Abstract
Opioid-dependent patients are highly sensitized to negative social feedback, and increased social rejection sensitivity was linked to adverse treatment outcome, but its neurobiological underpinnings have not been understood yet. The present study investigated gray matter (GM) volume differences between 19 opioid maintenance treatment (OMT) patients and 20 healthy controls using magnetic resonance imaging and voxel-based morphometry. Associations of GM volumes with subjective feelings of exclusion and inclusion during a social ostracism (Cyberball) paradigm, with rejection sensitivity, social interaction anxiety and social phobia were explored. OMT patients displayed smaller GM volume in the bilateral insula and inferior frontal gyri. Psychometric and task data showed that patients reported significantly higher rejection sensitivity, social anxiety and social phobia scores and felt more excluded and less included during the social ostracism paradigm. Smaller GM volume in the insula was associated with higher subjective exclusion, lower subjective inclusion and higher rejection sensitivity, social anxiety and social phobia scores. Findings indicate that structural deficits in emotion- and anxiety-processing brain regions in OMT patients are associated with increased social rejection sensitivity. As social rejection is a potential trigger for relapse, patients might benefit from therapeutic strategies that promote social integration.
Keywords: social rejection sensitivity, social exclusion, opioid addiction, gray matter volume, voxel-based morphometry, insula
Introduction
Opioid dependence is a chronic disease that is associated with social and physical impairments (Ward et al., 1999). Aside from cognitive dysfunction, opioid dependence is associated with changes in emotion processing and higher rates of depressive symptoms (Dole and Nyswander, 1965; Freedman and Senay, 1973). The underpinnings of those alterations are not fully understood, but studies indicate that, in part, constant negative social feedback might shape emotionality in patients toward a negative affective state and a higher rejection sensitivity (Frischknecht et al., 2011). Studies have shown that peer rejection contributes to latter social rejection sensitivity and increased social anxiety and withdrawal (London et al., 2007). Further studies confirmed the association between social rejection sensitivity and social anxiety (McDonald et al., 2010). Importantly, negative social interaction and poor social support are associated with negative treatment outcome in opioid-dependent patients (Termorshuizen et al., 2005). Attention has been drawn to the relevance of social context in addictive disorders with the insula being implicated as a key hub processing social inclusion and exclusion and drug craving (Heilig et al., 2016). Neuroimaging studies have identified structural deficits in patients on opioid maintenance therapy (OMT) in the insula and other areas associated with cognitive performance, emotion processing and social perception: Previous anatomical studies reported smaller gray matter (GM) volume in opioid-dependent patients in the bilateral insular gyrus; areas of the frontal lobe, such as the bilateral prefrontal cortex, inferior frontal cortex, and supplementary motor cortex; areas of the temporal lobe and thalamus; and right cerebellum (Lyoo et al., 2006; Liu et al., 2009; Yuan et al., 2009; Lin et al., 2012). It was hypothesized that these structural abnormalities result in behavioral and neuropsychological impairments. However, there is still little evidence on the association between structural changes and behavioral correlates. The study by Lin et al. (2012) investigated associations between GM volume changes and cognitive performance, as well as depressive symptoms and anxiety: While OMT patients showed little to no impairments with regard to memory performance, executive function and visual construction skills, significantly higher depression and anxiety scores were found that showed significant associations to smaller volume in the prefrontal cortex, cingulate, cerebellum and insula. It has been suggested that heroin-induced changes in regional cerebral blood flow with a resulting reduced perfusion of brain regions (Denier et al., 2013a; Denier et al., 2013b), in addition to an altered brain metabolism (Botelho et al., 2006), might be the underlying cause for structural abnormalities in opioid-dependent patients. In addition, the duration of heroin use has been suggested to be a critical factor for the extent of brain damage (Yuan et al., 2009). However, the impact of structural abnormalities on social perception remains to be elucidated.
The present analyses were performed on a dataset that was collected within the framework of a larger study that used psychometric assessment tools, an funtional magnetic resonance imaging (fMRI) social ostracism Cyberball task and structural MRI to investigate functional and structural brain correlates of social interaction and social rejection (Bach et al., 2019). The results of the social rejection functional correlates are reported in detail elsewhere (Bach et al., 2019). In short, results showed that patients on OMT felt less included and more excluded during fMRI Cyberball inclusion and control conditions and equally excluded during the social exclusion Cyberball condition. Furthermore, patients showed reduced pain sensitivity, but subjective pain was higher after social exclusion compared to social inclusion trials. When contrasting social exclusion and inclusion trials, healthy participants displayed significant activation in brain areas related to social feedback and emotion processing, whereas OMT patients showed no difference across conditions probably due to direct pharmacological inhibitory effects of the opioids.
Incremental to the previous publication (Bach et al., 2019), here we report data on the structural correlates of social anxiety and social rejection sensitivity. We investigated differences in GM volume between opioid-dependent patients and controls and their associations with social rejection sensitivity, social interaction anxiety and social phobia using structural MRI and voxel-based morphometry (VBM).
Hypotheses
Firstly, based on previous findings (Lyoo et al., 2006; Yuan et al., 2009; Wang et al., 2012), we hypothesized that OMT patients would show smaller GM volume in frontal cortical and subcortical areas, including the insula cortex.
Secondly, we hypothesized that patients on OMT show higher dispositional social rejection sensitivity, social phobia symptoms and social anxiety symptoms, when measured using established psychometric scales.
