Abstract
The association of chronic pain with depression is becoming increasingly recognized. Treating both the conditions together is essential for an effective treatment outcome. In this regard, it is important to identify a shared mechanism involved in the association of chronic pain with depression. Central serotonin (5-hydroxytryptamine; 5-HT) neurotransmission has long been known to participate in the processing of signals related to pain. It also plays a key role in the pathogenesis and treatment of depression. Although functional responses to serotonin are mediated via the activation of multiple receptor types and subtypes, the 5-HT1A subtype is involved in the processing of nociception as well as the pathogenesis and treatment of depression. This receptor is located presynaptically, as an autoreceptor, on the perikaryon and dendritic spines of serotonin-containing neurons. It is also expressed as a heteroreceptor on neurons receiving input from serotonergic neurons. This arti-cle targets the 5-HT1A receptors to show that indiscriminate activation of pre and postsynaptic 5-HT1A receptors is likely to produce no therapeutic benefits; biased activation of the 5-HT heteroreceptors may be a useful strategy for treating chronic pain and depression individually as well as in a comorbid condition.
Keywords: Chronic pain, depression, serotonin, 5-HT1A receptor, heteroreceptors, autoreceptors
1. INTRODUCTION
Chronic pain, defined as sustained or intermittent pain which lasts for more than 12 weeks, may arise as a consequence of injury such as a back sprain. It may be due to illness or even no evident reason. It is often categorized as neuropathic or nociceptive pain [1, 2]. Neuropathic pain is produced by a lesion/damage to the nervous system, while nociceptive pain is associated with a damage to non-neuronal tissues and sustained activation of nociceptors. Chronic pain conditions are highly prevalent and disabling [3], and are often associated with emotional disorders including anxiety and depression. Considering and treating these conditions with the associated emotional disorder is essential for effective and sustained treatment outcome [4-6].
The association of chronic pain with depression is becoming increasingly recognized. Prevalence studies show that the occurrence of a comorbid condition of chronic pain associated with depression is much higher than the individual occurrence of chronic pain or depression [7-9]. A meta-analysis shows that about 65% of patients with clinical symptom of depression have chronic pain, while a number of chronic pain patients (5-85%, depending on the severity of pain) have depression [10]. Moderate to severe pain highly impairs productivity and when it is associated with depression, the condition becomes worse and refractory to treatment [11]. An understanding of the mechanisms involved in comorbidity is therefore highly essential.
Chronic pain is considered as the expression of maladaptive plastic changes within the nociceptive pathway, such as ectopic generation of the action potential and facilitation or disinhibition of synaptic transmission [12]. It may also result because of the loss of synaptic connectivity or even the formation of new synaptic circuits and neuroimmune interactions. The activation of microglial cells in response to nerve injury is often implicated in the development of neuropathic pain [13]. These studies show that a variety of mediators are released from the injured tissue or neuron. These mediators have the ability to activate receptors on microglial cells to produce structural changes and the release of factors can lead to chronic pain. Thus, interaction between microglia, other glial cells and neuronal cells is involved in the development of chronic pain [14]. The role of glial cells, microglia and astrocytes, in neuronal plasticity related to depression is also becoming increasingly recognized [15, 16]. Investigations to characterize and portray common neuroplasticity changes shared by chronic pain and depression are also emerging [17, 18]. These efforts may help identify new drug targets for effectively treating chronic pain with depression. The focus of the present article is to understand serotonin1A receptor-dependent control of pain and depression for improving therapy in chronic pain with depression.
Failure to adapt to chronic stress may lead to chronic pain as well as depression [19, 20]. Central serotoninergic mechanisms playing a key role in responses to stress are also known to modulate pain transmission. Serotonin (5-Hydroxytryptamine; 5-HT) is the principal neurotransmitter involved in the pathophysiology as well as pharmacotherapy of depression. There is evidence that chronic pain patients with associated depression are at enhanced risk of addiction. These patients excessively use opioid drugs and benzodiazepines to manage chronic pain and the associated psychological condition [21, 22], which worsens the treatment. In this regard, it is important to point out that 5-HT1A receptors are also targeted for effectively modulating pathways involved in drug addiction [23, 24]. Buspirone, an antianxiety and antidepressant drug, and an agonist on 5-HT1A receptors, is shown to block addictive and hyperalgesic effects of morphine [25-27]. Moreover, buspirone itself can reduce pain perception [28]. Overall, these studies suggest that targeting 5-HT1A receptors can help to develop strategies for treating pain, depression and associated drug addiction, if any. The present article concerns a 5-HT1A receptor-mediated model that incorporates the treatment of chronic pain and depression, simultaneously.
2. SEROTONIN (5-HYDROXYTRYPTAMINE; 5-HT)
Serotonin, a biogenic amine, is present in animals as well as plants. It was identified as a gut stimulating factor (enteramine) in 1940 [29] and as a vasoconstrictor (serotonin) eight years later [30]. Both enteramine and serotoninwere chemically identified as 5-hydroxytryptamine (5-HT). The presence of this biogenic amine in the central nervous system (CNS) was reported soon thereafter [31, 32]. Although only a small amount of total body’s serotonin is synthesized in the CNS, as a neurotransmitter it is involved in almost every physiological function. It has a key role in the pathogenesis and pharmacotherapy of depression [23, 33, 34] and other psychiatric illnesses such as anxiety [35], migraine [36], anorexia [37, 38] and schizophrenia [39]. In addition, its functional significance in pain transmission is also well established [126, 40-42].
On the other hand, it is important to note that only about 5% of the total body 5-HT is present in the brain and most of it is produced and present peripherally. The peripheral 5-HT synthesized largely in the enterochromaffin cells of the gastrointestinal tract is secreted into the bloodstream. It is taken up by the blood platelets and stored there [43, 44]. Transported by blood platelets to various tissues, including immune cells and lymphatic system, serotonin is released upon activation [45]. Almost all the immune cells express 5-HT receptors, and evidence suggests that immune system communicates with the brain via humoral and neuronal mechanisms and that targeting the immune system for therapeutic development may provide an important opportunity to treat mental illness [46].
Neurons constituting serotonergic circuitry arise from the midbrain and brainstem raphe nuclei. Axons from the raphe extend rostrally and caudally to innervate, respectively, almost all brain regions and the spinal cord [47]. The functional responses to serotonin are mediated via seven different types of receptors which are further divided into at least 15 subtypes [48, 49]. All the types and subtypes of serotonin receptors, excluding 5-HT3, are G-protein coupled receptors [50]. Accumulating evidence suggests that activation of the 5-HT1A receptor subtype can modulate processing and control of signals associated with pain [26].
It is worth mentioning that serotonin is a precursor for melatonin, which is also implicated in pain reduction and mood elevation [51, 52]. It is, therefore, possible that some of the effects of increasing brain serotonin are processed via enhanced melatonin synthesis and function. However, the antinociceptive effects of 5-HT1A receptor and melatonin receptor activation do not seem to depend on each other. Thus pain-reducing effects of melatonin are antagonized by melatonin receptor antagonists [53] while antinociceptive effects of piromelatine, a multimodal sleep medication with agonist activity for melatonin as well as 5-HT1A receptors, are antagonized independently by melatonin as well as 5-HT1A receptor antagonists [54].
3. THE 5-HT1A RECEPTOR AND ITS LOCALIZATION
The 5-HT1A receptor is a G-Protein-coupled receptor Fig. (1). Activation of this receptor subtype reduces intracellular concentrations of cAMP. As a result, K+ ion channels open and Ca+2 channels are closed [55, 56] to inhibit neuronal firing Fig. (2). This receptor subtype is present on the presynaptic, as well as on the postsynaptic sites Fig. (3). As a presynaptic receptor, it is expressed on the cell soma and dendritic spines of neurons constituting serotonergic pathways. Low doses of 8-hydroxy-2-(di-n-propylamino) tetralin (8-OHDPAT) and buspirone preferentially activate 5-HT1A autoreceptors; consequently, the release of 5-HT from the serotonergic nerve endings is diminished [57-60]. The synthesis of 5-HT is reduced as a feedback mechanism. The 5-HT-1A heteroreceptors are expressed in many brain regions [61, 62], and the activation of these receptors inhibits the firing of neurons on which these receptors are located.
Fig. (1).
