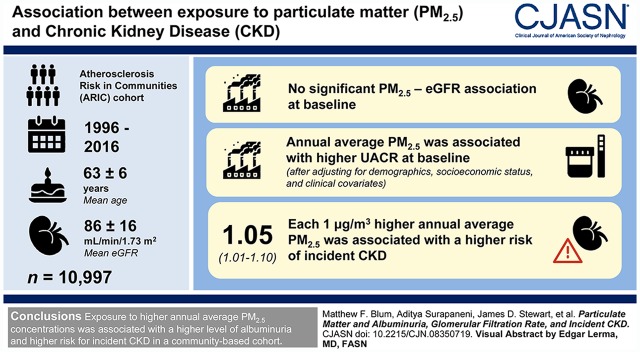
Visual Abstract
Keywords: chronic kidney disease, albuminuria, glomerular filtration rate, epidemiology and outcomes, ARIC, particulate matter, air pollution, humans, cross-sectional studies, creatinine, linear models, geographic information systems, International Classification of Diseases, follow-up studies, confidence intervals, chronic renal insufficiency, chronic kidney failure, kidney function tests, cohort studies, atherosclerosis, demography, hospitalization, social class, albumin
Abstract
Background and objectives
Exposure to particulate matter (PM) <2.5 μm in aerodynamic diameter (PM2.5) has been linked to detrimental health effects. This study aimed to describe the relationship between long-term PM2.5 exposure and kidney disease, including eGFR, level of albuminuria, and incident CKD.
Design, setting, participants, & measurements
The study included 10,997 participants from the Atherosclerosis Risk in Communities cohort who were followed from 1996–1998 through 2016. Monthly mean PM2.5 concentrations (μg/m3) were estimated at geocoded participant addresses using geographic information system–based, spatiotemporal generalized additive mixed models—including geospatial covariates such as land use—and then averaged over the 12-month period preceding participant examination. Covariate-adjusted, cross-sectional associations of PM2.5, baseline eGFR, and urinary albumin-creatinine ratio (UACR) were estimated using linear regression. PM2.5 and incident CKD (defined as follow-up eGFR <60 ml/min per 1.73 m2 with ≥25% eGFR decline relative to baseline, CKD-related hospitalization or death based on International Classification of Diseases 9/10 codes, or development of ESKD) associations were estimated using Cox proportional hazards regression. Modeling was stratified by study site, and stratum-specific estimates were combined using random-effects meta-analyses.
Results
Baseline mean participant age was 63 (±6) years and eGFR was 86 (±16) ml/min per 1.73 m2. There was no significant PM2.5-eGFR association at baseline. Each 1-μg/m3 higher annual average PM2.5 was associated with higher UACR after adjusting for demographics, socioeconomic status, and clinical covariates (percentage difference, 6.6%; 95% confidence interval [95% CI], 2.6% to 10.7%). Each 1-μg/m3 higher annual average PM2.5 was associated with a significantly higher risk of incident CKD (hazard ratio, 1.05; 95% CI, 1.01 to 1.10).
Conclusions
Exposure to higher annual average PM2.5 concentrations was associated with a higher level of albuminuria and higher risk for incident CKD in a community-based cohort.
Introduction
Particulate matter (PM) air pollution is a heterogeneous mixture of solid and liquid particles from various sources including fossil fuel combustion, road dust, industrial processes, and natural sources. Major components include sulfates, nitrates, ammonium, chloride, elemental and organic carbon, crustal and biologic materials, and trace metals; specific components vary by place and time (1–3). Fine particulate matter, which consists of particles <2.5 μm in aerodynamic diameter (PM2.5), is thought to be particularly harmful, because these small particles reach distal airways and alveoli where they may trigger a systemic inflammatory response and potentially enter the systemic vasculature (4). PM2.5 exposure has been linked to various adverse health outcomes including cardiovascular disease (5,6), respiratory disease (7), diabetes mellitus (8,9), and mortality (10–12).
Air pollution may also affect kidneys, which participate in the excretion of many systemically absorbed PM components. For example, heavy-metal components of PM2.5 such as lead, cadmium, and mercury may promote kidney injury through oxidative damage, causing tubular dysfunction and subsequent interstitial fibrosis (13,14). PM2.5-induced systemic inflammatory and dysautonomic effects (4) may affect kidneys as well (15). These effects may occur both acutely and through long-term exposure. A few studies have linked higher 1-year (16–18) or 2-year (19) PM2.5 exposure to higher prevalence of CKD (16), longitudinal eGFR decline (17,18), and higher incidence of eGFR <60 ml/min per 1.73 m2 (18,19), although the relationship between PM2.5 and prevalent eGFR has been mixed (17,20). One study has explored the association between PM exposure and albuminuria but found no relationship, despite the theoretic vulnerability of kidneys to toxin-mediated glomerular, tubulointerstitial, and vascular injury (21).
Featuring participants from the Atherosclerosis Risk in Communities study, a community-based cohort of adults, this study aimed to build upon previous work and examine the associations of PM2.5 exposure with kidney function, albuminuria, and incident CKD. We hypothesized that higher exposure to PM would be associated with higher burden of kidney disease, and that associations would be consistent across demographics and underlying comorbidities. As negative and positive cross-sectional controls, we evaluated the association of PM2.5 exposure with height and area deprivation index, respectively. As a negative and positive longitudinal control, we evaluated the association of PM2.5 exposure with incident cellulitis and mortality.
