Abstract
Mindfulness training ameliorates clinical and self-report measures of depression and chronic pain, but its use as an emotion regulation strategy—in individuals who do not meditate—remains understudied. As such, whether it (i) down-regulates early affective brain processes or (ii) depends on cognitive control systems remains unclear. We exposed meditation-naïve participants to two kinds of stimuli: negative vs. neutral images and painful vs. warm temperatures. On alternating blocks, we asked participants to either react naturally or exercise mindful acceptance. Emotion regulation using mindful acceptance was associated with reductions in reported pain and negative affect, reduced amygdala responses to negative images and reduced heat-evoked responses in medial and lateral pain systems. Critically, mindful acceptance significantly reduced activity in a distributed, a priori neurologic signature that is sensitive and specific to experimentally induced pain. In addition, these changes occurred in the absence of detectable increases in prefrontal control systems. The findings support the idea that momentary mindful acceptance regulates emotional intensity by changing initial appraisals of the affective significance of stimuli, which has consequences for clinical treatment of pain and emotion.
Keywords: mindfulness, acceptance, emotion regulation, fMRI, pain
Introduction
‘Let it be. Let it be. Let it be.’ (The Beatles)
The ability to regulate emotion is critical for success in workplace, family, and social settings. Indeed, emotional dysregulation is a core feature of disorders including depression, anxiety, addiction, and chronic pain (Gross, 2014) and predicts mortality (Steptoe et al., 2007; Pressman et al., 2013). Accordingly, there has been a tremendous rise in studies of emotion regulation. While this work has laid a strong foundation for understanding core regulatory processes, neuroscientific studies have focused almost entirely on strategies that control attention to or cognitively transform the meaning of emotional thoughts, stimuli, or events (e.g. distraction, reappraisal).
Such strategies are effective in regulating behavioral and brain correlates of negative emotions (Buhle et al., 2014) and are core components of established psychological treatments (Beck & Haigh, 2014; Gross, 2014). However, the cognitive control processes on which they depend may not operate effectively for all people (e.g. children, older adults, patient groups; Koenigsberg et al., 2009; Winecoff et al., 2011; Silvers et al., 2012) or in all situations (e.g. under stress; Raio et al., 2013; Troy et al., 2013). Therefore, it is important to ask: are there effective emotion regulation strategies that do not depend on top-down cognitive control? Identifying and understanding the mechanisms supporting such strategies could lead to improved treatments for emotionally vulnerable populations.
One candidate class of regulation strategies involves mindfully noticing and accepting one’s affective reactions. While rooted in ancient Buddhist traditions, modern scientific contexts operationalize mindfulness as involving attention to present moment experience—even if unpleasant—with an accepting attitude that lets it be exactly as it is, without reacting, judging, or avoiding it (Bishop et al., 2004). Unlike the traditional cognitive regulation approaches described above, such mindful acceptance may constitute a ‘mindset’ that can be applied across a variety of affective situations, even in individuals who do not meditate.
While this view of mindful acceptance suggests that it might depend upon different neural mechanisms than reappraisal and related strategies, this has not been well-studied. Instead, mindful acceptance has been largely studied in one of two ways. First, behavioral studies have shown that mindfulness- or acceptance-based treatments ameliorate depression (Ma & Teasdale, 2004), anxiety (Goldin & Gross, 2010; Hofmann et al., 2010), addiction (Brewer et al., 2011a), and chronic pain (Kabat-Zinn et al., 1985; Wetherell et al., 2011); improve functionality and quality of life in cancer and other conditions (Grossman et al., 2004; Carlson et al., 2013); and reduce pro-inflammatory gene expression (Creswell et al., 2012) as well as other physiological biomarkers associated with health and longevity (Jacobs et al., 2011). While such studies cannot illuminate the brain mechanisms underlying mindful acceptance as an emotion regulation strategy, they attest to the importance of understanding them.
Second, brain imaging studies have examined individuals who were trained or regularly engage in mindfulness meditation. Such studies have reported differences between long-term meditators and non-meditators in pain sensitivity and pain-related neural activity (Grant et al., 2011; Gard et al., 2012; Lutz et al., 2013). In one study, 4 days of mindfulness meditation training was associated with reduced pain unpleasantness and altered neural activity (Zeidan et al., 2011; Zeidan et al., 2012). While promising, such studies do not directly address the use of mindful acceptance as an emotion regulation strategy in individuals who do not practice meditation, and findings could depend on cumulative effects of training or characteristics of individuals who seek it (see Discussion for additional review).
We addressed this using functional magnetic resonance imaging (fMRI) and adapting a well-established emotion regulation task to assess the effects of mindful acceptance on affective and neural responses in meditation-naïve adults. Participants were briefly instructed to use mindful acceptance as a strategy in the presence of emotional stimuli. During the scanning session, they were exposed to aversive (vs. neutral) images and painful (vs. warm) heat while maintaining a mindset of mindful acceptance or reacting naturally. They also reported their negative emotion or pain after each trial. This design allowed us to address three main questions. First, we asked whether mindful acceptance is an effective regulation strategy for modulating negative emotion and pain. Second, we asked whether mindful acceptance modulates neural markers of negative emotion and pain. Addressing this question requires brain markers for primary affective representations. For this purpose, we tested whether mindful acceptance reduces responses to aversive images in the amygdala (Buhle et al., 2014) and to thermal pain in regions that encode the intensity of noxious heat and correlate with pain reports (Atlas et al., 2014), including the dorsal anterior cingulate, anterior insula, medial thalamus and somatosensory cortices. In addition, we tested for effects of mindful acceptance on pain-related responses in the Neurologic Pain Signature (NPS; Wager et al., 2013), an a priori multivariate pattern that has been validated across multiple studies as sensitive and specific to somatic pain (see Methods). Importantly, recent work has shown that the NPS is not affected by cognitive reappraisal (Woo et al., 2015). Therefore, influence of mindful acceptance on this biomarker would suggest that it has a more profound impact on pain processing (see Supplementary Materials).
Third, we tested two competing hypotheses regarding the neural mechanisms underlying mindful acceptance-based reductions in negative affect. Reappraisal is thought to depend on prefrontal cortical (PFC) regions implicated in cognitive control that modulate activity in affect-processing systems like the amygdala (Ochsner et al., 2012). For pain, reappraisal appears to engage similar prefrontal mechanisms, which are linked to changes in pain reports (Woo et al., 2015). Mindful acceptance could work via similar mechanisms. Alternatively, it may not depend on PFC recruitment, as it does not rely on verbal rehearsal, cognitive reframing, or response inhibition. If prefrontal mechanisms were not involved, it would suggest that mindful acceptance alters ‘bottom-up’ appraisals, rather than ‘top-down’ control like many other regulation strategies. We tested these hypotheses by comparing PFC recruitment in whole-brain, region-of-interest (ROI), and functional connectivity analyses.
Methods
Participants
Seventeen participants (5 women; ages 18–45, M = 31.75, SD = 5.18) were recruited via posters and electronic bulletin board ads in New York City. Recruitment materials (e.g. posters) described a study about emotion and/or pain perception. Upon arrival to the lab, participants were told that they would be asked to follow different types of instructions while looking at neutral and negative images and experiencing both neutral (warm) and hot (painful) temperatures. Then, participants gave written informed consent as approved by the Columbia University Institutional Review Board.