Thirdly, based on previous findings that demonstrated negative associations between GM volume in the limbic system, specifically the insula, and symptoms of social anxiety and depression (Lin et al., 2012; Syal et al., 2012; Kawaguchi et al., 2016), we hypothesize that GM volume in the limbic system correlates negatively with social rejection sensitivity, social phobia and social anxiety scores.
Materials and methods
Participants
Opioid-dependent patients were recruited in an outpatient opioid maintenance program of the Central Institute of Mental Health in Mannheim, Germany. The healthy control group consisted of volunteers that were recruited from newspaper and clinic homepage advertisement. The study was approved by the local ethics committee of the University of Heidelberg and performed in accordance with the Declaration of Helsinki, and informed written consent was obtained. All participants were required to be aged between 18 and 65 years. Opioid-dependent patients had to meet the diagnosis of opioid dependence according to the ICD10. Healthy participants were included only if no indication of substance abuse disorder or another mental illness was found during an interview with an experienced clinical psychiatrist. Exclusion criteria for both groups were: (i) substance use disorder other than nicotine (only for healthy controls), (ii) pregnancy, (iii) severe internal or neurological condition and (iv) contraindications for MRI scanning (e.g. tattoos, metal implants, pregnancy, pacemakers). A total of 25 patients and 22 healthy controls were included in the current study. 19 patients (18 males and 1 female) and 20 controls (18 males and 2 females) provided complete VBM datasets of sufficient quality. Patients did not undergo a formal screening for comorbid psychiatric disorders prior to being included in the current study; however, all patients were interviewed by two independent professional psychiatrists at the institute, in order to rule out relevant comorbid psychiatric diseases. In addition, patients were treated at the department’s opioid maintenance unit for several years with regular appointments with a specialized psychiatrist once a week and no clinical evidence for a comorbid psychiatric disorder. Patients were all HIV negative, but four were tested positive for hepatitis C virus (HCV) antibodies. Fourteen patients were treated with methadone and n = 5 with buprenorphine, the mean methadone equivalence dose was 63.35 mg/d (± 36.26 mg/d standard deviation (s.d.); Strain et al., 2000). Further clinical and social characteristics are displayed in Table 1. At the time of scanning, urine sampling was conducted to detect other (non-prescribed) psychoactive substances (see Table 1), and 9 out of 19 patients reported to use substances other than the prescribed opioid (methadone or buprenorphine), such as benzodiazepines, cocaine, alcohol or cannabis.
Table 1.
Clinical characteristics of study participants
| Variable | Opioid-dependent patients (N = 19) | Healthy controls (N = 20) | Statistics | P | ||
|---|---|---|---|---|---|---|
| N | N | |||||
| Sex (male/female) | 18:1 | 18:2 | Chi2(1) = 0.171 | 0.581 | ||
| Education (no post-secondary education/apprenticeship only/attended college or higher) | 13/6/0 | 2/9/9 | Chi2(2) = 17.653 | 0.001* | ||
| Medication (methadone/buprenorphine) | 15:4 | — | — | — | ||
| Substance use other than nicotine and methadone (yes/no) | 9:10 | — | — | — | ||
| Drugs used other than methadone (absolute numbers)a | BZD = 4, OPT = 7, ALC = 5, THC = 2, COC = 2 | — | — | — | ||
| Variable | Mean | s.d. | Mean | s.d. | ||
| Age (years) | 37.42 | 8.23 | 38.35 | 8.28 | t (37) = 0.351 | 0.727 |
| Duration of heroin abuse (years) | 14.4 | 7.10 | — | — | — | |
| Methadone equivalence dose (mg) | 63.35 | 36.26 | — | — | — | |
| SPS (sum score) | 14.11 | 19.76 | 4.00 | 3.67 | t (37) = 2.248 | 0.016*,#,† |
| RSQ (sum score) | 9.75 | 5.96 | 5.08 | 3.53 | t (37) = 4.670 | 0.003*,#,†,° |
| SIAS (sum score) | 22.00 | 17.76 | 13.45 | 7.22 | t (37) = 1.988 | 0.027*,# |
| BSI (global severity score) | 35.47 | 39.34 | 4.85 | 5.21 | t (37) = 3.452 | 0.001*,# |
| BSI—subscale interpersonal sensitivity (score) | 2.74 | 3.72 | 0.60 | 1.10 | t (37) = 2.137 | 0.019*,# |
| BSI—subscale anxiety (score) | 3.53 | 4.50 | 0.80 | 1.01 | t (37) = 2.726 | 0.012*,# |
| BSI—subscale phobic anxiety (score) | 2.63 | 4.51 | 0.15 | 0.49 | t (37) = 2.482 | 0.019*,# |
BZD = benzodiazepine, OPT = opiates other than methadone/buprenorphine, ALC = alcohol, THC = tetrahydrocannabinol, COC = cocaine. Methadone equivalence dose was calculated with 100 mg methadone = 16 mg buprenorphine (Strain et al., 2000).
a n = 8 patients consumed more than one drug other than methadone and nicotine concurrently.
*Significant group differences at P < 0.05.
#Significant group differences at P < 0.05 after controlling for partnership status.
†Significant group differences at P < 0.05 after controlling for education level.
°Significant group differences at P < 0.05 after controlling for partnership status and education level concurrently.