Materials and Methods
Study Population
The Atherosclerosis Risk in Communities study is a prospective community-based cohort following 15,792 adults across four sites in the United States: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. There have been six study visits: the first visit (1987–1990) was followed by triennial visits through visit 4 (1996–1998) and then, after a hiatus, visit 5 (2011–2013) and visit 6 (outcomes available through 2016). During follow-up, participant hospitalizations were tracked by active review of local hospital-discharge lists, and also through patient report in telephone surveys (initially annual, increased to semiannual in 2012), after which records of all reported hospitalizations were requested. The study was approved by the Institutional Review Boards of the University of North Carolina, Wake Forest University, the University of Mississippi Medical Center, the University of Texas Health Sciences Center at Houston, the University of Minnesota, and the Johns Hopkins University. Written informed consent was obtained. Further details about the Atherosclerosis Risk in Communities study have been published (22).
The study population included all participants in the Atherosclerosis Risk in Communities study with available plasma creatinine at visit 4 (n=11,560), which served as the baseline visit for this study. Individuals were excluded if they were missing annual PM2.5 exposures (n=476; Supplemental Table 1) or had ESKD at baseline (n=20). Black participants from Minneapolis (n=11) and Washington County (n=26) and nonblack, nonwhite individuals from all sites (n=30) were excluded due to small numbers (Supplemental Table 2). The final cohort consisted of 10,997 participants. For analyses of incident CKD, participants with eGFR <60 ml/min per 1.73 m2 at visit 4 also were excluded (final n=10,302).
Exposure
Monthly mean PM2.5 concentrations (μg/m3) were estimated (1988–2007) using a geographic information system–based, spatiotemporal generalized additive mixed model, which included meteorologic and geospatial covariates such as land use; this model had high predictive accuracy (crossvalidation R2 of 0.68–0.77 across regions). The exposure model was used to predict PM2.5 levels at accurately geocoded participant addresses, which were updated at visits 1 through 4 (23,24), and was based on US Environmental Protection Agency’s Air Quality System PM2.5 (25) and other monitoring data (26–28). Estimation before 1999 involved using the log-transformed ratio of PM2.5 to predicted PM10 (PM with aerodynamic diameter <10 μm) because PM2.5 monitoring data were not widely available until 1999. Before 1988, monthly concentrations were imputed using concentrations from corresponding months in 1988, given substantial correlation of monthly means over years. Monthly means were subsequently averaged over the 12-month period (annual average) ending on the visit 4 examination month, but only when data were available for ≥75% of months in the period. Annual average PM2.5 concentrations were missing in 4.1% of participants. In sensitivity analyses, we evaluated 60- and 120-month averaging periods, which had slightly higher missing data, up to 5.5% for the 120-month exposure.
Outcomes
Three outcomes were assessed: visit 4 eGFR, visit 4 albuminuria, and incident CKD after visit 4. eGFR was calculated using the creatinine-based CKD Epidemiology Collaboration equation and plasma creatinine measured at visit 4 (29). Creatinine was measured using the modified kinetic Jaffé method in plasma and urine specimens from visit 4 and measured using the Roche enzymatic method in serum specimens from visit 5 (Roche-Hitachi Modular P chemistry analyzer with Roche Creatininase Plus assay; Hoffman-La Roche) and visit 6 (Roche Cobas 6000 chemistry analyzer with Roche Creatininase Plus assay). Albumin was measured in urine specimens from visit 4 by a nephelometric method (Dade Behring BN100 or Beckman Image Nephelometer). Urinary albumin-creatinine ratio (UACR) was calculated at visit 4 and log-transformed to obtain a normal distribution. Incident CKD was a composite outcome defined as meeting any of the following: (1) development of eGFR <60 ml/min per 1.73 m2 at follow-up visit 5 or visit 6 accompanied by ≥25% eGFR decline relative to baseline; (2) CKD-related hospitalization or death based on International Classification of Diseases 9/10 (ICD-9/10) codes; or (3) development of ESKD, which was defined as United States Renal Data Systems–identified ESKD, eGFR <15 ml/min per 1.73 m2 at follow-up, or ICD-9/10 code for a kidney failure–related hospitalization or death (30,31). Participants were censored if lost to follow-up or death from a cause not related to CKD or ESKD (Supplemental Table 3).
Covariate Selection
Baseline covariate data were derived from visit 4. Demographic data included sex, age, and race along with socioeconomic factors including neighborhood socioeconomic score (32), family income, and education level. Clinical covariates included body mass index; smoking status; eGFR; UACR; C-reactive protein; systolic BP; and the presence of comorbid conditions including diabetes mellitus (defined as random blood glucose ≥200 mg/dl, fasting blood glucose ≥126 mg/dl, reported history of diabetes mellitus, or taking medications for diabetes mellitus), hypertension (defined as average of first two systolic BPs ≥140 mm Hg or diastolic BPs ≥90 mm Hg or taking antihypertensive medications), and a composite cardiovascular-disease covariate to reflect the presence of any prevalent coronary heart disease, stroke, heart failure, or peripheral artery disease (Supplemental Table 4). UACR and C-reactive protein were log-transformed. Daily mean ambient temperature (°C) across all National Climatic Data Center (33) monitoring stations ≤50 km from geocoded participant address was averaged over the same averaging periods used for PM2.5.
