Abstract
Advances in immunosuppressive therapy have drastically improved acute rejection rates in kidney transplant recipients over the past five decades. Nevertheless, it should remain high on any differential diagnosis of unexplained graft dysfunction because of the potential negative effect on graft longevity. Understanding the pre- and post-transplant risk factors for acute rejection can help estimate the probability of immunologic graft damage, and accurate identification of the type and severity of acute rejection will guide appropriate treatment. Tissue biopsy remains the gold standard for evaluating immunologic graft damage, and the histologic definition of acute rejection has evolved in recent years. Intravenous steroids and T cell depletion remain the standard therapy for T cell–mediated rejection and are effective in reversing most cases. Plasma exchange and intravenous Ig, with or without rituximab, are most commonly used for the treatment of antibody-mediated rejection and several newer agents have recently been investigated for severe cases. This review aims to provide the general nephrologist caring for transplant recipients with an approach to immunologic risk assessment and a summary of recent advances in the diagnosis and treatment of acute graft rejection.
Keywords: acute allograft rejection, renal transplantation, immunosuppression, Immunology and pathology, intravenous immunoglobulins, graft rejection, rituximab, plasma exchange, transplant recipients, kidney transplantation, nephrologists, risk factors, differential diagnosis, longevity, t-lymphocytes, plasmapheresis, antibodies, biopsy, risk assessment, allografts, humans
Introduction
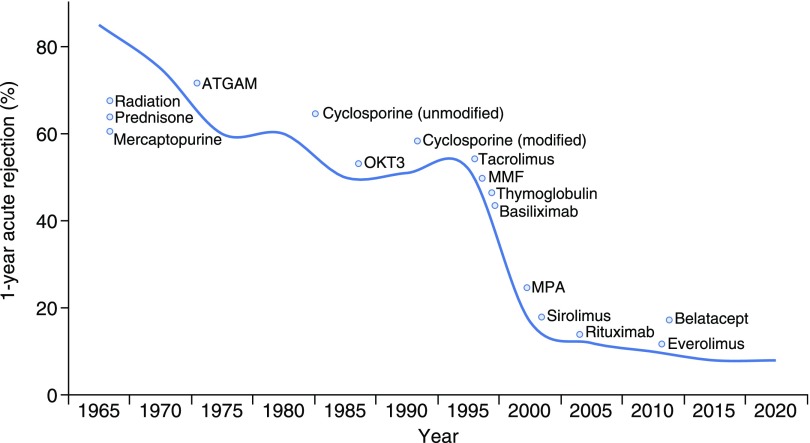
Transplantation of donor organs to non-HLA identical recipients introduces a stimulus for alloimmune responses, clinically referred to as graft rejection. Fifty years of research and development continues to elucidate the mechanisms underlying these responses, and has led to an evolution of immunosuppressive agents targeting these mechanisms. Over time these agents have become increasingly effective at inhibiting the transplant recipient’s immune response. As a result, acute rejection rates have steadily declined from nearly 100% in the first era of organ transplantation to approximately 10% more recently (1,2) (Figure 1). Not surprisingly this precipitous fall in acute rejection incidence has been mirrored by dramatic improvements in 1-year graft survival, especially after the introduction of cyclosporine in the mid-1980s and T cell–depleting induction in the mid-1990s (3). Nevertheless acute rejection, when it occurs, has the potential to significantly affect graft survival (4,5) and should remain high on the differential diagnosis for unexplained graft dysfunction in a transplant recipient.
Figure 1.
Decline of 1-year incidence of acute rejection over time with approximate date of immunosuppression medication introduction. Acute rejection rates have steadily declined over time with the introduction of increasingly effective immunosuppression. Modified and updated from Zand (3).
As our understanding of the alloimmune response has evolved, so has the classification of clinical acute rejection episodes. Initially characterized as “steroid responsive” and “steroid nonresponsive,” acute rejection is now broadly characterized as either “T cell mediated” or “antibody mediated,” respectively (6). These two forms of acute rejection result from separate mechanistic pathways, are associated with unique histologic findings and prognoses, and require distinct approaches to treatment. This review will summarize a modern approach to risk assessment, diagnosis, and treatment of acute rejection.
