more effective and leads to higher plasma values [42]. Flavonoids are generally excreted in urine or faeces Fig. (2). In case of flavonoid glycosides, the type of sugar attached plays an important role in determining the extent of absorption from small intestine. Generally, the absorption in small intestine can be up to 60% of the dose administered and elimination half-life lies in the range of 2-28 h [43].
Fig. (2).
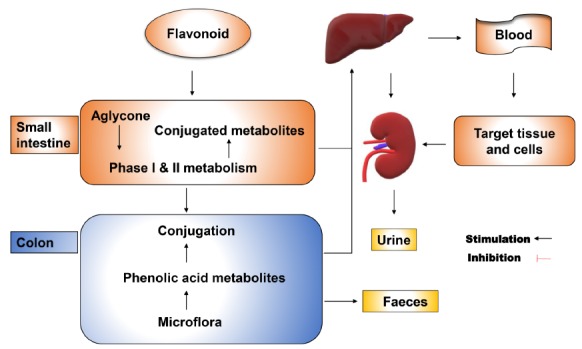
Pharmacokinetic profile of dietary flavonoids. Generally, flavonoids undergo glucuronidation/sulfation/O-methylation in the small intestine. Some flavonoids that are not absorbed in small intestine or secreted back with bile are metabolised by microorganisms in the colon. The main metabolites include phenylpropionic acid, hydroxyphenylpropionic acid, phenylvaleric acid, hydroxylated phenylvalerolactones, phenylacetic, benzoic acids and their derivatives. The metabolites are excreted out in urine or faeces. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
After flavonoids or their metabolites reach the systemic circulation, another challenge is to penetrate the blood-brain barrier (BBB) in order to have a direct effect on the brain. Several studies have demonstrated the brain bioavailability of flavonoids in different animal models [44]. Al Rahim et al. [45] showed that a metabolite of nobiletin, 40-demethylnobiletin, can be spotted in the brain after its single injection in rats. Clinical studies have also confirmed varied results regarding the absorption and the brain bioavailability of flavonoids. Although, quercetin-3-O-glucuronide is known to surpass the BBB, its metabolites were not found in the cerebrospinal fluid even after 3 h of green tea administration [38]. Mullen et al. [46] analysed the human plasma and urine samples collected after ingestion of 250 mL of orange juice. The juice was given with and without full fat yogurt and its impact on metabolism and bioavailability of orange juice was observed. HPLC-MS data revealed that the quantity of flavanone metabolites excreted after 0-5 h of orange juice consumption was expressively reduced by yogurt. However, for over the complete period of 24 h, the change was not significant. These findings suggested that bioavailability of dietary phytochemicals may be influenced by the food matrix in which they are consumed [46]. In spite of all these studies, continuous efforts are being made to understand the exact pharmacokinetic profile of flavonoids and to develop interventions to increase the bioavailability to such an extent so that effective therapeutic concentrations can be achieved in body tissues. Flavonoids are generally known to be safe to a great extent. The daily allowance suggested is around 250 to 400 mg/day [47].
Knowledge about drug- drug interactions is also very important in case of flavonoids as they are likely to achieve high intestinal concentration after ingestion of certain food products and health supplements [48]. Rajnarayana et al. [49] reported increased clearance of metronidazole and its major metabolite, hydroxy-metronidazole after silymarin administration in healthy volunteers. Baicalin was reported to increase the oral bioavailability of cyclosporine in rats [50]. Quercetin on the other hand increased the oral bioavailability of paclitaxel in rats and decreased the bioavailability of cyclosporine in pigs. A number of dietary supplements are prepared containing either glycoside or their aglycone part. These include milk thistle (Silybum marianum) and red clover (Trifolium pratense) extracts, etc. [51, 52]. The potential inhibition of CYPs by 44 different types of common flavonoids and their QSAR was studied by Li et al. [53]. The study showed that co-administration of flavonoids with clinical drugs that are eliminated by CYP3A4 like midazolam, nifedipine, tamoxifen, gefitinib, loratadine and diazepam can inhibit their metabolism in human liver microsomes. Different flavonoids were found to show competitive, non-competitive or mixed inhibition of CYP3A4 depending on their chemical structure [53].
Biological activities of flavonoids vary with the number and substitution positions of hydroxyl and/or methoxy groups [54]. Kakimoto et al. [55] showed that CYP2A6 and other human P450s play important roles in the metabolism of flavone and flavanones. According to Bojic et al. [56]. Cytochrome P450 1A2 is the most important enzyme involved in the metabolism of flavonoids, while others involve cytochromes P450 2D6, 2C19, and 3A4. P-glycoprotein (P-gp), Multidrug resistance-associated proteins (MRPs) and Breast cancer resistance protein (BCRP) are all plasma membrane efflux transporters, which pump their substrates out of the cells by utilizing energy derived from ATP hydrolysis. However, more precise studies need to be done to find out the interaction of flavonoid glucuronides and sulfate conjugates with these efflux transporters [57].
The overexpression of P-gp, at the capillary endothelium involving blood-brain barrier has also been suggested as a major reason responsible for drug resistant epilepsy. Flavonoids can be actively involved in the inhibition of P-gp. Ferreira et al. [58] proved that co-administration of baicalein, (-)-epigallocatechin gallate, kaempferol, quercetin and silymarin increases the intracellular concentration of AEDs and their metabolites in MDCK-MRD1 cells. The study also disclosed that flavonoids at a concentration of 200 µM were able to produce a marked increase in the intracellular accumulation of rhodamine-123 in MDCK-MDR1 cells. P-gp inhibition was the suggested mechanism of action behind the increased accumulation [58]. Bai et al. [59] reported that tangeretin, sinensetin, isosinensetin, sciadopitysin and oroxylin- A, exhibited substantial inhibitory effects on P-gp in MDR1-MDCK-II cells, which resulted in reduced P-gp-mediated efflux of paraquat and taxol leading to cell toxicity. Flavonoids might prove to be a useful intervention in the treatment of drug resistant epilepsy due to their P gp inhibitory action.
4. CREB-BDNF SIGNALLING PATHWAY IN EPILEPSY AND ASSOCIATED CONDITIONS
cAMP response element binding protein (CREB) is a key transcriptional regulator belonging to the family of leucine zipper transcription factors that recognises genes containing cyclic AMP response element (CRE) sequence in their upstream regulatory regions. It contains a leucine zipper domain and a C-terminal basic domain that facilitates DNA binding and dimerization. A variety of external signals which elicit the intracellular pathways that converge upon CREB results in phosphorylation at Serine 133 unit. The phosphorylated form of CREB regulates the transcription of various ion channels, neurotropic factors and synaptic proteins by binding to CRE promoter sites on the target genes Fig. (3). During the early developmental stages, CREB controls vital neuronal functions like cell proliferation, differentiation, growth and survival. However, in the adult brain it plays a crucial role in synaptic plasticity, learning and long-term memory formations [60-62].
Fig. (3).
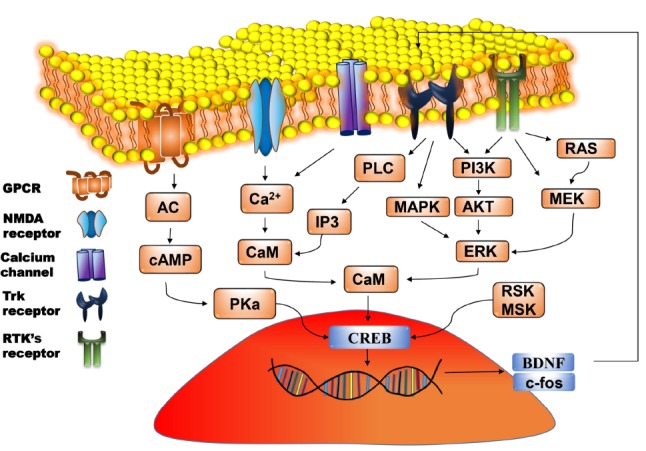
Molecular pathway of CREB-BDNF activation. Signals due to various stimuli arrive at the cell membrane and activates the corresponding receptors. This results in the activation of downstream signalling cascades and generation of second messengers which activates protein kinase. PKA translocates to the nucleus and causes phosphorylation of CREB, which then binds with the CRE region resulting in the transcription of genes such as BDNF and c-fos. BDNF binds with TrkB receptors and further enhances the phosphorylation of CREB. AC: Adenyl cyclase; AKT: Protein kinase B BDNF: Brain derived neurotrophic factor; CAM: Calmodulin; cAMP: Cyclic adenosine monophosphate; CREB: cAMP response element binding protein; ERK: Extracellular signal regulated kinase; GPCR: G protein coupled receptor; IP3: Inositol 1,4,5-triphosphate; MAPK: Mitogen activated protein kinase; MEK: Mitogen activated protein kinase; MSK: Mitogen and stress activated protein kinase; NMDA: N-methyl d-aspartate receptors; PI3K: Phosphoinositide 3-kinases; PKa: PLC: Phospholipase C; RTK’s: Receptor tyrosine kinase and TrkB: Tropomyosin receptor kinase/ tyrosine receptor kinases ; RSK: 90 kDa Ribosomal S6 kinase. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Mantamadiotis et al. [63] provided evidence that mouse with disrupted CREB1 and cAMP response element modulator (CREM) function in the postnatal forebrain showed neurodegeneration in the hippocampus and the dorsolateral striatum. It has also been found that the agents that enhance the activity of CREB, promote learning and memory [64]. CREB regulates the transcription of many pivotal genes that includes Brain-derived neurotrophic factor (BDNF), c-fos, tyrosine hydroxylase, neuropeptides (such as somatostatin, enkephalin, corticotropin-releasing hormone), Nerve growth factor (NGF) and genes involved in the mammalian circadian rhythm [65, 66]. CREB is also primarily associated with various neuroadaptation processes related to the usage of addictive substances, experiences such as physical/ emotional stress or exposure to a maze, etc. Moreover, studies have also revealed that memory allocation within the amygdala and the other brain regions is also dependent on CREB protein [67].
Previous studies have highlighted the role of CREB in epilepsy. Zhu et al. [68] demonstrated a 50% reduction in spontaneous seizures following pilocarpine administration in CREB αΔ mutant mice. Moreover, it was found that the mutants with reduced CREB level require more stimulation to be kindled electrically [68]. Using an antibody that detects Ser133-phosphorylated CREB protein, Moore et al. [69] reported that phosphorylation of CREB is maximum between 3 to 8 min after the onset of seizure activity in PTZ treated rats, and after that it drops slowly. The increased CREB phosphorylation can be blocked by sodium pentobarbital injection prior to PTZ administration [69]. In another study, it was observed that CREB phosphorylation gets upregulated up to 7 days following status epilepticus (SE) and returns to the basal levels within 14 to 60 days [70].