Statistical Analyses
Baseline characteristics were stratified above and below site-specific median annual average PM2.5 concentrations and pooled for comparison. Linear regression was used to estimate cross-sectional associations of PM2.5 with eGFR and UACR. Linear regressions were performed using log-transformed UACR; coefficients were expressed as a percentage difference ([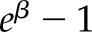 ]×100%) to aid interpretation. Cox proportional hazards regression was used to estimate associations between PM2.5 and incident CKD. All regressions were performed per 1-μg/m3 higher PM2.5
]×100%) to aid interpretation. Cox proportional hazards regression was used to estimate associations between PM2.5 and incident CKD. All regressions were performed per 1-μg/m3 higher PM2.5
Given the heterogeneity of PM2.5 concentrations among sites, analyses were stratified by site, and stratum-specific results were combined in random-effects meta-analyses with testing for heterogeneity of effect (34). Regressions were performed for the annual average PM2.5 exposures and sequentially adjusted using two models. The first model adjusted for sex, age, race, neighborhood socioeconomic score, family income, and education level. The second model incorporated the covariates of the first model and additionally included body mass index, diabetes mellitus, hypertension, systolic BP, composite cardiovascular disease, cigarette smoking, C-reactive protein, eGFR, UACR, and temperature. Linear splines were used for body mass index with a knot at 30 kg/m2 and systolic BP with a knot at 120 mm Hg. Multiple imputation was performed for missing covariate values, excluding eGFR and UACR. Models were stratified by sex, age, diabetes, hypertension, and site-specific median income, and tested for effect modification in the full model with interaction terms.
Exposure-control analyses for linear-regression models were performed using area deprivation index (35) and participant height as positive and negative controls, respectively, and for Cox proportional hazards models with mortality and cellulitis as positive and negative controls, respectively. Sensitivity analyses were performed excluding those with baseline UACR of at least 30 mg/g; excluding Jackson; in the full population using 60- and 120-month average PM2.5 exposures; and a competing risk analysis (modeling the competing event of non-CKD death). Analysis of the between- and within-site effects was performed for incident CKD using a Cox proportional hazards model for the association of incident CKD with the site mean PM2.5 as the exposure variable to assess between-site effect; another Cox proportional hazards model using both the site mean and individual PM2.5 exposure minus the site mean PM2.5 was used to assess within-site effect (36). Values were considered significant when P was <0.05. A Bonferroni-corrected P value of 0.01 was applied to the five interaction analyses. Statistical analysis was performed using Stata SE version 15.0 (StataCorp LLC, College Station, TX).
Results
Study Population
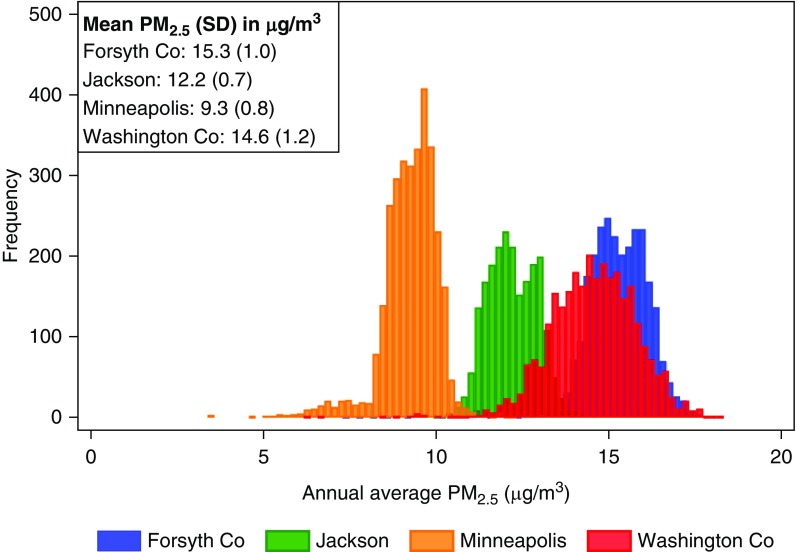
A total of 10,997 participants were included across the four sites. The population was 56% female. At visit 4 baseline, mean age was 63 (±6) years and mean eGFR was 86 (±16) ml/min per 1.73 m2. Table 1 shows demographic characteristics of participants stratified by site-specific annual average PM2.5. Missing covariates are listed in Supplemental Table 5. PM2.5 was highest in Forsyth County and lowest in Minneapolis (Figure 1).
Table 1.
