Abstract
Duchenne muscular dystrophy (DMD) is the most common paediatric muscular dystrophy and is caused by mutations in the DYSTROPHIN gene. We generated two induced pluripotent stem cell (iPSC) lines from DMD patients with nonsense mutations in exons 68 (UCLi011-A) or 70 (UCLi012-A) by transfecting reprogramming mRNAs. Both mutations affect expression of all dystrophin isoforms. iPSCs expressed pluripotency-associated markers, differentiated into cells of the three germ layers in vitro and had normal karyotypes. The selected mutations are potentially amenable to read-through therapies, exon-skipping and gene-editing. These new iPSCs are also relevant to study DYSTROPHIN role in tissues other than skeletal muscle.
Resource Table
| Unique stem cell lines identifier | UCLi011-A |
| UCLi012-A | |
| Alternative names of stem cell lines | DMD iPSCs ex.68 (UCLi011-A) |
| DMD iPSCs ex.70 (UCLi012-A) | |
| Institution | University College London (UCL), London, UK |
| Contact information of distributor | Dr Francesco Saverio Tedesco (f.s.tedesco@ucl.ac.uk) |
| Type of cell lines | iPSCs |
| Origin | Human |
| Cell Source | Fibroblasts |
| Clonality | Mixed |
| Method of reprogramming | Transgene free (mRNA transfection) |
| Multiline rationale | Same disease non-isogenic cell lines |
| Gene modification | Yes |
| Type of modification | Spontaneous mutation |
| Associated disease | Duchenne muscular dystrophy |
| Gene/locus | DMD Cells c.9851G>A (p.Trp3284X) in exon 68 |
| DMD Cells c.10141C>T (p.Arg3381X) in exon 70 | |
| Method of modification | N/A |
| Name of transgene or resistance | N/A |
| Inducible/constitutive system | N/A |
| Date archived/stock date | 15/04/2019 |
| Cell line repository/bank | Human Pluripotent Stem Cell Registry (hpscreg.eu): |
| • https://hpscreg.eu/cell-line/UCLi012-A (Biosample SAMEA5574041) | |
| • https://hpscreg.eu/cell-line/UCLi011-A (Biosample SAMEA5574032) | |
| Ethical approval | Fibroblasts were obtained from the MRC Neuromuscular center Biobank (UCL, London, UK; Research Ethics Committee reference no. 06/Q0406/33). Human cell work was conducted under the approval of the National Health Service (NHS) Health Research Authority Research Ethics Committee reference no. 13/LO/1826; Integrated Research Application System (IRAS) project no. 141,100. |
1. Resource utility
The new genomic-integration-free DMD iPSC lines UCLi011-A and UCLi012-A carry nonsense mutations beyond exon 63 of the dystrophin gene (Table 1). Although uncommon, mutations located between exon 63 and exon 79 cause loss of all the dystrophin isoforms including Dp71, the most abundant isoform in brain, and which deficiency is highly associated with cognitive impairment. These mutations are potentially amenable to therapeutic approaches based upon exon-skipping and read-through strategies (reviewed in Scoto et al., 2018) and are relevant to study dystrophin role both in muscle and extra-muscular tissues. Overall, UCLi011-A and UCLi012-A iPSCs will be useful to study the impact of dystrophin deficiency in multiple tissues and to screen possible therapies, particularly using recently-established platforms of complex muscle disease modelling in vitro (Maffioletti et al., 2018).
Table 1.
Summary of lines.
| iPSC line names | Abbreviation in figures | Gender | Age | Ethnicity | Genotype of locus | Disease |
|---|---|---|---|---|---|---|
| UCLi011-A | DMD iPSCs ex.68 | Male | 8 | N/A | DMD Cells c.9851G>A (p.Trp3284X) in exon 68 | DMD |
| UCLi012-A | DMD iPSCs ex.70 | Male | 3 | N/A | DMD Cells c.10141C>T (p.Arg3381X) in exon 70 | DMD |
2. Resource details
DMD is an inherited muscle-wasting disorder of childhood caused by mutations in the dystrophin gene (Mercuri and Muntoni 2013). Dystrophin is the largest gene in nature and has a very complex transcriptional regulation, with several tissue specific isoforms associated with their own promoter and unique first exon (Muntoni et al., 2003). The deficiency of Dp71, the shortest isoform, although ubiquitously expressed, has been linked to severe cognitive deficit, thus raising interest in the function it plays in the central nervous system. The promoter and unique first exons of this isoform is located in intron 62 of the dystrophin gene, so any mutations located towards the 3′ of exon 63 will affect Dp71 in addition to all the remaining isoforms.
