Abstract
Background
Heavy menstrual bleeding (HMB) is a significant health problem in premenopausal women; it can reduce their quality of life and can cause social disruption and physical problems such as iron deficiency anaemia. First‐line treatment has traditionally consisted of medical therapy (hormonal and non‐hormonal), but this is not always successful in reducing menstrual bleeding to acceptable levels. Hysterectomy is a definitive treatment, but it is more costly and carries some risk. Endometrial ablation may be an alternative to hysterectomy that preserves the uterus. Many techniques have been developed to 'ablate' (remove) the lining of the endometrium. First‐generation techniques require visualisation of the uterus with a hysteroscope during the procedure; although it is safe, this procedure requires specific technical skills. Newer techniques for endometrial ablation (second‐ and third‐generation techniques) have been developed that are quicker than previous approaches because they do not require hysteroscopic visualisation during the procedure.
Objectives
To compare the efficacy, safety, and acceptability of endometrial destruction techniques to reduce heavy menstrual bleeding (HMB) in premenopausal women.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE, Embase, CINAHL, and PsycInfo (from inception to May 2018). We also searched trials registers, other sources of unpublished or grey literature, and reference lists of retrieved studies, and we made contact with experts in the field and with pharmaceutical companies that manufacture ablation devices.
Selection criteria
Randomised controlled trials (RCTs) comparing different endometrial ablation or resection techniques for women reporting HMB without known uterine pathology, other than fibroids outside the uterine cavity and smaller than 3 centimetres, were eligible. Outcomes included improvement in HMB and in quality of life, patient satisfaction, operative outcomes, complications, and the need for further surgery, including hysterectomy.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trials for risk of bias, and extracted data. We contacted study authors for clarification of methods or for additional data. We assessed adverse events only if they were separately measured in the included trials. We undertook comparisons with individual techniques as well as an overall comparison of first‐ and second‐generation ablation methods.
Main results
We included in this update 28 studies (4287 women) with sample sizes ranging from 20 to 372. Most studies had low risk of bias for randomisation, attrition, and selective reporting. Less than half of these studies had adequate allocation concealment, and most were unblinded. Using GRADE, we determined that the quality of evidence ranged from moderate to very low. We downgraded evidence for risk of bias, imprecision, and inconsistency.
Overall comparison of second‐generation versus first‐generation (i.e. gold standard hysteroscopic ablative) techniques revealed no evidence of differences in amenorrhoea at 1 year and 2 to 5 years' follow‐up (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.78 to 1.27; 12 studies; 2145 women; I² = 77%; and RR 1.16, 95% CI 0.78 to 1.72; 672 women; 4 studies; I² = 80%; very low‐quality evidence) and showed subjective improvement at 1 year follow‐up based on a Pictorial Blood Assessment Chart (PBAC) (< 75 or acceptable improvement) (RR 1.03, 95% CI 0.98 to 1.09; 5 studies; 1282 women; I² = 0%; and RR 1.12, 95% CI 0.97 to 1.28; 236 women; 1 study; low‐quality evidence). Study results showed no difference in patient satisfaction between second‐ and first‐generation techniques at 1 year follow‐up (RR 1.01, 95% CI 0.98 to 1.04; 11 studies; 1750 women; I² = 36%; low‐quality evidence) nor at 2 to 5 years' follow‐up (RR 1.02, 95% CI 0.93 to 1.13; 672 women; 4 studies; I² = 81%).
Compared with first‐generation techniques, second‐generation endometrial ablation techniques were associated with shorter operating times (mean difference (MD) ‐13.52 minutes, 95% CI ‐16.90 to ‐10.13; 9 studies; 1822 women; low‐quality evidence) and more often were performed under local rather than general anaesthesia (RR 2.8, 95% CI 1.8 to 4.4; 6 studies; 1434 women; low‐quality evidence).
We are uncertain whether perforation rates differed between second‐ and first‐generation techniques (RR 0.32, 95% CI 0.10 to 1.01; 1885 women; 8 studies; I² = 0%).
Trials reported little or no difference between second‐ and first‐generation techniques in requirement for additional surgery (ablation or hysterectomy) at 1 year follow‐up (RR 0.72, 95% CI 0.41 to 1.26; 6 studies: 935 women; low‐quality evidence). At 5 years, results showed probably little or no difference between groups in the requirement for hysterectomy (RR 0.85, 95% CI 0.59 to 1.22; 4 studies; 758 women; moderate‐quality evidence).
Authors' conclusions
Approaches to endometrial ablation have evolved from first‐generation techniques to newer second‐ and third‐generation approaches. Current evidence suggests that compared to first‐generation techniques (endometrial laser ablation, transcervical resection of the endometrium, rollerball endometrial ablation), second‐generation approaches (thermal balloon endometrial ablation, microwave endometrial ablation, hydrothermal ablation, bipolar radiofrequency endometrial ablation, endometrial cryotherapy) are of equivalent efficacy for heavy menstrual bleeding, with comparable rates of amenorrhoea and improvement on the PBAC. Second‐generation techniques are associated with shorter operating times and are performed more often under local rather than general anaesthesia. It is uncertain whether perforation rates differed between second‐ and first‐generation techniques. Evidence was insufficient to show which second‐generation approaches were superior to others and to reveal the efficacy and safety of third‐generation approaches versus first‐ and second‐generation techniques.
Plain language summary
Are newer methods for destroying the lining of the uterus (endometrial ablation) more effective and safer compared to established methods?
Review question
This review compared the effectiveness, safety, acceptability, and complication rates of first‐, second‐ and third‐generation methods available to destroy the endometrium (lining of the uterus) for treatment of heavy menstrual bleeding (heavy periods) in premenopausal women.
Background
Medication and hysterectomy (surgery to remove the womb) used to be the main treatment options for heavy menstrual bleeding. Both are still effective and safe options, but available new treatments focus on removing the lining of the womb (endometrium) from which the bleeding comes. These procedures involve either removing the endometrium (resection) or destroying it with thermal (heat) energy from a laser, electrical instruments, or other devices (ablation). These treatments can stop or reduce menstrual bleeding.
Study characteristics
This review identified 28 randomised controlled trials undertaken in 4287 women. Most of the women knew which treatment they were receiving, which may have influenced their judgements about menstrual blood loss and satisfaction. Other aspects of study quality varied among trials. Evidence is current to May 2018. Nineteen of the 28 trials acknowledged that they received funding, supplies of equipment, or technical assistance from the pharmaceutical industry and from equipment manufacturers.
Key results
Moderate‐ to very low‐quality evidence suggests that first‐ and second‐generation approaches were equally effective in the treatment of HMB. Newer (second‐generation) treatment approaches were safer in terms of rate of fluid overload, cervical lacerations, and haematometra, with similar rates of uterine perforation. The newer approaches (second‐generation ablation) were quicker and were more likely to be done under local (rather than general) anaesthesia compared with first‐generation approaches. Most women in both groups were satisfied with results of the procedure. Not enough evidence is available to show which second‐generation approaches are superior to others, and information about third‐generation approaches is not available for comparison.
Quality of the evidence
Evidence ranged from moderate to very low quality. Few studies were blinded, data were limited, and heterogeneity was substantial for some outcomes, leading to downgrading of the quality of evidence.
Summary of findings
Summary of findings for the main comparison. Overall analyses: second‐generation endometrial ablation compared to first‐generation endometrial ablation for heavy menstrual bleeding.
| Overall analyses: second‐generation endometrial ablation compared to first‐generation endometrial ablation for heavy menstrual bleeding | |||||||
| Patient or population: heavy menstrual bleeding Setting: clinic Intervention: overall analyses: second‐generation endometrial ablation Comparison: first‐generation endometrial ablation | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with first‐generation endometrial ablation | Risk with overall analyses: second‐generation endometrial ablation | ||||||
| Bleeding | Amenorrhoea at 1 year follow‐up | 394 per 1000 | 390 per 1000 (307 to 501) | RR 0.99 (0.78 to 1.27) | 2145 (12 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | |
| PBAC < 75 or acceptable improvement at 12 months' follow‐up | 809 per 1000 | 833 per 1000 (793 to 882) | RR 1.03 (0.98 to 1.09) | 1282 (5 RCTs) | ⊕⊕⊝⊝ LOWd,e | ||
| Amenorrhoea at 2 to 5 years' follow‐up | 484 per 1000 | 561 per 1000 (377 to 832) | RR 1.16 (0.78 to 1.72) | 672 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWb,f | ||
| PBAC < 75 or acceptable improvement at 5 years' follow‐up | 537 per 1000 | 580 per 1000 (467 to 720) | RR 1.08 (0.87 to 1.34) | 263 (1 RCT) | ⊕⊕⊝⊝ LOWe,g | ||
| Satisfaction rate | At 1 year follow‐up | 898 per 1000 | 907 per 1000 (880 to 933) | RR 1.01 (0.98 to 1.04) | 1750 (11 RCTs) | ⊕⊕⊝⊝ LOWf,h | |
| At 2 to 5 years' follow‐up | 868 per 1000 | 886 per 1000 (808 to 981) | RR 1.02 (0.93 to 1.13) | 672 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWb,e,i | ||
| Duration of operation (minutes) | Mean duration of operation (minutes) was 27 | MD 13.52 lower (16.9 lower to 10.13 lower) | ‐ | 1822 (9 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d,e | ||
| Proportion given local anaesthesia (%) | 208 per 1000 | 578 per 1000 (366 to 915) | RR 2.78 (1.76 to 4.40) | 1434 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d,j | ||
| Complication rate ‐ perforation | 13 per 1000 | 4 per 1000 (1 to 13) | RR 0.32 (0.10 to 1.01) | 1885 (8 RCTs) | ⊕⊕⊝⊝ LOWe,k | ||
| Requirement for additional surgery | At 1 year follow‐up (ablation or hysterectomy) | 66 per 1000 | 47 per 1000 (27 to 83) | RR 0.72 (0.41 to 1.26) | 935 (6 RCTs) | ⊕⊕⊝⊝ LOWf,l | |
| At 2 to 5 years' follow‐up (hysterectomy) | 191 per 1000 | 162 per 1000 (113 to 233) | RR 0.85 (0.59 to 1.22) | 758 (4 RCTs) | ⊕⊕⊕⊝ MODERATEe | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PBAC: Pictorial Blood Assessment Chart; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aEight studies provided insufficient details for a judgement about allocation concealment; downgraded one level.
bHeterogeneity was high at I² > 75%; downgraded two levels.
cThe funnel plot suggested asymmetry; downgraded one level.
dOnly two studies provided sufficient details for a judgement about allocation concealment; no blinding of participants/researchers or outcome assessors; downgraded one level.
eNo blinding of participants/researchers or outcome assessors; downgraded one level.
fThree studies provided insufficient details for a judgement about allocation concealment; only one study provided adequate data on blinding of participants/researchers and outcome assessors; downgraded two levels.
gEvidence of imprecision based on one study with n < 300; downgraded one level.
hOnly one study provided adequate data on blinding of participants/researchers and outcome assessors; downgraded one level.
iOnly one study provided sufficient details for a judgement about allocation concealment; downgraded one level.
jThe confidence interval has a very wide range (1.76 to 4.40); downgraded one level.
kThe number of events is very low and the confidence interval is wide; downgraded one level.
lThe number of events is very low; downgraded one level.
Background
Description of the condition
Heavy menstrual bleeding (HMB), or menorrhagia, is a significant cause of ill health among women of reproductive age and can substantially impair their quality of life (NICE 2018).
Heavy menstrual bleeding has been classically defined as blood loss greater than or equal to 80 mL per menstrual cycle (Hallberg 1966). However, it is the woman's perception of her own menstrual loss that is the key determinant in her referral and subsequent treatment. According to the International Federation of Gynaecology and Obstetrics (FIGO), HMB is "an excessive menstrual blood loss that interferes with the woman's physical, emotional, social, and material quality of life, and can occur alone or in combination with other symptoms such as headache, fatigue, or dysmenorrhea" (Munro 2012). One in 20 women in the UK between 30 and 49 years of age consult their general practitioner (GP) each year with HMB (Grant 2000). According to a recent European survey, 27% of women over 18 years of age reported HMB in the previous 12‐month period (Fraser 2015). In New Zealand, for example, it is estimated that 1 in 50 GP consultations for women younger than 50 years are the result of HMB (NZ HMB Guideline 1998). In most cases, no pathology (abnormality) is found to explain the HMB (NICE 2018). Causes of HMB usually remain unknown, which limits the development of new non‐surgical therapies.
Surgical treatment for HMB often follows failed or ineffective medical therapy, although it is also used as first‐line therapy. Hysterectomy has traditionally been regarded as the definitive surgical treatment for HMB, but in spite of a 100% success rate (complete cessation of menstruation) and high levels of satisfaction (Middleton 2010), hysterectomy is a major surgical procedure with significant physical complications and social and economic costs. Almost half of the hysterectomies performed worldwide were carried out to treat women with HMB (Maresh 2002). However, many women prefer less invasive surgical treatment, even when they are made aware that the success of that treatment cannot be assured (Nagele 1998). A US review including 1169 women reported that 13.4% of those undergoing an endometrial ablation had a subsequent hysterectomy (mean follow‐up 39 months; standard deviation (SD) 19 months). The same study reported that the rate of hysterectomy was correlated with the age at which ablation was performed; in women younger than 36 at the time of ablation, the rate of hysterectomy was 21%, and among those 46 years of age or older at the time of ablation, the rate was 11% (Shavell 2012). A Scottish review of 14,078 women with endometrial ablation reported that 20% had a subsequent hysterectomy, and most of these procedures were performed within the first two years after ablation (Cooper 2011).
Description of the intervention
Endometrial destruction techniques, which aim to destroy or remove endometrial tissue, have become increasingly popular as less invasive alternatives over the past two decades; as a result, the number of hysterectomies in the UK declined by 64% between 1995 and 2002 (Reid 2005). The first effective ablation of the endometrium under hysteroscopic vision for treatment of HMB was performed via laser photo‐vaporisation (Goldrath 1981). Rollerball ablation with simple and cheap electrosurgical equipment rather than expensive lasers was performed a few years later (Lin 1988; Vaincaillie 1989). A method to excise rather than ablate the endometrium with an unmodified resectoscope (an instrument used for resection (excision)) was also developed and yielded good results (DeCherney 1983; DeCherney 1987). Transcervical resection of the endometrium (TCRE) is a technique that is often used in conjunction with rollerball ablation. These methods of ablation, also termed 'first‐generation methods', were the most commonly used and were widely regarded as the gold standard for endometrial ablation (Cooper 2000). All require direct visualisation by a hysteroscope (an instrument used to examine the uterine cavity), which may confer the additional advantage of diagnosis of polyps. Endometrial destruction techniques in use in the UK by 1995 included electrocautery ‐ either loop or rollerball (80%) ‐ laser (18%), and radiofrequency ‐ a procedure for which electromagnetic energy (2%) is used (RCOG 1995).
The expectation was that these first‐generation ablation methods would become an alternative to hysterectomy, but at least initially, the total number of operations for HMB increased (Bridgman 2000). More recent figures in the UK suggest that the rate of surgery for menorrhagia (based on data from 2004 to 2006) is 143 procedures per 100,000 premenopausal women (Cromwell 2009), of which approximately 60% are endometrial ablations. In a long‐term follow‐up (up to 25 years) study in the UK, only 25% of women with endometrial resection or ablation underwent a subsequent hysterectomy, and 75% of these surgeries were performed during the first 5 years of follow‐up (Kalampokas 2017), suggesting that endometrial ablation may have a role in limiting the number of hysterectomies. However, this may also reflect progression through menopause for many of these women.
Drawbacks of these first‐generation ablation techniques include the expertise needed and patient morbidity. A prospective national audit of hysteroscopic endometrial ablation and resection (10,686 cases) in England and Wales between 1993 and 1994 assessed the incidence of complications and reported a total complication rate of 4.4% (Overton 1997). Complications are thought to be avoidable with good surgical technique and adequate training. However, hysteroscopic endometrial ablation requires an operating room environment, a surgeon with specific technical skills, and use of general or regional anaesthesia.
Subsequently, second‐ and third‐generation non‐hysteroscopic techniques were developed; these are considered easier to perform and equally effective and safe (Madhu 2009), with lower complication rates,of around 1% for one second generation technique (bipolar) (Athanatos 2015; Laberge 2016). First‐generation ‐ commonly referred to as hysteroscopic ‐ techniques require hysteroscopic visualisation of the uterine cavity during the procedure. Examples in this group include endometrial laser ablation (ELA), transcervical resection of the endometrium (TCRE), and rollerball endometrial ablation. Second‐ and third‐generation approaches ‐ frequently referred to as non‐hysteroscopic techniques ‐ do not require direct visualisation of the uterine cavity during the procedure. Examples of second‐generation techniques include thermal balloon endometrial ablation (Cavaterm®, Thermachoice®), microwave endometrial ablation (MEA®, Microsulis®), hydrothermal ablation (Hydro ThermAblator®), bipolar radiofrequency endometrial ablation (Novasure®, Minerva®), and endometrial cryotherapy (Cerene®, Her Option®). An example of a third‐generation technique is Thermachoice III®. All of these second‐ and third‐generation techniques, with the exception of hydrothermal ablation and endometrial laser intrauterine thermal therapy, involve performing surgery without direct visualisation through a hysteroscope. They can be performed in outpatient settings and include cryoablation (Pitroff 1993), hot saline solution irrigation (Baggish 1995), diode laser hyperthermy (heating) (Donnez 1996), microwave ablation (Sharp 1995), a heated balloon system (Singer 1994), and photodynamic therapy (intrauterine light delivery) (Fehr 1995). Economic modelling suggests that second‐generation techniques may be more cost‐effective than first‐generation methods (Garside 2004). Third‐generation approaches have replaced the latex for silicone on the balloon and involve active fluid circulation, which enables the total endometrial surface to receive equal heat distribution (Cash 2012).
How the intervention might work
Endometrial destruction involves the removal of endometrial tissue. The endometrium can regenerate, and clinical improvement is predicated on removing the basal layer of the endometrium to prevent endometrial regrowth. The basal glands are believed to be the primary foci for endometrial regrowth. The endometrium can be removed under direct hysteroscopic view either by excision with an electrosurgical loop (one possible advantage of resection is that it yields a biopsy sample) or by ablation in which thermal energy of sufficient power is applied to its surface to produce necrosis (cell death) of the full thickness of the endometrium.
Why it is important to do this review
A wide range of techniques are available for ablating and destroying the endometrium to reduce HMB, and it is not clear which approaches offer the best options in terms of effectiveness and safety. The aim of this review is to assess the efficacy, safety, and acceptability of all methods, both by comparing individual techniques pairwise and by making overall comparisons between first‐ and second‐generation techniques. Other Cochrane reviews have compared endometrial ablation versus hysterectomy, and endometrial ablation versus medical therapies, for HMB (Lethaby 2009; Marjoribanks 2010).
Objectives
To compare the effectiveness, safety, and acceptability of endometrial destruction techniques to reduce heavy menstrual bleeding (HMB) in premenopausal women.
Methods
Criteria for considering studies for this review
Types of studies
We sought to include all randomised controlled trials (RCTs) comparing techniques for ablation or resection of the endometrium for treatment of HMB.
Types of participants
Source of recruitment
Primary care, family planning, or specialist clinics
Inclusion criteria
Women of reproductive years with regular heavy periods measured objectively or subjectively
Exclusion criteria
Postmenopausal bleeding (longer than 1 year from the last period)
Irregular menstruation and intermenstrual bleeding
Pathological causes of HMB (e.g. uterine cancer)
Iatrogenic causes of HMB (e.g. intrauterine coil devices)
Types of interventions
We included studies that compared endometrial resection and ablation techniques (TCRE, laser ablation, rollerball ablation, saline irrigation, microwave ablation, radiofrequency ablation, heated balloon, photodynamic therapy, cryoablation, and any other endometrial destruction techniques) against each other or grouped in the broad categories of first‐ or second‐generation techniques performed to reduce HMB.
Types of outcome measures
Assessment of most of the following outcomes was related to duration of follow‐up after the initial surgical procedure. Given that the aim of endometrial resection and ablation therapies is to induce permanent resolution of heavy menstrual bleeding, long‐term follow up of these treatments is needed to enable informed decision‐making between surgical options. Thus, for the following outcomes, evaluation at different time points is considered important for assessing effects over time: 6 months, 12 months, 2 years, 2 to 5 years, and longer than 5 years. When trials measured outcomes at two different follow‐up times within categories (e.g. at 3 years and at 5 years), they recorded longer follow‐up time only within the category of 2 to 5 years.
Primary outcomes
Menstrual bleeding
An objective measurement of menstrual blood loss (measured by the modified alkaline haematin method ‐ modified by Newton 1977 from the original technique of Hallberg 1964)
A semi‐objective or subjective assessment of improvement in menstrual blood loss (measured by the Pictorial Blood Assessment Chart (PBAC) as in Higham 1990, or by women's perception of improvement)
Rate of satisfaction
Assessment of satisfaction in terms of the outcome of the procedure (this outcome was moved from a secondary outcome to a primary outcome in the 2009 update)
Secondary outcomes
Operative outcomes
Duration of surgery (in minutes)
Operative difficulties (such as difficulty of surgery, technical complications, abandoning the procedure)
Proportion given local rather than general anaesthesia
Recovery
Length of hospital stay
Time or ability to return to normal activities or work
Quality of life
Women's perceived change in quality of life, when recorded in a reproducible and validated format
Improvement in menstrual symptoms such as premenstrual syndrome (PMS) and dysmenorrhoea
Adverse effects
-
Complication rate, frequency of specific adverse events both before and after discharge from hospital, divided into minor and major complications
-
Major complications
Perforation
Endometritis
Myometritis
Cervical laceration/tear or stenosis
Pelvic sepsis
Pelvic abscess
Pelvic inflammatory disease
Haematometra
Uterine tamponade
Blood transfusion
Glycine toxicity
Fluid overload
Fluid deficit
Bowel obstruction
Urinary incontinence
-
Minor complications
Skin rash and burning sensation
Headache
Nausea, vomiting, or severe pelvic pain
Weakness or fatigue during the first 24 hours
Backache during the first 24 hours
Bradycardia
Fever
Chills
Bloating
Abdominal tenderness
Dysuria
Urinary tract infection (UTI)
Hydrosalpinx
Spotting during the first 24 hours
Vaginal bleeding
Abdominal cramping
Infection (leucorrhoea)
First‐degree burn
-
Requirement for further surgery for menstrual symptoms (by duration of follow‐up)
Mortality as a direct result of surgery
Search methods for identification of studies
Electronic searches
The information specialist from the Cochrane Gynaecology and Fertility Group, Marian Showell, searched the following databases for the 2018 update.
Cochrane Gynaecology and Fertility (CGFG) Specialised Register (PROCITE platform); searched 22 May 2018 (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Register of Studies Online (CRSO) (Web platform); searched 22 May 2018 (Appendix 2).
MEDLINE (OVID platform); searched from 1946 to 22 May 2018 (Appendix 3).
Embase (OVID platform); searched from 1980 to 22 May 2018 (Appendix 4).
PsycINFO (OVID platform); searched from 1806 to 22 May 2018 (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO platform); searched from 1961 to 22 May 2018 (Appendix 6).
For the 2018 update, MB searched other electronic sources up to May 2018.
Trial registries for ongoing and registered trials: ClinicalTrials.gov, a service of the US National Institutes of Health (http://www.clinicaltrials.gov), and the World Health Organization International Trials Registry Platform search portal (http://www.who.int/trialsearch/Default.aspx).
The Cochrane Library (http://www.cochrane.org/index.htm) for Database of Abstracts of Reviews of Effects (DARE; reference lists from non‐Cochrane reviews on similar topics).
OpenGrey for unpublished literature from Europe (http://www.opengrey.eu/).
Latin American Caribbean Health Sciences Literature (LILACS) database: a source of trials from the Portuguese and Spanish‐speaking world (http://bvsalud.org/portal/?lang=en ‐ choose ‘LILACS’ in the ‘all sources’ dropdown box).
PubMed and Google for recent trials that have not yet been indexed in the major databases.
Searching other resources
We handsearched the reference lists of articles retrieved by the search.
Some of the newer second‐generation techniques are undergoing development and rigorous testing. For previous updates, we contacted expert researchers in the field and companies that manufacture the newer devices to try to locate ongoing trials and unpublished data. We contacted two experts in the field to ask about ongoing research on endometrial ablation techniques: Dr. David Parkin (Aberdeen Royal Infirmary, UK) and Dr. Jed Hawe (South Cleveland Hospital, UK). We identified descriptions of several ongoing trials but we found insufficient details for review authors to initiate contact with study authors.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
For the 2018 update, four review authors screened available abstracts (AL, MG, JB, MB). When the screened abstract presented a potentially eligible RCT, we obtained and inspected the full article to assess its relevance to this review based on the criteria for inclusion. We clarified uncertainty over eligibility through discussion between AL, MG, and JB or MB. We resolved disagreements as to study eligibility by consensus and found it was not necessary to involve another review author to arbitrate over selection.
Data extraction and management
Data extraction
Two of three review authors (MB, JB or MG) independently extracted study data using forms designed according to Cochrane guidelines. We collected the following details.
Trial characteristics
Method of randomisation
Presence or absence of blinding to treatment allocation
Quality of allocation concealment
Numbers of women randomised, excluded, and lost to follow‐up
Whether an intention‐to‐treat analysis was done
Whether a power calculation was done
Duration, timing, and location of the study
Source of funding
Characteristics of study participants
Age and any other recorded characteristics of women in the study
Other inclusion criteria
Exclusion criteria
Interventions used
Type of endometrial destruction technique performed
Outcomes
Methods used to measure menstrual blood loss
Methods used to evaluate participant satisfaction, change in quality of life, and menstrual symptoms
Assessment of risk of bias in included studies
For the 2018 update. three independent review authors (MG, MB, and JB) assessed the risk of bias of each study using the 'Risk of bias' tool developed by Cochrane (Higgins 2011).
We assessed the following domains.
Sequence generation (whether the allocation sequence was adequately generated, e.g. random numbers table, computer random numbers generator, coin tossing, throwing of dice).
Allocation concealment (whether the allocation was adequately concealed, e.g. sequentially numbered containers of identical appearance, central allocation, sequentially numbered opaque and sealed envelopes).
Blinding of participants, personnel, and outcome assessors (whether knowledge of the allocated intervention was adequately prevented during the study, e.g. by ensuring blinding of participants and key personnel; when there was no knowledge of blinding to the intervention, it was not likely to influence outcomes).
Incomplete outcome data (whether incomplete outcome data were adequately addressed, e.g. missing data balanced in numbers across intervention groups, proportion of missing outcomes insufficient to affect estimates, reasons for missing data unlikely to be related to outcomes).
Selective outcome reporting (whether reports of the study were free of suggestion of selective outcome reporting, e.g. previous publication of a study protocol, other evidence that the study contains all prespecified outcomes).
Other sources of bias (whether the study was apparently free of other problems that could put it at high risk of bias, e.g. baseline imbalance, bias related to study design, early termination of study).
We scored these domains as:
criterion met (i.e. low risk of bias);
unclear whether criterion met (i.e. uncertain risk of bias); or
criterion not met (i.e. high risk of bias).
Measures of treatment effect
Two review authors (MB, JB or MG) extracted data to enable calculation of risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data, together with 95% confidence intervals (CIs). Some outcomes such as satisfaction with treatment were measured by ordinal data. We dichotomised these data to represent satisfaction with surgery (highly satisfied and satisfied combined) versus no satisfaction (doubtful or dissatisfied) by collapsing categories. We inspected continuous data for evidence of skew, when possible, according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions, by calculating the observed mean minus the lowest (or highest) possible value divided by the standard deviation.
Unit of analysis issues
The unit of analysis and randomisation was women in all studies. Researchers individually randomised participants to groups and collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
We sought additional information on trial methods and trial results from the corresponding authors of some trials that appeared to meet eligibility criteria. We did this when aspects of methods were unclear or when data were provided in a form unsuitable for meta‐analysis. Authors of the following trials provided extra information: Abbott 2003; Athanatos 2015; Laberge 2016. Gynecare (pharmaceutical company) provided funding for Boujida 2002,Meyer 1998,Perino 2004, and van Zon‐Rabelink 2003. One of the study authors provided additional information for Penninx 2010 for a previous update of this review.
Assessment of heterogeneity
We analysed differences between studies in terms of methodological factors and variations between participants, interventions, and outcomes to determine whether it was appropriate to combine the studies in meta‐analysis. If studies were sufficiently homogeneous to consider pooling, we examined statistical heterogeneity between the results of different studies by inspecting scatter in data points on the graphs and the overlap in confidence intervals and, more formally, by checking the results of Chi² tests (with P < 0.1 considered evidence of significant heterogeneity) and the I² statistic. The I² statistic is a measure of consistency between trials in a meta‐analysis (Higgins 2011). As a general rule, I² values up to 25% provide evidence of low heterogeneity, values from 25% to 50% moderate heterogeneity, and 75% or above substantial heterogeneity.
Assessment of reporting biases
We undertook a comprehensive search, along with careful inspection of search results, to identify duplicates to reduce the risk of reporting bias. We also searched trial registers to ensure that all conducted trials were followed to locate publications. If we identified sufficient trials, we planned to investigate publication bias by preparing funnel plots of study results.
Data synthesis
When we found evidence of skewed data in the measurement of outcomes (e.g. summary trial results expressed as median and range), we did not pool the data for these outcomes in the meta‐analysis but included them in table format.
Before synthesis, we examined data for skew using the rough rule suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In addition, we noted whether summary trial results were expressed as medians together with ranges, or if data were analysed via non‐parametric methods, or both, which is also suggestive of skew. When we found no evidence of major skew in the data and no evidence of clinical heterogeneity (from inspection of trial characteristics), we pooled the outcomes statistically in a meta‐analysis using RevMan software. When data could not be pooled because of skew, we included the outcome data in table format.
We combined risk ratios (RRs) and 95% confidence intervals (CIs) for meta‐analysis using the Peto‐modified Mantel‐Haenszel method. For some dichotomous outcomes (e.g. the proportion of participants requiring further surgery), a higher proportion represented a negative consequence of that treatment, and for other outcomes (e.g. the proportion with improvement in menstrual blood loss), we considered a higher proportion as a benefit of treatment. This discrepancy in categorising of outcomes should be noted when summary graphs for the meta‐analysis are viewed for assessment of benefits as opposed to harms of treatment. Thus, for some dichotomous outcomes, treatment benefit is displayed as RRs and CIs to the left of the centre line, and for others, treatment benefit is displayed to the right of the centre line. We have clearly labelled the forest plot for each outcome for clarification.
We combined mean differences (MDs) and 95% CIs for meta‐analysis using the inverse variance method. For all continuous outcomes in this review, a high value represents a negative consequence of treatment, for example, duration of surgery, amount of fluid deficit (difference between input and output fluid during surgery), and PBAC score for menstrual blood loss. Thus, in evaluation of the summary graphs, means and CIs to the left are considered a benefit of the experimental or comparative treatment.
We used a fixed‐effect model to calculate summary effect measures. When we noted substantial statistical heterogeneity, we compared results from the fixed‐effect model against those from the random‐effects model to determine whether results were altered substantially by choice of model. A priori we expected that two of the outcomes ‐ duration of surgery and proportion ‐ would require local instead of general anaesthesia and would yield heterogeneous results regardless of comparison. For these comparisons, we used a random‐effects model. For all overall comparisons of first‐generation versus second‐generation methods, we used a random‐effects model because of expected clinical heterogeneity between trials.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for different times of follow‐up after surgery, in particular, for rates of amenorrhoea, satisfaction, and the requirement for additional surgery. We collected these outcomes at 6 months; at 1, 2, and 2 to 5 years; and longer than 5 years after surgery.
Sensitivity analysis
A priori we intended to perform sensitivity analysis to test the robustness of pooled results in the meta‐analysis based on:
trials with good methods (evidence of adequate allocation concealment and intention‐to‐treat analysis) versus all included trials;
trials with and without power calculations for sample size;
trials with participants who had confirmed objective HMB loss (more than 80 mL per cycle) versus all included trials; and
trials with participants who had initially failed medical treatment for HMB versus all included trials.
For most comparisons, we identified an insufficient number of studies for inclusion to perform any of these sensitivity analyses.
Overall quality of the body of evidence
We generated a 'Summary of findings' table for the overall outcome of first‐generation versus second‐generation ablation techniques using GRADEpro software. We used the outcomes of bleeding and satisfaction up to 5 years' follow‐up, duration of operation, proportion given local anaesthesia, complication rate from perforation, and requirement for additional surgery at 12 months' and up to 5 years' follow‐up. This table evaluates the overall quality of the body of evidence for each of the main review outcomes using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). We have documented judgements about evidence quality (high, moderate, low, or very low) and have incorporated them into reporting of results for each outcome.
Results
Description of studies
Results of the search
2005 update: Review authors excluded one study that compared two types of balloon ablation ‐ Menotreat and Cavaterm. A total of 19 studies, some of which provided several different publications describing longer‐term follow‐up or different outcomes, met the inclusion criteria of the review for this update.
2009 update: Two new trials (21 RCTs overall) were eligible for the 2009 update (Brun 2006; Onoglu 2007). Two studies provided additional follow‐up for previously included trials (Bongers 2004; Boujida 2002).
2013 update: Review authors included in the 2013 update four new trials (25 RCTs overall), one of which provided two publications (Clark 2011; Penninx 2010; Sambrook 2009; Thabet 2010). We have now excluded one study awaiting assessment since the 2009 update because it was not randomised (Feng 2006).
2018 update: Review authors determined that five additional trials were eligible for inclusion in the 2018 update and obtained the full texts of these papers (when available) for closer inspection. Review authors included four new trials in the 2018 update (Athanatos 2015: Ghazizadeh 2014; Laberge 2016: Penninx 2016), and we categorised one study as awaiting classification (Feng 2014). We added new data for previously included studies (Bongers 2004; Penninx 2010). One study that was ongoing in the previous update did not start recruitment (Cooper 2012); that study was stopped because of lack of funding and was moved to the excluded studies. We reviewed one trial that was previously included but excluded it at this update because it did not match our inclusion criteria (Soysal 2001).
Thus, a total of 28 trials (4287 women), with sample sizes ranging from 20 to 372, were eligible for this review. Full details of these studies can be found in the Characteristics of included studies table. We excluded a total of nine studies, and three are currently awaiting classification (see Characteristics of excluded studies). We identified one ongoing study (NCT02642926). We have presented details of the screening and selection process in Figure 1.
1.
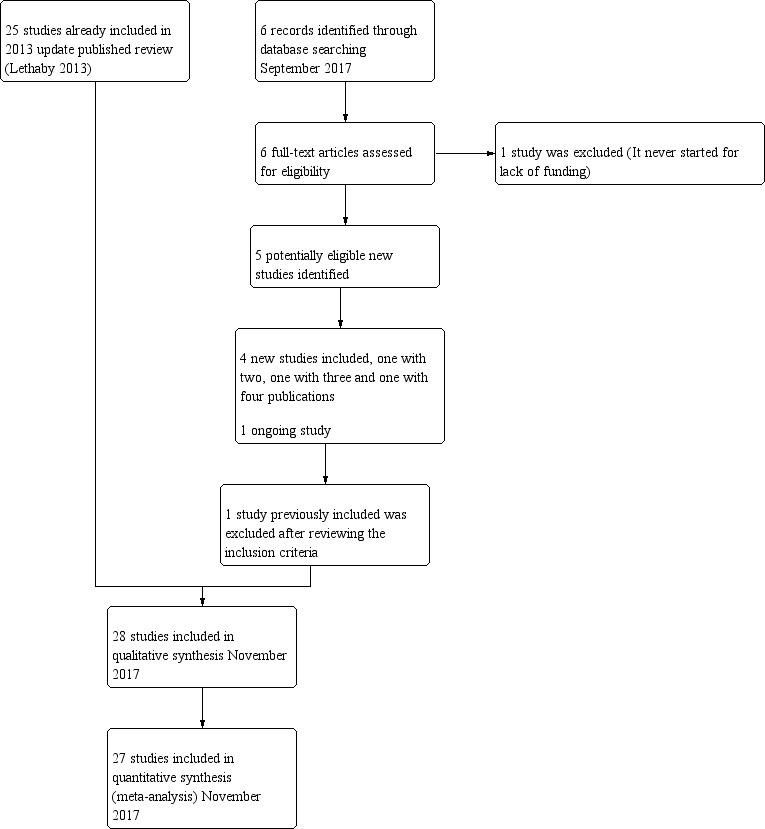
Study flow diagram.
Included studies
Study design and setting
All of the trials followed a parallel‐group design.
Twenty of the trials were single‐centre studies, one each from Germany (Romer 1998), Australia (McClure 1992), Egypt (Thabet 2010), Denmark (Boujida 2002), Greece (Athanatos 2015), Turkey (Onoglu 2007), and Iran (Ghazizadeh 2014); four from the Netherlands (Bongers 2004; Penninx 2010; Penninx 2016; van Zon‐Rabelink 2003); three from Italy (Pellicano 2002; Perino 2004: Vercellini 1999); and six from the UK (Abbott 2003; Bhattacharya 1997; Clark 2011; Cooper 1999; Hawe 2003; Sambrook 2009). We identified eight multi‐centre trials, two based in Canada, USA, and Mexico (Cooper 2002;Laberge 2016); one in USA‐Canada and UK (Cooper 2004); one in USA‐Australia (Corson 2000), one in USA‐Canada (Meyer 1998), and two in the USA (Corson 2001; Duleba 2003), and with three having additional centres in Canada, UK, or Australia; one multi‐centre trial had six centres, all based in France (Brun 2006).
Few of these studies used strict intention‐to‐treat (ITT) analyses or specified methods to deal with missing data. Twelve trials did not report an ITT analysis. Seven claimed that ITT analysis was performed but over time a percentage of participants were lost to follow‐up, so the claim of ITT was misleading. However, ITT analysis was usually performed in these studies when researchers assessed outcomes such as complication rates. Four trials performed true ITT analyses, and one had no reported dropouts. One other trial did not report ITT analysis and replaced dropouts with new cases.
Seventeen trials reported their recruiting time frame. One was recruited between 1989 and 1991 (McClure 1992), 12 between 1995 and 2002 (Abbott 2003; Bongers 2004; Brun 2006; Cooper 1999; Cooper 2004; Corson 2000; Hawe 2003; Meyer 1998; Pellicano 2002; Perino 2004; Thabet 2010; Vercellini 1999), and four between 2004 and 2010 (Athanatos 2015; Clark 2011; Penninx 2010; Sambrook 2009).
Participants
The 28 included studies included 4287 premenopausal participants, most within the age range 30 to 50 years. All of these studies recruited women from secondary or tertiary referral centres or clinics who described HMB.
The presence of fibroids was an exclusion criterion in 15 studies. All trials required that the uterine cavity be normal in size with no uterine pathology, except one (Laberge 2016), which excluded polyps larger than 2 cm. One trial excluded only submucous fibroids (Brun 2006), and another excluded both submucous fibroids and fibroids outside the the uterine cavity and greater than 3 cm (Clark 2011). One trial screened 637 women with self‐assessed HMB, but after applying exclusion criteria, enrolled and randomised less than half (n = 276) (Corson 2000). Almost half of the excluded women had uterine pathology in the form of fibroids or polyps.
Eighteen studies required women to have completed their families (Abbott 2003; Athanatos 2015; Bongers 2004; Boujida 2002; Brun 2006; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Hawe 2003; Laberge 2016; Meyer 1998; Penninx 2010; Penninx 2016; Sambrook 2009; Vercellini 1999), and 14 studies included women who previously had not tolerated or had received ineffective medical therapy for their heavy bleeding (Athanatos 2015; Brun 2006; Clark 2011; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Ghazizadeh 2014; Meyer 1998; Pellicano 2002; Perino 2004; Romer 1998; van Zon‐Rabelink 2003). Fourteen studies objectively confirmed the women's report of excessive bleeding by requiring them to record their blood loss (Abbott 2003; Athanatos 2015; Bongers 2004; Brun 2006; Cooper 2002; Cooper 2004; Corson 2000; Duleba 2003; Hawe 2003; McClure 1992; Meyer 1998; Penninx 2010; Penninx 2016; van Zon‐Rabelink 2003; Vercellini 1999). This occurred before surgery and before trial entry. Nine studies required women to have PBAC measurements of 150 or greater before entry (Abbott 2003; Athanatos 2015; Bongers 2004; Cooper 2002; Corson 2000; Duleba 2003; Meyer 1998; Penninx 2010; Penninx 2016), three required women to have PBAC measurements of 100 or greater before entry (Brun 2006; Hawe 2003; Vercellini 1999), and two required a blood loss score greater than 185 (Cooper 2004; van Zon‐Rabelink 2003). Two studies used the alkaline haematin method (Hallberg 1964): one included women if their blood loss exceeded 70 mL per cycle (McClure 1992), and the other used more than 160 mL per cycle as an inclusion criterion (Laberge 2016). All but one study reported comparable demographic characteristics between comparison groups at baseline (Brun 2006). In Brun 2006, women undergoing balloon ablation had significantly heavier blood loss than those undergoing TCRE at baseline (menstrual blood loss chart 400 vs 266; P = 0.002).
Interventions
Most of the included studies reported some kind of pretreatment before surgery (particularly first‐generation techniques). In 13 trials, participants had been given preoperative gonadotropin‐releasing hormone (GnRH) analogues to prepare and thin the endometrium before surgery (Athanatos 2015; Bhattacharya 1997; Cooper 1999; Cooper 2004; Corson 2001; Duleba 2003; Hawe 2003; Onoglu 2007; Pellicano 2002; Perino 2004; Romer 1998; van Zon‐Rabelink 2003; Vercellini 1999), although one of these studies provided pretreatment only to the TCRE group ‐ not to the balloon group (Pellicano 2002). Studies also provided preoperative treatment with progestogens for 3 months (McClure 1992), and for 2 weeks (Sambrook 2009). One study required 2 weeks of oral contraceptive therapy before surgery to ensure that women were scheduled at a similar time in their cycle (Corson 2000). Another study performed a loop resection of the endometrium before ablation only for the roller ball group ‐ not for the bipolar group (Laberge 2016). Three other trials used non‐steroidal anti‐inflammatory drugs (NSAIDs) to prevent uterine cramping (Clark 2011; Meyer 1998; Penninx 2016). The remaining nine trials provided no preoperative therapy (Abbott 2003; Bongers 2004; Boujida 2002; Brun 2006; Cooper 2002; Ghazizadeh 2014; Meyer 1998; Penninx 2010; Thabet 2010).
Five trials compared first‐generation ablation methods.
Two compared laser ablation versus TCRE (one using an argon laser, the other a neodymium yttrium aluminium garnet (Nd:YAG) laser) (Bhattacharya 1997;McClure 1992).
One compared a vaporising electrode procedure versus TCRE (Vercellini 1999).
Two compared rollerball versus TCRE (Boujida 2002; Onoglu 2007).
All TCRE comparison groups also underwent rollerball ablation to treat the uterine cornua (a horn‐like area within the uterus) and fundus (body of the uterus). It was claimed that the vaporising electrode (unlike rollerball) could be used to treat submucous fibroids.
Fifteen trials compared second‐generation methods versus first‐generation methods.
Three compared balloon ablation (three with Thermachoice, one with Cavaterm) versus rollerball (Meyer 1998; Romer 1998; van Zon‐Rabelink 2003).
One compared the Vesta system versus rollerball (Corson 2000).
Two compared microwave ablation versus TCRE and rollerball (Cooper 1999; Cooper 2004).
One compared heated saline (Hydro ThermAblator) versus rollerball (Corson 2001).
One compared cryoablation versus rollerball (Duleba 2003).
One compared laser versus TCRE (Perino 2004).
Two compared electrode ablation versus TCRE plus rollerball (Corson 2000; Cooper 2002).
One compared balloon (Cavaterm) versus laser (Nd:YAG) (Hawe 2003).
Two compared balloon (Cavaterm) versus TCRE plus rollerball (Brun 2006; Pellicano 2002).
One compared bipolar (Minerva) versus rollerball (Laberge 2016).
Seven trials compared second‐generation techniques.
Four compared bipolar electrode ablation (Novasure) versus balloon (Abbott 2003;Bongers 2004;Clark 2011;Penninx 2016).
One compared bipolar radiofrequency versus hydrothermal ablation (Penninx 2010).
One compared bipolar electrode ablation (Novasure) versus microwave (Athanatos 2015).
One compared microwave versus balloon ablation (Sambrook 2009).
All first‐generation techniques (laser, rollerball, vaporising electrode, and transcervical resection), which use the hysteroscope, were then combined and compared with all second‐generation techniques (balloon, microwave, Vesta system, cryoablation, thermal laser, bipolar electrode ablation, and hydrothermal ablation), which are blind techniques. An additional trial compared overcurettage versus ablative curettage (Thabet 2010).
Outcomes
Bleeding
Researchers measured bleeding as an outcome in 25 of the 28 trials (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Bongers 2004; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Ghazizadeh 2014; Hawe 2003; Laberge 2016; McClure 1992; Meyer 1998; Penninx 2010; Penninx 2016; Perino 2004; Romer 1998; Sambrook 2009; Thabet 2010; van Zon‐Rabelink 2003; Vercellini 1999). The most common way to describe bleeding was to report amenorrhoea. Twenty‐two trials reported amenorrhoea (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Bongers 2004; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Duleba 2003; Hawe 2003; Laberge 2016; McClure 1992; Meyer 1998; Penninx 2010; Penninx 2016; Perino 2004; Romer 1998; Sambrook 2009; Thabet 2010; Vercellini 1999). One reported PBAC < 100 (Corson 2001), and five reported PBAC < 75 (Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001).
Rate of satisfaction
Investigators in 19 of the 28 trials reported the rate of satisfaction with the procedure (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Bongers 2004; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Duleba 2003; Hawe 2003; Meyer 1998; Laberge 2016; Pellicano 2002; Penninx 2010; Penninx 2016; Perino 2004; Romer 1998; Sambrook 2009).
