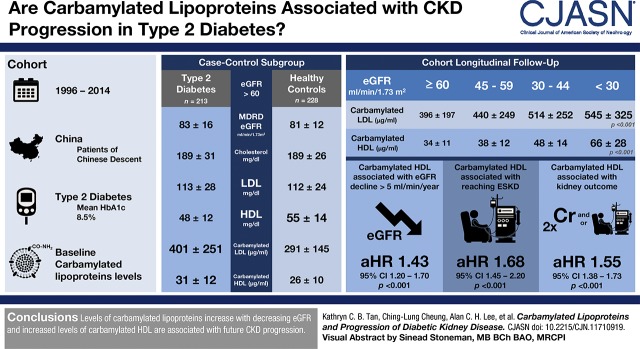
Visual Abstract
Keywords: diabetic nephropathy, progression of renal failure, humans, diabetic nephropathies, glomerular filtration rate, creatinine, case-control studies, HDL2 lipoproteins, HDL cholesterol, type 2 diabetes mellitus, urea, protein carbamylation, follow-up studies, LDL lipoproteins, chronic renal insufficiency, kidney, atherosclerosis, uremia, renal replacement therapy, regression analysis, enzyme-linked immunosorbent assay, cohort studies
Abstract
Background and objectives
Protein carbamylation is a consequence of uremia and carbamylated lipoproteins contribute to atherogenesis in CKD. Proteins can also be carbamylated by a urea-independent mechanism, and whether carbamylated lipoproteins contribute to the progression of CKD has not been investigated.
Design, setting, participants, & measurements
A case-control study was performed to determine whether there were changes in plasma levels of carbamylated lipoproteins in individuals with type 2 diabetes with eGFR >60 ml/min per 1.73 m2 compared with a group of age- and sex-matched healthy controls. A cohort of 1320 patients with type 2 diabetes with baseline eGFR ≥30 ml/min per 1.73 m2 was longitudinally followed up to evaluate the association between carbamylated lipoproteins and progression of CKD. The primary kidney outcome was defined as doubling of serum creatinine and/or initiation of KRT during follow-up. Plasma carbamylated LDLs and HDLs was measured by ELISA.
Results
In individuals with diabetes with eGFR >60 ml/min per 1.73 m2, both plasma carbamylated LDL and HDL levels were higher compared with healthy controls (P<0.001). After a mean follow-up of 9 years of the diabetic cohort, individuals in the top quartile of carbamylated LDL (hazard ratio, 2.21; 95% confidence interval, 1.42 to 3.46; P<0.001) and carbamylated HDL (hazard ratio, 4.53; 95% confidence interval, 2.87 to 7.13; P<0.001) had higher risk of deterioration of kidney function compared with those in the lowest quartile. On multivariable Cox regression analysis, plasma carbamylated LDL was no longer associated with kidney outcome after adjusting for baseline eGFR and potential confounding factors. However, the association between plasma carbamylated HDL and kidney outcome remained significant and was independent of HDL cholesterol.
Conclusions
Plasma carbamylated HDL but not carbamylated LDL was independently associated with progression of CKD in patients with type 2 diabetes.
Introduction
Carbamylation is a post-translational modification of proteins owing to the spontaneous nonenzymatic reaction between isocyanic acid and specific free functional groups of proteins. During the process, the “carbamoyl” moiety (−CONH2) is added to the amino terminus residues of protein like lysine, leading to the formation of ε-carbamyl-lysine (homocitrulline). This results in alterations in the structural and functional properties of proteins. Carbamylation of proteins can cause partial or complete loss of function and/or trigger specific inappropriate and potentially pathologic cellular responses (1). Because isocyanic acid is predominantly formed by the decomposition of urea, proteins are subjected to increased carbamylation in patients with CKD and plasma levels of isocyanic acid can be increased up to three-fold in patients with uremia (2,3). Wang et al. (4) have recently shown that proteins can also be carbamylated by a urea-independent mechanism. Decomposition of urea is not the only source of isocyanic acid formation and cyanate can be formed during the metabolism of thiocyanate by myeloperoxidase. In the presence of hydrogen peroxide, myeloperoxidase catalyzes thiocyanate oxidation to form cyanate, which is then rapidly converted to its reactive form, isocyanic acid. Myeloperoxidase is released from activated leukocytes during inflammation, and Wang et al. (4) showed that myeloperoxidase played a significant role in protein carbamylation at sites of inflammation and atherosclerotic plaques. It has been suggested that carbamylated proteins are involved in the progression of various chronic diseases, in particular kidney and cardiovascular disorders (5).