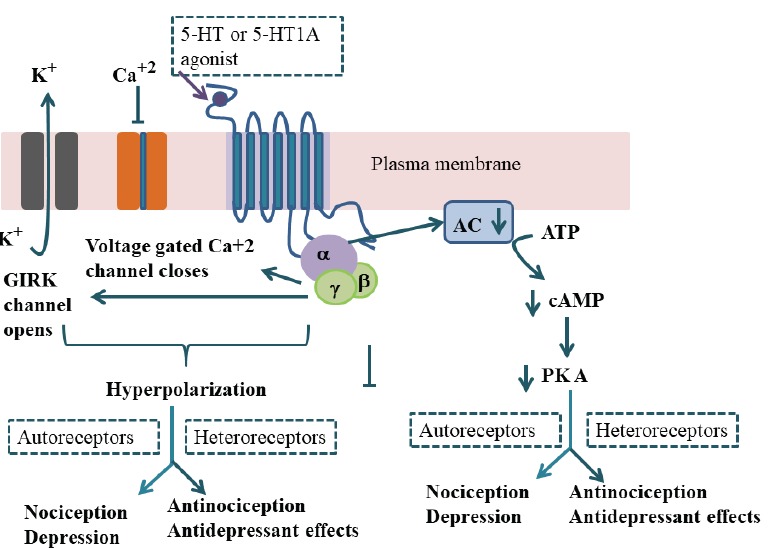
Diagrammatic sketch of 5-HT1A receptor and its signal transduction Pathway: Activation of 5-HT1A receptor which is coupled with Gi/o protein inhibits adenylate cyclase activity; cAMP formation and protein kinase-mediated protein phosphorylation are reduced. The activation of 5-HT1A receptors also opens G protein-gated K+ channels and inhibits voltage-gated calcium channels to lead to reduced neuronal firing. GIRK, G protein coupled inwardly-rectifying potassium; AC, adenylyl cyclase; cAMP, 3’, 5’-cyclic adenosine monophosphate; PKA, cAMP-dependent protein kinase. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
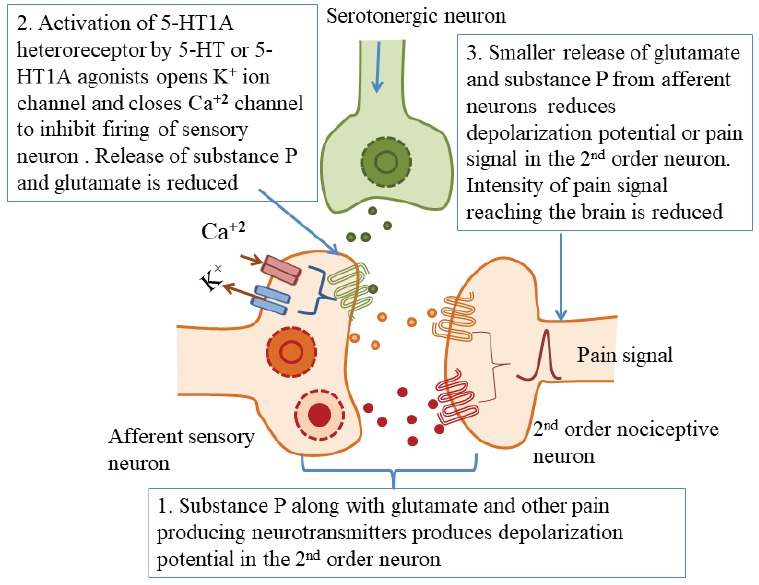
Serotonergic innervation of dorsal horn of the spinal cord showing localization of 5-HT1A heteroreceptors and inhibition of pain signals by the activation of these receptors. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (3).
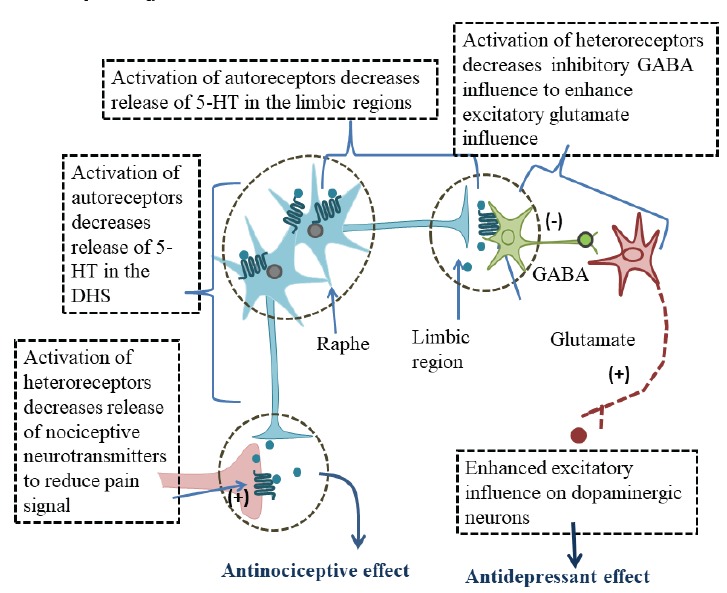
Diagrammatic sketch of serotonergic neurons arising from raphe and innervating DHS and limbic regions. Localization of 5-HT1A autoreceptors, heteroreceptors and functional responses to their activation are also depicted. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Differences in 5-HT1A autoreceptor and heteroreceptor coupling to G proteins have also been reported. The autoreceptors are mainly coupled with Gαi3; while heteroreceptors are preferentially coupled with Gαo in the hippocampus and equally with Gαo and Gαi3 in the cortex [63]. Differences in Gα coupling of 5-HT1A autoreceptors and heteroreceptors are thought to underlie differential signaling and desensitization in these cells. For example, long term increases of 5-HT upon chronic administration of antidepressant drugs produce greater desensitization of 5-HT1A autoreceptors than heteroreceptors [64].
In the raphe, 5-HT1A receptors are coupled via Gβγ subunits to inward rectifying potassium (GIRK) channels Fig. (1) to produce neuronal hyperpolarization [65-67]. The heteroreceptors of the hippocampus and cortex are also coupled to GIRK channels. Thus, the activation of 5-HT1A autoreceptors as well as of heteroreceptors of the hippocampus and the cortex increases GIRK current, leading to hyperpolarization [68]. The coupling of 5-HT1A autoreceptors and heteroreceptors in the hypothalamus via Gαo and Gβγ subunits resulting in the deactivation of voltage-dependent calcium channels is also reported [69]. In addition, the activation of 5-HT1A receptors in the raphe, hypothalamus and hippocampus can also increase the levels of phosphorylated mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) [70, 71]. There is evidence that 5-HT1A receptor-activated ERK is involved in hippocampal neurogenesis [72].
Buspirone is a partial agonist at the 5-HT1A heteroreceptor but a full agonist at 5-HT1A autoreceptors [73]. Administration of buspirone at low doses, therefore, activates 5-HT1A autoreceptors and serotonergic functions via heteroreceptors are diminished. Moreover, 8-OH-DPAT, which is a selective full agonist at 5-HT1A autoreceptors as well as heteroreceptors also preferentially acts via autoreceptors when administered at low doses [73]. This receptor type is known to have an important role in responses to stress [20, 38, 74] and in the pharmacotherapy of anxiety, depression and psychosis [34, 39, 75, 76].
Interestingly, 5-HT1A receptors are also expressed on the afferent nociceptive fibers in the dorsal horn of the spinal cord (DHS) [77] and their activation results in the diminished release of glutamate and substance P from the afferent fibers [25, 26, 78]. The release of serotonin from serotonergic projections arising from the brain stem and midbrain raphe [79] can activate 5-HT1A heteroreceptors located on the sensory neurons, resulting in an inhibition of nociceptive release from these fibers Fig. (2).
The dorsal raphe nucleus (DRN) has the highest density of 5-HT1A receptors [80]; it projects rostrally to innervate almost all the forebrain regions, including those which play an important role in mood, emotions and responses to stress [81, 82]. It also extends caudally to provide serotonergic projections to brain stem raphe magnus, and descends to the DHS [83]. Furthermore, the activation of somatodendritic receptors by 5-HT1A agonists produces a robust decrease of 5-HT release in terminal regions receiving input from DRN [84-86]. The 5-HT1A receptors are also highly expressed in the DHS, where they act as heteroreceptors to modulate the release of pain neurotransmitters from the first-order neurons [87]. They are also highly expressed in the brain regions involved in emotional control Fig. (3), where they play a key role in responses to stress and in the therapeutic effects of antidepressant drugs [34, 38].
It may be noted that 5-HT1A receptors are targeted for the pharmacotherapy of a number of brain disorders. However, indiscriminate activation of pre and postsynaptic 5-HT1A receptors is unlikely to produce any therapeutic benefits. Efforts made for preferentially increasing serotonergic activity via postsynaptic 5-HT1A receptors have led to the concept of ‘biased agonism’. Thus, drugs simultaneously blocking 5-HT1A receptors and serotonin transporters can produce a faster onset of antidepressant action compared to selective serotonin reuptake inhibitors (SSRIs) [88]. Long term administration of drugs preferentially activating 5-HT1A autoreceptors can desensitize feedback control over serotonergic activity to increase serotonin outflow towards postsynaptic 5-HT1A receptor to produce antidepressant effects [38].