Study design
Eligible participants were asked to complete several questionnaires before performing magnetic resonance imaging (MRI) with structural and functional scans. The psychometric measures included the social phobia scale (SPS; Mattick and Clarke, 1998), the social interaction anxiety scale (SIAS; Mattick and Clarke, 1998), the rejection sensitivity questionnaire (RSQ; Downey and Feldman, 1996; Berenson et al., 2009) and the brief symptom inventory (BSI; Derogatis and Melisaratos, 1983). All participants underwent structural MRI scanning and performed an fMRI Cyberball task that simulates social inclusion and social exclusion while applying painful and neutral reference temperature stimuli as used in previous studies (Domsalla et al., 2014).
MRI acquisition
High-resolution anatomical scans using a T1-weighted 3D magnetization-prepared rapid acquisition gradient-echo seq-uence (1 × 1 × 1 mm3 voxel size, slice thickness 1 mm, Field of view (FOV) 256 × 256 mm, Repetition Time (TR) = 2300 ms, Echo Time (TE) = 3.03 ms, Inversion Time (TI) = 900 ms) were performed using a 3 T whole-body tomograph (MAGNETOM Trio, Siemens Medical Systems, Erlangen, Germany).
VBM pre-processing and statistical analyses
All image analyses of structural data were conducted using the latest version of the statistical parametric mapping software (SPM) for Matlab (version 12, Wellcome Department of Cognitive Neurology, London, UK). Current methodological studies indicated that the Computational Anatomy Toolbox (CAT12, http://www.neuro.uni-jena.de/cat/index.html) for SPM12 provides a more accurate volumetric analysis of brain regions and is more robust and accurate against volumetric alterations than the VBM8 toolbox (Farokhian et al., 2017). Considering these findings, we performed our VBM analyses using the CAT12 toolbox for SPM12, applying the default settings and proceeding according to the authors’ manual (Gaser and Dahnke, 2016). Before image analyses, all image datasets were inspected by at least one medical technical radiology assistant to check for any gross structural abnormalities or imaging artifacts and referred to a neuroradiologist if necessary. All 3D T1-weighted MRI scans were normalized using an affine registration followed by non-linear registration, corrected for bias field inhomogeneity and then segmented into GM, white matter (WM) and cerebrospinal fluid components (Ashburner and Friston, 2005). We used the Diffeomorphic Anatomic Registration Through Exponentiated Lie (DARTEL) algebra algorithm to normalize the segmented scans into a standard Montreal Neurological Institute (MNI) space. Compared to the conventional algorithm, the DARTEL approach provides more precise spatial normalization to the template than standard registration methods (Yassa and Stark, 2009). Following this step, images were visually inspected for sample homogeneity of the unsmoothed data. This was evaluated using the procedure included in the CAT12 toolbox. Mahalanobis distance, combining the mean correlation and weighted overall image quality were inspected and outliers (>2 standard deviations) excluded from further analyses (n = 3). The remaining segmented, modulated and normalized datasets were smoothed using an 8 mm full-width half-maximum Gaussian smoothing kernel and then fed into second-level two-sample t-test analyses, considering total intracranial volume (TIV) and age as covariates. In order to control for multiple comparisons, a combined cluster-extent and voxel-wise threshold, corresponding to a family-wise error (FWE) rate of pFWE <0.05, was determined using the AlphaSim module of the NeuroElf toolbox (www.neuroelf.net) for Matlab. For a pre-set voxel-wise threshold of P < 0.001, the AlphaSim procedure determined a cluster-extent threshold of 103 voxels (10.000 Monte Carlo simulations, smoothing kernel of x = 8 mm, y = 8 mm, z = 8 mm). In order to follow up on the results of the whole-brain analyses, standardized anatomical masks were used to extract GM volume data from the respective brain regions that showed significant group effects (contrast: healthy participants > OMT patients). The masks that defined the regions of interest (ROI) were derived from the Hammers brain atlas (Hammers et al., 2003). Specifically, GM volume data were extracted from the (i) left insula [BA13], (ii) right insula [BA13], (iii) left inferior frontal gyrus [BA47] and (iv) right inferior frontal gyrus [BA47] using the standard procedure of the CAT12 toolbox for GM volume data extraction from ROIs. Data were imported into SPSS version 24.0 for further correlation analyses (SPSS, IBM Corp., Somers, NY, USA).
Statistical analyses
Demographical data and psychometric scales were analyzed using SPSS 24.0 and by applying two-sample t-tests and chi-squared tests. Group differences in GM volume were analyzed using two-sample t-tests correcting for TIV and age. Results are depicted in Table 1. Associations between GM volume in areas that displayed significant whole-brain differences in two-sample t-tests between opioid-dependent patients and healthy participants (i.e. bilateral insula and right inferior frontal gyrus) and psychometric scales covering social anxiety and rejection sensitivity (SIAS, RSQ, SPS) and Cyberball task-related changes in feelings of being excluded or included were investigated using partial correlation analyses, controlling for age and TIV (because both were related to GM volume variability in previous studies). Prior, exploratory data analyses were conducted to identify potential outliers. One patient had low values on the variable encoding left insula GM volume (>1.5× interquartile range). As the inspection of the raw imaging data and pre-processing quality indices indicated good quality of the anatomical data, we considered this value to reflect the true value of the participant and opted to include that participant in the correlation analyses. Partial correlations between (i) psychometric scales and (ii) subjective ratings of inclusion and exclusion during the Cyberball task inclusion condition and GM volume were corrected for multiple comparisons [i.e. (i) four independent partial correlations per brain area, left insula, right insula, inferior frontal gyrus, (ii) two independent partial correlations per brain area] using the Benjamini and Hochberg procedure as implemented in the Matlab ‘mafdr’ function (Benjamini and Hochberg, 1995).