Lipoproteins are subjected to carbamylation in the circulation and, in patients with ESKD, plasma levels of carbamylated LDLs and carbamylated HDLs are elevated and may cause detrimental cardiovascular effects (6,7). Carbamylation of lipoproteins is a major contributory factor of atherosclerosis in patients with CKD, and carbamylated LDL is a nontraditional risk factor for cardiovascular events in these patients (8). Although carbamylation of lipoproteins is a consequence of kidney disease, whether carbamylated lipoproteins may also contribute to the pathogenesis and progression of kidney disease has not been investigated. It has been suggested that changes in lipid metabolism may play a role in the development of kidney disease (9). We have previously shown that in patients with type 2 diabetes without kidney impairment, carbamylation of LDL was increased and carbamylation was mainly driven by elevated myeloperoxidase activity (10). However, whether plasma carbamylated HDL is higher in patients with type 2 diabetes without kidney impairment is unclear because patients with type 2 diabetes tend to have low plasma HDL and apoA concentrations. In this study, we aim to investigate whether carbamylated lipoproteins are elevated in patients with type 2 diabetes with and without kidney impairment and to determine whether carbamylated lipoproteins are associated with the progression of CKD.
Materials and Methods
To evaluate the relationship between plasma carbamylated lipoproteins and kidney function in patients with type 2 diabetes, plasma carbamylated LDL and carbamylated HDL was measured in baseline samples from a cohort of individuals with type 2 diabetes who were being prospectively followed up to study the pathogenesis and progression of complications in type 2 diabetes in Chinese patients. Details of the cohort has previously been reported (11). In brief, the cohort was recruited from 1996 to 2014 and individuals with type 2 diabetes were invited to participate on their first clinic visit and major exclusion criteria were non-Chinese descent, type 1 diabetes, malignancy or major illness with limited life expectancy, any hospitalization in the preceding 3 months, or unwilling to return for regular follow-up. Clinical data and fasting blood samples were collected at baseline. In this study, plasma carbamylated LDL and carbamylated HDL was determined in all participants with available stored blood samples and follow-up data. A subgroup of participants with type 2 diabetes with eGFR >60 ml/min per 1.73 m2 were randomly chosen and compared with a group of controls matched for age and sex, using a case-control design to address the issue whether plasma levels of carbamylated LDL and carbamylated HDL were higher in individuals with diabetes without kidney impairment. Healthy individuals without medical illness and not receiving medications were recruited as control from the community by advertisement. Informed consent was obtained from all participants and the study was approved by the Ethics Committee of the University of Hong Kong (approval number UW14–040).
Data collected from the longitudinal follow-up of the cohort were used to further investigate whether plasma carbamylated lipoproteins were associated with progression of kidney disease in patients with type 2 diabetes. Participants with eGFR <30 ml/min per 1.73 m2 and/or on KRT at baseline were excluded from the analysis because we intended to evaluate the association between carbamylated lipoproteins and kidney disease progression in patients with diabetes with less advanced kidney disease. Primary kidney outcome was defined as a doubling of serum creatinine from baseline and/or initiation of KRT. Rapid kidney progression was a secondary outcome and was defined as a rate of eGFR decline >5 ml/min per year. Length of follow-up was calculated as the time from baseline examination to the date of doubling of creatinine, date of dialysis or death, or last follow-up as per the censoring date of January 31, 2017, whichever was earliest. Serum creatinine was measured annually during follow-up and more frequently as clinically indicated in those with eGFR <60 ml/min per 1.73 m2.