Participants were excluded if they were left-handed, did not speak English fluently, had prior meditation experience or reported any of the following conditions: neurological or psychiatric disorders, use of psychoactive medications in the past 6 months, medical conditions that may alter cerebral function, cardiovascular disease, diabetes, prior head trauma with loss of consciousness >30 minutes, pregnancy, claustrophobia, or MRI contraindicated implants (Buhle et al., 2013). One participant was scanned but removed from the sample prior to analysis because he reported that he did not follow the task instruction during fMRI scanning. A priori sample size and data-collection stopping targets were based on sample and effect sizes reported at the time in the extant reappraisal literature (i.e. commonly around 16–18 participants; for a meta-analysis, see Buhle et al., 2014) and on availability of funding.
Stimuli
During the task, participants were exposed to 30 neutral and 30 negative images from the International Affective Picture System (see Supplementary Material for the full list) and experienced 30 warm and 30 painfully hot thermal stimulations, delivered to the non-dominant forearm using a Medoc TSA 2001 (Medoc Ltd, NC). Before scanning, warm and hot temperatures were chosen on an individualized basis for each participant. Warm temperatures (40.5–44°C) were chosen at calibration level 2 on a 10-point pain scale (described as ‘non-painful warmth’). Hot/painful temperatures (45–48°C) were chosen at calibration level 8, following our prior work (Buhle et al., 2013).
Procedure
Modeled after prior emotion regulation studies (e.g. Wager et al., 2008; Kober et al., 2010), participants received 30 minutes of task training prior to scanning. They were told that during the task, two instructional cues (ACCEPT and REACT) would direct them to think about subsequently presented images or thermal stimuli in one of two ways. REACT cues instructed participants to ‘react naturally, whatever your response might be’ (see Supplementary Materials for full instruction text). This served as the control condition, intended to provide a baseline measure of unregulated emotional responding. ACCEPT cues instructed participants to attend to and accept their experience as it is. This instruction was modeled after the two-component definition of mindfulness mentioned in the introduction (Bishop et al., 2004), including (i) attention to present moment sensation, coupled with (ii) non-judgmental acceptance of the sensation as it is, allowing it to exist without trying to avoid it or react to it. For example, participants were told ‘if you feel a sensation of warmth on your forearm, you should simply attend to what is felt, without making any judgment of the “goodness” or “badness” of that sensation.’ Importantly, these instructions are consistent with mindfulness-based intervention manuals (e.g. Kabat-Zinn, 1982; Grossman et al., 2004; Brewer et al., 2011a), Acceptance and Commitment Therapy (e.g. the bus mtaphore; Hayes, 2004) and Dialectical Behavior Therapy (e.g. the core mindfulness module, and ‘mindfulness of current emotion’ skill from the emotion regulation module; Linehan, 2015).
Once participants indicated that they understood the nature of mindful acceptance, and how to follow the ACCEPT and REACT instructions, they completed several sample trials during which they practiced following these instructions while looking at images that were not used during the scanning session. Participants were asked to verbalize their responses during the practice trials, just as they would do internally during the scan. The experimenter did not proceed, and participants did not begin the scanning session, until they demonstrated being able to follow the instruction cues and to use them appropriately (see Supplementary Materials for additional details).
During the scanning session, participants completed five functional runs. Each run consisted of eight task blocks; jittered fixation periods between blocks and between trial elements were explicitly included to avoid confounds and improve modeling (Ollinger et al., 2001a; Ollinger et al., 2001b; Wager & Nichols, 2003; Wager & Lindquist, 2015). As shown in Figure 1, each block began with a jittered fixation cross (~5.5 s), followed by a 3-s instruction cue that indicated that participants were to adopt either the REACT or ACCEPT mindset for the duration of the block (which lasted ~63 s). For the remainder of the block, a green- or blue-colored outline surrounding the screen served as a continuous reminder of the mindset (the assignment of colors was counterbalanced, and block order was randomized). Each block included three trials of images or thermal stimulations presented in a random order. Each trial began with a jittered fixation cross (~5.5 s), followed by an 8-s presentation of an image or thermal stimulus. Following another jittered fixation period (~5.5 s), participants indicated how negatively they felt by using an eight-point visual analogue scale that appeared onscreen for 3 s. In total, each run consisted of 24 trials consisting of 6 neutral images, 6 negative images, 6 warm temperatures and 6 hot temperatures.
Fig. 1.
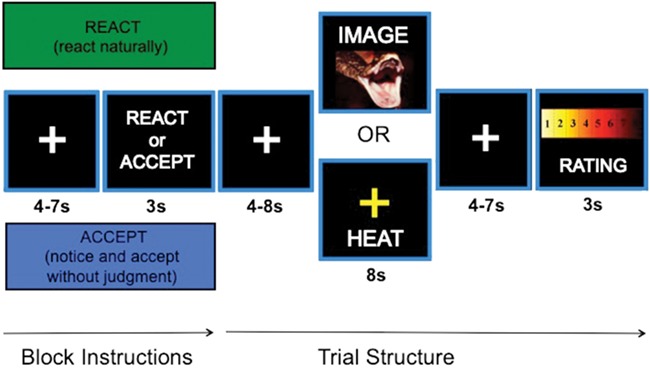
Task structure. REACT or ACCEPT instructions indicated which strategy participants should use when viewing negative/neutral images and experiencing hot/warm temperatures. Each instruction was associated with a unique outline color, which was counterbalanced across participants. After each stimulus period, participants rated their negative affect or pain.
Behavioral data acquisition and analysis
Stimulus presentation and behavioral data acquisition were controlled using E-Prime (Psychology Software Tools Inc.). Visual stimuli were presented via back-projection screen. Responses were made with the right hand using an MR-compatible trackball. Response data were subjected to a 2 (Strategy: ACCEPT vs. REACT) × 2 (Stimulus: Images vs. Temperatures) × 2 (Intensity: Neutral/Warm vs. Negative/Hot) repeated measures ANOVA, with an alpha level of p < .05. Effect sizes for main effects and interactions are reported as partial eta squared (ηp2). Post hoc paired t tests were conducted to further investigate significant effects; effect sizes for t tests are reported as Cohen’s d.
fMRI data acquisition and analysis
Data acquisition & preprocessing
Participants were scanned in a 1.5T GE Signa Twin Speed Excite HD scanner (GE Medical systems). Functional images were acquired with a T2*-weighed EPI BOLD sequence (TR/TE = 2000 ms/34 ms, flip angle = 0°, 64 × 64 in-plane matrix, 3.5 mm in-plane voxel size, FOV = 224 mm and 28 × 4.5 mm slices). High-resolution SPGR structural images were acquired for each subject (TR/TE = 9700 ms/2300 ms, flip angle = 20°, 256 × 256 in-plane matrix, FOV = 24 cm and ~182 × 1 mm slices), covering the entire brain. Functional images were subjected to standard preprocessing using SPM8 (Wellcome Department of Cognitive Neurology), including slice-time correction, motion correction, coregistration of functional to structural images, normalization to the Montreal Neurological Institute template using 3-mm isometric voxels, and spatial smoothing using a 6 mm FWHM Gaussian kernel. All data were examined for quality and motion. No participants’ data were excluded due to excessive motion (see Supplementary Materials).