We generated two iPSC lines starting from skin fibroblasts from two DMD patients aged 8 (iPSC UCLi011-A) and 3 (iPSC UCLi012-A) with nonsense mutations in exon 68 and 70, respectively (kindly provided by the MRC Neuromuscular Biobank, London). Fibroblasts were reprogrammed into iPSCs via serial transfections with a mix of mRNAs encoding the reprogramming factors OCT4, SOX2, KLF4, CMYC, NANOG and LIN28, as well as reprogramming-enhancing microRNAs (microRNA-enhanced mRNA reprogramming protocol; Stemgent, cat. No. 00–0071 and 00–0073).
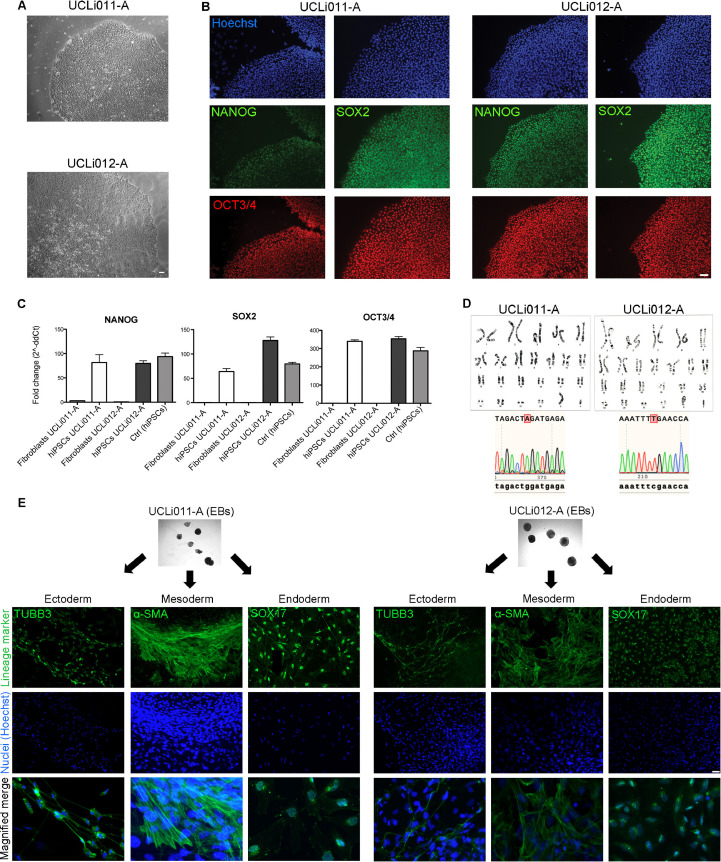
The resulting iPSC lines showed the expected morphology of human pluripotent colonies (Fig. 1A) and expressed the pluripotency-associated markers OCT4, NANOG and SOX2 at mRNA and protein levels (Fig. 1B,C). Both iPSCs UCLi011-A and UCLi012-A presented a normal karyotype (46,XY) with a correct ploidy and no major chromosomal abnormalities (Fig. 1D; UCLi011-A tested at passage 8; UCLi012-A tested at passage 6). Sanger sequencing confirmed that after reprogramming the DMD iPSCs still harbour the disease-causing mutations located in exon 68 (c.9851G>A (p.Trp3284X) and 70 c.10141C>T (p.Arg3381X) of the dystrophin gene (Fig. 1D). Functional pluripotency was demonstrated by differentiation into cell types of the three germ layers in embryoid body formation assays (Fig. 1E).
Fig. 1.