Plasma levels of lipids, carbamylated LDL and carbamylated HDL, glucose, hemoglobin A1c (HbA1c), and creatinine were measured in fasting blood samples taken at baseline. Serum creatinine was measured by the Jaffe method and eGFR was calculated by Modification of Diet in Renal Disease Study equation. Albuminuria status was determined by urine albumin-to-creatinine ratio from at least two random urine samples collected on two separate occasions within 6 months. Plasma total cholesterol and triglyceride was determined enzymatically, and HDL cholesterol was measured using a homogenous method with polyethylene glycol–modified enzymes and α-cyclodextrin. LDL cholesterol was calculated by the Friedewald equation or measured directly if plasma triglyceride was >399 mg/dl.
Plasma carbamylated LDL concentration was measured using an in-house sandwich ELISA with polyclonal rabbit anti-human carbamylated LDL antibody as the capture antibody, as previously described (10). Each sample was assayed in triplicates and results were expressed as microgram of carbamylated LDL per milliliter of plasma. The intra- and interassay coefficients of variation were 5.2% and 9.3%, respectively. To measure plasma level of carbamylated HDL, polyclonal rabbit anti-human carbamylated HDL antibody was raised with carbamylated HDL (Alfa Aesar, Lancashire, UK) as immunogen by a similar protocol used to develop polyclonal rabbit anti-human carbamylated LDL antibody (10). Plasma carbamylated HDL was assayed by sandwich ELISA using polyclonal rabbit anti-human carbamylated HDL antibody as the capture antibody and polyclonal goat anti-human ApoA1-HRP antibody as the secondary detecting antibody. A 100-µl aliquot of 2.5 µg/ml of polyclonal rabbit anti-human carbamylated HDL antibody was coated overnight at 4°C in pH 9.6 bicarbonate coating buffer. On the following day, the plates were rinsed five times with washing buffer (0.1% Tween 20 and PBS) followed by blockade of nonspecific binding with 5% (w/v) BSA/PBS for 2 hours at room temperature. A 100-µl aliquot at a 1:100 dilution of plasma was added for a 2-hour incubation at room temperature. After five washes, 100 µl of 1:1000 dilution of polyclonal goat anti-human ApoA1-horseradish peroxidase antibody (1:1000; Biodesign) was added and incubated for 1 hour at room temperature. At the end of the incubation, five washes were applied before 100 µl per well of 3,3′,5,5′-tetramethylbenzidine substrate (TMB substrate kit; Pierce) was added for 20 minutes followed with 50 μl per well of 2 M H2SO4 as a stop solution. The concentration of plasma carbamylated HDL was calculated using a 450 -nm absorbance standard curve of purified human carbamylated HDL purchased from Alfa Aesar. Each sample was assayed in triplicate, and results were expressed as microgram per milliliter carbamylated HDL. The intra- and interassay coefficients of variation were 6.8% and 9.6%, respectively.
Results were expressed as mean and SD or as median and interquartile range if the data were not normally distributed. Skewed data were logarithmically transformed before analyses were made. Comparisons between two groups and multiple groups were done using independent sample t test and ANOVA, respectively, and trend-test was used to look for a trend effect of the variable on the outcome. Pearson correlation coefficient was used to test the relationships between variables. Multivariable Cox regression analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs). The variables included in Cox regression models were those that were statistically significant in unadjusted analysis or were biologically relevant. Participants with no available baseline serum samples or those with missing follow-up data were excluded in the analysis.