Whole-brain GLM analysis
Following our prior work, functional images were subjected to first-level statistical analysis using outlier-resistant robust regression (e.g., Kober et al., 2014). We modeled eight conditions of interest crossing two instruction conditions (ACCEPT vs. REACT), two stimulus types (Images vs. Temperatures) and two levels within each (Neutral vs. Negative Images and Warm vs. Painful heat), and added six motion parameters, high-pass filter, and global white matter signal parameters as additional regressors of no interest. The conditions were: REACT-neutral images, ACCEPT-neutral images, REACT-negative images, ACCEPT-negative images, REACT-warm, ACCEPT-warm, REACT-hot, and ACCEPT-hot. We then performed second-level random effects analysis in NeuroElf (neuroelf.net) to localize regions of activation across participants in the contrasts of interest. Group results were family-wise error (FWE) corrected at p < .05 using the procedure first established in AFNI (‘AlphaSim’; Cox, 1996), implemented within NeuroElf. This process currently entails two steps. First, smoothness is estimated directly from the residual maps, then Monte Carlo simulation is used to estimate cluster size and intensity threshold to reach a combined corrected threshold (Xiong et al., 1995).
To identify regions responsive to negative images, we contrasted activity to Negative vs. Neutral Images. To identify regions modulated by mindful acceptance, we contrasted responses on ACCEPT vs. REACT trials for negative images only. To identify regions of overlap, we performed a formal conjunction analysis between Negative vs. Neutral Images contrast and the ACCEPT vs. REACT contrast. To identify regions responsive to painful heat, we contrasted Hot vs. Warm trials. To specifically identify pain-related regions modulated by mindful acceptance, we contrasted responses on ACCEPT vs. REACT trials with painful heat only. To identify regions of overlap, we performed a formal conjunction analysis between Hot vs. Warm temperatures and the ACCEPT vs. REACT contrast.
ROI analyses
To test whether mindful acceptance of negative affect or pain depended on prefrontal recruitment, we extracted % signal change from a-priori ROIs in bilateral dorsolateral and ventrolateral PFC and medial PFC, extracted from our previously published meta-analysis of reappraisal studies that revealed consistent activation during emotion regulation (Buhle et al., 2014); Table S1). We then conducted t tests between instruction conditions, echoing the imaging contrasts, to test specific hypotheses regarding PFC recruitment during mindful acceptance of negative affect and pain. Additional analyses for all seven regions reported in Buhle et al. (2014) as well as eight additional ROIs associated with cognitive control more broadly are reported in Supplementary Materials (Figures S1 and S2, Tables S1 and S2).
Functional connectivity
To further assess whether mindful acceptance of negative affect and pain depended on prefrontal recruitment, we conducted a standard functional connectivity analysis (psychophysiological interaction analysis; PPI; Friston et al., 1997). Seed regions were functionally defined as those regions identified in the GLM conjunction analyses as modulated by mindful acceptance of negative emotion in response to images and pain—namely, right amygdala (images), and right anterior insula, right posterior insula and dACC (pain). A separate GLM was then computed for each seed region incorporating regressors for (i) the within-participant coupling of activity between the seed region and other brain areas and (ii) an interaction term representing the coupling of the seed region with other brain areas modulated by ACCEPT vs. REACT difference (for images or pain). Participants’ six motion parameters, high-pass filter, and global white matter signal parameters were also entered as covariates of no interest. We then performed random effects analyses as described above, with contrasts performed for regions showing a significant PPI effect. Results were FWE corrected as described above.
Neurological Pain Signature (NPS)
Using machine learning, we previously created a multivariate spatial pattern of regression weights within and across brain regions targeted by primary nociceptive afferent fibers (including those listed above; e.g. dorsal anterior cingulate, anterior insula, medial thalamus, and somatosensory cortices). This pattern—named the NPS—reliably and selectively predicts physical pain intensity in data obtained in new participants (Wager et al., 2013), thereby constituting a signature of pain suitable for application to new individual datasets (see Supplementary Materials). Wager et al. (2013) validated the NPS across four studies and demonstrated that it (i) responds linearly to rising temperature and predicts pain self-reports, controlling for temperature; (ii) discriminates painful vs. non-painful conditions with 90–100% accuracy for individual participants; and (iii) responds specifically to somatic pain, and not to threat cues, retrospective pain judgments, or emotionally evocative images. Both its sensitivity and specificity to pain have been replicated in independent samples (Chang et al., 2015).
The NPS provides a weight at each voxel. Application of the NPS to a new dataset involves multiplying these weights with corresponding voxels in each activation map in the new dataset (within person and condition) and reducing the product to a weighed average. We first applied the NPS to each of the temperature condition activity maps (REACT-warm, ACCEPT-warm, REACT-hot, ACCEPT-hot). This yielded a single response value, the ‘NPS response,’ which quantifies the predicted pain for that condition. We then compared the NPS response between the two hot temperature conditions (ACCEPT-hot, REACT-hot), to assess whether the ACCEPT strategy led to significantly reduced pain response in the NPS.
Results
Reports of negative affect and pain
As expected, we found a main effect of stimulus intensity (F(1, 15) = 107.74, p < .001, ηp2 = .88) such that participants reported greater negative affect in response to negative vs. neutral images (t(15) = 10.41, p < .001, d = 2.60) and painfully hot vs. warm temperatures (t(15) = 7.88, p < .001, d = 1.97; Figure 2). Importantly, we found a main effect of instruction (F(1, 15) = 29.05, p < .001, ηp2 = .66); when participants followed instructions to ACCEPT vs. REACT to the stimuli, they reported significantly lower negative affect in response to both stimulus types (images: t(15) = 4.56, p < .001, d = 1.14; temperatures: t(15) = 6.05, p < .001, d = 1.51). Notably, there was no significant interaction of instruction and stimulus type, suggesting that the effects of mindful acceptance on affective responses were comparable for images and temperatures (see Supplementary Materials).
Fig. 2.
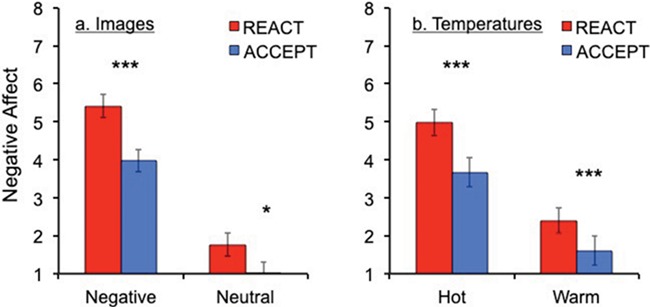
Self-reported negative affect for images and temperatures. Overall, N = 16 participants reported greater negative affect for negative images than neutral (a), and for hot temperatures compared to warm (b). Further, participants reported lower levels of negative affect on trials on which they practiced mindful acceptance compared to reacting naturally. T tests were performed between conditions: *P < .05, **P < .01, ***P < .001. Error bars represent standard errors.
fMRI results
Assessment of mindful acceptance as a regulation strategy
Negative images
A whole-brain Negative vs. Neutral Images contrast revealed greater activity for negative images in regions including bilateral amygdala, thalamus, midbrain (including periaqueductal gray), dorsal anterior cingulate (dACC), and dorsomedial prefrontal cortex (Table 1A, Figures 3a & S1). To identify the effects of mindful acceptance on neural responses, we computed a whole-brain ACCEPT vs. REACT contrast for negative image trials only. Right amygdala responses to negative images were significantly reduced during ACCEPT vs. REACT instructions (Table 1B, Figures 3b & S2).