Acute Rejection Risk
An individual’s immunologic risk at the time of transplant has conventionally been attributed to factors such as overall level of anti-HLA sensitization (panel reactive antibody), repeat transplant, black race, and recipient age. Wehmeier et al. (7) recently examined traditional risk factors in 527 kidney recipients, showing pretransplant donor-specific antibodies (DSA) and HLA A/B/DR mismatch to be the main predictors of antibody-mediated rejection and T cell–mediated rejection, respectively, whereas panel reactive antibody and repeat transplantation had no predictive effect. With this in mind, it is worth noting the degree of immunologic risk conferred by pretransplant DSA will depend on characteristics of the antibodies detected. Approximately 30%–50% of patients with pretransplant DSA at titers strong enough to warrant desensitization before transplant will experience acute antibody-mediated rejection (8), whereas lower-level antibodies do not appear to increase acute rejection risk or graft survival in the intermediate term (9).
In the post-transplant period, acute rejection risk is largely determined by immunosuppression regimen and exposure. Currently in the United States, 75% of kidney recipients receive rabbit anti-thymocyte globulin (rATG) induction and >90% receive maintenance immunosuppression consisting of tacrolimus and mycophenolate mofetil, with or without prednisone, as these regimens have historically been associated with lower rates of acute rejection (10). Strategies to reduce calcineurin inhibitor (CNI) exposure using mammalian target of rapamycin inhibitors (mTOR’s) have generally been met with higher rates of acute rejection and side effects (11). Calcineurin inhibitor-free maintenance immunosuppression with the newer agent belatacept has resulted in favorable, longer-term outcomes but with higher rates of T cell–mediated rejection (12); however, post hoc analysis has shown a significant reduction in DSA development in those receiving belatacept versus cyclosporine (1%–4% versus 12%, respectively) (13). Adams et al. (14) recently published their center’s early experience showing significant reduction in acute rejection in patients treated with belatacept by adding tacrolimus to the existing belatacept regimen followed by a steady taper over the first post-transplant year (acute rejection rates of 51% with belatacept alone versus 16% with belatacept plus tacrolimus taper).
Despite the prevalence of tacrolimus use for the prevention of acute rejection in transplant recipients, firm recommendations for appropriate dosing and exposure to prevent acute rejection have not been established. Recent data from our group and others have shown correlations with overall tacrolimus exposure and acute rejection risk (15–17). In a cohort of 538 consecutive transplant recipients initiated on tacrolimus-based triple immunosuppression at the University of Colorado, mean tacrolimus levels <8 ng/ml throughout the first year increased the risk of DSA development (odds ratio, 2.5 (95% CI 1.32–4.79); P<0.005 versus >8) and levels of 4–6 versus >8 ng/ml were associated with a 2.3-fold higher risk of acute rejection (16).
Thus, when considering the differential diagnosis of graft dysfunction, assessment of overall immunologic risk can help estimate acute rejection probability. A high index of suspicion, for example, would be warranted in a young patient with lower tacrolimus trough levels during the first post-transplant year and/or suspected immunosuppression non-adherence. Alternatively, nonimmunologic causes may first be considered in an older patient who received rATG induction with consistently therapeutic tacrolimus levels.
Acute Rejection Diagnosis
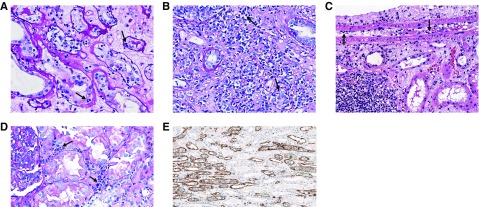
The gold standard for diagnosing acute rejection in kidney transplant recipients is tissue biopsy. Indications to pursue graft biopsy over concern for acute rejection include either an acute, otherwise unexplained deterioration in graft function or the presence of a biomarker consistent with acute rejection. As described in the preceding section, assessment of a patient’s immunologic risk at the time of and after transplant can help further define pretest probability of acute rejection when contemplating the utility of biopsy; however, allograft biopsy is generally considered a safe procedure and should be pursued without delay in patients with graft dysfunction that is not explained by other nonimmunologic causes. Allograft histology is interpreted using the Banff classification of kidney allograft pathology, which has undergone extensive updating and revision since its development in the 1990s (6) (Table 1). The diagnostic criteria for T cell–mediated rejection have undergone little change in recent years, and include lymphocytic infiltrate of tubules (tubulitis) and larger vessels (vasculitis), with the severity of these lesions depending on the degree of lymphocytic infiltrate per high-powered field (Figure 2, A–C).
Table 1.