Results
Group characteristics
Analyses of demographical data and psychometric scales indicated no significant deviation between groups with regard to age and gender distribution, but—as expected—more patients reported living without a partner and having fewer years of education. Patients also scored higher on psychometric scales measuring social anxiety (SIAS and BSI subscale anxiety) and symptoms of social phobia (SPS, BSI subscale phobic anxiety), as well as depressive symptoms (BDI, BSI subscale depression) and social rejection sensitivity (RSQ, BSI subscale interpersonal sensitivity).
Regional GM volume differences between groups
Overall TIV, GM and WM were descriptively smaller in OMT patients, but not statistically significant (tmax = 1.536, Pmin = 0.133). The location and extent of brain areas that showed significant local GM volume differences between groups are presented in Table 2 and Figure 1a. OMT patients showed significantly smaller GM volume in the left and right anterior insula (BA13); the left inferior frontal gyrus (BA47) and right frontal lobe, including the orbitofrontal parts of the right inferior frontal gyrus (BA47); and right inferior frontal gyrus (BA11). Additional, atlas-based (Hammers et al., 2003) analyses using CAT12 ROI analysis options corroborated these findings, indicating significant differences in GM volume in the left (t(37) = 3.245, P = 0.002) and right insula ROIs (t(37) = 3.452, P = 0.001) (Figure 1b and c), as well as in the left (t(37) = 2.396, P = 0.022) and right inferior frontal gyrus (t(37) = 2.583, P = 0.014).
Table 2.
Significant whole-brain GM volume differences between opioid maintenance patients (n = 19) and controls (n = 20) (N = 39, combined voxel-wise (P < 0.001) and cluster-extent (cluster size >103 voxel) threshold, corresponding to pFWE < 0.05 whole brain corrected)
| Side | Lobe | Brain areas | Cluster size (voxel) | MNI coordinates (x, y, z) | t max | ||
|---|---|---|---|---|---|---|---|
| a) Controls > OMT patients | |||||||
| R | Frontal | Anterior insula, lateral and posterior orbitofrontal cortex, inferior frontal gyrus | 1638 | 44 | 42 | −12 | 5.92 |
| L | Frontal, temporal | Anterior insula, superior temporal pole, inferior frontal gyrus | 234 | −38 | 14 | −14 | 4.27 |
| b) Controls < OMT patients | |||||||
| — | — | — | — | — | — | — | — |
Fig. 1.
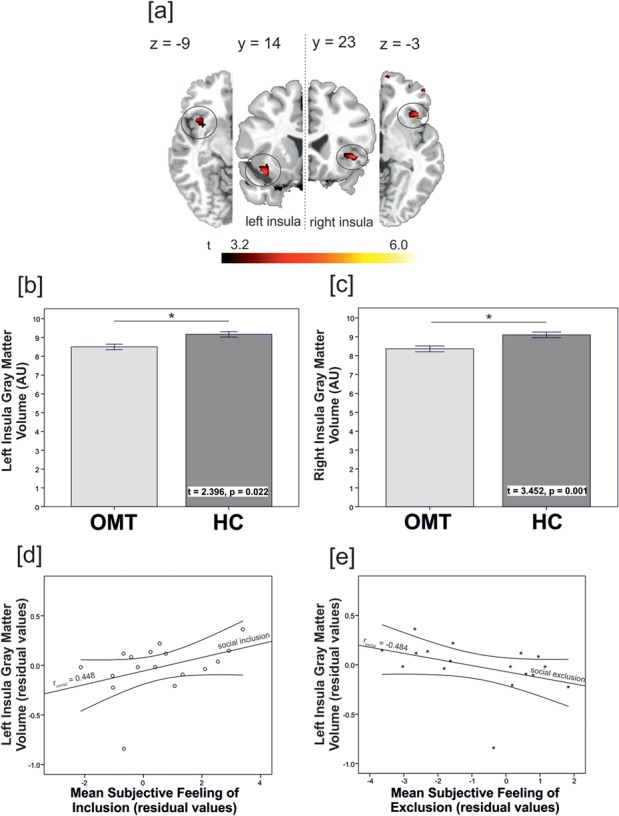
Depiction of (a) smaller GM volume in patients on opioid maintenance treatment, compared to healthy controls [combined voxel-wise (P < 0.001) and cluster-extent (cluster size >103 voxel) threshold, corresponding to pFWE < 0.05 whole brain corrected] in the (b) left and (c) right insula (HC = healthy controls). Illustration of the partial correlations between (d) the subjective feeling of inclusion and (e) the subjective feeling of exclusion during the social ostracism task with GM volume in the left insula in the patient group (rpartial(inclusion) = 0.448, P = 0.047, rpartial(exclusion) = −0.484, P = 0.034, partial correlations considered TIV and age as covariates, residual values are displayed).