Table 1.
Regions showing differential activation based on instruction. R/L/Bi refers to lateralization of activation. x, y and z are MNI peak coordinates. mm3 refers to the spatial extent of each cluster, expressed as cubic millimeters (3 × 3 × 3 mm/voxel * number of voxels). (A–D) Peak/mean statistics are t values. Results are whole-brain family-wise error-corrected at p < 0.05. (E–F) Peak statistics for conjunctions represent the minimum (less significant) t value of the two maps and average minimum t statistic in the cluster, following conjunction conventions (Nichols et al., 2005)
| Peak coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Regions of Activation | R/L/Bi | x | y | z | mm 3 | Peak statistic | Mean statistic |
| A. Negative images > neutral images | |||||||
| Temporal/occipital/parietal | Bi | −45 | −66 | 3 | 164 538 | 11.43 | 4.36 |
| Middle/inferior frontal/precentral gyrus | R | 45 | 36 | 9 | 33 966 | 8.68 | 4.17 |
| Dorsal anterior/posterior cingulate gyrus/dorsal medial frontal gyrus | L | −6 | 9 | 27 | 25 893 | 7.54 | 3.71 |
| Amygdala/thalamus/putamen/parahippoca mpal gyrus/midbrain | Bi | 24 | −30 | −9 | 18 495 | 6.83 | 3.66 |
| Postcentral gyrus | R | 15 | −48 | 72 | 5022 | −4.40 | −3.44 |
| Insula/inferior/middle frontal gyrus | L | −30 | 24 | −15 | 4995 | 5.44 | 3.66 |
| Superior temporal gyrus | R | 69 | −12 | −3 | 3537 | −5.38 | −3.57 |
| Middle frontal/precentral gyrus | L | −45 | 0 | 24 | 2781 | 4.77 | 3.54 |
| Middle/superior temporal gyrus | L | −66 | −39 | −3 | 2187 | −4.88 | −3.62 |
| Superior/middle temporal gyrus | L | −54 | −21 | −3 | 1620 | −4.68 | −3.39 |
| B. Accept > react for negative images | |||||||
| Superior/middle temporal gyrus | R | 42 | 21 | −24 | 4212 | −5.54 | −3.55 |
| Medial/superior frontal gyrus | Bi | −3 | 63 | −6 | 1890 | −5.43 | −3.53 |
| Amygdala/midbrain | R | 12 | −18 | −15 | 1647 | −5.09 | −3.53 |
| C. Painfully hot > warm temperatures | |||||||
| Dorsal anterior cingulate/medial frontal | |||||||
| Gyrus/precentral and postcentral gyrus/anterior and posterior insula/ thalamus/inferior parietal/parahippocampal gyrus/putamen/caudate/midbrain/cerebellum | Bi | 54 | −21 | 27 | 236 358 | 8.01 | 3.76 |
| Cuneus/lingual gyrus | L | −3 | −78 | 9 | 14 229 | 5.08 | 3.42 |
| Cerebellum | R | 39 | −42 | −36 | 2268 | 6.24 | 3.73 |
| Pre-SMA/middle frontal gyrus | R | 45 | 6 | 39 | 1782 | 4.07 | 3.24 |
| D. Accept > react for painful heat | |||||||
| Anterior and posterior insula/dorsal anterior cingulate/medial frontal gyrus/precentral and postcentral gyrus/inferior parietal/precuneus/posterior cingulate | Bi | 51 | −24 | 18 | 52 029 | −6.03 | −3.46 |
| Cerebellum/middle and inferior occipital | L | −45 | −81 | −21 | 4806 | −5.29 | −3.41 |
| Cerebellum/cuneus/posterior cingulate | R | 3 | −45 | 0 | 4698 | −4.61 | −3.49 |
| Cerebellum/middle and inferior occipital | R | 57 | −66 | −15 | 3996 | −5.17 | −3.39 |
| Thalamus | L | −12 | −27 | 9 | 2970 | −5.51 | −3.41 |
| Middle temporal gyrus/middle occipital | R | 45 | −72 | 15 | 2646 | −5.14 | −3.50 |
| Mid insula/precentral gyrus | R | 63 | 9 | 0 | 2484 | −5.89 | −3.54 |
| Inferior parietal lobule | R | 72 | −33 | 24 | 1836 | −4.32 | −3.29 |
| Caudate | R | 15 | 0 | 21 | 1620 | −5.56 | −3.75 |
| E. Conjunction: (A. negative images > neutral images) and (B. accept > react for negative images) | |||||||
| Amygdala | R | 21 | −9 | −12 | 567 | −4.46 | −3.57 |
| F. Conjunction: (C. painfully hot > warm temperatures) and (D. accept > react for painful heat) | |||||||
| Posterior insula/postcentral gyrus | L | −60 | −27 | 21 | 3456 | 4.67 | 3.41 |
| Caudate/thalamus | R | 15 | −3 | 21 | 1350 | 4.70 | 3.48 |
| Mid insula | L | −30 | −3 | 9 | 1242 | 5.19 | 3.53 |
| Posterior insula/postcentral gyrus | R | 54 | −24 | 18 | 1080 | 4.90 | 3.60 |
| Superior temporal gyrus | L | −57 | 0 | 0 | 999 | 3.75 | 3.21 |
| Paracentral lobule | R | 12 | −27 | 48 | 756 | 4.02 | 3.33 |
| Postcentral gyrus | R | 69 | −21 | 27 | 702 | 3.54 | 3.17 |
| Posterior cingulate | R | 6 | −33 | 24 | 621 | 3.65 | 3.23 |
Fig. 3.
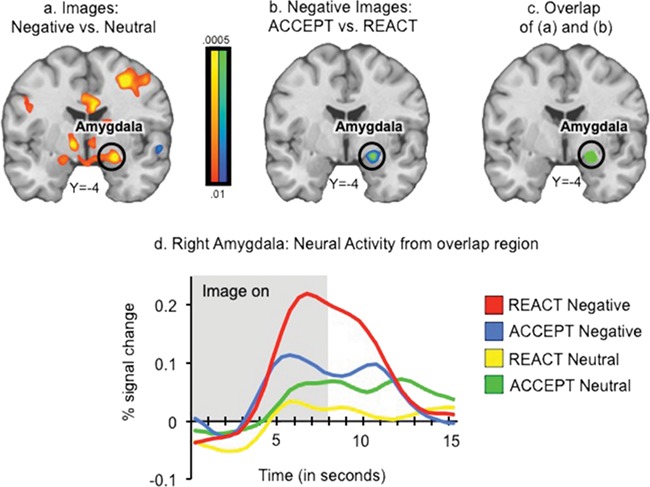
Mindful acceptance modulates response to negative images. (a) Regions responsive to negative images in the whole-brain contrast Negative Images vs. Neutral Images. Red/Yellow colors indicate greater activity during Negative Images than Neutral Images; yellow regions indicate the most significant differences. (b) Regions modulated by mindful acceptance in the whole-brain contrast ACCEPT vs. REACT for negative images only. Blue/green colors indicate greater activity in REACT than ACCEPT; green regions indicate the most significant differences. (c) Region of overlap found in conjunction of (a) and (b). (d) Extracted time courses from the amygdala region identified in the conjunction (c)—peak is shifted due to HRF/BOLD delay. Results are family-wise error corrected at p < .05. Right is displayed on the right.