Results
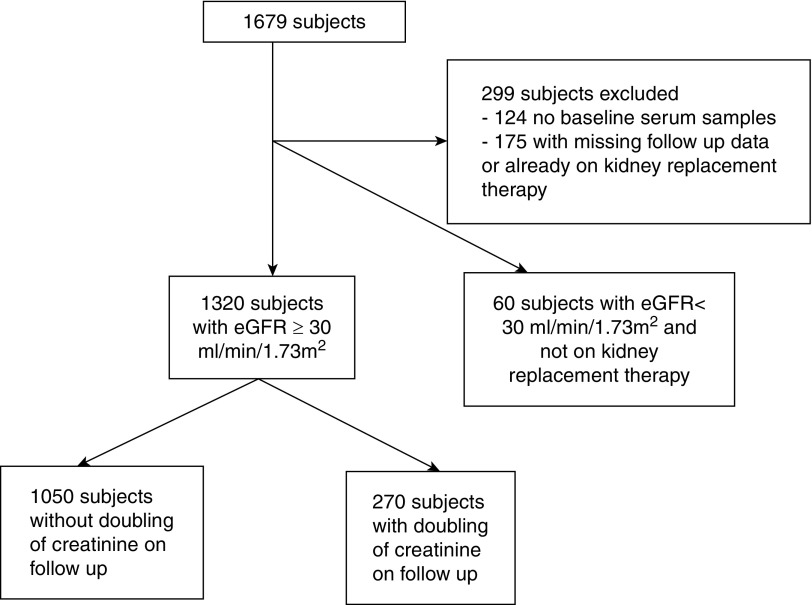
Out of the 1679 participants with diabetes who were recruited, plasma carbamylated LDL and carbamylated HDL was measured in 1380 patients with available blood samples and follow-up data (Figure 1). There was no sex difference in plasma carbamylated LDL but men had higher plasma carbamylated HDL than women (38±15 µg/ml versus 36±14 µg/ml; P=0.01). Participants were divided according to eGFR and the results are shown in Table 1. As expected, there was a progressive increase in plasma levels of carbamylated lipoproteins as kidney function worsened (Ptrend<0.001), and both plasma carbamylated LDL (r=−0.20; P<0.001) and carbamylated HDL (r=−0.29; P<0.001) correlated with eGFR. Plasma carbamylated LDL (r=0.13; P<0.001) and carbamylated HDL (r=0.18; P<0.001) also correlated with age but not with body mass index (BMI), HbA1c, or triglyceride. There were no significant correlations between plasma carbamylated LDL and LDL cholesterol or between carbamylated HDL and HDL cholesterol. To determine whether plasma levels of carbamylated LDL and carbamylated HDL were higher in patients with normal kidney function, plasma carbamylated LDL and carbamylated HDL levels in a random subgroup of patients with eGFR >60 ml/min per 1.73 m2 and not receiving any lipid-lowering therapy were compared with levels in a group of nondiabetic controls matched for age and sex using a case-control design. Although patients with diabetes had similar eGFR and LDL cholesterol and lower HDL cholesterol than controls (P<0.001), both plasma carbamylated LDL and carbamylated HDL was significantly higher (P<0.001) (Table 2). These differences remained significant even after adjusting for BMI.
Figure 1.
Flow chart describing the study participants.
Table 1.
Clinical characteristics and plasma carbamylated lipoproteins in individuals with diabetes according to baseline eGFR
| Characteristic | eGFR, ml/min per 1.73 m2 | |||
|---|---|---|---|---|
| ≥60 | ≥45 to <60 | ≥30 to <45 | <30 | |
| N | 1032 | 161 | 127 | 60 |
| Age, yr | 53±9 | 61±8 | 63±9 | 62±8 |
| Men/women, % | 51/49 | 54/46 | 65/35 | 72/28 |
| Duration of diabetes, yr | 12±7 | 14±8 | 16±9 | 15±8 |
| BMI, kg/m2 | 26±4 | 26±4 | 27±4 | 27±4 |
| Smoker, % | 10 | 7 | 8 | 13 |
| Hypertension, % | 59 | 83 | 99 | 98 |
| Normo/micro/macroalbuminuria, % | 60/31/9 | 32/39/29 | 10/30/60 | 0/13/87 |
| Retinopathy, % | 35 | 62 | 72 | 78 |
| Cardiovascular disease, % | 8 | 22 | 31 | 42 |
| ACEI/ARB, % | 41 | 54 | 70 | 53 |
| Lipid-lowering therapy, % | 21 | 38 | 58 | 68 |
| Systolic BP, mm Hg | 130±19 | 139±23 | 144±28 | 147±21 |
| Diastolic BP, mm Hg | 77±10 | 76±9 | 77±11 | 75±11 |
| Fasting glucose, mg/dl | 157±48 | 148±51 | 161±72 | 148±53 |
| HbA1c, % | 8.5±1.6 | 8.5±1.4 | 8.7±1.7 | 8.7±1.6 |
| eGFR, ml/min per 1.73 m2 | 83±16 | 54±4 | 38±4 | 22±6 |
| Total cholesterol, mg/dl | 188±39 | 187±46 | 185±42 | 180±42 |
| Triglyceride, mg/dl | 115 (80–177) | 133 (89–177) | 151 (106–221) | 142 (106–217) |
| LDL cholesterol, mg/dl | 112±74 | 110±38 | 105±38 | 101±36 |
| HDL cholesterol, mg/dl | 48±13 | 47±14 | 44±12 | 44±16 |
| Carbamylated LDL, µg/ml | 397 (269–409) | 433 (249–525) | 502 (306–612) | 535 (304–650) |
| Carbamylated HDL, µg/ml | 34±11 | 38±12 | 48±14 | 66±28 |
Data are expressed as mean±SD or median (interquartile range). BMI, body mass index; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HbA1c, hemoglobin A1c.