Associations between GM volume and social anxiety and social rejection sensitivity
Partial Pearson correlation analyses conducted separately for OMT patients and controls, considering age and TIV as covariates, showed significant associations between smaller left insula GM volume in OMT patients and diminished subjective feelings of inclusion during the inclusion condition of the fMRI Cyberball task [rpartial = 0.448, P = 0.047, pFDR = 0.047, see Figure 1d; details on the Cyberball task main effects on subjective feelings of in- and exclusion are reported in Bach et al., 2019]. In addition, analyses indicated a significant partial correlation between smaller left anterior insula GM volume in OMT patients and stronger subjective feelings of being excluded during the inclusion condition (rpartial = −0.484, P = 0.034, pFDR = 0.047, see Figure 1e). While associations for the right insula and left and right inferior frontal gyri GM volume in OMT patients pointed in the same direction, results did not yield statistical significance (rmin = −0.241, pmin = 0.103). Associations between GM volume in the insula and inferior frontal gyrus did not display significant associations between subjective feelings of exclusion or inclusion in the group of healthy participants (rmax = 0.155, pmin = 0.233). Further analyses in the whole study sample (patients and controls combined) showed that left anterior insula GM volume negatively correlated with social interaction anxiety symptoms, measured by the SIAS (rpartial = −0.277, P = 0.004, pFDR = 0.048); with global psychological distress levels, measured using the BSI (rpartial = −0.484, P = 0.001, pFDR = 0.048); with symptoms of social phobia, measured using the SPS (rpartial = −0.296, P = 0.038, pFDR = 0.048); and with social rejection sensitivity, measured using the RSQ (rpartial = −0.325, P = 0.025, pFDR = 0.048). In other words, smaller insula GM volume in the whole study sample was associated with higher (i) social interaction anxiety, (ii) global psychological distress levels, (iii) social phobia and (iv) social rejection sensitivity. Additional analyses without the patient who had extreme values on the variable encoding left insula GM volume showed that correlation coefficients (partial correlation) remained significant (rSIAS = −0.306 P = 0.035; rSPS = −0.319 P = 0.029; rBSI = −0.462 P = 0.002; and rRSQ = −0.299 P = 0.038).
In line with the results of previous studies (Mattick and Clarke, 1998), SIAS, SPS, RSQ and BSI scores showed significant intercorrelations (see Supplementary Table S1). In addition, all scales showed significant positive association with the extent of task-induced subjective feelings of exclusion and negative associations with task-induced subjective feelings of inclusion during the inclusion condition of the fMRI Cyberball task (see Supplementary Table S1).
Associations between GM volume and substance use patterns
Comparison between patients with and without concurrent substance use of either a single substance or a combination of alcohol, heroin, cocaine and benzodiazepines showed no significant differences with regard to total GM or local GM volume in the left and right anterior insula and bilateral inferior frontal gyri (tmax = 0.911, pmin = 0.375). In addition, comparisons of values of psychometric scales between patient groups with (n = 9) and without (n = 10) comorbid consumption of other substances revealed no significant difference between groups on any variable (SIAS, BSI, RSQ, SPS; tmax = 2.172, pmin = 0.054). While concurrent substance use did not show a significant effect on social rejection sensitivity, anxiety symptoms or social phobia symptoms or GM volume, partial correlations, considering age and TIV as covariates, showed a significant association between lower GM volume in the left inferior frontal gyrus (BA47) and the number of years patients took heroin without being on OMT (rpartial = −0.737, P = 0.007, M = 11.50 years, s.d. = 6.7, see Supplementary Figure S1).
Discussion
For the first time, we could show that smaller insula GM volume in OMT patients was associated with higher subjective feelings of exclusion and lower subjective inclusion during the social ostracism Cyberball task and smaller insula GM volume was associated with higher social anxiety scores.
Previous structural MRI studies that investigated more than 300 healthy participants found that GM volume in the region of the posterior cingulate cortex and precuneus was negatively associated with RSQ scores, while GM volume in the inferior temporal gyrus was positively correlated with RSQ scores (Sun et al., 2014). These large-scale data support the association between GM volume and social rejection sensitivity.
In line with previous work, current data demonstrate smaller GM volume in bilateral anterior insula and inferior frontal gyri in OMT patients (for review, see Wollman et al., 2017). A recent meta-analysis, investigating the role of different brain structures in interoception, emotion processing and social cognition, confirmed the role of the bilateral anterior insula (BA13) in emotion processing and interoception (Adolfi et al., 2017). Further, specifically the left insula seems to be involved in social cognition. The study by Adolfi and colleagues also indicated a contribution of the bilateral inferior frontal gyri (BA47) to social cognition and emotion processing, while no robust evidence was found for a role of this region in processing interoceptive information. This suggests that specifically the left insula is a hub where social, interoceptive and emotional perceptive components are processed. Further meta-analyses of fMRI data on social rejection confirmed the insula as one of three regions that was reliably recruited during experimental induction of social exclusion, in addition to the inferior frontal cortex and the left anterior cingulate cortex (Cacioppo et al., 2013). Further studies supported the involvement of the insula in the processing of social rejection (Kross et al., 2007). The prominent role of the insula is further underlined by its extensive connections to other brain areas. Specifically, the insula shows strong connections to ventral parts of the striatum. Greater anatomical connection strength between both areas (measured using MRI-based tractography) was related to less-risky gambling choices (Leong et al., 2016), stressing the role of the insula and its connections to addictive behavior. Further, the anterior insula is reciprocally connected with the amygdala (Nieuwenhuys, 2012; Cho et al., 2013), and it has been suggested that these connections might link social stress and drug seeking (Heilig et al., 2016). Heilig et al. (2016) proposed that the insula and connected brain circuits might be neural substrates for a social ‘spiral of distress’. The correlations between insula GM volume and specifically the left insula GM volume with subjective feelings of exclusion during a social ostracism Cyberball task, as well as social interaction anxiety scores, social phobia symptoms and, importantly, social rejection sensitivity, support the role of the insula as part of the neural basis of social rejection sensitivity and processing of negative social feedback. Previous studies also reported reduced GM volume in patients with social anxiety, supporting the idea that a loss of insula GM volume might play a role in social anxiety (Kawaguchi et al., 2016). The relevance of structural changes in the insula in OMT patients is further supported by evidence that demonstrates an association between negative social interaction and relapse risk in heroin addiction (Termorshuizen et al., 2005). The GM volume deficits in the insula and inferior frontal gyrus in the current patient sample might therefore influence social interaction and emotion processing and render OMT patients more vulnerable to psychosocial stressors and subsequent relapse.