Histologic criteria for diagnosing acute allograft rejection according to Banff 2017 guidelines (6)
| Acute T cell–mediated rejection (TCR) | |
| Ia | >25% Interstitial inflammation with moderate tubulitis (t2) |
| Ib | >25% Interstitial inflammation with severe tubulitis (t3) |
| IIa | Mild-to-moderate intimal arteritis (v1) |
| IIb | Severe intimal arteritis (v2) |
| III | Transmural arteritis and/or fibrinoid necrosis |
| Acute antibody-mediated rejection (AMR): all three criteria below required | |
| Histologic evidence of tissue injury including one or more of the following: | |
| Microvascular inflammation (g>0 and/or ptc>0) | |
| Arteritis (v>0) | |
| Thrombotic microangiopathy | |
| Acute tubular injury | |
| Evidence of current/recent antibody interaction with endothelium including one or more of the following: | |
| Positive C4d staining of peritubular capillaries | |
| Moderate microvascular inflammation (g+ptc ≥2) | |
| Increased expression of gene transcripts in biopsy tissue strongly associated with AMR | |
| Serologic evidence of donor-specific antibodies (DSA) | |
| Positive C4d staining or presence of AMR-associated gene transcripts may substitute for DSA | |
t, tubulitis; v, arteritis; g, glomerulitis; ptc, peritubular capillaritis.
Figure 2.
Histologic presentation of acute rejection. (A) Banff grade 1a with moderate lymphocytic infiltration of the tubules (arrows). (B) Banff grade 1b with severe lymphocytic interstitial infiltration and tubulitis (arrows). (C) Banff grade 2a with arterial intimal lymphocytic infiltration (arrows). (D) Peritubular lymphocytic infiltration characteristic of antibody-mediated rejection (arrows). (E) Positive C4d staining of the peritubular capillaries by immunohistochemistry.
In contrast, the Banff classification of acute antibody-mediated rejection continues to evolve with the ongoing recognition of its variable histologic presentation. Antibody-mediated rejection was first recognized within the Banff classification in the early and mid-2000s and required three features for diagnosis: (1) active tissue injury, (2) immunohistologic evidence of peritubular capillary complement split-product C4d deposition, and (3) circulating DSA. This relatively strict definition resulted in a problematic underdiagnosis of antibody-mediated rejection; especially concerning given the long-term clinical implications of antibody-mediated graft damage (18). Subsequent revisions have allowed for exceptions to these initial criteria, the most significant occurring in 2013 after several studies suggested an antibody-mediated rejection phenotype that lacks detectable C4d staining (19–21).
Microarray analysis of endothelial transcripts, work that has been largely pioneered by Halloran et al. (22), provides further evidence for C4d-negative antibody-mediated rejection. This technique applies a molecular phenotype to allograft tissue using extracted RNA to examine patterns of altered gene expression. Sis et al. (21) examined 173 for-cause biopsy specimens and showed poor prognosis in samples with DSA and endothelial transcript expression consistent with antibody-mediated rejection, only 40% of which showed C4d positivity. As a result of these studies and others, the revised 2013 Banff criteria for antibody-mediated rejection diagnosis removed the requirement for C4d detection and broadened this category to include “evidence of current/recent antibody interaction with vascular endothelium,” which may include either (1) positive C4d staining, (2) at least moderate microvascular inflammation, or (3) increased expression of endothelial gene transcripts (20).
The most recent Banff consensus notes studies showing a lack of DSA in patients with biopsy specimens demonstrating significant microvascular inflammation as well as a low false positive rate of C4d staining, and has now removed the requirement for documented circulating DSA in the setting of positive C4d staining and microvascular inflammation (6) (Figure 2, D and E, Table 1).
Although assessment of allograft tissue remains the gold standard for diagnosing acute rejection, tissue biopsy is resource intensive, presents a potential risk to patients, and has been associated with significant sampling error and variability in pathologic interpretation. Numerous studies of urine and blood biomarkers, such as CXCL9, CXCL10, granzyme B, perforin, and Fas ligand, have generally shown mixed sensitivity and specificity for identifying acute rejection, differentiating T cell–mediated rejection from antibody-mediated rejection, and distinguishing immunologic injury from other forms of graft damage (reviewed by Naesens and Anglicheau [23]). Short noncoding single-stranded microRNA have improved stability in urine compared with mRNA (24) and decreased urinary miR-210 levels have been associated with T cell–mediated rejection and subsequent 1-year GFR decline (25). Recently, donor-derived cellfree DNA (cf-DNA) profiling has been applied to the noninvasive diagnosis of antibody-mediated rejection, with results from the multicenter “Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients” (DART) study leading to a Medicare-reimbursable status in 2017. This study associated cf-DNA levels with 242 biopsy specimens (204 clinically indicated) and showed a negative predictive value for antibody-mediated rejection of 96% when using a cut-off value of 1% in recipient blood; however, positive predictive value was only 44% (26). A recent study from Huang et al. (27) applied a 0.74% cf-DNA cut-off to 63 for-cause biopsy samples and showed a positive predictive value for antibody-mediated rejection of 69% with a negative predictive value of 100%, but did not discriminate between those with and without T cell–mediated rejection.