Neurotrophins are closely related proteins which help in the survival, development and function of neurons. NGF, BDNF, Neurotrophin 3 (NT3) and Neurotrophin 4 (NT4) are the different types of neurotrophins. They are known to exert their effect by activating p75 neurotrophin receptor (p75NTR) or one or more of the tropomyosin-related kinase (Trk) receptors (TrkA, TrkB and TrkC) [71]. One of the most important neurotrophin whose transcription is dependent on CREB is BDNF that is encoded by BDNF gene. BDNF gene, which consists of five individual exons, has a promoter that contains a cAMP response element and a calcium response element, both of which can bind the transcriptional-activator CREB [72]. BDNF is considered to be the second member of the neurotrophin family that elicits its effects by TrkB- Extracellular signal regulated kinase (ERK) mediated phosphorylation of synapsin. Since, TrkB receptors are mainly located in the hippocampus, BDNF is having a direct effect on neural activity and cognition [73].
The secreted BDNF is capable of mediating various neuronal processes in mammalian brain, including survival of existing neurons, neuronal differentiation and growth, synaptogenesis, synaptic transmission, neuronal plasticity, excitability, as well as higher cognitive functions [74]. BDNF binds to TrkB receptors causing a cascade of events leading to activation of PI3K/Akt signalling pathway, phosphorylation of NMDA, mammalian target of rapamycin (mTOR) and release of neurotransmitters from pre-synaptic sites [75]. During normal physiological conditions, BDNF secretion is attributed to neurons, while astrocytes are related with its storage and secretion. Interestingly, some recent studies have implied that under conditions such as inflammation and neuronal lesions, astrocytes can also produce BDNF in functionally significant amount [76]. The role of BDNF in epileptogenesis and seizure progression has been explored by several researchers. Earlier studies showed that seizures significantly increase BDNF expression in the brain areas related to limbic seizures. Along with BDNF, TrkB is also increased following seizures in a pattern that mimics changes in BDNF level [77]. Heinrich et al. [78] showed that amplified BDNF level results in further hyperexcitability and epileptic activity. Seizures upregulate CREB/CREM activity in the brain for varied durations that modulates the transcription of trophic factors in the brain. Increased trophic factors like, BDNF can cause structural rearrangements in the hippocampal neurons that can lead to increased seizure activity [79]. Studies have also shown that infusion of BDNF in vivo can increase excitability and might induce seizures [80, 81]. BDNF can also cause reduction in GABAergic transmission and Potassium (K+)/Chloride (Cl−) symporter [82].
However, BDNF upregulation is also known to be a protective response against seizures, which is ultimately capable of counteracting the hippocampal epileptogenesis [83]. It is as well speculated that BDNF might regulate its own synthesis. A continuous infusion of BDNF in kindled rats was found to downregulate its own high-affinity TrkB receptors, which finally results in seizure suppression [84, 85]. BDNF also exerts protective role in several neurological conditions associated with epilepsy. BDNF acts as a target for various antidepressant drugs and actively participates in mood modulation. During epilepsy there is an impairment in the synaptic plasticity and long-term potentiation (LTP) formation, which results in learning and memory dysfunction along with other psychological problems [86].
BDNF infusion leads to increased expression of neuropeptide Y (NPY) in interneurons which gives protective effect against seizures [87]. BDNF is also known to cross the blood-brain barrier and thus levels in plasma may be correlated with the level in brain [88]. A study performed by Pan et al. [89] verified that BNDF showed rapid absorption across the BBB after 10 min. of i.v. infusion. The study also revealed that BDNF is stable for up to 60 min in blood. Almedia et al. [90] observed the effect of physical exercise in epileptic rats. Interestingly, it was detected that exercise causes a noteworthy increase in the level of hippocampal BDNF. Also, it was found that exercise restored the increased expression of full‐length fl-TrkB and truncated tr-TrkB isoforms to normal level in rats with epilepsy. The study concluded that epileptic seizures cause hypoactivation in the hippocampal ERK levels, which were restored by exercise [90]. Imbalance of TrkB receptor isoforms is correlated with epileptogenesis, excitotoxicity and neurodegeneration [91, 92].
Significant changes are also observed in the TrkB receptors of BDNF in relation to epilepsy. He et al. [93] studied the effect of kindling in BDNF-/- and TrkB-/- mice, and observed that no behavioural evidence of epileptogenesis was present in TrkB-/- mice in contrast to BDNF-/- mice. The study signifies that TrkB receptors play a very crucial role in the development of epilepsy [93]. CREB also leads to the expression of important inducible transcription factors including the immediate early gene (IEG) c-fos. Transcription of c-fos characterizes neuronal activity and is needed for LTP formation, while its deletion impairs learning and memory [94]. The immunohistochemical analysis of the brain sections of PTZ kindled mice showed increased collateral expression of c-fos in the cerebral cortex, the amygdala, the hippocampus and the thalamus [79]. BDNF and TrkB receptors also play a crucial role in the prevention of depression and pharmacological action of various antidepressant drugs. Post-mortem data from patients suffering from depression has shown a decline in BDNF level in the hippocampus and the prefrontal cortex, while its increased level was observed in the nucleus accumbens [80].
Carlezon et al. [95] specified that effects of Mediation of CREB dependent genes can have varied neurological effects depending on the region of brain involved. For example, the increased expression of CREB in the hippocampus is likely to contribute to trophic changes through the recruitment of BDNF and neuropeptide Y, resulting in enhanced neurogenesis and reversal of damage [96]. It still remains unclear that how CREB activation can take place extensively in different brain regions and yet in a very specific manner leading to specialized results. Hence more studies are required to understand these relationships.
5. MODULATION OF CREB-BDNF PATHWAY BY FLAVONOIDS
Extensive research has shown that flavonoids can alter several cell signalling pathways and gene expression to show therapeutic response [97]. Since flavonoids are capable of modulating the activation of CREB Fig. (4), it indicates that they influence the neuronal expression of various genes containing CRE sequences [98]. In general, it has been observed that in vivo administration of purified flavonoids leads to the phosphorylation of CREB in the hippocampus, which is followed by the increase in the ERK and BDNF [99]. A few polyphenolic compounds mimic the action of neurotrophins by directly acting as an agonist at TrkB receptors, while others stimulate the downstream pathways leading to the neurotrophic effects [100].
Fig. (4).
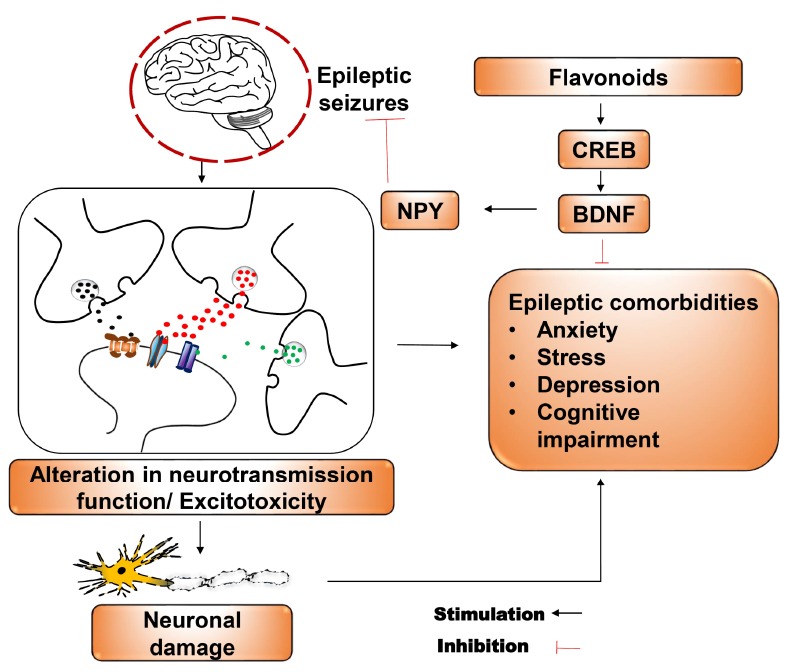
Role of dietary flavonoids in epilepsy via modulation of CREB-BDNF Pathway. Epileptic seizures result in enhanced neuronal discharges and alterations in neurotransmitter release which results in excitotoxicity that ultimately leads to various cognitive and behavioural impairments. Flavonoids interact with receptors like GABA, NMDA, Ion channels, TrkB etc and cause the upregulation of CREB-BDNF pathway. Enhanced BDNF shows reversal of neuronal damage and behavioural impairments caused by seizures and also BDNF potentiates the synthesis of NPY that further leads to the suppression of seizures. BDNF: Brain derived neurotrophic factor; CREB: cAMP response element binding protein and NPY: Neuropeptide Y. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Various in vitro and in vivo studies have demonstrated a rapid modulation of CREB related genes by flavonoids. In an experiment performed by Moghbelinejad et al. [101], rutin exhibited neuroprotective effects in rats preinjected with amyloid β (Aβ), bilaterally in the deep frontal cortex that is known to have a major role in development of Alzheimer's disease (AD). In comparison with the control, rutin administration significantly increased the expression of ERK1, CREB and BDNF in the rat hippocampus. Also, a marked increase in memory retrieval and decrease in MDA were observed following rutin treatment [101]. Likewise, apigenin and luteolin showed neuroprotective effects by increasing BDNF production, decreasing microglial activation, neuroinflammation and oxidative damage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced Parkinson’s disease [102].
Many polyphenolic compounds exhibited antidepressant effects that can be linked with augmented BDNF levels. Su et al. [103] showed that Xiao chai hu tang (a mixture of seven Chinese herbs) used by Chinese population for treatment of depression, works by increasing BDNF, TrkB, TrkA and NGF expressions in the hippocampus, and also mitigates depression-like symptoms in rats in chronic unpredictable mild stress model. Fisetin and Butein isolated from the flavonoid-rich fraction of Rhus verniciflua bark extract presented memory-enhancing effect by the activation of ERK-CREB-BDNF pathway in the hippocampus in mice model of scopolamine-induced amnesia [104]. Maher et al. [105], exposed the rat brain slices (400 µm) to Fisetin (1 mM), a flavonol found in strawberries. Fisetin exposure increased ERK activation and CREB phosphorylation within 5 min. It also facilitated LTP to a weak stimulus in the rat hippocampal slices. However, these effects were blocked by MAPK/ERK inhibitors PD98059 and U0126 [105].