Operative outcomes
A total of 19 trials compared the duration of surgery (in minutes) (Abbott 2003; Bhattacharya 1997; Bongers 2004; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Corson 2000; Laberge 2016; McClure 1992; Meyer 1998; Onoglu 2007; Pellicano 2002; Penninx 2010; Penninx 2016; Perino 2004; Sambrook 2009; van Zon‐Rabelink 2003; Vercellini 1999). Twelve trials reported operative difficulties such as difficulty of surgery, technical complications, and abandoning the procedure (Abbott 2003; Bhattacharya 1997; Boujida 2002; Brun 2006; Cooper 1999; Corson 2000; Hawe 2003; Pellicano 2002; Perino 2004; Sambrook 2009; van Zon‐Rabelink 2003; Vercellini 1999). Only six trials compared the proportion given local rather than general anaesthesia (Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Sambrook 2009). Six trials reported length of hospital stay and time or ability to return to normal activities or work (Brun 2006; Clark 2011; Cooper 1999; Pellicano 2002; Sambrook 2009; Thabet 2010).
Quality of life
Six trials recorded women's perceived change in quality of life in a reproducible and validated format (Abbott 2003; Bongers 2004; Clark 2011; Cooper 1999; Hawe 2003; Sambrook 2009).
Improvement in other menstrual symptoms
Five trials reported on improvement in premenstrual syndrome (PMS) (Abbott 2003; Cooper 1999; Hawe 2003; Laberge 2016; Meyer 1998), and nine reported on improvement in dysmenorrhoea (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Cooper 1999; Cooper 2004; Hawe 2003; Laberge 2016; Meyer 1998; Penninx 2010).
Complication rate
Fourteen trials reported the frequency of specific adverse events both before and after discharge from the hospital (Athanatos 2015; Bhattacharya 1997; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Laberge 2016; Meyer 1998; Pellicano 2002; Penninx 2010; Thabet 2010; van Zon‐Rabelink 2003).
We have divided complications into major and minor complications.
Major complications
Perforation
Endometritis
Myometritis
Cervical laceration/tear or stenosis
Pelvic sepsis
Pelvic abscess
Pelvic inflammatory disease
Haematometra
Uterine tamponade
Blood transfusion
Glycine toxicity
Fluid overload
Fluid deficit
Bowel obstruction
Urinary incontinence
Minor complications
Skin rash and burning sensation
Headache
Nausea, vomiting or severe pelvic pain
Weakness or fatigue during the first 24 hours
Backache during the first 24 hours
Bradycardia
Fever
Chills
Bloating
Abdominal tenderness
Dysuria
Urinary tract infection (UTI)
Hydrosalpinx
Spotting during the first 24 hours
Vaginal bleeding
Abdominal cramping
Infection (leucorrhoea)
First‐degree burn
Requirement for further surgery
A total of 23 trials reported on the requirement for further surgery (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Bongers 2004; Boujida 2002; Brun 2006; Clark 2011; Cooper 1999; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Hawe 2003; Laberge 2016; McClure 1992; Meyer 1998; Pellicano 2002; Penninx 2010; Penninx 2016; Perino 2004; Sambrook 2009; Thabet 2010; van Zon‐Rabelink 2003).
Sixteen trials reported on the requirement for further endometrial ablation or hysterectomy (Abbott 2003; Bhattacharya 1997; Boujida 2002; Brun 2006; Clark 2011; Cooper 1999; Cooper 2004; Corson 2001; Duleba 2003; Hawe 2003; McClure 1992; Meyer 1998; Pellicano 2002; Penninx 2010; Penninx 2016; van Zon‐Rabelink 2003).
Nineteen trials reported on the requirement for further hysterectomy (Athanatos 2015; Bongers 2004; Boujida 2002; Brun 2006; Clark 2011; Cooper 1999; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Laberge 2016; Meyer 1998; Pellicano 2002; Penninx 2010; Penninx 2016; Perino 2004; Sambrook 2009; Thabet 2010; van Zon‐Rabelink 2003).
Mortality as a direct result of surgery
No trials reported mortality as a result of surgery.
Follow‐up
Eight trials followed up on women at 12 months (Cooper 2004; Corson 2001; Duleba 2003; Laberge 2016; Meyer 1998; Penninx 2016; Perino 2004; Vercellini 1999). Seven trials followed up on women at 3 and/or 6 months and at 12 months (Abbott 2003; Athanatos 2015; Brun 2006; Clark 2011; Cooper 2002; Corson 2000; Hawe 2003). One trial provided 6 months' follow‐up (McClure 1992), and another provided 9 and 15 months' follow‐up (Romer 1998).
One trial reported 3, 12, and 24 months' follow‐up (Pellicano 2002). Two provided follow‐up at different times and up to 5 years (Penninx 2010; Sambrook 2009). Three trials followed up at different times and up to 10 years (Bongers 2004; Boujida 2002; Cooper 1999).
One trial did not follow up on women, and all outcomes were related to the procedure (van Zon‐Rabelink 2003).
Three trials described unclear follow‐up time (Ghazizadeh 2014; Onoglu 2007; Thabet 2010).
Funding and conflicts of interest
In terms of funding, four trials reported institutional or government funding: from the Chief Scientist Office at the Scottish Department of Health (Bhattacharya 1997), from the Research Foundation of the County of West Zealand (Boujida 2002), from Akdeniz University (Onoglu 2007), and from the Chief Scientist Office at the Scottish Government Health Directorates (Sambrook 2009).
Fourteen trials reported that funding was received from industry, that study authors were associated with industry, or that equipment was provided by industry (Abbott 2003; Bongers 2004; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Hawe 2003; Laberge 2016; Meyer 1998; Pellicano 2002; Vercellini 1999). One trial acknowledged a medical equipment company for technical assistance, but it is unknown whether or not the trial received funding (Brun 2006).
Two trials reported no external funding (Ghazizadeh 2014; Penninx 2016).
Seven trials did not report details on the source of funding (Athanatos 2015; McClure 1992; Penninx 2010; Perino 2004; Romer 1998; Thabet 2010; van Zon‐Rabelink 2003).
Four trials reported conflicts of interest.
Cooper 1999: one study author was funded in part by industry as a research fellow, other study authors had received travel and accommodation support from industry for attending conferences and training courses, and one study author is director and a stock shareholder and receives travel grants from industry.
Duleba 2003: study authors are consultants for industry.
Penninx 2010: one study author received an unconditional grant from industry for another research project.
Sambrook 2009: two study authors received financial support from industry for travel and for attending meetings.
Three studies declared that authors had no conflicts of interest (Abbott 2003; Laberge 2016; Penninx 2016).
Twenty‐one trials provided no details on conflicts of interest (Athanatos 2015; Bhattacharya 1997; Bongers 2004; Boujida 2002; Brun 2006; Clark 2011; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Ghazizadeh 2014; Hawe 2003; McClure 1992; Meyer 1998; Onoglu 2007; Pellicano 2002; Perino 2004; Romer 1998; Thabet 2010; van Zon‐Rabelink 2003; Vercellini 1999).
Excluded studies
We excluded six studies.
One compared different waveforms for rollerball ablation (Chang 2009).
One was not randomised (El‐Nashar 2009).
Two compared similar types of endometrial ablation with or without a co‐intervention (Abd Ek Hameed 2012; Cash 2012).
One did not take place (Cooper 2012).
One included a population that does not meet review criteria (Soysal 2001).
Risk of bias in included studies
We have provided information on risk of bias in the included studies in the Characteristics of included studies table, and we have summarised this information in Figure 2 and Figure 3.
2.
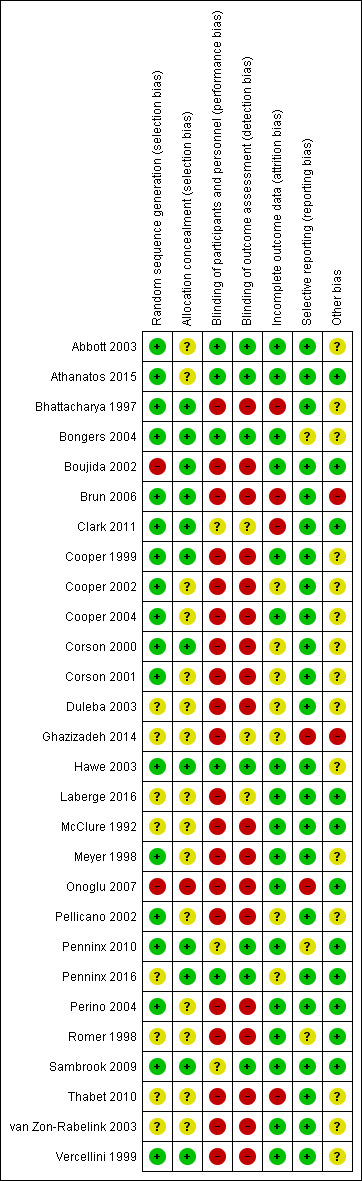
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
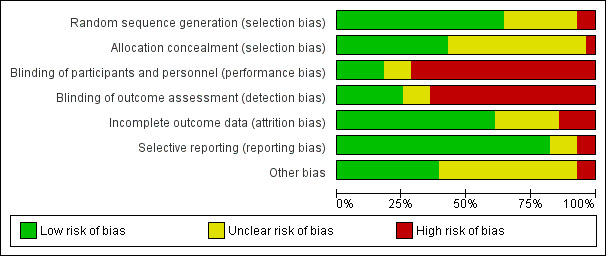
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation method
Eighteen studies described adequate randomisation methods, and we judged them to be at low risk of selection bias. They used either computer‐generated numbers or lists of random numbers (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Bongers 2004; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Hawe 2003; Meyer 1998; Pellicano 2002; Penninx 2010; Perino 2004; Sambrook 2009; Vercellini 1999). We judged eight studies to be at unclear risk of selection bias; two reported unclear data about the random sequence generation (Laberge 2016; Thabet 2010), and six provided no details on the randomisation method (Duleba 2003; Ghazizadeh 2014; McClure 1992; Penninx 2016; Romer 1998; van Zon‐Rabelink 2003). Two studies provided details of an inadequate randomisation method (Boujida 2002; Onoglu 2007); Onoglu 2007 reported that researchers allocated participants to treatment in the order in which they came into the clinic. Boujida 2002 reported using odd and even numbers. We judged these studies to be at high risk of bias.
Allocation concealment
Thirteen studies provided evidence of adequate allocation concealment, and we judged them to be at low risk of bias. These studies used either sequentially numbered opaque envelopes or a central method for allocation to groups (Bhattacharya 1997; Bongers 2004; Boujida 2002; Brun 2006; Clark 2011; Cooper 1999; Cooper 2004; Corson 2000; Hawe 2003; Penninx 2010; Penninx 2016; Sambrook 2009; Vercellini 1999).
We judged that 14 studies were at unclear risk of bias because they did not provide details as to whether allocation was concealed (Abbott 2003; Athanatos 2015; Cooper 2002; Corson 2001; Duleba 2003; Ghazizadeh 2014; Laberge 2016; McClure 1992; Meyer 1998; Pellicano 2002; Perino 2004; Romer 1998; Thabet 2010; van Zon‐Rabelink 2003). We scored the remaining study as having no concealment and judged it to be at high risk of bias (Onoglu 2007).
Blinding
Performance bias
Most of the studies did not specifically undertake or report blinding; for all these studies, blinding was unlikely due to the nature of the interventions. Three studies that compared second‐generation techniques (bipolar radiofrequency vs balloon) (Abbott 2003; Bongers 2004; Penninx 2016), along with another comparing balloon versus laser (Hawe 2003), described triple blinding (patients, investigators, and assessors), and two studies on second‐generation approaches reported double blinding (patients and assessors) (Athanatos 2015; Penninx 2016). Women were blinded to allocation in Clark 2011, although they were likely to have guessed allocation; we judged this study to be at unclear risk of bias. Two other studies blinded women but not investigators (Penninx 2010; Sambrook 2009).
Detection bias
We judged seven studies to be at low risk of detection bias (Abbott 2003; Athanatos 2015; Bongers 2004; Hawe 2003; Penninx 2010; Penninx 2016; Sambrook 2009). We judged three studies to be at unclear risk of detection bias because they provided insufficient details (Clark 2011; Ghazizadeh 2014; Laberge 2016). For the remaining trials, we considered risk of detection bias to be high.
Incomplete outcome data
For assessments regarding incomplete outcome data, we scored 17 studies as having adequately addressed their missing data (if any) because they reported no dropouts, missing data were balanced between groups, or they had minimal loss to follow‐up that was unlikely to affect the calculation of estimates (Abbott 2003; Athanatos 2015; Bongers 2004; Boujida 2002; Cooper 1999; Cooper 2004; Hawe 2003; Laberge 2016; McClure 1992; Meyer 1998; Onoglu 2007; Penninx 2010; Perino 2004; Romer 1998; Sambrook 2009;; van Zon‐Rabelink 2003; Vercellini 1999); we judged these studies to be at low risk of attrition bias. For seven studies, it was unclear whether their missing data could cause bias (Cooper 2002; Corson 2000; Corson 2001; Duleba 2003; Ghazizadeh 2014; Pellicano 2002; Penninx 2016), and we judged them to be at unclear risk of bias. Most of them reported dropouts without reasons or details on the distribution per group. Four studies had high risk of attrition bias: one for differences in the number of participants providing data for different outcomes (Bhattacharya 1997), one for differences in the number lost at assessment at 12 months for different outcomes (Clark 2011), one because withdrawals were unbalanced between groups (Brun 2006), and another because dropouts were replaced by other cases, which is likely to cause major bias (Thabet 2010).
Selective reporting
We judged 23 out of 28 studies to have low risk of reporting bias; study authors reported all prespecified outcomes in the results sections (Abbott 2003; Athanatos 2015; Bhattacharya 1997; Boujida 2002; Brun 2006; Clark 2011; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Hawe 2003; Laberge 2016; McClure 1992; Meyer 1998; Pellicano 2002; Penninx 2016; Perino 2004; Sambrook 2009; Thabet 2010; van Zon‐Rabelink 2003; Vercellini 1999).
Three studies had unclear risk of selective reporting ‐ two because they did not report complications (Penninx 2010; Romer 1998), and one because study authors did not report or prespecify adverse effects (Bongers 2004).
We judged only two studies as having high risk of selective reporting ‐ one because it reported no quantification of bleeding (Ghazizadeh 2014), and the other because study authors described prespecified bleeding patterns but did not report the data (Onoglu 2007).
Other potential sources of bias
Four studies had other potential sources of bias: one recruited participants over two different time periods and comparison of the two groups indicated substantial differences (Bhattacharya 1997); in another, numbers in the two randomised groups differed substantially with no explanation given (van Zon‐Rabelink 2003); in another, past medical history was significantly different between groups (Ghazizadeh 2014); and in another, one woman receiving cryoablation had higher PBAC scores than the others (Duleba 2003).
Effects of interventions
See: Table 1
First‐generation technique comparisons
1. Laser ablation versus transcervical resection of the endometrium (TCRE) (Comparison 1)
Two studies with a total of 176 women reported laser versus transcervical resection of the endometrium (Bhattacharya 1997; McClure 1992) .
Primary outcomes
1.1 and 1.2 Bleeding
No clear evidence showed any differences between laser ablation and TCRE groups in the rate of amenorrhoea at 6 months (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.66 to 1.45; 348 women; 2 studies; I² = 28%), the combined rate of amenorrhoea and hypomenorrhoea at 6 months (RR 0.97, 95% CI 0.89 to 1.05; 326 women; 1 study) or at 12 months (RR 1.06, 95% CI 0.92 to 1.22; 306 women; 1 study), or mean blood loss at 6 months (mean difference (MD) 23.60 mL, 95% CI ‐8.32 to 55.52; 22 women; 1 study). See Analysis 1.2 and Analysis 1.1.
1.2. Analysis.
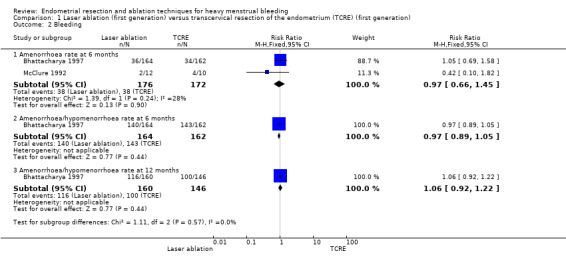
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 2 Bleeding.
1.1. Analysis.
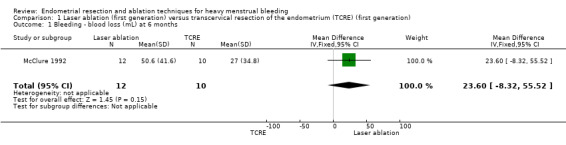
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 1 Bleeding ‐ blood loss (mL) at 6 months.
1.3 Rate of satisfaction
One trial provided no clear evidence of a difference between laser ablation and TCRE groups in the rate of satisfaction at 12 months (RR 0.99, 95% CI 0.92 to 1.06; 321 women; 1 study). See Analysis 1.3.
1.3. Analysis.
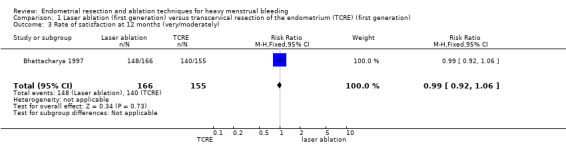
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 3 Rate of satisfaction at 12 months (very/moderately).
Secondary outcomes
1.4 Duration of surgery
Duration of laser ablation surgery was on average 9 minutes longer than for TCRE (MD 9.15 minutes, 95% CI 7.2 to 11.1; 386 women; 2 studies; I² = 74%). See Analysis 1.4.
1.4. Analysis.
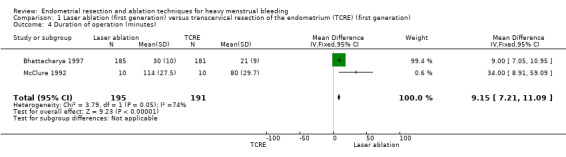
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 4 Duration of operation (minutes).
1.5 Operative difficulties
Risks of equipment failure were greater among women who had laser ablation than among those with TCRE (RR 5.54, 95% CI 1.65 to 18.60; 366 women; 1 study). Trials found no clear evidence of differences between groups for abandonment of procedure (RR 1.47, 95% CI 0.61 to 3.51; 366 women; 1 study), instrument failure (RR 0.20, 95% CI 0.01 to 4.05; 366 women; 1 study), or need for immediate hysterectomy (RR 0.33, 95% CI 0.01 to 7.95; 366 women; 1 study). See Analysis 1.5.
1.5. Analysis.
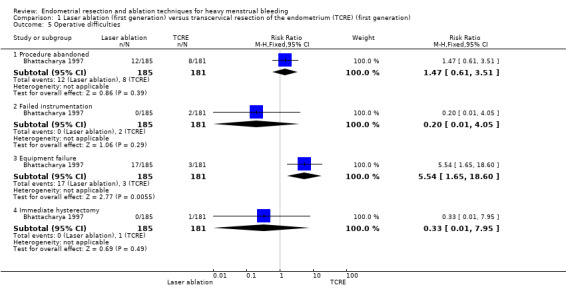
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 5 Operative difficulties.
1.6 Women's perceived change in quality of life
Researchers found no clear evidence of a difference between laser ablation and TRCE at 12 months for the proportion of women reporting good general health (RR 1.03, 95% CI 0.95 to 1.12; 321 women). See Analysis 1.6.
1.6. Analysis.
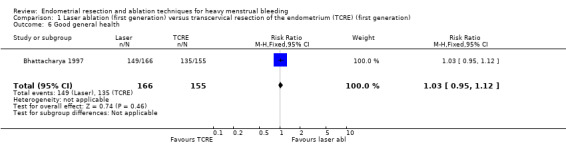
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 6 Good general health.
1.7 Improvement in other menstrual symptoms
We found no clear evidence of differences between laser ablation and TRCE for improvement in general symptoms (RR 1.03, 95% CI 0.87 to 1.21; 321 women; 1 study) or for improvement in dysmenorrhoea at 6 months' (RR 1.17, 95% CI 1.00 to 1.38; 253 women; 1 study) or 12 months' follow‐up (RR 1.00, 95% CI 0.87 to 1.15; 218 women; 1 study). See Analysis 1.7.
1.7. Analysis.
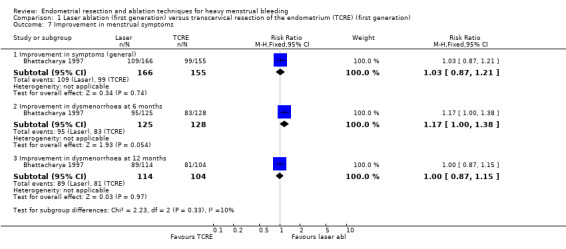
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 7 Improvement in menstrual symptoms.
1.8 Complication rate: major complications
No clear evidence showed a difference between laser ablation and TRCE in major complication rates including the following (see Analysis 1.8).
1.8. Analysis.
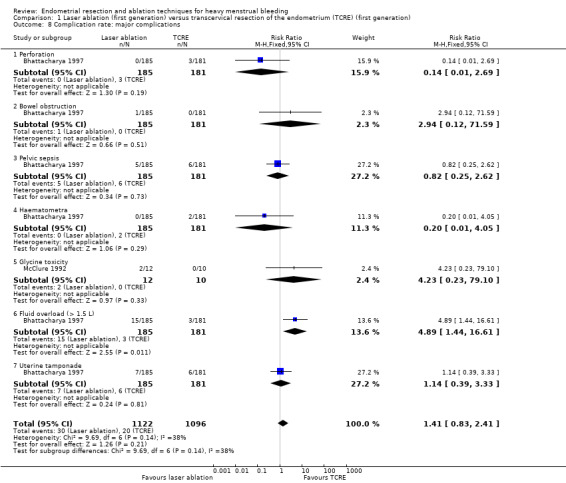
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 8 Complication rate: major complications.
Perforation (RR 0.14, 95% CI 0.01 to 2.69; 366 women; 1 study).
Bowel obstruction (RR 2.94, 95% CI 0.12 to 71.59; 366 women; 1 study).
Pelvic sepsis (RR 0.82, 95% CI 0.25 to 2.62; 366 women; 1 study).
Haematometra (RR 0.20, 95% CI 0.01 to 4.05; 366 women; 1 study).
Glycine toxicity (RR 4.23, 95% CI 0.23 to 79.10 ; 22 women; 1 study).
Fluid overload >1.5 L (RR 4.89, 95% CI 1.44 to 16.61; 366 women; 1 study).
Uterine tamponade (RR 1.14, 95% CI 0.39 to 3.33; 366 women; 1 study).
1.9 Complication rate: minor complications
No clear evidence showed a difference between laser ablation and TRCE in minor complication rates including the following (see Analysis 1.9).
1.9. Analysis.
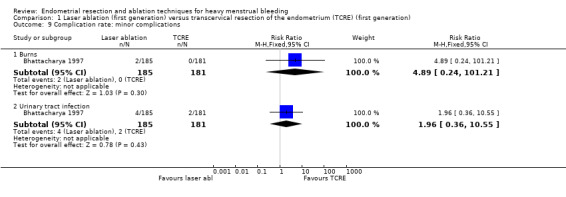
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 9 Complication rate: minor complications.
Burns (RR 4.89, 95% CI 0.24 to 101.21; 366 women; 1 study).
Urinary tract infection (RR 1.96, 95% CI 0.36 to 10.55; 366 women; 1 study).
1.10 Requirement for further surgery
Trials have provided no clear evidence of a difference between laser ablation and TRCE in the requirement of further surgery up to 12 months' follow‐up (RR 0.84, 95% CI 0.55 to 1.29; 388 women; 2 studies; I² = 0%). See Analysis 1.10.
1.10. Analysis.
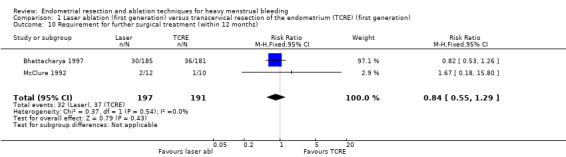
Comparison 1 Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation), Outcome 10 Requirement for further surgical treatment (within 12 months).
Researchers have provided no data on the proportion of women given local rather than general anaesthesia, length of hospital stay, and time or ability to return to normal activities or work.
2. Vaporising electrode ablation versus TCRE (Comparison 2)
One study with 91 women reported on vaporising electrode ablation versus TCRE (Vercellini 1999).
Primary outcomes
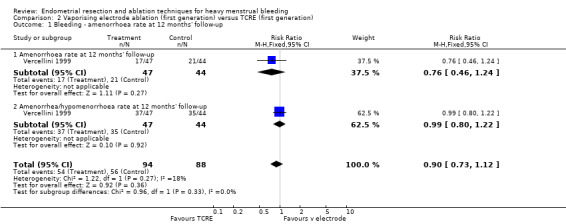
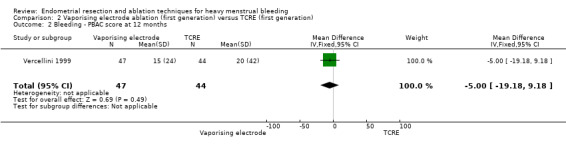
2.1 and 2.2 Bleeding
Studies have provided no clear evidence of a difference between vaporising electrode ablation and TCRE for bleeding as measured by amenorrhoea (RR 0.90, 95% CI 0.73 to 1.12; 182 women; 1 study), hypomenorrhoea (scanty menstruation) rate (RR 0.99, 95% CI 0.80 to 1.22; 91 women; 1 study), or pictorial chart method (PBAC) score at 12 months (MD ‐5.00 units, 95% CI ‐19.18 to 9.18; 91 women; 1 study). See Analysis 2.1 and Analysis 2.2.
2.1. Analysis.
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 1 Bleeding ‐ amenorrhoea rate at 12 months' follow‐up.
2.2. Analysis.
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 2 Bleeding ‐ PBAC score at 12 months.
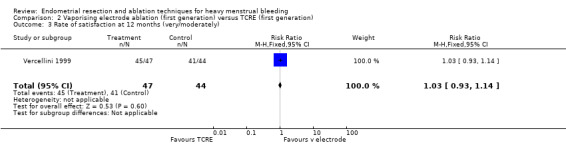
2.3 Rate of satisfaction
We found no clear evidence of a difference between vaporising electrode ablation and TCRE in the rate of satisfaction (very/moderately) with treatment at 12 months (RR 1.03, 95% CI 0.93 to 1.14; 91 women; 1 study). See Analysis 2.3.
2.3. Analysis.
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 3 Rate of satisfaction at 12 months (very/moderately).
Secondary outcomes
2.4 Duration of operation
The duration of the operation/procedure was shorter with vaporising electrode ablation than with TRCE (MD ‐1.50 minutes, 95% CI ‐2.65 to ‐0.35; 91 women; 1 study). See Analysis 2.4.
2.4. Analysis.
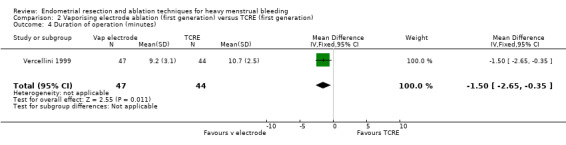
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 4 Duration of operation (minutes).
2.5 Operative difficulties
Vaporising electrode ablation was associated with a reduction in difficulty with surgery, reported as moderate or severe, compared with TCRE (RR 0.29, 95% CI 0.10 to 0.82; 91 women; 1 study). See Analysis 2.5.
2.5. Analysis.
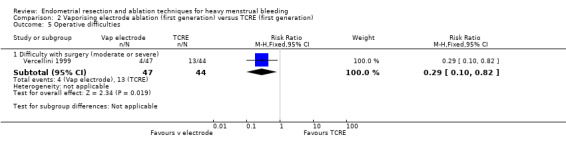
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 5 Operative difficulties.
2.6 Complication rate: major complications
The extent of fluid deficit was greater in the TCRE group than in the vaporising electrode ablation group (MD ‐258.00, 95% CI ‐342.05 to ‐173.95; 91 women; 1 study). See Analysis 2.6.
2.6. Analysis.
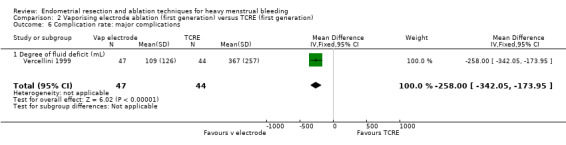
Comparison 2 Vaporising electrode ablation (first generation) versus TCRE (first generation), Outcome 6 Complication rate: major complications.
Researchers have provided no data on the proportion of women given local rather than general anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, complication rates, requirement for further surgery, or mortality as a direct result of surgery.
3. Rollerball versus TCRE (Comparison 3)
Two trials with a total of 165 women reported on rollerball versus TCRE (Boujida 2002; Onoglu 2007).
Primary outcomes
Researchers have provided no data on bleeding or satisfaction rates.
Secondary outcomes
3.1 Duration of surgery
No clear evidence showed a difference between rollerball and TCRE for duration of surgery (MD ‐1.10 minutes, 95% CI ‐2.92 to 0.72; 45 women; 1 study). Boujida 2002 provided data as median (range) values that we did not include in the meta‐analysis. These data suggest that the duration of surgery was shorter with rollerball than with TCRE, median 13 minutes with rollerball (range 6 to 105 minutes) in 61 women versus 20 minutes (range 4 to 45 minutes) with TCRE in 59 women. See Analysis 3.1.
3.1. Analysis.
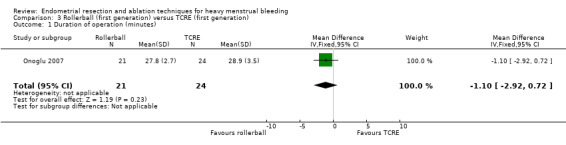
Comparison 3 Rollerball (first generation) versus TCRE (first generation), Outcome 1 Duration of operation (minutes).
3.2 Complication rate
No clear evidence showed a difference in major complication rates between rollerball and TCRE such as the following (see Analysis 3.2).
3.2. Analysis.
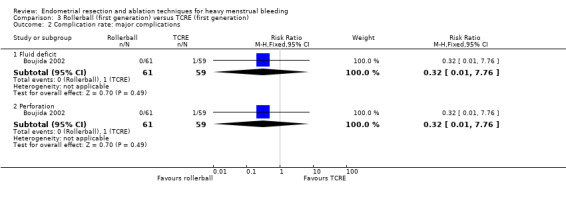
Comparison 3 Rollerball (first generation) versus TCRE (first generation), Outcome 2 Complication rate: major complications.
Fluid deficit (RR 0.32, 95% CI 0.01 to 7.76; 120 women; 1 study).
Perforation (RR 0.32, 95% CI 0.01 to 7.76; 120 women; 1 study).
3.3 Requirement for further surgery
Trials have provided no evidence of any differences between rollerball and TCRE in the number of women requiring either hysterectomy or any surgical intervention up to 10 years' follow‐up, including the following (see Analysis 3.3).
3.3. Analysis.
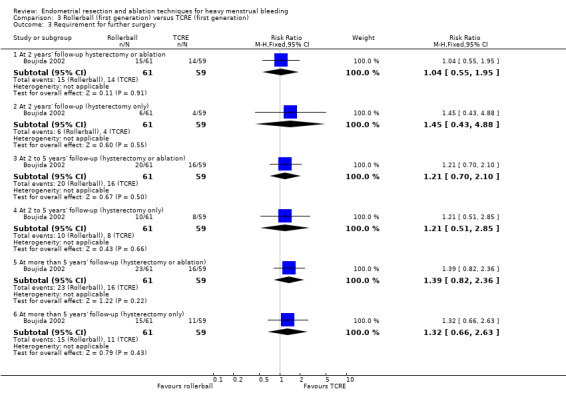
Comparison 3 Rollerball (first generation) versus TCRE (first generation), Outcome 3 Requirement for further surgery.
2 years' follow‐up (hysterectomy and ablation) (RR 1.04, 95% CI 0.55 to 1.95; 120 women; 1 study).
2 years' follow‐up (hysterectomy only) (RR 1.45, 95% CI 0.43 to 4.88; 120 women; 1 study).
2 to 5 years' follow‐up (hysterectomy and ablation) (RR 1.21, 95% CI 0.70 to 2.10; 120 women; 1 study).
2 to 5 years' follow‐up (hysterectomy only) (RR 1.21, 95% CI 0.51 to 2.85; 120 women; 1 study).
More than 5 years' follow‐up (hysterectomy and ablation) (RR 1.39, 95% CI 0.82 to 2.36; 120 women; 1 study).
More than 5 years' follow‐up (hysterectomy only) (RR 1.32, 95% CI 0.66 to 2.63; 120 women; 1 study).
Researchers have provided no data for operative difficulties, the proportion of women given local rather than general anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, complication rates, or mortality as a direct result of surgery.
Second‐generation versus first‐generation technique comparisons
4. Thermal laser versus TCRE (Comparison 4)
One study with 111 women reported on thermal laser versus TCRE (Perino 2004).
Primary outcomes
4.1 Bleeding
Rates of amenorrhoea at 1 and 3 years after surgery were greater for women in the thermal laser group than in the TCRE group (RR 2.46, 95% CI 1.50 to 4.03; 111 women; 1 study; RR 2.49, 95% CI 1.48 to 4.21; 111 women; 1 study, respectively). See Analysis 4.1.
4.1. Analysis.
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 1 Bleeding ‐ amenorrhoea rate.
4.2 Rate of satisfaction
Trials showed no clear evidence of a difference in satisfaction rates between thermal laser and TCRE at 1 year (RR 1.04, 95% CI 0.94 to 1.16; 111 women; 1 study) and 5 years' (RR 1.02, 95% CI 0.91 to 1.14; 111 women; 1 study) follow‐up. See Analysis 4.2.
4.2. Analysis.
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
4.3 Duration of operation
Mean length of surgery was shorter for women in the thermal laser group than in the TCRE group (MD ‐9.30, 95% CI ‐11.36 to ‐7.24; 111 women; 1 study). See Analysis 4.3.
4.3. Analysis.
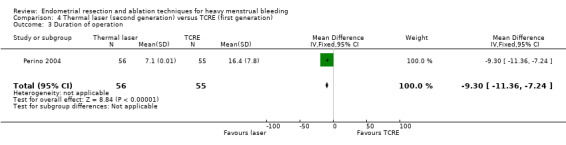
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 3 Duration of operation.
4.4 Complication rate: major complications
Researchers have provided no evidence of differences in the major complication rate between thermal laser and TCRE such as perforation (no events in either group). See Analysis 4.4.
4.4. Analysis.
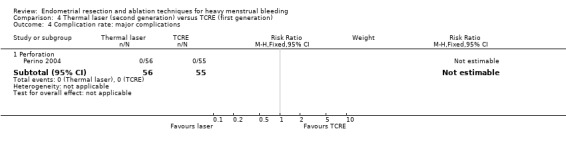
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 4 Complication rate: major complications.
4.5 Complication rate: minor complications
Studies have reported no evidence of differences in the minor complication rate between thermal laser and TCRE such as urinary tract infection (RR 0.49, 95% CI 0.05 to 5.26; 111 women; 1 study). See Analysis 4.5.
4.5. Analysis.
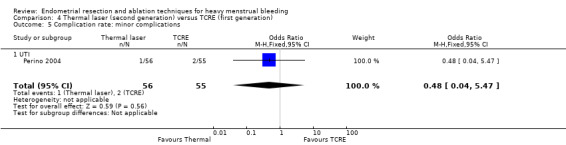
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 5 Complication rate: minor complications.
4.6 Requirement for further surgery
No clear evidence showed a difference in the requirement for hysterectomy at 2 to 5 years' follow‐up between thermal laser and TCRE groups (RR 0.59, 95% CI 0.15 to 2.35; 111 women; 1 study). See Analysis 4.6.
4.6. Analysis.
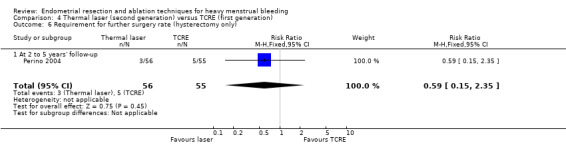
Comparison 4 Thermal laser (second generation) versus TCRE (first generation), Outcome 6 Requirement for further surgery rate (hysterectomy only).
Trials have provided no data for operative difficulties, the proportion of women given local rather than general anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
5. Hydro ThermAblator (HTA) versus rollerball (Comparison 5)
One study with 276 women compared Hydro ThermAblator (HTA) versus rollerball (Corson 2001).
Primary outcomes
5.1 Bleeding
We assessed bleeding at 1 year, 2 years', and up to 5 years' follow‐up in three different ways: PBAC score up to and including 75; or PBAC score up to and including 100; or by reporting of amenorrhoea. Trials provided no clear evidence of a difference between HTA and rollerball, as shown by the following (see Analysis 5.1).
5.1. Analysis.
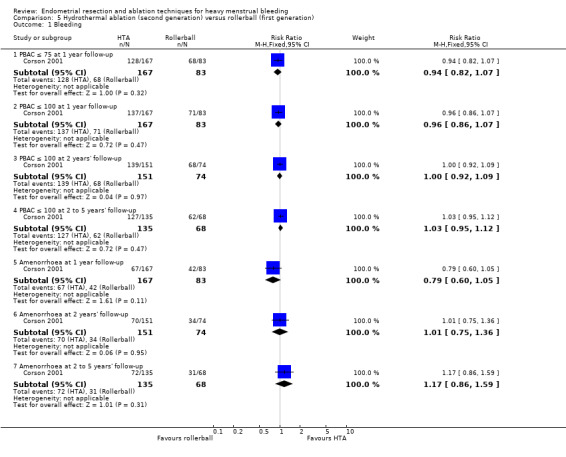
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 1 Bleeding.
PBAC ≤ 75 at 1 year follow‐up (RR 0.94, 95% CI 0.82 to 1.07; 250 women; 1 study).
PBAC ≤ 100 at 1 year follow‐up (RR 0.96, 95% CI 0.86 to 1.07; 250 women; 1 study).
PBAC ≤ 100 at 2 years' follow‐up (RR 1.00, 95% CI 0.92 to 1.09; 225 women; 1 study).
PBAC ≤ 100 at 2 to 5 years' follow‐up (RR 1.03, 95% CI 0.95 to 1.12; 203 women; 1 study).
Amenorrhoea at 1 year follow‐up (RR 0.79, 95% CI 0.60 to 1.05; 250 women; 1 study).
Amenorrhoea at 2 years' follow‐up (RR 1.01, 95% CI 0.75 to 1.36; 225 women; 1 study).
Amenorrhoea at 2 to 5 years' follow‐up (RR 1.17, 95% CI 0.86 to 1.59; 203 women; 1 study).
5.2 Rate of satisfaction
We noted no clear evidence of a difference in the rate of satisfaction with treatment at 2 to 5 years' follow‐up between HTA and rollerball groups (RR 1.01, 95% CI 0.96 to 1.06; 203 women; 1 study). See Analysis 5.2.
5.2. Analysis.
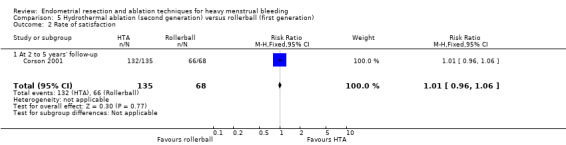
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
5.3 Proportion given local rather than general anaesthesia
Women undergoing HTA ablation were almost twice as likely as those with TRCE to require only a local anaesthetic (RR 2.02, 95% CI 1.32 to 3.09; 269 women; 1 study). See Analysis 5.3.
5.3. Analysis.
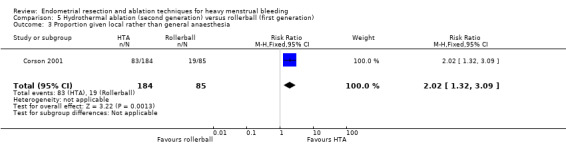
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 3 Proportion given local rather than general anaesthesia.
5.4 Complication rate: major complications
Women in the HTA group were less likely to experience the adverse event of haematometra (haemorrhage in the uterus) from surgery (RR 0.18, 95% CI 0.04 to 0.93). See Analysis 5.4. However, results showed no clear differences in other major complications such as the following.
5.4. Analysis.
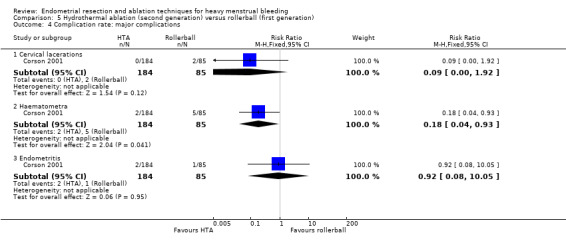
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 4 Complication rate: major complications.
Cervical lacerations (RR 0.09, 95% CI 0.00 to 1.92; 269 women; 1 study).
Endometritis (RR 0.92, 95% CI 0.08 to 10.05; 269 women; 1 study).
5.5 Complication rate: minor complications
Women with HTA were more likely to experience abdominal pain (RR 1.40, 95% CI 1.03 to 1.90; 269 women; 1 study) and nausea and vomiting after surgery (RR 3.08, 95% CI 1.36 to 6.98; 269 women; 1 study). See Analysis 5.5. Study results showed no clear evidence of differences in other minor complications such as the following.
5.5. Analysis.
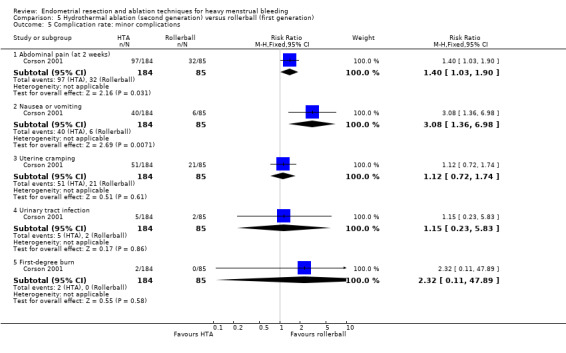
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 5 Complication rate: minor complications.
Uterine cramping (RR 1.12, 95% CI 0.72 to 1.74; 269 women; 1 study).
Urinary tract infection (RR 1.15, 95% CI 0.23 to 5.83; 269 women; 1 study).
First‐degree burn (RR 2.32, 95% CI 0.11 to 47.89; 269 women; 1 study).
5.6 Requirement for further surgery
We found no clear evidence of differences between groups in the requirement for further surgery, including any surgery at 1 year follow‐up (RR 2.32, 95% CI 0.11 to 47.89; 269 women; 1 study); any surgery at 2 to 5 years' follow‐up (RR 1.26, 95% CI 0.58 to 2.73; 269 women; 1 study); or hysterectomy at 5 years' follow‐up (RR 1.54, 95% CI 0.58 to 4.06; 269 women; 1 study). See Analysis 5.6.
5.6. Analysis.
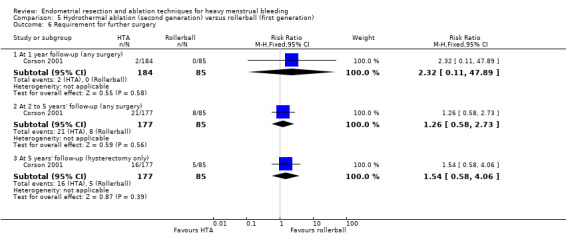
Comparison 5 Hydrothermal ablation (second generation) versus rollerball (first generation), Outcome 6 Requirement for further surgery.
Researchers provided no data for duration of surgery, operative difficulties, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
6. Cryoablation versus rollerball (Comparison 6)
One study with 279 women compared cryoablation and rollerball (Duleba 2003).
Primary outcomes
6.1 Bleeding
Women undergoing cryoablation were less likely to have amenorrhoea 1 year after surgery than women receiving rollerball treatment (odds ratio (OR) 0.5, 95% CI 0.36 to 0.69; 279 women; 1 study). See Analysis 6.1.
6.1. Analysis.
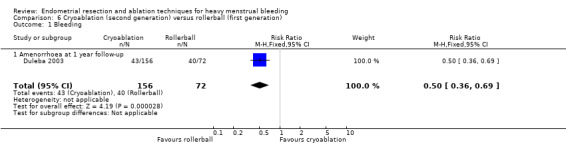
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 1 Bleeding.
6.2 Rate of satisfaction
We found no evidence of clear differences between groups for satisfaction with treatment at 1 year (RR 1.06, 95% CI 0.96 to 1.17; 279 women; 1 study) or 2 years' follow‐up (RR 1.04, 95% CI 0.91 to 1.17; 279 women; 1 study). See Analysis 6.2.
6.2. Analysis.
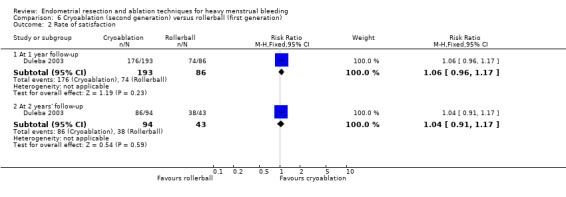
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
Operative outcomes
6.3 Proportion given local anaesthesia
Women undergoing cryoablation were more likely to receive local rather than general anaesthesia than women undergoing rollerball ablation (RR 6.6, 95% CI 3.2 to 13.6; 279 women; 1 study). See Analysis 6.3.
6.3. Analysis.
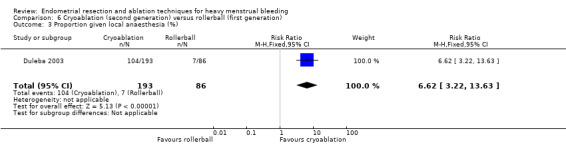
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 3 Proportion given local anaesthesia (%).
6.4 Complication rate: major complications
No evidence showed clear differences between groups for major complications such as the following (see Analysis 6.4).
6.4. Analysis.
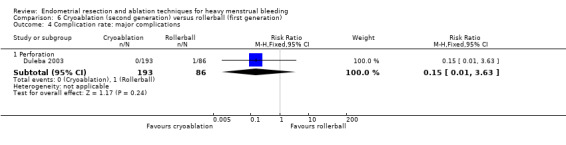
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 4 Complication rate: major complications.
Perforation (RR 0.15, 95% CI 0.01 to 3.63; 279 women; 1 study).