The observation of a significant intercorrelation between the psychometric scales and associations with the extent of task-induced subjective feelings of exclusion during social ostracism task indicate that the different psychometric scales (SPS, SIAS, RSQ, BSI) and the social ostracism task measure, at least in part, a common construct. Previous research investigated whether the intercorrelation between SPS and SIAS and other scales measuring anxiety and depressive symptoms reflect a measure of ‘general emotional distress’ or if high levels of ‘fear of negative evaluation’ mediate the associations (the latter was measured using the specific ‘Fear of Negative Evaluation Scale’ (FNES; Leary, 1983). The results indicated that when the effects of the general emotional distress measures were controlled, FNES remained a significant predictor of both SPS and SIAS scores. This suggests that fear of negative evaluation might be an underlying construct of the associations between anxiety symptoms and social rejection sensitivity. Longitudinal studies also confirmed that social rejection sensitivity was associated with social anxiety and social withdrawal (London et al., 2007). While social anxiety and social rejection seem to be interconnected and both contribute to subjective experience of loneliness and dysfunctional social interaction patterns, it has been suggested that social rejection sensitivity is qualitatively different from social anxiety (Marin and Miller, 2013). The disentanglement of social rejection and social anxiety is beyond the scope of the current study, but future research seems warranted as both factors are likely to contribute to dysfunctional behavioral patterns that are observed in opioid-dependent patients.
Previous studies have indicated that heroin-induced changes in cerebral blood flow and brain metabolism, especially in frontal lobe regions, might be the cause for neurobiological structural changes (Denier et al., 2013a; Denier et al., 2013b). Importantly, heroin, but not methadone, has been associated with such unfavorable changes in perfusion, measured using brain spectroscopy and transcranial sonography, and brain metabolism, suggesting that OMT might not contribute to the progress of impairments (Danos et al., 1998; Herning et al., 2003). In contrast, studies suggested that patients that remained on stable OMT without relapse to heroin showed an amelioration of structural abnormalities in the superior frontal gyrus, while differences in the middle frontal gyrus, occipital gyrus and cingulate persisted (Wang et al., 2012). However, it should be noted that a study in Asian population investigating former heroin-dependent individuals (mean heroin use 20.43 years) without OMT that were in drug rehabilitation and remained abstinent for 5 months from any drugs still showed GM volume deficits in the insula and inferior frontal gyrus (Liu et al., 2009). Importantly, structural deficits were replicated in opioid-dependent patients with no concurrent use of other psychoactive substances (Yuan et al., 2009). This suggests that current or past use of psychoactive substances other than heroine might not be the only relevant factor contributing to GM differences. This is corroborated by the data of our study that show no differences between groups with or without current use of psychoactive substances. Furthermore, differences in social anxiety scores, higher social phobia scores and increased rejection sensitivity could not be explained by absence or presence of comorbid substance consumption. Based on previous literature, we speculate that the frequent experience of stigmatization and social rejection in OMT patients might have fostered negative expectations and beliefs regarding social interactions and might have triggered social anxiety and social phobia (Frischknecht et al., 2011). Additionally, current results may implicate that GM volume differences in limbic brain areas might also contribute to an increased social anxiety, social phobia and rejection sensitivity.
Limitations
Current data do not allow statements about the chronology and causality of the development of opioid dependence and GM volume deficits. However, based on previous studies that established a negative association between heroin intake and both brain perfusion and brain metabolism, and associations between duration of heroin abuse and extent of GM deficits, it seems likely that heroin consumption contributed to the deficits that were found in the current study. In addition, the temporal connection between social rejection sensitivity and social anxiety and opioid dependence could not be determined based on current data. We speculate that there is a bidirectional interdependency between learning history and structural deficits in OMT patients that cannot be disentangled, based on the correlational design of the current study. Further research is warranted, in order to determine the relative contribution and temporal alignment of both factors. However, even without this information, the association between the extent of social rejection sensitivity and GM volume reductions in brain regions that are prominently involved in social cognition and emotion processing seems relevant and should be considered in treatment. A limitation of the current study is the small sample size that might have limited the possibility to detect smaller effects (e.g. associations between right insula GM volume and social perception). Additionally, the consideration of education level (which could be regarded as proxy for the socioeconomic status) as covariate in the group comparisons of psychometric data rendered some of the group differences on the SIAS, SPS, RSQ and BSI scales insignificant. This suggests that socioeconomic factors contribute significantly to the observed differences between patients and controls. This suggests that not patient status per se determines the extent of social rejection sensitivity and anxiety but that socioeconomic status—independent of group—might account for a relevant proportion of the inter-individual differences. While inferences should be drawn very carefully, the finding points toward the relevance of therapeutic strategies that aim at social rehabilitation of patients, which might contribute to an amelioration of social rejection sensitivity and social anxiety. The control of current partnership status in the group comparisons did not change the significance of most results (RSQ, SPS, BSI) with the exception of the SIAS scale that did not show significant difference after controlling for partnership status. While the current sample showed a relevant consumption of substances other than methadone and buprenorphine (e.g. benzodiazepines), the replication of GM volume deficits in brain areas that were implicated by recent meta-analyses (e.g. insula and inferior frontal gyrus) and the fact that statistical correction of concurrent substance use did not change significance of these results support the notion that the current sample might still reflect a representative sample of OMT patients. Some patients presented with positive HCV antibodies. Previous studies showed that HCV-infected patients displayed GM atrophy in the bilateral insula and thalamus and increasing atrophy over a 7-year follow-up in the left amygdala and left parahippocampal regions (Prell et al., 2019). While we cannot rule out the possibility that HCV infection might have contributed to smaller GM volume in the OMT patient sample, exploratory two-sample t-test did not show a significant effect of HCV antibody status on any GM volume measure. Additionally, all patients that were included in the current analyses did not present with suprathreshold HCV load at the time of the experiment.