Baseline characteristics of the Atherosclerosis Risk in Communities study population, stratified above and below site-specific median annual average PM2.5 concentration
| Characteristic | Annual Average PM2.5 (μg/m3) | |
|---|---|---|
| ≤Site-Specific Median | >Site-Specific Median | |
| Number of participants | 5499 | 5498 |
| Age, mean (SD), yr | 63 (6) | 63 (6) |
| Female, n | 3033 (55%) | 3128 (57%) |
| Black, n | 1177 (21%) | 1231 (22%) |
| Smoker, n | ||
| Never | 2332 (43%) | 2211 (41%) |
| Former | 2362 (43%) | 2420 (44%) |
| Current | 790 (14%) | 822 (15%) |
| eGFR, mean (SD), ml/min per 1.73 m2 | 86 (16) | 86 (16) |
| eGFR<60 ml/min per 1.73 m2, n | 346 (6%) | 349 (6%) |
| UACR, median (IQR), mg/g | 3.4 (5.8) | 4.0 (6.4) |
| C-reactive protein, median (IQR), mg/L | 2.5 (4.4) | 2.5 (4.4) |
| Body mass index, mean (SD), kg/m2 | 28.8 (5.5) | 28.8 (5.7) |
| Systolic BP, mean (SD), mm Hg | 127 (19) | 128 (19) |
| Hypertension, n | 2546 (46%) | 2678 (49%) |
| Diabetes mellitus, n | 872 (16%) | 943 (17%) |
| Composite cardiovascular disease, n | 1026 (19%) | 1090 (20%) |
| Neighborhood socioeconomic score (SD) | 0.4 (5.4) | −0.3 (5.5) |
| Annual household income, n | ||
| <$25,000 | 1591 (30%) | 1699 (32%) |
| ≥$25,000 | 3676 (68%) | 3536 (66%) |
| Refused | 124 (2%) | 126 (2%) |
| Education, n | ||
| ≤11 yr | 1045 (19%) | 1042 (19%) |
| 12–16 yr | 2273 (41%) | 2358 (43%) |
| 17–21 yr | 2171 (40%) | 2091 (38%) |
| Temperature, mean (SD), °C | 12.4 (4.3) | 13.0 (4.0) |
Median annual average PM2.5 concentration for each site: Forsyth County, 15.3 μg/m3; Jackson, 12.2 μg/m3; Minneapolis, 9.4 μg/m3; and Washington County, 14.6 μg/m3. PM2.5, particulate matter <2.5 μm in aerodynamic diameter; UACR, urinary albumin-creatinine ratio; IQR, interquartile range.
Figure 1.
Annual average PM2.5 concentration varies across sites. Co, county; PM2.5, particulate matter <2.5 μm in aerodynamic diameter.
Cross-Sectional Associations between Fine Particulate Matter and Estimated Glomerular Filtration Rate and Urinary Albumin-Creatinine Ratio
There was no statistically significant cross-sectional association between PM2.5 and eGFR in either adjustment model, nor across any stratifying variable (Table 2). In contrast, a 1-μg/m3 higher annual average PM2.5 was significantly associated with higher UACR when adjusting for demographics and socioeconomic status in model 1 (percentage difference, 8.5%; 95% confidence interval [95% CI], 3.6% to 13.7%) and when additionally adjusting for clinical covariates in model 2 (percentage difference, 6.6%; 95% CI, 2.6% to 10.7%). There were no significant interactions for any eGFR or UACR subgroup analysis. Associations between PM2.5 and UACR were significant at each site except Jackson in models 1 and 2 (Supplemental Figure 1), and the overall I2 was 50.4% (P=0.11; Supplemental Table 6), indicating possible heterogeneity. Results were consistent in analyses excluding Jackson (Supplemental Table 7) with a lower I2 (Supplemental Table 8), and were weaker when using longer averaging periods.
Table 2.
Associations of annual average PM2.5 with baseline eGFR and urinary albumin-creatinine ratio
| Characteristic | Number of Eligible Participants | eGFR | UACRa | ||||
|---|---|---|---|---|---|---|---|
| ml/min per 1.73 m2 (95% CI) | P Value | Interaction P Value | % Difference (95% CI) | P Value | Interaction P Value | ||
| Overall | |||||||
| Model 1b | 10,997 | 0.14 (−0.32 to 0.60) | 0.55 | 8.5 (3.6 to 13.7) | <0.001 | ||
| Model 2c | 0.07 (−0.28 to 0.41) | 0.71 | 6.6 (2.6 to 10.7) | 0.001 | |||
| Sex | |||||||
| Male | 4836 | 0.16 (−0.25 to 0.56) | 0.46 | 0.56 | 10.3 (5.9 to 14.9) | <0.001 | 0.09 |
| Female | 6161 | −0.00 (−0.52 to 0.52) | 1.00 | 3.7 (−1.7 to 9.4) | 0.18 | ||
| Age, yr | |||||||
| <65 | 6597 | 0.03 (−0.40 to 0.46) | 0.90 | 0.95 | 7.0 (0.8 to 13.7) | 0.03 | 0.32 |
| ≥65 | 4400 | 0.17 (−0.26 to 0.60) | 0.44 | 5.9 (1.7 to 10.3) | 0.005 | ||
| History of diabetes | |||||||
| No | 9135 | 0.06 (−0.36 to 0.48) | 0.78 | 0.67 | 6.0 (2.8 to 9.4) | <0.001 | 0.44 |
| Yes | 1815 | 0.46 (−0.43 to 1.35) | 0.31 | 7.6 (−8.6 to 26.7) | 0.38 | ||
| History of hypertension | |||||||
| No | 5729 | −0.05 (−0.49 to 0.39) | 0.81 | 0.61 | 7.5 (1.0 to 14.4) | 0.02 | 0.75 |
| Yes | 5224 | 0.24 (−0.21 to 0.70) | 0.29 | 5.2 (−0.3 to 11.1) | 0.07 | ||
| Income above mediand | |||||||
| No | 6043 | 0.09 (−0.28 to 0.47) | 0.64 | 0.90 | 6.5 (1.4 to 11.8) | 0.01 | 0.80 |
| Yes | 4459 | 0.03 (−0.55 to 0.60) | 0.93 | 7.5 (3.5 to 11.8) | <0.001 | ||
PM2.5, particulate matter <2.5 μm in aerodynamic diameter; UACR, urinary albumin-creatinine ratio.