6.5 Complication rate: minor complications
No evidence showed clear differences between groups for minor complications such as the following (See Analysis 6.5)
6.5. Analysis.
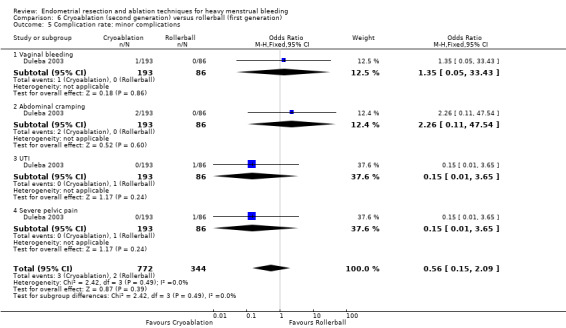
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 5 Complication rate: minor complications.
Vaginal bleeding (RR 1.35, 95% CI 0.06 to 32.70; 279 women; 1 study).
Abdominal cramping (RR 2.24, 95% CI 0.11 to 46.21; 279 women; 1 study).
Urinary tract infection (RR 0.15, 95% CI 0.01 to 3.63; 279 women; 1 study).
Severe pelvic pain (RR 0.15, 95% CI 0.01 to 3.63; 279 women; 1 study).
6.6 Requirement for further surgery
Researchers showed no clear evidence of differences between groups in the requirement for further surgery at 2 years after ablation treatment for any surgery (RR 1.00, 95% CI 0.45 to 2.22; 279 women; 1 study) or for hysterectomy only (RR 0.83, 95% CI 0.34 to 2.00; 279 women; 1 study). See Analysis 6.6.
6.6. Analysis.
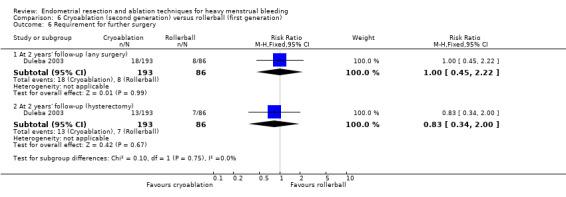
Comparison 6 Cryoablation (second generation) versus rollerball (first generation), Outcome 6 Requirement for further surgery.
Researchers provided no data for duration of surgery, operative difficulties, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
7. Electrode ablation (balloon or mesh) versus TCRE (Comparison 7)
Two studies with a total of 541 women compared electrode ablation (balloon or mesh) versus TCRE. Corson 2000 compared electrode ablation with a balloon system, and Cooper 2002 compared an electrode balloon system versus mesh.
Primary outcomes
7.1 , 7.2, and 7.3 Bleeding
Trial results showed no clear evidence of differences between groups for bleeding.
Amenorrhoea rate with the balloon system (RR 0.89, 95% CI 0.62 to 1.29; 234 women; 1 study) versus the mesh system (RR 1.16, 95% CI 0.82 to 1.64; 236 women; 1 study). See Analysis 7.1.
PBAC score < 75 with the balloon system (RR 1.05, 95% CI 0.94 to 1.17; 234 women; 1 study) versus the mesh system (RR 1.08, 95% CI 0.96 to 1.22; 236 women; 1 study). See Analysis 7.2.
PBAC score at 12 months' follow‐up. See Analysis 7.3.
7.1. Analysis.
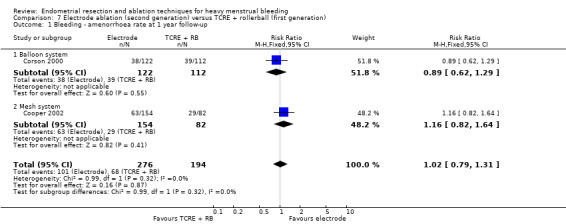
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 1 Bleeding ‐ amenorrhoea rate at 1 year follow‐up.
7.2. Analysis.
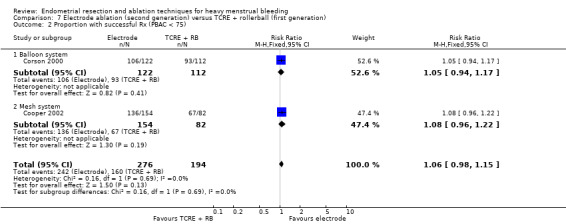
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 2 Proportion with successful Rx (PBAC < 75).
7.3. Analysis.
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 3 PBAC score 12 months after treatment.
| PBAC score 12 months after treatment | |||
|---|---|---|---|
| Study | Electrode system | TCRE + RB | Stat test for diff |
| Balloon system | |||
| Corson 2000 | N=122 Mean PBAC (SD): 18 (37) | N=112 Mean PBAC (SD): 28 (70) | Not significantly different |
| Mesh system | |||
| Cooper 2002 | N=154 Mean PBAC (SD): 26.8 (57.4) | N=82 Mean PBAC (SD): 36.4 (66.3) | No reported difference |
7.4 Rate of satisfaction
Upon assessing rate of satisfaction with treatment after 1 year, study authors did not report clear differences between groups comparing the mesh system to TCRE (RR 0.99, 95% CI 0.92 to 1.06; 236 women; 1 study). See Analysis 7.4.
7.4. Analysis.
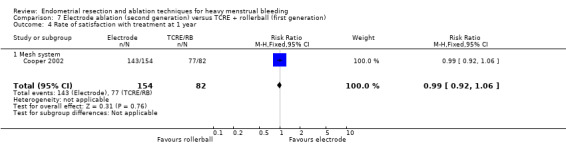
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 4 Rate of satisfaction with treatment at 1 year.
Secondary outcomes
Operative outcomes
7.5 Duration of surgery
The duration of the procedure was significantly longer for women undergoing TCRE compared with ablation (MD 18.7 minutes, 95% CI 16.8 to 20.7; 520 women; 2 studies; I² = 69%). See Analysis 7.5.
7.5. Analysis.
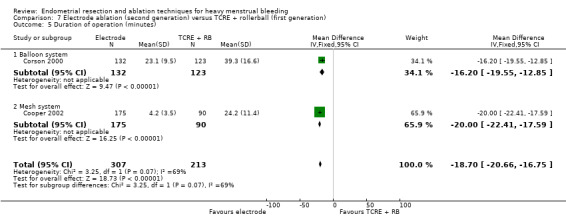
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 5 Duration of operation (minutes).
7.6 Procedure abandonment
We found no evidence of a clear difference between groups for abandonment of the procedure (RR 2.57, 95% CI 0.11 to 62.41; 267 women; 1 study). See Analysis 7.6.
7.6. Analysis.
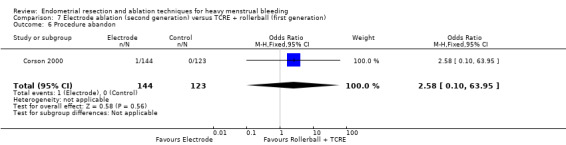
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 6 Procedure abandon.
7.7 Proportion given general versus local anaesthesia
Women undergoing electrode ablation were more likely to receive local rather than general anaesthesia compared with women having TCRE (RR 3.9, 95% CI 2.9 to 5.0; 520 women; 2 studies; I² = 0%). See Analysis 7.7.
7.7. Analysis.
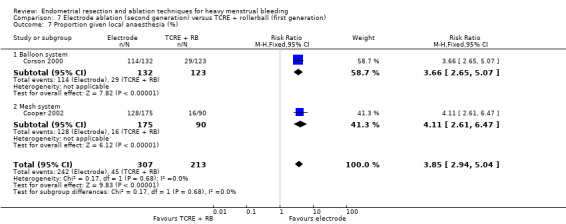
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 7 Proportion given local anaesthesia (%).
7.8 Complication rate: major complications
Clear evidence showed differences in major complications such as perforation and cervical tears or lacerations between groups. Perforation (RR 0.13, 95% CI 0.02 to 1.01; 532 women; 2 studies; I² = 0%) and cervical tears or lacerations (RR 0.11, 95% CI 0.01 to 0.87; 532 women; 2 studies; I² = 0%) were less likely with electrode ablation than with TCRE. See Analysis 7.8.
7.8. Analysis.
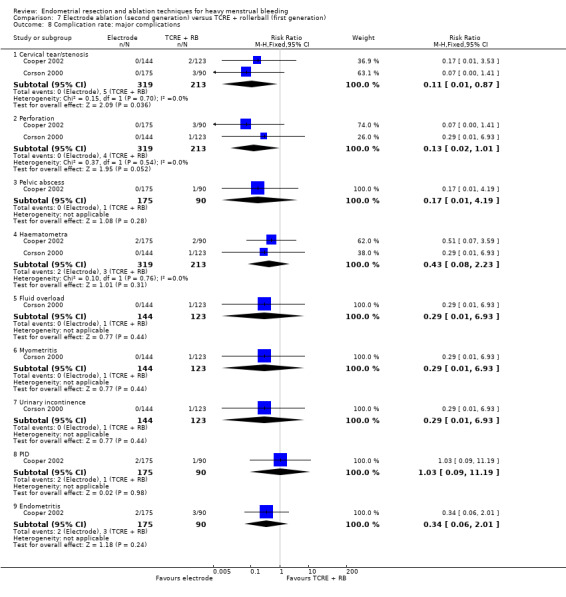
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 8 Complication rate: major complications.
We found no report of clear evidence of differences in other major complications such as the following.
Pelvic abscess (RR 0.17, 95% CI 0.01 to 4.19; 267 women; 1 study).
Haematometra (RR 0.43, 95% CI 0.08 to 2.23; 267 women; 1 study).
Fluid overload (RR 0.29, 95% CI 0.01 to 6.93; 267 women; 1 study).
Myometritis (RR 0.29, 95% CI 0.01 to 6.93; 267 women; 1 study).
Urinary incontinence (RR 0.29, 95% CI 0.01 to 6.93; 267 women; 1 study).
Pelvic inflammatory disease (RR 1.03, 95% CI 0.09 to 11.19; 267 women; 1 study).
Endometritis (RR 0.34, 95% CI 0.06 to 2.01; 267 women; 1 study).
7.9 Complication rate: minor complications
The minor complication rate did not show clear evidence of differences between groups for minor complications such as the following (see Analysis 7.9).
7.9. Analysis.
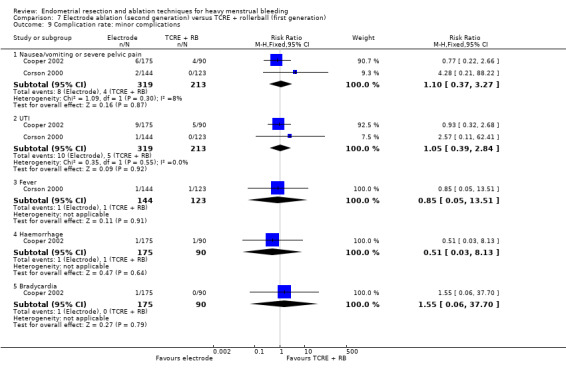
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 9 Complication rate: minor complications.
Nausea/vomiting or severe pelvic pain (RR 1.10, 95% CI 0.37 to 3.27; 267 women; 1 study).
Urinary tract infection (RR 1.05, 95% CI 0.39 to 2.84; 267 women; 1 study).
Fever (RR 0.85, 95% CI 0.05 to 13.51; 267 women; 1 study).
Haemorrhage (RR 0.51, 95% CI 0.03 to 8.13; 267 women; 1 study).
Bradycardia (RR 1.55, 95% CI 0.06 to 37.70; 267 women; 1 study).
7.10 Requirement for further surgery
At two years' follow‐up, comparison of the balloon system versus TCRE + roller ball provided no clear evidence of differences between groups for hysterectomy rate (RR 0.52, 95% CI 0.18 to 1.50; 255 women; 1 study). See Analysis 7.10.
7.10. Analysis.
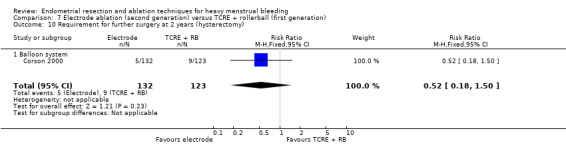
Comparison 7 Electrode ablation (second generation) versus TCRE + rollerball (first generation), Outcome 10 Requirement for further surgery at 2 years (hysterectomy).
Researchers provided no data for operative difficulties, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
8. Microwave versus TCRE plus rollerball (Comparison 8)
Two studies with a total of 585 women compared microwave versus TCRE plus rollerball (Cooper 1999; Cooper 2004).
Primary outcomes
8.1 Bleeding
No evidence showed differences between groups in primary outcomes measuring menstrual blood loss (see Analysis 8.1). Bleeding was measured by:
8.1. Analysis.
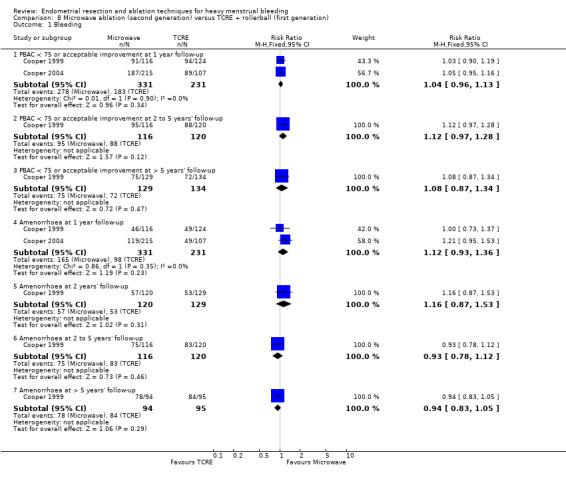
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 1 Bleeding.
PBAC < 75 or acceptable improvement at 1 year follow‐up (RR 1.04, 95% CI 0.96 to 1.13; 562 women; 2 studies; I² = 0%);
PBAC < 75 or acceptable improvement at 2 to 5 years' follow‐up (RR 1.12, 95% CI 0.97 to 1.28; 236 women; 1 study);
PBAC < 75 or acceptable improvement at > 5 years' follow‐up (RR 1.08, 95% CI 0.87 to 1.34; 263 women; 1 study);
Amenorrhoea at 1 year follow‐up (RR 1.12, 95% CI 0.93 to 1.36; 562 women; 2 studies; I² = 0%);
Amenorrhoea at 2 years' follow‐up (RR 1.16, 95% CI 0.87 to 1.53; 249 women; 1 study);
Amenorrhoea at 2 to 5 years' follow‐up (RR 0.93, 95% CI 0.78 to 1.12; 236 women; 1 study); and
Amenorrhoea at > 5 years' follow‐up (RR 0.94, 95% CI 0.83 to 1.05; 189 women; 1 study).
8.2 Rate of satisfaction
Results of the comparison vary over time. At 2 years' follow‐up, results showed benefit for microwave ablation in terms of satisfaction with treatment when compared with TCRE (RR 1.01, 95% CI 0.95 to 1.07; 533 women; 2 studies; I² = 0%), and this benefit was maintained at 5 years' (RR 1.19, 95% CI 1.02 to 1.38; 249 women; 1 study) but not at 10 years' follow‐up in the same study (RR 1.14, 95% CI 0.92 to 1.42; participants = 263; studies = 1). See Analysis 8.2.
8.2. Analysis.
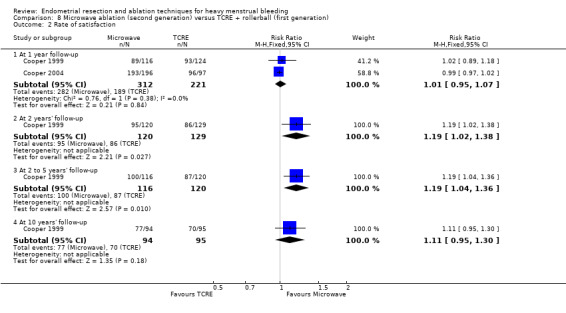
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
Operative outcomes
8.3 Duration of surgery
In one study, the duration of the procedure was significantly shorter with microwave than with TCRE (MD 3.6, 95% CI ‐5.7 to ‐1.4; P = 0.001). See Analysis 8.3.
8.3. Analysis.
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 3 Duration of operation (minutes).
| Duration of operation (minutes) | |||
|---|---|---|---|
| Study | Microwave | TCRE | Results |
| Cooper 1999 | N=129 Mean duration of procedure (SD): 11.4 (10.5) mins | N=134 Mean duration of procedure (SD): 15.0 (7.2) mins | Mann Whitney U test Mean difference: 3.6 (‐5.7, ‐1.4); P=0.001 |
8.4 Surgery difficulties
In one study, risk of equipment failure was higher in the microwave group than in the TCRE group (RR 3.81, 95% CI 1.09 to 13.34; 263 women; 1 study), and results did not show clear evidence of differences between groups in abandoning the procedure (RR 1.04, 95% CI 0.31 to 3.50; 263 women; 1 study). See Analysis 8.4.
8.4. Analysis.
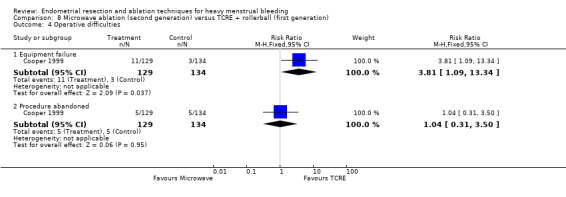
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 4 Operative difficulties.
8.5 Proportion given general versus local anaesthesia
Participants undergoing microwave ablation were more likely to receive local anaesthesia than those undergoing TCRE (RR 2.54, 95% CI 1.73 to 3.72; 315 women; 1 study). See Analysis 8.5.
8.5. Analysis.
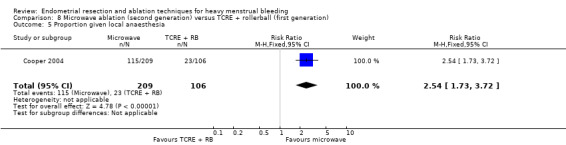
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 5 Proportion given local anaesthesia.
8.6 Duration of hospital stay
We found no clear evidence of a difference between groups in terms of hours spent in the hospital (no differences; P = 0.17). See Analysis 8.6.
8.6. Analysis.
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 6 Duration of hospital stay (hours).
| Duration of hospital stay (hours) | |||
|---|---|---|---|
| Study | Microwave | TCRE | Results |
| Cooper 1999 | N=129 Mean duration of hospital stay (SD): 13.4 (17.6) hours | N=134 Mean duration of hospital stay (SD): 16.7 (21.2) hours | Mann Whitney U test No differences between groups; P=0.17 |
8.7 Inability to work
Researchers provided no clear evidence of differences between groups in the proportion of women with inability to work at 12 months' (RR 0.53, 95% CI 0.17 to 1.73; 240 women; 1 study) and 5 years' follow‐up (RR 1.52, 95% CI 0.26 to 8.87; 189 women; 1 study). See Analysis 8.7.
8.7. Analysis.
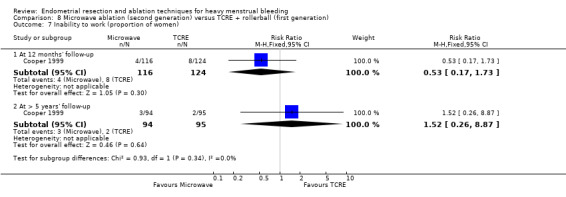
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 7 Inability to work (proportion of women).
8.8 Quality of life
We found no clear evidence of differences between groups on Short Form‐36 (SF‐36) after treatment at 12 months, and at 2, 5, and 10 years. See Analysis 8.8.
8.8. Analysis.
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 8 Quality of life ‐ change in SF‐36 score after treatment.
| Quality of life ‐ change in SF‐36 score after treatment | |||
|---|---|---|---|
| Study | MEA | TCRE | Results |
| Physical functioning | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD):
0.7 (18.9)
AT 2 YEARS:
N=120
Mean change (SD):
2.3 (21.3)
AT 5 YEARS:
N=116
Mean change (SD): 0.2 (24) At 10 YEARS: N=94 Mean change (SD): ‐4.4 (27) |
AT 1 YEAR:
N=124
Mean change (SD):
2.4 (16.8)
AT 2 YEARS:
N=129
Mean change (SD):
0.9 (20.4)
AT 5 YEARS:
N=120
Mean change (SD): ‐1.2 (21) At 10 YEARS: N=95 Mean change (SD): ‐3.0 (25) |
AT 1 YEAR:
t test:
CI (‐6.4, 2.9); P=0.45
Ancova: P=0.58
AT 2 YEARS:
t test:
P=0.28 (95% CI ‐3.8, 6.6)
AT 5 YEARS:
t test:
NS (95% CI ‐4.5 to 7.3) At 10 YEARS: t test: NS (95% CI ‐8.9 to 6.1) |
| Social functioning | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 20.6 (26.5)
AT 2 YEARS:
N=120
Mean change (SD): 10.1 (27.5)
AT 5 YEARS:
N=116
Mean change (SD): 7.7 (30) At 10 YEARS: N=94 Mean change (SD): 10.1 (30) |
At 1 YEAR: N=124 Mean change (SD): 16.2 (24.4) AT 2 YEARS: N=129 Mean change (SD): 6.2 (23.7) AT 5 YEARS: Mean change (SD): 9.7 (25) At 10 YEARS: N=95 Mean change (SD): 9.9 (26) |
AT 1 YEAR:
t test:
CI (‐2.1, 10.90): P=0.18
Ancova:
P=0.12
AT 2 YEARS:
t test:
P=0.33 (95% CI ‐2.5, 10.3)
AT 5 YEARS:
t test:
NS (95% CI ‐9.0 to 5.0) At 10 YEARS: t test: NS (95% CI ‐7.9 to 8.3) |
| Physical role | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 23.9 (49.4)
AT 2 YEARS:
N=120
Mean change (SD): 18.5 (53.7)
AT 5 YEARS:
N=116
Mean change (SD): 17 (54) At 10 YEARS: N=94 Mean change (SD): 15.0 (53) |
AT 1 YEAR:
N=124
Mean change (SD): 11.3 (41.7)
AT 2 YEARS:
N=129
Mean change (SD): 6.1 (43.8)
AT 5 YEARS:
N=120
Mean change (SD): 11 (43) At 10 YEARS: N=95 Mean change (SD): 10.9 (47) |
AT 1 YEAR:
t test:
CI (1.0 to 24.3);
P=0.03
Ancova:
P=0.03
AT 2 YEARS:
t test:
P=0.06 (95% CI ‐0.2, 24.6)
AT 5 YEARS:
t test:
NS, 95% CI ‐5.8 to 19 At 10 YEARS: t test: NS, 95% CI ‐10.3 to 18.5 |
| Emotional role | |||
| Cooper 1999 | AT ONE YEAR:
N=116
Mean change (SD): 17.0 (48.5)
AT 2 YEARS:
N=120
Mean change (SD): 17.8 (47.5)
AT 5 YEARS:
N=116
Mean change (SD): 19 (48) At 10 YEARS: N=94 Mean change (SD): 21.1 (50) |
AT 1 YEAR:
N=124
Mean change (SD): 13.7 (47.9)
AT 2 YEARS:
N=129
Mean change (SD): 4.2 (40.1)
AT 5 YEARS: N=120 Mean change (SD): 20 (41) At 10 YEARS: N=95 Mean change (SD): 13.5 (47) |
AT 1 YEAR:
t test:
CI (‐9.1 to 15.6);
P=0.59
Ancova:
P=0.38
AT 2 YEARS:
t test
P=0.17 (95% CI ‐3.6, 23.5)
AT 5 YEARS:
t test:
NS, 95% CI ‐13 to 10 At 10 YEARS: t test: NS, 95% CI 6.3 to 21.5 |
| Mental health | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 6.3 (19.5)
AT 2 YEARS:
N=120
Mean change (SD): 6.0 (21.6)
AT 5 YEARS:
N=116
Mean change (SD): 1.4 (21) At 10 YEARS: N=94 Mean change (SD): 7.2 (21) |
AT 1 YEAR:
N=124
Mean change (SD): 6.0 (22.2)
AT 2 YEARS:
N=129
Mean change (SD): 4.1 (19.8)
AT 5 YEARS N=120 Mean change (SD): 1.2 (21) At 10 YEARS: N=95 Mean change (SD): 7.9 (25) |
AT 1 YEAR:
t test:
CI (‐4.9 to 5.7);
P=0.89
Ancova:
P=0.83
AT 2 YEARS:
t test:
P=0.44 (95% CI ‐3.3, 6.9)
AT 5 YEARS:
t test:
NS, 95% CI ‐5.2 to 5.6 At 10 YEARS: t test: NS, 95% CI ‐7.3 to 5.9 |
| Energy/fatigue | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 12.8 (21.7)
AT 2 YEARS:
N=120
Mean change (SD): 11.4 (25.1)
AT 5 YEARS: N=116 Mean change (SD): 9.3 (25) At 10 YEARS: N=94 Mean change (SD): 12.9 (29) |
AT 1 YEAR:
N=124
Mean change (SD): 12.1 (23.0)
AT 2 YEARS:
N=129
Mean change (SD): 11.8 (22.6)
AT 5 YEARS: N=120 Mean change (SD): 12 (26) At 10 YEARS: N=95 Mean change (SD): 15.3 (27) |
AT 1 YEAR:
t test:
CI (‐4.9 to 6.5);
p=0.80
Ancova:
p=0.58
AT 2 YEARS:
t test:
P=0.90 (95% CI ‐6.4, 5.5)
AT 5 YEARS:
t test:
NS, 95% CI ‐9.1 to 4.2 At 10 YEARS: t test: NS, 95% CI ‐10.4 to 5.6 |
| Pain | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 14.8 (31.0)
AT 2 YEARS:
N=120
Mean change (SD): 13.5 (31.7)
AT 5 YEARS: N=116 Mean change (SD): 9.3 (35) At 10 YEARS: N=94 Mean change (SD): 11.6 (37) |
AT 1 YEAR:
N=124
Mean change (SD): 7.2 (31.1)
AT 2 YEARS:
N=129
Mean change (SD): 3.0 (29.8)
AT 5 YEARS: N=120 Mean change (SD): 6.4 (31) At 10 YEARS: N=95 Mean change (SD): 12.3 (35) |
AT 1 YEAR:
t test:
CI (‐0.2 to 15.5);
P=0.06
Ancova:
P=0.54
AT 2 YEARS:
t test:
P=0.02 (95% CI 2.9, 18.2)
AT 5 YEARS:
t test:
NS, 95% CI ‐5.7 to 12 At 10 YEARS: t test: NS, 95% CI ‐11.0 to 9.6 |
| General health | |||
| Cooper 1999 | AT 1 YEAR:
N=116
Mean change (SD): 2.4 (20.3)
AT 2 YEARS:
N=120
Mean change (SD): 0.0 (24.4)
AT 5 YEARS: N=116 Mean change (SD): ‐3.3 (26) At 10 YEARS: N=94 Mean change (SD): 0.94 (23) |
AT 1 YEAR:
N=124
Mean change (SD): ‐2.9 (20.0)
AT 2 YEARS:
N=129
Mean change (SD): ‐2.9 (19.0)
AT 5 YEARS: N=120 Mean change (SD): ‐2.4 (19) At 10 YEARS: N=95 Mean change (SD): 2.8 (22) |
AT 1 YEAR:
t test:
CI (0.2 to 10.5);
P=0.04
Ancova:
P=0.06
AT 2 YEARS:
t test:
P=0.29 (95% CI ‐2.5, 8.4)
AT 5 YEARS:
t test:
NS, 95% CI ‐6.5 to 4.9 At 10 YEARS: t test: NS, 95% CI ‐8.3 to 4.6 |
8.9 Improvement in other menstrual symptoms: PMS
We found no clear evidence of differences in PMS improvement between groups at 1 year follow‐up (RR 0.98, 95% CI 0.89 to 1.09; 533 women; 2 studies; I² = 0%) or at 2 years' follow‐up (RR 1.05, 95% CI 0.93 to 1.19; 249 women; 1 study). See Analysis 8.9.
8.9. Analysis.
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 9 Improvement in other menstrual symptoms: PMS.
8.10 and 8.11 Improvement in other menstrual symptoms: dysmenorrhoea
Trial results showed no clear evidence of differences in improvement in the rate of dysmenorrhoea between groups at 1 year follow‐up (RR 0.98, 95% CI 0.89 to 1.09; 533 women; 2 studies; I² = 0%) nor at 2 years' follow‐up (RR 1.05, 95% CI 0.93 to 1.19; 249 women; 1 study). See Analysis 8.10. They also provided no clear evidence of differences in reduction in pain score at 5 years' follow‐up (MD ‐0.80, 95% CI ‐4.32 to 2.72; 189 women; 1 study). See Analysis 8.11.
8.10. Analysis.
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 10 Improvement in other menstrual symptoms.
8.11. Analysis.
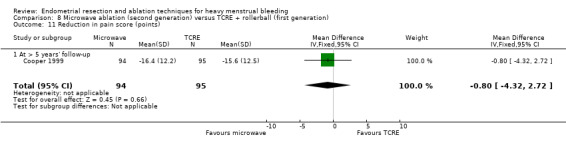
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 11 Reduction in pain score (points).
8.12 Postoperative analgesia rate
Researchers provided no clear evidence of differences between groups (RR 0.94, 95% CI 0.81 to 1.10; 263 women; 1 study). See Analysis 8.12.
8.12. Analysis.
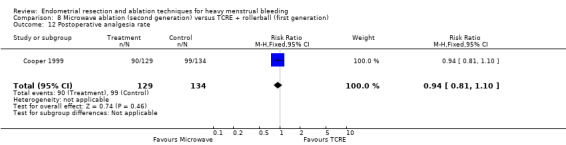
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 12 Postoperative analgesia rate.
8.13 Complication rate: major complications
We found no clear evidence of differences between groups in the incidence of major complications such as haemorrhage (RR 0.09, 95% CI 0.01 to 1.69; 263 women; 1 study). See Analysis 8.13.
8.13. Analysis.
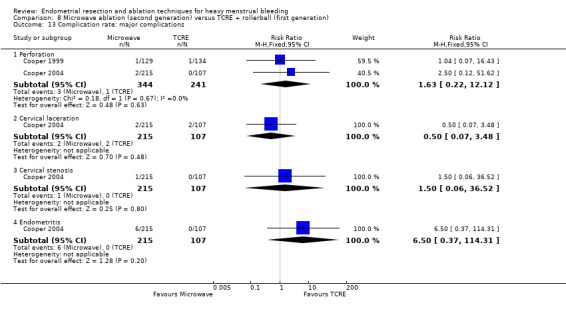
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 13 Complication rate: major complications.
Perforation (RR 1.63, 95% CI 0.22 to 12.12; 585 women; 2 studies; I² = 0%).
Cervical laceration (RR 0.50, 95% CI 0.07 to 3.48; 322 women; 1 study).
Cervical stenosis (RR 1.50, 95% CI 0.06 to 36.52; 322 women; 1 study).
Endometritis (RR 6.50, 95% CI 0.37 to 114.31; 322 women; 1 study).
8.14 Complication rate: minor complications
We found no clear evidence of differences between groups in the incidence of minor complications such as the following (see Analysis 8.14).
8.14. Analysis.
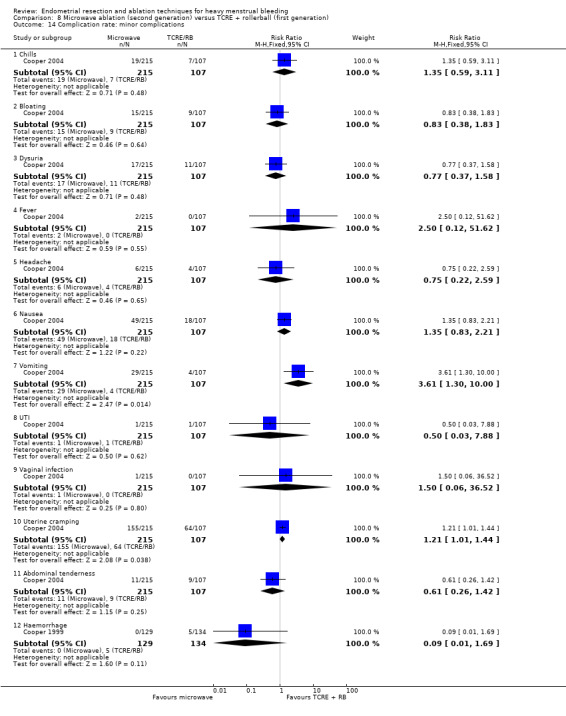
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 14 Complication rate: minor complications.
Chills (RR 1.35, 95% CI 0.59 to 3.11; 322 women; 1 study).
Bloating (RR 0.83, 95% CI 0.38 to 1.83; 322 women; 1 study).
Dysuria (RR 0.77, 95% CI 0.37 to 1.58; 322 women; 1 study).
Fever (RR 2.50, 95% CI 0.12 to 51.62; 322 women; 1 study).
Headache (RR 0.75, 95% CI 0.22 to 2.59; 322 women; 1 study).
Nausea (RR 1.35, 95% CI 0.83 to 2.21; 322 women; 1 study).
Vomiting (RR 3.61, 95% CI 1.30 to 10.00; 322 women; 1 study).
Urinary tract infection (RR 0.50, 95% CI 0.03 to 7.88; 322 women; 1 study).
Vaginal infection (RR 1.50, 95% CI 0.06 to 36.52; 322 women; 1 study).
Uterine cramping (RR 1.21, 95% CI 1.01 to 1.44; 322 women; 1 study).
Abdominal tenderness (RR 0.61, 95% CI 0.26 to 1.42; 322 women; 1 study).
8.15 Requirement for further surgery
At 10 years' follow‐up, risk of hysterectomy was reduced with microwave ablation compared with TCRE plus rollerball (RR 0.60, 95% CI 0.38 to 0.96; n = 263; 1 study). See Analysis 8.15. Caution is advised when these results are interpreted, as evidence is based on a single study that reported loss to follow‐up greater than 25%.
8.15. Analysis.
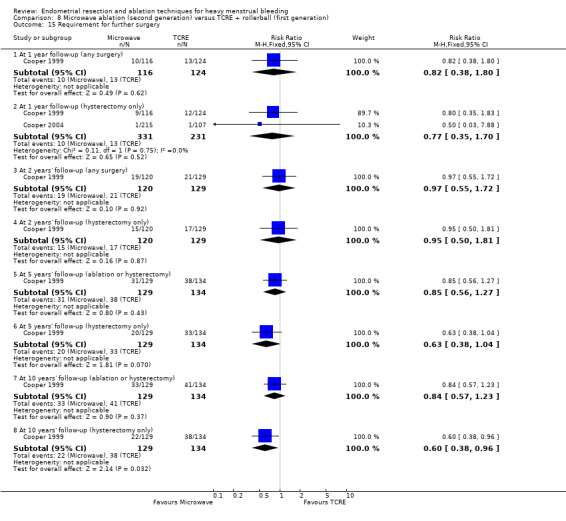
Comparison 8 Microwave ablation (second generation) versus TCRE + rollerball (first generation), Outcome 15 Requirement for further surgery.
Investigators reported no data for operative difficulties nor for mortality as a direct result of surgery.
9. Balloon versus rollerball (Comparison 9)
Three studies with a total of 414 women compared balloon versus rollerball (Meyer 1998; Romer 1998; van Zon‐Rabelink 2003).
Primary outcomes
9.1 to 9.4 Bleeding
9.1 Amenorrhoea was less likely after balloon ablation than after rollerball ablation at 1 year follow‐up (RR 0.62, 95% CI 0.39 to 1.00; 259 women; 2 studies; I² = 41%), but results showed no significant differences between groups at 2 years (RR 0.60, 95% CI 0.33 to 1.07; 227 women; 1 study) and up to 5 years (RR 0.70, 95% CI 0.39 to 1.25; 122 women; 1 study) after treatment, although a strong trend favoured rollerball ablation. See Analysis 9.1. No evidence showed significant differences between groups for rate of amenorrhoea/eumenorrhoea at 1 year follow‐up (RR 0.95, 95% CI 0.86 to 1.06; 259 women; 2 studies; I² = 0%), at 2 years' follow‐up (RR 0.99, 95% CI 0.91 to 1.08; 227 women; 1 study), or at 2 to 5 years' follow‐up (RR 0.98, 95% CI 0.91 to 1.06; 122 women; 1 study). See Analysis 9.1.
9.1. Analysis.
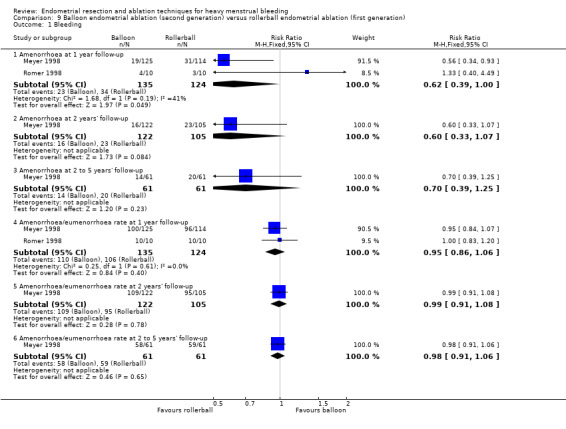
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 1 Bleeding.
9.2 Results showed no clear differences in PBAC score at 1 year follow‐up, but one study (van Zon‐Rabelink 2003) found a significantly lower PBAC at 2 years' follow‐up in women treated with balloon (median 33.5, SD 0.905; vs median 73, SD 0.585; P = 0.01; 111 women). See Analysis 9.2.
9.2. Analysis.
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 2 PBAC score after treatment.
| PBAC score after treatment | |||
|---|---|---|---|
| Study | Balloon | Rollerball | Results |
| At 1 year follow‐up | |||
| Meyer 1998 | N=125 Mean PBAC (SD): 52.2 (85.2) | N=114 Mean PBAC (SD): 39.6 (86.4) | No statistical test performed of these outcomes |
| van Zon‐Rabelink 2003 | N=74 Median PBAC (range): 70 (0, 2265) | N=55 Median PBAC (range): 73 (0, 535) | Wilcoxon test: P=0.90 |
| At 2 years' follow‐up | |||
| van Zon‐Rabelink 2003 | N=66 Median PBAC (range): 33.5 (0, 905) | N=55 Median PBAC (range): 73 (0, 585) | Wilcoxon test: P=0.01 |
9.3 Results showed no clear differences between groups in terms of success of treatment measured as lighter periods and no need for further surgery at 2 to 5 years' follow‐up (RR 0.98, 95% CI 0.80 to 1.20; 170 women; 1 study). See Analysis 9.3.
9.3. Analysis.
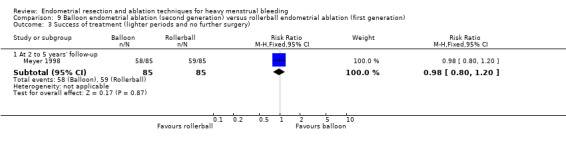
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 3 Success of treatment (lighter periods and no further surgery).
9.4 Researchers reported no clear differences between groups in terms of success of treatment measured as menstrual score < 185 at 1 year follow‐up (RR 1.00, 95% CI 0.83 to 1.20; 129 women; 1 study) nor at 2 years' follow‐up (RR 1.01, 95% CI 0.83 to 1.23; 121 women; 1 study).
9.5 Rate of satisfaction
No evidence showed clear differences between groups in terms of satisfaction with treatment at 1 year follow‐up (RR 0.97, 95% CI 0.93 to 1.01; 259 women; 2 studies; I² = 0%), at 2 years' follow‐up (RR 1.02, 95% CI 0.93 to 1.12; 348 women; 2 studies; I² = 0%), or at 2 to 5 years' follow‐up (RR 0.93, 95% CI 0.87 to 1.01; 122 women; 1 study). See Analysis 9.5.
9.5. Analysis.
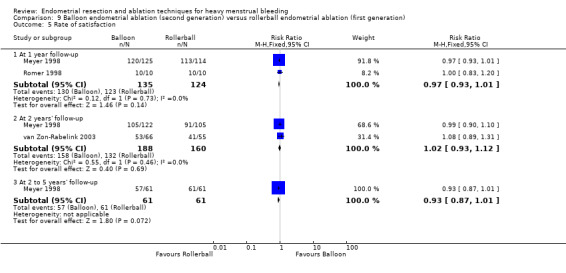
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 5 Rate of satisfaction.
Secondary outcomes
Operative outcomes
9.6 Duration of surgery
The mean difference between duration of surgery for women in the balloon group and those in the rollerball group was 15 minutes (MD ‐14.58, 95% CI ‐17.00 to ‐12.17; participants = 378; 2 studies; I² = 74%). See Analysis 9.6.
9.6. Analysis.
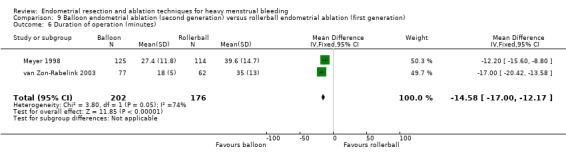
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 6 Duration of operation (minutes).
9.7 Operative difficulties
We found no evidence of significant differences between groups in terms of technical complication rates (RR 1.05, 95% CI 0.49 to 2.22; 139 women; 1 study). See Analysis 9.7.
9.7. Analysis.
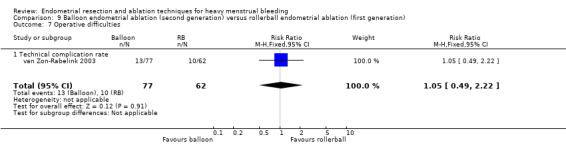
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 7 Operative difficulties.
9.8 Inability to work
Trials did not present clear evidence of differences between groups for ability to work at 1 year follow‐up (RR 1.52, 95% CI 0.37 to 6.22; 239 women; 1 study), at 2 years' follow‐up (RR 0.29, 95% CI 0.03 to 2.72; 227 women; 1 study), or at 2 to 5 years' follow‐up (RR 0.87, 95% CI 0.26 to 2.93; 210 women; 1 study). See Analysis 9.8.
9.8. Analysis.
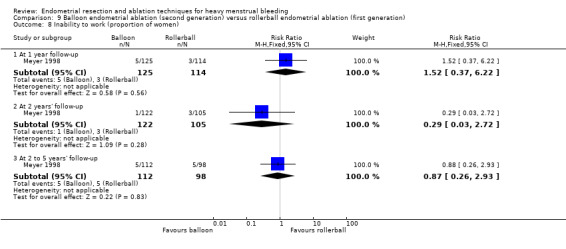
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 8 Inability to work (proportion of women).
9.9 Improvement in other menstrual symptoms
Trials did not present clear evidence of differences between groups in terms of improvement in other menstrual symptoms for dysmenorrhoea at 12 months (RR 0.93, 95% CI 0.80 to 1.09; 239 women; 1 study) and in premenstrual symptoms from moderate to severe at 1 year (RR 0.94, 95% CI 0.74 to 1.19; 185 women; 1 study), at 2 years' (RR 1.03, 95% CI 0.82 to 1.29; 177 women; 1 study), and at 2 to 5 years' follow‐up (RR 0.99, 95% CI 0.75 to 1.30; 166 women; 1 study). See Analysis 9.9.
9.9. Analysis.
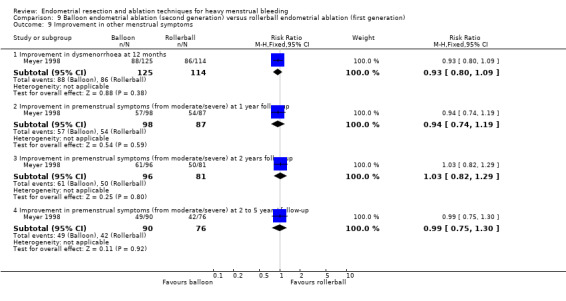
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 9 Improvement in other menstrual symptoms.
9.10 Complication rate: major complications
We found no clear evidence of differences between groups in major complications such as the following (see Analysis 9.10).
9.10. Analysis.
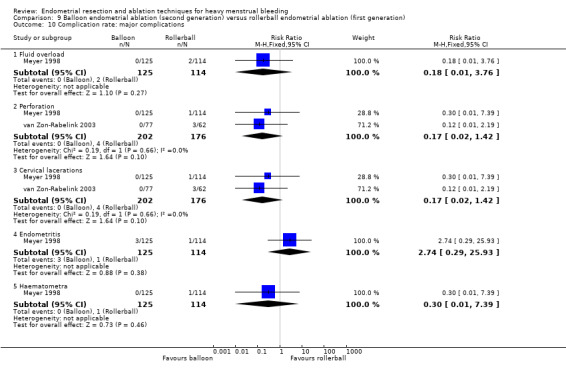
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 10 Complication rate: major complications.
Fluid overload (RR 0.18, 95% CI 0.01 to 3.76; 239 women; 1 study).
Perforation (RR 0.17, 95% CI 0.02 to 1.42; 378 women; 2 studies; I² = 0%).
Cervical lacerations (RR 0.17, 95% CI 0.02 to 1.42; 378 women; 2 studies; I² = 0%).
Endometritis (RR 2.74, 95% CI 0.29 to 25.93; 239 women; 1 study).
Haematometra (RR 0.30, 95% CI 0.01 to 7.39; 239 women; 1 study).
9.11 Complication rate: minor complications
We found no clear evidence of differences between groups in minor complications such as the following (see Analysis 9.11).
9.11. Analysis.
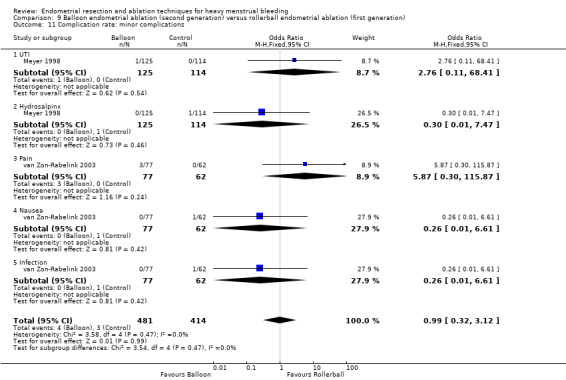
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 11 Complication rate: minor complications.
Urinary tract infection (RR 2.74, 95% CI 0.11 to 66.54; 239 women; 1 study).
Hydrosalpinx (RR 0.30, 95% CI 0.01 to 7.39; 239 women; 1 study).
Pain (RR 5.65, 95% CI 0.30 to 107.43; 139 women; 1 study).