Table 2.
Clinical characteristics and plasma carbamylated lipoproteins in individuals with diabetes with eGFR >60 ml/min per 1.73 m2 and the nondiabetic control group
| Characteristic | Control | Diabetes |
|---|---|---|
| N | 228 | 213 |
| Age, yr | 48±7 | 48±6 |
| Men/women, % | 50/50 | 49/51 |
| BMI, kg/m2 | 25±3 | 26±4 |
| Smoker, % | 14 | 12 |
| Duration of diabetes, yr | — | 9 (6–14) |
| Lipid-lowering therapy, % | — | 0 |
| Fasting glucose, mg/dl | 87±9 | 158±55 |
| HbA1c, % | 5.6±0.5 | 8.5±1.6 |
| eGFR, ml/min per 1.73 m2 | 81±12 | 83±16 |
| Total cholesterol, mg/dl | 189±26 | 189±31 |
| Triglyceride, mg/dl | 89 (62–124) | 115 (80–177) |
| LDL cholesterol, mg/dl | 112±24 | 113±28 |
| HDL cholesterol, mg/dl | 55±14 | 48±12 |
| Carbamylated LDL, µg/ml | 267 (171–388) | 340 (214–548) |
| Carbamylated HDL, µg/ml | 26±10 | 31±12 |
Data are expressed as mean±SD or median (interquartile range). BMI, body mass index; —, not applicable; HbA1c, hemoglobin A1c.
To investigate whether plasma lipids and plasma carbamylated lipoproteins were associated with progression of kidney disease, the diabetic cohort was prospectively followed up. Participants with eGFR <30 ml/min per 1.73 m2 and/or on KRT at baseline and those with missing follow-up data were excluded (n=299), and data from 1320 individuals were available for analysis (Figure 1). Doubling of serum creatinine was observed in 270 individuals over a mean follow-up period of 9±5 years (71 patients required dialysis and six received kidney transplant). The mortality rate of the whole cohort was 8.3 per 1000 patient-years. Patients with doubling of serum creatinine had higher baseline plasma triglyceride (142 mg/dl [97–216], median [interquartile range], versus 115 mg/dl [80–168]; P<0.001) and LDL cholesterol (119±38 mg/dl versus 109±35 mg/dl; P<0.001) but lower HDL cholesterol (45±12 mg/dl versus 48±13 mg/dl; P=0.002) than those without doubling of creatinine. Both baseline plasma carbamylated LDL (455±222 µg/ml versus 398±201 µg/ml; P<0.001) and carbamylated HDL (46±13 µg/ml versus 33±11 µg/ml; P<0.001) was significantly higher in the group with doubling of serum creatinine even after adjusting for age, sex, BMI, HbA1c, eGFR, smoking, duration of diabetes, albuminuria status, and lipid-lowering therapy.