Thus, despite its downfalls, tissue biopsy remains the gold standard for diagnosing acute rejection in transplant recipients and noninvasive biomarkers have failed to completely replace tissue diagnosis due in part to inconsistent performance between studies. However, normal results from assays with high negative predictive value, such as donor-derived cf-DNA, may offer a level of reassurance to providers and patients with abnormal clinical findings (DSA, graft dysfunction) in whom tissue biopsy is either not feasible or considered too high risk.
Acute Rejection Treatment
The approach to treatment of the transplant recipient with acute rejection relies on accurate diagnosis and classification of the immunologic pathology. Treatment strategies differ between T cell–mediated rejection and antibody-mediated rejection, and aggressiveness of treatment generally follows severity of lesions that are diagnosed. Graft prognosis after treated acute rejection also depends on type and severity (4); however, any untreated clinical acute rejection episode will ultimately result in accelerated graft loss. Thus timely recognition and diagnosis of acute rejection is crucial to promptly initiate appropriate treatment. Treatment options for acute rejection are listed in Table 2, and a suggested algorithm for treating acute rejection is presented in Figure 3.
Table 2.
Treatment options for acute allograft rejection
| Treatment | Indication | Mechanism | Adverse Effects |
|---|---|---|---|
| Methylprednisolone | TCR: Banff Ia, Ib | Multiple, anti-inflammatory glucocorticoid | Hyperglycemia, hypertension, other metabolic effects |
| rATG | TCR: Banff Ib, IIa, IIb, III | T cell depletion | Fever, chills, hypertension, hypotension, leukopenia, infusion reaction, serum sickness |
| Plasma exchange | AMR | Antibody removal | Fevera, chillsa, urticariaa, TRALIa, bleeding |
| IVIG | AMR | Multiple “immunomodulatory” effects including antibody clearance, neutralization, and inhibited production, Fc receptor saturation, complement inhibition | Infusion reaction including headache, fever, chills, urticaria, back pain, abdominal pain, nausea, vomiting |
| Rituximab | AMR | Anti-CD20 B cell depletion | Infusion reaction, HBV reactivation, PML |
| Bortezomib | AMR | Plasma cell apoptosis via proteasome inhibition | Peripheral neuropathy, fatigue, generalized weakness |
| Eculizumab | AMR | Terminal complement C5 inhibition | Meningococcal infection, influenza, peritonitis |
| C1-INH | AMR | Classic complement pathway inhibition | Headache |
TCR, T cell–mediated rejection; rATG, rabbit anti-thymocyte globulin; AMR, antibody-mediated rejection; TRALI, transfusion-related acute lung injury; IVIG, intravenous immunoglobulin; HBV, hepatitis B virus; PML, progressive multifocal leukoencephalopathy; C1-INH, C1-esterase inhibitors.
Associated more with plasma as replacement fluid.
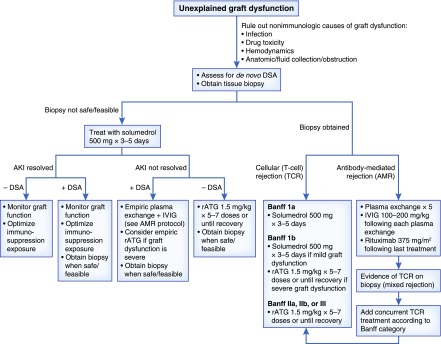
Figure 3.
Proposed algorithm for treatment of presumed and biopsy-proven acute kidney allograft rejection. Once non-immunologic etiologies of graft dysfunction are ruled out, allograft biopsy and assessment of DSA should be pursued with treatment dictated by biopsy findings. In cases where biopsy is not feasible, empirical treatment is indicated and can be tailored based on clinical response and results of DSA screening. DSA, donor-specific antibody; rATG, rabbit anti-thymocyte globulin; IVIG, intravenous IG.
T Cell–Mediated Rejection
The treatment of T cell–mediated rejection has changed little over time and few data exist comparing one strategy to another. Initial treatment conventionally includes pulse methylprednisolone at 250–500 mg daily for 3–5 days, as recommended by international guidelines (28). Treatment is ultimately guided by biopsy findings, with the majority of Banff class 1 lesions responding to methylprednisolone alone. T cell–mediated rejection involving lymphocytic infiltrate of the vasculature (Banff II and III lesions) generally requires T cell–depleting therapy, most commonly rATG dosed at 1.5 mg/kg for five to seven doses. One of the few randomized, controlled trials in this field compared rATG with horse anti-thymocyte globulin, showing superior effectiveness of rATG with a reversal rate of 88% versus 76% and an average total dose of 10 mg/kg (29). An updated Cochrane Database review published in 2017 concluded antibody therapy was superior to steroid therapy in reversing T cell–mediated rejection with no effect on subsequent acute rejection incidence or patient survival, noting most data were derived from studies during older immunosuppression eras where cyclosporine and azathioprine use was standard (30).