Nausea (RR 0.27, 95% CI 0.01 to 6.50; 139 women; 1 study).
Infection (RR 0.27, 95% CI 0.01 to 6.50; 139 women; 1 study).
9.12 Requirement for further surgery
Trials provided no evidence of clear differences between groups in the requirement for further surgery including the following (see Analysis 9.12).
9.12. Analysis.
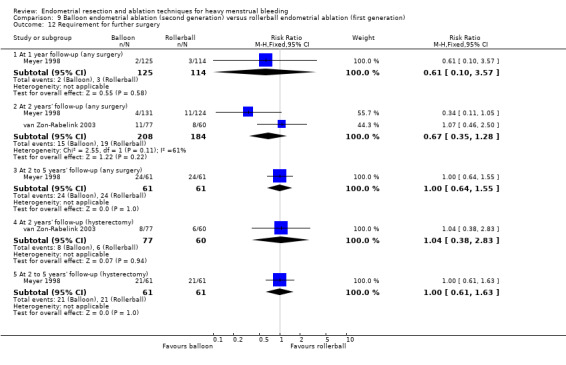
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 12 Requirement for further surgery.
Any surgery at 1 year follow‐up (RR 0.61, 95% CI 0.10 to 3.57; 239 women; 1 study), at 2 years' follow‐up (RR 0.67, 95% CI 0.35 to 1.28; 392 women; 2 studies; I² = 61%), and at 2 to 5 years' follow‐up (RR 1.00, 95% CI 0.64 to 1.55; 122 women; 1 study). .
Hysterectomy at 2 years' follow‐up (RR 1.04, 95% CI 0.38 to 2.83; 137 women; 1 study) or at 2 to 5 years' follow‐up (RR 1.00, 95% CI 0.61 to 1.63; 122 women; 1 study).
Researchers provided no data for the proportion having general versus local anaesthesia, time or ability to return to normal activities or work, or mortality as a direct result of surgery.
10. Balloon versus laser (Comparison 10)
One study with 70 women compared balloon versus laser (Hawe 2003).
Primary outcomes
10.1 and 10.2 Bleeding
Researchers measured bleeding as rate of amenorrhoea and PBAC score after treatment. Evidence showed no clear differences between groups, including the following (see Analysis 10.1 and Analysis 10.2).
10.1. Analysis.
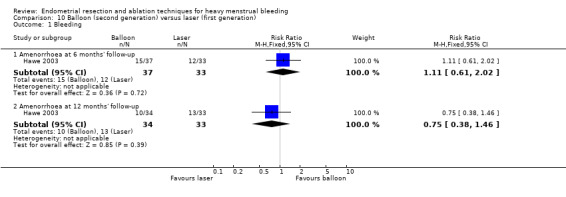
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 1 Bleeding.
10.2. Analysis.
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 2 PBAC score after treatment.
| PBAC score after treatment | |||
|---|---|---|---|
| Study | Balloon | Laser | Statistical test |
| At 6 months' follow‐up | |||
| Hawe 2003 | N=37 Mean PBAC (SD): 28.8 (59.6) | N=33 Mean PBAC (SD): 27.4 (57.6) | Significance not reported |
Amenorrhoea at 6 months' follow‐up (RR 1.11, 95% CI 0.61 to 2.02; 70 women; 1 study).
Amenorrhoea at 12 months' follow‐up (RR 0.75, 95% CI 0.38 to 1.46; 67 women; 1 study).
PBAC score at 6 months' follow‐up (mean (SD), 28.8 (59.6)/27.4 (57.6)); study authors did not report significance.
10.3 Rate of satisfaction
Trials provided no clear evidence of differences between groups in rate of satisfaction with treatment at 6 months' (RR 1.04, 95% CI 0.91 to 1.20; 69 women; 1 study) and at 12 months' follow‐up (RR 0.97, 95% CI 0.86 to 1.09; 57 women; 1 study). See Analysis 10.3.
10.3. Analysis.
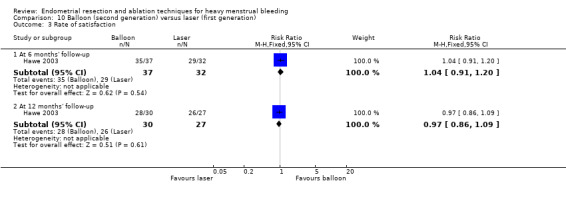
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 3 Rate of satisfaction.
Secondary outcomes
Operative outcomes
10.4 Operative difficulties
No evidence showed clear differences between groups in the rate of equipment failure (RR 4.47, 95% CI 0.22 to 89.94; 70 women; 1 study). See Analysis 10.4.
10.4. Analysis.
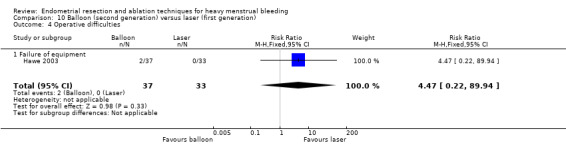
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 4 Operative difficulties.
10.5 Post‐procedure pain
Participants completed a visual analogue scale (VAS) 4 hours postoperatively and indicated that the laser was significantly less painful than the balloon (mean (SD), 63.6 (17.6) vs 30.9 (20.4); MD 32.7, 95% CI 14.0 to 51.4; P = 0.002). See Analysis 10.5.
10.5. Analysis.
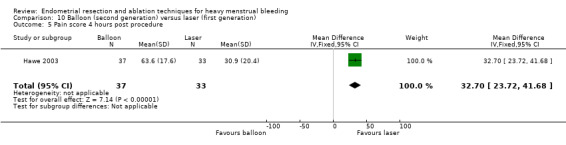
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 5 Pain score 4 hours post procedure.
10.5 Quality of life
Women receiving balloon treatment had a significantly greater pain score than women receiving laser treatment (MD 32.7, 95% CI 23.7 to 41.7; 1 study). At 12 months after treatment, women in the balloon group had higher scores on the EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D) VAS than women in the laser group (MD 10.1, 95% CI 2.4 to 17.8; 1 study); this was not found at earlier follow‐up nor for other quality of life scores. See Analysis 10.6.
10.6. Analysis.
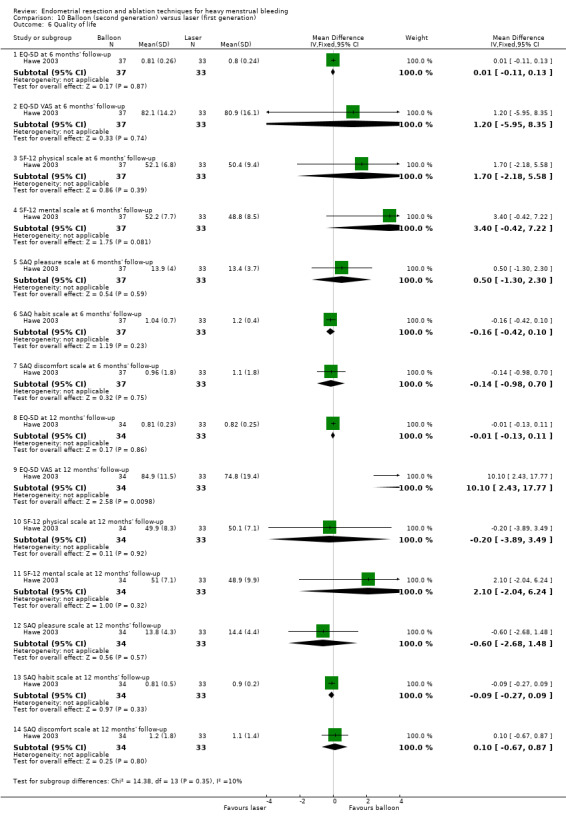
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 6 Quality of life.
10.7 Improvement in other menstrual symptoms: PMS
In one study with 70 women, researchers reported on improvement in PMS for the balloon group versus the laser group at 6 months (mean (SD), 24.6 (33) for balloon and 30.5 (36) for laser) and at 12 months (mean (SD), 21.9 (26.9) for balloon and 30.5 (34.7) for laser) but did not report on the significance of differences between groups in improvement in PMS. See Analysis 10.7.
10.7. Analysis.
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 7 Improvement in other menstrual symptoms.
| Improvement in other menstrual symptoms | |||
|---|---|---|---|
| Study | Balloon | Laser | Statistical test |
| PMS at 6 months' follow‐up | |||
| Hawe 2003 | N=37 Mean score (SD): 24.6 (33) | N=33 Mean score (SD): 34.8 (36) | Not reported |
| PMS at 12 months' follow‐up | |||
| Hawe 2003 | N=34 Mean score (SD): 21.9 (26.9) | N=33 Mean score (SD): 30.5 (34.7) | Not reported |
10.8 Improvement in other menstrual symptoms: dysmenorrhoea
One study with 70 women reported on improvement in dysmenorrhoea for the balloon versus the laser at 6 months (mean (SD), 24 (30.9) for the balloon vs 23 (33.9) for the laser) and at 12 months (mean (SD), 25.2 (31.5) for the balloon and 16.5 (22.3) for the laser) but did not report on the significance of differences between groups in improvement of dysmenorrhoea. See Analysis 10.8.
10.8. Analysis.
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 8 Improvement in other menstrual symptoms: dysmenorrhoea (visual analogue).
| Improvement in other menstrual symptoms: dysmenorrhoea (visual analogue) | |||
|---|---|---|---|
| Study | Balloon | Laser | Statistical test |
| Dysmenorrhoea at 6 months' follow‐up | |||
| Hawe 2003 | N=37 Mean score (SD): 24 (30.9) | N=33 Mean score (SD): 23 (33.9) | Not reported |
| Dysmenorrhoea at 12 months' follow‐up | |||
| Hawe 2003 | N=34 Mean score (SD): 25.2 (31.5) | N=33 Mean score (SD): 16.5 (22.3) | Not reported |
10.9 Requirement for further surgery
One study with 67 women found no clear differences between groups in the requirement for further surgery up to 12 months' follow‐up (RR 0.78, 95% CI 0.23 to 2.64). See Analysis 10.9.
10.9. Analysis.
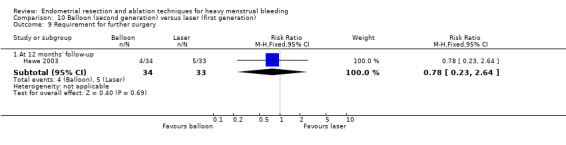
Comparison 10 Balloon (second generation) versus laser (first generation), Outcome 9 Requirement for further surgery.
Study authors provided no data for duration of surgery, proportion given general versus local anaesthesia, length of hospital stay, time or ability to return to normal activities or work, complication rates, or mortality as a direct result of surgery.
11. Balloon versus TCRE (Comparison 11)
Two studies with a total of 133 women compared balloon and TCRE (Brun 2006; Pellicano 2002).
Primary outcomes
11.1 Bleeding
We found no evidence of a clear difference between groups in rates of amenorrhoea at 6 months' (RR 0.95, 95% CI 0.31 to 2.93; 49 women; 1 study) and 12 months' follow‐up after surgery (RR 1.21, 95% CI 0.50 to 2.95; 45 women; 1 study). See Analysis 11.1.
11.1. Analysis.
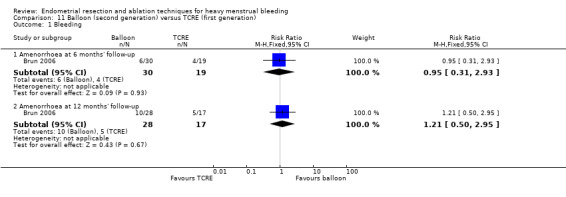
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 1 Bleeding.
11.2 Rate of satisfaction
Satisfaction with treatment was greater in the balloon group than in the TCRE group 2 years after surgery (RR 1.4, 95% CI 1.1 to 1.7; 69 women; 1 study), but this difference was not evident at 6 months (RR 1.06, 95% CI 0.93 to 1.20; 50 women; 1 study) or 1 year after surgery (RR 1.06, 95% CI 0.96 to 1.18; 122 women; 2 studies; I² = 0%). See Analysis 11.2.
11.2. Analysis.
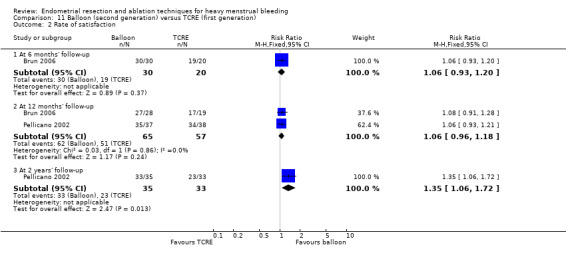
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
Operative outcomes
11.3 and 11.4 Duration of surgery
One trial with 82 women found that surgical time was significantly shorter (35%) with balloon than with TCRE treatment (MD ‐13.00, 95% CI ‐15.20 to ‐10.80) (see Analysis 11.3), but the second trial did not confirm this finding (mean (SD) 48 (24 to 150)/45 (23 to 105); no statistical test reported, but significant difference unlikely). See Analysis 11.4.
11.3. Analysis.
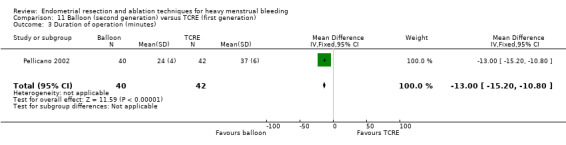
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 3 Duration of operation (minutes).
11.4. Analysis.
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 4 Duration of operation (minutes).
| Duration of operation (minutes) | |||
|---|---|---|---|
| Study | Cavaterm balloon | TCRE | Comments |
| Brun 2006 | n=31 Median (range): 48 (24‐150) |
n=20 Median (range): 45 (23‐105) |
No statistical test reported ‐ unlikely to be a difference |
11.5 Operative difficulties
Equipment failure was not clearly different between groups (RR 7.22, 95% CI 0.42 to 123.83; 51 women; 1 study). See Analysis 11.5.
11.5. Analysis.
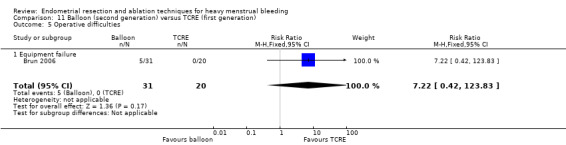
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 5 Operative difficulties.
11.6 and 11.7 Intraoperative complications
Mean intraoperative blood loss (measured in millilitres) was significantly less for balloon treatment than for laser treatment in one small trial ((MD ‐81.80, 95% CI ‐93.33 to ‐70.27; 82 women; 1 study). See Analysis 11.13. Study authors reported that fluid overload (RR 0.10, 95% CI 0.01 to 1.67; 82 women; 1 study), cervical tear (RR 0.35, 95% CI 0.01 to 8.34; 82 women; 1 study), and rate of conversion to hysterectomy (RR 0.24, 95% CI 0.01 to 4.84; 88 women; 1 study) did not show clear differences between groups. See Analysis 11.12.
11.13. Analysis.
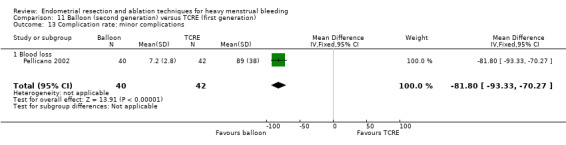
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 13 Complication rate: minor complications.
11.12. Analysis.
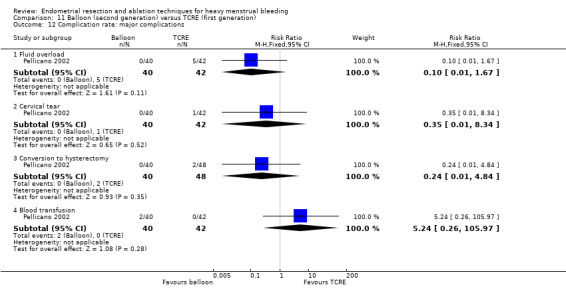
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 12 Complication rate: major complications.
11.9 and 11.10 Postoperative pain
Postoperative pain (as measured by a continuous VAS score) was significantly greater for women in the TCRE group than in the balloon group in both trials (MD ‐0.60, 95% CI ‐0.88 to ‐0.32; 82 women; 1 study; see Analysis 11.6; mean (SD), 45 (1 to 100)/10 (0 to 90); P = 0.012; see Analysis 11.7).
11.6. Analysis.
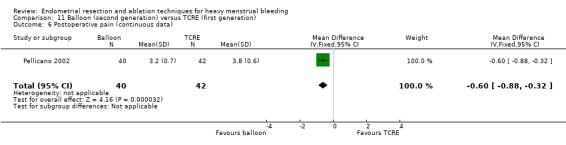
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 6 Postoperative pain (continuous data).
11.7. Analysis.
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 7 Postoperative pain (descriptive data).
| Postoperative pain (descriptive data) | |||
|---|---|---|---|
| Study | Cavaterm balloon | TCRE | Comments |
| Brun 2006 | n=31 Pain score (VAS scale 0‐100): median (range): 45 (1‐100) |
n=20 Pain score (VAS scale 0‐100): median (range): 10 (0‐90) |
Mann Whitney rank sum test: P=0.012 |
11.10 and 11.11 Recovery
Length of the stay in hospital was shorter in the balloon group than in the TCRE group (MD ‐0.30 days, 95% CI ‐0.52 to ‐0.08; 82 women; 1 study). See Analysis 11.8.
11.8. Analysis.
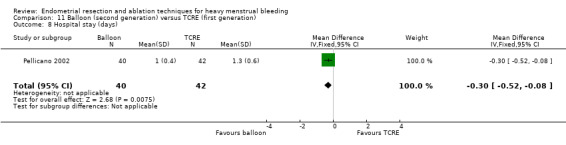
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 8 Hospital stay (days).
Time until return to normal activities was significantly shorter for the balloon group in one study (MD ‐2.10 days, 95% CI ‐3.38 to ‐0.82; 82 women; 1 study; see Analysis 11.10), but it was not clearly different in the second study (mean (SD) 4 days (1 to 20) and 2 days (1 to 30); 49 women;1 study; see Analysis 11.11).
11.10. Analysis.
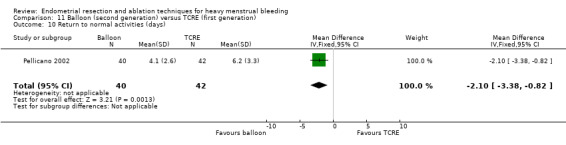
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 10 Return to normal activities (days).
11.11. Analysis.
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 11 Return to normal activities (days).
| Return to normal activities (days) | |||
|---|---|---|---|
| Study | Cavaterm balloon | TCRE | Comments |
| Brun 2006 | n=31 Median (range): 4 (1‐20) |
n=20 Median (range): 2 (1‐30) |
Mann Whitney rank test ‐ not significantly different |
11.12 Complication rate: major complications
Trial results showed no evidence of clear differences between groups for major complications such as the following (Analysis 11.12).
Fluid overload (RR 0.10, 95% CI 0.01 to 1.67; 82 women; 1 study).
Cervical tear (RR 0.35, 95% CI 0.01 to 8.34; 82 women; 1 study).
Conversion to hysterectomy (RR 0.24, 95% CI 0.01 to 4.84; 88 women; 1 study).
Blood transfusion (RR 5.24, 95% CI 0.26 to 105.97; 82 women; 1 study).
11.13 and 11.14 Complication rate: minor complications
Blood loss during the procedure was clearly less in the balloon group (MD ‐81.80 mL, 95% CI ‐93.33 to ‐70.27; 82 women; 1 study). See Analysis 11.13.
We found no evidence of clear differences between groups for other minor complications such as the following (see Analysis 11.14).
11.14. Analysis.
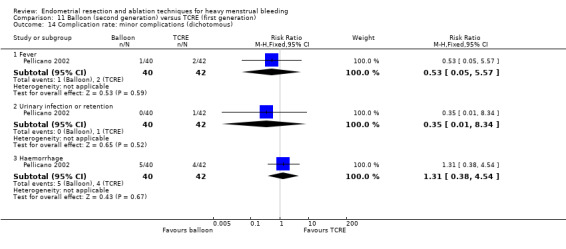
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 14 Complication rate: minor complications (dichotomous).
Fever (RR 0.53, 95% CI 0.05 to 5.57; 82 women; 1 study).
Urinary tract infection or retention (RR 0.35, 95% CI 0.01 to 8.34; 82 women; 1 study).
Haemorrhage (RR 1.31, 95% CI 0.38 to 4.54; 82 women; 1 study).
11.15 Requirement for further surgery
No evidence showed any differences between groups at 12 months' follow‐up after any surgery (RR 0.51, 95% CI 0.10 to 2.64; 75 women; 1 study) or after hysterectomy (RR 0.12, 95% CI 0.01 to 2.44; 45 women; 1 study), nor at 2 years' follow‐up, for any surgery (RR 0.38, 95% CI 0.08 to 1.81; 68 women; 1 study). See Analysis 11.15.
11.15. Analysis.
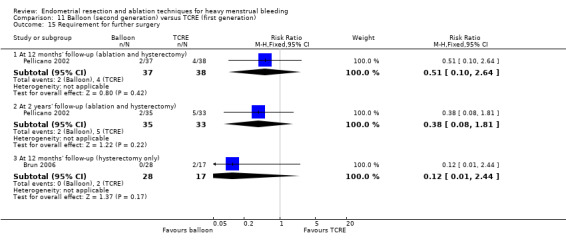
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 15 Requirement for further surgery.
Researchers provided no data for proportion given general versus local anaesthesia, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
Second‐generation ablation comparisons
12. Bipolar electrode ablation (second generation) versus balloon (second generation) (Comparison 12)
Four studies with a total of 366 women compared bipolar electrode ablation versus balloon (Abbott 2003; Bongers 2004; Clark 2011; Penninx 2016).
Primary outcomes
12.1 and 12.2 Bleeding
Amenorrhoea was more likely for women in the electrode ablation group than in the balloon group, both at 6 months (RR 3.37, 95% CI 2.09 to 5.44; 283 women; 3 studies; I² = 0%) and at 12 months after treatment (RR 3.12, 95% CI 2.06 to 4.72; 335 women; 4 studies; I² = 0%). Trial results showed no clear differences between groups at longer follow‐up: at 2 to 5 years' (RR 1.56, 95% CI 0.93 to 2.64;120 women; 1 study) nor at 10 years' follow‐up (RR 1.10, 95% CI 0.83 to 1.46; 104 women; 1 study). See Analysis 12.1.
In terms of PBAC, results differed between studies. One trial at 12 months' follow‐up did not find a difference between groups in PBAC scores after treatment (median (SD), 3(0.720)/21(0.157); 55 women; 1 study), but a second trial reported a clear difference favouring bipolar ablation in light of PBAC < 100 at 12 months' follow‐up (RR 0.4, 95% CI 0.2 to 0.8; 104 women; 1 study). See Analysis 12.2.
12.1. Analysis.
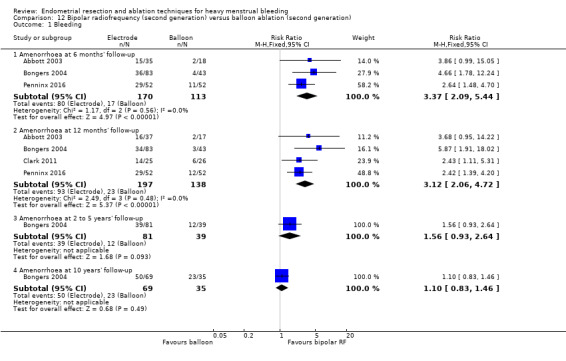
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 1 Bleeding.
12.2. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 2 PBAC score after treatment.
| PBAC score after treatment | |||
|---|---|---|---|
| Study | Electrode | Balloon | Statistical test |
| At 6 months' follow‐up | |||
| Penninx 2016 | |||
| At 12 months' follow‐up | |||
| Abbott 2003 | N=37 Median PBAC (range): 3 (0, 720) | N=18 Median PBAC (range): 21 (0, 157) | Mann Whitney P=0.2 |
| Penninx 2016 | N=52 PBAC<100 at 12 months: 44 |
N=52 PBAC<100 at 12 months: 31 |
RR=0.4 95% CI=0.2‐0.8 |
12.3 Rate of satisfaction
The rate of satisfaction was variable over time. We found no evidence of significant differences between groups in rates of satisfaction after treatment at 6 months' (RR 1.08, 95% CI 0.94 to 1.24; 181 women; 2 studies; I² = 90%) or at 10 years' follow‐up, but at 12 months' follow‐up, a clear difference favoured bipolar.ablation (RR 1.14, 95% CI 1.04 to 1.26; 334 women; 4 studies; I² = 0%).
Secondary outcomes
Operative outcomes
12.4 Duration of surgery
The duration of the procedure was 2 to 19 minutes shorter with bipolar ablation than with balloon ablation in four studies (two of which recorded significant differences). See Analysis 12.4.
12.4. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 4 Duration of operation.
| Duration of operation | |||
|---|---|---|---|
| Study | Electrode | Balloon | Statistical test |
| Abbott 2003 | N=37 Mean time in mins (range): 4 (2, 8) | N=18 Mean time in mins (range): 23 (19, 29) | t test P=0.0001 |
| Bongers 2004 | N=82 Mean time in mins (range): 9 (5, 32) | N=43 Mean time in mins (range): 14 (9, 40) | Not reported |
| Clark 2011 | N=42 Mean time in mins (SD): 5.7 (2.1) |
N=39 Mean time in mins (SD): 12.5 (2.3) |
MD=6.7 mins (95% CI 5.8 to 7.7); p<0.001 Note: this is an office procedure in both arms) |
| Penninx 2016 | N=52 Mean time in mins (range) 10.4 min (6‐30) |
N=52 Mean time in mins (range) 12.1 (5‐45) |
p=0.34 |
12.5 Operative difficulties
Only one trial reported these without significant differences (Abbott 2003).
12.6 Completion of the procedure
Only one trial reported on this without significant differences (Clark 2011).
12.7 Time taken off work and 12.8 Time to return to work
One trial reported on this but did not provide data in a format that could be entered into meta‐analysis (see Analysis 12.7 and Analysis 12.8) (Clark 2011).
12.7. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 7 Time taken off work (days).
| Time taken off work (days) | |||
|---|---|---|---|
| Study | Bipolar RF ablation | Thermal ablation | Results |
| Clark 2011 | N=42 Mean: 6.4 days |
N=39 Mean: 6.6 days |
No significant difference between groups: 0.2 days difference (95% CI ‐5.9 to 6.2) |
12.8. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 8 Time to resume normal activities (days).
| Time to resume normal activities (days) | |||
|---|---|---|---|
| Study | Bipolar RF ablation | Balloon ablation | Results |
| Clark 2011 | N=42 Mean (days): 4.9 |
N=39 Mean (days): 8.1 |
No significant difference between groups: 3.2 days difference (95% CI ‐1.6 to 8.1) |
12.9 Quality of life
Most quality of life scores revealed no significant differences between groups. However, women undergoing balloon ablation had significantly higher scores on the SF‐36 emotional role domain than those having bipolar ablation 5 years after treatment (MD ‐9.0 points, 95% CI ‐3.6 to ‐14.5), but not at other follow‐up times. See Analysis 12.9.
12.9. Analysis.
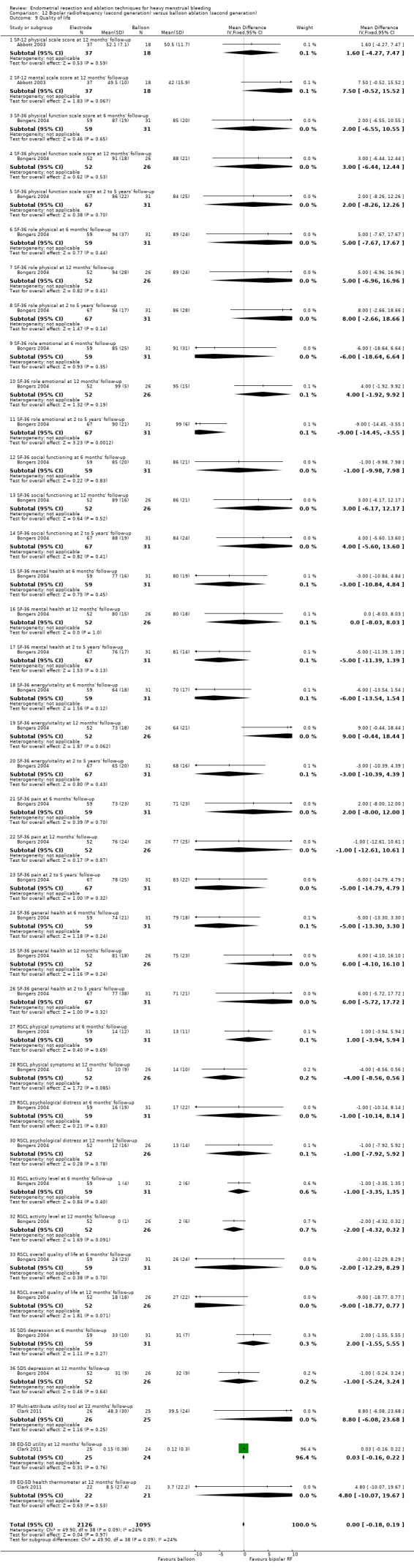
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 9 Quality of life.
12.10 Menorrhagia outcome questionnaire
Trial results showed no evidence of a difference between groups (MD ‐0.60, 95% CI ‐3.87 to 2.67; 51 women; 1 study).
12.11 Dysmenorhoea rate (VAS score)
One trial reported this but did not provide data in a format that could be entered into meta‐analysis (see Analysis 12.11) (Abbott 2003).
12.11. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 11 Dysmenorrhoea rate (VAS score).
| Dysmenorrhoea rate (VAS score) | |||
|---|---|---|---|
| Study | Electrode | Balloon | Statistical test |
| At 12 months' follow‐up | |||
| Abbott 2003 | N=37 Median score (range): 0 (0, 96) | N=18 Median score (range): 29 (0, 77) | Mann Whitney P=0.008 |
12.12 Improvement in other menstrual symptoms
Results were inconsistent for rates of dysmenorrhoea and PMS: two trials found no evidence of a difference in rates of dysmenorrhoea between groups, and one trial found no evidence of a difference in PMS symptoms. However, another trial found that bipolar ablation was associated with improved dysmenorrhoea and PMS symptoms (summary figures not provided).
12.14 Complication rate and 12.15 Requirement for further surgery
One trial showed no evidence of significant differences between groups for complications or requirement for further surgery up to 10 years' follow‐up.
13. Microwave ablation (MEA) (second generation) versus balloon ablation (second generation) (Comparison 13)
One trial with 320 women compared microwave ablation versus balloon ablation (Sambrook 2009).
Primary outcomes
13.1 Bleeding
Researchers reported bleeding as rates of amenorrhoea and PBAC scores. Microwave ablation was associated with higher rates of amenorrhoea than balloon ablation at 6 months' follow‐up (RR 1.50, 95% CI 1.07 to 2.12; 277 women; 1 study). Trial results showed no clear differences between groups at 12 months' follow‐up (RR 1.10, 95% CI 0.82 to 1.47; n = 282; trials = 1) nor at 5 years' follow‐up (RR 1.03, 95% CI 0.86 to 1.23; 217 women; 1 study). See Analysis 13.1. Study authors reported PBAC scores as means with an interquartile range. No clear difference between groups was evident. See Analysis 13.2.
13.1. Analysis.
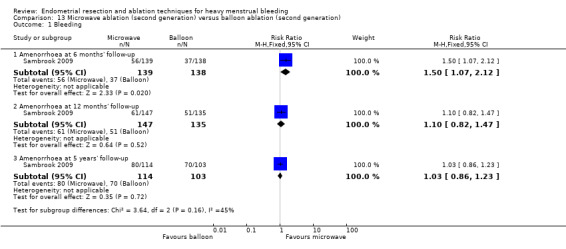
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 1 Bleeding.
13.2. Analysis.
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 2 PBAC score at 12 months' follow‐up.
| PBAC score at 12 months' follow‐up | ||||
|---|---|---|---|---|
| Study | Follow up | Microwave ablation | Balloon ablation | Results |
| Sambrook 2009 | 12 months | N=143 Mean PBAC score (interquartile range): 3.0 (0.0 to 14.0) |
N=135 Mean PBAC score (interquartile range): 4.0 (0.0 to 14.0) |
Incidence rate ratio (95% CI): 0.91 (0.6 to 1.5) |
13.3 Rate of satisfaction
Satisfaction rates were not clearly different between groups at 12 months' (RR 1.00, 95% CI 0.88 to 1.14; 278 women; 1 study) nor at 5 years' follow‐up (RR 0.99, 95% CI 0.87 to 1.13; 217 women; 1 study). See Analysis 13.3.
13.3. Analysis.
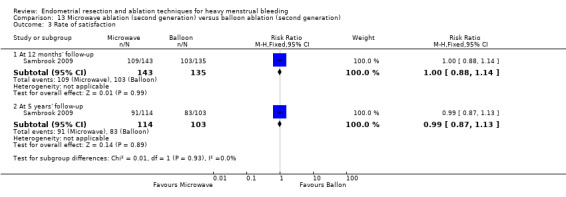
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 3 Rate of satisfaction.
Secondary outcome
13.4 Duration of surgery
Microwave ablation led to reduced operation time by almost 7 minutes compared to balloon ablation (MD ‐6.6 minutes, 95% CI ‐5.8 to ‐7.4; 314 women; 1 study). See Analysis 13.4.
13.4. Analysis.
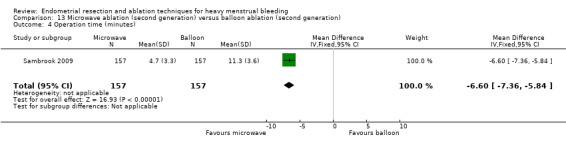
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 4 Operation time (minutes).
13.5 Surgery difficulties causing failure
The microwave device was less likely to fail than the balloon (RR 0.09, 95% CI 0.01 to 0.70; 314 women; 1 study). Researchers did not report clear differences between difficulties causing failure such as unsuitable cavity (RR 0.75, 95% CI 0.17 to 3.30; 314 women; 1 study) or use of a non‐sterile device (RR 5.00, 95% CI 0.24 to 103.32; 314 women; 1 study). See Analysis 13.5.
13.5. Analysis.
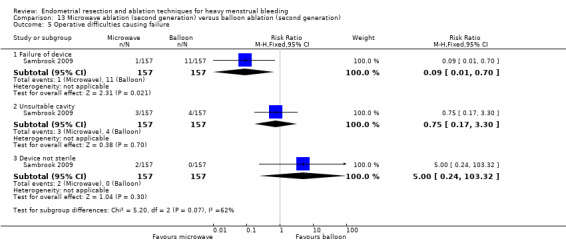
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 5 Operative difficulties causing failure.
13.6 Proportion given local anaesthesia
We found no evidence of clear differences between groups in the proportion of women choosing local or general anaesthesia (RR 1.01, 95% CI 0.79 to 1.31; 314 women; 1 study). See Analysis 13.6.
13.6. Analysis.
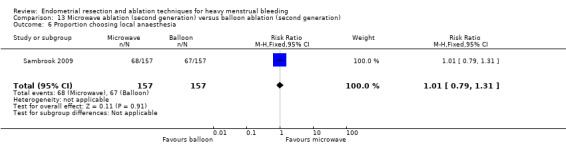
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 6 Proportion choosing local anaesthesia.
13.7 Proportion requiring opiate analgesia
No evidence showed clear differences between groups in the requirement for opiate analgesia (RR 0.92, 95% CI 0.83 to 1.01; 314 women; 1 study). See Analysis 13.7.
13.7. Analysis.
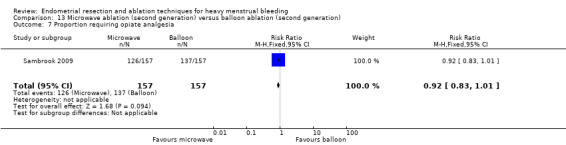
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 7 Proportion requiring opiate analgesia.
13.8 Recovery: proportion requiring overnight stay
No evidence showed clear differences between groups in the requirement for overnight stay (RR 0.66, 95% CI 0.42 to 1.04; 314 women; 1 study). See Analysis 13.8.
13.8. Analysis.
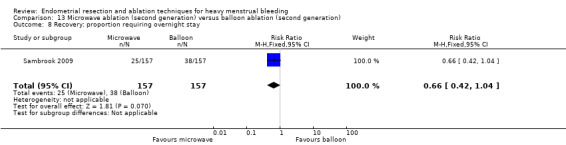
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 8 Recovery: proportion requiring overnight stay.
13.9 Quality of life
Researchers measured quality of life using EQ‐5D and SF‐12 physical and mental scores. Results provide no evidence of clear differences between groups at any time point. Test scales range from 0 to 100: results at 12 months are presented here for EQ‐5D (MD 0.02 points, 95% CI ‐0.04 to 0.08; 285 women; 1 study), SF‐12 physical (MD ‐0.70 points, 95% CI ‐2.64 to 1.24; 285 women; 1 study), and SF‐12 mental (MD ‐1.20 points, 95% CI ‐3.67 to 1.27; 285 women; 1 study); and at 5 years for EQ‐5D (MD 0.00 points, 95% CI ‐0.07 to 0.07; 217 women; 1 study), SF‐12 physical (MD ‐1.50 points, 95% CI ‐3.99 to 0.99; 217 women; 1 study), and SF‐12 mental (MD ‐0.30 points, 95% CI ‐2.90 to 2.30; 217 women; 1 study). See Analysis 13.9.
13.9. Analysis.
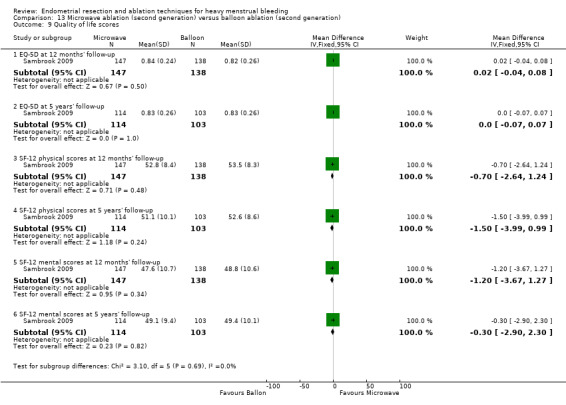
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 9 Quality of life scores.
13.10 Requirement for further surgery
We found no evidence of clear differences in the requirement for further hysterectomy between groups at 12 months' (RR 0.94, 95% CI 0.31 to 2.84; 285 women; 1 study) and up to 5 years' follow‐up (RR 1.29, 95% CI 0.51 to 3.27; 217 women; 1 study). See Analysis 13.10.
13.10. Analysis.
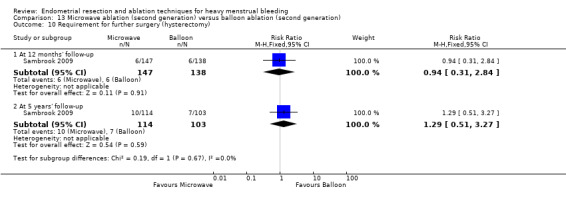
Comparison 13 Microwave ablation (second generation) versus balloon ablation (second generation), Outcome 10 Requirement for further surgery (hysterectomy).
Study authors provided no data for time or ability to return to normal activities or work, improvement in menstrual symptoms, complication rates, or mortality as a direct result of surgery.
14. Bipolar electrode ablation (second generation) versus hydrothermal ablation (second generation) (Comparison 14)
One study with 160 women compared bipolar electrode ablation versus hydrothermal ablation (Penninx 2010).
Primary outcomes
14.1 Bleeding
Amenorrhoea rates were significantly increased with bipolar ablation when compared to hydrothermal ablation at all time points: at 6 months' (RR 2.27, 95% CI 1.25 to 4.12; 150 women; 1 study), 12 months' (RR 1.95, 95% CI 1.21 to 3.15; 146 women; 1 study), or up to 2 to 5 years' follow‐up (RR 1.57, 95% CI 1.06 to 2.31; 139 women; 1 study). See Analysis 14.1.
14.1. Analysis.
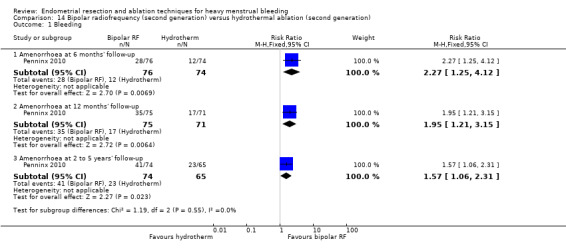
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 1 Bleeding.
14.2 Rate of satisfaction
Satisfaction rates were higher in the bipolar group than in the hydrothermal balloon group at 6 months' (RR 1.44, 95% CI 1.17 to 1.77; 150 women; 1 study), 12 months' (RR 1.11, 95% CI 1.02 to 1.21; 146 women; 1 study), and up to 2 to 5 years' follow‐up (RR 1.62, 95% CI 1.23 to 2.13; 139 women; 1 study). See Analysis 14.2.
14.2. Analysis.
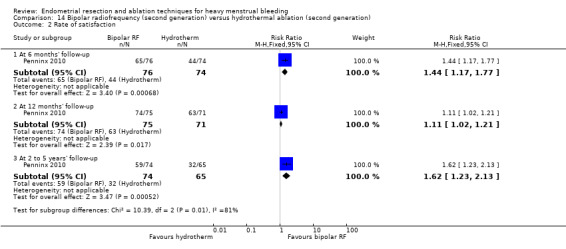
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
Operative outcomes
14.3 Duration of surgery
The duration of the procedure was significantly shorter with bipolar ablation (median (range), 11.8 minutes (5 to 40) with bipolar vs 27.8 (14 to 55) minutes with hydrothermal ablation; 156 women; 1 study). See Analysis 14.3.
14.3. Analysis.
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 3 Duration of procedure (minutes).
| Duration of procedure (minutes) | |||
|---|---|---|---|
| Study | Bipolar RF | Hydrotherm ablation | Results |
| Penninx 2010 | N=82 Median (range): 11.8 (5 to 40) |
N=74 Median (range): 27.8 (14 to 55) |
Test used not stated p<0.001 |
14.4 Improvement in other menstrual symptoms: dysmenorrhoea
The chance of eliminating dysmenorrhoea symptoms was greater with bipolar ablation than with hydrothermal ablation at 5 years' follow‐up (RR 1.32, 95% CI 1.00 to 1.74; 139 women; 1 study), but no clear difference was evident at 12 months' follow‐up (RR 0.92, 95% CI 0.79 to 1.06; 146 women; 1 study). See Analysis 14.4.
14.4. Analysis.
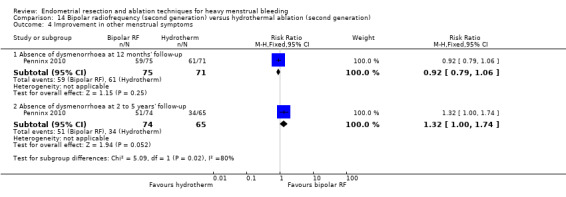
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 4 Improvement in other menstrual symptoms.
14.5 Surgical complications: major complications
We found no evidence of clear differences between groups for major complications including the following (see Analysis 14.4).
Uterine perforation (RR 2.71, 95% CI 0.11 to 65.54; 156 women; 1 study).
Saline leakage (RR 0.13, 95% CI 0.01 to 2.46; 156 women; 1 study).
14.6 Requirement for further surgery
Risk of requiring any surgery (ablation or hysterectomy) was reduced with bipolar ablation compared to hydrothermal ablation both at 12 months' (RR 0.28, 95% CI 0.11 to 0.72; 160 women; 1 study) and up to 5 years' follow‐up (RR 0.44, 95% CI 0.23 to 0.83; 136 women; 1 study). The difference is not clear when the risk of requiring a hysterectomy was compared at 12 months' (RR 0.42, 95% CI 0.14 to 1.32; 160 women; 1 study) and up to 2 to 5 years' follow‐up (RR 0.63, 95% CI 0.29 to 1.38; 136 women; 1 study). See Analysis 14.6.
14.6. Analysis.
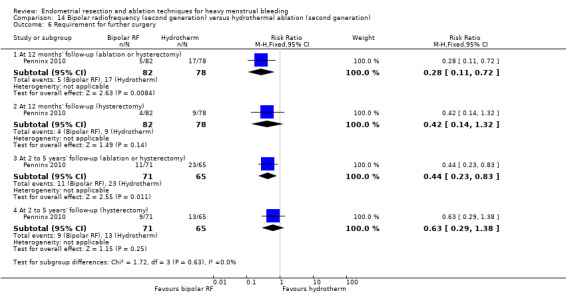
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 6 Requirement for further surgery.
Researchers provided no data for operative difficulties, proportion given general versus local anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, or mortality as a direct result of surgery.
15. Ablative curettage versus overcurettage (Comparison 15)
One study with 100 women compared ablative curettage versus overcurettage (Thabet 2010).
Primary outcomes
15.1 Bleeding
Researchers measured bleeding as amenorrhoea or eumenorrhoea at 3 years' follow‐up. Ablative curettage resulted in significantly higher rates of amenorrhoea compared with overcurettage (RR 4.50, 95% CI 2.33 to 8.69; 100 women; 1 study) and higher rates of amenorrhoea and normal menses combined (RR 1.9, 95% CI 1.3 to 2.7; 100 women; 1 study). See Analysis 15.1.
15.1. Analysis.
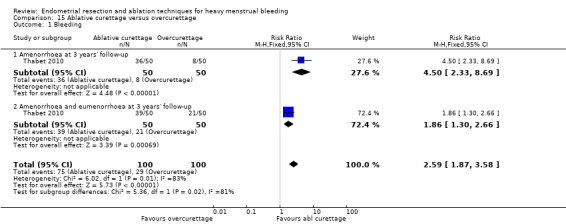
Comparison 15 Ablative curettage versus overcurettage, Outcome 1 Bleeding.
Secondary outcomes
Operative outcomes
15.2 Surgery difficulties
Failure of the procedure was less likely with ablative curettage than with overcurettage (RR 0.29, 95% CI 0.12 to 0.74; 100 women; 1 study). See Analysis 15.2.