Several results demonstrated that the effect of mindful-acceptance on amygdala activity was specific to negative images. First, data extracted from the significant cluster in right amygdala did not show an ACCEPT vs. REACT effect for neutral images (p > .1; Figure S3a). Second, although there were modest increases in amygdala activity for Hot vs. Warm Temperatures (p < .01), activity for hot trials was comparable to that for neutral images (Hot Temperatures vs. Neutral Images, p > .1), and there were no mindful-acceptance effects on Hot trials in the amygdala (p > .1; Figure S3b). ACCEPT instructions also reduced activity in right midbrain areas contiguous with the amygdala, right lateral and medial (opercular) anterior temporal cortex, temporal pole, and frontopolar cortex (Table 1B, Figure S2). Conjunction analysis between Negative vs. Neutral Images contrast and the ACCEPT vs. REACT contrast revealed a single area of overlap in the right amygdala (Figure 3c and d, Table 1E). This region was both responsive to negative images and modulated by mindful acceptance (see Supplementary Materials for additional analyses).
Pain
A whole-brain Hot vs. Warm contrast revealed greater activity in a large set of regions, including bilateral dACC and medial frontal gyrus, sensorimotor regions including precentral and postcentral gyri, pre-SMA, anterior and posterior insula, thalamus, midbrain (including periaqueductal gray), and cerebellum (Table 1C, Figures 4a & S4). These regions were previously shown to be responsive to painful stimuli and correlate with pain intensity across subjects (Coghill et al., 1999; Apkarian et al., 2005) and within subjects when temperatures are matched (e.g. Atlas et al., 2014).
Fig. 4.
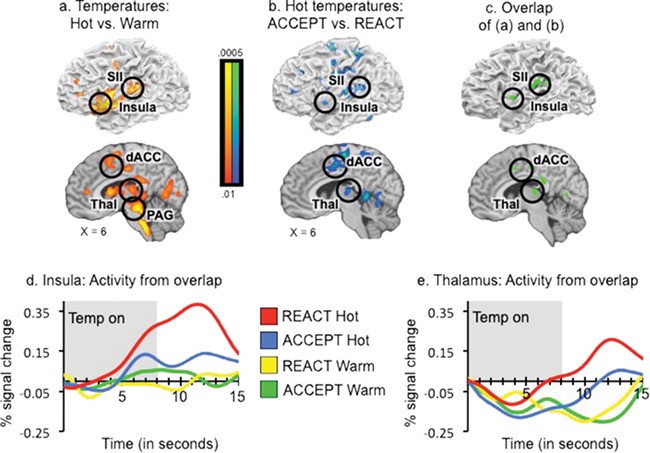
Mindful acceptance modulates response to painful heat. (a) Regions responsive to painful heat identified in the contrast Hot vs. Warm Temperatures. Red/yellow colors indicate greater activity during Hot than Warm Temperatures; yellow regions indicate the most significant differences. (b) Regions modulated by mindful acceptance in the contrast ACCEPT vs. REACT for hot temperatures only. Blue/green colors indicate greater activity in REACT than ACCEPT; green regions indicate the most significant differences. (c) Regions of overlap found in conjunction of (a) and (b). (d–e) Extracted time courses from insula and thalamus regions identified in the conjunction (b)—peaks are shifted due to HRF/BOLD delay. All results are family-wise error corrected at P < .05. Right is displayed on the right.
Importantly, the ACCEPT vs. REACT contrast during painful heat revealed significantly reduced activity during ACCEPT in many of the same regions, including dACC and medial frontal gyrus, sensorimotor regions including precentral and postcentral gyri, pre-SMA, anterior and posterior insula, thalamus, and cerebellum (Table 1D, Figures 4b & S5), as well as posterior regions such as posterior cingulate, cuneus, precuneus, and lateral occipital cortex (see Supplementary Materials for additional analyses; Table S2). Conjunction analysis between Hot vs. Warm temperatures and the ACCEPT vs. REACT contrast revealed areas of overlap in dACC, thalamus, anterior and posterior insula, and SII (Figure 4c–e, Table 1F). These regions were both responsive to painful stimulation and modulated by mindful acceptance.
NPS
We calculated NPS responses (Figure 5a) in each of the four temperature conditions (REACT-warm, ACCEPT-warm, REACT-hot, ACCEPT-hot). The NPS responded more strongly to Hot vs. Warm temperatures in both REACT (t(15) = 4.58, p < .001, d = 1.15) and ACCEPT (t(15) = 3.86, p = .002, d = .97) instruction conditions. It accurately predicted which condition was Hot in 93.75% of participants, showing strong positive effects consistent with results in previous NPS studies. Responses to Warm conditions were not significantly different from baseline (Figure 5b). In addition, the NPS did not respond to either negative or neutral images, and there was no effect of Negative vs. Neutral images. These results validate the sensitivity and specificity of the NPS for pain in this sample and serve as a positive control that demonstrates the validity and quality of imaging data in this sample. Critically, comparing ACCEPT-Hot vs. REACT-Hot revealed a significantly lower NPS response for the ACCEPT-hot condition (t(15) = 2.59, p = .02, d = .65; 26% drop). Importantly, this effect of the ACCEPT strategy on the NPS is larger than 19 of 20 recently reviewed placebo studies (Zunhammer et al., 2018; see Figure 5c). These results demonstrate that the ACCEPT instruction meaningfully reduced NPS responses and validate the modulating effect of mindful acceptance on the neural signature of pain.
Fig. 5.
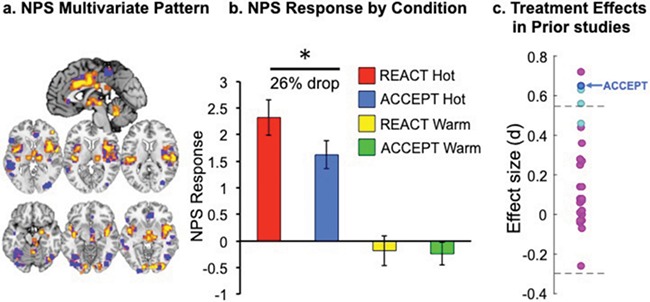
Neurologic pain signature (NPS) prediction of pain intensity. The NPS biomarker (a) predicts strong pain response to REACT-Hot, and a significantly lower pain response to ACCEPT-Hot (b) two conditions in which the temperatures were objectively identical (*p < .05). This, in turn, suggests that mindful acceptance modulates the intensity of experienced pain, including physiological aspects above and beyond judgments and self-report of pain. Right is displayed on the right.
Assessment of PFC involvement in mindful acceptance
Notably, the above contrasts did not reveal any increased recruitment of prefrontal regions in mindful-acceptance-based emotion-regulation. To provide additional strong tests of the potential involvement of PFC (which is implicated in reappraisal-based emotion regulation), we performed two types of targeted analyses.