Antibody-Mediated Rejection
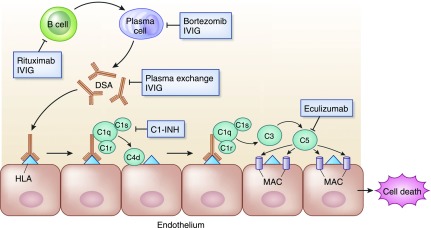
Similar to T cell–mediated rejection, few high-quality, randomized trials exist comparing treatment regimens for antibody-mediated rejection. In contrast to T cell–mediated rejection, however, several new therapeutic treatment options have been studied in recent years. Antibody-mediated rejection treatments are directed at removing antibody-producing B cells or plasma cells, removing antibodies (DSA), and/or inhibiting the subsequent complement-regulated graft damage (Figure 4). A systematic review by Roberts et al. (31) identified 12 comparative trials of antibody-mediated rejection treatment from 1950 to 2011, only five of which were randomized and three published in abstract form. These trials are small with a mean of 13 patients per arm with large degrees of heterogeneity including patients with both acute and chronic lesions. The authors report low-quality evidence supporting antibody removal therapies (plasma exchange, immunoabsorption), and very low-quality evidence for all other treatments. A recently updated analysis included nine additional studies, four of which were randomized and three in abstract form, with similar conclusions (32). Nevertheless, plasma exchange and intravenous Ig (IVIG), with or without rituximab, was the most commonly used strategy and is generally considered standard of care for antibody-mediated rejection treatment (28,30,31). A typical regimen includes daily or every other day plasma exchange consisting of 1.5 plasma volume removal with each treatment followed by IVIG at 100–200 mg/kg, with or without a single dose of rituximab at 3.75 mg/m2.
Figure 4.
Target sites for current available and experimental therapeutic agents for antibody-mediated allograft rejection. B cells (inhibited by rituximab) differentiate to plasma cells (inhibited by bortezomib), which generate donor-specific anti-HLA antibodies (removed by plasma exchange, modulated by IVIG). Upon binding to HLA molecules on graft endothelium, donor-specific antibodies (DSA) fix complement (inhibited by C1-esterase inhibitors [C1-INH]) and initiate a cascade resulting in C5 cleavage (inhibited by eculizumab) and formation of the membrane attack complex (MAC), leading to apoptosis and tissue damage.
Despite the limited data quality in this field, several studies are worth pointing out. Lefaucheur et al. (33) randomized 24 patients with antibody-mediated rejection to receive either monotherapy with IVIG at 2 gm/kg every 3 weeks× four doses or a more intensive regimen consisting of plasma exchange, IVIG, and two doses of rituximab. Patients receiving more intensive therapy experienced greater reduction in DSA with 92% graft survival at 3 years compared with 50% in those receiving IVIG alone. Although this study is small and does not necessarily confirm combination therapy with plasma exchange, IVIG, and rituximab as the best available therapy, the high rate of graft loss in the IVIG arm suggests a treatment regimen consisting only of IVIG is inadequate for most cases of acute antibody-mediated rejection. Sautenet et al. (34) attempted to clarify the utility of rituximab in combination with standard plasma exchange/IVIG therapy with the multicenter, blinded, randomized, placebo-controlled “Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation” (RITUX-ERAH) trial. Thirty eight patients with antibody-mediated rejection received three doses of plasma exchange plus IVIG before undergoing randomization to rituximab 375 mg/m2 (n=19) or placebo (n=19), followed by two additional plasma exchange/IVIG treatments in each group. There was no difference in the composite primary outcome of graft loss or improvement in graft function at day 12; however, a large crossover between groups limits accurate interpretation of these data, with eight of 19 patients in the placebo arm receiving rituximab as rescue therapy for insufficient treatment efficacy.