15.2. Analysis.
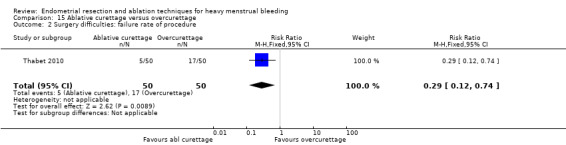
Comparison 15 Ablative curettage versus overcurettage, Outcome 2 Surgery difficulties: failure rate of procedure.
15.3 Recovery hospital stay
Overcurettage was associated with a significantly reduced hospital stay in comparison to ablative curettage (MD 1.6 days, 95% CI 1.2 to 2.0; 100 women; 1 study). See Analysis 15.3;
15.3. Analysis.
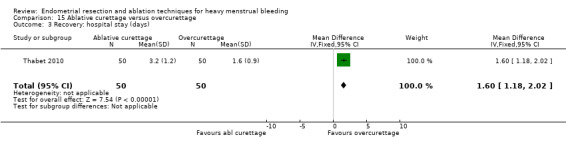
Comparison 15 Ablative curettage versus overcurettage, Outcome 3 Recovery: hospital stay (days).
15.4 Complication rate: major complications
Evidence showed no clear difference in the rate of perforation between groups (RR 0.14, 95% CI 0.01 to 2.70; 100 women; 1 study).
15.5 Complication rate: minor complications
Bleeding complications were significantly less likely with ablative curettage than with overcurettage (RR 0.21, 95% CI 0.07 to 0.70; 100 women; 1 study); study authors provided no evidence of clear differences in the rate of infection or vaginal discharge (leucorrhoea) between groups (RR 0.80, 95% CI 0.23 to 2.81; 100 women; 1 study). See Analysis 15.4.
15.4. Analysis.
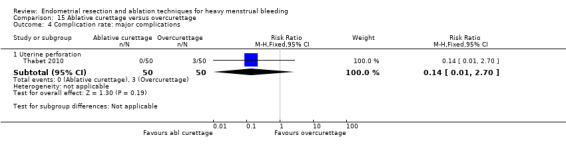
Comparison 15 Ablative curettage versus overcurettage, Outcome 4 Complication rate: major complications.
15.6 Requirement for further surgery
Trial results showed no evidence of clear differences between groups in the requirement for hysterectomy up to 3 years' follow‐up (RR 0.42, 95% CI 0.16 to 1.10; 100 women; 1 study). See Analysis 15.6.
15.6. Analysis.
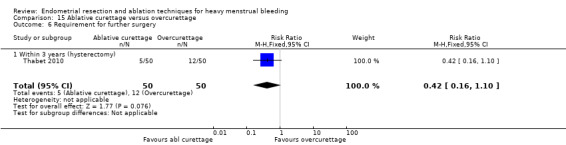
Comparison 15 Ablative curettage versus overcurettage, Outcome 6 Requirement for further surgery.
Study authors provided no data on rate of satisfaction, duration of surgery, proportion given general versus local anaesthesia, time or ability to return to normal activities or work, women's perceived change in quality of life, improvement in menstrual symptoms, or mortality as a direct result of surgery.
16. Microwave ablation (second generation) versus bipolar radiofrequency ablation (second generation) (Comparison 16)
One trial with a total of 66 women compared microwave ablation versus bipolar radiofrequency ablation (Athanatos 2015).
Primary outcomes
16.1 and 16.2 Bleeding
Amenorrhoea rates were increased in the microwave ablation group when compared to the bipolar radiofrequency ablation group at 3 months (RR 0.19, 95% CI 0.07 to 0.54; 66 women; 1 study) and at 12 months (RR 0.10, 95% CI 0.03 to 0.32; 66 women; 1 study). See Analysis 16.1. The PBAC at 12 months showed a clear difference favouring the bipolar group (RR ‐57.42, 95%CI ‐108.41 to ‐6.43; 66 women; 1 study). See Analysis 16.2.
16.1. Analysis.
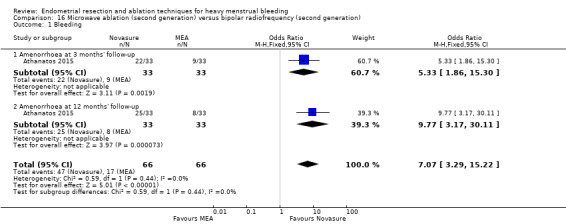
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 1 Bleeding.
16.2. Analysis.
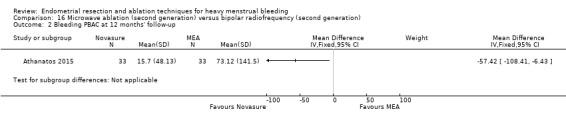
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 2 Bleeding PBAC at 12 months' follow‐up.
16.3 Rate of satisfaction
Researchers measured rate of satisfaction as satisfaction with treatment and as improvement in everyday life. No evidence showed a clear difference at 3 months (RR 0.97, 95% CI 0.89 to 1.05; 66 women; 1 study); results indicated that microwave ablation may decrease the rate of satisfaction compared with bipolar frequency ablation at 12 months (RR 0.85, 95% CI 0.73 to 0.99; 66 women; 1 study). See Analysis 16.3. For improvement in everyday life, study authors did not provide clear evidence of differences in both groups at 12 months' follow‐up (RR 0.91, 95% CI 0.81 to 1.03; 66 women; 1 study).
16.3. Analysis.
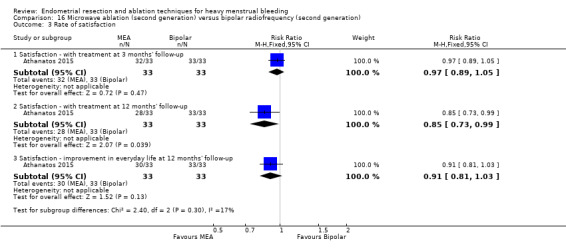
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 3 Rate of satisfaction.
Secondary outcomes
Operative outcomes
16.4 Duration of surgery
Surgical duration was measured in seconds, so even though results show a clear difference (MD 9.80, 95% CI 2.63 to 16.97; 66 women; 1 study), both procedures took less than 2 minutes to perform. See Analysis 16.4.
16.4. Analysis.
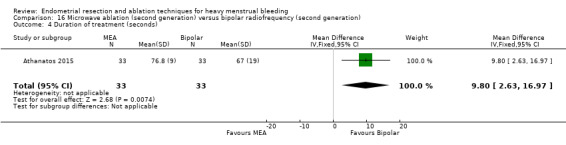
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 4 Duration of treatment (seconds).
16.5 Improvement in other menstrual symptoms: dysmenorrhoea
The dysmenorrhoea rate did not show clear differences between groups at 3 months' (RR 2.00, 95% CI 0.39 to 10.18; 66 women; 1 study) nor at 12 months' (RR 4.00, 95% CI 0.92 to 17.44; 66 women; 1 study) follow‐up. See Analysis 16.5.
16.5. Analysis.
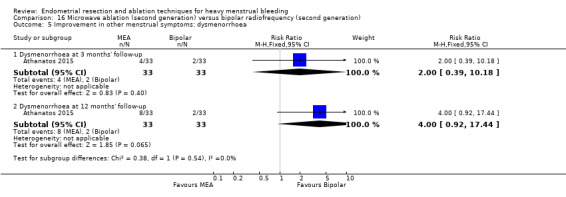
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 5 Improvement in other menstrual symptoms: dysmenorrhoea.
16.6 Complication rate: major and minor complications
Researchers reported no complications in either group. See Analysis 16.6.
16.6. Analysis.
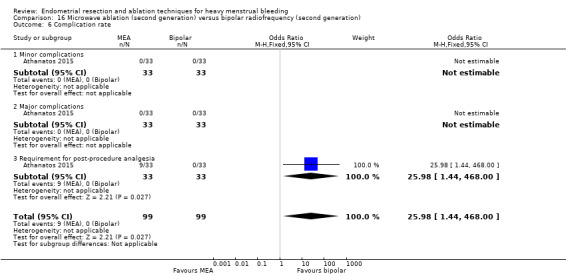
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 6 Complication rate.
The risk of requiring post‐procedure analgesia was significantly higher in the microwave endometrial ablation group (RR 25.98, 95% CI 1.44 to 468.00). See Analysis 16.6.
16.7 Requirement for further surgery
Trial results did show a clear difference between groups in the requirement for hysterectomy at 12 months' follow‐up (RR 5.00, 95% CI 0.25 to 100.32; 66 women; 1 study). See Analysis 16.7.
16.7. Analysis.
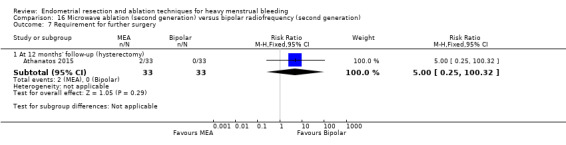
Comparison 16 Microwave ablation (second generation) versus bipolar radiofrequency (second generation), Outcome 7 Requirement for further surgery.
Study authors provided no data for operative difficulties, proportion given general versus local anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, or mortality as a direct result of surgery.
17. Bipolar (Minerva) (second generation) versus rollerball ablation (first generation) (Comparison 17)
One study with 153 women compared bipolar an endometrial ablation system (Minerva) versus rollerball ablation (Laberge 2016).
Primary outcome
17.1 Bleeding
Researchers reported using haematin alkaline < 80 mL/cycle at 12 months and rate of amenorrhoea at 12 months as dichotomous outcomes. Results for women having haematin alkaline less than 80 mL/cycle at 12 months did not show a clear difference between groups (RR 1.16, 95% CI 1.00 to 1.34), even though data showed a trend towards bipolar. The amenorrhoea rate at 12 months was clearly higher in the bipolar group (RR 1.46, 95% CI 1.08 to 1.98; 153 women; 1 study). See Analysis 17.1.
17.1. Analysis.
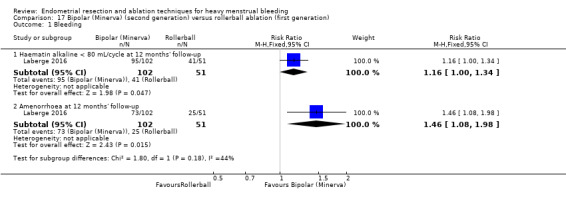
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 1 Bleeding.
17.2 Rate of satisfaction
The rate of satisfaction did not show clear differences between groups at 12 months' follow‐up. See Analysis 17.2.
17.2. Analysis.
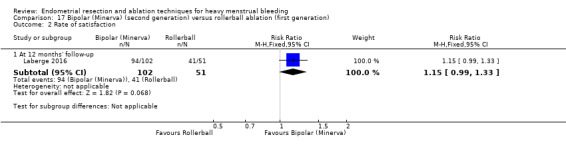
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 2 Rate of satisfaction.
Secondary outcomes
Operative outcomes
17.3 Duration of surgery
The duration of the procedure was significantly shorter in the bipolar group than in the rollerball group (MD ‐14.10 minutes, 95% CI ‐15.94 to ‐12.26; 153 women; 1 study). See Analysis 17.3.
17.3. Analysis.
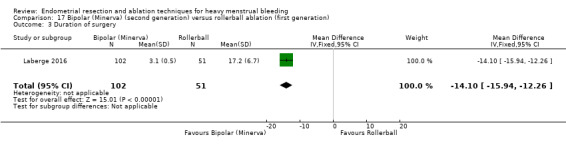
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 3 Duration of surgery.
17.4 Improvement in other menstrual symptoms: dysmenorrhoea
The rate of improvement in dysmenorrhoea did not show clear differences between groups (RR 1.02, 95% CI 0.71 to 1.48; 153 women; 1 study). See Analysis 17.4.
17.4. Analysis.
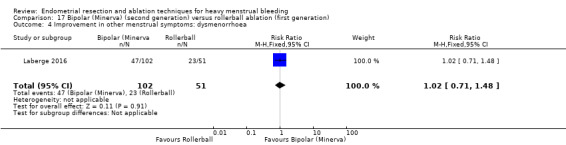
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 4 Improvement in other menstrual symptoms: dysmenorrhoea.
17.5 Improvement in other menstrual symptoms: PMS
The rate of improvement in PMS did not show clear differences between groups (RR 1.25, 95% CI 0.87 to 1.80; 153 women; 1 study). See Analysis 17.5.
17.5. Analysis.
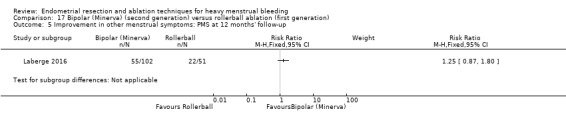
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 5 Improvement in other menstrual symptoms: PMS at 12 months' follow‐up.
17.6 Complication rate: major complications
No evidence showed clear differences between groups in major complications such as the following (see Analysis 17.6).
17.6. Analysis.
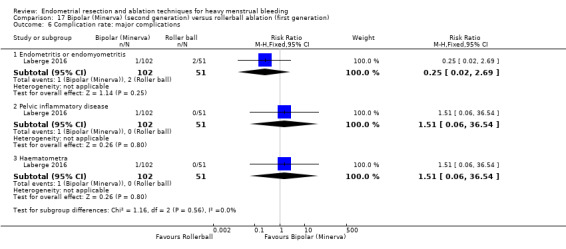
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 6 Complication rate: major complications.
Endometritis or endomyometritis (RR 0.25, 95% CI 0.02 to 2.69; 153 women; 1 study).
Pelvic inflammatory disease (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Haematometra (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
17.7 Complication rate: minor complications
Studies have provided no evidence of clear differences between groups for minor complications such as the following (see Analysis 17.7).
17.7. Analysis.
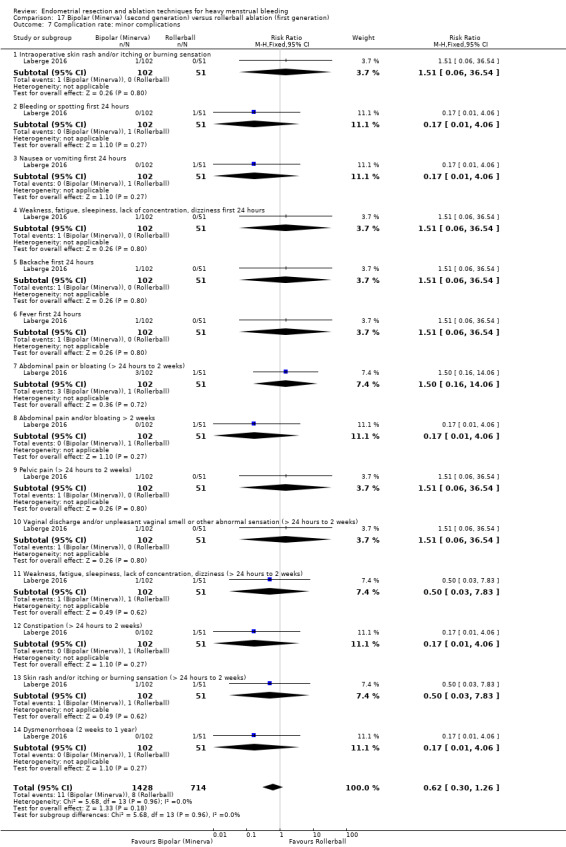
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 7 Complication rate: minor complications.
Intraoperative skin rash and/or itching or burning sensation (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Bleeding or spotting first 24 hours (RR 0.17, 95% CI 0.01 to 4.06; 153 women; 1 study).
Nausea or vomiting first 24 hours (RR 0.17, 95% CI 0.01 to 4.06; 153 women; 1 study).
Weakness, fatigue, sleepiness, lack of concentration, dizziness first 24 hours (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Backache first 24 hours (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Fever first 24 hours (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Abdominal pain or bloating up to 2 weeks (RR 1.50, 95% CI 0.16 to 14.06; 153 women; 1 study).
Abdominal pain and/or bloating for more than 2 weeks (RR 0.17, 95% CI 0.01 to 4.06 ; 153 women; 1 study)
Pelvic pain for up to 2 weeks (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Vaginal discharge and/or unpleasant vaginal smell or other abnormal sensation for up to 2 weeks (RR 1.51, 95% CI 0.06 to 36.54; 153 women; 1 study).
Weakness, fatigue, sleepiness, lack of concentration, dizziness for up to 2 weeks (RR 0.50, 95% CI 0.03 to 7.83; 153 women; 1 study).
Constipation for up to 2 weeks (RR 0.17, 95% CI 0.01 to 4.06; 153 women; 1 study).
Skin rash and/or itching or burning sensation for up to 2 weeks (RR 0.50, 95% CI 0.03 to 7.83; 153 women; 1 study).
Dysmenorrhea for up to 1 year (RR 0.17, 95% CI 0.01 to 4.06; 153 women; 1 study).
17.8 Requirement for further surgery
Trial results showed no clear evidence of differences between groups in the rate of hysterectomy up to 1 year (RR 0.33, 95% CI 0.06 to 1.93; 153 women; 1 study). See Analysis 17.8.
17.8. Analysis.
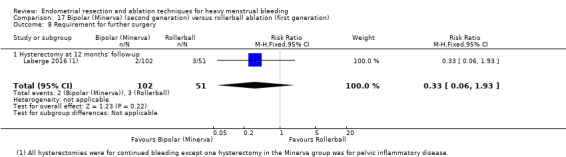
Comparison 17 Bipolar (Minerva) (second generation) versus rollerball ablation (first generation), Outcome 8 Requirement for further surgery.
Study authors provided no data for duration of surgery, operative difficulties, proportion given general versus local anaesthesia, length of hospital stay, time or ability to return to normal activities or work, women's perceived change in quality of life, or mortality as a direct result of surgery.
18 Second‐generation ablative techniques versus first‐generation ablation techniques (overall)
Thirteen studies with a total of 2368 women compared first‐ versus second‐generation ablation techniques (Brun 2006; Cooper 1999; Cooper 2002; Cooper 2004; Corson 2000; Corson 2001; Duleba 2003; Hawe 2003; Laberge 2016; Meyer 1998; Perino 2004; Romer 1998; van Zon‐Rabelink 2003).
Primary outcomes
18.1 Bleeding
We found no evidence of clear differences in bleeding parameters such as the following (see Analysis 18.1).
18.1. Analysis.
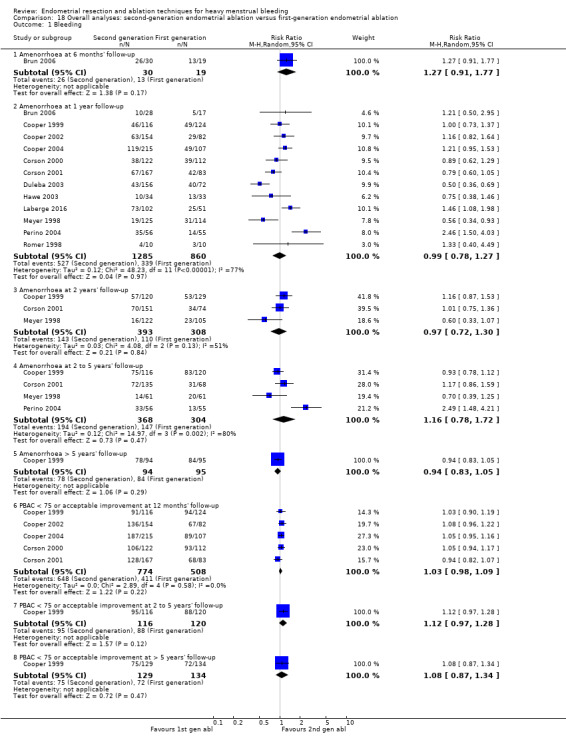
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 1 Bleeding.
Amenorrhoea at 6 months' follow‐up (RR 1.27, 95% CI 0.91 to 1.77; 49 women; 1 study).
Amenorrhoea at 2 years' follow‐up (RR 0.97, 95% CI 0.72 to 1.30; 701 women; 3 studies; I² = 51%).
Amenorrhoea at 2 to 5 years' follow‐up (RR 1.16, 95% CI 0.78 to 1.72; 672 women; 4 studies; I² = 80%).
Amenorrhoea at up to 10 years' follow‐up (RR 0.94, 95% CI 0.83 to 1.05; 189 women; 1 study).
PBAC < 75 or acceptable improvement at 12 months' follow‐up (RR 1.03, 95% CI 0.98 to 1.09; 1282 women; 5 studies; I² = 0%).
PBAC < 75 or acceptable improvement at 2 to 5 years' follow‐up (RR 1.12, 95% CI 0.97 to 1.28; 236 women; 1 study).
PBAC < 75 or acceptable improvement at up to 10 years' follow‐up (RR 1.11, 95% CI 0.95 to 1.30; 189 women; 1 study).
18.2 Amenorrhoea at 1 year follow‐up
Trials provided no evidence of clear differences in the rate of amenorrhoea between groups at 12 months' follow‐up (RR 0.99, 95% CI 0.78 to 1.27; 2145 women; 12 studies; I² = 77%). See Analysis 18.2. See the funnel plot for this comparison in Figure 4.
18.2. Analysis.
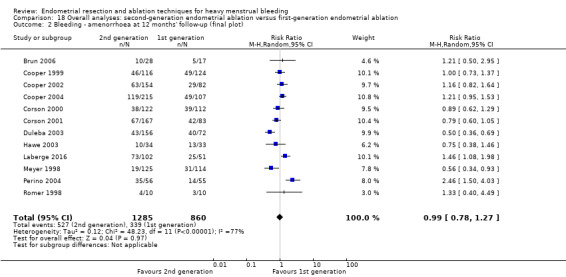
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 2 Bleeding ‐ amenorrhoea at 12 months' follow‐up (final plot).
4.
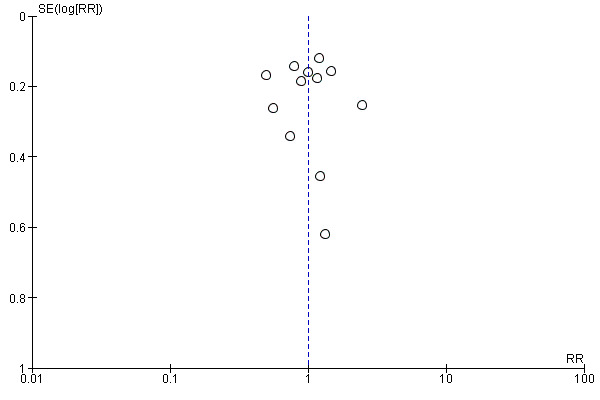
Funnel plot of comparison: 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, outcome: 18.2 Bleeding ‐ amenorrhoea at 12 months (final plot).
18.3 Rate of satisfaction
We found no evidence of clear differences in satisfaction rates up to 10 years' follow‐up, including the following (see Analysis 18.3).
18.3. Analysis.
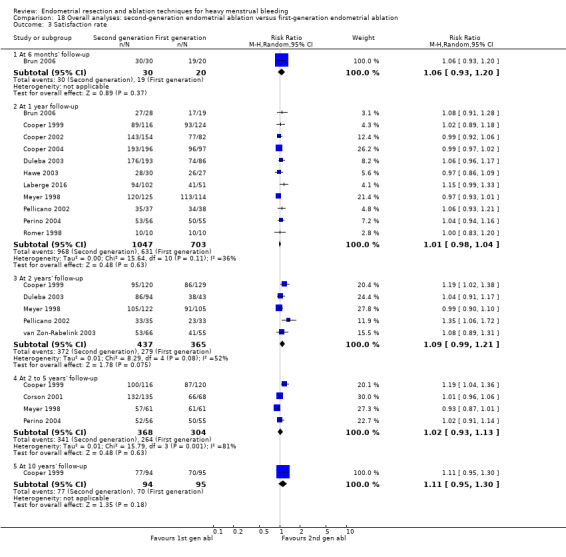
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 3 Satisfaction rate.
Satisfaction rate at 6 months' follow‐up (RR 1.06, 95% CI 0.93 to 1.20; 50 women; 1 study).
Satisfaction rate at 2 years' follow‐up (RR 1.09, 95% CI 0.99 to 1.21; 802 women; 5 studies; I² = 52%).
Satisfaction rate at 2 to 5 years' follow‐up (RR 1.02, 95% CI 0.93 to 1.13; 672 women; 4 studies; I² = 81%).
Satisfaction rate at 10 years' follow‐up (RR 1.11, 95% CI 0.95 to 1.30; 189 women; 1 study).
18.4 Satisfaction rate at 12 months' follow‐up
Study results showed no evidence of clear differences in rates of amenorrhoea between groups at 12 months' follow‐up (RR 1.01, 95% CI 0.98 to 1.04; 1750 women; 11 studies; I² = 36%). See Analysis 18.4. See the funnel plot for this comparison in Figure 5.
18.4. Analysis.
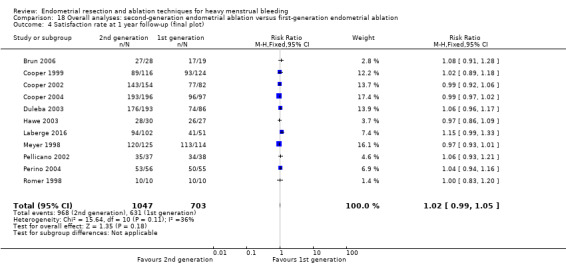
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 4 Satisfaction rate at 1 year follow‐up (final plot).
5.
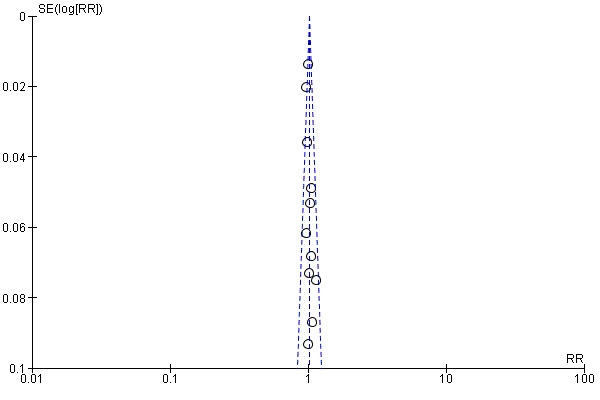
Funnel plot of comparison: 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, outcome: 18.4 Satisfaction rate at 1 year follow‐up (final plot).
Secondary outcomes
Operative outcomes
18.5 Duration of surgery
The mean difference in average surgical time between first‐ and second‐generation techniques was 13 minutes, ranging between 17 and 10 minutes. Heterogeneity was very high (94%), so we could not pool the analysis; we found that removing studies with high risk of allocation bias did not make any difference. See Analysis 18.5.
18.5. Analysis.
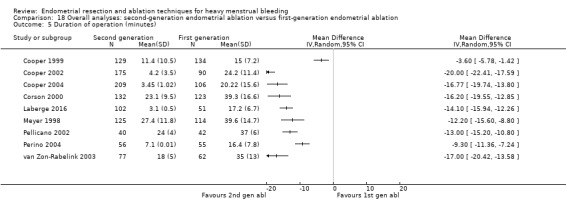
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 5 Duration of operation (minutes).
18.6 Operative difficulties
Risk of equipment failure was greater with second‐generation devices (RR 4.26, 95% CI 1.46 to 12.43; 384 women; 3 studies; I² = 0%). See Analysis 18.6. It is important to mention here that only 3 of 10 studies comparing first‐ versus second‐generation ablation techniques reported equipment failure. Lack of reporting of treatment failure does not necessarily mean that it did not happen. The theory that treatment failure could be associated with the beginning of the technique does not explain it; only one of the remaining seven studies is newer than the ones reporting equipment failure. We found no evidence of clear differences between groups in terms of abandoning the procedure (RR 1.18, 95% CI 0.38 to 3.67; 629 women; 3 studies; I² = 0%).
18.6. Analysis.
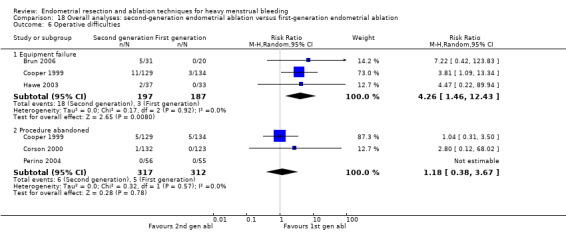
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 6 Operative difficulties.
18.7 Proportion given local anaesthesia
The chance that local rather than general anaesthesia would be used was greater with second‐generation devices (RR 2.78, 95% CI 1.76 to 4.40; I² = 85%). This must be carefully interpreted because heterogeneity was high. See Analysis 18.7.
18.7. Analysis.
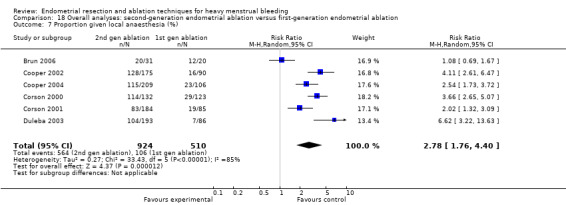
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 7 Proportion given local anaesthesia (%).
18.8 Inability to work
We noted no evidence of a clear difference between groups in inability to work (RR 0.84, 95% CI 0.30 to 2.30; 279 women; 2 studies; I² = 20%). See Analysis 18.8.
18.8. Analysis.
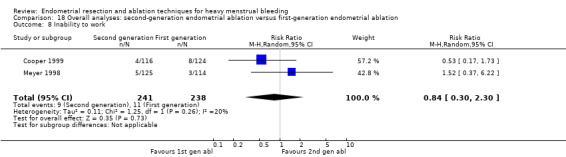
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 8 Inability to work.
18.9 Complication rate: major complications
Regarding major complications, women undergoing second‐generation ablation procedures, when compared to the group having first‐generation procedures, were less likely to have the following major complications.
Cervical lacerations (RR 0.21, 95% CI 0.07 to 0.61; 1583 women; 7 studies; I² = 0%).
Haematometra (RR 0.34, 95% CI 0.12 to 0.95; 1193 women; 5 studies; I² = 0%).
Fluid overload (RR 0.16, 95% CI 0.03 to 0.94; 588 women; 3 studies; I² = 0%).
We found no clear evidence of differences between groups in other major complications such as the following (see Analysis 18.9).
18.9. Analysis.
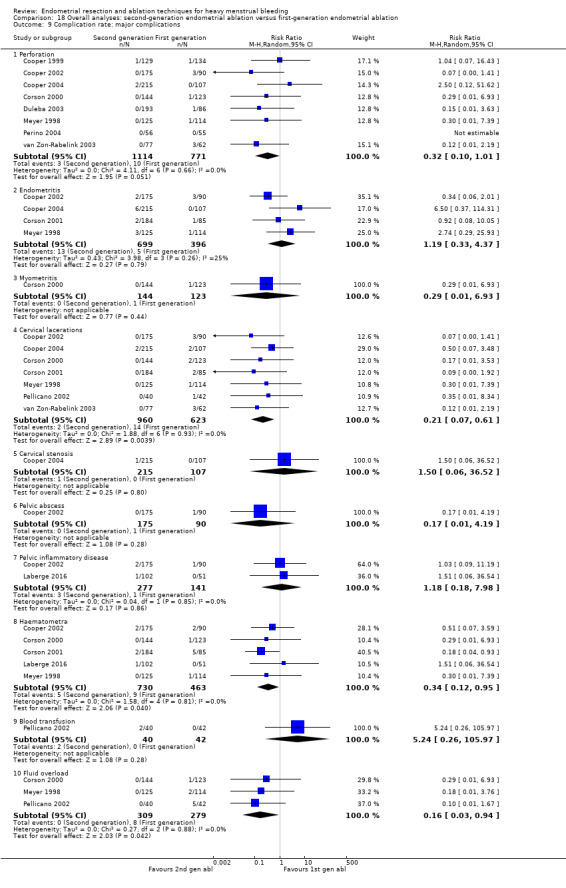
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 9 Complication rate: major complications.
Perforation (RR 0.32, 95% CI 0.10 to 1.01; 1885 women; 8 studies; I² = 0%).
Endometritis (RR 1.19, 95% CI 0.33 to 4.37; 1095 women; 4 studies; I² = 25%).
Myometritis (RR 0.29, 95% CI 0.01 to 6.93; 267 women; 1 study).
Cervical stenosis (RR 1.50, 95% CI 0.06 to 36.52; 322 women; 1 study).
Pelvic abscess (RR 0.17, 95% CI 0.01 to 4.19; 265 women; 1 study).
Pelvic inflammatory disease (RR 1.18, 95% CI 0.18 to 7.98; 418 women; 2 studies; I² = 0%).
Blood transfusion (RR 5.24, 95% CI 0.26 to 105.97; 82 women; 1 study).
18.10 Complication rate: minor complications
Regarding minor complications, women undergoing first‐generation ablation procedures, when compared to those having second‐generation procedures, were less likely to have the following minor complications.
Nausea and vomiting (RR 2.01, 95% CI 1.40 to 2.88; 997 women; 4 studies; I² = 0%).
Uterine cramping (RR 1.21, 95% CI 1.02 to 1.45; 601 women; 2 studies; I² = 0%).
Trial results provided no clear evidence of differences between groups for other minor complications such as the following (see Analysis 18.10).
18.10. Analysis.
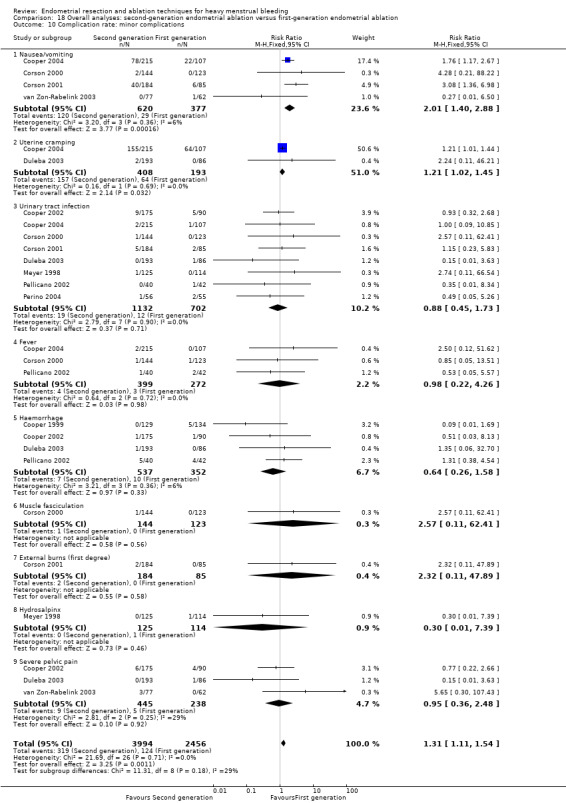
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 10 Complication rate: minor complications.
Urinary tract infection (RR 0.88, 95% CI 0.45 to 1.73; 1834 women; 4 studies; I² = 0%).
Fever (RR 0.98, 95% CI 0.22 to 4.26; 671 women; 3 studies; I² = 0%).
Haemorrhage (RR 0.64, 95% CI 0.26 to 1.58; 889 women; 4 studies; I² = 4%).
Muscle fasciculation (RR 2.57, 95% CI 0.11 to 62.41; 267 women; 1 study).
External burns (first degree) (RR 2.32, 95% CI 0.11 to 47.89; 269 women; 1 study).
Hydrosalpinx (RR 0.30, 95% CI 0.01 to 7.39; 239 women; 1 study).
Severe pelvic pain (RR 0.95, 95% CI 0.36 to 2.48; OR 0.95, 95% CI 0.35 to 2.60; 683 women; 3 studies; I² = 30%).
18.11 Requirement for further surgery
We found no evidence of significant differences in the requirement for any additional surgery (hysterectomy or ablation) or hysterectomy in both groups up to 5 years' follow‐up, including the following (see Analysis 18.11).
18.11. Analysis.
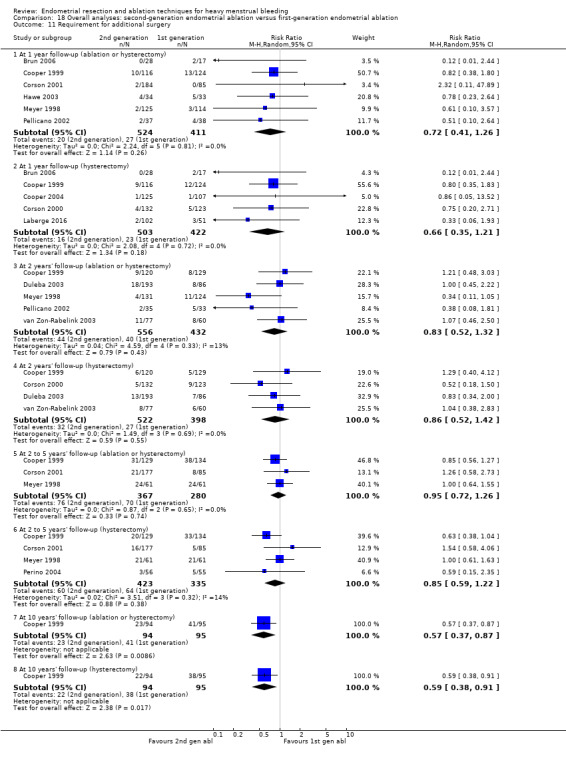
Comparison 18 Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation, Outcome 11 Requirement for additional surgery.
Requirement for any additional surgery (hysterectomy or ablation) at 1 year follow‐up (RR 0.72, 95% CI 0.41 to 1.26; 935 women; 6 studies; I² = 0%).
Requirement for any additional surgery (hysterectomy or ablation) at 2 years' follow‐up (RR 0.83, 95% CI 0.52 to 1.32; 988 women; 5 studies; I² = 13%).
Requirement for any additional surgery (hysterectomy or ablation) at 2 to 5 years' follow‐up (RR 0.95, 95% CI 0.72 to 1.26; 647 women; 3 studies; I² = 0%).
Requirement for hysterectomy at 1 year follow‐up (RR 0.66, 95% CI 0.35 to 1.21; (RR 0.66, 95% CI 0.35 to 1.21; 925 women ; 5 studies; I2 = 0%).
Requirement for hysterectomy at 2 years' follow‐up (RR 0.86, 95% CI 0.52 to 1.42; 920 women; 4 studies; I² = 0%).
Requirement for hysterectomy at 2 to 5 years' follow‐up (RR 0.85, 95% CI 0.59 to 1.22; 758 women; 4 studies; I² = 14%).
At 10 years' follow‐up, women undergoing second‐generation techniques have reduced possibilities of undergoing any further surgery (ablation or hysterectomy) (RR 0.57, 95% CI 0.37 to 0.87; 189 women; 1 study) or a subsequent hysterectomy (RR 0.60, 95% CI 0.38 to 0.96; 189 women; 1 study). These results must be interpreted cautiously; they reflect only one trial, in which more than 25% of participants were lost to follow‐up. Study authors also reported 9% requiring further hysteroscopies with the second‐generation technique but did not provide further details.
The main outcomes for this overall comparison can be viewed in Table 1.
Heterogeneity
1. Specific types of endometrial resection or ablation
Most of the forest plots comparing specific types of endometrial ablation showed comparisons between groups in individual studies or pooled two or four studies at most, and they provided little evidence of statistical heterogeneity. However, we found substantial statistical heterogeneity (I² > 50%) for the following forest plots.
Comparison 1.4 (Analysis 1.4): duration of operation (laser vs TCRE).
Comparison 7.5 (Analysis 7.5): duration of operation (electrode ablation vs TCRE + rollerball).
Comparison 9.6 (Analysis 9.6): duration of operation (balloon vs rollerball).
Comparison 9.12 (Analysis 9.12): requirement for further surgery (2 years' follow‐up) (balloon vs rollerball).
Comparison 12.3 (Analysis 12.3): satisfaction rate (6 months' follow‐up) (bipolar radiofrequency ablation vs balloon).
12.3. Analysis.
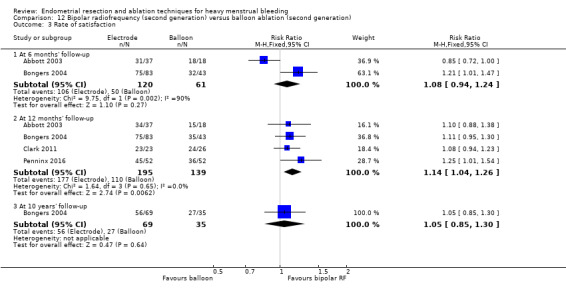
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 3 Rate of satisfaction.
Comparison 12.15 (Analysis 12.15): requirement for further surgery (bipolar radiofrequency vs balloon).
12.15. Analysis.
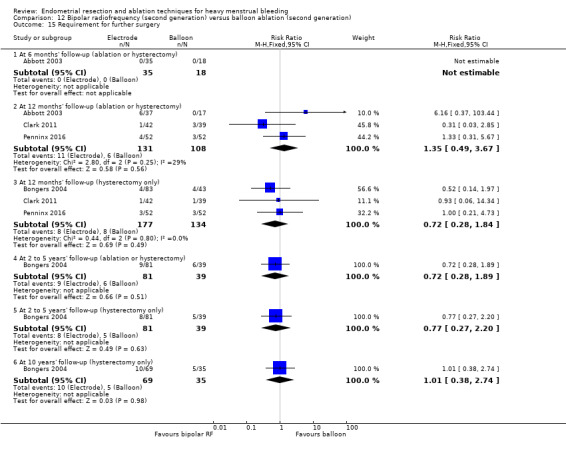
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 15 Requirement for further surgery.
Duration of operation was affected by numerous confounding factors such as expertise of individual surgeons, hospital type and procedures, and differences between groups of women. For the comparison laser versus TCRE, the Bhattacharya study did not include total time spent in theatre, and the McClure study recorded induction and reversal of anaesthesia in the estimation of operation time, which resulted in much larger estimates. In this latter trial, temporary laser malfunction prolonged two laser cases to 240 minutes. For the comparison electrode ablation versus TCRE + rollerball, differences between studies were likely to be explained by the two different systems used: the Corson study used the Vesta balloon ablation, and the Cooper study used Novasure. In the comparison balloon versus rollerball, all three pooled studies used the Thermachoice balloon system. The operation time recorded for rollerball ablation was similar in the three trials, but times differed between studies for balloon ablation. The Meyer study provided no preoperative treatment to thin the endometrium, whereas the other two studies provided 2 months of gonadotropin‐releasing hormone (GnRH) agonist pretreatment. Other factors such as cavity length were correlated with operation time, and it is not clear whether these were similarly distributed between participants in the three trials. Another major confounding factor was the ability to use local rather than general anaesthesia, which was more likely in trials comparing second‐generation versus first‐generation ablation methods.
Satisfaction is also likely to have varied because of different methods of measurement used. In the comparison bipolar radiofrequency ablation versus balloon, satisfaction rates at 6 months in the small Abbott trial may have been related to the technical failure rate for the Novasure procedure, but rates at 12 months' follow‐up were similar and were not significantly different.
Significant heterogeneity was evident for the outcome requirement for further surgery in the comparisons of balloon versus rollerball and bipolar electrode ablation versus balloon. Different results in the two pooled trials for either comparison could not be explained by examining their characteristics. Neither trial reported a significant difference in outcomes by ablation technique.
2. Overall analyses comparing first‐ and second‐generation techniques
Substantial heterogeneity was evident for many outcomes when researchers compared first‐generation procedures versus second‐generation procedures (Comparison 18), in particular, rate of amenorrhoea, duration of operation, and proportion given local as opposed to general anaesthesia. The I² value for the outcome amenorrhoea at 1 year after surgery was 77%, at 2 years 51%, and at 2 to 5 years 80%. Rates of amenorrhoea ranged widely in the included trials, and study authors reported no statistical differences between groups. When we compared estimates calculated with the fixed‐effect model versus estimates calculated with a random‐effects model, we found that estimates did not change markedly, but confidence intervals (CIs) were wider with the latter approach. Thus no evidence shows that amenorrhoea rates varied according to whether first‐ or second‐generation techniques were used to ablate the endometrium.
Forest plots for the outcomes duration of surgery and local versus general anaesthesia also indicated substantial heterogeneity. Given that these two categories were very broad and included several different ablative techniques, we expected to find heterogeneity, and we used a random‐effects model to display results. As previously explained, apart from differences between techniques, duration of surgery was likely to be affected by extraneous factors such as skill and expertise of the surgeon, hospital policy, and the operating environment. However, each of the included trials reported separately that second‐generation techniques took significantly less time to perform than first‐generation techniques, regardless of the procedures compared. A random‐effects model approach indicated significantly less time required for second‐generation procedures; each of the trials individually showed a statistically significant difference. The other comparison ‐ proportion of women given local as opposed to general anaesthesia ‐ also showed highly significant heterogeneity. For all trials in the meta‐analysis, the proportions of women undergoing ablation with first‐generation techniques under local anaesthesia (either TCRE + rollerball or rollerball alone) ranged from 8% to 23%, and the proportion undergoing second‐generation ablation under local anaesthesia (Vesta, HTA, Novasure, cryoablation, or microwave) ranged from 45% to 86%. All trials separately reported large significant differences between first‐ and second‐generation techniques. A random‐effects model confirmed these differences in pooled results.
To sum up, random‐effects model analyses confirmed the following.
Evidence showing no difference in rates of amenorrhoea when first‐generation techniques were compared with second‐generation techniques.
Evidence suggesting that duration of surgery with second‐generation techniques overall was less than with first‐generation techniques (average of 14 minutes less); however, due to high levels of heterogeneity, we were unable to pool the data for meta‐analysis.
Women undergoing ablation with second‐generation techniques were more likely to be given local anaesthesia than those undergoing ablation with first‐generation techniques.
Sensitivity analyses
We performed sensitivity analyses only on comparisons for which five or more trials were pooled, specifically for the comparison of rates of satisfaction and amenorrhoea at 1 year follow‐up between first‐ and second‐generation ablation. We found no significant differences reported between randomised groups, and planned sensitivity analyses did not substantially change the results of included trials, although heterogeneity was reduced.
Discussion
Summary of main results
See Table 1.
This review has assessed a wide range of efficacy, satisfaction, and safety outcomes related to different techniques for ablation or resection of the endometrium for women with heavy menstrual bleeding.