UACR coefficient underwent exponentiation using the formula (eβ−1)×100%.
Model 1: sex, age, race, neighborhood socioeconomic score, family income, education level.
Model 2: model 1 plus body mass index, diabetes mellitus, hypertension, systolic BP, composite cardiovascular disease, cigarette smoking, eGFR,* UACR,** C-reactive protein, temperature. Covariate omitted for eGFR analyses (*) and UACR analyses (**). Model 2 adjustment used for stratified analyses. Analyses performed per 1-μg/m3 higher PM2.5.
Median income was $16,000–$24,999 for Jackson, $25,000–$34,999 for Washington County, and $35,000–$49,999 for Forsyth County and Minneapolis.
Associations between Fine Particulate Matter and Incident CKD
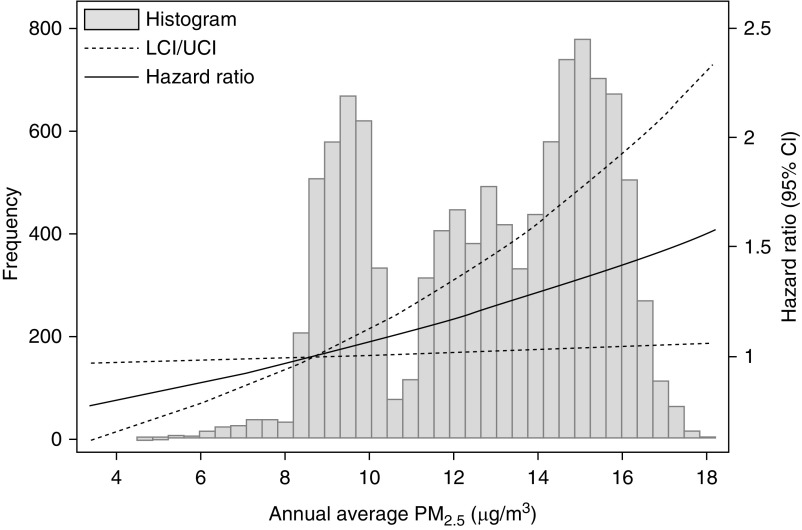
Out of 10,302 participants with eGFR ≥60 ml/min per 1.73 m2, there were 2816 cases of incident CKD over a median 17.7 years follow-up through visit 6 (Table 3). The overall CKD incidence was 17.8 events per 1000 person-years (Supplemental Table 9). Annual average PM2.5 was significantly associated with increased risk of CKD in model 1 (hazard ratio per 1-μg/m3 higher PM2.5, 1.07; 95% CI, 1.03 to 1.11) and model 2 (hazard ratio, 1.05; 95% CI, 1.01 to 1.10). Figure 2 shows the model 2 exposure response function. There were no significant interactions for any subgroup. There was no significant heterogeneity (I2=6.8%, P=0.36). Supplemental Table 10 describes the within- and between-site effects. Results were directionally similar but weaker when excluding Jackson or using longer averaging periods (Supplemental Figures 2 and 3), and consistent when excluding participants with UACR ≥30 mg/g at baseline (Supplemental Table 11). In the competing risk analysis, annual average PM2.5 was significantly associated with incident CKD in model 1 but not model 2 (Supplemental Table 12).
Table 3.
Association of annual average PM2.5 with incident CKD in participants with eGFR ≥60 ml/min per 1.73 m2 at baseline
| Characteristic | Number of Eligible Participants | Number of CKD Events | Person-Years | CKD Incidence per 1000 Person-Years (95% CI) | Incident CKD, HR (95% CI) | P Value | Interaction P Value |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| Model 1a | 10,302 | 2816 | 158,199 | 17.8 (17.2 to 18.5) | 1.07 (1.03 to 1.11) | 0.001 | |
| Model 2b | 1.05 (1.01 to 1.10) | 0.03 | |||||
| Sex | |||||||
| Male | 4518 | 1359 | 65,511 | 20.7 (19.7 to 21.9) | 1.04 (0.98 to 1.10) | 0.20 | 0.54 |
| Female | 5784 | 1457 | 92,687 | 15.7 (14.9 to 16.5) | 1.10 (1.00 to 1.21) | 0.05 | |
| Age, yr | |||||||
| <65 | 6385 | 1559 | 104,501 | 14.9 (14.2 to 15.7) | 1.03 (0.97 to 1.10) | 0.31 | 0.63 |
| ≥65 | 3917 | 1257 | 53,697 | 23.4 (22.2 to 24.7) | 1.09 (0.99 to 1.20) | 0.07 | |
| History of diabetes | |||||||
| No | 8623 | 2105 | 136,223 | 15.5 (14.8 to 16.1) | 1.08 (1.01 to 1.16) | 0.02 | 0.13 |
| Yes | 1635 | 698 | 21,387 | 32.6 (30.3 to 35.2) | 0.98 (0.89 to 1.08) | 0.73 | |
| History of hypertension | |||||||
| No | 5536 | 1177 | 89,278 | 13.2 (12.5 to 14.0) | 1.02 (0.96 to 1.09) | 0.55 | 0.15 |
| Yes | 4726 | 1629 | 68,339 | 23.8 (22.7 to 25.0) | 1.09 (1.02 to 1.16) | 0.02 | |
| Income above medianc | |||||||
| No | 5581 | 1616 | 81,926 | 19.7 (18.8 to 20.7) | 1.05 (0.98 to 1.12) | 0.21 | 0.58 |
| Yes | 4253 | 1072 | 69,603 | 15.4 (14.5 to 16.4) | 1.06 (0.99 to 1.14) | 0.07 |
PM2.5, particulate matter <2.5 μm in aerodynamic diameter; HR, hazard ratio.