4. 5-HT1A RECEPTOR-DEPENDENT CONTROL OF PAIN TRANSMISSION
Preclinical studies support the notion that the activation of 5-HT1A heteroreceptors in the DHS decreases pain transmission. These studies show that the administration of 8-OHDPAT in the DHS produces a marked reduction in the activity of afferent sensory neurons [89], which is associated with a dose-dependent decrease in NMDA receptor-dependent glutamate response [90]. These studies suggest that 5-HT1A receptor-dependent inhibition of nociceptive signals is due to the inhibition of glutamate release.
It was shown in a previous study that intrathecal and intracerebral administration of 8-OH-DPAT produced opposite effects on nociceptive behavior [91]. The drug injected systemically in low doses was found to enhance pain perception while high doses attenuated it. We now know that low doses of 8-OH-DPAT preferentially activate 5-HT1A autoreceptors Fig. (3). The associated decrease in the firing of serotonergic neurons and diminished 5-HT availability in the DHS can reduce 5-HT1A heteroreceptor-mediated inhibitory control over nociceptive signals to facilitate pain perception [25, 26]. On the other hand, higher doses of 8-OHDPAT activate 5-HT1A autoreceptors in the raphe region as well as heteroreceptors in the DHS. Adequate heteroreceptor activation can counteract autoreceptor-mediated enhancement in pain perception; it can also produce an additional antinociceptive effect. Likewise, intrathecally injected 8-OH-DPAT activates 5-HT1A heteroreceptors to reduce pain. Conversely, intracerebral administration preferentially activates autoreceptors to produce nociceptive effects. Nociception, due to an electrical stimulus, is also attenuated by intrathecally administered 8-OH-DPAT [92], while the antinociceptive effect of 8-OHDPAT is antagonized by a 5-HT1A antagonist. Moreover, the antinociceptive effects of piromelatine in mice with partial sciatic nerve ligation are also blocked by 5-HT1A receptor antagonism [93]. Transcription regulation studies of 5-HT1A receptor expression show that the transcription factor deformed epidermal autoregulatory factor-1 (Deaf-1) represses 5-HT1A autoreceptors expression, but enhances the 5-HT1A promoter activity for the expression of 5-HT1A heteroreceptors [94, 95]. These studies show that in Deaf-1 knockout mice, 5-HT1A heteroreceptors were knocked out. Studies have been performed on 5-HT1A heteroreceptor knockout mice. In these mice, 5-HT1A autoreceptors were over-expressed and central serotonin levels were reduced [96]. A higher nociceptive response to pain-producing stimuli also occurred in these mice [97].
Overall, these findings support the notion that the activation of 5-HT1A heteroreceptors in the DHS reduces pain transmission. Conversely, greater activity of 5-HT1A autoreceptors can diminish heteroreceptor-mediated antinociception because the availability of 5-HT at functional antinociceptive sites is attenuated Fig. (3).
Preclinical research on the effects of buspirone is also consistent. Buspirone is an FDA- approved prescription medication for treating depression and anxiety. It is an agonist for 5-HT1A heteroreceptors as well autoreceptors but exhibits full agonist activity at autoreceptors and only partial activity at heteroreceptors [98, 99]. Some previous studies show that systemically injected buspirone reduces pain perception [100, 101]. Recent studies show that low and high doses of buspirone produce opposite effects on pain perception [25, 26]. The perception of pain is enhanced and attenuated, respectively, in rats injected with low doses and high doses of buspirone. The opposite effects of low and high doses of buspirone on pain perception are also explicable in terms of preferential stimulation of autoreceptors at low doses while high doses stimulate autoreceptors as well as heteroreceptors. Autoreceptor occupancy is expected to decrease 5-HT release; effects of 5-HT via 5-HT1A heteroreceptors on afferent sensory fibers are reduced to facilitate pain transmission (Fig. (2). On the other hand, the activation of 5-HT1A heteroreceptors in the DHS following the administration of high doses of buspirone can counteract pain facilitatory effects of autoreceptor activation. It has been also shown that repeated administration of buspirone produces hypoalgesia [25] because the efficacy of autoreceptor-mediated control of the firing of serotoninergic neurons is diminished.
5. 5-HT1A RECEPTORS IN DEPRESSION AND ANTIDEPRESSANT ACTION
The role of 5-HT1A receptors in depression and antidepressant action has been addressed in many studies [20, 102]. These preclinical studies show that the activation of 5-HT1A receptors by the selective agonist 8-OH-DPAT produces antidepressant-like effects [103-105]. It has been also shown that these effects are produced because of the activation of 5-HT1A heteroreceptors in the limbic pathway [20, 106]. Located on the adjacent neurons, these heteroreceptors inhibit the activity of GABA interneurons Fig. (3); glutamate input to VTA dopamine neurons is enhanced to elevate mood [26]. Conversely, the activation of 5-H1A autoreceptors decreases the firing of serotonergic neurons and diminished activation of 5-HT1A heteroreceptors elicits depression-like behavior [20, 107]. Exposure to uncontrollable stress produces depression-like behavior in rats [108] and this is associated with an upregulation of 5-HT1A autoreceptors [58]. Conversely, adaptation to repeated predictable stress downregulates 5-HT1A receptors, and extracellular 5-HT concentration increases to produce antidepressant-like effects.
The antidepressant effects of SSRIs are also explained on the same lines. These drugs inhibit high affinity reuptake of serotonin to increase extracellular 5-HT, which activates autoreceptors to produce a feedback effect on 5-HT release. The availability of serotonin at postsynaptic receptors, including 5-HT1A receptors, is reduced. Ineffectiveness of SSRIs for treating depression after single or short term administration is often explained on the same lines. Repeated or long term administration desensitizes autoreceptors and the flow of serotonin towards postsynaptic receptors is enhanced, leading to the antidepressant effect [109]. Therefore, blocking 5-HT1A autoreceptors is often used as adjunctive therapy for improving acute antidepressant effects of SSRIs [110, 111].
6. CLINICAL RESEARCH ON THE USE OF ANTIDEPRESSANTS FOR TREATING PAIN
Although antidepressants drugs were designed for the treatment of depression, interest in their analgesic effect emerged because of the association of chronic pain with depression. Some, but not all antidepressant drugs are reported to produce the desired effect in relieving chronic pain [112, 113]. SSRIs are at present the most commonly prescribed first line agents for treating depression largely, as these drugs have fewer side effects. Despite the significant role of 5-HT1A heteroreceptors as well as autoreceptors in the mechanism of action of SSRIs and in the modulation of pain; there are limited studies justifying the treatment efficacy of SSRIs for chronic pain conditions and the reported results are not conclusive.
Fluoxetine is one of the prototype SSRIs approved as a prescription medication for depression. Treatment with fluoxetine is reported to be effective in tension headache [114] but not in diabetic neuropathic pain [115]. However, it is effective in ameliorating somatoform pain disorder in depressed patients [115, 116]. Fluvoxamine, another SSRI, is also effective in tension-type headache [117], post-stroke pain and osteoarthritis [118, 119] but not in chronic cancer pain [120]. Sertraline is shown to be effective in non-cardiac chronic chest pain [121]. Paroxetine is shown to have efficiency for treating pain associated with diabetic neuropathy [122]. Treatment with citalopram produces a moderate analgesic effect in somatoform pain disorder [123-125]. Escitalopram has, however, been shown to be useful for a number of chronic pain conditions including diabetic neuropathy, somatoform disorder and pain associated with depression [126-128].
On the other hand, it is widely believed that tricyclic antidepressants and serotonin-noradrenaline reuptake inhibitors (SNRIs) are more effective than SSRIs in treating chronic/neuropathic pain [129]. In this context, it is important to note that SSRIs do not selectively target 5-HT1A heteroreceptors. Preferential activation of 5-HT1A heteroreceptors by a co-drug can potentially enhance the efficacy and potency of SSRIs in treating depression, chronic pain and comorbidity.
CONCLUSION: OVERLAPPING PHARMACOTHERAPY OF PAIN AND DEPRESSION
Accumulated evidence as described above suggests that the activation of 5-HT1A heteroreceptors in the DHS and in the limbic region produces, respectively, an inhibition of nociceptive signal and antidepressant-like effect Fig. (3). These studies also show that desensitization of 5-HT autoreceptors increases the flow of serotonin towards 5-HT1A heteroreceptors to produce antidepressant as well as antinociceptive effects. The overlapping pharmacotherapy of chronic pain and depression is indicative of a causal relationship between chronic pain and depression. It tends to suggest that an overexpression of 5-HT1A autoreceptors decreasing 5-HT outflow towards the DHS and limbic region may lead to chronic pain and depression, respectively. Drugs producing indiscriminate activation of pre and postsynaptic 5-HT1A receptors are likely to produce no therapeutic benefits. Long-term administration of drugs preferentially activating 5-HT1A autoreceptors rather than heteroreceptors can increase serotonin outflow towards 5-HT1A heteroreceptors. These drugs can produce a delayed, but long-lasting effect in reducing pain as well as depression. On the other hand, simultaneous blockade of the 5-HT1A autoreceptor and serotonin transporter can produce a faster onset of analgesic as well as antidepressant effect. Targeting 5-HT1A receptors for particularly biased activation of heteroreceptors may be a useful strategy for treating chronic pain and depression as well as comorbid pain with depression. These strategies may also help to develop novel agents for treating chronic pain and depression.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The study was supported by a grant from Higher Education Commission Pakistan (No. 20-3997/NRPU/R&D/HEC/14).
