Significant disparities exist in kidney disease, with blacks facing a higher burden of CKD and kidney failure. In addition to socioeconomic/health system factors, these disparities are linked to two sequence variants (G1 and G2) in the apoL1 (APOL1) gene. The high-risk APOL1 genotype (two copies of variants), present in up to 16% of blacks but <1% of Europeans (http://apol1.org/), is associated with an increased risk for CKD (1,2). However, the APOL1 high-risk genotype has incomplete penetrance and only some individuals with the high risk genotype develop overt kidney disease, indicating the presence of genetic/environmental modifiers (i.e., “second hits”). Thus, investigations into nontraditional/emerging risk factors are necessary to explore what modifies genetic risk.
Air pollution measured by fine particulate matter <2.5 µm (PM2.5) is an emerging robust risk factor and CKD/kidney failure risk increases linearly with PM2.5 (3). We hypothesized that like other chronic diseases, environmental risk (PM2.5) interacts with genetic risk (APOL1) to increase kidney disease. We utilized a large, biobanked cohort (BioMe Biobank at Mount Sinai) with linked genetic, clinical, and residential history information to reconstruct environmental exposures. We included 4800 participants self-identifying as black enrolled at a large, quaternary care health system in New York City serving an urban population from all boroughs and New Jersey. The mean age was 51 years, 63% were women, 29% had a history of type 2 diabetes (T2D), and 62% had a history of hypertension. APOL1 high-risk genotype was defined as two risk variants and low-risk genotype was defined as one or zero risk variants. We mapped the residences of participants to city blocks and estimated average PM2.5 exposure in the year before enrollment, using a previously developed model combining satellite-derived aerosol optical depth retrieval with land use, meteorology, and spatially derived features (4). Finally, we identified CKD stage 3 or higher using a validated algorithm (5), and kidney failure using US Renal Data System linkage over follow-up. We defined a composite kidney outcome as either CKD stage 3 or higher or kidney failure (Figure 1).
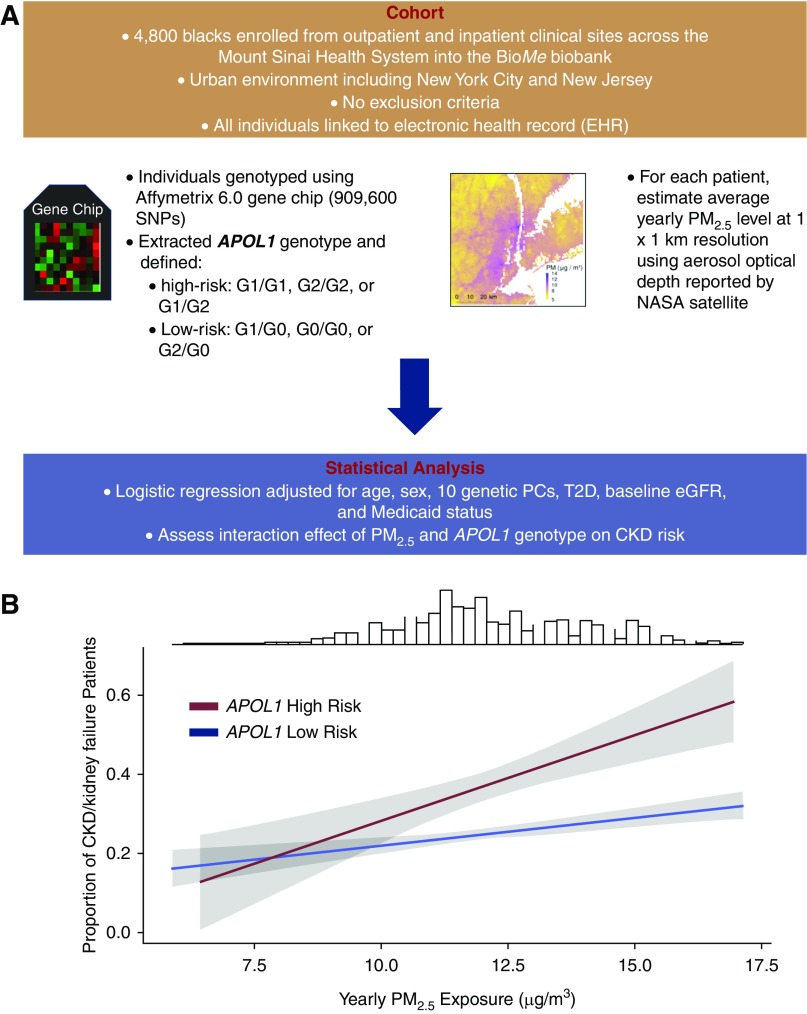
Figure 1.
Study methodology and APOL1 interaction for CKD risk. (A) Flowchart of methodology used in this study. (B) Proportion of CKD/kidney failure stratified by APOL1 genotype with higher average PM2.5 exposure in the year before enrollment. Shaded gray regions represent 95% confidence intervals obtained from a logistic regression model adjusted for age, sex, body mass index, 10 genetic principal components, history of type 2 diabetes, Medicaid status, and baseline eGFR. For purposes of visualization of the slopes, the values of continuous predictors are computed at the cohort mean of each covariate. Frequency distribution at top represents the distribution of mean PM2.5 in our cohort. NASA, National Aeronautics and Space Administration; PC, principal component; PM2.5, fine particulate matter <2.5 µm; T2D, type 2 diabetes.