Overall comparison of first‐generation versus second‐generation techniques
Some types of intraoperative and postoperative complications such as fluid overload, cervical lacerations, and haematometra were more common with first‐generation ablation; other types of complications, nausea and vomiting, and uterine cramping and pain were more common with second‐generation techniques. No clear evidence shows differences in perforation rates between first‐ and second‐generation techniques. Concerns about these 'blind' methods leading to bowel injuries from undetected uterine perforation did not seem to be confirmed in published studies. However, many anecdotal examples indicate that such events can occur, and great care must be taken to minimise the risk of such potentially serious complications.
Trial results showed no differences in rates of re‐intervention ‐ either repeat ablation or hysterectomy or both ‐ between first‐ and second‐generation ablation up to 5 years' follow‐up. Only one small trial reported a clear difference at 10 years, but this should be interpreted cautiously because if repeated hysteroscopy is considered a surgical procedure, the difference is not significant, and no report provided the number of women transitioned through menopause. A recurrent comment about newer techniques that rely on 'devices' inserted into the uterine cavity to destroy the endometrium involved the incidence of equipment failure. This may represent expected 'teething problems' associated with new equipment. However, given that the older methods are extremely simple (a loop, laser, or diathermy to destroy the endometrium below it) and that newer techniques are potentially complex (microwaves, bags of fluid, etc.), the potential remains for mechanical breakdown to occur. In addition, considerable experience in intrauterine cavity assessment and manipulation is required for safe use any of these devices.
Comparison of different types of first‐generation ablation techniques
First‐generation ablation techniques have been acknowledged traditionally as the 'gold standard' by which other, newer procedures were judged (Papadopoulos 2007). Improvement in menstrual bleeding and satisfaction seems to be similar between first‐generation techniques. The complication profile between techniques is slightly different; for example, fluid overload was more likely with laser ablation than with transcervical resection of the endometrium (TCRE) and was more likely with TCRE than with vaporising electrode ablation. However, it is likely that operator safety is a much more important arbiter of patient safety than the instrument itself. Duration of surgery was longer with the laser than with TCRE and was longer with TCRE than with vaporising electrode ablation. Equipment failure was more likely with laser ablation than with TCRE, and the procedure was more difficult with TCRE than with vaporising electrode ablation.
Comparison of different types of second‐generation ablation techniques
Bipolar radiofrequency ablation was associated with significantly higher rates of amenorrhoea than was balloon ablation up to 12 months' follow‐up, but researchers report no significant differences at 2, 5, and 10 years' follow‐up. In accordance with the amenorrhoea report, the satisfaction rate is higher at 12 months for bipolar radiofrequency ablation but trials show no significant differences at 6 months' or 10 years' follow‐up. Surgery was shorter with bipolar ablation, and premenstrual syndrome (PMS) scores were reduced. No evidence shows that bipolar radiofrequency ablation resulted in lower rates of further surgery for heavy menstrual bleeding when compared to balloon ablation.
Bipolar ablation also increased rates of amenorrhoea and satisfaction when compared with hydrothermal ablation. Procedure time was shorter with bipolar ablation and women were less likely to require additional surgery at later follow‐up when compared to hydrothermal ablation. Amenorrhoea rates appeared to be increased with microwave when compared with balloon, but trials reported no differences in Pictorial Blood Assessment Chart (PBAC) scores or satisfaction. Operation time was also reduced with microwave ablation.
Comparison of different types of first‐generation and second‐generation techniques
With reference to comparisons of different types of second‐generation techniques versus first‐generation techniques, thermal laser was more effective than TCRE in reducing blood loss (as measured by rates of amenorrhoea), but research shows no differences in patient satisfaction between approaches (using the same measurement tools). Although rollerball ablation was more likely to result in amenorrhoea when compared to cryoablation, trial results showed no difference in patient satisfaction between approaches. Patients appeared to be more satisfied with microwave than with TCRE at 2 and 5 years after surgery, but these findings were not significant at 1 and 10 years' follow‐up. With regards to secondary outcomes, duration of surgery was consistently shorter with second‐generation ablation, and procedures were more likely to be performed with the patient under local anaesthesia. Post‐surgical pain was also more likely with some types of second‐generation techniques such as thermal laser, balloon, and Hydro ThermAblator (HTA), but not all trials measured this outcome. Data show no significant differences between procedures in terms of improvement in dysmenorrhoea.
Overall completeness and applicability of evidence
The diagnosis of HMB is based on subjective complaints and its impact on quality of life ‐ not on objective measures of blood loss (Munroe 2006; NICE 2018). However, many women with heavy menstrual bleeding (HMB) referred from primary to tertiary care do not describe HMB when directly questioned, suggesting a tendency for broad description of menstrual characteristics to be reframed as excessive bleeding at referral and during management (Warner 2001). This is likely to result in women receiving inappropriate care and will influence the actual and perceived efficacy of treatment modalities for HMB.
Published literature on endometrial destruction techniques for HMB covers a wide range of surgical methods and uses a variety of outcome measures to assess treatment success, making clear comparisons between studies difficult. Participant groups showed varied and often potentially important clinical factors such as the presence of uterine fibroids or a perimenopausal state, which were not mentioned in the inclusion or exclusion criteria. This is particularly important with longer follow‐up studies. Current clinical approaches to HMB advise that medical therapy should be offered in the first instance, and it would be unusual in normal practice to advise endometrial resection or ablation without trying any medical therapies. Indeed, medical treatment with the levonorgestrel‐releasing intrauterine system (Mirena, Schering) reduces menstrual blood loss (MBL) by 94% at 3 months (Irvine 1998), and it is equally effective as thermal balloon ablation (de Souza 2010; Shaw 2007), rollerball endometrial ablation (Ergun 2012), and endometrial ablation. Surgical approaches to resect or ablate the endometrium are generally second‐line after medical therapies. Fourteen published studies focussed on women with failed medical management of HMB.
Published studies show wide variation in the outcome criteria used to assess the efficacy of endometrial ablation and resection techniques. No studies have used women's perceptions of HMB as an inclusion criterion nor women's perception of improvement as an outcome, even though this is the main diagnostic criterion. Several studies used the PBAC (Higham 1990), but entry and success criteria for PBAC score varied widely between studies. It is important to identify core outcomes for future trials on treatments for HMB for better comparisons. The COMET initiative (Core Outcome Measures in Effectiveness Trials) is working towards this objective; it is hoped that this initiative will help to improve study outcomes for HMB (COMET 2018).
Quality of the evidence
The evidence base on which this review is based was of variable quality. In particular, few studies were blinded, and for most comparisons between individual techniques, a limited number of studies provided data. Lack of blinding is likely to influence more subjective outcomes such as satisfaction rates, so findings of these types of outcomes should be viewed with caution.
We identified substantial heterogeneity in some outcomes in the overall comparison between first‐ and second‐generation techniques, and we have downgraded the quality of evidence to reflect the uncertainty around summary effect estimates. See Table 1.
Potential biases in the review process
A comprehensive search for relevant studies, together with duplicate and independent study selection, data extraction, and quality assessment of studies, has minimised the chance of potential bias in the review process.
Agreements and disagreements with other studies or reviews
It is surprising that although numerous randomised controlled trials (RCTs) and observational studies have examined specific types of endometrial ablation techniques, few systematic reviews have made overall comparisons of specific endometrial ablation techniques for reduction of HMB. Numerous narrative reviews have been published, together with comprehensive audits for first‐generation techniques. Upon comparing first‐generation methods of endometrial ablation versus resection, the MISTLETOE study concluded that methods produced similar outcomes in terms of bleeding and participant satisfaction, but that resection methods are associated with significantly more complications, suggesting that ablation should be used for all women with a non‐fibroid uterus (Overton 1997).
Systematic reviews ‐ one with individual participant data ‐ have not been able to determine major differences between first‐ and second‐generation techniques in terms of effectiveness or satisfaction with treatment (Garside 2005; Middleton 2010). However, Middleton has confirmed the findings of this review that second‐generation techniques are faster, local anaesthesia is more likely to be used, and some complications are less frequent. The suggestion in this review that additional surgery may be less likely with second‐generation techniques at longer follow‐up (10 years) is based on only one trial and needs confirmation from further research. On the other hand, at 2 to 5 years' follow‐up, researchers found no significant difference in the requirement for further surgery ‐ hysterectomy or ablation (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.72 to 1.26; 647 women; 3 studies) or only hysterectomy (RR 0.85, 95% CI 0.59 to 1.22; 758 women; 4 studies). According to a Scottish review of 14,078 women with endometrial ablation having a subsequent hysterectomy, the median time interval between surgeries was 15 months (range 8 to 32 months) (Cooper 2011).
Among second‐generation techniques, the most studied have been Novasure, balloon, and microwave ablation (NHS 2011). A recent network meta‐analysis reported that bipolar radiofrequency and microwave ablation resulted in higher rates of amenorrhoea than thermal balloon ablation at 12 months after treatment (Daniels 2012), but no evidence shows a convincing difference between the three techniques in terms of satisfaction rates or the number of women still experiencing heavy bleeding. Researchers did not assess other outcomes. However, lack of a consistent measure of effectiveness has made it difficult to adequately compare techniques and reach conclusions on the technique of choice. Other study authors have suggested that there might be commercial resistance to comparing devices, given the likely effect on the market share for the inferior treatment (McGurgan 2007). It has also been suggested that a potential limitation of second‐generation devices involves restrictions on size and configuration of the endometrial cavity that may prevent general application of any device to the HMB population (Munroe 2006). Many of the included studies that evaluated these devices in this review applied fairly strict inclusion criteria, limiting the applicability of results to women with large or distorted uteri. Thus, not all women with HMB may be candidates for second‐generation ablation, and it has been suggested that gynaecologists should retain their skills in hysteroscopic surgery for certain types of intrauterine pathology (Papadopoulos 2007).
An additional issue is the role of patient preferences in decision‐making regarding treatments for HBS. A recent review suggested that reaching a decision on a 'one size fits all' approach may be elusive, and that eliciting patient preferences, based on the evidence, is required to reach the decision on the 'best' approach (Roberts 2011).
Authors' conclusions
Implications for practice.
Second‐generation techniques are safer, quicker, and equally effective when compared with first‐generation techniques for treatment of HMB; also, the potential for second‐generation methods to be performed under local anaesthesia offers a considerable advantage.
Satisfaction rates and reduction in HMB are similar with both approaches.
Second‐generation endometrial ablation should be considered for women with a normal uterus presenting with heavy menstrual bleeding, who are not planning a present or future pregnancy.
Implications for research.
Future studies should focus on comparing different second‐generation approaches to clarify real advantages are associated with one method over the others; researchers should also compare third‐generation versus second‐generation approaches to assess which are better.
Future research should use as inclusion criteria women’s reports of heavy menstrual bleeding, according to International Federation of Gynaecology and Obstetrics (FIGO) and National Institute for Health and Care Excellence (NICE) guidelines (Munro 2012; NICE 2018). One alternative involves using a questionnaire to evaluate the woman's menstrual bleeding such as "the menstrual bleeding questionnaire", which has been developed and validated to improve the assessment of women with self‐reported HMB in both clinical practice and research (Matteson 2015). At this point, research shows no significant differences in bleeding outcomes between second‐generation techniques; therefore it will be important to evaluate the cost of different techniques for both women and the healthcare system.
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2018 | New citation required but conclusions have not changed | The addition of new studies has not led to a change in the conclusions of this review |
| 22 May 2018 | New search has been performed | Four new trials were added (Athanatos 2015; Ghazizadeh 2014; Laberge 2016; Penninx 2016), as well as 1 ongoing study (NCT02642926) |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 31 July 2013 | New citation required but conclusions have not changed | Six new publications were added but no change was made to the conclusions |
| 31 July 2013 | New search has been performed | Review has been updated. Six new publications were added: 1 reported longer follow‐up of a study already included, 4 were new trials, and 1 additional publication reported longer follow‐up of one of the 4 new trials. Summary measures for dichotomous data changed from odds ratios to risk ratios because for some outcomes, event rates were large (satisfaction, amenorrhoea rates) |
| 6 August 2009 | New citation required but conclusions have not changed | Review has been updated with 2 new citations but conclusions have not changed. A new review author has been added |
| 17 December 2008 | Amended | Title has been changed from "Endometrial destruction techniques for heavy menstrual bleeding" to "Endometrial resection and ablation techniques for heavy menstrual bleeding" |
| 10 December 2008 | Amended | Review has been converted to new review format |
| 23 August 2005 | New citation required and conclusions have changed | Substantive amendments have been made |
Acknowledgements
The authors of the 2018 update of this review thank Dr Jane Thomas and Dr Shantini Paranjothy for providing peer review comments. They also thank Marian Showell (Information Specialist) and Helen Nagels (Managing Editor) at the Cochrane Gynaecology and Fertility Group's editorial base for their time and support, and Dolores Matthews for comprehensive copy editing of their draft.
The authors of the 2013 review acknowledge and thank the Cochrane Menstrual Disorders and Subfertility Group for extensive support in the preparation of this review. Special thanks are due to Shauna Sylvester, Sarah Hetrick, Michelle Proctor, Jane Clarke, and Helen Nagels (Managing Editors during the lifecycle of this review); Sue Furness, Ruth Withers, and Marian Showell (Trials Search Co‐ordinators or Information Specialists); Neil Johnson (Editor); and Sue Hall (who provided secretarial assistance). The review authors also thank Amy Goodwin, Manager of Clinical Research, Gynecare, for extra data and for answering queries on the Meyer trial; authors from some of the other trials (Abbott 2003; Boujida 2002; Perino 2004; van Zon‐Rabelink 2003); and Joerg Neumann for translating relevant sections of the Romer trial. The review authors are also indebted to Sarah Hetrick of the Australasian Cochrane Centre, who helped with update searching in 2004, as well as extraction of data and addition of entries to the Characteristics of included studies.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
Searched 22 May 2018
PROCITE platform
Keywords CONTAINS "heavy bleeding"or"heavy menstrual bleeding"or "heavy menstrual loss"or "menometrorrhagia"or "menorrhagia"or "menorrhagia‐outcome"or "Menorrhagia‐Symptoms"or "abnormal bleeding"or "abnormal uterine bleeding"or "abnormal vaginal bleeding"or"excessive menstrual bleeding"or "excessive menstrual loss"or "dysfunctional bleeding"or "dysfunctional uterine bleeding" or Title CONTAINS "heavy bleeding"or"heavy menstrual bleeding"or "heavy menstrual loss"or "menometrorrhagia"or "menorrhagia"or "menorrhagia‐outcome"or "Menorrhagia‐Symptoms"or "abnormal bleeding"or "abnormal uterine bleeding"or "abnormal vaginal bleeding"or"excessive menstrual bleeding"or "excessive menstrual loss"or "dysfunctional bleeding"or "dysfunctional uterine bleeding"
AND
Keywords CONTAINS "endometrial ablation" or "endometrial ablation, bipolar radiofrequency" or "Endometrial ablation, chemical" or "endometrial ablation, laser" or "endometrial ablation, microwave" or "endometrial ablation, Novasure" or "endometrial ablation, rollerball" or "endometrial ablation, thermal balloon" or "transcervical endometrial resection" or "transcervical hysteroresection" or "transcervical hysteroscopic endometrial coagulation" or "Laser Ablation" or "rollerball" or "rollerball electro‐ablation" or "balloon endometrial ablation" or "microwave endometrial ablation" or "microwave" or "photoablation" or "cryoblation therapy" or "NovaSure" or "endometrial cryoblation" or "endometrial resection" or "endometrial resection, transcervical" or "ablation" (278 hits)
Appendix 2. CENTRAL via CENTRAL Register of Studies Online (CRSO) search strategy
Searched 22 May 2018
Web platform
#1 MESH DESCRIPTOR Menorrhagia EXPLODE ALL TREES 319
#2 (menstrua* adj5 disorder*):TI,AB,KY 306
#3 (heavy adj5 menstrua* ):TI,AB,KY 228
#4 (iron adj5 anaem*):TI,AB,KY 467
#5 menorrhag*:TI,AB,KY 704
#6 hypermenorr*:TI,AB,KY 23
#7 (dysfunction* adj2 uter*):TI,AB,KY 150
#8 (excessive* adj2 menstru*):TI,AB,KY 25
#9 (heavy adj2 mense*):TI,AB,KY 4
#10 (abnormal* adj2 uterine):TI,AB,KY 280
#11 (excessive adj2 uter*):TI,AB,KY 29
#12 (heavy adj2 period*):TI,AB,KY 13
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 1914
#14 MESH DESCRIPTOR Ablation Techniques EXPLODE ALL TREES 5093
#15 TCRE:TI,AB,KY 28
#16 (resect* or ablat*):TI,AB,KY 22615
#17 rollerball*:TI,AB,KY 37
#18 balloon:TI,AB,KY 8225
#19 microwave:TI,AB,KY 521
#20 electrosurg*:TI,AB,KY 444
#21 hypertherm*:TI,AB,KY 1450
#22 photodynam*:TI,AB,KY 1447
#23 thermotherap*:TI,AB,KY 358
#24 phototherap*:TI,AB,KY 2112
#25 cryoablat*:TI,AB,KY 215
#26 radiofreq*:TI,AB,KY 2742
#27 laser*:TI,AB,KY 13532
#28 Thermachoice*:TI,AB,KY 26
#29 Cavaterm*:TI,AB,KY 14
#30 Elitt*:TI,AB,KY 1
#31 Vesta*:TI,AB,KY 15
#32 Novasure*:TI,AB,KY 24
#33 Cryogen*:TI,AB,KY 49
#34 (endometr* adj2 destr*):TI,AB,KY 4
#35 (saline adj2 irrigat*):TI,AB,KY 292
#36 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 48759
#37 #13 AND #36 330
Appendix 3. MEDLINE search strategy
Searched from 1946 to 22 May 2018
OVID platform
1 (menstrua$ adj5 disorder$).tw. (2660) 2 (heavy adj5 menstrua$).ti,ab,hw,tn,mf. (898) 3 (iron adj5 anaem$).ti,ab,hw,tn,mf. (3454) 4 menorrhag$.ti,ab,hw,tn,mf. (5518) 5 hypermenorr$.ti,ab,hw,tn,mf. (285) 6 exp menorrhagia/ (4030) 7 (dysfunction$ adj2 uter$).tw. (1134) 8 (excessive$ adj2 menstru$).tw. (198) 9 (heavy adj2 menses).tw. (42) 10 (abnormal$ adj2 uterine).tw. (2815) 11 (excessive$ adj2 uter$).tw. (196) 12 (heavy adj2 period$).tw. (441) 13 or/1‐12 (15769) 14 exp Endometrial Ablation Techniques/ (328) 15 TCRE.tw. (97) 16 resect$.tw. (310456) 17 ablat$.tw. (95314) 18 (rollerball$ or bipolar or monopolar).tw. (58787) 19 microwav$.tw. (30965) 20 electrosurg$.tw. (3287) 21 hypertherm$.tw. (32047) 22 photodynam$.tw. (20312) 23 thermotherap$.tw. (2188) 24 phototherap$.tw. (7735) 25 cryoablat$.tw. (3059) 26 radiofreq$.tw. (29118) 27 laser*.tw. (239302) 28 Thermachoice.tw. (44) 29 Cavaterm.tw. (18) 30 Elitt.tw. (5) 31 Vesta.tw. (72) 32 Novasure.tw. (58) 33 Microsoulis.tw. (0) 34 Cryogen.tw. (382) 35 (endometr$ adj2 destr$).tw. (125) 36 (saline adj2 irrigat$).tw. (1245) 37 balloon.tw. (57900) 38 or/14‐37 (820849) 39 randomized controlled trial.pt. (462119) 40 controlled clinical trial.pt. (92434) 41 randomized.ab. (413162) 42 placebo.tw. (194596) 43 clinical trials as topic.sh. (183822) 44 randomly.ab. (291200) 45 trial.ti. (183145) 46 (crossover or cross‐over or cross over).tw. (76588) 47 or/39‐46 (1181233) 48 exp animals/ not humans.sh. (4462498) 49 47 not 48 (1086655) 50 13 and 38 and 49 (252)
Appendix 4. Embase search strategy
Searched from 1980 to 22 May 2018
OVID platform
1 (menstrua$ adj5 disorder$).tw. (3137) 2 (heavy adj5 menstrua$).ti,ab,hw,tn,mf. (1518) 3 (iron adj5 anaem$).ti,ab,hw,tn,mf. (5005) 4 menorrhag$.ti,ab,hw,tn,mf. (9711) 5 hypermenorr$.ti,ab,hw,tn,mf. (657) 6 menstruation disorder/ or exp "menorrhagia and metrorrhagia"/ or exp hypermenorrhea/ or exp menorrhagia/ or exp metrorrhagia/ (21442) 7 (dysfunction$ adj2 uter$).tw. (1441) 8 (excessive$ adj2 menstru$).tw. (257) 9 (heavy adj2 menses).tw. (76) 10 (abnormal$ adj2 uterine).tw. (4338) 11 (excessive$ adj2 uter$).tw. (265) 12 (heavy adj2 period$).tw. (583) 13 or/1‐12 (34189) 14 exp endometrium ablation/ (2421) 15 ablat$.tw. (138348) 16 TCRE.tw. (159) 17 resect$.tw. (426396) 18 (rollerball$ or bipolar or monopolar).tw. (81906) 19 electrosurg$.tw. (4197) 20 hypertherm$.tw. (36664) 21 photodynam$.tw. (23701) 22 thermotherap$.tw. (2768) 23 phototherap$.tw. (10396) 24 cryoablat$.tw. (5252) 25 radiofreq$.tw. (43255) 26 (laser adj3 interstit$).tw. (1020) 27 Thermachoice.tw. (109) 28 Cavaterm.tw. (26) 29 Elitt.tw. (7) 30 Vesta.tw. (69) 31 Novasure.tw. (201) 32 Microsoulis.tw. (0) 33 Cryogen.tw. (442) 34 (endometr$ adj2 destr$).tw. (185) 35 microwave.tw. (31370) 36 (saline adj2 irrigat$).tw. (1637) 37 (heat$ adj2 balloon).tw. (31) 38 or/14‐37 (741288) 39 Clinical Trial/ (964217) 40 Randomized Controlled Trial/ (499723) 41 exp randomization/ (78253) 42 Single Blind Procedure/ (31333) 43 Double Blind Procedure/ (147013) 44 Crossover Procedure/ (55360) 45 Placebo/ (311217) 46 Randomi?ed controlled trial$.tw. (181155) 47 Rct.tw. (28426) 48 random allocation.tw. (1779) 49 randomly allocated.tw. (29590) 50 allocated randomly.tw. (2315) 51 (allocated adj2 random).tw. (796) 52 Single blind$.tw. (20811) 53 Double blind$.tw. (181979) 54 ((treble or triple) adj blind$).tw. (775) 55 placebo$.tw. (267842) 56 prospective study/ (448348) 57 or/39‐56 (1902190) 58 case study/ (54421) 59 case report.tw. (353224) 60 abstract report/ or letter/ (1037032) 61 or/58‐60 (1436046) 62 57 not 61 (1854050) 63 13 and 38 and 62 (744)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 22 May 2018
OVID platform
1 exp Menstrual Disorders/ (1180) 2 menorrhag$.tw. (83) 3 (menstrua$ adj5 disorder$).tw. (382) 4 (heavy adj5 menstrua$).tw. (28) 5 (iron adj5 anaem$).tw. (43) 6 hypermenorr$.tw. (2) 7 (dysfunction$ adj2 uter$).tw. (31) 8 (excessive$ adj2 menstru$).tw. (8) 9 (heavy adj2 menses).tw. (1) 10 (abnormal$ adj2 uterine).tw. (28) 11 (excessive$ adj2 uter$).tw. (5) 12 (heavy adj2 period$).tw. (78) 13 or/1‐12 (1645) 14 TCRE.tw. (2) 15 (transcerv$ adj3 resect$).tw. (1) 16 rollerball$.tw. (1) 17 electrosurg$.tw. (13) 18 hypertherm$.tw. (1492) 19 photodynam$.tw. (35) 20 thermotherap$.tw. (11) 21 phototherap$.tw. (353) 22 cryoablat$.tw. (12) 23 radiofreq$.tw. (420) 24 (laser adj3 interstit$).tw. (15) 25 Thermachoice.tw. (0) 26 Cavaterm.tw. (0) 27 Elitt.tw. (0) 28 Vesta.tw. (12) 29 Novasure.tw. (1) 30 Microsoulis.tw. (0) 31 Cryogen.tw. (0) 32 (endometr$ adj2 destr$).tw. (0) 33 (endometr$ adj2 resection$).tw. (6) 34 (saline adj2 irrigat$).tw. (10) 35 (heat$ adj2 balloon).tw. (1) 36 ablat$.tw. (5042) 37 or/14‐36 (7315) 38 13 and 37 (12) 39 random.tw. (52808) 40 control.tw. (407679) 41 double‐blind.tw. (21474) 42 clinical trials/ (10902) 43 placebo/ (5096) 44 exp Treatment/ (711851) 45 or/39‐44 (1108122) 46 38 and 45 (10)
Appendix 6. CINAHL search strategy
Searched from 1961 to 22 May 2018
EBSCO platform
| # | Query | Results |
| S54 | S41 AND S53 | 105 |
| S53 | S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 | 1,234,529 |
| S52 | TX allocat* random* | 8,702 |
| S51 | (MH "Quantitative Studies") | 19,585 |
| S50 | (MH "Placebos") | 10,775 |
| S49 | TX placebo* | 50,943 |
| S48 | TX random* allocat* | 8,702 |
| S47 | (MH "Random Assignment") | 47,415 |
| S46 | TX randomi* control* trial* | 149,041 |
| S45 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 957,459 |
| S44 | TX clinic* n1 trial* | 224,755 |
| S43 | PT Clinical trial | 86,361 |
| S42 | (MH "Clinical Trials+") | 240,916 |
| S41 | S13 AND S40 | 369 |
| S40 | S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 32,031 |
| S39 | TX heat* N3 balloon | 7 |
| S38 | TX saline N2 irrigat* | 304 |
| S37 | TX electrode* N2 ablat* | 136 |
| S36 | TX endometr* N3 destr* | 26 |
| S35 | TX Cryogen* | 131 |
| S34 | TX Novasure | 31 |
| S33 | TX vesta | 70 |
| S32 | TX Cavaterm | 4 |
| S31 | TX Thermachoice | 15 |
| S30 | TX laser* N5 interstit* | 63 |
| S29 | TX radiofreq* | 7,661 |
| S28 | TX cryoablat* | 953 |
| S27 | TX phototherap* | 3,269 |
| S26 | TX thermotherap* | 212 |
| S25 | TX photodynam* | 1,903 |
| S24 | TX hypertherm* | 3,005 |
| S23 | TX electrosurg* | 1,177 |
| S22 | TX microwav* N5 ablat* | 458 |
| S21 | TX balloon N5 ablat* | 147 |
| S20 | TX rollerball* | 22 |
| S19 | TX laser N5 ablat* | 1,092 |
| S18 | TX transcerv* N3 resect* | 53 |
| S17 | TX TCRE | 7 |
| S16 | TX endometr* N3 resect* | 221 |
| S15 | TX endometr* N2 ablat* | 477 |
| S14 | (MM "Endometrial Ablation Techniques") OR (MM "Ablation Techniques+") | 18,318 |
| S13 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 4,085 |
| S12 | TX heavy N2 period* | 124 |
| S11 | TX excessiv* N3 uter* | 54 |
| S10 | TX abnormal* N2 uterine | 603 |
| S9 | TX heavy N2 mense* | 16 |
| S8 | TX excessiv* N2 menstru* | 42 |
| S7 | TX dysfunction* N2 uter* | 159 |
| S6 | TX hypermenorr* | 15 |
| S5 | TX iron N5 anaem* | 617 |
| S4 | TX heavy N5 menstrua* | 340 |
| S3 | TX menstrua* N5 disorder* | 1,562 |
| S2 | TX menorrhag* | 1,126 |
| S1 | (MM "Menorrhagia") | 599 |
Data and analyses
Comparison 1. Laser ablation (first generation) versus transcervical resection of the endometrium (TCRE) (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding ‐ blood loss (mL) at 6 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 23.6 [‐8.32, 55.52] |
| 2 Bleeding | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Amenorrhoea rate at 6 months | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.45] |
| 2.2 Amenorrhoea/hypomenorrhoea rate at 6 months | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 2.3 Amenorrhoea/hypomenorrhoea rate at 12 months | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| 3 Rate of satisfaction at 12 months (very/moderately) | 1 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
| 4 Duration of operation (minutes) | 2 | 386 | Mean Difference (IV, Fixed, 95% CI) | 9.15 [7.21, 11.09] |
| 5 Operative difficulties | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Procedure abandoned | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.61, 3.51] |
| 5.2 Failed instrumentation | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.05] |
| 5.3 Equipment failure | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.54 [1.65, 18.60] |
| 5.4 Immediate hysterectomy | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 6 Good general health | 1 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 7 Improvement in menstrual symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Improvement in symptoms (general) | 1 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.21] |
| 7.2 Improvement in dysmenorrhoea at 6 months | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.38] |
| 7.3 Improvement in dysmenorrhoea at 12 months | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 8 Complication rate: major complications | 2 | 2218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.83, 2.41] |
| 8.1 Perforation | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.69] |
| 8.2 Bowel obstruction | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 71.59] |
| 8.3 Pelvic sepsis | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.25, 2.62] |
| 8.4 Haematometra | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.05] |
| 8.5 Glycine toxicity | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [0.23, 79.10] |
| 8.6 Fluid overload (> 1.5 L) | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [1.44, 16.61] |
| 8.7 Uterine tamponade | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.33] |
| 9 Complication rate: minor complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Burns | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.21] |
| 9.2 Urinary tract infection | 1 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.36, 10.55] |
| 10 Requirement for further surgical treatment (within 12 months) | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.29] |
Comparison 2. Vaporising electrode ablation (first generation) versus TCRE (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding ‐ amenorrhoea rate at 12 months' follow‐up | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 1.1 Amenorrhoea rate at 12 months' follow‐up | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.24] |
| 1.2 Amenorrhea/hypomenorrhoea rate at 12 months' follow‐up | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 2 Bleeding ‐ PBAC score at 12 months | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐19.18, 9.18] |
| 3 Rate of satisfaction at 12 months (very/moderately) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 4 Duration of operation (minutes) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.65, ‐0.35] |
| 5 Operative difficulties | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Difficulty with surgery (moderate or severe) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.82] |
| 6 Complication rate: major complications | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐258.0 [‐342.05, ‐173.95] |
| 6.1 Degree of fluid deficit (mL) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐258.0 [‐342.05, ‐173.95] |
Comparison 3. Rollerball (first generation) versus TCRE (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of operation (minutes) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.92, 0.72] |
| 2 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Fluid deficit | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.76] |
| 2.2 Perforation | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.76] |
| 3 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 2 years' follow‐up hysterectomy or ablation | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.55, 1.95] |
| 3.2 At 2 years' follow‐up (hysterectomy only) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.43, 4.88] |
| 3.3 At 2 to 5 years' follow‐up (hysterectomy or ablation) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.70, 2.10] |
| 3.4 At 2 to 5 years' follow‐up (hysterectomy only) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.51, 2.85] |
| 3.5 At more than 5 years' follow‐up (hysterectomy or ablation) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.82, 2.36] |
| 3.6 At more than 5 years' follow‐up (hysterectomy only) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.66, 2.63] |
Comparison 4. Thermal laser (second generation) versus TCRE (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding ‐ amenorrhoea rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1 year follow‐up | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.50, 4.03] |
| 1.2 At 2 to 5 years' follow‐up | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.48, 4.21] |
| 2 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 year follow‐up | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 2.2 At 2 to 5 years' follow‐up | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
| 3 Duration of operation | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐9.30 [‐11.36, ‐7.24] |
| 4 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Perforation | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Complication rate: minor complications | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.47] |
| 5.1 UTI | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.47] |
| 6 Requirement for further surgery rate (hysterectomy only) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.35] |
| 6.1 At 2 to 5 years' follow‐up | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.35] |
Comparison 5. Hydrothermal ablation (second generation) versus rollerball (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PBAC ≤ 75 at 1 year follow‐up | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.07] |
| 1.2 PBAC ≤ 100 at 1 year follow‐up | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.07] |
| 1.3 PBAC ≤ 100 at 2 years' follow‐up | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 1.4 PBAC ≤ 100 at 2 to 5 years' follow‐up | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 1.5 Amenorrhoea at 1 year follow‐up | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.05] |
| 1.6 Amenorrhoea at 2 years' follow‐up | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.75, 1.36] |
| 1.7 Amenorrhoea at 2 to 5 years' follow‐up | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Rate of satisfaction | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 2.1 At 2 to 5 years' follow‐up | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 3 Proportion given local rather than general anaesthesia | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.32, 3.09] |
| 4 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cervical lacerations | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.92] |
| 4.2 Haematometra | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.93] |
| 4.3 Endometritis | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.08, 10.05] |
| 5 Complication rate: minor complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Abdominal pain (at 2 weeks) | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.03, 1.90] |
| 5.2 Nausea or vomiting | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [1.36, 6.98] |
| 5.3 Uterine cramping | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.72, 1.74] |
| 5.4 Urinary tract infection | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.23, 5.83] |
| 5.5 First‐degree burn | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.11, 47.89] |
| 6 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 1 year follow‐up (any surgery) | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.11, 47.89] |
| 6.2 At 2 to 5 years' follow‐up (any surgery) | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.58, 2.73] |
| 6.3 At 5 years' follow‐up (hysterectomy only) | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.58, 4.06] |
Comparison 6. Cryoablation (second generation) versus rollerball (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 1.1 Amenorrhoea at 1 year follow‐up | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 2 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 year follow‐up | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.17] |
| 2.2 At 2 years' follow‐up | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.17] |
| 3 Proportion given local anaesthesia (%) | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.62 [3.22, 13.63] |
| 4 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Perforation | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 3.63] |
| 5 Complication rate: minor complications | 1 | 1116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.15, 2.09] |
| 5.1 Vaginal bleeding | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.05, 33.43] |
| 5.2 Abdominal cramping | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.11, 47.54] |
| 5.3 UTI | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 3.65] |
| 5.4 Severe pelvic pain | 1 | 279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 3.65] |
| 6 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 2 years' follow‐up (any surgery) | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.22] |
| 6.2 At 2 years' follow‐up (hysterectomy) | 1 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.34, 2.00] |
Comparison 7. Electrode ablation (second generation) versus TCRE + rollerball (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding ‐ amenorrhoea rate at 1 year follow‐up | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.31] |
| 1.1 Balloon system | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.29] |
| 1.2 Mesh system | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.64] |
| 2 Proportion with successful Rx (PBAC < 75) | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.15] |
| 2.1 Balloon system | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.17] |
| 2.2 Mesh system | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.22] |
| 3 PBAC score 12 months after treatment | Other data | No numeric data | ||
| 3.1 Balloon system | Other data | No numeric data | ||
| 3.2 Mesh system | Other data | No numeric data | ||
| 4 Rate of satisfaction with treatment at 1 year | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
| 4.1 Mesh system | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.06] |
| 5 Duration of operation (minutes) | 2 | 520 | Mean Difference (IV, Fixed, 95% CI) | ‐18.70 [‐20.66, ‐16.75] |
| 5.1 Balloon system | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐16.20 [‐19.55, ‐12.85] |
| 5.2 Mesh system | 1 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐22.41, ‐17.59] |
| 6 Procedure abandon | 1 | 267 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.10, 63.95] |
| 7 Proportion given local anaesthesia (%) | 2 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [2.94, 5.04] |
| 7.1 Balloon system | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.66 [2.65, 5.07] |
| 7.2 Mesh system | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [2.61, 6.47] |
| 8 Complication rate: major complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Cervical tear/stenosis | 2 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.87] |
| 8.2 Perforation | 2 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.01] |
| 8.3 Pelvic abscess | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.19] |
| 8.4 Haematometra | 2 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.08, 2.23] |
| 8.5 Fluid overload | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.93] |
| 8.6 Myometritis | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.93] |
| 8.7 Urinary incontinence | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.93] |
| 8.8 PID | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.09, 11.19] |
| 8.9 Endometritis | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.01] |
| 9 Complication rate: minor complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Nausea/vomiting or severe pelvic pain | 2 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.37, 3.27] |
| 9.2 UTI | 2 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.39, 2.84] |
| 9.3 Fever | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.05, 13.51] |
| 9.4 Haemorrhage | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.03, 8.13] |
| 9.5 Bradycardia | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.06, 37.70] |
| 10 Requirement for further surgery at 2 years (hysterectomy) | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.18, 1.50] |
| 10.1 Balloon system | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.18, 1.50] |
Comparison 8. Microwave ablation (second generation) versus TCRE + rollerball (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PBAC < 75 or acceptable improvement at 1 year follow‐up | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.96, 1.13] |
| 1.2 PBAC < 75 or acceptable improvement at 2 to 5 years' follow‐up | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.28] |
| 1.3 PBAC < 75 or acceptable improvement at > 5 years' follow‐up | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 1.4 Amenorrhoea at 1 year follow‐up | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.93, 1.36] |
| 1.5 Amenorrhoea at 2 years' follow‐up | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.53] |
| 1.6 Amenorrhoea at 2 to 5 years' follow‐up | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.12] |
| 1.7 Amenorrhoea at > 5 years' follow‐up | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Rate of satisfaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 1 year follow‐up | 2 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 2.2 At 2 years' follow‐up | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.02, 1.38] |
| 2.3 At 2 to 5 years' follow‐up | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.04, 1.36] |
| 2.4 At 10 years' follow‐up | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.30] |
| 3 Duration of operation (minutes) | Other data | No numeric data | ||
| 4 Operative difficulties | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Equipment failure | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.81 [1.09, 13.34] |
| 4.2 Procedure abandoned | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.31, 3.50] |
| 5 Proportion given local anaesthesia | 1 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.73, 3.72] |
| 6 Duration of hospital stay (hours) | Other data | No numeric data | ||
| 7 Inability to work (proportion of women) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 12 months' follow‐up | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.73] |
| 7.2 At > 5 years' follow‐up | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.87] |
| 8 Quality of life ‐ change in SF‐36 score after treatment | Other data | No numeric data | ||
| 8.1 Physical functioning | Other data | No numeric data | ||
| 8.2 Social functioning | Other data | No numeric data | ||
| 8.3 Physical role | Other data | No numeric data | ||
| 8.4 Emotional role | Other data | No numeric data | ||
| 8.5 Mental health | Other data | No numeric data | ||
| 8.6 Energy/fatigue | Other data | No numeric data | ||
| 8.7 Pain | Other data | No numeric data | ||
| 8.8 General health | Other data | No numeric data | ||
| 9 Improvement in other menstrual symptoms: PMS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 At 1 year follow‐up | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.19] |
| 9.2 At 2 to 5 years' follow‐up | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.28] |
| 10 Improvement in other menstrual symptoms | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Improvement in dysmenorrhoea at 1 year follow‐up | 2 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.09] |
| 10.2 Improvement in dysmenorrhoea at 2 years' follow‐up | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.19] |
| 11 Reduction in pain score (points) | 1 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.32, 2.72] |
| 11.1 At > 5 years' follow‐up | 1 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.32, 2.72] |
| 12 Postoperative analgesia rate | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.10] |
| 13 Complication rate: major complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Perforation | 2 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.22, 12.12] |
| 13.2 Cervical laceration | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.07, 3.48] |
| 13.3 Cervical stenosis | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.52] |
| 13.4 Endometritis | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.5 [0.37, 114.31] |
| 14 Complication rate: minor complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Chills | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.59, 3.11] |
| 14.2 Bloating | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.38, 1.83] |
| 14.3 Dysuria | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.58] |
| 14.4 Fever | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.12, 51.62] |
| 14.5 Headache | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.59] |
| 14.6 Nausea | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.83, 2.21] |
| 14.7 Vomiting | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.61 [1.30, 10.00] |
| 14.8 UTI | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.03, 7.88] |
| 14.9 Vaginal infection | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.52] |
| 14.10 Uterine cramping | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.01, 1.44] |
| 14.11 Abdominal tenderness | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.42] |
| 14.12 Haemorrhage | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.69] |
| 15 Requirement for further surgery | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 At 1 year follow‐up (any surgery) | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.38, 1.80] |
| 15.2 At 1 year follow‐up (hysterectomy only) | 2 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.70] |
| 15.3 At 2 years' follow‐up (any surgery) | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.55, 1.72] |
| 15.4 At 2 years' follow‐up (hysterectomy only) | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.81] |
| 15.5 At 5 years' follow‐up (ablation or hysterectomy) | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.27] |
| 15.6 At 5 years' follow‐up (hysterectomy only) | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.04] |
| 15.7 At 10 years' follow‐up (ablation or hysterectomy) | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.23] |
| 15.8 At 10 years' follow‐up (hysterectomy only) | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
Comparison 9. Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 1 year follow‐up | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.39, 1.00] |
| 1.2 Amenorrhoea at 2 years' follow‐up | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.33, 1.07] |
| 1.3 Amenorrhoea at 2 to 5 years' follow‐up | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.39, 1.25] |
| 1.4 Amenorrhoea/eumenorrhoea rate at 1 year follow‐up | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 1.5 Amenorrhoea/eumenorrhoea rate at 2 years' follow‐up | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 1.6 Amenorrhoea/eumenorrhoea rate at 2 to 5 years' follow‐up | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 2 PBAC score after treatment | Other data | No numeric data | ||
| 2.1 At 1 year follow‐up | Other data | No numeric data | ||
| 2.2 At 2 years' follow‐up | Other data | No numeric data | ||
| 3 Success of treatment (lighter periods and no further surgery) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 2 to 5 years' follow‐up | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.20] |
| 4 Success of treatment (menstrual score < 185) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 year follow‐up | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 4.2 At 2 years' follow‐up | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.23] |
| 5 Rate of satisfaction | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 1 year follow‐up | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 5.2 At 2 years' follow‐up | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 5.3 At 2 to 5 years' follow‐up | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.01] |
| 6 Duration of operation (minutes) | 2 | 378 | Mean Difference (IV, Fixed, 95% CI) | ‐14.58 [‐15.00, ‐12.17] |
| 7 Operative difficulties | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.49, 2.22] |
| 7.1 Technical complication rate | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.49, 2.22] |
| 8 Inability to work (proportion of women) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 At 1 year follow‐up | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.37, 6.22] |
| 8.2 At 2 years' follow‐up | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.03, 2.72] |
| 8.3 At 2 to 5 years' follow‐up | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.26, 2.93] |
| 9 Improvement in other menstrual symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Improvement in dysmenorrhoea at 12 months | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.09] |
| 9.2 Improvement in premenstrual symptoms (from moderate/severe) at 1 year follow‐up | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |
| 9.3 Improvement in premenstrual symptoms (from moderate/severe) at 2 years follow up | 1 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 9.4 Improvement in premenstrual symptoms (from moderate/severe) at 2 to 5 years' follow‐up | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.75, 1.30] |
| 10 Complication rate: major complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Fluid overload | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.76] |
| 10.2 Perforation | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.42] |
| 10.3 Cervical lacerations | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.42] |
| 10.4 Endometritis | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.29, 25.93] |
| 10.5 Haematometra | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.39] |
| 11 Complication rate: minor complications | 2 | 895 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.32, 3.12] |
| 11.1 UTI | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.11, 68.41] |
| 11.2 Hydrosalpinx | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.47] |
| 11.3 Pain | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.87 [0.30, 115.87] |
| 11.4 Nausea | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 6.61] |
| 11.5 Infection | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 6.61] |
| 12 Requirement for further surgery | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 At 1 year follow‐up (any surgery) | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.10, 3.57] |
| 12.2 At 2 years' follow‐up (any surgery) | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.28] |
| 12.3 At 2 to 5 years' follow‐up (any surgery) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.64, 1.55] |
| 12.4 At 2 years' follow‐up (hysterectomy) | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.38, 2.83] |
| 12.5 At 2 to 5 years' follow‐up (hysterectomy) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.61, 1.63] |
9.4. Analysis.
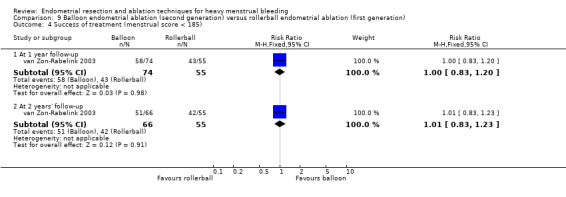
Comparison 9 Balloon endometrial ablation (second generation) versus rollerball endometrial ablation (first generation), Outcome 4 Success of treatment (menstrual score < 185).