All of these studies strongly supported the interaction of flavonoids with brain CREB-BDNF pathway. Although a number of flavonoids demonstrate neuroprotective effect by modulation of CREB-BDNF pathway but their antiepileptic potential is not fully explored. Table 2 summarizes some of the important flavonoids that have been reported to interact with the brain CREB-BDNF pathway.
Table 2. Major dietary flavonoids showing neuropharmacological effects via interacting with CREB/BDNF pathway and their antiepileptic status.
| S. No. | Flavonoid | CREB/BDNF Interaction | Antiepileptic Effect | |||||
|---|---|---|---|---|---|---|---|---|
| Dose/Conc. Species/ Cell line |
Observed neuropharmacological effect | Dose/Conc. Species |
Observed neuropharmacological effect | |||||
| 1. | Apigenin | 10, 20 mg/kg; p.o., Swiss albino mice 5, 10, 20 mg/kg; p.o., Swiss albino mice |
Improved memory impairment by upregulation of CREB-BDNF pathway in the hippocampus. Sharma et al. [35] Attenuation in the locomotor and muscular activities in MPTP treated mice by upregulation of BDNF in substantia nigra. Su et al. [103] |
22, 50 mg/kg; i.p., ICR mice and SD rats |
Reduction in kainic acid-induced seizures. Han et al. [118] | |||
| 2. | Baicalein | 20 mg/kg and 1 µM, SD rats |
Increased LTP formation and CREB phosphorylation in CA1 region of hippocampus in rats (Fear conditioning task) and hippocampal brain slices (High frequency stimulation). Wang et al. [132] | 100 mg/kg; i.p., SD rats |
Anticonvulsant and neuroprotective effect in pilcocarpine induced SE . Liu et al. [119] | |||
| 3. | Calycopterin | 25, 50, 100 mM; Differentiated PC12 cells | Inhibition of reduction in CREB in H2O2-induced oxidative stress. Savestani et al. [133] | NE | - | |||
| 4. | Dihydromyricetin | 10, 20 mg/kg; i.p. Male C57BL/6J mice |
Activation of ERK1/2, CREB and inhibition of GSK-3β. Ren et al. [134] | NE | ||||
| 5. | Epicatechin | 100-300 nmol/L; 15–16 day‐old Swiss mouse embryos |
Increase in CRE-luciferase activity in primary cortical neurons by ERK- CREB activation. Schroeter et al. [135] | NE | - | |||
| 6. | Fisetin | 40 mg/kg; Male ICR mice 1 µM |
Memory enhancing effect via activation of CREB-BDNF pathway in scopolamine-induced amnesia. Maher et al. [105] Facilitation of LTP formation and enhanced object recognition by activation of ERK-CREB phosphorylation. Wang et al. [132] |
NE | - | |||
| 7. | Hyperoside | 2.5, 5 and 10 μg/mL; PC12 cells |
Increase in CREB-BDNF activation in corticosterone treated (10 µM) PC12 cells as an in vitro. Zheng et al. [136] | NE | - | |||
| 8. | Isorhamnetin | Heterozygous APPswe/PS1D9 transgenic (TgAPP/PS1) founder mice 50 mg/kg; p.o |
Stimulation of CREB-BDNF pathway in depression. Huo et al. [137] | NE | - | |||
| 9. | Kaempferol | 10 mΜ; Human islets INS-1E cells |
Reversal of decreased CREB activation in hyperlipidemia induced by palmitate exposure in Human islets (INS-1E cells). Zhang et al. [137]. | 12.5, 25, 50 mg/kg; p.o., Swiss albino mice | Suppression of PTZ induced kindling by upregulation of GABAergic transmission. Taiwe et al. [123] | |||
| Heterozygous APPswe/PS1D9 transgenic (TgAPP/PS1) founder mice 50 mg/kg; p.o |
Stimulation of CREB-BDNF pathway in depression. Hou et al., 2010 [139] | - | ||||||
| S. No. | Flavonoid | CREB/BDNF Interaction | Antiepileptic Effect | |||||
| 10. | Luteolin | 10, 20 mg/kg; p.o.
Swiss-albino mice |
Enhanced BDNF in MPTP induced Parkinson’s disease Su et al. [103]. | 50,100 mg/kg; p.o., SD rats | Protection from seizures in PTZ induced kindling via activation of CREB-BDNF pathway. Zhen et al. [125] |
|||
| 11. | Quercetin | 8.5 & 17 mg/kg; male Chinese Kunming (KM) mice |
reversal of cognitive decline in mice exposed to high fat diet. Xia et al. [138] | 5, 10, 20, 40 mg/kg; i.p., Wistar rats | Inhibition of PTZ and PTX induced seizures by GABA modulation | |||
| Heterozygous APPswe/PS1D9 transgenic (TgAPP/PS1) founder mice 50 mg/kg p.o | Stimulation of CREB-BDNF pathway in depression. Hou et al. [139] | - | ||||||
| 12. | Rutin | 100 mg/kg; i.p. male Wistar rats |
Enhanced memory retrival in β-amyloid (Aβ) treated rats (deep frontal cortex). Patil et al. [102] | 10, 50, 100 mg/kg; i.p., Wistar rats | Inhibition of seizures in PTZ kindled rats. Nassiri-Asl et al. [131] | |||
| 13. | Silibinin | (100, 200, 400 mg/kg) i.g. | Antidepressant like effect through BDNF/TrkB signalling pathway. Li et al. [140] | NE | ||||
| 14. | Spinosin | 5 mg/kg; i.p, Male ICR mice |
Enhanced neuronal survival and proliferation by activation of ERK-CREB-BDNF pathway in passive avoidance test. Lee et al. [141] | NE | - | |||
Abbrevations: BDNF: Brain derived neurotropic growth factor; CREB: Cyclic-AMP response element binding; CA1: Cornu ammonis1; ERK: Extracellular signal-regulated kinase; GABA: Gaba amino butyric acid; LTP: Long term potentiation; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NE: Not explored; PTX: Picrotoxin; PTZ: Pentylenetetrazol; SD: Sprague Dawley and SE: Status epilepticus; TrkB: Tropomysin related kinase B; GSK 3 β: Glycogen synthase kinase 3 beta.
6. CLINICAL STUDIES
A massive increase has been noticed in publications on flavonoids in the last few decades. However, future research will be fruitful only when it provides solid information that can be translated to clinical practice [106]. A recent randomised controlled trial addressed the therapeutic potential of a herbal extract of Maritime pine bark (pycnogenol) in Attention deficit hyperactivity disorder [107]. Herrlinger et al. [108] demonstrated in their study that spearmint extract improved working and spatial memory in older patients with age-associated memory impairments. Patients who were administered spearmint extract (900 mg/day) reported improvement in their ability to fall asleep in comparison to placebo group [108]. Hyperammonemia plays an important role in the pathophysiology of minimal hepatic encephalopathy (MHE) and contributes to neurotransmission abnormalities, injury to astrocytes and microglia activation. Recent clinical evidence shows that resveratrol improves depression, anxiety and serum ammonia levels in MHE patients [109]. In another study Blueberry supplements were administered to elderly patients with mild cognitive impairments. Blueberry containing anthocyanin and cyanidin 3-glucoside were found to increase the blood oxygen level dependent (BOLD) signal activation in the left pre-central gyrus, the left middle frontal gyrus and the left inferior parietal lobe during working memory load conditions. Thus, blueberry can be effective for improving brain function and cognitive behaviour, especially in late-life dementia [110]. A randomized, double-blind, placebo controlled clinical trial on tart cherry (Prunus cerasus) juice containing procynidin was found to be effective for the treatment of insomnia. Sleep time, as well as sleep quality was improved in human subjects older than 50 years on tart cherry juice administration. Procyanidin B-2 can be considered as the likely active ingredient which acts through plasma kynurenine reduction, tryptophan enhancement and inhibition of serum indoleamine 2,3-dioxygenase [111]. Hirose et al. [112] demonstrated that a low dose (25 mg/day) of isoflavone aglycone was found to alleviate the symptoms of depression and insomnia related to menopause in Japanese women. Overall, despite the promising outcomes in preclinical studies, there is still a need for extensive research in future providing strong clinical evidence related to the use of flavonoids alone or as an add on therapy for the comprehensive management of epilepsy and associated conditions. More precise and planned clinical studies having adequate sample size and control groups with special attention to the challenges presented by flavonoids need to be performed in
the future. We hope that future endeavours in flavonoid research will come out with conclusive endpoints related to their use in epilepsy.
CONCLUSION
Current research strategies have changed the approach towards disease management as now more attention is given in targeting the underlying aetiologies of a disorder rather than providing symptomatic relief only. The aim of the current AED therapy is to abolish seizures, without altering the normal functions. It is evident from the previous studies that several members of flavonoid family are capable of modulating the CREB-BDNF pathway and influence various neurological processes. Since, CREB is found within almost all neural circuits and influences the expression of a wide spectrum of genes, general alterations in CREB function cannot be expected to produce uniform effects throughout the central nervous system. It has been observed that seizure activity increases progressively in some patients suffering from recurrent seizures, therefore, Sir William Gowers postulated that “seizures beget seizures”. This signifies that there are some mediators in the pathogenesis of epilepsy which can act as a cause, as well as a consequence to epileptic seizures [113]. Considering the fact that the different brain regions and neural circuits are associated with distinct physiological functions, CREB activation in specific areas of the brain can be utilized for the effective management of epilepsy and associated complications. Moreover, as the maximum number of studies are performed on the whole hippocampus, the precise cell type and localization studies can give valuable information about the therapeutic mechanism of neuroactive compounds.
The studies involving direct interaction of flavonoids with the downstream targets of CREB are scanty in literature. Such studies are important as the downstream targets are expressed more narrowly in the brain regions associated with specialized functions, BDNF is one such potential target. Some studies have revealed that the brain tissue isolated from patients with intractable temporal lobe epilepsy (TLE) show considerably high amount of BDNF [114]. Also, it was observed that polymorphism in BDNF gene can lead to partial epilepsy [115]. Targeting TrkB and BDNF can be a fruitful approach in understanding the complications in the epileptic brain. Moreover, the role of CREB/BDNF pathway in neuroprotection, cell survival, growth, neuronal plasticity and LTP formation is well established in various experimental studies, whereas the impact of this pathway on seizures progression is still ambiguous to a great extent and need further systematic investigations.