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Colloca L., Ludman T., Bouhassira D., Baron R., Dickenson A.H., Yarnitsky D., Freeman R., Truini A., Attal N., Finnerup N.B., Eccleston C., Kalso E., Bennett D.L., Dworkin R.H., Raja S.N. Neuropathic pain. Nat. Rev. Dis. Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [http://dx.doi.org/10.1038/nrdp.2017.2]. [PMID: 28205574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St. John S.E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018;265(2):231–238. doi: 10.1007/s00415-017-8641-6. [http://dx.doi.org/10.1007/s00415-017-8641-6]. [PMID: 29032407]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Häuser W., Wolfe F., Henningsen P., Schmutzer G., Brähler E., Hinz A. Untying chronic pain: prevalence and societal burden of chronic pain stages in the general population - a cross-sectional survey. BMC Public Health. 2014;14:352. doi: 10.1186/1471-2458-14-352. [http://dx.doi.org/10.1186/1471-2458-14-352]. [PMID: 24725286]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinrib A.Z., Azam M.A., Birnie K.A., Burns L.C., Clarke H., Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br. J. Pain. 2017;11(4):169–177. doi: 10.1177/2049463717720636. [http://dx.doi.org/10.1177/2049463717720636]. [PMID: 29123661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belayev L.Y., Mor M.K., Sevick M.A., Shields A.M., Rollman B.L., Palevsky P.M., Arnold R.M., Fine M.J., Weisbord S.D. Longitudinal associations of depressive symptoms and pain with quality of life in patients receiving chronic hemodialysis. Hemodial. Int. 2015;19(2):216–224. doi: 10.1111/hdi.12247. [http://dx.doi.org/10.1111/hdi.12247]. [PMID: 25403142]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooten W.M. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin. Proc. 2016;91(7):955–970. doi: 10.1016/j.mayocp.2016.04.029. [http://dx.doi.org/10.1016/j.mayocp.2016.04.029]. [PMID: 27344405]. [DOI] [PubMed] [Google Scholar]
- 7.Mossey J.M., Gallagher R.M. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5(4):335–348. doi: 10.1111/j.1526-4637.2004.04041.x. [http://dx.doi.org/10.1111/j.1526-4637.2004.04041.x]. [PMID: 15563319]. [DOI] [PubMed] [Google Scholar]
- 8.Polatin P., Bevers K., Gatchel R.J. Pharmacological treatment of depression in geriatric chronic pain patients: a biopsychosocial approach integrating functional restoration. Expert Rev. Clin. Pharmacol. 2017;10(9):957–963. doi: 10.1080/17512433.2017.1339602. [http://dx.doi.org/10.1080/17512433.2017.1339602]. [PMID: 28590144]. [DOI] [PubMed] [Google Scholar]
- 9.Hassett A.L., Marshall E., Bailey A.M., Moser S., Clauw D.J., Hooten W.M., Urquhart A., Brummett C.M. Changes in anxiety and depression are mediated by changes in pain severity in patients undergoing lower-extremity total joint arthroplasty. Reg. Anesth. Pain Med. 2018;43(1):14–18. doi: 10.1097/AAP.0000000000000682. [http://dx.doi.org/10.1097/AAP.0000000000000682]. [PMID: 29077589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bair M.J., Robinson R.L., Katon W., Kroenke K. Depression and pain comorbidity: a literature review. Arch. Intern. Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [http://dx.doi.org/10.1001/archinte.163.20.2433]. [PMID: 14609780]. [DOI] [PubMed] [Google Scholar]
- 11.Cabrera-León A., Cantero-Braojos M.Á., Garcia-Fernandez L., Guerra de Hoyos J.A. Living with disabling chronic pain: results from a face-to-face cross-sectional population-based study. BMJ Open. 2018;8(11):e020913. doi: 10.1136/bmjopen-2017-020913. [http://dx.doi.org/10.1136/bmjopen-2017-020913]. [PMID: 30420342]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costigan M., Scholz J., Woolf C.J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [http://dx.doi.org/10.1146/annurev.neuro.051508.135531]. [PMID: 19400724]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo M., Bennett D.L. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp. Neurol. 2012;234(2):271–282. doi: 10.1016/j.expneurol.2011.08.018. [http://dx.doi.org/10.1016/j.expneurol.2011.08.018]. [PMID: 21893056]. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H., Alam A., Chen Q.A., Eusman M., Pal A., Eguchi S., Wu L., Ma D. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br. J. Anaesth. 2017;118(4):504–516. doi: 10.1093/bja/aex006. [http://dx.doi.org/10.1093/bja/aex006]. [PMID: 28403399]. [DOI] [PubMed] [Google Scholar]
- 15.Rial D., Lemos C., Pinheiro H., Duarte J.M., Gonçalves F.Q., Real J.I., Prediger R.D., Gonçalves N., Gomes C.A., Canas P.M., Agostinho P., Cunha R.A. Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 2016;9:521. doi: 10.3389/fncel.2015.00521. [http://dx.doi.org/10.3389/fncel.2015.00521]. [PMID: 26834566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno Y., Kinboshi M., Shimizu S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int. J. Mol. Sci. 2018;19(11):E3313. doi: 10.3390/ijms19113313. [http://dx.doi.org/10.3390/ijms19113313]. [PMID: 30356026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doan L., Manders T., Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691. doi: 10.1155/2015/504691. [http://dx.doi.org/10.1155/2015/504691]. [PMID: 25810926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng J., Liu S., Wang Y., Cui R., Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi: 10.1155/2017/9724371. [http://dx.doi.org/10.1155/2017/9724371]. [PMID: 28706741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdallah C.G., Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress (Thousand Oaks) 2017;1:1–10. doi: 10.1177/2470547017704763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabeen H.D. Raphe-hippocampal serotonin neurotransmission in the sex related differences of adaptation to stress: focus on serotonin-1a receptor. Curr. Neuropharmacol. 2011;9(3):512–521. doi: 10.2174/157015911796558019. [http://dx.doi.org/10.2174/157015911796558019]. [PMID: 22379463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer B., Lusted A., Roerecke M., Taylor B., Rehm J. The prevalence of mental health and pain symptoms in general population samples reporting nonmedical use of prescription opioids: a systematic review and meta-analysis. J. Pain. 2012;13(11):1029–1044. doi: 10.1016/j.jpain.2012.07.013. [http://dx.doi.org/10.1016/j.jpain.2012.07.013]. [PMID: 23040158]. [DOI] [PubMed] [Google Scholar]
- 22.Morley K.I., Ferris J.A., Winstock A.R., Lynskey M.T. Polysubstance use and misuse or abuse of prescription opioid analgesics: a multi-level analysis of international data. Pain. 2017;158(6):1138–1144. doi: 10.1097/j.pain.0000000000000892. [http://dx.doi.org/10.1097/j.pain.0000000000000892]. [PMID: 28267061]. [DOI] [PubMed] [Google Scholar]
- 23.Haleem D.J. Extending therapeutic use of psychostimulants: focus on serotonin-1A receptor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:170–180. doi: 10.1016/j.pnpbp.2013.07.015. [http://dx.doi.org/10.1016/j.pnpbp.2013.07.015]. [PMID: 23906987]. [DOI] [PubMed] [Google Scholar]
- 24.Devinsky O., Cilio M.R., Cross H., Fernandez-Ruiz J., French J., Hill C., Katz R., Di Marzo V., Jutras-Aswad D., Notcutt W.G., Martinez-Orgado J., Robson P.J., Rohrback B.G., Thiele E., Whalley B., Friedman D. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [http://dx.doi.org/10.1111/epi.12631]. [PMID: 24854329]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haleem D.J., Nawaz S. Inhibition of reinforcing, hyperalgesic, and motor effects of morphine by buspirone in rats. J. Pain. 2017;18(1):19–28. doi: 10.1016/j.jpain.2016.10.001. [http://dx.doi.org/10.1016/j.jpain.2016.10.001]. [PMID: 27742411]. [DOI] [PubMed] [Google Scholar]