Model 1: sex, age, race, neighborhood socioeconomic score, family income, education level.
Model 2: model 1 plus body mass index, diabetes mellitus, hypertension, systolic BP, composite cardiovascular disease, cigarette smoking, eGFR, urinary albumin-creatinine ratio, C-reactive protein, temperature. Model 2 adjustment used for stratified analyses. Analyses performed per 1-μg/m3 higher PM2.5.
Median income was $16,000–$24,999 for Jackson, $25,000–$34,999 for Washington County, and $35,000–$49,999 for Forsyth County and Minneapolis.
Figure 2.
Higher annual average PM2.5 concentration associates with higher risk of incident CKD. Exposure response function created using model 2. The fifth percentile PM2.5 value of 8.7 μg/m3 was selected as the reference. CI, confidence interval; LCI, lower CI; PM2.5, particulate matter <2.5 μm in aerodynamic diameter; UCI, upper CI.
Control Analyses
In analyses of negative cross-sectional and longitudinal controls, there was no association between PM2.5 and baseline height or between PM2.5 and incident cellulitis (Supplemental Table 13). In analyses of positive cross-sectional and longitudinal controls, higher PM2.5 was significantly associated with higher area deprivation index and increased mortality.
Discussion
In this study of older adults at four sites followed for a median 17.7 years, higher annual average PM2.5 exposure was associated with increased albuminuria and a higher risk of CKD. There were no associations between PM2.5 and baseline eGFR. These findings support the role of PM2.5 exposure as a potential risk factor for CKD and suggest PM2.5 mitigation efforts as a potential avenue for reducing CKD burden.
Animal models support a causal link between PM2.5 exposure and kidney injury. Healthy rats exposed to 8 weeks of PM2.5 experienced a variety of physiologic changes including increased BP, increased levels of angiotensin-converting enzyme and angiotensin II receptor type 1, a depleted antioxidant response, hematuria, and reduced GFR, but no change in urinary albumin (15). Histologic changes after PM2.5 exposure included increased kidney fibrosis, mesangial expansion, and reduced glomerular and tubular lumen volumes (37). TNF-α signaling pathways may be important contributors to PM2.5-induced oxidative kidney injury. Prolonged PM2.5 exposure may promote sodium retention by reducing expression of the D1 receptor, contributing to hypertension (38). A study of rats exposed to PM2.5 showed a direct relationship between the concentrations of metals measured in the air and those measured in kidney tissue, supporting the hypothesis that systemically absorbed PM2.5 components directly damage kidney tissue (37). Overall, these mechanistic studies suggest the presence of a complex, multifaceted response to PM2.5, which is likely affected by the timing, duration, and specific components of exposure.
This study adds to the current body of literature by providing further support for the link between long-term PM2.5 exposure and CKD incidence and by demonstrating an association between PM2.5 and albuminuria. A previous study in a large Veterans Affairs (VA) cohort found 10-μg/m3 higher PM2.5 averaged over 1 year to be associated with a 27% (95% CI, 17%–38%) higher risk of incident CKD (18). A Taiwanese study in the general population showed a smaller magnitude of association, with a 10-μg/m3 higher PM2.5 averaged over 2 years associated with a 6% (95% CI, 2%–10%) higher risk of CKD (19). The overall PM2.5 concentration was much higher in the Taiwanese study (mean, 27.1 μg/m3) compared with the VA study (median, 11.8 μg/m3) and the cohorts differed in study design: the Taiwanese population was younger (38.9 versus 62.5 years old), had higher baseline eGFR (87.0 versus 76.3 ml/min per 1.73 m2), was followed at standardized medical exam rather than clinically, and had lower incidence of CKD (6 versus 41 events per 1000 person-years). In the single previous study of PM exposure and UACR, neither short-term (1- and 2-month) nor long-term (20-year) PM2.5 exposure period was associated with cross-sectional UACR or change in UACR over time in the Multi-Ethnic Study of Atherosclerosis cohort (21). In our study, we found an association when looking at different length exposures (12- and 60-month). These time frames, which are long enough to account for seasonal variations, were not previously used in UACR analyses and may account for our distinct findings, as could the lower baseline cardiovascular disease burden in the Multi-Ethnic Study of Atherosclerosis cohort.