Limited absorption, rapid metabolism, selective permeability across the BBB and stability aspects are some issues which need to be addressed fully before establishing the therapeutic potential of flavonoids. It is apparent from the observed neurological effects that flavonoids can cross the BBB. However, the extent to which this passage takes place with respect to the administered dose needs more emphasis. Furthermore, the information about the biological fate of dietary flavonoids is highly indispensable when studying the pharmacological effects. In many cases the bioavailability of flavonoids remains unquantified because methods are not optimized for metabolites. Likewise, there are a lot of flavonoids which have shown neuroprotective effects in numerous studies, but their antiepileptic potential need to be explored. These concerns must be addressed in order to get better bioavailability and efficacy of flavonoids. Future studies utilizing radiolabelled flavonoids may be supportive in investigating the pharmacokinetics of various metabolites generated in vivo.
Although, there is data in support of a relationship between the consumption of flavonoids and substantial therapeutic outcomes in animal models of epilepsy, there is still a need of rigorous studies to establish a clear relationship to achieve more significant clinical outcomes. Extensive pharmacokinetic and pharmacodynamic studies are necessary to approve the therapeutic success of flavonoid rich compounds in the management of epilepsy. If significant results can be confirmed at clinical level in future, it can be expected that this class of bioactive molecules will emerge out as a novel and effective approach in comprehensive management of epilepsy and its associated comorbid conditions. A number of dietary supplements consisting of flavonoids are already in market and are consumed greatly for their pharmacological benefits, with no toxicity [116]. Considering the fact that flavonoids like quercetin, kaempferol, naringenin and others have already shown effectiveness in neurological disorders, well-designed clinical trials in the future can prove their efficacy in refractory epilepsy as well as associated comorbid conditions [117].
ACKNOWLEDGEMENTS
The authors are thankful to the Director, CSIR-IHBT, Palampur (HP), for his constant support. The institute communication number for this manuscript is 4387.
List of Abbreviations
- 4-AP
4 Aminopyridine
- AEDs
Antiepileptic drugs
- AGS
Genetically audiogenic seizures
- AMP
Adenosine monophosphate
- ATP
Adenosine triphosphate
- Aβ
Amyloid beta
- BBB
Blood brain barrier
- Bcl-2
B-cell lymphoma 2
- BCRP
Breast cancer resistance protein
- BDNF
Brain derived neurotrophic factor
- BOLD
Blood oxygen level dependent
- CA1
Cornu ammonis1
- CAT
Catalase
- COX
Cyclooxygenase
- CREB
cAMP response element binding protein
- CREM
cAMP response element modulator
- CYPs
Cytochromes P450
- ERK
Extracellular signal-regulated kinase
- GABA
Gamma amino butyric acid
- GR
Glutathione reductase
- GSH
Glutathione
- GSK 3 β
Glycogen synthase kinase 3 beta
- HPLC MS
High performance liquid chromatography mass spectrometry
- i.c.v.
Intracerebroventricularly
- i.p.
Intraperitonial
- ILAE
International league against epilepsy
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LTP
Long term potentiation
- MAPK
Mitogen-activated protein kinase
- MDA
Malondialdehyde
- MDCK-MRD1
Madin-Darby canine kidney cells transfected with the human MDR1 gene
- MES
Maximal electroshock
- MHE
Minimal hepatic encephalopathy
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRPs
Multidrug resistance-associated proteins
- mTOR
Mammalian target of rapamycin
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NE
Not explored
- NF-κB
Nuclear factor kappa B
- NGF
Nerve growth factor
- NMDA
N-methyl-D-aspartate
- NO
Nitric oxide
- NPY
Neuropeptide Y
- NTs
Neurotrophins
- P-gp
P glycoprotein
- PI3K
Phosphoinositide 3-kinases
- PTX
Picrotoxin
- PTZ
Pentylenetetrazole
- QSAR
Quantitative structure activity relationship
- ROS
Reactive oxygen species
- SD
Sprague Dawley
- SE
Status epilepticus
- SOD
Superoxide dismutase
- TEA
Tetraethylammonium
- TLR4
Toll-like receptor 4
- TNF-α
Tumour necrosis factor alpha
- TrkB
Tropomysin related kinase B
- WHO
World Health Organisation
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The financial support of CSIR, New Delhi for infrastructural support in the form of project MLP:0204 is highly acknowledged. Pallavi Sharma is grateful to Indian Council of Medical Research for providing ICMR-SRF fellowship for pursing PhD (File no: 5/3/8/38/ITR-F/2018-ITR).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Moshé S.L., Perucca E., Ryvlin P., Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–898. doi: 10.1016/S0140-6736(14)60456-6. [http://dx.doi.org/10.1016/S0140-6736(14)60456-6]. [PMID: 25260236]. [DOI] [PubMed] [Google Scholar]
- 2.Van Diessen E., Van Der Maas F., Cabral V., Otte W.M. Community-based rehabilitation offers cost-effective epilepsy treatment in rural Guinea-Bissau. Epilepsy Behav. 2018;79:23–25. doi: 10.1016/j.yebeh.2017.11.009. [http://dx.doi.org/10.1016/j.yebeh.2017.11.009]. [DOI] [PubMed] [Google Scholar]
- 3.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., Nordli D.R., Perucca E., Tomson T., Wiebe S., Zhang Y.H., Zuberi S.M. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [http://dx.doi.org/10.1111/epi.13709]. [PMID: 28276062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivathamboo S., Perucca P., Velakoulis D., Jones N.C., Goldin J., Kwan P., O’Brien T.J. Sleep-disordered breathing in epilepsy: epidemiology, mechanisms, and treatment. Sleep (Basel) 2018;41(4):41. doi: 10.1093/sleep/zsy015. [http://dx.doi.org/10.1093/sleep/zsy015]. [PMID: 29394413]. [DOI] [PubMed] [Google Scholar]
- 5.Dalic L., Cook M.J. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr. Dis. Treat. 2016;12:2605–2616. doi: 10.2147/NDT.S84852. [http://dx.doi.org/10.2147/NDT.S84852]. [PMID: 27789949]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin G., Yao G., Zhang K., Li B. Recent advances in pathophysiological studies and treatment of epilepsy. Curr. Neuropharmacol. 2018;16(1):3–4. doi: 10.2174/1570159X1601171214093823. [PMID: 29301484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aneja S., Sharma S. Newer anti-epileptic drugs. Indian Pediatr. 2013;50(11):1033–1040. doi: 10.1007/s13312-013-0284-9. [http://dx.doi.org/10.1007/s13312-013-0284-9]. [PMID: 24382900]. [DOI] [PubMed] [Google Scholar]
- 8.Manford M. Recent advances in epilepsy. J. Neurol. 2017;264(8):1811–1824. doi: 10.1007/s00415-017-8394-2. [http://dx.doi.org/10.1007/s00415-017-8394-2]. [PMID: 28120042]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodie M.J., Zuberi S.M., Scheffer I.E., Fisher R.S. The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epileptic Disord. 2018;20(2):77–87. doi: 10.1684/epd.2018.0957. [http://dx.doi.org/10.1684/epd.2018.0957]. [PMID: 29620013]. [DOI] [PubMed] [Google Scholar]
- 10.Neal E.G., Cross J.H. Efficacy of dietary treatments for epilepsy. J. Hum. Nutr. Diet. 2010;23(2):113–119. doi: 10.1111/j.1365-277X.2010.01043.x. [http://dx.doi.org/10.1111/j.1365-277X.2010.01043.x]. [PMID: 20487176]. [DOI] [PubMed] [Google Scholar]
- 11.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [http://dx.doi.org/10.1016/j.mam.2017.11.003]. [PMID: 29117513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3-4):115–126. doi: 10.1007/s12263-008-0091-4. [http://dx.doi.org/10.1007/s12263-008-0091-4]. [PMID: 18937002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [http://dx.doi.org/10.1155/2013/162750]. [PMID: 24470791]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey A., Kang X., Qiu J., Du Y., Jiang J. Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol. Sci. 2016;37(6):463–484. doi: 10.1016/j.tips.2016.03.001. [http://dx.doi.org/10.1016/j.tips.2016.03.001]. [PMID: 27062228]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diniz T.C., Silva J.C., de Lima-Saraiva S.R., Ribeiro F.P., Pacheco A.G., de Freitas R.M., Quintans-Júnior L.J. Quintans, Jde.S.; Mendes, R.L.; Almeida, J.R. The role of flavonoids on oxidative stress in epilepsy. Oxid. Med. Cell. Longev. 2015;2015:171756. doi: 10.1155/2015/171756. [http://dx.doi.org/10.1155/2015/171756]. [PMID: 25653736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh P., Singh D., Goel R.K. Phytoflavonoids: antiepileptics for the future. Int. J. Pharm. Pharm. Sci. 2014;6:51–66. [Google Scholar]
- 17.Copmans D., Orellana-Paucar A.M., Steurs G., Zhang Y., Ny A., Foubert K., Exarchou V., Siekierska A., Kim Y., Borggraeve W.D., Dehaen W. Pieters; de Witte, P.A.M. Methylated flavonoids as anti-seizure agents: Naringenin 40,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018;112:124–133. doi: 10.1016/j.neuint.2017.11.011. [http://dx.doi.org/10.1016/j.neuint.2017.11.011]. [PMID: 29174382]. [DOI] [PubMed] [Google Scholar]