Neither baseline LDL cholesterol nor triglyceride was associated with doubling of serum creatinine. Serum HDL cholesterol was associated with kidney outcome and individuals with diabetes with HDL cholesterol in the top quartile had significantly lower risk of doubling of serum creatinine than those in the first quartile (HR, 0.63; 95% CI, 0.45 to 0.90; P=0.01). However, the association was no longer significant after adjusting for baseline eGFR, albuminuria status, age, sex, BMI, duration of diabetes, HbA1c, smoking, systolic BP, ACEI/ARB, and lipid-lowering therapy. Baseline clinical characteristics of the patients with diabetes, stratified by quartiles of carbamylated LDL and HDL, are shown in Supplemental Tables 1 and 2, respectively. There was a graded association between increasing quartiles of carbamylated LDL and carbamylated HDL and kidney outcome, and individuals in the top quartile had the highest risk (Table 3). Per 1-SD change in carbamylated LDL and carbamylated HDL, the HR was 1.29 (95% CI, 1.17 to 1.43; P<0.001) and 1.94 (95% CI, 1.78 to 2.12; P<0.001), respectively, for the primary kidney outcome. On multivariable Cox regression analysis, the association between plasma carbamylated LDL and doubling of serum creatinine was no longer significant after adjusting for baseline clinical characteristics, eGFR, and potential confounding factors (Supplemental Table 3). Nevertheless, the association between plasma carbamylated HDL and kidney outcome was still significant, and further adjusting for HDL cholesterol and LDL cholesterol did not change the result (Table 4). This would suggest that the association between carbamylated HDL and kidney outcome was independent of HDL cholesterol. Inclusion of carbamylated LDL in the model also did not affect the result and there was no interaction between carbamylated HDL and carbamylated LDL on kidney outcome.
Table 3.
Associations of quartiles of plasma carbamylated lipoproteins with primary and secondary kidney outcomes
| Quartile | Doubling of Serum Creatinine | Rapid Kidney Progression | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Events | HR (95% CI) | P Value | Ptrend | No. of Events | HR (95% CI) | P Value | Ptrend | |
| Carbamylated LDL | ||||||||
| <274 µg/ml | 43 | Reference | <0.001 | 36 | Reference | 0.07 | ||
| ≥274 and <397 µg/ml | 57 | 1.25 (0.77 to 2.03) | 0.37 | 42 | 0.86 (0.58 to 1.26) | 0.43 | ||
| ≥397 and <459 µg/ml | 78 | 1.96 (1.25 to 3.09) | 0.004 | 51 | 1.29 (0.71 to 2.47) | 0.10 | ||
| ≥459 µg/ml | 92 | 2.21 (1.42 to 3.46) | <0.001 | 54 | 1.38 (0.93 to 2.05) | 0.11 | ||
| Carbamylated HDL | ||||||||
| <29 µg/ml | 39 | Reference | <0.001 | 35 | Reference | 0.005 | ||
| ≥29 and <33 µg/ml | 43 | 1.04 (0.59 to 1.84) | 0.69 | 40 | 0.97 (0.60 to 1.42) | 0.65 | ||
| ≥33 and <42 µg/ml | 70 | 1.65 (1.06 to 2.76) | 0.02 | 47 | 1.13 (0.66 to 1.94) | 0.35 | ||
| ≥42 µg/ml | 118 | 4.53 (2.87 to 7.13) | <0.001 | 61 | 1.98 (1.19 to 3.29) | 0.01 | ||
HR, hazard ratio; 95% CI, 95% confidence interval.
Table 4.
Associations between plasma carbamylated HDL and primary and secondary kidney outcomes
| Parameter | Doubling of Serum Creatinine | Rapid Kidney Progression | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Carbamylated HDL, µg/ml | 1.55 (1.39 to 1.73) | <0.001 | 1.55 (1.38 to 1.73) | <0.001 | 1.43 (1.20 to 1.70) | <0.001 | 1.43 (1.20 to 1.70) | <0.001 |
| Age, yr | 1.34 (1.12 to 1.59) | 0.001 | 1.34 (1.12 to 1.60) | 0.001 | 1.22 (0.96 to 1.55) | 0.10 | 1.23 (0.97 to 1.57) | 0.09 |