Interestingly, previous cross-sectional studies between PM2.5 and eGFR have yielded variable results. In an analysis of the Medicare population, higher PM2.5 was associated with higher CKD prevalence defined by ICD-9, Clinical Modification diagnosis codes (16), consistent with a VA study in which PM2.5 exposure was associated with lower eGFR (17). Our results, and those of a large Taiwanese cohort, showed no association between PM2.5 and eGFR (20). Potential reasons underlying these distinct findings include differences in baseline eGFR, albuminuria, or racial makeup of these populations. Furthermore, PM2.5 components vary by region, with certain regions containing concentrations of components that could be more detrimental to glomerular or proximal tubular function (39).
Study strengths include the use of cohort data with exposures and outcomes measured in a research setting with active ascertainment, the high percentage of patients with UACR measurement, and sensitivity analyses using various PM2.5 averaging periods. Weaknesses include the prolonged period of time between visits 4 and 5, limiting the ability to trend biomarker changes over time; absence of dietary data; use of spot UACR instead of first morning void or 24-hour urine albumin collections; absence of indoor air pollution exposure; lack of hemoglobin A1C as an available covariate for visit 4; analysis limited to one pollutant; and the possibility of residual confounding. The relatively small cohort size reduced analytical power resulting in wide CIs. All but one site predominantly enrolled a single race, precluding tests of interaction by race. The relatively advanced cohort age may reflect a selection bias in which the highest risk participants may not have survived to the baseline visit (40). There was substantial heterogeneity in the eGFR and UACR models, which was driven by Jackson, Mississippi. In the most highly adjusted models, PM2.5 was not associated with incident CKD in the analysis that excluded Jackson, although this may reflect a lack of power. Assigning PM2.5 by home address provided an incomplete characterization of the participants’ true exposure.
In summary, this study found that exposure to higher annual average concentration of PM2.5 was associated with increased albuminuria and increased risk of incident CKD. This finding may be especially important for parts of the world with higher air pollution burden, such as China and India, where PM2.5 concentrations are five to ten times higher than in the United States (41). Future work should quantify whether efforts to improve air quality yield health benefits, including reducing the burden of CKD.
Disclosures
Dr. Grams reports receiving nonfinancial travel support from Dialysis Clinic, Inc. and Kidney Disease Improving Global Outcomes as well as grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Kidney Foundation, outside of the submitted work. Dr. Blum, Dr. Liao, Dr. Power, Mr. Stewart, Dr. Surapaneni, Dr. Whitsel, and Dr. Yanosky have nothing to disclose.
Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part by the National Heart, Lung, and Blood Institute; National Institutes of Health (NIH); US Department of Health and Human Services federal grants HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Funding for laboratory testing and biospecimen collection at Atherosclerosis Risk in Communities visit 6 was supported by NIDDK grant R01DK089174. Dr. Grams is supported by NIDDK grant 5R01DK115534. Dr. Yanosky is supported by NIH grant R01ES020836-02 (PI: Dr. Whitsel).
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Air Pollution and Kidney Disease,” on pages 301–303.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08350719/-/DCSupplemental.
Supplemental Table 1. Missing PM2.5.
Supplemental Table 2. Excluded participants.
Supplemental Table 3. Participants censored.
Supplemental Table 4. Variable parameterization.
Supplemental Table 5. Missing covariates.
Supplemental Table 6. Heterogeneity of effect (I2) for associations of annual average PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD.
Supplemental Table 7. Sensitivity analyses for associations of annual average PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD when omitting Jackson and testing 60- and 120-month PM2.5 exposures.
Supplemental Table 8. Heterogeneity of effect for associations of annual average PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD when excluding Jackson.
Supplemental Table 9. CKD incidence in participants with eGFR ≥60 ml/min per 1.73 m2 at baseline by site, stratified by median annual average PM2.5.
Supplemental Table 10. Between and within site effects for Cox proportional hazards models of annual average PM2.5 and incident CKD.
Supplemental Table 11. Sensitivity analyses for association of annual average PM2.5 with incident CKD when excluding those with urinary albumin to creatinine ratio ≥30 mg/g.
Supplemental Table 12. Competing risk analysis for association of annual average PM2.5 with incident CKD with a competing risk of non-CKD death.
Supplemental Table 13. Positive and negative control models for the associations of annual average PM2.5 with height, area deprivation index, incident cellulitis, and incident mortality.
Supplemental Figure 1. Forest plots describing overall and site-specific associations of annual average PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD.
Supplemental Figure 2. Forest plots describing overall and site-specific associations of 60-month PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD.
Supplemental Figure 3. Forest plots describing overall and site-specific associations of 120-month PM2.5 with baseline eGFR, urinary albumin to creatinine ratio, and incident CKD.