Several small series have evaluated alternative agents for antibody-mediated rejection refractory to “standard” treatment regimens. Bortezomib, an US Food and Drug Administration approved proteasome inhibitor for treatment of multiple myeloma, has received special focus because of its ability to induce apoptosis in antibody-producing plasma cells; however, there is minimal effect on DSA burden when used as a sole agent (35,36), with mixed results when used in combination with plasma exchange/IVIG (37–39). A recent case series describes successful reduction in plasma cell infiltrate and stabilization of graft function after treatment with bortezomib in several patients with plasma cell-rich acute rejection, a rare histologic finding historically associated with poor outcomes despite aggressive treatment (40). The humanized mAb eculizumab has been targeted in the prevention (41) and treatment (42) of antibody-mediated injury because of its mechanism of complement component C5 inhibition. In a small series, Orandi et al. (42) evaluated outcomes of 24 patients with severe antibody-mediated rejection after desensitization for positive crossmatch kidney transplant treated with either splenectomy (n=14), eculizumab (n=5), or combination splenectomy and eculizumab (n=5). At 1 year, four of 14 and four of five patients experienced graft loss in the splenectomy and eculizumab groups, respectively, whereas those treated with combination splenectomy and eculizumab experienced no graft loss and minimal transplant glomerulopathy on protocol biopsy. Not surprisingly this aggressive regimen is not without risk, with high rates of infection (urinary tract infection, bacteremia/sepsis, pneumonia) in those undergoing combination therapy. A pilot study of the anti-IL six receptor antibody tocilizumab has shown promising results for patients with chronic antibody-mediated rejection (43), a disease for which there is currently no proven treatment; however, this agent has not been studied in the treatment of acute antibody-mediated rejection.
Lastly, C1-esterase inhibitors (C1-INH) have recently been studied in two small studies for the treatment of acute antibody-mediated rejection (44,45). C1-INH inhibits proximal enzymes in the classic complement pathway including C1q, and reports of poor outcomes after detection of C1q-binding DSA (46) provide further rational for its use in antibody-mediated rejection. In a single-arm pilot study, Viglietti et al. (45) treated six patients with antibody-mediated rejection deemed non-responsive to conventional treatment, with all patients showing improvement in GFR at 6 months and a decrease in C4d deposition from baseline (five of six patients) to month 6 (one of six patients). Montgomery et al. (44) randomized 18 patients with antibody-mediated rejection to either C1-INH at 20,000 U every other day for 2 weeks or placebo, in addition to standard therapy including plasma exchange, IVIG, and rituximab. There was no difference in the primary end point of graft loss or histology at 20 days; however, out of 14 patients with biopsies at 6 months, three of seven patients receiving placebo versus zero of seven patients receiving C1-INH showed transplant glomerulopathy. There is currently one ongoing randomized, clinical trial comparing C1-INH with placebo in patients with antibody-mediated rejection receiving standard of care (Clinicaltrials.gov identifier NCT03221842); however, another was recently terminated because of lack of efficacy (Clinicaltrials.gov identifier NCT02547220).
Subclinical Rejection
The identification and treatment of alloimmune responses before the onset of clinical graft dysfunction may theoretically minimize the development of chronic lesions that ultimately lead to graft loss (47,48). This approach involves protocol biopsy of stable grafts or the use of screening biomarkers such as DSA to identify patients at risk for subclinical immunologic lesions. The incidence of subclinical T cell–mediated rejection in the modern era of immunosuppression is low (49), and long-term outcomes do not appear to be as severely affected compared with subclinical antibody-mediated rejection (50). In 121 patients treated with tacrolimus, mycophenolate mofetil, and prednisone and randomized to protocol versus indication-only biopsies, the incidence of subclinical T cell–mediated rejection was only 5%, with no difference in graft function at 6 months (49) despite those with subclinical rejection receiving treatment. Subclinical antibody-mediated rejection, in contrast, represents an attractive therapeutic target given the attributed poor long-term outcomes (50), likely via progression to chronic antibody-mediated rejection over time (47,51). Using DSA as a biomarker, studies have shown approximately 50% of patients with stable function and de novo DSA will show evidence of subclinical antibody-mediated rejection on biopsy (47,51–53). Despite the risk of poor outcomes associated with subclinical antibody-mediated rejection, few data exist to suggest treatment intervention will alter the clinical course. Orandi et al. (54) published a retrospective analysis of 77 patients with subclinical antibody-mediated rejection diagnosed by protocol biopsy, 54% of which received treatment with various combinations of plasma exchange, IVIG, rituximab, and eculizumab. With a mean follow-up of 5.2 years, overall rates of graft loss were similar between those treated and untreated. However, when compared with matched controls, treated patients experienced a lower risk of graft loss versus untreated patients (hazard ratio, 1.73 [P=0.21] versus 3.34 [P=0.01], respectively). Thus, although the prospect of identifying subclinical antibody-mediated rejection before clinical dysfunction remains attractive, more data are needed before concluding that treatment of this population will improve the long-term clinical course.