Conclusion
In summary, current data show GM volume reductions in the bilateral insulae and bilateral inferior frontal gyri in OMT patients. Especially the GM volume in the left insula was related to lower subjective feelings of inclusion during a social ostracism ‘Cyberball’ task and in addition associated with increased social rejection sensitivity, social anxiety and social phobia. As negative social interaction is a potential trigger for relapse, this is a relevant finding, and patients might benefit from therapeutic strategies that aim at enhancing social coping skills by means of how to cope with negative social feedback. Although conclusions on causality cannot be drawn from our findings, they underline the need for additional support of OMT patients.
Acknowledgements
We would like to thank Michael Rieß and Oliver Klein for their assistance in data collection. We acknowledge financial support by Deutsche Forschungsgemeinschaft (DFG) within the funding program Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Funding
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Cooperative Research Center TRR 265/1 2019). C.V. was a consultant to and received honoraria from Indivior and Mundipharma. Outside the submitted work, D.H. received honoraria for participating in advisory boards of the pharmaceutical companies Indivior, Camurus and Servier.
Conflict of interest
None declared.
Authors contribution
D.H., F.K., S.L. and S.V.K. were responsible for study design. All authors contributed to the revision of the study concept. C.V., P.B. and D.K. performed clinical experiments. P.B., M.B., D.K., D.H., S.K. and S.V.K. analyzed the data. P.B., U.F., D.H., M.B., F.K., S.V.K., S.K., S.L. and M.B. drafted the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version.
References
- Adolfi F., Couto B., Richter F., et al. (2017). Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex, 88, 124–42. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. (2005). Unified segmentation. NeuroImage, 26(3), 839–51. [DOI] [PubMed] [Google Scholar]
- Bach P., Frischknecht U., Bungert M., et al. (2019). Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. European Neuropsychopharmacology, 29(2), 291–305. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Berenson K.R., Gyurak A., Ayduk Ö., et al. (2009). Rejection sensitivity and disruption of attention by social threat cues. Journal of Research in Personality, 43(6), 1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho M.F., Relvas J.S., Abrantes M., et al. (2006). Brain blood flow SPET imaging in heroin abusers. Annals of the New York Academy of Sciences, 1074, 466–77. [DOI] [PubMed] [Google Scholar]
- Cacioppo S., Frum C., Asp E., Weiss R.M., Lewis J.W., Cacioppo J.T. (2013). A quantitative meta-analysis of functional imaging studies of social rejection. Scientific Reports, 3, 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.T., Ernst M., Fudge J.L. (2013). Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. The Journal of Neuroscience, 33(35), 14017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos P., Kasper S., Grunwald F., et al. (1998). Pathological regional cerebral blood flow in opiate-dependent patients during withdrawal: a HMPAO-SPECT study. Neuropsychobiology, 37(4), 194–9. [DOI] [PubMed] [Google Scholar]
- Denier N., Gerber H., Vogel M., et al. (2013a). Reduction in cerebral perfusion after heroin administration: a resting state arterial spin labeling study. PLoS One, 8(9), e71461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier N., Schmidt A., Gerber H., et al. (2013b). Association of frontal gray matter volume and cerebral perfusion in heroin addiction: a multimodal neuroimaging study. Frontiers in Psychiatry, 4, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L.R., Melisaratos N. (1983). The brief symptom inventory: an introductory report. Psychological Medicine, 13(3), 595–605. [PubMed] [Google Scholar]
- Dole V.P., Nyswander M. (1965). A medical treatment for diacetylmorphine (heroin) addiction: a clinical trial with methadone hydrochloride. JAMA, 193(8), 646–50. [DOI] [PubMed] [Google Scholar]
- Domsalla M., Koppe G., Niedtfeld I., et al. (2014). Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience, 9(11), 1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G., Feldman S.I. (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology, 70(6), 1327. [DOI] [PubMed] [Google Scholar]
- Farokhian F., Beheshti I., Sone D., Matsuda H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Frontiers in Neurology, 8, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D.X., Senay E.C. (1973). Methadone treatment of heroin addiction. Annual Review of Medicine, 24(1), 153–64. [DOI] [PubMed] [Google Scholar]
- Frischknecht U., Beckmann B., Heinrich M., et al. (2011). The vicious circle of perceived stigmatization, depressiveness, anxiety, and low quality of life in substituted heroin addicts. European Addiction Research, 17(5), 241–9. [DOI] [PubMed] [Google Scholar]