(A) Phase contrast images showing colonies of both DMD iPSC lines. Scale bar 100 μm. (B) Immunofluorescence staining showing pluripotency-associated markers (NANOG, SOX2 and OCT3/4) in UCLi011-A and UCLi012-A iPSCs. Hoechst: nuclei. Scale bar: 75 μm. (C) Quantitative real-time PCR analysis showing expression of mRNAs of pluripotency-associated factors (NANOG, SOX2 and OCT3/4) in UCLi011-A and UCLi012-A iPSCs and their absence in parental fibroblasts. (D) Upper images: normal karyotype of UCLi011-A and UCLi012-A iPSCs (46,XY). Lower images: electropherograms confirming presence of pathogenic mutations in exon 68 (c.9851G>A (p.Trp3284X) and 70 c.10141C>T (p.Arg3381X) of the dystrophin gene. (E) Embryoid body formation assay. Upper phase contract images show morphology of UCLi011-A and UCLi012-A embryoid bodies. Lower panels show immunofluorescence staining of the same embryoid bodies with lineage-specific markers: α-smooth muscle actin (mesoderm), βIII-tubulin (ectoderm) and SOX17 (endoderm). Hoechst: nuclei. Scale bar: 75 μm. Bottom images: merged pictures showing magnified areas of each lineage-specific staining.
Additionally, cell identity was confirmed by STR analysis, which demonstrated a 100% match in the analysed alleles of parental fibroblasts and derived iPSCs (available with the authors). Finally, both iPSC lines were negative for Mycoplasma contamination (characterization and validation summarized in Table 2).
Table 2.
Characterization and validation.
| Classification | Test | Result | Data |
|---|---|---|---|
| Morphology | Photography (phase contrast microscopy) | Adherent colonies with epithelial morphology and high nuclear-cytoplasmic ratio. | Fig. 1 panel A |
| Phenotype | Qualitative analysis (Immunofluorescence) | Positive staining for pluripotency-associated markers: OCT4, NANOG, SOX2 | Fig. 1 panel B |
| Quantitative analysis (RT-qPCR) | Positive expression of OCT4, NANOG AND SOX2 transcripts | Fig. 1 panel C | |
| Genotype | Karyotype (G-banding) and resolution | 46XY, Resolution 6–10 MB | Fig. 1 panel F |
| Identity | STR analysis | 16 loci tested; 100%match | Summary table available with authors |
| Mutation analysis (IF APPLICABLE) | Sequencing | X-linked mutations: DMD c.9851G>A (p.Trp3284X) in exon 68; DMD c.10141C>T (p.Arg3381X) in exon 70 | Fig. 1 panel D |
| Microbiology and virology | Mycoplasma | Mycoplasma testing by luminescence: Negative | Materials and methods |
| Differentiation potential | Embryoid body formation | Embryoid bodies spontaneous differentiation: α-smooth muscle actin (mesoderm), βIII-tubulin (ectoderm) and SOX17 (endoderm) | Fig. 1 panel E |
3. Materials and methods
3.1. Cell culture
DMD fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (FBS; Sigma) and 1% penicillin-streptomycin antibiotics (PS) (Sigma). 5 × 104 fibroblasts were plated onto 6-cm dishes coated with Matrigel™. After 24 h, media was changed to NuFF-conditioned Pluriton media supplemented with B18R (eBioscience) and Pluriton supplement (Stemgent). Subsequently fibroblasts were transfected with a mix of mRNA reprogramming factors (OCT4, SOX2, KLF4, cMYC, NANOG and LIN28; Stemgent, cat.no 00–0071) (Warren et al. 2010), following manufacturer instructions. Transfections were performed daily for 11 days with Stemgent Stemfect RNA Transfection Kit (Stemgent 00–0069) in NuFF-conditioned Pluriton media; cells were not split/passaged until appearance of colonies. On days 1 and 5, MicroRNAs (Stemgent, cat.no. 00–0073) were added to the mRNA cocktail to enhance reprogramming efficiency, following manufacturer's instructions.