- 26.Haleem D.J. Serotonin-1A receptor dependent modulation of pain and reward for improving therapy of chronic pain. Pharmacol. Res. 2018;134:212–219. doi: 10.1016/j.phrs.2018.06.030. [http://dx.doi.org/10.1016/j.phrs.2018.06.030]. [PMID: 29969666]. [DOI] [PubMed] [Google Scholar]
- 27.Haleem D.J., Nawaz S., Salman T. Dopamine and serotonin metabolism associated with morphine reward and its inhibition with buspirone: A study in the rat striatum. Pharmacol. Biochem. Behav. 2018;170:71–78. doi: 10.1016/j.pbb.2018.05.010. [http://dx.doi.org/10.1016/j.pbb.2018.05.010]. [PMID: 29782941]. [DOI] [PubMed] [Google Scholar]
- 28.Haleem D.J., Nawaz S., Salman T. Dose related effects of buspirone on pain, learning / memory and food intake. Regul. Toxicol. Pharmacol. 2018;99:182–190. doi: 10.1016/j.yrtph.2018.09.017. [http://dx.doi.org/10.1016/j.yrtph.2018.09.017]. [PMID: 30244043]. [DOI] [PubMed] [Google Scholar]
- 29.Erspamer V. Pharmakologische studien uber enteramin: Uber die, wirkung von acetonextrkten der kaninchenmagenschleimhaut auf den blutdruck und auf isoliert uberlebende organe. Arch Exp Path Pharmakol. 1940;196:343–407. [http://dx.doi.org/10.1007/BF01861121]. [Google Scholar]
- 30.Rapport M.M., Green A.A., Page I.H. Crystalline serotonin. Science. 1948;108(2804):329–330. doi: 10.1126/science.108.2804.329. [http://dx.doi.org/10.1126/science.108.2804.329]. [PMID: 17748034]. [DOI] [PubMed] [Google Scholar]
- 31.Amin A.H., Crawford T.B., Gaddum J.H. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J. Physiol. 1954;126(3):596–618. doi: 10.1113/jphysiol.1954.sp005229. [http://dx.doi.org/10.1113/jphysiol.1954.sp005229]. [PMID: 13222357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodie B.B., Pletscher A., Shore P.A. Evidence that serotonin has a role in brain function. Science. 1955;122(3177):968. doi: 10.1126/science.122.3177.968. [http://dx.doi.org/10.1126/science.122.3177.968]. [PMID: 13274056]. [DOI] [PubMed] [Google Scholar]
- 33.Nemeroff C.B., Owens M.J. The role of serotonin in the pathophysiology of depression: as important as ever. Clin. Chem. 2009;55(8):1578–1579. doi: 10.1373/clinchem.2009.123752. [http://dx.doi.org/10.1373/clinchem.2009.123752]. [PMID: 19498050]. [DOI] [PubMed] [Google Scholar]
- 34.Haleem D.J. Drug targets for obesity and depression: from serotonin to leptin. Curr. Drug Targets. 2016;17(11):1282–1291. doi: 10.2174/1389450117666151209123049. [http://dx.doi.org/10.2174/1389450117666151209123049]. [PMID: 26648065]. [DOI] [PubMed] [Google Scholar]
- 35.Akimova E., Lanzenberger R., Kasper S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry. 2009;66(7):627–635. doi: 10.1016/j.biopsych.2009.03.012. [http://dx.doi.org/10.1016/j.biopsych.2009.03.012]. [PMID: 19423077]. [DOI] [PubMed] [Google Scholar]
- 36.Goadsby P.J. Emerging therapies for migraine. Nat. Clin. Pract. Neurol. 2007;3(11):610–619. doi: 10.1038/ncpneuro0639. [http://dx.doi.org/10.1038/ncpneuro0639]. [PMID: 17982431]. [DOI] [PubMed] [Google Scholar]
- 37.Haleem D.J. Serotonin neurotransmission in anorexia nervosa. Behav. Pharmacol. 2012;23(5-6):478–495. doi: 10.1097/FBP.0b013e328357440d. [http://dx.doi.org/10.1097/FBP.0b013e328357440d]. [PMID: 22854305]. [DOI] [PubMed] [Google Scholar]
- 38.Haleem D.J. Improving therapeutics in anorexia nervosa with tryptophan. Life Sci. 2017;178:87–93. doi: 10.1016/j.lfs.2017.04.015. [http://dx.doi.org/10.1016/j.lfs.2017.04.015]. [PMID: 28438641]. [DOI] [PubMed] [Google Scholar]
- 39.Haleem D.J. 5-HT1A receptor-dependent control of nigrostriatal dopamine neurotransmission in the pharmacotherapy of Parkinson’s disease and schizophrenia. Behav. Pharmacol. 2015;26(1-2):45–58. doi: 10.1097/FBP.0000000000000123. [http://dx.doi.org/10.1097/FBP.0000000000000123]. [PMID: 25503261]. [DOI] [PubMed] [Google Scholar]
- 40.Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [http://dx.doi.org/10.1016/S0301-0082(02)00009-6]. [PMID: 12034378]. [DOI] [PubMed] [Google Scholar]
- 41.Sagalajev B., Viisanen H., Wei H., Pertovaara A. Descending antinociception induced by secondary somatosensory cortex stimulation in experimental neuropathy: role of the medullospinal serotonergic pathway. J. Neurophysiol. 2017;117(3):1200–1214. doi: 10.1152/jn.00836.2016. [http://dx.doi.org/10.1152/jn.00836.2016]. [PMID: 28053243]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ossipov M.H., Morimura K., Porreca F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care. 2014;8(2):143–151. doi: 10.1097/SPC.0000000000000055. [PMID: 24752199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gershon M.D., Ross L.L. Location of sites of 5-hydroxytryptamine storage and metabolism by radioautography. J. Physiol. 1966;186(2):477–492. doi: 10.1113/jphysiol.1966.sp008047. [http://dx.doi.org/10.1113/jphysiol.1966.sp008047]. [PMID: 5298337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sikander A., Rana S.V., Prasad K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta. 2009;403(1-2):47–55. doi: 10.1016/j.cca.2009.01.028. [http://dx.doi.org/10.1016/j.cca.2009.01.028]. [PMID: 19361459]. [DOI] [PubMed] [Google Scholar]
- 45.Ahern G.P. 5-HT and the immune system. Curr. Opin. Pharmacol. 2011;11(1):29–33. doi: 10.1016/j.coph.2011.02.004. [http://dx.doi.org/10.1016/j.coph.2011.02.004]. [PMID: 21393060]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mawe G.M., Hoffman J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [http://dx.doi.org/10.1038/nrgastro.2013.105]. [PMID: 23797870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charnay Y., Léger L. Brain serotonergic circuitries. Dialogues Clin. Neurosci. 2010;12(4):471–487. doi: 10.31887/DCNS.2010.12.4/ycharnay. [PMID: 21319493]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pytliak M., Vargová V., Mechírová V., Felšöci M. Serotonin receptors - from molecular biology to clinical applications. Physiol. Res. 2011;60(1):15–25. doi: 10.33549/physiolres.931903. [PMID: 20945968]. [DOI] [PubMed] [Google Scholar]
- 49.Nichols D.E., Nichols C.D. Serotonin receptors. Chem. Rev. 2008;108(5):1614–1641. doi: 10.1021/cr078224o. [http://dx.doi.org/10.1021/cr078224o]. [PMID: 18476671]. [DOI] [PubMed] [Google Scholar]
- 50.Peroutka S.J. Molecular biology of serotonin (5-HT) receptors. Synapse. 1994;18(3):241–260. doi: 10.1002/syn.890180310. [http://dx.doi.org/10.1002/syn.890180310]. [PMID: 7855737]. [DOI] [PubMed] [Google Scholar]
- 51.Comai S., Lopez-Canul M., De Gregorio D., Posner A., Ettaoussi M., Guarnieri F.C., Gobbi G. Melatonin MT1 receptor as a novel target in neuropsychopharmacology: MT1 ligands, pathophysiological and therapeutic implications, and perspectives. Pharmacol. Res. 2019;144:343–356. doi: 10.1016/j.phrs.2019.04.015. [http://dx.doi.org/10.1016/j.phrs.2019.04.015]. [PMID: 31029764]. [DOI] [PubMed] [Google Scholar]
- 52.Caumo W., Hidalgo M.P., Souza A., Torres I.L.S., Antunes L.C. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J. Pain Res. 2019;12:545–556. doi: 10.2147/JPR.S176857. [http://dx.doi.org/10.2147/JPR.S176857]. [PMID: 30787633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srinivasan V., Lauterbach E.C., Ho K.Y., Acuña-Castroviejo D., Zakaria R., Brzezinski A. Melatonin in antinociception: its therapeutic applications. Curr. Neuropharmacol. 2012;10(2):167–178. doi: 10.2174/157015912800604489. [http://dx.doi.org/10.2174/157015912800604489]. [PMID: 23204986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y.Y., Yin D., Chen L., Qu W.M., Chen C.R., Laudon M., Cheng N.N., Urade Y., Huang Z.L. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology (Berl.) 2014;231(20):3973–3985. doi: 10.1007/s00213-014-3530-5. [http://dx.doi.org/10.1007/s00213-014-3530-5]. [PMID: 24700387]. [DOI] [PubMed] [Google Scholar]