Comparison 10. Balloon (second generation) versus laser (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.61, 2.02] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.46] |
| 2 PBAC score after treatment | Other data | No numeric data | ||
| 2.1 At 6 months' follow‐up | Other data | No numeric data | ||
| 3 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months' follow‐up | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.20] |
| 3.2 At 12 months' follow‐up | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.09] |
| 4 Operative difficulties | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [0.22, 89.94] |
| 4.1 Failure of equipment | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [0.22, 89.94] |
| 5 Pain score 4 hours post procedure | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 32.7 [23.72, 41.68] |
| 6 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 EQ‐5D at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.13] |
| 6.2 EQ‐5D VAS at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐5.95, 8.35] |
| 6.3 SF‐12 physical scale at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐2.18, 5.58] |
| 6.4 SF‐12 mental scale at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐0.42, 7.22] |
| 6.5 SAQ pleasure scale at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.30, 2.30] |
| 6.6 SAQ habit scale at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.42, 0.10] |
| 6.7 SAQ discomfort scale at 6 months' follow‐up | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.98, 0.70] |
| 6.8 EQ‐5D at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.13, 0.11] |
| 6.9 EQ‐5D VAS at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [2.43, 17.77] |
| 6.10 SF‐12 physical scale at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.89, 3.49] |
| 6.11 SF‐12 mental scale at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐2.04, 6.24] |
| 6.12 SAQ pleasure scale at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.68, 1.48] |
| 6.13 SAQ habit scale at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.27, 0.09] |
| 6.14 SAQ discomfort scale at 12 months' follow‐up | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.67, 0.87] |
| 7 Improvement in other menstrual symptoms | Other data | No numeric data | ||
| 7.1 PMS at 6 months' follow‐up | Other data | No numeric data | ||
| 7.2 PMS at 12 months' follow‐up | Other data | No numeric data | ||
| 8 Improvement in other menstrual symptoms: dysmenorrhoea (visual analogue) | Other data | No numeric data | ||
| 8.1 Dysmenorrhoea at 6 months' follow‐up | Other data | No numeric data | ||
| 8.2 Dysmenorrhoea at 12 months' follow‐up | Other data | No numeric data | ||
| 9 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 At 12 months' follow‐up | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.23, 2.64] |
Comparison 11. Balloon (second generation) versus TCRE (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.93] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.95] |
| 2 Rate of satisfaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.20] |
| 2.2 At 12 months' follow‐up | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.18] |
| 2.3 At 2 years' follow‐up | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.06, 1.72] |
| 3 Duration of operation (minutes) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐15.20, ‐10.80] |
| 4 Duration of operation (minutes) | Other data | No numeric data | ||
| 5 Operative difficulties | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.22 [0.42, 123.83] |
| 5.1 Equipment failure | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.22 [0.42, 123.83] |
| 6 Postoperative pain (continuous data) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐0.88, ‐0.32] |
| 7 Postoperative pain (descriptive data) | Other data | No numeric data | ||
| 8 Hospital stay (days) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.52, ‐0.08] |
| 9 Duration of hospital stay (hours) | Other data | No numeric data | ||
| 10 Return to normal activities (days) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.38, ‐0.82] |
| 11 Return to normal activities (days) | Other data | No numeric data | ||
| 12 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Fluid overload | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.67] |
| 12.2 Cervical tear | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.34] |
| 12.3 Conversion to hysterectomy | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.84] |
| 12.4 Blood transfusion | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [0.26, 105.97] |
| 13 Complication rate: minor complications | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐81.8 [‐93.33, ‐70.27] |
| 13.1 Blood loss | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐81.8 [‐93.33, ‐70.27] |
| 14 Complication rate: minor complications (dichotomous) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Fever | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.57] |
| 14.2 Urinary infection or retention | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.34] |
| 14.3 Haemorrhage | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.38, 4.54] |
| 15 Requirement for further surgery | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 At 12 months' follow‐up (ablation and hysterectomy) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.64] |
| 15.2 At 2 years' follow‐up (ablation and hysterectomy) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.81] |
| 15.3 At 12 months' follow‐up (hysterectomy only) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.44] |
11.9. Analysis.
Comparison 11 Balloon (second generation) versus TCRE (first generation), Outcome 9 Duration of hospital stay (hours).
| Duration of hospital stay (hours) | |||
|---|---|---|---|
| Study | Cavaterm balloon | TCRE | Comments |
| Brun 2006 | n=31 Median (range): 21 (0‐36) |
n=20 Median (range): 30 (6‐72) |
Mann Whitney rank sum test P=0.012 |
Comparison 12. Bipolar radiofrequency (second generation) versus balloon ablation (second generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 3 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.37 [2.09, 5.44] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 4 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [2.06, 4.72] |
| 1.3 Amenorrhoea at 2 to 5 years' follow‐up | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.93, 2.64] |
| 1.4 Amenorrhoea at 10 years' follow‐up | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.83, 1.46] |
| 2 PBAC score after treatment | Other data | No numeric data | ||
| 2.1 At 6 months' follow‐up | Other data | No numeric data | ||
| 2.2 At 12 months' follow‐up | Other data | No numeric data | ||
| 3 Rate of satisfaction | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months' follow‐up | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.24] |
| 3.2 At 12 months' follow‐up | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.04, 1.26] |
| 3.3 At 10 years' follow‐up | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.30] |
| 4 Duration of operation | Other data | No numeric data | ||
| 5 Operative difficulties | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Technical complication rate | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.13, 3.99] |
| 6 Completion of procedure | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.97, 1.15] |
| 7 Time taken off work (days) | Other data | No numeric data | ||
| 8 Time to resume normal activities (days) | Other data | No numeric data | ||
| 9 Quality of life | 3 | 3221 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.18, 0.19] |
| 9.1 SF‐12 physical scale score at 12 months' follow‐up | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐4.27, 7.47] |
| 9.2 SF‐12 mental scale score at 12 months' follow‐up | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [‐0.52, 15.52] |
| 9.3 SF‐36 physical function scale score at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.55, 10.55] |
| 9.4 SF‐36 physical function scale score at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐6.44, 12.44] |
| 9.5 SF‐36 physical function scale score at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐8.26, 12.26] |
| 9.6 SF‐36 role physical at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐7.67, 17.67] |
| 9.7 SF‐36 role physical at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐6.96, 16.96] |
| 9.8 SF‐36 role physical at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐2.66, 18.66] |
| 9.9 SF‐36 role emotional at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐18.64, 6.64] |
| 9.10 SF‐36 role emotional at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐1.92, 9.92] |
| 9.11 SF‐36 role emotional at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐14.45, ‐3.55] |
| 9.12 SF‐36 social functioning at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐9.98, 7.98] |
| 9.13 SF‐36 social functioning at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐6.17, 12.17] |
| 9.14 SF‐36 social functioning at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐5.60, 13.60] |
| 9.15 SF‐36 mental health at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐10.84, 4.84] |
| 9.16 SF‐36 mental health at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐8.03, 8.03] |
| 9.17 SF‐36 mental health at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐11.39, 1.39] |
| 9.18 SF‐36 energy/vitality at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐13.54, 1.54] |
| 9.19 SF‐36 energy/vitality at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐0.44, 18.44] |
| 9.20 SF‐36 energy/vitality at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐10.39, 4.39] |
| 9.21 SF‐36 pain at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.00, 12.00] |
| 9.22 SF‐36 pain at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐12.61, 10.61] |
| 9.23 SF‐36 pain at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐14.79, 4.79] |
| 9.24 SF‐36 general health at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐13.30, 3.30] |
| 9.25 SF‐36 general health at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐4.10, 16.10] |
| 9.26 SF‐36 general health at 2 to 5 years' follow‐up | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.72, 17.72] |
| 9.27 RSCL physical symptoms at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐3.94, 5.94] |
| 9.28 RSCL physical symptoms at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐8.56, 0.56] |
| 9.29 RSCL psychological distress at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐10.14, 8.14] |
| 9.30 RSCL psychological distress at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.92, 5.92] |
| 9.31 RSCL activity level at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.35, 1.35] |
| 9.32 RSCL activity level at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐4.32, 0.32] |
| 9.33 RSCL overall quality of life at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐12.29, 8.29] |
| 9.34 RSCL overall quality of life at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐18.77, 0.77] |
| 9.35 SDS depression at 6 months' follow‐up | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐1.55, 5.55] |
| 9.36 SDS depression at 12 months' follow‐up | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐5.24, 3.24] |
| 9.37 Multi‐attribute utility tool at 12 months' follow‐up | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 8.80 [‐6.08, 23.68] |
| 9.38 EQ‐5D utility at 12 months' follow‐up | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.16, 0.22] |
| 9.39 EQ‐5D health thermometer at 12 months' follow‐up | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 4.8 [‐10.07, 19.67] |
| 10 Menorrhagia Outcome Questionnaire | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.87, 2.67] |
| 10.1 At 12 months' follow‐up | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.87, 2.67] |
| 11 Dysmenorrhoea rate (VAS score) | Other data | No numeric data | ||
| 11.1 At 12 months' follow‐up | Other data | No numeric data | ||
| 12 Improvement in other menstrual symptoms | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.20] |
| 12.1 Improvement in dysmenorrhoea at 12 months' follow‐up | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.89, 2.10] |
| 12.2 Improvement in PMS (emotional) at 12 months' follow‐up | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.45, 1.43] |
| 12.3 Improvement in PMS (physical) at 12 months' follow‐up | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.72, 2.34] |
| 12.4 Dysmenorrhoea rate at 6 months' follow‐up | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.26, 1.86] |
| 12.5 Dysmenorrhoea rate at 12 months' follow‐up | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.18, 1.51] |
| 12.6 Dysmenorrhoea rate at 2 to 5 years' follow‐up | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.44] |
| 13 PMS rate (VAS score) | Other data | No numeric data | ||
| 13.1 At 12 months' follow‐up | Other data | No numeric data | ||
| 14 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Infection (endometritis) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.42] |
| 15 Requirement for further surgery | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 At 6 months' follow‐up (ablation or hysterectomy) | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 At 12 months' follow‐up (ablation or hysterectomy) | 3 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.49, 3.67] |
| 15.3 At 12 months' follow‐up (hysterectomy only) | 3 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.84] |
| 15.4 At 2 to 5 years' follow‐up (ablation or hysterectomy) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.89] |
| 15.5 At 2 to 5 years' follow‐up (hysterectomy only) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.27, 2.20] |
| 15.6 At 10 years' follow‐up (hysterectomy only) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.38, 2.74] |
12.5. Analysis.
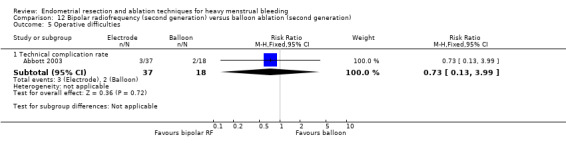
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 5 Operative difficulties.
12.6. Analysis.
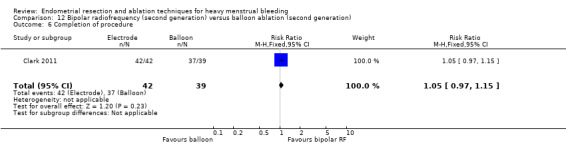
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 6 Completion of procedure.
12.10. Analysis.
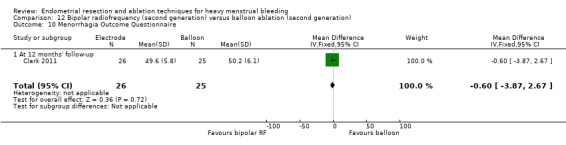
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 10 Menorrhagia Outcome Questionnaire.
12.12. Analysis.
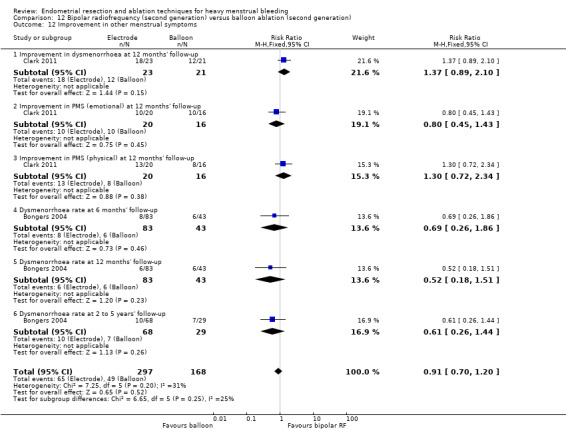
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 12 Improvement in other menstrual symptoms.
12.13. Analysis.
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 13 PMS rate (VAS score).
| PMS rate (VAS score) | |||
|---|---|---|---|
| Study | Electrode | Balloon | Statistical test |
| At 12 months' follow‐up | |||
| Abbott 2003 | N=37 Median score (range): 0 (0, 100) | N=18 Median score (range): 32 (0, 100) | Mann Whitney P=0.007 |
12.14. Analysis.
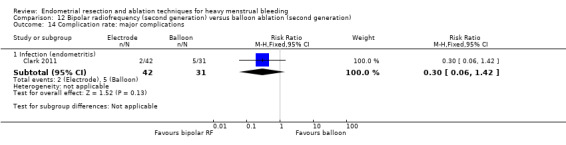
Comparison 12 Bipolar radiofrequency (second generation) versus balloon ablation (second generation), Outcome 14 Complication rate: major complications.
Comparison 13. Microwave ablation (second generation) versus balloon ablation (second generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 1 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.07, 2.12] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.47] |
| 1.3 Amenorrhoea at 5 years' follow‐up | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.23] |
| 2 PBAC score at 12 months' follow‐up | Other data | No numeric data | ||
| 3 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 12 months' follow‐up | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 3.2 At 5 years' follow‐up | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.13] |
| 4 Operation time (minutes) | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐7.36, ‐5.84] |
| 5 Operative difficulties causing failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Failure of device | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.70] |
| 5.2 Unsuitable cavity | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.30] |
| 5.3 Device not sterile | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 103.32] |
| 6 Proportion choosing local anaesthesia | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.31] |
| 7 Proportion requiring opiate analgesia | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 8 Recovery: proportion requiring overnight stay | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.04] |
| 9 Quality of life scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 EQ‐5D at 12 months' follow‐up | 1 | 285 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 9.2 EQ‐5D at 5 years' follow‐up | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 9.3 SF‐12 physical scores at 12 months' follow‐up | 1 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.64, 1.24] |
| 9.4 SF‐12 physical scores at 5 years' follow‐up | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐3.99, 0.99] |
| 9.5 SF‐12 mental scores at 12 months' follow‐up | 1 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.67, 1.27] |
| 9.6 SF‐12 mental scores at 5 years' follow‐up | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.90, 2.30] |
| 10 Requirement for further surgery (hysterectomy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 At 12 months' follow‐up | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.84] |
| 10.2 At 5 years' follow‐up | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.27] |
Comparison 14. Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [1.25, 4.12] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.21, 3.15] |
| 1.3 Amenorrhoea at 2 to 5 years' follow‐up | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.06, 2.31] |
| 2 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 6 months' follow‐up | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.17, 1.77] |
| 2.2 At 12 months' follow‐up | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.02, 1.21] |
| 2.3 At 2 to 5 years' follow‐up | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.23, 2.13] |
| 3 Duration of procedure (minutes) | Other data | No numeric data | ||
| 4 Improvement in other menstrual symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Absence of dysmenorrhoea at 12 months' follow‐up | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 4.2 Absence of dysmenorrhoea at 2 to 5 years' follow‐up | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.00, 1.74] |
| 5 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Uterine perforation | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 65.54] |
| 5.2 Saline leakage | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.46] |
| 6 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 12 months' follow‐up (ablation or hysterectomy) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.72] |
| 6.2 At 12 months' follow‐up (hysterectomy) | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.32] |
| 6.3 At 2 to 5 years' follow‐up (ablation or hysterectomy) | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.23, 0.83] |
| 6.4 At 2 to 5 years' follow‐up (hysterectomy) | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.38] |
14.5. Analysis.
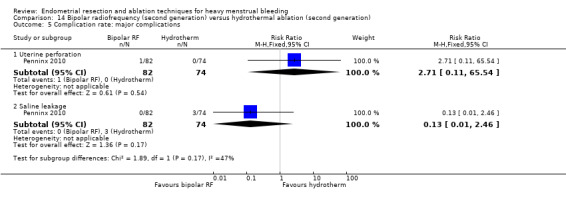
Comparison 14 Bipolar radiofrequency (second generation) versus hydrothermal ablation (second generation), Outcome 5 Complication rate: major complications.
Comparison 15. Ablative curettage versus overcurettage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.87, 3.58] |
| 1.1 Amenorrhoea at 3 years' follow‐up | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [2.33, 8.69] |
| 1.2 Amenorrhoea and eumenorrhoea at 3 years' follow‐up | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.30, 2.66] |
| 2 Surgery difficulties: failure rate of procedure | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.74] |
| 3 Recovery: hospital stay (days) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [1.18, 2.02] |
| 4 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Uterine perforation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.70] |
| 5 Complication rate: minor complications | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.16, 0.84] |
| 5.1 Bleeding | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.70] |
| 5.2 Infection/leucorrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| 6 Requirement for further surgery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.10] |
| 6.1 Within 3 years (hysterectomy) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.10] |
15.5. Analysis.
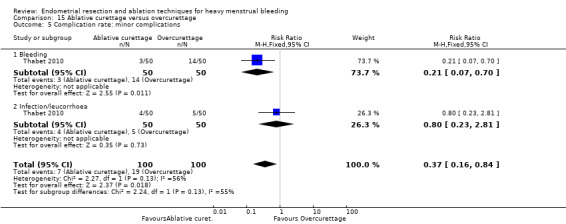
Comparison 15 Ablative curettage versus overcurettage, Outcome 5 Complication rate: minor complications.
Comparison 16. Microwave ablation (second generation) versus bipolar radiofrequency (second generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.07 [3.29, 15.22] |
| 1.1 Amenorrhoea at 3 months' follow‐up | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.33 [1.86, 15.30] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.77 [3.17, 30.11] |
| 2 Bleeding PBAC at 12 months' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Satisfaction ‐ with treatment at 3 months' follow‐up | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.05] |
| 3.2 Satisfaction ‐ with treatment at 12 months' follow‐up | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 3.3 Satisfaction ‐ improvement in everyday life at 12 months' follow‐up | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 4 Duration of treatment (seconds) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 9.80 [2.63, 16.97] |
| 5 Improvement in other menstrual symptoms: dysmenorrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Dysmenorrhoea at 3 months' follow‐up | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.39, 10.18] |
| 5.2 Dysmenorrhoea at 12 months' follow‐up | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.92, 17.44] |
| 6 Complication rate | 1 | 198 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.98 [1.44, 468.00] |
| 6.1 Minor complications | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Major complications | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Requirement for post‐procedure analgesia | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.98 [1.44, 468.00] |
| 7 Requirement for further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 12 months' follow‐up (hysterectomy) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.32] |
Comparison 17. Bipolar (Minerva) (second generation) versus rollerball ablation (first generation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Haematin alkaline < 80 mL/cycle at 12 months' follow‐up | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.00, 1.34] |
| 1.2 Amenorrhoea at 12 months' follow‐up | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.08, 1.98] |
| 2 Rate of satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 12 months' follow‐up | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 3 Duration of surgery | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐14.1 [‐15.94, ‐12.26] |
| 4 Improvement in other menstrual symptoms: dysmenorrhoea | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.48] |
| 5 Improvement in other menstrual symptoms: PMS at 12 months' follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Complication rate: major complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Endometritis or endomyometritis | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.02, 2.69] |
| 6.2 Pelvic inflammatory disease | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 6.3 Haematometra | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7 Complication rate: minor complications | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.26] |
| 7.1 Intraoperative skin rash and/or itching or burning sensation | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.2 Bleeding or spotting first 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 7.3 Nausea or vomiting first 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 7.4 Weakness, fatigue, sleepiness, lack of concentration, dizziness first 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.5 Backache first 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.6 Fever first 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.7 Abdominal pain or bloating (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.16, 14.06] |
| 7.8 Abdominal pain and/or bloating > 2 weeks | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 7.9 Pelvic pain (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.10 Vaginal discharge and/or unpleasant vaginal smell or other abnormal sensation (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.06, 36.54] |
| 7.11 Weakness, fatigue, sleepiness, lack of concentration, dizziness (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 7.83] |
| 7.12 Constipation (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 7.13 Skin rash and/or itching or burning sensation (> 24 hours to 2 weeks) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 7.83] |
| 7.14 Dysmenorrhoea (2 weeks to 1 year) | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.06] |
| 8 Requirement for further surgery | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.93] |
| 8.1 Hysterectomy at 12 months' follow‐up | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.93] |
Comparison 18. Overall analyses: second‐generation endometrial ablation versus first‐generation endometrial ablation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Amenorrhoea at 6 months' follow‐up | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.91, 1.77] |
| 1.2 Amenorrhoea at 1 year follow‐up | 12 | 2145 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.27] |
| 1.3 Amenorrhoea at 2 years' follow‐up | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.30] |
| 1.4 Amenorrhoea at 2 to 5 years' follow‐up | 4 | 672 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.78, 1.72] |
| 1.5 Amenorrhoea > 5 years' follow‐up | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.05] |
| 1.6 PBAC < 75 or acceptable improvement at 12 months' follow‐up | 5 | 1282 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.98, 1.09] |
| 1.7 PBAC < 75 or acceptable improvement at 2 to 5 years' follow‐up | 1 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.97, 1.28] |
| 1.8 PBAC < 75 or acceptable improvement at > 5 years' follow‐up | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.34] |
| 2 Bleeding ‐ amenorrhoea at 12 months' follow‐up (final plot) | 12 | 2145 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.27] |
| 3 Satisfaction rate | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 At 6 months' follow‐up | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.20] |
| 3.2 At 1 year follow‐up | 11 | 1750 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.98, 1.04] |
| 3.3 At 2 years' follow‐up | 5 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.99, 1.21] |
| 3.4 At 2 to 5 years' follow‐up | 4 | 672 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.93, 1.13] |
| 3.5 At 10 years' follow‐up | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.95, 1.30] |
| 4 Satisfaction rate at 1 year follow‐up (final plot) | 11 | 1750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 5 Duration of operation (minutes) | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Operative difficulties | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Equipment failure | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 4.26 [1.46, 12.43] |
| 6.2 Procedure abandoned | 3 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.38, 3.67] |
| 7 Proportion given local anaesthesia (%) | 6 | 1434 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.76, 4.40] |
| 8 Inability to work | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.30, 2.30] |
| 9 Complication rate: major complications | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Perforation | 8 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.10, 1.01] |
| 9.2 Endometritis | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.33, 4.37] |
| 9.3 Myometritis | 1 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 6.93] |
| 9.4 Cervical lacerations | 7 | 1583 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 9.5 Cervical stenosis | 1 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.06, 36.52] |
| 9.6 Pelvic abscess | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 4.19] |
| 9.7 Pelvic inflammatory disease | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.18, 7.98] |
| 9.8 Haematometra | 5 | 1193 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.12, 0.95] |
| 9.9 Blood transfusion | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 5.24 [0.26, 105.97] |
| 9.10 Fluid overload | 3 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.03, 0.94] |
| 10 Complication rate: minor complications | 10 | 6450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.11, 1.54] |
| 10.1 Nausea/vomiting | 4 | 997 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.40, 2.88] |
| 10.2 Uterine cramping | 2 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.02, 1.45] |
| 10.3 Urinary tract infection | 8 | 1834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.45, 1.73] |
| 10.4 Fever | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.26] |
| 10.5 Haemorrhage | 4 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.26, 1.58] |
| 10.6 Muscle fasciculation | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.11, 62.41] |
| 10.7 External burns (first degree) | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.11, 47.89] |
| 10.8 Hydrosalpinx | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.39] |
| 10.9 Severe pelvic pain | 3 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.36, 2.48] |
| 11 Requirement for additional surgery | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 At 1 year follow‐up (ablation or hysterectomy) | 6 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.26] |
| 11.2 At 1 year follow‐up (hysterectomy) | 5 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.21] |
| 11.3 At 2 years' follow‐up (ablation or hysterectomy) | 5 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.32] |
| 11.4 At 2 years' follow‐up (hysterectomy) | 4 | 920 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.52, 1.42] |
| 11.5 At 2 to 5 years' follow‐up (ablation or hysterectomy) | 3 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 11.6 At 2 to 5 years' follow‐up (hysterectomy) | 4 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.22] |
| 11.7 At 10 years' follow‐up (ablation or hysterectomy) | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.37, 0.87] |
| 11.8 At 10 years' follow‐up (hysterectomy) | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbott 2003.
| Methods | Parallel randomised controlled trial. Study authors did not report the number of centres involved in the study | |
| Participants | 57 women with unstated ages
Inclusion criteria:
Exclusion criteria: none reported Setting: James Cook University Hospital in the UK Timing: all surgical procedures were performed between July 1999 and May 2000 |
|
| Interventions | Novasure endometrial ablation (n = 37) vs Cavaterm endometrial ablation (n = 18) Duration: 6 months' follow‐up and 12 months' follow‐up |
|
| Outcomes |
Primary:
|
|
| Notes |
Source of funding: Novacept Power calculation performed; study authors reported use of intention‐to‐treat analysis Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequences in balanced blocks of 5 |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes but no details if these were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, nursing staff, and GP all blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in each group withdrew after randomisation and before surgery |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Unclear risk | Groups appeared balanced at baseline, but medical equipment company provided funding |
Athanatos 2015.
| Methods | Parallel randomised controlled trial. Single centre | |
| Participants | 66 women recruited Inclusion criteria:
Exclusion criteria:
Setting: Department of Obstetrics and Gynaecology, Papageorgiou University of Thessaloniki, Greece Timing: January 2008 to December 2010 |
|
| Interventions | Pretreatment GnRH for 3 months for all participants Novasure impedance control system (n = 33) vs Microwave endometrial ablation (n = 33) |
|
| Outcomes |
At 3 months:
At 12 months:
|
|
| Notes |
Conflicts of interest: not reported Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Table was not disclosed to recruiting or follow‐up physicians; the women were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | On 12 months' follow‐up, doctors assessing patients were unaware of patient allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 66 patients had 3 and 12 months' follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and registered |
| Other bias | Low risk | Groups balanced at baseline; no other evidence of bias |
Bhattacharya 1997.
| Methods | Single‐centre parallel randomised controlled trial (Scotland) | |
| Participants | 372 women; mean age 41 years
Inclusion criteria:
Exclusion criteria: not reported Setting: gynaecology clinics at Aberdeen Royal Infirmary, Scotland Timing: not reported |
|
| Interventions | All women underwent clinical assessment and endometrial biopsy before treatment, as well as endometrial preparation with a single injection of goserelin 3.6 mg subcutaneously 5 weeks before surgery
Laser ablation (n = 188)
vs TCRE with rollerball (n = 184) Duration: 12 months |
|
| Outcomes |
Outcomes Operative complications:
|
|
| Notes | Recruitment of participants took place over 2 different time periods. 105 women were randomised to ELA or TCRE for an earlier study. After a gap of 8 months, an additional 267 women were recruited Power calculation was performed for sample size, and study authors reported intention‐to‐treat analysis (although because of dropouts, this was impossible) Source of funding: Chief Scientist Office at the Scottish Department of Health Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes stratified per consultant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Different numbers of participants provided data for different outcomes; 366/372 for operative details, 321/372 for satisfaction, 306/372 for menstrual loss |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Recruitment of participants over 2 different time periods, and the 2 groups differed in baseline characteristics. 15% of one group crossed over to the other treatment, but analyses were undertaken according to randomised group |
Bongers 2004.
| Methods | Single‐centre parallel randomised controlled trial (Netherland) | |
| Participants | 126 women; mean age 43 years Inclusion criteria:
Exclusion criteria:
Setting: large teaching hospital (500 beds) in the Netherlands Timing: 1 November 1999 to 1 July 2001 |
|
| Interventions | Novasure endometrial ablation (n = 83)
vs Thermachoice endometrial ablation (n = 43) Follow‐up at 3, 6, and 12 months |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | A technical failure with the Novasure generator part way during the trial. As a result, 2 analyses were performed:
Power calculation for sample size performed and study authors claimed analysis by intention‐to‐treat Source of funding: Novasure devices provided by Novacept; Thermachoice devices discounted Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and co‐ordinator of follow‐up blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment by either patients or doctors, so blinding was followed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up over 5 years (6/126); 18% lost to follow‐up at 10 years |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not prespecified or reported |
| Other bias | Unclear risk | Support to trial by medical equipment company. At baseline, more women (16%) in the bipolar group had a retroverted uterus than women (9%) in the balloon group |
Boujida 2002.
| Methods | Parallel randomised controlled trial. Study authors did not report the number or locations of the centres involved | |
| Participants | 120 women; aged > 35 years; mean coagulation 42.6; mean resection 44.8
Inclusion criteria:
Exclusion criteria:
Timing: not specified |
|
| Interventions | Transcervical hysteroscopic endometrial coagulation (n = 61) vs Endometrial resection (n = 59) Duration: clinical exam 2 years post questionnaire and 5 years' follow‐up |
|
| Outcomes |
Primary:
|
|
| Notes | A power calculation was performed; not reported Intention‐to‐treat analysis was performed, but no dropouts were reported for primary outcomes Source of funding: Research Foundation of the County of West Zealand Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Documented Geigy random numbers; even numbers rollerball, odd numbers TCRE |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes opened just before surgery |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | True intention‐to‐treat analysis. No dropouts for assessment of primary outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups balanced at baseline |
Brun 2006.
| Methods | Parallel randomised controlled trial at 6 centres in France | |
| Participants | 62 women; median age 45 years (Cavaterm) and 46 years (TCRE) Inclusion criteria:
Exclusion criteria:
Setting: Departments of Obstetrics and Gynaecology at university hospitals in France (6 centres) Timing: February 2000 and December 2001 |
|
| Interventions | Cavaterm thermal balloon ablation (n = 31) vs Transcervical resection of the endometrium (TCRE) (n = 20) Duration: 6 months' and 12 months' follow‐up |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Power calculation for sample size (26 participants in each arm for 80% power to detect 42% difference in amenorrhoea rate between groups). Analysis not by intention‐to‐treat and randomisation unbalanced after withdrawals Source of funding: Wallsten (a medical equipment company in Switzerland) acknowledged for technical assistance ‐ unknown whether funding was provided Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated telephone number sequence at 1:1 allocation ratio |
| Allocation concealment (selection bias) | Low risk | Centralised system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals unbalanced between groups ‐ created unbalanced randomisation |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | High risk | Menstrual blood loss greater in the Cavaterm group at baseline; medical equipment company acknowledged ‐ not sure if company provided funding |
Clark 2011.
| Methods | Single‐centre parallel‐group trial | |
| Participants | 81 women with heavy menstrual bleeding randomised; mean age 42 and 44 years; recruited from gynaecology outpatient clinic, at Birmingham Women's Hospital, in Birmingham, UK Inclusion criteria:
Exclusion criteria:
Duration of follow‐up: 3, 6, and 12 months |
|
| Interventions | All women had preoperative endometrial biopsy and imaging of the uterine cavity by transvaginal US or office diagnostic hysteroscopy before randomisation. Surgery was performed in an office setting and local anaesthetics were used
|
|
| Outcomes |
Primary: Amenorrhoea rate at 6 months Secondary: Satisfaction, QOL, technical feasibility (failed procedure, operative complications, duration of Rx), acceptability, improvement in dysmenorrhoea, improvement in premenstrual syndrome |
|
| Notes |
Prespecified subgroups: age (< 40 years, ≥ 40 years) and uterine cavity length (≤ 8 cm or > 8 cm) Power calculation for sample size, allowing for dropouts Intention‐to‐treat analysis for feasibility, pain, and acceptability ‐ not for amenorrhoea and menstrual data Source of funding: first study author received funding from Cytyc, which manufactures the Novasure ablation system Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified block randomisation with variable block size |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation with variable block size |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women not told of allocation ‐ no details on how blinding was maintained; study authors acknowledged that women could have guessed their allocation Surgeons not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessment by patient questionnaire, so unclear if blinding was broken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For primary outcome, significant dropout RFA group: n = 17 at 12 months; no outcome data available TBA group: n = 13 at 12 months; no outcome data available |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No significant differences at baseline, except that women in TEA group were slightly older and were more likely to be sexually active |
Cooper 1999.
| Methods | Single‐centre parallel‐group design | |
| Participants | 263 women randomised with mean age 41 years; recruited from gynaecology outpatient department at Aberdeen Royal Infirmary (referred for surgery) between September 1996 and February 1998
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Endometrial thinning with goserelin 3.6 mg 5 weeks before surgery for all women
Duration: 12 months' and 5 and 10 years' follow‐up |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Power calculation for sample size (230 women required to have power of 80% to detect a minimum 15% difference in satisfaction, significant at 0.5 level)
Analysis by intention‐to‐treat but loss to follow‐up of 23 women not included
Funding: support received from Microsulis (microwave equipment and salary support) Conflicts of interest: Dr. C. Brain was funded in part by Microsulis as a research fellow. Drs. Cooper and Parkin have received travel and accommodation support from Microsulis for attending conferences and training courses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables in balanced blocks of 20 |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes opened by an independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total dropouts 23/263 for menstrual and satisfaction outcomes ‐ balanced between groups and unlikely to affect estimates |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Funding provided by medical equipment company |
Cooper 2002.
| Methods | Multi‐centre (9) parallel randomised controlled trial Timing: not specified | |
| Participants | 265 women randomised; aged 25 to 50; recruited from centres in the USA
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Duration: follow‐up at 3, 6, and 12 months (randomised using ratio of 2:1 Novasure:rollerball) |
|
| Outcomes |
|
|
| Notes | No power calculation performed; study authors did not report intention‐to‐treat analysis (except for safety results)
Funding: in part by unrestricted grant from Novacept Inc. (Dr. Cooper is a stockholder, and Dr. Laberge a consultant) Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers for each site (separate for < 40 and ≥ 40) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants contributed data for safety outcomes, but for other outcomes, 13% were lost to follow‐up with no details reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Groups appeared balanced at baseline, but funding provided by medical equipment company |
Cooper 2004.
| Methods | 8 centres, parallel‐group design Timing: between April 2000 and September 2001 |
|
| Participants | 322 women randomised; mean age 41 years; recruited from 5 centres in the USA, 2 centres in Canada, and 1 centre in the UK (academic medical centres and private medical practices) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
All women had prior investigations with ultrasound, endometrial biopsy, and Pap smear All women had pretreatment with GnRHa for 1 month Duration: 12 months |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Women were stratified into 2 groups: < 40 years and ≥ 40 years Power calculation for sample size and intention‐to‐treat analysis (evaluable patient analysis also performed) Funding: all study authors are associated with the company that produces the microwave device Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in a 2:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | True intention‐to‐treat analysis for primary outcomes; dropouts regarded as failures |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Study authors were employees, consultants/speakers for or owned stock in a medical equipment company that produced one of the interventions |
Corson 2000.
| Methods | Multi‐centre study (n = 8), parallel‐group design Timing: November 1995 to June 1997 |
|
| Participants | 276 women randomised; aged 30 to 49 years; recruited from 8 centres (7 in the USA, 1 in Australia)
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All participants were initially treated with 2 weeks of oral contraceptive pills and their randomised treatment and were followed immediately after withdrawal bleeding
Duration: 12 months (follow‐up at 2 weeks; at 3, 6, and 12 months) |
|
| Outcomes |
|
|
| Notes | No power calculation for sample size reported
Analysis was not by intention‐to‐treat
Funding: supported by Vesta Medical, Colorado Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number sequence |
| Allocation concealment (selection bias) | Low risk | Sealed individual envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 42/276 (15%) participants lost at assessment of outcomes at 12 months' follow‐up ‐ no reasons given; for assessment of operative outcomes, 21/276 participants lost |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Funding by medical equipment company that produces one of the interventions; some study authors received stocks in the company |
Corson 2001.
| Methods | Multi‐centre study (n = 9), parallel‐group design Timing: not specified |
|
| Participants | 276 women randomised; aged 30 to 50 years; recruited from 9 private practice and university centres in the USA
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All participants had endometrial biopsy and cervical cytology to exclude pathology and endometrial preparation (single injection of depot leuprolide acetate 7.5 mg on day 21 of cycle) and a pregnancy test
Duration: 1 year |
|
| Outcomes |
|
|
| Notes | Power calculation for sample size 276 required, assuming success in rollerball arm 80%, and rates not differing by more than 20%: a = 0.05, b = 0.10, dropout rate = 12%
Analyses both intention‐to‐treat and per‐protocol
Funding: Dr. Corson is medical director and a stock shareholder and receives travel grants from B.E.I. Medical Systems Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation blocks of 12 stratified by site with a 2:1 ratio, and stratified into 2 groups by age |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26/276 (9%) lost at 12 months ‐ unbalanced between groups and no reasons given. Study authors claimed intention‐to‐treat analyses but not for all randomised participants Dropouts regarded as failures Number of exclusions before treatment: 3 (HTA), 4 (balloon) Number of dropouts/losses to follow‐up after treatment by 1 year: 17 (HTA) ‐ 7 of these for equipment failure; 2 (balloon) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Groups appeared balanced at baseline, but funding provided by medical equipment company that produced one of the interventions |
Duleba 2003.
| Methods | Multi‐centre (10), parallel prospective randomised design Timing: not specified |
|
| Participants | 279 women randomised; aged 30 to 50 years (mean EC 41.2 (5.1) and RBE 41.1 (4.8)); recruited from university and private medical centres in the USA
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Power calculation performed; study authors did not state intention‐to‐treat analysis
Funding: Cryogen Inc. (Duleba, Soderstrom, and Townsend all consultants to Cryogen Inc.) Conflicts of interest: not reported "those randomised to cryoablation had significantly worse menorrhagia" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but no other details of how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 51/279 (18%) dropouts for outcomes measured at 12 months ‐ no reasons given nor details on distribution per group |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Participants receiving cryoablation had higher PBAC scores at baseline; study authors were consultants for the medical equipment company that provided funding for cryoablation |
Ghazizadeh 2014.
| Methods | Parallel randomised controlled trial | |
| Participants | Department of Obstetrics and Gynecology and Maternal‐Fetal and Neonatal Research Center and Breastfeeding Research Center, Tehran University of Medical Sciences Inclusion criteria:
Exclusion criteria:
Setting: Tehran, Iran Time frame: October 2009 to November 2010 |
|
| Interventions | Bipolar endometrial ablation (Novasure) (n = 30) Hysteroscopic endometrial resection (HER) (n = 32) Mirena (lg‐IUS) (n = 48) |
|
| Outcomes | Decreased menstrual blood loss Interaction between bleeding and normal activity Anaemia (estimated 6.8 mg/dL as cut‐off for anaemia) Patients’ satisfaction (checklist 6 months' follow‐up; some up to 12) |
|
| Notes |
Funding: not specified; includes the statement "No competing financial interest" Outcomes do not match correctly on the report. Study authors contacted for more details November 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The titles says RCT, but no data provided on the randomisation process |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers in tables do not match numbers in text; study authors have been contacted for confirmation |
| Selective reporting (reporting bias) | High risk | No quantification of bleeding; not specified at what time satisfaction was measured |
| Other bias | High risk | Past medical history was positive in 12% Mirena, 13.3% Novasure, and 53.1% of HER (P < 0.0001) Ultrasonography was performed in 35.4% of patients in the Mirena group, 66.7% in the Nova‐Sure group, and 96.8% in the hysteroscopic endometrial resection group (P < 0.0001) |
Hawe 2003.
| Methods | Single‐centre study, randomised controlled trial Timing: recruited between August 1997 and April 2000 |
|
| Participants | 72 women randomised; aged 29 to 51 years (mean cav 41.4, mean laser 41.1); recruited from a minimal access gynaecological surgery unit at a district general hospital
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Duration: preop 6 and 12 months for questionnaire; pictorial blood loss assessment 6 months |
|
| Outcomes |
Primary • Amenorrhoea rate, then effect on menstrual status • Questionnaire assessing menstrual symptoms • QOL • Sexual activity • Procedure satisfaction and acceptability ‐ included questionnaires EQ‐5D, SF‐12, SAQ; VAS; pain VAS • Operative details and morbidity |
|
| Notes | Power calculation performed; study authors did not report intention‐to‐treat analysis
Funding: not reported but Wallsten Medical, which supplied the Cavaterm equipment Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutated blocks predetermined by computer‐generated random number tables (blocks of 4 sequentially numbered envelopes) |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, nursing staff, and GP blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor of outcomes blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant excluded after randomisation because she did not meet inclusion criteria; 4 other participants lost by 12 months ‐ unlikely to affect assessment of outcomes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Groups appeared balanced at baseline except for cavity length, but differences unlikely to be clinically significant; a medical equipment company provided one of the interventions |
Laberge 2016.
| Methods | Parallel randomised controlled trial | |
| Participants | Premenopausal women + HMB (AH > 160 mL/cycle) Inclusion criteria:
Exclusion criteria:
Setting: academic and private medical settings (USA, Canada, and Mexico) Timing: not specified |
|
| Interventions | Bipolar endometrial ablation (Minerva) (n = 102) Rollerball endometrial ablation (n = 51) |
|
| Outcomes |
|
|
| Notes |
Funding: sponsored by Minerva Surgical Inc. Conflicts of interest: study authors declare that they have no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were block randomised from a centralised electronic patient database in a 2:1 scheme to the test group or the control group, but no details were provided |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details in the paper, but on the clinical trial register says "open label" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6.5% dropout after 1 year; used ITT analysis for all participants |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Groups are balanced at baseline; no other sources of bias were identified |
McClure 1992.
| Methods | Single‐centre study, parallel‐group design with unclear blinding | |
| Participants | 38 women initially recruited for trial; mean age 42 years; from tertiary referral centre at University Department, Monash University
12 excluded before randomisation because of prior MBL measurements < 70 mL; 4 dropped out because of dissatisfaction with operative delay
Inclusion criteria:
Exclusion criteria:
Setting: tertiary referral centre, University department Timing: between May 1989 and July 1991 |
|
| Interventions | All participants had pelvic examination and transvaginal ultrasonography Those randomised also received preoperative treatment with 10 mg MPA 3 times/d for 3 months to thin the endometrium
Duration: 6 months |
|
| Outcomes | • Reduction in MBL • Duration of surgery • Postoperative complications and requirement for analgesia • Need for further surgery • Amenorrhoea rate |
|
| Notes | No power calculation for sample size Source of funding: not stated Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported but very small study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No evidence of other biases; source of funding not reported; groups appeared balanced at baseline |
Meyer 1998.
| Methods | Multi‐centre (n = 14), parallel‐group trial Setting: 12 investigative centres in the USA and 2 in Canada Timing: recruiting between January and September 1996 |
|
| Participants | 275 women; aged 29 to 50 years; recruited from 12 investigative centres in USA and 2 in Canada
Inclusion criteria:
Exclusion criteria: • Submucous fibroids • Suspected genital tract infection or malignancy • Previous endometrial ablation |
|
| Interventions |
Duration: 12 months' follow‐up |
|
| Outcomes |
|
|
| Notes | Power calculation for sample size performed (108 participants required per group (assuming response rate of 85% for those treated with rollerball) to detect if balloon therapy more than 20% less effective at a 5% level of significance with 90% power) Source of funding: Gynecare Ltd., USA Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/275 (7%) withdrew before surgery; 239/275 (87%) provided data at 12 months' follow‐up; study authors compared characteristics of original randomised group vs the group that provided 6 and 12 months' data and found no differences Reasons for loss to follow‐up not provided |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Funding provided by medical equipment company |
Onoglu 2007.
| Methods | Single‐centre parallel‐group study | |
| Participants | 48 women with average age 48 years and 47 years; recruited from a hospital clinic in Turkey Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Duration: follow‐up every 3 months up to 38.4 ± 2.4 months |
|
| Outcomes | Duration of surgery Menstrual blood loss |
|
| Notes | No power calculation for sample size; intention‐to‐treat analysis not reported Source of funding: Akdeniz University Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants randomised in the order they were seen in the clinic ‐ this has the potential for significant bias |
| Allocation concealment (selection bias) | High risk | Not reported but very likely allocation was known to investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts ‐ unlikely to affect calculation of estimates |
| Selective reporting (reporting bias) | High risk | Bleeding patterns prespecified but figures not reported |
| Other bias | Low risk | No evidence of other significant bias ‐ groups appeared balanced at baseline and funding was not reported |
Pellicano 2002.
| Methods | Single‐centre parallel‐group trial Setting: Department of Ob Gyn, University of Naples Timing: recruitment from May 1998 to June 1999 |
|
| Participants | 82 women; mean age 43 years; recruited from University of Naples Obs and Gyn Department (Italy) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Duration: 3 months', 1 year, and 2 years' follow‐up |
|
| Outcomes |
Primary: Satisfaction rate at 3 months, 1 year, and 2 years Secondary: Duration of surgery Intraoperative blood loss Requirement for further surgery Postoperative pain Hospital stay Complications Resumption of normal activity |
|
| Notes |
Power calculation: not reported Source of funding: surgical equipment provided by Wolf Germany and Wallsten Medical SA, Morges, Switzerland Conflict of interest: no data provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Assessment of some outcomes such as requirement for further surgery and satisfaction included all randomised participants. 8.5% had dropped out for assessment of year 1 outcomes; 17% had dropped out for assessment of year 2 outcomes; no reasons given |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Groups appeared balanced at baseline; funding provided by medical equipment company |
Penninx 2010.
| Methods | Single‐centre parallel‐group design, with double‐blinding Setting: teaching hospital in the Netherlands Timing: recruitment between March 2005 and August 2007 |
|
| Participants | 160 women with menorrhagia and heavy menstrual bleeding; mean age 45 years; recruited from Maxima Medical Centre, Veldhoven, the Netherlands, between March 2005 and August 2007 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Saline infusion sonography or diagnostic hysteroscopy required to confirm normal uterine cavity (6 to 12 cm) Surgery in days 3 to 8 of the menstrual cycle; no endometrial pretreatment
Duration of follow‐up: 1 month, 6 months, 12 months, 5 years |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Time power calculation for sample size Source of funding: not stated Conflict of interest: Dr. Bongers has received an unconditional grant from Hologic for another research project Women who chose to have a hysterectomy after ablation were considered amenorrhoeic. This may have inflated the rates of amenorrhoea among women who felt their ablation had not been successful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated" |
| Allocation concealment (selection bias) | Low risk | Doctors were masked |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were unaware of their treatment but doctors performing the surgery were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal dropouts (7 lost in each group) |
| Selective reporting (reporting bias) | Unclear risk | Complications not reported |
| Other bias | Low risk | Groups comparable at baseline; no other potential bias |
Penninx 2016.
| Methods | Parallel randomised controlled trial Setting: multi‐centre (outpatient clinic at 3 different hospitals in the Netherlands) Time framing: between June 2009 and December 2011; 104 women were included in the study |
|
| Participants | Women with heavy menstrual bleeding Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Balloon endometrial ablation (n = 52) Bipolar endometrial ablation (Novasure) (n = 52) Follow‐up: 12 months |
|
| Outcomes |
|
|
| Notes | No external funding provided for this study Conflicts of interest: J. Penninx received an unconditional grant from Hologic for writing her thesis. Prof. Bongers is a member of the Dutch advisory board of Hologic. The other study authors did not report any potential conflicts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided; "Women were randomly allocated to bipolar or balloon ablation" |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope 1:1 was taken to each centre just before treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and doctors performing follow‐up were blinded to the device used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doctors doing follow‐up were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45 and 40 patients at 12 months Balloon – 52 randomised: 3 had bipolar ablation, 2 had no intervention, 6 were lost to follow‐up at 6 weeks, and 1 was lost to follow‐up at 12 months |
| Selective reporting (reporting bias) | Low risk | Outcomes listed were reported |
| Other bias | Low risk | Groups appear balanced at baseline; no other sources of bias identified |
Perino 2004.
| Methods | Single‐centre parallel‐group design; blinding not reported but unlikely Setting: academic teaching hospital Timing: January 1998 to July 1999 |
|
| Participants | 116 women; age range 36 to 48 years (mean 41 to 42); recruited from a university clinic in Italy Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
All women had investigations before treatment: ultrasound, hysteroscopy with endometrial biopsy, blood tests for clotting defects, FSH/E2 serum sampling. All received pretreatment with 1 dose of GnRHa |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Power calculation for sample size
No intention‐to‐treat analysis performed
Source of funding: not mentioned Conflicts of interest: no data provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/116 (4%) dropped out and no reasons given, but proportion was balanced between randomised groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Groups appeared balanced at baseline but characteristics reported for only 96% of those randomised (minimal dropout). No source of funding reported. No evidence of significant other bias |
Romer 1998.
| Methods | Single‐centre parallel‐group trial; blinding not reported | |
| Participants | 20 women; aged 35 to 52; recruited
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All women were pretreated with 2 injections (4 weeks apart) of leuprorelin acetate depot. Treatment followed 2 weeks after the last injection
Duration: follow‐up 9 to 15 months |
|
| Outcomes |
|
|
| Notes | No power calculation for sample size reported
Analysis by intention‐to‐treat (no dropouts)
Source of funding: not reported Paper in German language; study author contacted for clarification but no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported but very small study |
| Selective reporting (reporting bias) | Unclear risk | Complications of procedures not reported |
| Other bias | Low risk | Groups appear balanced at baseline; no source of funding reported. No evidence of other significant bias |
Sambrook 2009.
| Methods | Single‐centre parallel‐group design; blinding of assessors but not patients or investigators Setting: UK teaching hospital Time frame: January 2003 to January 2005 |
|
| Participants | 320 women requesting endometrial ablation; mean age 43 years; recruited from the Gynaecology Department of Aberdeen Royal Infirmary in the UK Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Patients did not routinely undergo hysteroscopy. Treatment was undertaken in the postmenstrual phase, under general or local anaesthetic, according to patient preference
Follow‐up at 2 weeks, 6 months, 1 year, and 5 years following surgery |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Power calculation for sample size Stated as intention‐to‐treat analysis but no imputation made for dropouts Source of funding: Chief Scientists Office, Scottish Government Health Directorates Conflicts of interest: Dr. Cooper has received financial support from Microsulis and Gynecare for travel and meeting attendance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated random blocks" |
| Allocation concealment (selection bias) | Low risk | "Telephone randomisation service based on a separate site" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding for patients but not investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding for assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for dropouts given – similar for 2 groups and not likely to cause major bias |
| Selective reporting (reporting bias) | Low risk | All likely outcomes reported |
| Other bias | Low risk | Groups similar at baseline; no evidence of any other potential bias |
Thabet 2010.
| Methods | Single centre; unclear if blinding used Setting: teaching hospital in Egypt Time frame: February 2000 to October 2002 |
|
| Participants | 100 women; recruited from outpatient clinic at Kasr El‐Aini School of Medicine, Cairo University, Egypt Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Duration of follow‐up: not clear, although study authors reported follow‐up time of 3 years |
|
| Outcomes | Intraoperative complications Amenorrhoea Normal menstruation Sexual function |
|
| Notes | No power calculation reported; intention‐to‐treat analysis not reported Source of funding: not reported Conflicts of interest: not reported. Follow‐up in the trial unclear; potential bias from replacing dropouts with new cases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "true random bases" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Replacement of dropouts with new cases likely to cause bias in the results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Baseline comparability not reported |
van Zon‐Rabelink 2003.
| Methods | Single‐centre randomised controlled trial; use of blinding unclear Setting: teaching hospital in the Netherlands Time frame: not reported |
|
| Participants | 139 women; unreported ages; recruited from a teaching hospital in the Netherlands
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
No follow‐up reported |
|
| Outcomes | Technical safety aspects Reduction in menstrual bleeding Success rate (PBAC < 185) Satisfaction |
|
| Notes | Power calculation was performed; study authors did not state intention‐to‐treat analysis
Source of funding: not reported Conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | Groups appeared balanced at baseline but only for age and cavity length; other characteristics were not reported. No source of funding was identified. Numbers in the randomised groups differed substantially |
Vercellini 1999.
| Methods | Single‐centre parallel‐group trial; no blinding
Number of women randomised = 91
Number of dropouts/losses to follow up = 1 Setting: teaching hospital in Milan Timing: March 1996 to February 1997 |
|
| Participants | 91 women; mean age 46 years; recruited from an outpatient clinic in Milan, Italy
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | All participants underwent complete clinical examination, transvaginal ultrasonography, diagnostic hysteroscopy, and endometrial biopsy before treatment. They also received preoperative treatment with depot GnRH agonist triptorelin for 2 months
Duration: 12 months |
|
| Outcomes |
|
|
| Notes | Power calculation for sample size performed (40 women per treatment arm required to find a difference of 200 mL in fluid absorption with 80% power at 5% significance level) Intention‐to‐treat analysis performed for satisfaction rate and menstrual pattern Source of funding: Circum Acmi (supply of vaporising electrodes) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes kept secure in another location |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Immediate postoperative outcomes included all randomised participants; for outcomes assessed at 1 year, 1 woman was lost to follow‐up and did not contribute data to the PBAC |
| Selective reporting (reporting bias) | Low risk | All prespecifed outcomes reported |
| Other bias | Unclear risk | Groups were balanced at baseline, but a medical equipment company provided funding. This study received no external funding |
AH: alkaline haematin.