ROI analyses
To directly test whether mindful acceptance of negative emotion and pain was associated with increased recruitment of PFC regions, we extracted neural activity from a priori ROIs defined as consistently activated during cognitive reappraisal by our published meta-analysis of emotion regulation studies (Buhle et al., 2014). These four regions included right vlPFC, right dlPFC, left dlPFC/vlPFC, and mPFC (Table S1). Following the main fMRI analyses, we compared extracted neural activity between ACCEPT vs. REACT instructions during negative image viewing, and ACCEPT vs. REACT during painful heat. No tests were significant, suggesting that neural activity in these regions was not significantly greater during mindful-acceptance of negative emotion or pain (see Supplementary Materials for additional details, and additional analyses within other emotion regulation and cognitive control ROIs; Figures S1 and S2, Tables S1 and S2).
Functional connectivity
This analysis was designed to further assess whether mindful acceptance depended on recruitment of prefrontal regions. The right amygdala region modulated by mindful acceptance identified in the conjunction analysis was used as a seed region in a PPI analysis, which identifies regions with differential functional connectivity with amygdala under ACCEPT vs. REACT conditions. The conjunction analysis focusing on mindful acceptance of painful heat identified regions of dACC and posterior and anterior insula, which are regions within pain intensity-coding regions that were modulated by mindful acceptance; they were therefore used as seed regions in the PPI analysis for painful heat. Across both analyses, we found no prefrontal or cingulate regions with a significant PPI effect. Taken together, these analyses suggest that mindful-acceptance-based modulation of negative emotion and pain does not depend on top-down prefrontal regions previously associated with cognitive control.
Discussion
Mindful acceptance is an ingredient of several treatments that confer psychological and physiological benefits on both healthy individuals and those with psychopathology. Here, we aimed to provide novel evidence that mindful acceptance can be effective as an emotion regulation strategy for meditation-naïve adults. We found that it reduced behavioral and neural markers of negative emotion associated with aversive images and painful heat. Importantly, these reductions occurred in the absence of detectable PFC recruitment. This is particularly striking because this pattern is unlike many forms of emotion regulation, such as reappraisal (Ochsner et al., 2012), which generally depend on PFC regions related to cognitive control. Critically, mindful acceptance also reduced pain-related activity in the NPS, an independently validated biomarker of pain experience (Wager et al., 2013). This is important because prior work has shown that reappraisal of pain does not affect the NPS (Woo et al., 2015). This suggests that mindful acceptance has more pervasive effects on pain processing than does reappraisal. The NPS finding also dovetails with the absence of PFC involvement to support the idea that mindful acceptance changes early affective appraisals in ways that differ from other emotion regulation strategies. As such, the present findings have implications for basic models of emotion regulation, our understanding of the neural mechanisms that support mindfulness-based regulation, and treatments for clinical disorders.
Implications for models of emotion regulation
Mindful acceptance can be viewed within the broad space of emotion regulation strategies (Gross, 2014), which are known to modulate negative affect and pain (Petrovic & Ingvar, 2002; Wager et al., 2008; Lapate et al., 2012; Woo et al., 2015). Neurobiological models of such strategies typically describe interactions between prefrontal cognitive control systems that support ‘top-down’ modulation and subcortical systems that support ‘bottom-up’ generation of affective responses (Ochsner et al., 2012). Mindful acceptance, in contrast, was shown to operate via a different neural mechanism in two important ways: it did not recruit PFC, and it modulated the NPS.
Across whole-brain GLM, ROI, and functional connectivity analyses, we showed that mindful-acceptance modulated regions associated with negative affect and pain, but did not recruit prefrontal regions. While surprising, this finding is consistent with theoretical models positing that mindful acceptance does not involve effortfully ignoring or cognitively changing one’s mental representation (Bishop et al., 2004; Hayes, 2004; Teasdale & Chaskalson, 2011b) and might depend on ‘bottom up’ processes (e.g. Chambers et al., 2009; Farb et al., 2012; Chiesa et al., 2013; Guendelman et al., 2017; see Supplementary Material for additional discussion). It also is consistent with behavioral data showing that acceptance-based regulation is less mentally depleting than cognitive strategies (Alberts et al., 2012), and with imaging data showing that mindful acceptance decreases cigarette craving-related neural activity without PFC engagement (Westbrook et al., 2013).
Although it is possible that additional yet-unexplored analyses could reveal recruitment of top-down control mechanisms, at this time, the absence of PFC involvement can be cautiously interpreted in at least three ways. One possibility is that mindful acceptance changes one’s primary appraisal of the affective significance of a stimulus (Lazarus, 1991). A second possibility is that acceptance of an aversive stimulus increases confidence in one’s coping ability, leading to a secondary appraisal of challenge rather than threat. A potential consequence of this shift is reduced cognitive elaboration of the aversive appraisal, which is consistent with the Buddhist view that mindful acceptance prevents amplification of affect at an early stage of affect-generation (‘the second arrow’; Teasdale & Chaskalson, 2011a).
A third possibility is that mindful acceptance operates at an earlier stage of the emotion generation sequence, modifying one’s mental representation of the eliciting stimulus itself. In this view, mindful acceptance is a form of ‘situation modification’ that involves representing the perceptual properties of a stimulus in a less aversive format (e.g. as temporary physical sensation rather than injurious stimulus; Gross & John, 2003; Gross, 2014). This is important because strategies that are deployed earlier in the emotion-generation process are considered more effective (Gross, 2014). Consistent with this interpretation, mindful acceptance reduced the NPS response, unlike reappraisal. This suggests that mindful acceptance modulates the same nociceptive and affective components of pain that are modulated by stimulus intensity (i.e. temperature), rendering the same hot stimulus, in effect, less intense, unlike some forms of reappraisal (Woo et al., 2015) or placebo (Wager et al., 2013). These interpretations are not mutually exclusive, and it remains for future research to clarify how shifts in patterns of appraisal or perceptual representation can be enacted without involvement of PFC-based control systems.
Implications for understanding mindfulness
Several recent studies have demonstrated emotional and health benefits following multi-week mindfulness training programs (e.g. mindfulness-based stress reduction; Hölzel et al., 2011). However, while this work has established mindfulness meditation as an effective intervention, and has suggested mechanisms by which it may exert its beneficial effects over time (e.g. Hölzel et al., 2011; Creswell & Lindsay, 2014; Sayers et al., 2015; Tang et al., 2015), it has not elucidated the mechanisms by which mindfulness operates as an emotion regulation strategy. One reason is that mindfulness-based programs are long, include several other components (e.g. yoga), entail effortful practice, and can lead to changes in appraisal biases as well as tendencies to notice, experience, and report certain kinds of experiences (e.g. pain; Vago & Silbersweig, 2012). Similarly, several studies have shown differences between long-term meditators and healthy controls (Brewer et al., 2011b), including in pain processing and experience (Brown & Jones, 2010; Grant et al., 2010; Grant et al., 2011). However, long-term meditators are a self-selecting group, who often practice multiple types of meditation (e.g. loving-kindness) and may differ from non-meditators in several ways, thus confounding group differences with pre-existing individual differences in cross-sectional designs (Davidson & Kaszniak, 2015; Josipovic & Baars, 2015).