Conclusions
Despite historically low acute rejection rates thanks to increasingly effective immunosuppressive protocols, acute rejection episodes continue to affect graft survival and prompt recognition and treatment is crucial. When feasible tissue biopsy should be performed in any patient with unexplained acute graft dysfunction, and accurate assessment of immunologic risk can assist in determining the need for tissue diagnosis. The Banff criteria for diagnosing antibody-mediated rejection continue to evolve and no longer require the combination of DSA and C4d deposition, with several new agents under investigation for treatment. Identifying subclinical antibody-mediated rejection provides an opportunity to intervene before the onset of clinical dysfunction; however, further clinical data are needed to determine if treatment will alter clinical outcomes.
Disclosures
Dr. Cooper has nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Starzl TE: Personal reflections in transplantation. Surg Clin North Am 58: 879–893, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Kucheryavaya A, Woodbury M, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2015 Annual data report: Kidney. Am J Transplant 17[Suppl 1]: 21–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zand MS: Immunosuppression and immune monitoring after renal transplantation. Semin Dial 18: 511–519, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bouatou Y, Viglietti D, Pievani D, Louis K, Duong Van Huyen JP, Rabant M, Aubert O, Taupin JL, Glotz D, Legendre C, Loupy A, Lefaucheur C: Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell-mediated rejection. Am J Transplant 19: 1972–1988, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, van Huyen JPD, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehmeier C, Hönger G, Cun H, Amico P, Hirt-Minkowski P, Georgalis A, Hopfer H, Dickenmann M, Steiger J, Schaub S: Donor specificity but not broadness of sensitization is associated with antibody-mediated rejection and graft loss in renal allograft recipients. Am J Transplant 17: 2092–2102, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Marfo K, Lu A, Ling M, Akalin E: Desensitization protocols and their outcome. Clin J Am Soc Nephrol 6: 922–936, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Adebiyi OO, Gralla J, Klem P, Freed B, Davis S, Wiseman AC, Cooper JE: Clinical significance of pretransplant donor-specific antibodies in the setting of negative cell-based flow cytometry crossmatching in kidney transplant recipients. Am J Transplant 16: 3458–3467, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Robinson A, Wainright JL, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2017 Annual data report: Kidney. Am J Transplant 19[Suppl 2]: 19–123, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Sawinski D, Trofe-Clark J, Leas B, Uhl S, Tuteja S, Kaczmarek JL, French B, Umscheid CA: Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: A systematic review and meta-analysis. Am J Transplant 16: 2117–2138, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Vincenti F: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 2600–2601, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, Meier-Kriesche HU, Larsen CP: De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant 18: 1783–1789, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams AB, Goldstein J, Garrett C, Zhang R, Patzer RE, Newell KA, Turgeon NA, Chami AS, Guasch A, Kirk AD, Pastan SO, Pearson TC, Larsen CP: Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long-term renal allograft function. Am J Transplant 17: 2922–2936, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis S, Gralla J, Klem P, Stites E, Wiseman A, Cooper JE: Tacrolimus intra-patient variability, time in therapeutic range, and risk of de novo donor-specific antibodies [published online ahead of print August 9, 2019]. Transplantation doi:10.1097/TP.0000000000002913 [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Gralla J, Klem P, Tong S, Wedermyer G, Freed B, Wiseman A, Cooper JE: Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transplant 18: 907–915, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, Goldberg A, Ho J, Karpinski M, Pochinco D, Sharma A, Storsley L, Matas AJ, Nickerson PW: Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol 28: 3353–3362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH: Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M; Banff meeting report writing committee: Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, Famulski KS: Review: The transcripts associated with organ allograft rejection. Am J Transplant 18: 785–795, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Naesens M, Anglicheau D: Precision transplant medicine: Biomarkers to the rescue. J Am Soc Nephrol 29: 24–34, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Vrie M, Deegens JK, Eikmans M, van der Vlag J, Hilbrands LB: Urinary MicroRNA as biomarker in renal transplantation. Am J Transplant 17: 1160–1166, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, Gwinner W, Thum T: Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant 11: 2221–2227, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Bloom RD, Bromberg JS, Poggio ED, Bunnapradist S, Langone AJ, Sood P, Matas AJ, Mehta S, Mannon RB, Sharfuddin A, Fischbach B, Narayanan M, Jordan SC, Cohen D, Weir MR, Hiller D, Prasad P, Woodward RN, Grskovic M, Sninsky JJ, Yee JP, Brennan DC; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators: Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 28: 2221–2232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang E, Sethi