- 18.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardenas-Rodriguez N., Huerta-Gertrudis B., Rivera-Espinosa L., Montesinos-Correa H., Bandala C., Carmona-Aparicio L., Coballase-Urrutia E. Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. Int. J. Mol. Sci. 2013;14(1):1455–1476. doi: 10.3390/ijms14011455. [http://dx.doi.org/10.3390/ijms14011455]. [PMID: 23344052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tambe R., Patil A., Jain P., Sancheti J., Somani G., Sathaye S. Assessment of luteolin isolated from Eclipta alba leaves in animal models of epilepsy. Pharm. Biol. 2017;55(1):264–268. doi: 10.1080/13880209.2016.1260597. [http://dx.doi.org/10.1080/13880209.2016.1260597]. [PMID: 27927066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Citraro R., Navarra M., Leo A., Donato Di Paola E., Santangelo E., Lippiello P., Aiello R., Russo E., De Sarro G. The anticonvulsant activity of a flavonoid-rich extract from orange juice involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules. 2016;21(9):E1261. doi: 10.3390/molecules21091261. [http://dx.doi.org/10.3390/molecules21091261]. [PMID: 27657037]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C.Y., Lin T.Y., Lu C.W., Huang S.K., Wang Y.C., Chou S.S., Wang S.J. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;50:157–169. doi: 10.1016/j.neuro.2015.08.014. [http://dx.doi.org/10.1016/j.neuro.2015.08.014]. [PMID: 26342684]. [DOI] [PubMed] [Google Scholar]
- 23.Abbasi E., Nassiri-Asl M., Shafeei M., Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012;80(2):274–278. doi: 10.1111/j.1747-0285.2012.01400.x. [http://dx.doi.org/10.1111/j.1747-0285.2012.01400.x]. [PMID: 22554436]. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary N., Bijjem K.R., Kalia A.N. Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J. Ethnopharmacol. 2011;135(2):238–242. doi: 10.1016/j.jep.2011.02.019. [http://dx.doi.org/10.1016/j.jep.2011.02.019]. [PMID: 21354295]. [DOI] [PubMed] [Google Scholar]
- 25.Vezzani A., Sperk G., Colmers W.F. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22(1):25–30. doi: 10.1016/s0166-2236(98)01284-3. [http://dx.doi.org/10.1016/S0166-2236(98)01284-3]. [PMID: 10088996]. [DOI] [PubMed] [Google Scholar]
- 26.Wu L.H., Lin C., Lin H.Y., Liu Y.S., Wu C.Y., Tsai C.F., Chang P.C., Yeh W.L., Lu D.Y. Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expressions. Mol. Neurobiol. 2016;53(2):1080–1091. doi: 10.1007/s12035-014-9042-9. [http://dx.doi.org/10.1007/s12035-014-9042-9]. [PMID: 25579382]. [DOI] [PubMed] [Google Scholar]
- 27.Khan H., Perviz S., Sureda A., Nabavi S.M., Tejada S. Current standing of plant derived flavonoids as an antidepressant. Food Chem. Toxicol. 2018;119:176–188. doi: 10.1016/j.fct.2018.04.052. [http://dx.doi.org/10.1016/j.fct.2018.04.052]. [PMID: 29704578]. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y., Chen M., Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. doi: 10.1016/j.mito.2019.07.003. [http://dx.doi.org/10.1016/j.mito.2019.07.003]. [PMID: 31288090]. [DOI] [PubMed] [Google Scholar]
- 29.Folbergrová J., Kunz W.S. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12(1):35–40. doi: 10.1016/j.mito.2011.04.004. [http://dx.doi.org/10.1016/j.mito.2011.04.004]. [PMID: 21530687]. [DOI] [PubMed] [Google Scholar]
- 30.Gibellini L., Bianchini E., De Biasi S., Nasi M., Cossarizza A., Pinti M. Natural compounds modulating mitochondrial functions. Evid. Based Complement. Alternat. Med. 2015;2015:527209. doi: 10.1155/2015/527209. [http://dx.doi.org/10.1155/2015/527209]. [PMID: 26167193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagoa R., Graziani I., Lopez-Sanchez C., Garcia-Martinez V., Gutierrez-Merino C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta. 2011;1807(12):1562–1572. doi: 10.1016/j.bbabio.2011.09.022. [http://dx.doi.org/10.1016/j.bbabio.2011.09.022]. [PMID: 22015496]. [DOI] [PubMed] [Google Scholar]
- 32.Gao J., Chi Z.F., Liu X.W., Shan P.Y., Wang R. Mitochondrial dysfunction and ultrastructural damage in the hippocampus of pilocarpine-induced epileptic rat. Neurosci. Lett. 2007;411(2):152–157. doi: 10.1016/j.neulet.2006.10.022. [http://dx.doi.org/10.1016/j.neulet.2006.10.022]. [PMID: 17092649]. [DOI] [PubMed] [Google Scholar]
- 33.Avallone R., Zanoli P., Puia G., Kleinschnitz M., Schreier P., Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem. Pharmacol. 2000;59(11):1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [http://dx.doi.org/10.1016/S0006-2952(00)00264-1]. [PMID: 10751547]. [DOI] [PubMed] [Google Scholar]
- 34.Kavvadias D., Sand P., Youdim K.A., Qaiser M.Z., Rice-Evans C., Baur R., Sigel E., Rausch W.D., Riederer P., Schreier P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br. J. Pharmacol. 2004;142(5):811–820. doi: 10.1038/sj.bjp.0705828. [http://dx.doi.org/10.1038/sj.bjp.0705828]. [PMID: 15231642]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma P., Sharma S., Singh D. Apigenin reverses behavioural impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr. Neurosci. 2018;30:1–10. doi: 10.1080/1028415X.2018.1478653. [http://dx.doi.org/10.1080/1028415X.2018.1478653]. [PMID: 29847220]. [DOI] [PubMed] [Google Scholar]
- 36.Rahman H., Eswaraiah M.C., Duttal A.M. Neuropharmacological activities of ethanolic extract of Citrus macroptera (Varannamensis) fruit peels. Glob. J. Pharmacol. 2014;8:609–616. [Google Scholar]
- 37.Bakoyiannis I., Daskalopoulou A., Pergialiotis V., Perrea D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019;109:1488–1497. doi: 10.1016/j.biopha.2018.10.086. [http://dx.doi.org/10.1016/j.biopha.2018.10.086]. [PMID: 30551400]. [DOI] [PubMed] [Google Scholar]
- 38.Thilakarathna S.H., Rupasinghe H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5(9):3367–3387. doi: 10.3390/nu5093367. [http://dx.doi.org/10.3390/nu5093367]. [PMID: 23989753]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viskupicova J., Ondrejovic M., Sturdik E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008;47:151–162. [Google Scholar]
- 40.Passamonti S., Terdoslavich M., Franca R., Vanzo A., Tramer F., Braidot E., Petrussa E., Vianello A. Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr. Drug Metab. 2009;10(4):369–394. doi: 10.2174/138920009788498950. [http://dx.doi.org/10.2174/138920009788498950]. [PMID: 19519345]. [DOI] [PubMed] [Google Scholar]
- 41.Watson R.R., Preedy V.R., Zibadi S. Polyphenols in Human Health and Disease. 2014. [Google Scholar]
- 42.Hollman P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004;42:74–83. [http://dx.doi.org/10.3109/13880200490893492]. [Google Scholar]
- 43.Manach C., Donovan J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004;38(8):771–785. doi: 10.1080/10715760410001727858. [http://dx.doi.org/10.1080/10715760410001727858]. [PMID: 15493450]. [DOI] [PubMed] [Google Scholar]
- 44.Krasieva T.B., Ehren J., O’Sullivan T., Tromberg B.J., Maher P. Cell and brain tissue imaging of the flavonoid fisetin using label-free two-photon microscopy. Neurochem. Int. 2015;89:243–248. doi: 10.1016/j.neuint.2015.08.003. [http://dx.doi.org/10.1016/j.neuint.2015.08.003]. [PMID: 26271433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Rahim M., Nakajima A., Saigusa D., Tetsu N., Maruyama Y., Shibuya M., Yamakoshi H., Tomioka Y., Iwabuchi Y., Ohizumi Y., Yamakuni T. 4′-Demethylnobiletin, a bioactive metabolite of nobiletin enhancing PKA/ERK/CREB signaling, rescues learning impairment associated with NMDA receptor antagonism via stimulation of the ERK cascade. Biochemistry. 2009;48(32):7713–7721. doi: 10.1021/bi901088w. [http://dx.doi.org/10.1021/bi901088w]. [PMID: 19601643]. [DOI] [PubMed] [Google Scholar]
- 46.Mullen W., Archeveque M.A., Edwards C.A., Matsumoto H., Crozier A. Bioavailability and metabolism of orange juice flavanones in humans: impact of a full-fat yogurt. J. Agric. Food Chem. 2008;56(23):11157–11164. doi: 10.1021/jf801974v. [http://dx.doi.org/10.1021/jf801974v]. [PMID: 19007165]. [DOI] [PubMed] [Google Scholar]
- 47.Peluso I., Palmery M. Flavonoids at the pharma-nutrition interface: Is a therapeutic index in demand? Biomed. Pharmacother. 2015;71:102–107. doi: 10.1016/j.biopha.2015.02.028. [http://dx.doi.org/10.1016/j.biopha.2015.02.028]. [PMID: 25960223]. [DOI] [PubMed] [Google Scholar]
- 48.Morris M.E., Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78(18):2116–2130. doi: 10.1016/j.lfs.2005.12.003. [http://dx.doi.org/10.1016/j.lfs.2005.12.003]. [PMID: 16455109]. [DOI] [PubMed] [Google Scholar]
- 49.Rajnarayana K., Reddy M.S., Vidyasagar J., Krishna D.R. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneimittelforschung. Drug Res. 2004;54(2):109–113. doi: 10.1055/s-0031-1296944. [PMID: 15038460]. [DOI] [PubMed] [Google Scholar]
- 50.Lai M.Y., Hsiu S.L., Hou Y.C., Tsai S.Y., Chao P.D. Significant decrease of cyclosporine bioavailability in rats caused by a decoction of the roots of Scutellaria baicalensis. Planta Med. 2004;70(2):132–137. doi: 10.1055/s-2004-815489. [http://dx.doi.org/10.1055/s-2004-815489]. [PMID: 14994190]. [DOI] [PubMed] [Google Scholar]
- 51.Choi J.S., Jo B.W., Kim Y.C. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur. J. Pharm. Biopharm. 2004;57(2):313–318. doi: 10.1016/j.ejpb.2003.11.002. [http://dx.doi.org/10.1016/j.ejpb.2003.11.002]. [PMID: 15018990]. [DOI] [PubMed] [Google Scholar]