From day 19, the first colonies were picked and plated onto 6-well dishes. iPSCs UCLi011-A were plated directly on Vitronectin XF™(Stemcell Technologies), and maintained in feeder-free, chemically defined TeSR™-E8™ medium (Stemcell Technologies, cat.no. 05,940) at 37 °C with 5% CO2 and 3% O2. Approximately every 6 days, iPSCs were passaged via either manual picking or gentle cell dissociation reagent at a 1:8 ratio (Stemcell Technologies, cat. no.07174), following manufacturer's instructions. iPSCs UCLi012-A were first expanded on feeder cells (mouse embryonic fibroblasts) to increase their attachment and viability. After two passages on feeders, iPSCs UCLi012-A were stabilised in feeder-free conditions (~2/3 passages) as described for iPSCs UCLi011-A. Mycoplasma contamination was ruled out by MycoAlert™ kit, following manufacturer's instructions (Lonza); a ratio <0.9 is considered negative: UCLi011-A = 0.48; UCLi012-A = 0.71.
3.2. Immunofluorescence
Cells were washed with PBS, fixed with 4% (w/v) PFA for 5 min, followed by a further PBS wash. Fixed cells were permeabilized for 1 hour with permeabilization solution (1% bovine serum albumin (BSA) + 0.2% Triton in PBS) at room temperature. Cells were then blocked for 30 min with 10% donkey or goat serum diluted in permeabilizing solution at room temperature. Cells were then incubated overnight at 4 °C with the primary antibodies (Table 3) diluted in permeabilization solution. Unbound primary antibody was removed with three washes of 0.2% Triton in PBS. Cells were then incubated for 1 hour with secondary antibodies and Hoechst 33342 diluted in 0.2% Triton in PBS. Unbound secondary antibody was washed away with two washes of 0.2% Triton in PBS, followed by one rinse with PBS. Cells were imaged with an inverted fluorescence microscope (Leica DM16000B).
Table 3.
Reagents details.
| Antibodies used for immunocytochemistry/flow-citometry | |||
|---|---|---|---|
| Antibody | Dilution | Company Cat # and RRID | |
| Pluripotency Markers | Mouse anti-OCT4 | 1:100 | Santa Cruz Biotechnology Cat# sc-5279, RRID:AB_628,051 |
| Pluripotency Markers | Rabbit anti-SOX2 | 1:200 | Abcam cat# ab97959 AB_2,341,193 |
| Pluripotency Markers | Rabbit anti-NANOG | 1:200 | Abcam cat# ab80892 RRID: AB_2,150,114 |
| Differentiation Markers | Rabbit anti- SOX17 | 1:100 | Millipore cat#09–038 RRID: AB_1,587,525 |
| Differentiation Markers | Mouse anti-Actin, α-smooth muscle | 1:300 | Sigma cat# A2547 RRID: AB_476,701 |
| Differentiation Markers | Mouse anti class III beta-Tubulin | 1:100 | Stemcell Technologies cat# 1409 RRID: AB_215,509 |
| Secondary antibodies | Donkey Anti-Mouse IgG, Alexa Fluor 546 | 1:500 | Thermo Fisher Scientific Cat# A10036, RRID:AB_2,534,012 |
| Secondary antibodies | Donkey Anti-Rabbit IgG, Alexa Fluor 647 | 1:500 | Molecular Probes Cat# A-31,573, RRID:AB_2,536,183 |
| Secondary antibodies | Donkey Anti-Mouse IgG, Alexa Fluor 488 | 1:500 | Molecular Probes Cat# A-21,202, RRID:AB_141,607 |
| Primers | |||
|---|---|---|---|
| Target | Forward/Reverse primer (5′−3′) | ||
| Targeted mutation analysis/sequencing | DMD c.9851G>A (p.Trp3284X) in exon 68 | CCAGCCTAGCTTTGCAACCAT / CCCGTGAAGACACGCACT | |
| Targeted mutation analysis/sequencing | DMD c.10141C>T (p.Arg3381X) in exon 70 | CCTGGTTTCAGAGCCCCATT / TGGCAACTGGACATCAGCTT | |
| House-Keeping Gene (qPCR) | GAPDH | TTCACCACCATGGAGAAGGC/ GGCATGGACTGTGGTCATGA | |
| Reprogramming factor (qPCR) | NANOG | CAATGGTGTGACGCAGGGAT/ CCAAGTCACTGGCAGGAGAAT | |
| Reprogramming factor (qPCR) | SOX2 | AACCAGCGCATGGACAGTTA/ GACTTGACCACCGAACCCAT | |
| Reprogramming factor (qPCR) | OCT3/4 | AGGTTTCTCACCTGTGTGGGTT/ CTTTGTGTTCCCAATTCCTTCC | |
3.3. qPCR analysis
RNA was isolated from cell pellets using RNeasy Mini kit (Qiagen; 74,104) according to manufacturer's instructions. A DNase step was included to eliminate genomic contamination. RNA quality and yield was assessed using a Nanodrop. Retro-transcription to cDNA was conducted with the ImProm-II™ Reverse Transcription System kit (Promega; A3800) following manufacturer's instructions. qPCRs were performed with SYBR-Green Real Time Master Mix (Promega; A600A) according to manufacturer instructions using the BioRad CFX96 machine. A house keeping gene (GAPDH) reaction was included on each plate for all samples. The ΔCT method has been used to analyse the experimental CT values. A commercially-available human iPSC line (Gibco; cat. no. A13777) has been included as a positive control and human myoblasts provided a negative control. Primer sequences are listed in Table 3.