Of 4800 blacks with 5.4 median follow-up years, 675 (14%) had APOL1 high-risk. There were 1286 blacks with the composite outcome and 293 with kidney failure; 253 (37%) individuals in the APOL1 high-risk group and 1033 (25%) in the low-risk group had the composite kidney outcome. Compared with low-risk APOL1 individuals, high-risk APOL1 individuals had a significantly lower eGFR at enrollment (75 versus 82 ml/min per 1.73 m2; P<0.001), higher proportion of kidney outcome (37% versus 25%; P<0.001), and hypertension (66% versus 61%; P=0.03). We evaluated statistical significance using the chi-squared test and t test for categorical and continuous variables, respectively. There were no significant differences in BMI (31.3 versus 30.7; P=0.08), age (51 versus 51 years; P=0.45), sex (63% versus 63% female; P=0.98), T2D (31% versus 29%; P=0.43), or PM2.5 (12.1 versus 12.2 μg/m3; P=0.61) concentrations between APOL1 high- and low-risk.
We investigated the APOL1–PM2.5 interaction using a logistic regression model adjusted for age, sex, ten genetic principal components, Medicaid status, T2D, and enrollment eGFR with an APOL1–PM2.5 interaction term to generate adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs). First, there was a significant association with outcome for PM2.5 and APOL1 individually. We found an increased aOR of 1.07 (95% CI, 1.01 to 1.15; P=0.02) for every 10 µg/m3 increase in PM2.5 and 1.12 for APOL1 high-risk (95% CI, 1.09 to 1.16; P=1.3×10−11). We also found a significant interaction between PM2.5 and APOL1 (P<0.001). In individuals with APOL1 high-risk, we observed increased aOR of 1.54 (95% CI, 1.32% to 1.80%; P=1.1×10−7) for every 10 µg/m3 increase in PM2.5. In contrast, in APOL1 low-risk, we found an increased aOR of 1.11 of kidney outcome (95% CI, 1.05 to 1.17; P=1.9×10−4) for every 10 µg/m3 increase in PM2.5. Thus, slope of PM2.5 exposure for APOL1 high-risk is steeper than that for low-risk individuals, suggesting a multiplicative interaction (Figure 1B).
This suggests that, although both PM2.5 and APOL1 independently increase kidney disease in blacks, the effect of PM2.5 is worse in APOL1 high-risk individuals. Thus, although APOL1 high-risk genetically predisposes individuals for kidney disease, environmental exposures may serve as a second hit that accentuates this. This may partially explain the incomplete penetrance of APOL1 wherein interplay between genetic and environmental factors influence kidney risk. Limitations include lack of replication, complete personal exposure data, enrollment albuminuria and detailed socioeconomic information. Additionally the cross-sectional nature of our study may not capture the effect of varying PM2.5 exposure over time. Further, as we defined the composite outcome as both prevalent/incident CKD and kidney failure, it is possible that PM2.5 exposure in the year before enrollment contributes more to incident than prevalent CKD. If replicated, it would represent the first example where a common genotype interacts with a common environmental exposure for kidney disease and exacerbates ethnic disparities.
In conclusion, in a cohort of 4800 blacks we demonstrated a significant interaction effect between APOL1 high-risk genotype and PM2.5 for kidney disease.
Disclosures
Dr. Nadkarni is cofounder of and owns equity in Pensieve Health and is a cofounder and member of scientific advisory board of and owns equity and options in RenalytixAI. Dr. Nadkarni also reports receiving consulting fees from AstraZeneca, BioVie, Inc., GLG Consulting, and Reata Pharmaceuticals. Dr. Bottinger, Dr. Chaudhary, Dr. Cooper, Dr. DeFelice, Dr. Glicksberg, Dr. Horowitz, Mr. Jaladanki, Dr. Just, Mr. Kapoor, Dr. Manna, Mr. O’Hagan, Mr. I. Paranjpe, and Mr. M. Paranjpe have nothing to disclose.
Funding
Dr. DeFelice is supported by grants from National Aeronautics and Space Administration, National Institute of Environmental Health Sciences (NIEHS), and NIEHS Environmental Influences on Child Health Outcomes. Dr. Just is supported by NIEHS grants P30ES023515 and R00ES023450. Dr. Nadkarni is supported by National Institute for Diabetes and Digestive and Kidney Diseases career development award K23DK107908.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Just AC, De Carli MM, Shtein A, Dorman M, Lyapustin A, Kloog I: Correcting measurement error in satellite aerosol optical depth with machine learning for modeling PM2.5 in the Northeastern USA. Remote Sens (Basel) 10: 803, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadkarni GN, Gottesman O, Linneman JG, Chase H, Berg RL, Farouk S, Nadukuru R, Lotay V, Ellis S, Hripcsak G, Peissig P, Weng C, Bottinger EP: Development and validation of an electronic phenotyping algorithm for chronic kidney disease. AMIA Annu Symp Proc 2014: 907–916, 2014 [PMC free article] [PubMed] [Google Scholar]