- Gaser C., Dahnke R. (2016, 2016). CAT-a computational anatomy toolbox for the analysis of structural MRI data. Human Brain Mapping, 336–48. [Google Scholar]
- Hammers A., Allom R., Koepp M.J., et al. (2003). Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping, 19(4), 224–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M., Epstein D.H., Nader M.A., Shaham Y. (2016). Time to connect: bringing social context into addiction neuroscience. Nature Reviews. Neuroscience, 17(9), 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning R.I., Better W.E., Tate K., Umbricht A., Preston K.L., Cadet J.L. (2003). Methadone treatment induces attenuation of cerebrovascular deficits associated with the prolonged abuse of cocaine and heroin. Neuropsychopharmacology, 28(3), 562–8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Nemoto K., Nakaaki S., et al. (2016). Insular volume reduction in patients with social anxiety disorder. Frontiers in Psychiatry, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E., Egner T., Ochsner K., Hirsch J., Downey G. (2007). Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience, 19(6), 945–56. [DOI] [PubMed] [Google Scholar]
- Leary M.R. (1983). A brief version of the fear of negative evaluation scale. Personality and Social Psychology Bulletin, 9(3), 371–5. [Google Scholar]
- Leong J.K., Pestilli F., Wu C.C., Samanez-Larkin G.R., Knutson B. (2016). White-matter tract connecting anterior insula to nucleus Accumbens correlates with reduced preference for positively skewed gambles. Neuron, 89(1), 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.C., Chou K.H., Chen H.L., et al. (2012). Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry Research, 201(2), 89–97. [DOI] [PubMed] [Google Scholar]
- Liu H., Hao Y., Kaneko Y., et al. (2009). Frontal and cingulate gray matter volume reduction in heroin dependence: optimized voxel-based morphometry. Psychiatry and Clinical Neurosciences, 63(4), 563–8. [DOI] [PubMed] [Google Scholar]
- London B., Downey G., Bonica C., Paltin I. (2007). Social causes and consequences of rejection sensitivity. Journal of Research on Adolescence, 17(3), 481–506. [Google Scholar]
- Lyoo I.K., Pollack M.H., Silveri M.M., et al. (2006). Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology, 184(2), 139–44. [DOI] [PubMed] [Google Scholar]
- Marin T.J., Miller G.E. (2013). The interpersonally sensitive disposition and health: an integrative review. Psychological Bulletin, 139(5), 941–84. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Clarke J.C. (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36(4), 455–70. [DOI] [PubMed] [Google Scholar]
- McDonald K.L., Bowker J.C., Rubin K.H., Laursen B., Duchene M.S. (2010). Interactions between rejection sensitivity and supportive relationships in the prediction of adolescents' internalizing difficulties. Journal of Youth and Adolescence, 39(5), 563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. (2012). The insular cortex: a review. Progress in Brain Research, 195, 123–63. [DOI] [PubMed] [Google Scholar]
- Prell T., Dirks M., Arvanitis D., et al. (2019). Cerebral patterns of neuropsychological disturbances in hepatitis C patients. Journal of Neurovirology, 25(2), 229–38. [DOI] [PubMed] [Google Scholar]
- Strain E.C., Stoller K., Walsh S.L., Bigelow G.E. (2000). Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology, 148(4), 374–83. [DOI] [PubMed] [Google Scholar]
- Sun J., Li H., Li W., et al. (2014). Regional gray matter volume is associated with rejection sensitivity: a voxel-based morphometry study. Cognitive, Affective, & Behavioral Neuroscience, 14(3), 1077–85. [DOI] [PubMed] [Google Scholar]
- Syal S., Hattingh C.J., Fouché J.-P., et al. (2012). Grey matter abnormalities in social anxiety disorder: a pilot study. Metabolic Brain Disease, 27(3), 299–309. [DOI] [PubMed] [Google Scholar]
- Termorshuizen F., Krol A., Prins M., Geskus R., Brink W., Ameijden E.J.C. (2005). Prediction of relapse to frequent heroin use and the role of methadone prescription: an analysis of the Amsterdam cohort study among drug users. Drug and Alcohol Dependence, 79(2), 231–40. [DOI] [PubMed] [Google Scholar]
- Wang X., Li B., Zhou X., et al. (2012). Changes in brain gray matter in abstinent heroin addicts. Drug and Alcohol Dependence, 126(3), 304–8. [DOI] [PubMed] [Google Scholar]
- Ward J., Hall W., Mattick R.P. (1999). Role of maintenance treatment in opioid dependence. The Lancet, 353(9148), 221–6. [DOI] [PubMed] [Google Scholar]
- Wollman S.C., Alhassoon O.M., Hall M.G., et al. (2017). Gray matter abnormalities in opioid-dependent patients: a neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse, 43(5), 505–17. [DOI] [PubMed] [Google Scholar]
- Yassa M.A., Stark C.E. (2009). A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage, 44(2), 319–27. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhu Z., Shi J., et al. (2009). Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain and Cognition, 71(3), 223–8. [DOI] [PubMed] [Google Scholar]