S, Peng A, Najjar R, Mirocha J, Haas M, Vo A, Jordan SC: Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am J Transplant 19: 1663–1670, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Gaber AO, First MR, Tesi RJ, Gaston RS, Mendez R, Mulloy LL, Light JA, Gaber LW, Squiers E, Taylor RJ, Neylan JF, Steiner RW, Knechtle S, Norman DJ, Shihab F, Basadonna G, Brennan DC, Hodge EE, Kahan BD, Kahan L, Steinberg S, Woodle ES, Chan L, Ham JM, Schroeder TJ: Results of the double-blind, randomized, multicenter, phase III clinical trial of thymoglobulin versus atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation 66: 29–37, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Webster AC, Wu S, Tallapragada K, Park MY, Chapman JR, Carr SJ: Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochrane Database Syst Rev 7: CD004756, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts DM, Jiang SH, Chadban SJ: The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 94: 775–783, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ: The treatment of antibody-mediated rejection in kidney transplantation: An updated systematic review and meta-analysis. Transplantation 102: 557–568, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C: Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9: 1099–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Sautenet B, Blancho G, Büchler M, Morelon E, Toupance O, Barrou B, Ducloux D, Chatelet V, Moulin B, Freguin C, Hazzan M, Lang P, Legendre C, Merville P, Mourad G, Mousson C, Pouteil-Noble C, Purgus R, Rerolle JP, Sayegh J, Westeel PF, Zaoui P, Boivin H, Le Gouge A, Lebranchu Y: One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 100: 391–399, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Kwun J, Burghuber C, Manook M, Iwakoshi N, Gibby A, Hong JJ, Knechtle S: Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol 28: 1991–1996, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno Gonzales MA, Gandhi MJ, Schinstock CA, Moore NA, Smith BH, Braaten NY, Stegall MD: 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation 101: 1222–1227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waiser J, Budde K, Schütz M, Liefeldt L, Rudolph B, Schönemann C, Neumayer HH, Lachmann N: Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant 27: 1246–1251, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Walsh RC, Alloway RR, Girnita AL, Woodle ES: Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int 81: 1067–1074, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Flechner SM, Fatica R, Askar M, Stephany BR, Poggio E, Koo A, Banning S, Chiesa-Vottero A, Srinivas T: The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation 90: 1486–1492, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Tasaki M, Saito K, Nakagawa Y, Ikeda M, Imai N, Ito Y, Sudo M, Ikezumi Y, Yamada T, Hasegawa H, Kobayashi T, Miura K, Narita I, Takahashi K, Tomita Y: Bortezomib eliminates plasma cells from a renal graft in plasma cell-rich acute rejection. Transplant Proc 51: 1732–1738, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD: Positive crossmatch kidney transplant recipients treated with eculizumab: Outcomes beyond 1 year. Am J Transplant 15: 1293–1302, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA: Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98: 857–863, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, Kim I, Louie S, Kang A, Peng A, Kahwaji J, Reinsmoen N, Toyoda M, Jordan SC: Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant 17: 2381–2389, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, Woodle ES, Sommerer C, Fitts D, Rockich K, Zhang P, Uknis ME: Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: Results of a randomized double-blind placebo-controlled pilot study. Am J Transplant 16: 3468–3478, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Viglietti D, Gosset C, Loupy A, Deville L, Verine J, Zeevi A, Glotz D, Lefaucheur C: C1 inhibitor in acute antibody-mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: A pilot study. Am J Transplant 16: 1596–1603, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, Knoll G, Lachance JG, Landsberg D, Shapiro J, Shoker A, Yilmaz S: Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: A randomized study. Am J Transplant 7: 2538–2545, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Loupy A, Vernerey D, Tinel C, Aubert O, Duong van Huyen JP, Rabant M, Verine J, Nochy D, Empana JP, Martinez F, Glotz D, Jouven X, Legendre C, Lefaucheur C: Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol 26: 1721–1731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, Karpinski M, Goldberg A, Storsley L, Rush DN, Nickerson PW: Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 15: 2921–2930, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Viglietti D, Loupy A, Vernerey D, Bentlejewski C, Gosset C, Aubert O, Duong van Huyen JP, Jouven X, Legendre C, Glotz D, Zeevi A, Lefaucheur C: Value of donor-specific anti-HLA antibody monitoring and characterization for risk stratification of kidney allograft loss. J Am Soc Nephrol 28: 702–715, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schinstock CA, Cosio F, Cheungpasitporn W, Dadhania DM, Everly MJ, Samaniego-Picota MD, Cornell L, Stegall MD: The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transplant 17: 1574–1584, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, Montgomery JR, Wickliffe C, Lonze BE, Bagnasco SM, Alachkar N, Kraus ES, Jackson AM, Montgomery RA, Segev DL: Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant 15: 489–498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]