- 52.Chen H.Y., Wu T.S., Su S.F., Kuo S.C., Chao P.D. Marked decrease of cyclosporin absorption caused by phellamurin in rats. Planta Med. 2002;68(2):138–141. doi: 10.1055/s-2002-20244. [http://dx.doi.org/10.1055/s-2002-20244]. [PMID: 11859464]. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Ning J., Wang Y., Wang C., Sun C., Huo X., Yu Z., Feng L., Zhang B., Tian X., Ma X. Drug interaction study of flavonoids toward CYP3A4 and their quantitative structure activity relationship (QSAR) analysis for predicting potential effects. Toxicol. Lett. 2018;294:27–36. doi: 10.1016/j.toxlet.2018.05.008. [http://dx.doi.org/10.1016/j.toxlet.2018.05.008]. [PMID: 29753067]. [DOI] [PubMed] [Google Scholar]
- 54.Shimada T., Kakimoto K., Takenaka S., Koga N., Uehara S., Murayama N., Yamazaki H., Kim D., Guengerich F.P., Komori M. Roles of human CYP2A6 and monkey CYP2A24 and 2A26 cytochrome P450 enzymes in the oxidation of 2,5,2´,5´-tetrachlorobiphenyl. Drug Metab. Dispos. 2016;44(12):1899–1909. doi: 10.1124/dmd.116.072991. [http://dx.doi.org/10.1124/dmd.116.072991]. [PMID: 27625140]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakimoto K., Murayama N., Takenaka S., Nagayoshi H., Lim Y.R., Kim V., Kim D., Yamazaki H., Komori M., Guengerich F.P., Shimada T. Cytochrome P450 2A6 and other human P450 enzymes in the oxidation of flavone and flavanone. Xenobiotica. 2019;49(2):131–142. doi: 10.1080/00498254.2018.1426133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bojic M., Benkovic G., Males Z., Zulj R.T., Tomic S. Cytochromes P450 involved in metabolism of flavonoid aglycones. FASEB J. 2018;32(1):564–567. [Google Scholar]
- 57.Litman T., Druley T.E., Stein W.D., Bates S.E. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell. Mol. Life Sci. 2001;58(7):931–959. doi: 10.1007/PL00000912. [http://dx.doi.org/10.1007/PL00000912]. [PMID: 11497241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira A., Rodrigues M., Fortuna A., Falcão A., Alves G. Flavonoid compounds as reversing agents of the P-glycoprotein-mediated multidrug resistance: An in vitro evaluation with focus on antiepileptic drugs. Food Res. Int. 2018;103:110–120. doi: 10.1016/j.foodres.2017.10.010. [http://dx.doi.org/10.1016/j.foodres.2017.10.010]. [PMID: 29389596]. [DOI] [PubMed] [Google Scholar]
- 59.Bai J., Zhao S., Fan X., Chen Y., Zou X., Hu M., Wang B., Jin J., Wang X., Hu J., Zhang D., Li Y. Inhibitory effects of flavonoids on P-glycoprotein in vitro and in vivo: Food/herb-drug interactions and structure-activity relationships. Toxicol. Appl. Pharmacol. 2019;369:49–59. doi: 10.1016/j.taap.2019.02.010. [http://dx.doi.org/10.1016/j.taap.2019.02.010]. [PMID: 30790579]. [DOI] [PubMed] [Google Scholar]
- 60.Lonze B.E., Ginty D.D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [http://dx.doi.org/10.1016/S0896-6273(02)00828-0]. [PMID: 12194863]. [DOI] [PubMed] [Google Scholar]
- 61.Ponti C., Gibellini D., Boin F., Melloni E., Manzoli F.A., Cocco L., Zauli G., Vitale M. Role of CREB transcription factor in c-fos activation in natural killer cells. Eur. J. Immunol. 2002;32(12):3358–3365. doi: 10.1002/1521-4141(200212)32:12<3358::AID-IMMU3358>3.0.CO;2-Q. [http://dx.doi.org/10.1002/1521-4141(200212)32:12<3358:AID-IMMU3358>3.0.CO;2-Q]. [PMID: 12432566]. [DOI] [PubMed] [Google Scholar]
- 62.Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015;8:46. doi: 10.3389/fnmol.2015.00046. [http://dx.doi.org/10.3389/fnmol.2015.00046]. [PMID: 26379491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mantamadiotis T., Lemberger T., Bleckmann S.C., Kern H., Kretz O., Martin Villalba A., Tronche F., Kellendonk C., Gau D., Kapfhammer J., Otto C., Schmid W., Schütz G. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 2002;31(1):47–54. doi: 10.1038/ng882. [http://dx.doi.org/10.1038/ng882]. [PMID: 11967539]. [DOI] [PubMed] [Google Scholar]
- 64.Tully T., Bourtchouladze R., Scott R., Tallman J. Targeting the CREB pathway for memory enhancers. Nat. Rev. Drug Discov. 2003;2(4):267–277. doi: 10.1038/nrd1061. [http://dx.doi.org/10.1038/nrd1061]. [PMID: 12669026]. [DOI] [PubMed] [Google Scholar]
- 65.Dale A., George J., David F., William C., Anthony-Samuel L., McNamara J.O., White L.E. Neuroscience. 4th ed. Sinauer Associates; 2008. pp. 170–176. [Google Scholar]
- 66.Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [http://dx.doi.org/10.1146/annurev-physiol-021909-135821]. [PMID: 20148687]. [DOI] [PubMed] [Google Scholar]
- 67.Rogerson T., Jayaprakash B., Cai D.J., Sano Y., Lee Y.S., Zhou Y., Bekal P., Deisseroth K., Silva A.J. Molecular and cellular mechanisms for trapping and activating emotional memories. PLoS One. 2016;11(8):e0161655. doi: 10.1371/journal.pone.0161655. [http://dx.doi.org/10.1371/journal.pone.0161655]. [PMID: 27579481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X., Han X., Blendy J.A., Porter B.E. Decreased CREB levels suppress epilepsy. Neurobiol. Dis. 2012;45(1):253–263. doi: 10.1016/j.nbd.2011.08.009. [http://dx.doi.org/10.1016/j.nbd.2011.08.009]. [PMID: 21867753]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore A.N., Waxham M.N., Dash P.K. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J. Biol. Chem. 1996;271(24):14214–14220. doi: 10.1074/jbc.271.24.14214. [http://dx.doi.org/10.1074/jbc.271.24.14214]. [PMID: 8662977]. [DOI] [PubMed] [Google Scholar]
- 70.Guo J., Wang H., Wang Q., Chen Y., Chen S. Expression of p-CREB and activity-dependent miR-132 in temporal lobe epilepsy. Int. J. Clin. Exp. Med. 2014;7(5):1297–1306. [PMID: 24995086]. [PMC free article] [PubMed] [Google Scholar]
- 71.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [http://dx.doi.org/10.1098/rstb.2006.1894]. [PMID: 16939974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tabuchi A., Sakaya H., Kisukeda T., Fushiki H., Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J. Biol. Chem. 2002;277(39):35920–35931. doi: 10.1074/jbc.M204784200. [http://dx.doi.org/10.1074/jbc.M204784200]. [PMID: 12114522]. [DOI] [PubMed] [Google Scholar]
- 73.Nair A., Vaidya V.A. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: molecules that modulate our mood? J. Biosci. 2006;31(3):423–434. doi: 10.1007/BF02704114. [http://dx.doi.org/10.1007/BF02704114]. [PMID: 17006024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [http://dx.doi.org/10.1038/nrn3379]. [PMID: 23254191]. [DOI] [PubMed] [Google Scholar]
- 75.Spencer J.P. Flavonoids: modulators of brain function? Br. J. Nutr. 2008;99(Suppl. 1):ES60–ES77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 76.Giralt A., Friedman H.C., Caneda-Ferrón B., Urbán N., Moreno E., Rubio N., Blanco J., Peterson A., Canals J.M., Alberch J. BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington’s disease. Gene Ther. 2010;17(10):1294–1308. doi: 10.1038/gt.2010.71. [http://dx.doi.org/10.1038/gt.2010.71]. [PMID: 20463759]. [DOI] [PubMed] [Google Scholar]
- 77.Scharfman H.E. Brain-derived neurotrophic factor and epilepsy--a missing link? Epilepsy Curr. 2005;5(3):83–88. doi: 10.1111/j.1535-7511.2005.05312.x. [http://dx.doi.org/10.1111/j.1535-7511.2005.05312.x]. [PMID: 16145610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heinrich C., Lähteinen S., Suzuki F., Anne-Marie L., Huber S., Häussler U., Haas C., Larmet Y., Castren E., Depaulis A. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2011;42(1):35–47. doi: 10.1016/j.nbd.2011.01.001. [http://dx.doi.org/10.1016/j.nbd.2011.01.001]. [PMID: 21220014]. [DOI] [PubMed] [Google Scholar]
- 79.Malhi S.M., Jawed H., Hanif F., Ashraf N., Zubair F., Siddiqui B.S., Begum S., Kabir N., Simjee S.U. Modulation of c-Fos and BDNF protein expression in pentylenetetrazole-kindled mice following the treatment with novel antiepileptic compound HHL-6. BioMed Res. Int. 2014;2014:876712. doi: 10.1155/2014/876712. [http://dx.doi.org/10.1155/2014/876712]. [PMID: 24605339]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J.C., Yao W., Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016;14(7):721–731. doi: 10.2174/1570159X14666160119094646. [http://dx.doi.org/10.2174/1570159X14666160119094646]. [PMID: 26786147]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scharfman H.E., Goodman J.H., Sollas A.L., Croll S.D. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp. Neurol. 2002;174(2):201–214. doi: 10.1006/exnr.2002.7869. [http://dx.doi.org/10.1006/exnr.2002.7869]. [PMID: 11922662]. [DOI] [PubMed] [Google Scholar]
- 82.Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., Kokaia Z., Airaksinen M.S., Voipio J., Kaila K., Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002;159(5):747–752. doi: 10.1083/jcb.200209011. [http://dx.doi.org/10.1083/jcb.200209011]. [PMID: 12473684]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reibel S., Larmet Y., Carnahan J., Marescaux C., Depaulis A. Endogenous control of hippocampal epileptogenesis: a molecular cascade involving brain-derived neurotrophic factor and neuropeptide Y. Epilepsia. 2000;41(Suppl. 6):S127–S133. doi: 10.1111/j.1528-1157.2000.tb01571.x. [http://dx.doi.org/10.1111/j.1528-1157.2000.tb01571.x]. [PMID: 10999534]. [DOI] [PubMed] [Google Scholar]