References
- 1.Valavanidis A, Fiotakis K, Vlachogianni T: Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26: 339–362, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Harrison RM, Yin J: Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ 249: 85–101, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Solomon PA, Sioutas C: Continuous and semicontinuous monitoring techniques for particulate matter mass and chemical components: A synthesis of findings from EPA’s Particulate Matter Supersites Program and related studies. J Air Waste Manag Assoc 58: 164–195, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Chin MT: Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 101: 253–256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr., Tager I; Expert Panel on Population and Prevention Science of the American Heart Association: Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism: Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J: The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med 351: 1057–1067, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Copes R: Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 121: 804–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen AB, Ravnskjær L, Loft S, Andersen KK, Bräuner EV, Baastrup R, Yao C, Ketzel M, Becker T, Brandt J, Hertel O, Andersen ZJ: Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int 91: 243–250, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., Speizer FE: An association between air pollution and mortality in six U.S. cities. N Engl J Med 329: 1753–1759, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Pope CA 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW Jr.: Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med 151: 669–674, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Laden F, Schwartz J, Speizer FE, Dockery DW: Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med 173: 667–672, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS: Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis 17: 254–264, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Afsar B, Elsurer Afsar R, Kanbay A, Covic A, Ortiz A, Kanbay M: Air pollution and kidney disease: Review of current evidence. Clin Kidney J 12: 19–32, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aztatzi-Aguilar OG, Uribe-Ramírez M, Narváez-Morales J, De Vizcaya-Ruiz A, Barbier O: Early kidney damage induced by subchronic exposure to PM2.5 in rats. Part Fibre Toxicol 13: 68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bragg-Gresham J, Morgenstern H, McClellan W, Saydah S, Pavkov M, Williams D, Powe N, Tuot D, Hsu R, Saran R; Centers for Disease Control and Prevention CKD Surveillance System: County-level air quality and the prevalence of diagnosed chronic kidney disease in the US Medicare population. PLoS One 13: e0200612, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, Vokonas PS, Schwartz JD: Long-term exposure to ambient fine particulate matter and renal function in older men: The Veterans Administration Normative Aging Study. Environ Health Perspect 124: 1353–1360, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan TC, Zhang Z, Lin BC, Lin C, Deng HB, Chuang YC, Chan JWM, Jiang WK, Tam T, Chang LY, Hoek G, Lau AKH, Lao XQ: Long-term exposure to ambient fine particulate matter and chronic kidney disease: A cohort study. Environ Health Perspect 126: 107002, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YR, Chen YM, Chen SY, Chan CC: Associations between long-term particulate matter exposure and adult renal function in the taipei metropolis. Environ Health Perspect 125: 602–607, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill MS, Diez-Roux AV, Auchincloss AH, Franklin TG, Jacobs DR Jr., Astor BC, Dvonch JT, Kaufman J: Airborne particulate matter exposure and urinary albumin excretion: The Multi-Ethnic Study of Atherosclerosis. Occup Environ Med 65: 534–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 23.Whitsel EA, Rose KM, Wood JL, Henley AC, Liao D, Heiss G: Accuracy and repeatability of commercial geocoding. Am J Epidemiol 160: 1023–1029, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, Heiss G: Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov 3: 8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Environmental Protection Agency: Air Quality System (AQS). Available at: https://www.epa.gov/aqs. Accessed Apr 29, 2019 [PubMed]
- 26.Paciorek CJ, Yanosky JD, Puett RC, Laden F, Suh HH: Practical large-scale spatio-temporal modeling of particulate matter concentrations. Ann Appl Stat 3: 370–397, 2009 [Google Scholar]
- 27.Yanosky JD, Paciorek CJ, Schwartz J, Laden F, Puett R, Suh HH: Spatio-temporal modeling of chronic PM10 exposure for the Nurses’ Health Study. Atmos Environ (1994) 42: 4047–4062, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, Suh HH: Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13: 63, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, Wruck L, Coresh J: Identification of incident CKD stage 3 in research studies. Am J Kidney Dis 64: 214–221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebholz CM, Coresh J, Ballew SH, McMahon B, Whelton SP, Selvin E, Grams ME: Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis 66: 231–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL: Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345: 99–106, 2001 [DOI] [PubMed] [Google Scholar]
- 33.National Oceanic and Atmospheric Administration; National Centers for Environmental Information: Global surface summary of the day (GSOD). Available at: https://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.ncdc:C00516. Accessed Apr 29, 2019
- 34.Power MC, Lamichhane AP, Liao D, Xu X, Jack CR, Gottesman RF, Mosley T, Stewart JD, Yanosky JD, Whitsel EA: The association of long-term exposure to particulate matter air pollution with brain MRI findings: The ARIC study. Environ Health Perspect 126: 027009, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M: Neighborhood socioeconomic disadvantage and 30-day rehospitalization: A retrospective cohort study. Ann Intern Med 161: 765–774, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD: Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356: 447–458, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Tavera Busso I, Mateos AC, Juncos LI, Canals N, Carreras HA: Kidney damage induced by sub-chronic fine particulate matter exposure. Environ Int 121: 635–642, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Lu X, Ye Z, Zheng S, Ren H, Zeng J, Wang X, Jose PA, Chen K, Zeng C: Long‐term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G‐protein-coupled receptor kinase type 4 expression in sprague‐dawley rats. J Am Heart Assoc 7: e007185, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF: Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 27: 3739–3746, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Hao H, Yu W, Wu X, Zhou H: Association of elevated glycosylated hemoglobin A1c with hyperfiltration in a middle-aged and elderly Chinese population with prediabetes or newly diagnosed diabetes: a cross-sectional study. BMC Endocr Disord 15: 47, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Jiang L, Zhao W, Xiong Q, Zhao W, Yan X: Comparison of ground-based PM2.5 and PM10 concentrations in China, India, and the U.S. Int J Environ Res Public Health 15: 1382, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.