- 55.Rojas P.S., Fiedler J.L. What do we really know about 5-ht1a receptor signaling in neuronal cells? Front. Cell. Neurosci. 2016;10:272. doi: 10.3389/fncel.2016.00272. [http://dx.doi.org/10.3389/fncel.2016.00272]. [PMID: 27932955]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [http://dx.doi.org/10.1016/S0028-3908(99)00010-6]. [PMID: 10462127]. [DOI] [PubMed] [Google Scholar]
- 57.Hjorth S., Magnusson T. The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 1988;338(5):463–471. doi: 10.1007/BF00179315. [http://dx.doi.org/10.1007/BF00179315]. [PMID: 2469021]. [DOI] [PubMed] [Google Scholar]
- 58.Haleem D.J. Attenuation of 8-OH-DPAT-induced decreases in 5-Ht synthesis in brain regions of rats adapted to a repeated stress schedule. Stress. 1999;3(2):123–129. doi: 10.3109/10253899909001117. [http://dx.doi.org/10.3109/10253899909001117]. [PMID: 10938574]. [DOI] [PubMed] [Google Scholar]
- 59.Pazos A., Palacios J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346(2):205–230. doi: 10.1016/0006-8993(85)90856-x. [http://dx.doi.org/10.1016/0006-8993(85)90856-X]. [PMID: 4052776]. [DOI] [PubMed] [Google Scholar]
- 60.Riad M., Garcia S., Watkins K.C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417(2):181–194. [http://dx.doi.org/10.1002/(SICI)1096-9861(20000207)417:2<181:AID-CNE4>3.0.CO;2-A]. [PMID: 10660896]. [PubMed] [Google Scholar]
- 61.Pompeiano M., Palacios J.M., Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J. Neurosci. 1992;12(2):440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [http://dx.doi.org/10.1523/JNEUROSCI.12-02-00440.1992]. [PMID: 1531498]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doherty M.D., Pickel V.M. Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. J. Comp. Neurol. 2001;433(3):390–400. doi: 10.1002/cne.1147. [http://dx.doi.org/10.1002/cne.1147]. [PMID: 11298363]. [DOI] [PubMed] [Google Scholar]
- 63.Mannoury la Cour C., El Mestikawy S., Hanoun N., Hamon M., Lanfumey L. Regional differences in the coupling of 5-hydroxytryptamine-1A receptors to G proteins in the rat brain. Mol. Pharmacol. 2006;70(3):1013–1021. doi: 10.1124/mol.106.022756. [http://dx.doi.org/10.1124/mol.106.022756]. [PMID: 16772521]. [DOI] [PubMed] [Google Scholar]
- 64.Altieri S.C., Garcia-Garcia A.L., Leonardo E.D., Andrews A.M. Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem. Neurosci. 2013;4(1):72–83. doi: 10.1021/cn3002174. [http://dx.doi.org/10.1021/cn3002174]. [PMID: 23336046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ehrengruber M.U., Doupnik C.A., Xu Y., Garvey J., Jasek M.C., Lester H.A., Davidson N. Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc. Natl. Acad. Sci. USA. 1997;94(13):7070–7075. doi: 10.1073/pnas.94.13.7070. [http://dx.doi.org/10.1073/pnas.94.13.7070]. [PMID: 9192693]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penington N.J., Kelly J.S., Fox A.P. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J. Physiol. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [http://dx.doi.org/10.1113/jphysiol.1993.sp019819]. [PMID: 8271204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okuhara D.Y., Beck S.G. 5-HT1A receptor linked to inward-rectifying potassium current in hippocampal CA3 pyramidal cells. J. Neurophysiol. 1994;71(6):2161–2167. doi: 10.1152/jn.1994.71.6.2161. [http://dx.doi.org/10.1152/jn.1994.71.6.2161]. [PMID: 7931509]. [DOI] [PubMed] [Google Scholar]
- 68.Béïque J.C., Campbell B., Perring P., Hamblin M.W., Walker P., Mladenovic L., Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: Coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 2004;24(20):4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [http://dx.doi.org/10.1523/JNEUROSCI.5113-03.2004]. [PMID: 15152041]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee J.S., Ishibashi H., Akaike N. Serotonin modulates high-voltage-activated Ca2+ channels in rat ventromedial hypothalamic neurons. Neuropharmacology. 1996;35(8):1093–1100. doi: 10.1016/s0028-3908(96)00052-4. [http://dx.doi.org/10.1016/S0028-3908(96)00052-4]. [PMID: 9121612]. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan N.R., Crane J.W., Damjanoska K.J., Carrasco G.A., D’Souza D.N., Garcia F., Van de Kar L.D. Tandospirone activates neuroendocrine and ERK (MAP kinase) signaling pathways specifically through 5-HT1A receptor mechanisms in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2005;371(1):18–26. doi: 10.1007/s00210-004-1005-7. [http://dx.doi.org/10.1007/s00210-004-1005-7]. [PMID: 15655673]. [DOI] [PubMed] [Google Scholar]
- 71.Crane J.W., Shimizu K., Carrasco G.A., Garcia F., Jia C., Sullivan N.R., D’Souza D.N., Zhang Y., Van de Kar L.D., Muma N.A., Battaglia G. 5-HT1A receptors mediate (+)8-OH-DPAT-stimulation of extracellular signal-regulated kinase (MAP kinase) in vivo in rat hypothalamus: time dependence and regional differences. Brain Res. 2007;1183:51–59. doi: 10.1016/j.brainres.2007.07.101. [http://dx.doi.org/10.1016/j.brainres.2007.07.101]. [PMID: 17976547]. [DOI] [PubMed] [Google Scholar]
- 72.Mogha A., Guariglia S.R., Debata P.R., Wen G.Y., Banerjee P. Serotonin 1A receptor-mediated signaling through ERK and PKCα is essential for normal synaptogenesis in neonatal mouse hippocampus. Transl. Psychiatry. 2012 doi: 10.1038/tp.2011.58. 2e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peroutka S.J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [http://dx.doi.org/10.1016/0166-2236(88)90011-2]. [PMID: 2469177]. [DOI] [PubMed] [Google Scholar]
- 74.Artigas F., Bel N., Casanovas J.M., Romero L. Adaptative changes of the serotonergic system after antidepressant treatments. Adv. Exp. Med. Biol. 1996;398:51–59. doi: 10.1007/978-1-4613-0381-7_6. [http://dx.doi.org/10.1007/978-1-4613-0381-7_6]. [PMID: 8906240]. [DOI] [PubMed] [Google Scholar]
- 75.Nash J.R., Sargent P.A., Rabiner E.A., Hood S.D., Argyropoulos S.V., Potokar J.P., Grasby P.M., Nutt D.J. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br. J. Psychiatry. 2008;193(3):229–234. doi: 10.1192/bjp.bp.107.041186. [http://dx.doi.org/10.1192/bjp.bp.107.041186]. [PMID: 18757983]. [DOI] [PubMed] [Google Scholar]
- 76.Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr. Opin. Investig. Drugs. 2010;11(7):802–812. [PMID: 20571976]. [PubMed] [Google Scholar]
- 77.Perrin F.E., Gerber Y.N., Teigell M., Lonjon N., Boniface G., Bauchet L., Rodriguez J.J., Hugnot J.P., Privat A.M. Anatomical study of serotonergic innervation and 5-HT(1A) receptor in the human spinal cord. Cell Death Dis. 2011 doi: 10.1038/cddis.2011.98. 2e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bardin L., Tarayre J.P., Malfetes N., Koek W., Colpaert F.C. Profound, non-opioid analgesia produced by the high-efficacy 5-HT(1A) agonist F 13640 in the formalin model of tonic nociceptive pain. Pharmacology. 2003;67(4):182–194. doi: 10.1159/000068404. [http://dx.doi.org/10.1159/000068404]. [PMID: 12595749]. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki R., Rahman W., Hunt S.P., Dickenson A.H. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004;1019(1-2):68–76. doi: 10.1016/j.brainres.2004.05.108. [http://dx.doi.org/10.1016/j.brainres.2004.05.108]. [PMID: 15306240]. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs B.L., Azmitia E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [http://dx.doi.org/10.1152/physrev.1992.72.1.165]. [PMID: 1731370]. [DOI] [PubMed] [Google Scholar]