Against this backdrop, the present study addressed a single, fundamental component of mindfulness practices—mindful-acceptance as an emotion regulation strategy, applied in the moment—and offers new insights into the neural mechanisms by which it reduces negative affect and pain, in the absence of meditation training. This raises the question of how a brief instruction in mindful-acceptance (as in the present study) compares to longer courses of mindfulness meditation training in terms of impact on behavioral and neural responses to aversive stimuli. We hope that future work will address the impact of increasing the training ‘dose’ of mindful acceptance as a strategy and link it with studies of mindfulness-based training and treatments, as well as long-term meditation practitioners who have cultivated mindful acceptance over many hours of practice (for recent discussions of potential ‘dose’ effects, see Tang et al., 2015; Zeidan, 2015).
Clinical/translational implications
The present findings suggest a neural mechanism by which the mindful-acceptance component of treatments may be beneficial. Further, the finding that beneficial effects of mindful-acceptance were observed in participants who were not trained previously to meditate suggests that mindful acceptance can be taught as an emotion regulation strategy to broad audiences in a single session. Importantly, mindful acceptance may be particularly useful for those who lack the capacity to generate and implement cognitively demanding regulatory strategies that depend on PFC, or in situations where effortful, attention-demanding regulation is not possible or weakened. This is important in light of lower PFC recruitment reported in many forms of psychopathology, children, the elderly, and under conditions of stress (Arnsten, 2009; Ochsner et al., 2012; Kober et al., 2014), and in light of the absence of PFC recruitment observed during mindful-acceptance in the current study. Future work could elucidate the boundary conditions surrounding these basic mechanisms in healthy and clinical populations. For example, studies could directly compare mindful acceptance and reappraisal, asking whether certain individuals may be more effective at deploying one strategy vs. another, or whether mindful acceptance is better suited for certain situations or particular kinds of affective responses (e.g. Sheppes et al., 2011).
Limitations & conclusion
The present study has several limitations. First, the sample size is relatively small. However, it was the a priori sample size based on emotion regulation sample sizes that were common at the time of study initiation (typically 16–18 participants; for a meta-analysis, see Buhle et al., 2014) and on availability of funding. Notably, our findings are consistent with prior work on mindfulness as a strategy to regulate craving, which was conducted with a much larger sample (Westbrook et al., 2013). Further, several analyses focused on ROIs and on the NPS, which are sensitive tests that were also defined a priori. Ultimately, we hope that this novel study will serve to motivate increased investment in the resources needed to run large-scale studies of mindful acceptance, and we are working towards replicating and extending the findings in a larger study.
In addition, we note that the mindful-acceptance strategy we used may not be representative of all mindfulness or acceptance practices. Indeed, the definition of mindfulness is a topic of current debate (e.g. Van Dam et al., 2018). To address this limitation, we provided our own definition in the introduction, based on several clinical protocols involving mindfulness and acceptance-based strategies.
Another limitation is that the study did not explicitly compare mindful acceptance to reappraisal, and future work would need to compare these strategies within the same study to explicitly test for differences in efficacy and underlying neural mechanisms. Finally, we acknowledge that we rely on a series of null results in reporting that mindful acceptance does not recruit PFC. It is possible that future analyses might reveal different findings, especially with larger samples. We are currently working towards such future studies.
Nevertheless, the present findings suggest that mindful acceptance is a powerful emotion-regulation strategy, that can be learned quickly, deployed effectively, and that may not depend on PFC to profoundly alter the psychological and neural consequences of negative affect and pain.
Author Contributions
H.K., J.B., T.D.W. and K.N.O. contributed to the study design. Testing and data collection were performed by H.K. and J.B. H.K., J.W. and T.D.W. performed the data analysis. H.K. and K.N.O. drafted the manuscript, and T.D.W. provided critical revisions. All authors approved the final version of the manuscript for submission.
Conflicts of Interest
The authors declare no conflicts of interest. H.K. has provided consulting for Indivior Inc., on topics entirely unrelated to this manuscript.
Supplementary Material
Acknowledgements
We thank Brent Hughes and Diego Berman for help in conceptualization; Ethan Kross and Chas DiCapua for comments on instructions; Peter Mende-Siedlecki for data collection; Alan Anticevic and Xoli Redmond for comments on the data; and Bethany Goodhue, Maggie Mae Mell, Matthew Schafer, and Shosuke Suzuki for help preparing the manuscript. This work was funded by the Mind and Life Institute, K12DA00167, P50DA09241, R01MH076136 and R01DA035484.
References
- Alberts H.J.E.M., Schneider F., Martijn C. (2012). Dealing efficiently with emotions: acceptance-based coping with negative emotions requires fewer resources than suppression. Cognition & Emotion, 26(5), 863–70. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463–3. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas L.Y., Lindquist M.A., Bolger N., Wager T.D. (2014). Brain mediators of the effects of noxious heat on pain. Pain, 155(8), 1632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Haigh E.A. (2014). Advances in cognitive theory and therapy: the generic cognitive model. Annual Review of Clinical Psychology, 10, 1–24. [DOI] [PubMed] [Google Scholar]
- Bishop S.R., Lau M., Shapiro S., Carlson L., Anderson N.D., Carmody J., Devins G. (2004). Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice, 11(3), 230–41. [Google Scholar]
- Brewer J.A., Mallik S., Babuscio T.A., Nich C., Johnson H.E., Deleone C.M., Rounsaville B.J. (2011a). Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence, 119(1–2), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.A., Worhunsky P.D., Gray J.R., Tang Y.Y., Weber J., Kober H. (2011b). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences, 108(50), 20254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.A., Jones A.K.P. (2010). Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain, 150(3), 428–38. [DOI] [PubMed] [Google Scholar]
- Buhle J.T., Kober H., Ochsner K.N., Mende-Siedlecki P., Weber J., Hughes B.L., Wager T.D. (2013). Common representation of pain and negative emotion in the midbrain periaqueductal gray. Social Cognitive and Affective Neuroscience, 8(6), 609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J., Wager T.D., Lopez R., Onyemekwu C., Kober H., Ochsner K.N. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L.E., Doll R., Stephen J., Faris P., Tamagawa R., Drysdale E., Speca M. (2013). Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer (MINDSET). Journal of Clinical Oncology, 31(25), 3119–26. [DOI] [PubMed] [Google Scholar]
- Chambers R., Gullone E., Allen N.B. (2009). Mindful emotion regulation: an integrative review. Clinical Psychology Review, 29(6), 560–72. [DOI] [PubMed] [Google Scholar]
- Chang L.J., Gianaros P.J., Manuck S.B., Krishnan A., Wager T.D. (2015). A sensitive and specific neural signature for picture-induced negative affect. PLoS Biology, 13(6), e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A., Serretti A., Jakobsen J.C. (2013). Mindfulness: top–down or bottom–up emotion regulation strategy? Clinical Psychology Review, 33(1), 82–96. [DOI] [PubMed] [Google Scholar]
- Coghill R.C., Sang C.N., Maisog J.M., Iadarola M.J. (1999). Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of Neurophysiology, 82(4), 1934–43. [DOI] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Creswell J.D., Lindsay E.K. (2014). How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science, 23(6), 401–7. [Google Scholar]