BP: Blood pressure.
CIN: cervical endothelial neoplasia.
CS: caesarean section.
E2: Estradiol.
EC: electrocoagulation.
ELA: endometrial laser ablation.
ELITT: endometrial laser intrauterine thermal therapy.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions.
GnRH: gonadotropin‐releasing hormone.
GP: general practitioner.
HER: hysteroscopic endometrial resection.
HMB: heavy menstrual bleeding.
HTA: Hydro ThermAblator.
ITT: intention‐to‐treat.
IUD: intrauterine device.
MBL: menstrual blood loss.
MPA: Medroxyprogesterone acetate.
Nd:YAG: neodymium yttrium‐aluminium‐garnet.
PBAC: Pictorial Blood Assessment Chart.
PBLAC: Pictorial Blood Loss Assessment Chart.
PID: pelvic inflammatory disease.
PMS: premenstrual syndrome.
QOL: quality of life.
RBE: rollerball electrocoagulation.
RCT: randomised controlled trial.
RFA: radiofrequency ablation.
SAQ: Seattle Angina Questionnaire.
SF‐36: Short Form‐36.
TBA: thermal balloon ablation.
TCRE: transcervical resection of the endometrium.
TSS: toxic shock syndrome.
US: ultrasound.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd Ek Hameed 2012 | RCT of endometrial thermal balloon ablation with or without a co‐intervention (pre‐ablation curettage) |
| Cash 2012 | RCT of third‐generation thermal uterine balloon therapy with or without a co‐intervention (post‐ablation curettage); includes non‐randomised comparison with first‐generation balloon ablation |
| Chang 2009 | Comparison of different waveforms for rollerball ablation ‐ not different ablation methods |
| Cooper 2012 | Never started for lack of funds |
| El‐Nashar 2009 | Cohort study ‐ not randomised |
| Feng 2006 | Not a randomised study |
| Shokeir 2013 | RCT of hysteroscopic endometrial resection with or without a co‐intervention (long‐acting gestagen) |
| Soysal 2001 | Did not match the inclusion criteria |
| Vihko 2003 | Excluded as it compared 2 types of balloon ablation ‐ Menotreat and Cavaterm |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Feng 2014.
| Methods | Prospective randomised multi‐centre clinical trial |
| Participants | 60 pre‐menopausal patients with abnormal uterine bleeding from benign causes who have completed childbirth |
| Interventions | Cardea GEA system vs transcervical resection of the endometrium (TCRE) combined with rollerball ablation for treatment of abnormal uterine bleeding |
| Outcomes | Bleeding reduction at 6 months Time of operation Patient satisfaction rate and amenorrhoea rate |
| Notes | No published results (2018) |
Hamza 2005.
| Methods | Not clear whether randomised |
| Participants | Participants had dysfunctional uterine bleeding |
| Interventions | Rollerball ablation TCRE |
| Outcomes | Complications Menstrual blood loss |
| Notes | Attempt being made to contact study authors |
NCT00549159.
| Methods | Randomised parallel‐group single‐blind (patient) |
| Participants | Women with dysfunctional uterine bleeding |
| Interventions | Cavaterm thermal balloon endometrial ablation vs transcervical resection of the endometrium (TCRE) |
| Outcomes |
Primary: Reduction in uterine bleeding |
| Notes | Unclear whether trial completed; results not published; attempt being made to contact study authors |
TCRE: transcervical resection of the endometrium.
Characteristics of ongoing studies [ordered by study ID]
NCT02642926.
| Trial name or title | Randomized Controlled Trial Comparing the Efficiency of the Bipolar Energy Compared With the Monopolar Energy in Endometrial Ablation in Women Having Menorrhagia |
| Methods | Randomised parallel trial |
| Participants | Women older than 18 years with heavy menstrual bleeding Inclusion criteria:
Exclusion criteria:
|
| Interventions | Bipolar ablation Monopolar ablation |
| Outcomes |
Primary: Bleeding abundance (at 12 months) will be measured by the Higham score, on a questionnaire sent to the patient Secondary:
|
| Starting date | December 2015 |
| Contact information | Andre Nazac |
| Notes | Attempts made to contact study author during 2017 and 2018 |
AVK: anti‐vitamin K.
Differences between protocol and review
In December 2008, we changed the title from "Endometrial destruction techniques for heavy menstrual bleeding" to "Endometrial resection and ablation techniques for heavy menstrual bleeding".
In 2018, we divided the complication rate into major and minor categories to distinguish common adverse effects of surgery such as nausea and vomiting from more serious post‐procedure complications.
Contributions of authors
For the 2018 update:
Magdalena Bofill performed selection of trials, data extraction, and data entry and prepared all versions of drafts and the final version of the review for comments from the other review authors.
Anne Lethaby performed selection of trials and commented on all versions of drafts and the final version of the review.
Mihaela Grigore performed selection of trials and data extraction.
Julie Brown performed selection of trials and data extraction and checked data entry; she also commented on all versions of drafts and the final version of the review.
Cindy Farquhar and Martha Hickey contributed clinical knowledge and commented on the final version of the review.
All review authors approved the final version.
Jane Marjoribanks helped update the search for the 2013 update.
Josien Penninx performed independent data extraction and assessment of risk of bias for the 2009 and 2013 updates and commented on the final version of the review.
Julie Brown performed independent selection of trials for the 2009 update.
Anne Lethaby wrote the original protocol, searched for relevant trials, assessed trials for eligibility for inclusion, extracted data from the included trials, assessed trials for risk of bias, compared independent data extraction and clarified points of disagreement, entered data, and wrote and commented on the final review (excluding the discussion and conclusion).
Martha Hickey commented on the final list of included trials, extracted data from the included trials for earlier versions of the review, wrote the discussion and conclusion, and commented on the draft of the protocol and an earlier version of the full review.
Ray Garry commented on the final draft of an earlier version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
UK NHS, Other.
The update in 2009 was funded by Dept of Health (England) Incentive Scheme 2008
Declarations of interest
MB, AL, CF, MG and JB did not report any conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abbott 2003 {published data only}
- Abbott J, Hawe J, Hunter D, Garry R. A double‐blind randomized trial comparing the Cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertility and Sterility 2003;80(1):203‐8. [DOI] [PubMed] [Google Scholar]
Athanatos 2015 {published data only}
- Athanatos D, Pados G, Venetis C, Stamatopoulos P, Rousso D, Tsolakidis D, et al. Novasure impedance control system versus microwave endometrial ablation for the treatment of dysfunctional uterine bleeding: a double‐blind, randomized controlled trial. Clinical and Experimental Obstetrics & Gynecology 2015;42(3):347‐51. [PMID: 26152008] [PubMed] [Google Scholar]
- Pados G. Treatment of dysfunctional uterine bleeding with second generation ablation devices: microwaves (MEAA) vs Bipolar Impedance Control System (Novasure). http://clinicaltrials.gov/show/NCT01173965.
Bhattacharya 1997 {published data only}
- Bhattacharya S, Cameron IM, Parkin DE, Abramovich DR, Mollison J, Pinion SB, et al. A pragmatic randomised comparison of transcervical resection of the endometrium with endometrial laser ablation for the treatment of menorrhagia. British Journal of Obstetrics and Gynaecology 1997;104:601‐7. [DOI] [PubMed] [Google Scholar]
Bongers 2004 {published data only}
- Bongers M, Herman M, Josien P, Mol BW. Ten‐year follow‐up of a randomized controlled trial comparing NovaSure and Thermachoice in endometrial ablation for dysfunctional uterine bleeding. Journal of Minimally Invasive Gynecology 2011;18(6 Suppl 1):S127. [Google Scholar]
- Bongers MY, Bourdrez P, Heintz PM, Brolmann HAM, Mol BWJ. Bipolar radio frequency endometrial ablation compared with balloon endometrial ablation in dysfunctional uterine bleeding: impact on patients' health‐related quality of life. Fertility and Sterility 2005;83(3):724‐34. [DOI] [PubMed] [Google Scholar]
- Bongers MY, Bourdrez P, Mol BWJ, Heintz APM, Brolmann HAM. Randomised controlled trial of bipolar radio‐frequency endometrial ablation and balloon endometrial ablation. British Journal of Obstetrics and Gynaecology 2004;111:1095‐102. [DOI] [PubMed] [Google Scholar]
- Herman M, Penninx M, Mol B, Bongers M. Ten‐year follow‐up of a randomised controlled trial comparing bipolar endometrial ablation with balloon ablation for heavy menstrual bleeding. British Journal Obstetrics and Gynaecology 2013;120:966‐970. [DOI: 10.1111/1471-0528.12213] [DOI] [PubMed] [Google Scholar]
- Kleijn JH, Engels R, Bourdrez P, Mol BWJ, Bongers MY. Five‐year follow up of a randomised controlled trial comparing NovaSure and ThermaChoice endometrial ablation. British Journal of Obstetrics and Gynaecology 2008;115:193‐8. [DOI] [PubMed] [Google Scholar]
Boujida 2002 {published data only}
- Boujida VH, Philipsen T, Pelle J, Joergensen JC. Five‐year follow‐up of endometrial ablation: endometrial coagulation versus endometrial resection. Obstetrics and Gynecology 2002;99:988‐92. [DOI] [PubMed] [Google Scholar]
- Furst SN, Philipsen T, Joergensen JC. Ten‐year follow‐up of endometrial ablation. Acta Obstetricia et Gynecologica 2007;86:334‐8. [DOI] [PubMed] [Google Scholar]
Brun 2006 {published data only}
- Brun J‐L, Raynal J, Burlet G, Galand B, Quereux C, Bernard P. Cavaterm thermal balloon endometrial ablation versus hysteroscopic endometrial resection to treat menorrhagia: the French, multicenter, randomized study. Journal of Minimally Invasive Gynecology 2006;13:424‐30. [DOI] [PubMed] [Google Scholar]
Clark 2011 {published data only}
- Clark TJ, Samuel N, Malick S, Middleton LJ, Daniels J, Gupta JK. Bipolar radiofrequency compared with thermal balloon endometrial ablation in the office. Obstetrics and Gynecology 2011;117:109‐18. [DOI] [PubMed] [Google Scholar]
Cooper 1999 {published data only}
- Bain C, Cooper KG, Parkin DE. Microwave endometrial ablation versus endometrial resection: a randomized controlled trial. Obstetrics and Gynecology 2002;99:983‐7. [DOI] [PubMed] [Google Scholar]
- Cooper KG, Bain C, Lawrie L, Parkin DE. A randomised comparison of microwave endometrial ablation with transcervical resection of the endometrium: follow up at a minimum of five years. British Journal of Obstetrics and Gynaecology 2005;112:470‐5. [DOI] [PubMed] [Google Scholar]
- Cooper KG, Bain C, Parkin DE. Comparison of microwave endometrial ablation and transcervical resection of the endometrium for treatment of heavy menstrual loss: a randomised trial. Lancet 1999;354:1859‐63. [DOI] [PubMed] [Google Scholar]
- Sambrook AM, Bain C, Parkin DE, Cooper KG. A randomised comparison of microwave endometrial ablation with transcervical resection of the endometrium: follow up at a minimum of 10 years. British Journal of Obstetrics and Gynaecology 2009;116:1033‐7. [DOI] [PubMed] [Google Scholar]
Cooper 2002 {published data only}
- Cooper J, Gimpelson R, Laberge P, Galen D, Garza‐Leal JG, Scott J, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure system in the treatment of menorrhagia. Journal of the American Association of Gynecologic Laparoscopists 2002;9(4):418‐28. [DOI] [PubMed] [Google Scholar]
Cooper 2004 {published data only}
- Cooper JM, Anderson TL, Fortin CA, Jack SA, Plentl MB. Microwave endometrial ablation vs rollerball electroablation for menorrhagia: a multicenter randomized trial. Journal of the American Association of Gynecologic Laparoscopists 2004;11(3):394‐403. [DOI] [PubMed] [Google Scholar]
Corson 2000 {published data only}
- Corson SL, Brill AI, Brooks PG, Cooper JM, Indman PD, Liu JH, et al. Interim results of the American Vesta trial of endometrial ablation. Journal of the American Association of Gynecologic Laparoscopists 1999;6(1):45‐9. [DOI] [PubMed] [Google Scholar]
- Corson SL, Brill AI, Brooks PG, Cooper JM, Indman PD, Liu JH, et al. One‐year results of the Vesta system for endometrial ablation. Journal of the American Association of Gynecologic Laparoscopists 2000;7(4):489‐97. [DOI] [PubMed] [Google Scholar]
Corson 2001 {published data only}
- Corson SL. A multicenter evaluation of endometrial ablation by hydrothermablator and rollerball for treatment of menorrhagia. Journal of the American Association of Gynecologic Laparoscopists 2001;8(3):359‐67. [DOI] [PubMed] [Google Scholar]
- Goldrath MH. Evaluation of hydrothermablator and rollerball endometrial ablation for menorrhagia 3 years after treatment. Journal of the American Association of Gynecologic Laparoscopists 2003;10(4):505‐11. [DOI] [PubMed] [Google Scholar]
- Loffer F. A clinical comparison of hydrothermablation (HTA) and rollerball for endometrial ablation to treat menorrhagia: a randomized multicenter clinical trial. XVI FIGO World Congress of Obstetrics & Gynecology. 2000; Vol. Abstract Book 2:95.
Duleba 2003 {published data only}
- Duleba AJ, Heppard MC, Soderstrom RM, Townsend DE. A randomized study comparing endometrial cryoablation and rollerball electroablation for treatment of dysfunctional uterine bleeding. Journal of the American Association of Gynecologic Laparoscopists 2003;10(1):17‐26. [DOI] [PubMed] [Google Scholar]
- Townsend DE, Duleba AJ, Wilkes MM, et al. Durability of treatment effects after cryoablation versus rollerball electroablation for abnormal uterine bleeding: two‐year results of a multicenter randomized trial. American Journal of Obstetrics and Gynecology 2003;188:699‐701. [DOI] [PubMed] [Google Scholar]
Ghazizadeh 2014 {published data only}
- Ghazizadeh S, Panahi Z, Ghanbari Z, Tarafdari Menshadi A, Farahmandian T, Javadian P. Comparative efficacy of NovaSure, the levonorgestrel‐releasing intrauterine system, and hysteroscopic endometrial resection in the treatment of menorrhagia: a randomized clinical trial. Journal of Gynecologic Surgery 2014;30(4):215‐8. [DOI: 10.1089/gyn.2012.0041] [DOI] [Google Scholar]
Hawe 2003 {published data only}
- Hawe J, Abbott J, Hunter D, Phillips G, Garry R. A randomised controlled trial comparing the Cavaterm endometrial ablation system with the Nd:YAG laser for the treatment of dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology 2003;110:350‐7. [PubMed] [Google Scholar]
Laberge 2016 {published data only}
- Laberge P, Garza‐Leal J, Fortin C, Basinski C, Thiel J, Leyland N, et al. A prospective, randomized, multi‐center, controlled, international clinical study of the safety and efficacy of the Minerva endometrial ablation system: 6 and 12‐month follow‐up results. Journal of Minimally Invasive Gynecology. 2014. [DOI] [PubMed]
- Laberge P, Garza‐Leal J, Fortin C, Grainger D, Johns DA, Adkins RT, et al. A randomized controlled multicenter US Food and Drug Administration trial of the safety and efficacy of the Minerva Endometrial Ablation System: one‐year follow‐up results. Journal of Minimally Invasive Gynecology 2017;24(1):124‐32. [DOI: 10.1016/j.jmig.2016.09.009] [DOI] [PubMed] [Google Scholar]
- Laberge P, Garza‐Leal J, Fortin C, Thiel J, Johns D, Grainger D, et al. A randomized, controlled, multi‐center trial of the safety and efficacy of the Minerva Endometrial Ablation System: one‐year follow‐up results. Journal of Minimally Invasive Gynecology. 2016; Vol. 23:S1‐S252. [DOI] [PubMed]
McClure 1992 {published data only}
- McClure N, Marners M, Healy DL, Hill DJ, Lawrence AS, Wingfield M, et al. A quantitative assessment of endometrial electrocautery in the management of menorrhagia and a comparative report of argon laser endometrial ablation. Gynaecological Endoscopy 1992;1:199‐202. [Google Scholar]
Meyer 1998 {published data only}
- Grainger DA, Tjaden DO, Rowland C, Meyer WR. Thermal balloon and rollerball ablation to treat menorrhagia: two‐year results of a multicenter, prospective, randomized clinical trial. Journal of the American Association of Gynecologic Laparoscopists 2000;7(2):175‐9. [DOI] [PubMed] [Google Scholar]
- Loffer FD. Five year post‐procedure follow‐up of patients participating in a randomised trial of uterine balloon therapy vs rollerball ablation for the treatment of menorrhagia. Journal of the American Association of Gynecologic Laparoscopy 2001;8(1):48‐54. [Google Scholar]
- Loffer FD. Three‐year comparison of thermal balloon and rollerball ablation in treatment of menorrhagia. Journal of the American Association of Gynecologic Laparoscopists 2001;8(1):48‐54. [DOI] [PubMed] [Google Scholar]
- Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffer FD, Steege JF. Thermal balloon and rollerball ablation to treat menorrhagia: a multicenter comparison. Obstetrics and Gynecology 1998;92:98‐103. [DOI] [PubMed] [Google Scholar]
Onoglu 2007 {published data only}
- Onoglu A, Taskin O, Inal M, Sadik S, Simsek M, Akar M, et al. Comparison of the long‐term histopathologic and morphologic changes after endometrial rollerball ablation and resection: a prospective randomized trial. Journal of Minimally Invasive Gynecology 2007;14:39‐42. [DOI] [PubMed] [Google Scholar]
Pellicano 2002 {published data only}
- Pellicano M, Guida M, Acunzo G, Cirillo D, Bifulco G, Nappi C. Hysteroscopic transcervical endometrial resection versus thermal destruction for menorrhagia: a prospective randomized trial on satisfaction rate. American Journal of Obstetrics and Gynecology 2002;187:545‐50. [DOI] [PubMed] [Google Scholar]
Penninx 2010 {published data only}
- Bongers M, Herman M, Penninx J, Mol BW. Longterm follow‐up of a randomized controlled trial comparing NovaSure and hydrothermablation for dysfunctional uterine bleeding. Journal of Minimally Invasive Gynecology 2011;18(6 Suppl 1):S34. [Google Scholar]
- Penninx JPM, Herman MC, Mol BW, Bongers MY. Five‐year follow‐up after comparing bipolar endometrial ablation with hydrothermablation for menorrhagia. Obstetrics and Gynecology 2011;118:1287‐92. [DOI] [PubMed] [Google Scholar]
- Penninx JPM, Mol BW, Engels R, Rumste MME, Kleijn C, Koks CAM, et al. Bipolar radiofrequency endometrial ablation compared with hydrothermablation for dysfunctional uterine bleeding. Obstetrics and Gynecology 2010;116:819‐26. [DOI] [PubMed] [Google Scholar]
Penninx 2016 {published data only}
- Penninx J, Bongers M. Bipolar radiofrequency endometrial ablation versus thermablate balloon ablation for dysfunctional bleeding in the outpatient clinic: a randomized controlled trial. www.controlled‐trials.com/ISRCTN17974690.
- Penninx J, Herman M, Kruitwagen R, Ter Haar A, Mol B, Bongers M. Bipolar versus balloon endometrial ablation in the office: a randomized controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;196:52‐6. [DOI: 10.1016/j.ejogrb.2015.10.010] [DOI] [PubMed] [Google Scholar]
- Penninx J, Herman M, Mol B, Kruitwagen R, Bongers M. Bipolar radiofrequency endometrial ablation versus Thermablate balloon ablation for dysfunctional bleeding in the outpatient clinic: a randomized controlled trial. Journal of Minimally Invasive Gynecology 2012;19(6):S21‐S22. [DOI: 10.1016/j.jmig.2012.08.076] [DOI] [Google Scholar]
- Penninx JPM, Herman MC, Mol BW, Bongers MY. Bipolar radiofrequency endometrial ablation versus thermablate balloon ablation for dysfunctional bleeding in the outpatient clinic: a randomized controlled trial. Journal of Minimally Invasive Gynecology 2012;19(6 Suppl 1):S21‐2. [Google Scholar]
Perino 2004 {published data only}
- Perino A, Castelli A, Cucinella G, Biondo A, Pane A, Venezia R. A randomized comparison of endometrial laser intrauterine thermotherapy and hysteroscopic endometrial resection. Fertility and Sterility 2004;82:731‐4. [DOI] [PubMed] [Google Scholar]
Romer 1998 {published data only}
- Romer T. The treatment of recurrent menorrhagias ‐ Cavaterm‐balloon‐coagulation versus Rollerball‐endometrial ablation ‐ a prospective randomized comparative study [Die therapie rezidivierender Menorrhagien ‐ Cavaterm‐Ballon‐Koagulatioon versus Roller‐Ball‐Endometriumkoagulation ‐ eine prospektive randomisierte Vergleichsstudie]. Zentralblatt fur Gynakologie 1998;120(10):511‐4. [PubMed] [Google Scholar]
Sambrook 2009 {published data only}
- Sambrook A, Elders A, Cooper K. Microwave endometrial ablation versus thermal balloon endometrial ablation (MEATBall): 5‐year follow up of a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2014;121:748‐54. [DOI: 10.1111/1471-0528.12585] [DOI] [PubMed] [Google Scholar]
- Sambrook AM, Cooper KG, Campbell MK, Cook JA. Clinical outcomes from a randomised comparison of microwave endometrial ablation with thermal balloon endometrial ablation for the treatment of heavy menstrual bleeding. British Journal of Obstetrics and Gynaecology 2009;116:1038‐45. [DOI] [PubMed] [Google Scholar]
Thabet 2010 {published data only}
- Thabet SMA. New attempt using ablative curettage technique for managing benign premenopausal uterine bleeding. Obstetrics and Gynaecology Research 2010;36(4):803‐9. [DOI] [PubMed] [Google Scholar]
van Zon‐Rabelink 2003 {published data only}
- Zon‐Rabelink IAA, Vleugels MPH. Treating menorrhagia with endometrial ablation: rollerball electrocoagulation versus thermal ablation with the uterine balloon. Gynaecological Endoscopy Abstract from the 6th Annual Congress of the European Society for Gynaecological Endoscopy 1997;6 Suppl 2:41. [Google Scholar]
- Zon‐Rabelink IAA, Vleugels MPH, Merkus HMWM, Graaf R. Efficacy and satisfaction rate comparing endometrial ablation by rollerball electrocoagulation to uterine balloon thermal ablation in a randomised controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2004;114(1):97‐103. [DOI] [PubMed] [Google Scholar]
- Zon‐Rabelink IAA, Vleugels MPH, Merkus HMWM, Graaf R. Endometrial ablation by rollerball electrocoagulation compared to uterine balloon thermal ablation. Technical and safety aspects. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;110:220‐3. [DOI] [PubMed] [Google Scholar]
Vercellini 1999 {published data only}
- Vercellini P, Oldani S, Yaylayan L, Zaina B, Giorgi O, Crosignani PG. Randomised comparison of vaporising electrode and cutting loop for endometrial ablation. Obstetrics and Gynecology 1999;94:521‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd Ek Hameed 2012 {published data only}
- Abd Ek Hammeed AA. Endometrial thermal balloon ablation by a simple technique using Foley’s catheter with or without pre ablation endometrial curettage to treat cases with intractable menorrhagia. Middle East Fertility Society Journal 2012;17(2):116‐21. [Google Scholar]
Cash 2012 {published data only}
- Cash C, Garza‐Leal J, Donovan A, Guidry C, Romanowski C, Patel B. Clinical evaluation of long‐term safety and effectiveness of a third‐generation thermal uterine balloon therapy system for heavy menstrual bleeding. Journal of Minimally Invasive Gynecology 2012;19(4):469‐76. [DOI] [PubMed] [Google Scholar]
Chang 2009 {published data only}
- Chang P, Vilos G, Abu‐Rafea B, Hollett‐Caines J, Abyaneh Z, Edris F. Comparison of clinical outcomes with low‐voltage (cut) versus high‐voltage (coag) waveforms during hysteroscopic endometrial ablation with the rollerball: a pilot study. Journal of Minimally Invasive Gynecology 2009;1:350‐4. [DOI] [PubMed] [Google Scholar]
Cooper 2012 {unpublished data only}
- Cooper K. Blind versus visual endometrial ablation: a randomised controlled trial. http://www.controlled‐trials.com/mrct/trial/2292665/. [this trial did not take place due to lack of funding]
El‐Nashar 2009 {published data only}
- El‐Nashar SA, Hopkins MR, Creedon DJ, Cliby WA, Famuyide AO. Efficacy of bipolar radiofrequency endometrial ablation vs thermal balloon ablation for management of menorrhagia: a population‐based cohort. Journal of Minimally Invasive Gynecology 2009;16:692‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2006 {published data only}
- Feng LM, Gao WL. Clinical analysis of abnormal uterine bleeding treatment with Thermablate EAS. Beijing da Xue Xue Bao 2006;38(4):432‐5. [PubMed] [Google Scholar]
Shokeir 2013 {published data only}
- Shokeir T, Eid M, Abdel‐Hady ES. Does adjuvant long‐acting gestagen therapy improve the outcome of hysteroscopic endometrial resection in women of low‐resource settings with heavy menstrual bleeding?. Journal of Minimally Invasive Gynecology 2013;201(2):222‐6. [DOI] [PubMed] [Google Scholar]
Soysal 2001 {published data only}
- Soysal ME, Soysal SK, Vicdan K. Thermal balloon ablation in myoma‐induced menorrhagia under local anaesthesia. Gynecologic and Obstetric Investigation 2001;51:128‐33. [DOI] [PubMed] [Google Scholar]
Vihko 2003 {published data only}
- Vihko KK, Raitala R, Taina E. Endometrial thermoablation for treatment of menorrhagia: comparison of two methods in outpatient setting. Acta Obstetricia et Gynecologica Scandinavian 2003;82:269‐74. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Feng 2014 {published data only}
- Feng L, Zhang Z, Yang Q, Chen Q. Preliminary results of a randomized controlled trial of the Cardea GEA System versus transcervical resection of the endometrium (TCRE) combined with roller‐ball ablation for the treatment of abnormal uterine bleeding. Journal of Minimally Invasive Gynecology 2014;21:S139. [Google Scholar]
Hamza 2005 {published data only}
- Hamza A, Ismail MT, Abu Shady Y, Hawas NG. Resection versus coagulation techniques of ablation in the management of dysfunctional uterine bleeding. Royal College of Obstetricians and Gynaecologists 6th International Scientific Meeting, Cairo Egypt. 27‐30 September 2005.
NCT00549159 {unpublished data only}
- Yu Y, Liu N. Multicenter randomized clinical trial to evaluate the safety and effectiveness of Cavaterm TM thermal balloon endometrial ablation in women with dysfunctional uterine bleeding compared to transcervical resection of the endometrium (TCRE). http://clinicaltrials.gov/show/NCT00549159 accessed May 2018.
References to ongoing studies
NCT02642926 {published data only}
- Nazac A. Comparison of the efficiency of bipolar energy versus monopolar energy in endometrial ablation in women having menorrhagia. Clinicaltrials.gov accessed May 2018. [https://clinicaltrials.gov/ct2/show/NCT02642926]
Additional references
Baggish 1995
- Baggish MS, Paraiso M, Brexnock EM, Griffey S. A computer‐controlled, continuously circulating hot irrigating system for endometrial ablation. American Journal of Obstetrics and Gynecology 1995;173:1842‐8. [PMID: 8610773] [DOI] [PubMed] [Google Scholar]
Bridgman 2000
- Bridgman SA, Dunn KM. Has endometrial ablation replaced hysterectomy for the treatment of dysfunctional uterine bleeding? National figures. British Journal of Obstetrics and Gynaecology. 2000;107:531‐4. [PMID: 10759274] [DOI] [PubMed] [Google Scholar]
COMET 2018
- Cooper N, Khan K. Defining core outcomes for clinical trials of heavy menstrual bleeding: a Core Outcome Sets for Gynaecological conditions (COGS) project. COMET Initiative accessed May 2018. [http://www.comet‐initiative.org/studies/details/789]
Cooper 2011
- Cooper K, Lee AJ, Chien P, Raja EA, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. British Journal of Obstetrics and Gynaecology 2011;118(10):1171‐9. [DOI: 10.1111/j.1471-0528.2011.03011.x] [DOI] [PubMed] [Google Scholar]
Cooper 2000
- Cooper JM, Erickson ML. Global endometrial technologies. Obstetrics and Gynecology Clinics of North America 2000;27(2):385‐96. [PMID: 10857128] [DOI] [PubMed] [Google Scholar]
Cromwell 2009
- Cromwell DA, Mahmood TA, Templeton A, Meulen JH. Surgery for menorrhagia within English regions: variation in rates of endometrial ablation and hysterectomy. British Journal of Obstetrics and Gynaecology 2009;116:1373‐9. [DOI: 10.1111/j.1471-0528.2009.02284.x] [DOI] [PubMed] [Google Scholar]
Daniels 2012
- Daniels JP, Middleton LJ, Champaneria R, Khan KS, Cooper K, Mol BWJ, et al. on behalf of the International Heavy Menstrual Bleeding IPG Meta‐analysis Collaborative Group. Second generation endometrial ablation techniques for heavy menstrual bleeding: network meta‐analysis. British Medical Journal 2012;344:e2564. [PMID: 22529302] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Souza 2010
- Souza S, S.Camargos A, Rezende C, Pereira F, Araujo C, Silva Filho A. A randomized prospective trial comparing the levonorgestrel‐releasing intrauterine system with thermal balloon ablation for the treatment of heavy menstrual bleeding. Contraception 2010;81(3):226‐31. [DOI: 10.1016/j.contraception.2009.09.012] [DOI] [PubMed] [Google Scholar]
DeCherney 1983
- DeCherney AH, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstetrics & Gynecology 1983;61:392‐7. [PMID: 6823383] [PubMed] [Google Scholar]
DeCherney 1987
- DeCherney AH, Diamond MP, Lavey G, Polan ML. Endometrial ablation for intractable uterine bleeding: hysteroscopic resection. Obstetrics and Gynecology 1987;70:668‐70. [PMID: 3627634] [PubMed] [Google Scholar]
Donnez 1996
- Donnez J, Polet R, Mathieu PE, Konwitz E, Nisolle M, Casanas‐Roux F. Endometrial laser interstitial hyperthermy: a potential modality for endometrial ablation. Obstetrics and Gynecology 1996;87:459‐64. [PMID: 8598976] [DOI] [PubMed] [Google Scholar]
Ergun 2012
- Ergun B, Kuru O, Sen S, Kilic Y, Bastu E. Rollerball endometrial ablation versus levonorgestrel releasing intrauterine system in the management of abnormal uterine bleeding. Journal Romanian Society of Ultrasonography in Obstetrics and Gynecology 2012;8(30):199‐201. [CRSREF 3289369] [Google Scholar]
Fehr 1995
- Fehr MK, Madsen SJ, Svaasand LO, Tromberg BJ, Eusebio J, Berns MW, et al. Intrauterine light delivery for photodynamic therapy of the human endometrium. Human Reproduction 1995;10:3067‐72. [PMID: 8747076] [DOI] [PubMed] [Google Scholar]
Fraser 2015
- Fraser I, Mansour S, Breymann D, Hoffman C, Mezzacasa C, Petraglia A. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey [Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey]. International Journal of Gynaecology & Obstetrics 2015;128(3):196‐200. [DOI: 10.1016/j.ijgo.2014.09.027] [DOI] [PubMed] [Google Scholar]
Garside 2004
- Garside R, Stein K, Wyatt K, Round A, Price A. The effectiveness and cost‐effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling. Health Technology Assessment 2004; Vol. 8, issue 3:1‐155. [PMID: 14754561] [DOI] [PubMed]
Garside 2005
- Garside R, Stein K, Wyatt K, Round A. Microwave and thermal balloon ablation for heavy menstrual bleeding: a systematic review. British Journal of Obstetrics and Gynaecology 2005;112:12‐23. [PMID: 15663392] [DOI] [PubMed] [Google Scholar]
Goldrath 1981
- Goldrath MH, Fuller TA, Segal S. Laser photo‐vaporization of endometrium for the treatment of menorrhagia. American Journal of Obstetrics and Gynecology 1981;140:14‐9. [PMID: 7223809] [DOI] [PubMed] [Google Scholar]
Grant 2000
- Grant C, Gallier L, Fahey T, Pearson N, Sarangi J. Management of menorrhagia in primary care ‐ impact on referral and hysterectomy: data from the Somerset Morbidity Project. Journal of Epidemiology and Community Health 2000;54:709‐13. [PMID: 10942454] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallberg 1964
- Hallberg L, Nilsson L. Determination of menstrual blood loss. Scandinavian Journal of Clinical and Laboratory Investigation 1964;16:244‐8. [PMID: 14161862] [PubMed] [Google Scholar]
Hallberg 1966
- Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss ‐ a population study. Acta Obstetricia et Gynecologica Scandinavian 1966;45:320‐51. [PMID: 5922481] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011, 2011. [http://training.cochrane.org/handbook] [Google Scholar]
Higham 1990
- Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology 1990;97:734‐9. [PMID: 2400752] [DOI] [PubMed] [Google Scholar]
Irvine 1998
- Irvine GA, Campbell‐Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for the treatment of idiopathic menorrhagia. British Journal of Obstetrics and Gynaecology 1998;105:592‐8. [PMID: 9647148] [DOI] [PubMed] [Google Scholar]
Kalampokas 2017
- Kalampokas E, McRobbie S, Payne F, Parkin D. Long‐term incidence of hysterectomy following endometrial resection or endometrial ablation for heavy menstrual bleeding. International Journal of Gynaecology & Obstetrics 2017;139(1):61‐64. [DOI: 10.1002/ijgo.12259] [DOI] [PubMed] [Google Scholar]
Lethaby 2009
- Lethaby A, Shepperd S, Cooke I. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding (Cochrane Review). Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000329] [DOI] [PubMed] [Google Scholar]
Lin 1988
- Lin BL, Miyamoto N, Tomomatu M. The development of a new hysteroscopic resectoscope and its clinical applications on transcervical resection and endometrial ablation. Japanese Journal of Gynecologic and Obstetric Endoscopy 1988;4:56‐9. [Google Scholar]
Madhu 2009
- Madhu CK, Nattey J, Naeem T. Second generation endometrial ablation techniques: an audit of clinical practice. Archives of Gynaecology and Obstetrics 2009;280:599‐602. [DOI: 10.1007/s00404-009-0982-7] [DOI] [PubMed] [Google Scholar]
Maresh 2002
- Maresh MJA, Metcalfe MA, McPherson K, Overton C, Hall V, Hargreaves J. The VALUE national hysterectomy study: description of the patients and their surgery. British Journal of Obstetrics and Gynaecology 2002;109:302‐12. [PMID: 11950186] [DOI] [PubMed] [Google Scholar]
Marjoribanks 2010
- Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD003855.pub2] [DOI] [Google Scholar]
Matteson 2015
- Matteson K, Scott D, Raker C, Clark M. The menstrual bleeding questionnaire: development and validation of a comprehensive patient‐reported outcome instrument for heavy menstrual bleeding. British Journal of Obstetrics and Gynaecology 2015;122(5):681‐9. [DOI: 10.1111/1471-0528.13273] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGurgan 2007
- McGurgan P, O'Donovan P. Second‐generation endometrial ablation ‐ an overview. Best Practice and Research. Clinical Obstetrics and Gynaecology 2007;21(6):931‐45. [DOI: 10.1016/j.bpobgyn.2007.03.0151] [DOI] [PubMed] [Google Scholar]
Middleton 2010
- Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. for the International Heavy Menstrual Bleeding Individual Patient Data Meta‐analysis Collaborative Group. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta‐analysis of data from individual patients. British Medical Journal 2010;341:c3929. [DOI: 10.1136/bmj.c3929] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 2012
- Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them?. Americam Journal of Obstetrics & Gynecology 2012;207(4):259‐65. [DOI: 10.1016/j.ajog.2012.01.046] [DOI] [PubMed] [Google Scholar]
Munroe 2006
- Munroe MG. Endometrial ablation: where have we been? Where are we going?. Clinical Obstetrics and Gynecology 2006;49(4):736‐66. [DOI: 10.1097/01.grf.0000211947.28842.93] [DOI] [PubMed] [Google Scholar]
Nagele 1998
- Nagele F, Rubinger T, Magos A. Why do women choose endometrial ablation rather than hysterectomy?. Fertility and Sterility 1998;69(6):1063‐6. [PMID: 9627293] [DOI] [PubMed] [Google Scholar]
Newton 1977
- Newton J, Barnard G, Collins W. A rapid method for measuring menstrual blood loss using automatic extraction. Contraception 1977;16:269‐82. [DOI: 10.1016/0010-7824(77)90026-9] [DOI] [Google Scholar]
NHS 2011
- NHS Information Centre. Hospital episode statistics 2011. NHS 2011.
NICE 2018
- National Institute of Health and Care Excellence (NICE). Heavy menstrual bleeding. National Institute for Health and Care Excellence (2018) Heavy menstrual bleeding (NICE Guideline 88). Available at: https://www.nice.org.uk/guidance/conditions‐and‐diseases/gynaecological‐conditions/heavy‐menstrual‐bleeding [Accessed 22 May 2018]. [https://www.nice.org.uk/guidance/conditions‐and‐diseases/gynaecological‐conditions/heavy‐menstrual‐bleeding]
NZ HMB Guideline 1998
- Working Party on behalf of the National Health Committee of New Zealand. Guidelines for the management of heavy menstrual bleeding. Guidelines for the management of heavy menstrual bleeding in New Zealand. Available at file:///P:/Cochrane/various%20cochrane/guidelines‐management‐heavy‐menstrual‐bleeding%20NZ%201998.pdf accessed Nov 2017 1998.
Overton 1997
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. British Journal of Obstetrics and Gynaecology 1997;104(12):1351‐9. [DOI] [PubMed] [Google Scholar]
Papadopoulos 2007
- Papadopoulos NP, Magos A. First‐generation endometrial ablation: rollerball vs loop vs laser. Best Practice and Research. Clinical Obstetrics and Gynaecology 2007;21(6):915‐29. [DOI: 10.1016/j.bpobgyn.2007.03.014] [DOI] [PubMed] [Google Scholar]
Pitroff 1993
- Pitroff R, Majia S, Murray A. Initial experience with transcervical cryoablation using saline as a uterine distension medium. Minimally Invasive Therapy 1993;2:69‐73. [DOI: 10.3109/13645709309152670] [DOI] [Google Scholar]
RCOG 1995
- RCOG Medical Audit Unit. Mistletoe Report for the Fifth Bulletin. Manchester: RCOG, 1995. [Google Scholar]
Reid 2005
- Reid PC, Mukri F. Trends in number of hysterectomies performed in England for menorrhagia: examination of health episode statistics, 1989 to 2002‐3. British Medical Journal 2005;330:938‐9. [DOI: 10.1136/bmj.38376.505382.AE; PMCID: PMC556338 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2011
- Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. British Medical Journal 2011;342:d2202. [PMCID: PMC3082380] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 1995
- Sharp NC, Cronin N, Feldberg I, Evans M, Hodgson D, Ellis S. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet 1995;346(8981):1003‐4. [PMID: 7475547] [DOI] [PubMed] [Google Scholar]
Shavell 2012
- Shavell V, Diamond M, Senter J, Kruger M, Johns A. Hysterectomy subsequent to endometrial ablation. Journal of Minimally Invasive Gynecology 2012;19(4):459‐64. [DOI: ] [DOI] [PubMed] [Google Scholar]
Shaw 2007
- Shaw RW, Symonds IM, Tamizian O, Chaplain J, Mukhopadhyay S. Randomised comparative trial of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Australia and New Zealand Journal of Obstetrics and Gynaecology 2007;47(4):335‐40. [DOI: 10.1111/j.1479-828X.2007.00747.x] [DOI] [PubMed] [Google Scholar]
Singer 1994
- Singer A, Almanza R, Gutierrez A, Haber G, Bolduc L, Neuwirth R. Preliminary clinical experience with thermal balloon endometrial ablation method to treat menorrhagia. Obstetrics and Gynecology 1994;83:732‐7. [PMID: 8164933] [PubMed] [Google Scholar]
Vaincaillie 1989
- Vaincaillie TG. Electrocoagulation of the endometrium with the ball‐ended resectoscope. Obstetrics and Gynecology 1989;74:425‐7. [PMID: 2761921] [PubMed] [Google Scholar]
Warner 2001
- Warner P, Critchley HO, Lumsden MA, Campbell‐Brown M, Douglas A, Murray G. Referral for menstrual problems: cross sectional survey of symptoms, reasons for referral and management. British Medical Journal 2001;323:2‐8. [DOI: 10.1136/bmj.323.7303.24] [DOI] [PMC free article] [PubMed] [Google Scholar]