- 81.Nishitani N., Nagayasu K., Asaoka N., Yamashiro M., Andoh C., Nagai Y., Kinoshita H., Kawai H., Shibui N., Liu B., Hewinson J., Shirakawa H., Nakagawa T., Hashimoto H., Kasparov S., Kaneko S. Manipulation of dorsal raphe serotonergic neurons modulates active coping to inescapable stress and anxiety-related behaviors in mice and rats. Neuropsychopharmacology. 2019;44(4):721–732. doi: 10.1038/s41386-018-0254-y. [http://dx.doi.org/10.1038/s41386-018-0254-y]. [PMID: 30377380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teissier A., Chemiakine A., Inbar B., Bagchi S., Ray R.S., Palmiter R.D., Dymecki S.M., Moore H., Ansorge M.S. Activity of raphé serotonergic neurons controls emotional behaviors. Cell Rep. 2015;13(9):1965–1976. doi: 10.1016/j.celrep.2015.10.061. [http://dx.doi.org/10.1016/j.celrep.2015.10.061]. [PMID: 26655908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huo F.Q., Huang F.S., Lv B.C., Chen T., Feng J., Qu C.L., Tang J.S., Li Y.Q. Activation of serotonin 1A receptors in ventrolateral orbital cortex depresses persistent nociception: a presynaptic inhibition mechanism. Neurochem. Int. 2010;57(7):749–755. doi: 10.1016/j.neuint.2010.08.011. [http://dx.doi.org/10.1016/j.neuint.2010.08.011]. [PMID: 20813144]. [DOI] [PubMed] [Google Scholar]
- 84.Clark M.S., McDevitt R.A., Neumaier J.F. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J. Comp. Neurol. 2006;498(5):611–623. doi: 10.1002/cne.21073. [http://dx.doi.org/10.1002/cne.21073]. [PMID: 16917826]. [DOI] [PubMed] [Google Scholar]
- 85.Hervás I., Bel N., Fernández A.G., Palacios J.M., Artigas F. In vivo control of 5-hydroxytryptamine release by terminal autoreceptors in rat brain areas differentially innervated by the dorsal and median raphe nuclei. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358(3):315–322. doi: 10.1007/pl00005259. [http://dx.doi.org/10.1007/PL00005259]. [PMID: 9774218]. [DOI] [PubMed] [Google Scholar]
- 86.Hillegaart V., Hjorth S., Ahlenius S. Effects of 5-HT and 8-OH-DPAT on forebrain monoamine synthesis after local application into the median and dorsal raphe nuclei of the rat. J. Neural Transm. (Vienna) 1990;81(2):131–145. doi: 10.1007/BF01245833. [http://dx.doi.org/10.1007/BF01245833]. [PMID: 2141990]. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y.Q., Gao X., Ji G.C., Huang Y.L., Wu G.C., Zhao Z.Q. Expression of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain. 2002;98(3):287–295. doi: 10.1016/S0304-3959(02)00026-X. [http://dx.doi.org/10.1016/S0304-3959(02)00026-X]. [PMID: 12127030]. [DOI] [PubMed] [Google Scholar]
- 88.Newman-Tancredi A., Kleven M.S. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl.) 2011;216(4):451–473. doi: 10.1007/s00213-011-2247-y. [http://dx.doi.org/10.1007/s00213-011-2247-y]. [PMID: 21394633]. [DOI] [PubMed] [Google Scholar]
- 89.Gjerstad J., Tjolsen A., Hole K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996;318(2-3):315–321. doi: 10.1016/s0014-2999(96)00819-9. [http://dx.doi.org/10.1016/S0014-2999(96)00819-9]. [PMID: 9016920]. [DOI] [PubMed] [Google Scholar]
- 90.Mjellem N., Lund A., Eide P.K., Størkson R., Tjølsen A. The role of 5-HT1A and 5-HT1B receptors in spinal nociceptive transmission and in the modulation of NMDA induced behaviour. Neuroreport. 1992;3(12):1061–1064. doi: 10.1097/00001756-199212000-00007. [http://dx.doi.org/10.1097/00001756-199212000-00007]. [PMID: 1337283]. [DOI] [PubMed] [Google Scholar]
- 91.Fasmer O.B., Berge O.G., Post C., Hole K. Effects of the putative 5-HT1A receptor agonist 8-OH-2-(di-n-propylamino)tetralin on nociceptive sensitivity in mice. Pharmacol. Biochem. Behav. 1986;25(4):883–888. doi: 10.1016/0091-3057(86)90402-8. [http://dx.doi.org/10.1016/0091-3057(86)90402-8]. [PMID: 2947249]. [DOI] [PubMed] [Google Scholar]
- 92.Nadeson R., Goodchild C.S. Antinociceptive role of 5-HT1A receptors in rat spinal cord. Br. J. Anaesth. 2002;88(5):679–684. doi: 10.1093/bja/88.5.679. [http://dx.doi.org/10.1093/bja/88.5.679]. [PMID: 12067006]. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y.Y., Yin D., Chen L., Qu W.M., Chen C.R., Laudon M., Cheng N.N., Urade Y., Huang Z.L. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology (Berl.) 2014;231(20):3973–3985. doi: 10.1007/s00213-014-3530-5. [http://dx.doi.org/10.1007/s00213-014-3530-5]. [PMID: 24700387]. [DOI] [PubMed] [Google Scholar]
- 94.Lemonde S., Turecki G., Bakish D., Du L., Hrdina P.D., Bown C.D., Sequeira A., Kushwaha N., Morris S.J., Basak A., Ou X.M., Albert P.R. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 2003;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [http://dx.doi.org/10.1523/JNEUROSCI.23-25-08788.2003]. [PMID: 14507979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Czesak M., Lemonde S., Peterson E.A., Rogaeva A., Albert P.R. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J. Neurosci. 2006;26(6):1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI.2643-05.2006]. [PMID: 16467535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czesak M., Le François B., Millar A.M., Deria M., Daigle M., Visvader J.E., Anisman H., Albert P.R. Increased serotonin-1A (5-HT1A) autoreceptor expression and reduced raphe serotonin levels in deformed epidermal autoregulatory factor-1 (Deaf-1) gene knock-out mice. J. Biol. Chem. 2012;287(9):6615–6627. doi: 10.1074/jbc.M111.293027. [http://dx.doi.org/10.1074/jbc.M111.293027]. [PMID: 22232550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kayser V., Elfassi I.E., Aubel B., Melfort M., Julius D., Gingrich J.A., Hamon M., Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-, 5-HT3A-/- and 5-HTT-/- knock-out male mice. Pain. 2007;130(3):235–248. doi: 10.1016/j.pain.2006.11.015. [http://dx.doi.org/10.1016/j.pain.2006.11.015]. [PMID: 17250964]. [DOI] [PubMed] [Google Scholar]
- 98.Haleem D.J., Shireen E., Haleem M.A. Somatodendritic and postsynaptic serotonin-1A receptors in the attenuation of haloperidol-induced catalepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28(8):1323–1329. doi: 10.1016/j.pnpbp.2004.08.003. [http://dx.doi.org/10.1016/j.pnpbp.2004.08.003]. [PMID: 15588759]. [DOI] [PubMed] [Google Scholar]
- 99.Celada P., Bortolozzi A., Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs. 2013;27(9):703–716. doi: 10.1007/s40263-013-0071-0. [http://dx.doi.org/10.1007/s40263-013-0071-0]. [PMID: 23757185]. [DOI] [PubMed] [Google Scholar]
- 100.Galeotti N., Ghelardini C., Bartolini A. 5-HT1A agonists induce central cholinergic antinociception. Pharmacol. Biochem. Behav. 1997;57(4):835–841. doi: 10.1016/s0091-3057(96)00401-7. [http://dx.doi.org/10.1016/S0091-3057(96)00401-7]. [PMID: 9259013]. [DOI] [PubMed] [Google Scholar]
- 101.Belcheva S., Petkov V.D., Konstantinova E., Petkov V.V., Boyanova E. Effects on nociception of the Ca2+ and 5-HT antagonist dotarizine and other 5-HT receptor agonists and antagonists. Acta Physiol. Pharmacol. Bulg. 1995;21(4):93–98. [PMID: 8830881]. [PubMed] [Google Scholar]
- 102.Savitz J., Lucki I., Drevets W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [http://dx.doi.org/10.1016/j.pneurobio.2009.01.009]. [PMID: 19428959]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kennett G.A., Dourish C.T., Curzon G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur. J. Pharmacol. 1987;134(3):265–274. doi: 10.1016/0014-2999(87)90357-8. [http://dx.doi.org/10.1016/0014-2999(87)90357-8]. [PMID: 2883013]. [DOI] [PubMed] [Google Scholar]
- 104.Detke M.J., Wieland S., Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berl.) 1995;119(1):47–54. doi: 10.1007/BF02246053. [http://dx.doi.org/10.1007/BF02246053]. [PMID: 7675949]. [DOI] [PubMed] [Google Scholar]
- 105.Mayorga A.J., Dalvi A., Page M.E., Zimov-Levinson S., Hen R., Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J. Pharmacol. Exp. Ther. 2001;298(3):1101–1107. [PMID: 11504807]. [PubMed] [Google Scholar]