- Creswell J.D., Irwin M.R., Burklund L.J., Lieberman M.D., Arevalo J.M.G., Ma J., Cole S.W. (2012). Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain, Behavior, and Immunity, 26(7), 1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Kaszniak A.W. (2015). Conceptual and methodological issues in research on mindfulness and meditation. American Psychologist, 70(7), 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Segal Z.V. (2012). The mindful brain and emotion regulation in mood disorders. The Canadian Journal of Psychiatry, 57(2), 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Gard T., Hölzel B.K., Sack A.T., Hempel H., Lazar S.W., Vaitl D., Ott U. (2012). Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral Cortex, 22(11), 2692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Gross J.J. (2010). Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion, 10(1), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J.A., Courtemanche J., Duerden E.G., Duncan G.H., Rainville P. (2010). Cortical thickness and pain sensitivity in zen meditators. Emotion, 10(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Grant J.A., Courtemanche J., Rainville P. (2011). A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain, 152, 150–6. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (2014). Emotion Regulation: conceptual and empirical foundations In: Gross J.J., editor. Handbook of Emotion Regulation, 2nd edn, New York, NY: Guilford Press, pp. 3–20. [Google Scholar]
- Gross J.J., John O.P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–62. [DOI] [PubMed] [Google Scholar]
- Grossman P., Niemann L., Schmidt S., Walach H. (2004). Mindfulness-based stress reduction and health benefits: a meta-analysis. Journal of Psychosomatic Research, 57(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Guendelman S., Medeiros S., Rampes H. (2017). Mindfulness and emotion regulation: insights from neurobiological, psychological, and clinical studies. Frontiers in Psychology, 8(220). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S.C. (2004). Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behavior Therapy, 35(4), 639–65. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Sawyer A.T., Witt A.A., Oh D. (2010). The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6(6), 537–59. [DOI] [PubMed] [Google Scholar]
- Jacobs T.L., Epel E.S., Lin J., Blackburn E.H., Wolkowitz O.M., Bridwell D.A., MacLean K.A. (2011). Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology, 36(5), 664–81. [DOI] [PubMed] [Google Scholar]
- Josipovic Z., Baars B.J. (2015). What can neuroscience learn from contemplative practices? Frontiers in Psychology, 6, 1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry, 4(1), 33–47. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J., Lipworth L., Burney R. (1985). The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine, 8(2), 163–90. [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., Weber J., Mischel W., Hart C.L., Ochsner K.N. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, 107(33), 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., DeVito E.E., DeLeone C.M., Carroll K.M., Potenza M.N. (2014). Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology, 39(10), 2288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., Liu X., Guise K.G., Pizzarello S., Goodman M. (2009). Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry, 66(9), 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapate R.C., Lee H., Salomons T.V., Reekum C.M., Greischar L.L., Davidson R.J. (2012). Amygdalar function reflects common individual differences in emotion and pain regulation success. Journal of Cognitive Neuroscience, 24(1), 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R.S. (1991). Progress on a cognitive-motivational-relational theory of emotion. American Psychologist, 46(8), 819. [DOI] [PubMed] [Google Scholar]
- Linehan M.M. (2015). DBT Skills Training Manual, 2nd edn, New York, NY: Guilford Press. [Google Scholar]
- Lutz A., McFarlin D.R., Perlman D.M., Salomons T.V., Davidson R.J. (2013). Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. NeuroImage, 64, 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.H., Teasdale J.D. (2004). Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology, 72(1), 31–40. [DOI] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.-B. (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–60. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger J.M., Corbetta M., Shulman G.L. (2001a). Separating processes within a trial in event-related functional MRI: II analysis. NeuroImage, 13(1), 218–29. [DOI] [PubMed] [Google Scholar]
- Ollinger J.M., Shulman G.L., Corbetta M. (2001b). Separating processes within a trial in event-related functional MRI: I. The method. NeuroImage, 13(1), 210–7. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Ingvar M. (2002). Imaging cognitive modulation of pain processing. Pain, 95(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Pressman S.D., Gallagher M.W., Lopez S.J. (2013). Is the emotion-health connection a “first-world problem”? Psychological Science, 24(4), 544–9. [DOI] [PubMed] [Google Scholar]
- Raio C.M., Orederu T.A., Palazzolo L., Shurick A.A., Phelps E.A. (2013). Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences, 110(37), 15139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers W.M., Creswell J.D., Taren A. (2015). The emerging neurobiology of mindfulness and emotion processing In: Handbook of Mindfulness and Self-Regulation, Springer, pp. 9–22. [Google Scholar]
- Sheppes G., Scheibe S., Suri G., Gross J.J. (2011). Emotion-regulation choice. Psychological Science, 22(11), 1391–6. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D.E., Gross J.J., Remy K.A., Ochsner K.N. (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Hamer M., Chida Y. (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–12. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Hölzel B.K., Posner M.I. (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16(4), 213–25. [DOI] [PubMed] [Google Scholar]
- Teasdale J.D., Chaskalson M. (2011a). How does mindfulness transform suffering? I: the nature and origins of dukkha. Contemporary Buddhism, 12(1), 89–102. [Google Scholar]
- Teasdale J.D., Chaskalson M. (2011b). How does mindfulness transform suffering? II: the transformation of dukkha. Contemporary Buddhism, 12(1), 103–24. [Google Scholar]
- Troy A.S., Shallcross A.J., Mauss I.B. (2013). A person-by-situation approach to emotion regulation cognitive reappraisal can either help or hurt, depending on the context. Psychological Science, 24(12), 2505–14. [DOI] [PubMed] [Google Scholar]
- Vago D.R., Silbersweig D.A. (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6Article 296, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam N.T., van Vugt M.K., Vago D.R., Schmalzl L., Saron C.D., Olendzki A., Gorchov J. (2018). Mind the hype: a critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspectives on Psychological Science, 13(1), 36–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Lindquist M.A. (2015). Principles of fMRI In: Leanpub.
- Wager T.D., Nichols T.E. (2003). Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage, 18(2), 293–309. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.-W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine, 368(15), 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook C., Creswell J.D., Tabibnia G., Julson E., Kober H., Tindle H.A. (2013). Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social, Cognitive, and Affective Neuroscience, 8(1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell J.L., Afari N., Rutledge T., Sorrell J.T., Stoddard J.A., Petkus A.J., Hampton Atkinson J. (2011). A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain, 152(9), 2098–107. [DOI] [PubMed] [Google Scholar]
- Winecoff A., LaBar K.S., Madden D.J., Cabeza R., Huettel S.A. (2011). Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience, 6(2), 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.-W., Roy M., Buhle J.T., Wager T.D. (2015). Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biology, 13(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Gao J.H., Lancaster J.L., Fox P.T. (1995). Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping, 3(4), 287–301. [Google Scholar]
- Zeidan F. (2015). The Neurobiology of Mindfulness Meditation: Handbook of Mindfulness: Theory, Research, and Practice, New York: The Guilford Press. [Google Scholar]
- Zeidan F., Martucci K.T., Kraft R.A., Gordon N.S., McHaffie J.G., Coghill R.C. (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. The Journal of Neuroscience, 31(14), 5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Grant J.A., Brown C., McHaffie J., Coghill R. (2012). Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neuroscience Letters, 520(2), 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunhammer M., Bingel U., Wager T.D. (2018). Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurology, 75(11), 1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