3.4. Embryoid body formation assay
iPSCs were dissociated into clumps using gentle cell dissociation reagent and embryoid bodies (EBs) were allowed to form and grow in suspension in TeSR™-E6 medium (Stemcell Technologies) in non-adhesive dishes. After 7 days EBs were transferred to standard 10 cm tissue cultures dishes to allow adhesion in DMEM (Sigma) with 20%(v/v) FBS (Life technologies), 1% l-glutamine (Sigma), 1% PS (Sigma) in 3% O2 and 5% CO2 to induce spontaneous differentiation. Media was changed every other day and plates were fixed in 4% PFA after 14–20 days of differentiation.
3.5. Sequencing, STR profiling and karyotype analysis
Genomic DNA was extracted from each cell line by DNeasy kit (Quiagen). 100 ng/μl of Gotaq® DNA polymerase (Promega) was used for amplification (35 cycles using a BioRad T100™ Thermal cycler). DMD specific primers upstream and downstream the point mutations were designed (Table 3) and purified PCR reactions sequenced via dideoxynucleoside Sanger sequencing by Source Biosciences (Cambridge). iPSCs UCLi011-A and UCLi012-A were authenticated by STR analysis performed by Source Biosciences (Nottingham) using Promega PowerPlex 16 HS assay (available with the authors). For each cell line karyotyped, a T25 flask of 80% confluent cells was sent to The Doctors Laboratory (TDL, London, UK) were G-band analysis was performed at a resolution of 6–10 MB (UCLi011-A: passage 8, 10 metaphase spreads analysed; UCLi012-A: passage 6, 20 metaphase spreads analysed).
Acknowledgements
The authors thank the MRC Centre for Neuromuscular Diseases Biobank at UCL for providing samples and Silvia Torelli for helpful discussions. This work was funded by Muscular Dystrophy UK (RA4/3023/1 with Duchenne Children's Trust / Duchenne UK and Duchenne Research Fund; 17GRO-PS48- 0093–1), the European Research Council (7591108 – HISTOID), Takeda New Frontiers Science, the EU 7th Framework Program project no. 602423 (PluriMes), the MRC, BBSRC, Duchenne Parent Project, AFM-Telethon and the National Institute for Health Research (NIHR); the views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
References
- Maffioletti S.M., Sarcar S., Henderson A.B.H., Mannhardt I., Pinton L., Moyle L.A., Steele-Stallard H., Cappellari O., Wells K.E., Ferrari G., Mitchell J.S., Tyzack G.E., Kotiadis V.N., Khedr M., Ragazzi M., Wang W., Duchen M.R., Patani R., Zammit P.S., Wells D.J., Eschenhagen T., Tedesco F.S. Three-Dimensional human iPSC-Derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep. 2018 Apr 17;23(3):899–908. doi: 10.1016/j.celrep.2018.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri E., Muntoni F. Muscular dystrophies. The Lancet. 2013;381(9869):845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- Muntoni F., Torelli S., Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. The Lancet Neurology. 2003;2(12):731–740. doi: 10.1016/s1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- Scoto M., Finkel R., Mercuri E., Muntoni F. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018 Aug;2(8):600–609. doi: 10.1016/S2352-4642(18)30140-8. [DOI] [PubMed] [Google Scholar]
- Warren L. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]