- 84.Xu B., Michalski B., Racine R.J., Fahnestock M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, Trk expression and seizure-related morphological changes. Neuroscience. 2004;126(3):521–531. doi: 10.1016/j.neuroscience.2004.03.044. [http://dx.doi.org/10.1016/j.neuroscience.2004.03.044]. [PMID: 15183502]. [DOI] [PubMed] [Google Scholar]
- 85.Quesseveur G., David D.J., Gaillard M.C., Pla P., Wu M.V., Nguyen H.T., Nicolas V., Auregan G., David I., Dranovsky A., Hantraye P., Hen R., Gardier A.M., Déglon N., Guiard B.P. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry. 2013;3e:253. doi: 10.1038/tp.2013.30. [http://dx.doi.org/10.1038/tp.2013.30]. [PMID: 23632457]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mazumder A.G., Sharma P., Patial V., Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-kB activation. Basic Clin. Pharmacol. Toxicol. 2017;120(5):426–433. doi: 10.1111/bcpt.12694. [http://dx.doi.org/10.1111/bcpt.12694]. [PMID: 27800651]. [DOI] [PubMed] [Google Scholar]
- 87.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [http://dx.doi.org/10.1038/nrneurol.2010.178]. [PMID: 21135885]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N., Kumakiri C., Nakazato M., Watanabe H., Shinoda N., Okada S., Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54(1):70–75. doi: 10.1016/s0006-3223(03)00181-1. [http://dx.doi.org/10.1016/S0006-3223(03)00181-1]. [PMID: 12842310]. [DOI] [PubMed] [Google Scholar]
- 89.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [http://dx.doi.org/10.1016/S0028-3908(98)00141-5]. [PMID: 9886678]. [DOI] [PubMed] [Google Scholar]
- 90.de Almeida A.A., Gomes da Silva S., Lopim G.M., Vannucci Campos D., Fernandes J., Cabral F.R., Arida R.M. Physical exercise alters the activation of downstream proteins related to BDNF-TrkB signaling in male Wistar rats with epilepsy. J. Neurosci. Res. 2018;96(5):911–920. doi: 10.1002/jnr.24196. [http://dx.doi.org/10.1002/jnr.24196]. [PMID: 29098710]. [DOI] [PubMed] [Google Scholar]
- 91.Gu B., Huang Y.Z., He X.P., Joshi R.B., Jang W., McNamara J.O. A peptide uncoupling BDNF receptor TrkB from phospholipase Cγ1 prevents epilepsy induced by status epilepticus. Neuron. 2015;88(3):484–491. doi: 10.1016/j.neuron.2015.09.032. [http://dx.doi.org/10.1016/j.neuron.2015.09.032]. [PMID: 26481038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danelon V., Montroull L.E., Unsain N., Barker P.A., Mascó D.H. Calpain-dependent truncated form of TrkB-FL increases in neurodegenerative processes. Mol. Cell. Neurosci. 2016;75:81–92. doi: 10.1016/j.mcn.2016.07.002. [http://dx.doi.org/10.1016/j.mcn.2016.07.002]. [PMID: 27449758]. [DOI] [PubMed] [Google Scholar]
- 93.He X.P., Kotloski R., Nef S., Luikart B.W., Parada L.F., McNamara J.O. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43(1):31–42. doi: 10.1016/j.neuron.2004.06.019. [http://dx.doi.org/10.1016/j.neuron.2004.06.019]. [PMID: 15233915]. [DOI] [PubMed] [Google Scholar]
- 94.Gandolfi D., Cerri S., Mapelli J., Polimeni M., Tritto S., Fuzzati-Armentero M.T., Bigiani A., Blandini F., Mapelli L., D’Angelo E. Activation of the CREB/c-fos pathway during long-term synaptic plasticity in the cerebellum granular layer. Front. Cell. Neurosci. 2017;11:184. doi: 10.3389/fncel.2017.00184. [http://dx.doi.org/10.3389/fncel.2017.00184]. [PMID: 28701927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlezon W.A., Jr, Duman R.S., Nestler E.J. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [http://dx.doi.org/10.1016/j.tins.2005.06.005]. [PMID: 15982754]. [DOI] [PubMed] [Google Scholar]
- 96.Hansel D.E., Eipper B.A., Ronnett G.V. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410(6831):940–944. doi: 10.1038/35073601. [http://dx.doi.org/10.1038/35073601]. [PMID: 11309620]. [DOI] [PubMed] [Google Scholar]
- 97.Spencer J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010;104(Suppl. 3):S40–S47. doi: 10.1017/S0007114510003934. [http://dx.doi.org/10.1017/S0007114510003934]. [PMID: 20955649]. [DOI] [PubMed] [Google Scholar]
- 98.Conkright M.D., Guzmán E., Flechner L., Su A.I., Hogenesch J.B., Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol. Cell. 2003;11(4):1101–1108. doi: 10.1016/s1097-2765(03)00134-5. [http://dx.doi.org/10.1016/S1097-2765(03)00134-5]. [PMID: 12718894]. [DOI] [PubMed] [Google Scholar]
- 99.Jaeger B.N., Parylak S.L., Gage F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Aspects Med. 2018;61:50–62. doi: 10.1016/j.mam.2017.11.003. [http://dx.doi.org/10.1016/j.mam.2017.11.003]. [PMID: 29117513]. [DOI] [PubMed] [Google Scholar]
- 100.Moosavi F., Hosseini R., Saso L., Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Devel. Ther. 2015;10:23–42. doi: 10.2147/DDDT.S96936. [PMID: 26730179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moghbelinejad S., Nassiri-Asl M., Farivar T.N., Abbasi E., Sheikhi M., Taghiloo M., Farsad F., Samimi A., Hajiali F. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014;224(1):108–113. doi: 10.1016/j.toxlet.2013.10.010. [http://dx.doi.org/10.1016/j.toxlet.2013.10.010]. [PMID: 24148604]. [DOI] [PubMed] [Google Scholar]
- 102.Patil S.P., Jain P.D., Sancheti J.S., Ghumatkar P.J., Tambe R., Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2014.07.012. [http://dx.doi.org/10.1016/j.neuropharm.2014.07.012]. [PMID: 25087727]. [DOI] [PubMed] [Google Scholar]
- 103.Su G.Y., Yang J.Y., Wang F., Ma J., Zhang K., Dong Y.X., Song S.J., Lu X.M., Wu C.F. Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J. Ethnopharmacol. 2014;152(1):217–226. doi: 10.1016/j.jep.2014.01.006. [http://dx.doi.org/10.1016/j.jep.2014.01.006]. [PMID: 24440317]. [DOI] [PubMed] [Google Scholar]
- 104.Cho N., Lee K.Y., Huh J., Choi J.H., Yang H., Jeong E.J., Kim H.P., Sung S.H. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem. Toxicol. 2013;58:355–361. doi: 10.1016/j.fct.2013.05.007. [http://dx.doi.org/10.1016/j.fct.2013.05.007]. [PMID: 23688860]. [DOI] [PubMed] [Google Scholar]
- 105.Maher P., Akaishi T., Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA. 2006;103(44):16568–16573. doi: 10.1073/pnas.0607822103. [http://dx.doi.org/10.1073/pnas.0607822103]. [PMID: 17050681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perez-Vizcaino F., Fraga C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018;646:107–112. doi: 10.1016/j.abb.2018.03.022. [http://dx.doi.org/10.1016/j.abb.2018.03.022]. [PMID: 29580946]. [DOI] [PubMed] [Google Scholar]
- 107.Verlaet A.A., Ceulemans B., Verhelst H., Van West D., De Bruyne T., Pieters L., Savelkoul H.F., Hermans N. Effect of Pycnogenol® on attention-deficit hyperactivity disorder (ADHD): study protocol for a randomised controlled trial. Trials. 2017;18(1):145. doi: 10.1186/s13063-017-1879-6. [http://dx.doi.org/10.1186/s13063-017-1879-6]. [PMID: 28351412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrlinger K.A., Nieman K.M., Sanoshy K.D., Fonseca B.A., Lasrado J.A., Schild A.L., Maki K.C., Wesnes K.A., Ceddia M.A. Spearmint extract improves working memory in men and women with age-associated memory impairment. J. Altern. Complement. Med. 2018;24(1):37–47. doi: 10.1089/acm.2016.0379. [http://dx.doi.org/10.1089/acm.2016.0379]. [PMID: 29314866]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malaguarnera G., Pennisi M., Bertino G., Motta M., Borzì A.M., Vicari E., Bella R., Drago F., Malaguarnera M. Resveratrol in patients with minimal hepatic encephalopathy. Nutrients. 2018;10(3):E329. doi: 10.3390/nu10030329. [http://dx.doi.org/10.3390/nu10030329]. [PMID: 29522439]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boespflug E.L., Eliassen J.C., Dudley J.A., Shidler M.D., Kalt W., Summer S.S., Stein A.L., Stover A.N., Krikorian R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018;21(4):297–305. doi: 10.1080/1028415X.2017.1287833. [http://dx.doi.org/10.1080/1028415X.2017.1287833]. [PMID: 28221821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Losso J.N., Finley J.W., Karki N., Liu A.G., Prudente A., Tipton R., Yu Y., Greenway F.L. John, W.; Finley, Namrata, Karki.; Ann, G.; Liu, Alfredo.; Prudente, Russell.; Tipton, Ying Yu.; Frank, L.; Greenway. Pilot study of the Tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Ther. 2018;25(2):e194–e201. doi: 10.1097/MJT.0000000000000584. [http://dx.doi.org/10.1097/MJT.0000000000000584]. [PMID: 28901958]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirose A., Terauchi M., Akiyoshi M., Owa Y., Kato K., Kubota T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: a randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016;293(3):609–615. doi: 10.1007/s00404-015-3849-0. [http://dx.doi.org/10.1007/s00404-015-3849-0]. [PMID: 26294070]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karoly P.J., Nurse E.S., Freestone D.R., Ung H., Cook M.J., Boston R. Bursts of seizures in long-term recordings of human focal epilepsy. Epilepsia. 2017;58(3):363–372. doi: 10.1111/epi.13636. [http://dx.doi.org/10.1111/epi.13636]. [PMID: 28084639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murray K.D., Isackson P.J., Eskin T.A., King M.A., Montesinos S.P., Abraham L.A., Roper S.N. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J. Comp. Neurol. 2000;418(4):411–422. doi: 10.1002/(sici)1096-9861(20000320)418:4<411::aid-cne4>3.0.co;2-f. [http://dx.doi.org/10.1002/(SICI)1096-9861(20000320) 418:4<411:AID-CNE4>3.0.CO;2-F]. [PMID: 10713570]. [DOI] [PubMed] [Google Scholar]