- 106.Blier P., de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15(7):220–226. doi: 10.1016/0165-6147(94)90315-8. [http://dx.doi.org/10.1016/0165-6147(94)90315-8]. [PMID: 7940983]. [DOI] [PubMed] [Google Scholar]
- 107.Albert P.R., Lembo P., Storring J.M., Charest A., Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14(1):19–25. doi: 10.1016/S0893-133X(96)80055-8. [http://dx.doi.org/10.1016/S0893-133X(96)80055-8]. [PMID: 8719026]. [DOI] [PubMed] [Google Scholar]
- 108.Haleem D.J., Parveen T. Brain regional serotonin synthesis following adaptation to repeated restraint. Neuroreport. 1994;5(14):1785–1788. doi: 10.1097/00001756-199409080-00025. [http://dx.doi.org/10.1097/00001756-199409080-00025]. [PMID: 7827332]. [DOI] [PubMed] [Google Scholar]
- 109.Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/s0006-3223(02)01643-8. [http://dx.doi.org/10.1016/S0006-3223(02)01643-8]. [PMID: 12559651]. [DOI] [PubMed] [Google Scholar]
- 110.Artigas F., Celada P., Laruelle M., Adell A. How does pindolol improve antidepressant action? Trends Pharmacol. Sci. 2001;22(5):224–228. doi: 10.1016/s0165-6147(00)01682-5. [http://dx.doi.org/10.1016/S0165-6147(00)01682-5]. [PMID: 11339972]. [DOI] [PubMed] [Google Scholar]
- 111.Sahli Z.T., Banerjee P., Tarazi F.I. The preclinical and clinical effects of vilazodone for the treatment of major depressive disorder. Expert Opin. Drug Discov. 2016;11(5):515–523. doi: 10.1517/17460441.2016.1160051. [http://dx.doi.org/10.1517/17460441.2016.1160051]. [PMID: 26971593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janakiraman R., Hamilton L., Wan A. Unravelling the efficacy of antidepressants as analgesics. Aust. Fam. Phys. 2016;45(3):113–117. [PMID: 27052046]. [PubMed] [Google Scholar]
- 113.Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H., Gilron I., Haanpää M., Hansson P., Jensen T.S., Kamerman P.R., Lund K., Moore A., Raja S.N., Rice A.S., Rowbotham M., Sena E., Siddall P., Smith B.H., Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [http://dx.doi.org/10.1016/S1474-4422(14)70251-0]. [PMID: 25575710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walker Z., Walker R.W., Robertson M.M., Stansfeld S. Antidepressant treatment of chronic tension-type headache: a comparison between fluoxetine and desipramine. Headache. 1998;38(7):523–528. doi: 10.1046/j.1526-4610.1998.3807523.x. [http://dx.doi.org/10.1046/j.1526-4610.1998.3807523.x]. [PMID: 15613168]. [DOI] [PubMed] [Google Scholar]
- 115.Max M.B., Lynch S.A., Muir J., Shoaf S.E., Smoller B., Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N. Engl. J. Med. 1992;326(19):1250–1256. doi: 10.1056/NEJM199205073261904. [http://dx.doi.org/10.1056/NEJM199205073261904]. [PMID: 1560801]. [DOI] [PubMed] [Google Scholar]
- 116.Luo Y.L., Zhang M.Y., Wu W.Y., Li C.B., Lu Z., Li Q.W. A randomized double-blind clinical trial on analgesic efficacy of fluoxetine for persistent somatoform pain disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(8):1522–1525. doi: 10.1016/j.pnpbp.2009.08.013. [http://dx.doi.org/10.1016/j.pnpbp.2009.08.013]. [PMID: 19733606]. [DOI] [PubMed] [Google Scholar]
- 117.Manna V., Bolino F., Di Cicco L. Chronic tension-type headache, mood depression and serotonin: therapeutic effects of fluvoxamine and mianserine. Headache. 1994;34(1):44–49. doi: 10.1111/j.1526-4610.1994.hed3401044.x. [http://dx.doi.org/10.1111/j.1526-4610.1994.hed3401044.x]. [PMID: 8132440]. [DOI] [PubMed] [Google Scholar]
- 118.Shimodozono M., Kawahira K., Kamishita T., Ogata A., Tohgo S., Tanaka N. Reduction of central poststroke pain with the selective serotonin reuptake inhibitor fluvoxamine. Int. J. Neurosci. 2002;112(10):1173–1181. doi: 10.1080/00207450290026139. [http://dx.doi.org/10.1080/00207450290026139]. [PMID: 12587520]. [DOI] [PubMed] [Google Scholar]
- 119.Xiao Y., Liu J., Huang X.E., Ca L.H., Ma Y.M., Wei W., Zhang R.X., Huang X.H., Chang J., Wu Y.J. Clinical study on fluvoxamine combined with oxycodone prolonged-release tablets in treating patients with moderate to severe cancer pain. Asian Pac. J. Cancer Prev. 2014;15(23):10445–10449. doi: 10.7314/apjcp.2014.15.23.10445. [http://dx.doi.org/10.7314/APJCP.2014.15.23.10445]. [PMID: 25556490]. [DOI] [PubMed] [Google Scholar]
- 120.Kane C.M., Mulvey M.R., Wright S., Craigs C., Wright J.M., Bennett M.I. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: Systematic review and meta-analysis. Palliat. Med. 2018;32(1):276–286. doi: 10.1177/0269216317711826. [http://dx.doi.org/10.1177/0269216317711826]. [PMID: 28604172]. [DOI] [PubMed] [Google Scholar]
- 121.Keefe F.J., Shelby R.A., Somers T.J., Varia I., Blazing M., Waters S.J., McKee D., Silva S., She L., Blumenthal J.A., O’Connor J., Knowles V., Johnson P., Bradley L. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain. 2011;152(4):730–741. doi: 10.1016/j.pain.2010.08.040. [http://dx.doi.org/10.1016/j.pain.2010.08.040]. [PMID: 21324590]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sindrup S.H., Gram L.F., Brøsen K., Eshøj O., Mogensen E.F. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42(2):135–144. doi: 10.1016/0304-3959(90)91157-E. [http://dx.doi.org/10.1016/0304-3959(90)91157-E]. [PMID: 2147235]. [DOI] [PubMed] [Google Scholar]
- 123.Viazis N., Katopodi K., Karamanolis G., Denaxas K., Varytimiadis L., Galanopoulos M., Tsoukali E., Kamberoglou D., Christidou A., Karamanolis D.G., Papatheodoridis G., Mantzaris G.J. Proton pump inhibitor and selective serotonin reuptake inhibitor therapy for the management of noncardiac chest pain. Eur. J. Gastroenterol. Hepatol. 2017;29(9):1054–1058. doi: 10.1097/MEG.0000000000000925. [http://dx.doi.org/10.1097/MEG.0000000000000925]. [PMID: 28628496]. [DOI] [PubMed] [Google Scholar]
- 124.Giannopoulos S., Kosmidou M., Sarmas I., Markoula S., Pelidou S.H., Lagos G., Kyritsis A.P. Patient compliance with SSRIs and gabapentin in painful diabetic neuropathy. Clin. J. Pain. 2007;23(3):267–269. doi: 10.1097/AJP.0b013e31802fc14a. [http://dx.doi.org/10.1097/AJP.0b013e31802fc14a]. [PMID: 17314587]. [DOI] [PubMed] [Google Scholar]
- 125.Roohafza H., Pourmoghaddas Z., Saneian H., Gholamrezaei A. Citalopram for pediatric functional abdominal pain: A randomized, placebo-controlled trial. Neurogastroenterol. Motil. 2014;26(11):1642–1650. doi: 10.1111/nmo.12444. [http://dx.doi.org/10.1111/nmo.12444]. [PMID: 25244442]. [DOI] [PubMed] [Google Scholar]
- 126.Otto M., Bach F.W., Jensen T.S., Brøsen K., Sindrup S.H. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139(2):275–283. doi: 10.1016/j.pain.2008.04.012. [http://dx.doi.org/10.1016/j.pain.2008.04.012]. [PMID: 18547727]. [DOI] [PubMed] [Google Scholar]
- 127.Muller J.E., Wentzel I., Koen L., Niehaus D.J., Seedat S., Stein D.J. Escitalopram in the treatment of multisomatoform disorder: a double-blind, placebo-controlled trial. Int. Clin. Psychopharmacol. 2008;23(1):43–48. doi: 10.1097/YIC.0b013e32825ea301. [http://dx.doi.org/10.1097/YIC.0b013e32825ea301]. [PMID: 18090507]. [DOI] [PubMed] [Google Scholar]
- 128.Jaracz J., Gattner K., Jaracz K., Górna K., Moczko J., Hauser J. Is venlafaxine more effective than escitalopram and nortriptyline in the management of painful symptoms in patients with major depression? Pharmacopsychiatry. 2018;51(4):148–152. doi: 10.1055/s-0043-122077. [http://dx.doi.org/10.1055/s-0043-122077]. [PMID: 29141255]. [DOI] [PubMed] [Google Scholar]
- 129.Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017;18(11):E2483. doi: 10.3390/ijms18112483. [http://dx.doi.org/10.3390/ijms18112483]. [PMID: 29160850]. [DOI] [PMC free article] [PubMed] [Google Scholar]