- 115.Kanemoto K., Kawasaki J., Tarao Y., Kumaki T., Oshima T., Kaji R., Nishimura M. Association of partial epilepsy with brain-derived neurotrophic factor (BDNF) gene polymorphisms. Epilepsy Res. 2003;53(3):255–258. doi: 10.1016/s0920-1211(03)00032-9. [http://dx.doi.org/10.1016/S0920-1211(03)00032-9]. [PMID: 12694935]. [DOI] [PubMed] [Google Scholar]
- 116.Ferreira A., Pousinho S., Fortuna A., Falcão A., Alves G. Flavonoid compounds as reversal agents of the P-glycoprotein-mediated multidrug resistance: biology, chemistry and pharmacology. Phytochem. Rev. 2015;14:233–272. [http://dx.doi.org/10.1007/s11101-014-9358-0]. [Google Scholar]
- 117.Panda S.S., Jhanji N. Natural products as potential anti-Alzheimer Agents. Curr. Med. Chem. 2019 doi: 10.2174/0929867326666190618113613. [Epub ahead of print]. [http://dx.doi.org/10.2174/0929867326666190618113613]. [PMID: 31215372]. [DOI] [PubMed] [Google Scholar]
- 118.Han J.Y., Ahn S.Y., Kim C.S., Yoo S.K., Kim S.K., Kim H.C., Hong J.T., Oh K.W. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol. Pharm. Bull. 2012;35(9):1440–1446. doi: 10.1248/bpb.b110686. [http://dx.doi.org/10.1248/bpb.b110686]. [PMID: 22975493]. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y.F., Gao F., Li X.W., Jia R.H., Meng X.D., Zhao R., Jing Y.Y., Wang Y., Jiang W. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem. Res. 2012;37(8):1670–1680. doi: 10.1007/s11064-012-0771-8. [http://dx.doi.org/10.1007/s11064-012-0771-8]. [PMID: 22528832]. [DOI] [PubMed] [Google Scholar]
- 120.Rasilingam D., Duraisamy S., Subramanian R. Anticonvulsant activity of bioflavonoid gossypin. Bangladesh J. Pharmacol. 2009;4:51–54. [Google Scholar]
- 121.Dimpfel W. Different anticonvulsive effects of hesperidin and its aglycone hesperetin on electrical activity in the rat hippocampus in-vitro. J. Pharm. Pharmacol. 2006;58(3):375–379. doi: 10.1211/jpp.58.3.0012. [http://dx.doi.org/10.1211/jpp.58.3.0012]. [PMID: 16536905]. [DOI] [PubMed] [Google Scholar]
- 122.Kumar A., Lalitha S., Mishra J. Hesperidin potentiates the neuroprotective effects of diazepam and gabapentin against pentylenetetrazole-induced convulsions in mice: Possible behavioral, biochemical and mitochondrial alterations. Indian J. Pharmacol. 2014;46(3):309–315. doi: 10.4103/0253-7613.132180. [http://dx.doi.org/10.4103/0253-7613.132180]. [PMID: 24987179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taiwe G.S., Tchoya T.B., Menanga J.R., Dabole B., De Waard M. Anticonvulsant activity of an active fraction extracted from Crinum jagus L. (Amaryllidaceae), and its possible effects on fully kindled seizures, depression-like behaviour and oxidative stress in experimental rodent models. J. Ethnopharmacol. 2016;194:421–433. doi: 10.1016/j.jep.2016.10.023. [http://dx.doi.org/10.1016/j.jep.2016.10.023]. [PMID: 27725241]. [DOI] [PubMed] [Google Scholar]
- 124.Busquets O., Ettcheto M., Verdaguer E., Castro-Torres R.D., Auladell C., Beas-Zarate C., Folch J., Camins A. JNK1 inhibition by Licochalcone A leads to neuronal protection against excitotoxic insults derived of kainic acid. Neuropharmacology. 2018;131:440–452. doi: 10.1016/j.neuropharm.2017.10.030. [http://dx.doi.org/10.1016/j.neuropharm.2017.10.030]. [PMID: 29111385]. [DOI] [PubMed] [Google Scholar]
- 125.Zhen J.L., Chang Y.N., Qu Z.Z., Fu T., Liu J.Q., Wang W.P. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;57:177–184. doi: 10.1016/j.yebeh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 126.Jang H., Jeong K.H., Kim S.R. Naringin attenuates granule cell dispersion in the dentate gyrus in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2016;123:6–10. doi: 10.1016/j.eplepsyres.2016.03.001. [http://dx.doi.org/10.1016/j.eplepsyres.2016.03.001]. [PMID: 27040812]. [DOI] [PubMed] [Google Scholar]
- 127.Shakeel S., Rehman M.U., Tabassum N., Amin U., Mir M.U.R. Effect of naringenin (a naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn. Mag. 2017;13(Suppl. 1):S154–S160. doi: 10.4103/0973-1296.203977. [http://dx.doi.org/10.4103/0973-1296.203977]. [PMID: 28479741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Copmans D., Orellana-Paucar A.M., Steurs G., Zhang Y., Ny A., Foubert K., Exarchou V., Siekierska A., Kim Y., De Borggraeve W., Dehaen W., Pieters L., de Witte P.A.M. Methylated flavonoids as anti-seizure agents: Naringenin 4′,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018;112:124–133. doi: 10.1016/j.neuint.2017.11.011. [http://dx.doi.org/10.1016/j.neuint.2017.11.011]. [PMID: 29174382]. [DOI] [PubMed] [Google Scholar]
- 129.Zhen J., Qu Z., Fang H., Fu L., Wu Y., Wang H., Zang H., Wang W. Effects of grape seed proanthocyanidin extract on pentylenetetrazole-induced kindling and associated cognitive impairment in rats. Int. J. Mol. Med. 2014;34(2):391–398. doi: 10.3892/ijmm.2014.1796. [http://dx.doi.org/10.3892/ijmm.2014.1796]. [PMID: 24912930]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sefil F., Kahraman I., Dokuyucu R., Gokce H., Ozturk A., Tutuk O., Aydin M., Ozkan U., Pinar N. Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int. J. Clin. Exp. Med. 2014;7(9):2471–2477. [PMID: 25356099]. [PMC free article] [PubMed] [Google Scholar]
- 131.Nassiri-Asl M., Mortazavi S.R., Samiee-Rad F., Zangivand A.A., Safdari F., Saroukhani S., Abbasi E. The effects of rutin on the development of pentylenetetrazole kindling and memory retrieval in rats. Epilepsy Behav. 2010;18:50–53. doi: 10.1016/j.yebeh.2010.03.005. [http://dx.doi.org/10.1016/j.yebeh.2010.03.005]. [DOI] [PubMed] [Google Scholar]
- 132.Wang W., Wang F., Yang Y.J., Hu Z.L., Long L.H., Fu H., Xie N., Chen J.G. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br. J. Pharmacol. 2011;162(6):1364–1379. doi: 10.1111/j.1476-5381.2010.01143.x. [http://dx.doi.org/10.1111/j.1476-5381.2010.01143.x]. [PMID: 21133890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sarvestani N.N., Khodagholi F., Ansari N., Farimani M.M. Involvement of p-CREB and phase II detoxifying enzyme system in neuroprotection mediated by the flavonoid calycopterin isolated from Dracocephalum kotschyi. Phytomedicine. 2013;20(10):939–946. doi: 10.1016/j.phymed.2013.03.013. [http://dx.doi.org/10.1016/j.phymed.2013.03.013]. [PMID: 23639191]. [DOI] [PubMed] [Google Scholar]
- 134.Ren Z., Yan P., Zhu L., Yang H., Zhao Y., Kirby B.P., Waddington J.L., Zhen X. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology (Berl.) 2018;235(1):233–244. doi: 10.1007/s00213-017-4761-z. [http://dx.doi.org/10.1007/s00213-017-4761-z]. [PMID: 29058041]. [DOI] [PubMed] [Google Scholar]
- 135.Schroeter H., Bahia P., Spencer J.P., Sheppard O., Rattray M., Cadenas E., Rice-Evans C., Williams R.J. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J. Neurochem. 2007;101(6):1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.04434.x]. [PMID: 17298385]. [DOI] [PubMed] [Google Scholar]
- 136.Zheng M., Liu C., Pan F., Shi D., Zhang Y. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine. 2012;19(2):145–149. doi: 10.1016/j.phymed.2011.06.029. [http://dx.doi.org/10.1016/j.phymed.2011.06.029]. [PMID: 21802268]. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y., Zhen W., Maechler P., Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J. Nutr. Biochem. 2013;24(4):638–646. doi: 10.1016/j.jnutbio.2012.03.008. [http://dx.doi.org/10.1016/j.jnutbio.2012.03.008]. [PMID: 22819546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xia S.F., Xie Z.X., Qiao Y., Li L.R., Cheng X.R., Tang X., Shi Y.H., Le G.W. Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol. Behav. 2015;138:325–331. doi: 10.1016/j.physbeh.2014.09.008. [http://dx.doi.org/10.1016/j.physbeh.2014.09.008]. [PMID: 25447470]. [DOI] [PubMed] [Google Scholar]
- 139.Hou Y., Aboukhatwa M.A., Lei D.L., Manaye K., Khan I., Luo Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 2010;58(6):911–920. doi: 10.1016/j.neuropharm.2009.11.002. [http://dx.doi.org/10.1016/j.neuropharm.2009.11.002]. [PMID: 19917299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y.J., Li Y.J., Yang L.D., Zhang K., Zheng K.Y., Wei X.M., Yang Q., Niu W.M., Zhao M.G., Wu Y.M. Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behav. Brain Res. 2018;348:184–191. doi: 10.1016/j.bbr.2018.04.025. [http://dx.doi.org/10.1016/j.bbr.2018.04.025]. [PMID: 29680784]. [DOI] [PubMed] [Google Scholar]
- 141.Lee Y., Jeon S.J., Lee H.E., Jung I.H., Jo Y.W., Lee S., Cheong J.H., Jang D.S., Ryu J.H. Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol. Biochem. Behav. 2016;145:9–16. doi: 10.1016/j.pbb.2016.03.007. [http://dx.doi.org/10.1016/j.pbb.2016.03.007]. [PMID: 26997033]. [DOI] [PubMed] [Google Scholar]