| Sex, men/women | 0.68 (0.52 to 0.89) | 0.005 | 0.69 (0.53 to 0.91) | 0.009 | 1.09 (0.74 to 1.59) | 0.67 | 1.16 (0.78 to 1.71) | 0.46 |
| BMI, kg/m2 | 1.27 (1.12 to 1.44) | <0.001 | 1.26 (1.10 to 1.43) | 0.001 | 0.99 (0.82 to 1.19) | 0.90 | 0.94 (0.78 to 1.14) | 0.50 |
| Duration of diabetes, yr | 0.97 (0.86 to 1.10) | 0.62 | 0.97 (0.86 to 1.10) | 0.68 | 0.95 (0.79 to 1.15) | 0.60 | 0.96 (0.79 to 1.16) | 0.96 |
| Smoker, no/yes | 1.38 (0.89 to 2.14) | 0.15 | 1.38 (0.89 to 2.15) | 0.15 | 1.55 (0.86 to 2.86) | 0.15 | 1.47 (0.81 to 2.66) | 0.21 |
| Systolic BP, mm Hg | 1.18 (1.04 to 1.34) | 0.01 | 1.18 (1.04 to 1.34) | 0.01 | 1.52 (1.24 to 1.85) | <0.001 | 1.50 (1.22 to 1.83) | <0.001 |
| HbA1c, % | 1.29 (1.14 to 1.47) | <0.001 | 1.29 (1.14 to 1.47) | <0.001 | 1.41 (1.19 to 1.67) | <0.001 | 1.43 (1.21 to 1.70) | <0.001 |
| Baseline eGFR, ml/min per 1.73 m2 | 0.52 (0.43 to 0.63) | <0.001 | 0.53 (0.44 to 0.63) | <0.001 | 0.84 (0.71 to 0.98) | 0.03 | 0.85 (0.72 to 0.99) | 0.04 |
| Normoalbuminuria | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 | Reference | <0.001 |
| Microalbuminuria | 2.64 (1.85 to 3.78) | <0.001 | 2.65 (1.84 to 3.80) | <0.001 | 3.20 (2.01 to 5.09) | <0.001 | 3.27 (2.04 to 5.24) | <0.001 |
| Macroalbuminuria | 8.13 (5.47 to 12.07) | <0.001 | 8.29 (5.55 to 12.35) | <0.001 | 15.12 (8.79 to 26.0) | <0.001 | 16.05 (9.25 to 27.85) | <0.001 |
| ACEI/ARB, no/yes | 1.11 (0.86 to 1.45) | 0.42 | 1.12 (0.85 to 1.46) | 0.43 | 1.11 (0.77 to 1.61) | 0.58 | 1.11 (0.76 to 1.61) | 0.61 |
| Lipid-lowering therapy, no/yes | 1.28 (0.96 to 1.52) | 0.11 | 1.23 (0.97 to 1.56) | 0.08 | 1.13 (0.81 to 1.58) | 0.47 | 1.13 (0.80 to 1.61) | 0.49 |
| LDL cholesterol, mg/dl | — | 1.04 (0.92 to 1.19) | 0.54 | — | 1.03 (0.85 to 1.24) | 0.80 | ||
| HDL cholesterol, mg/dl | — | 0.95 (0.83 to 1.09) | 0.49 | — | 0.82 (0.63 to 1.01) | 0.05 | ||
The given hazard ratio is for 1-SD change in plasma carbamylated HDL and continuous covariates at baseline. Model 1: adjusted for carbamylated HDL, age, sex, BMI, duration of diabetes, smoking, systolic BP, HbA1c, baseline eGFR, albuminuria status, ACEI/ARB therapy, and lipid-lowering therapy. Model 2: further adjusted for LDL cholesterol and HDL cholesterol. HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index; HbA1c, hemoglobin A1c; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; —, not applicable.
In addition, we evaluated whether carbamylated lipoproteins were associated with rapid kidney progression (defined as a rate of eGFR decline >5 ml/min per year) that was observed in 183 participants. Carbamylated HDL was significantly associated with rapid progression in the fully adjusted model (HR, 1.43; 95% CI, 1.20 to 1.70; P<0.001) (Table 4). Furthermore, 77 participants needed KRT and carbamylated HDL was also associated with the risk of entering ESKD requiring KRT (HR, 1.68; 95% CI, 1.45 to 2.20; P<0.001). There was no relationship between carbamylated LDL and rapid kidney progression or the risk of entering ESKD. The mean HbA1c level of our diabetic cohort was 8.5% and glycemic control was suboptimal. To determine whether the relationship between carbamylated HDL and the primary kidney outcome was still significant in patients with good glycemic control, the data were analyzed after excluding patients with HbA1c>7.5% and the HR was 1.60 (95% CI, 1.26 to 2.03; P<0.001) in the fully adjusted model.
Discussion
Several studies have shown that elevated levels of carbamylated proteins are associated with adverse prognosis and higher mortality in patients with and without impaired kidney function and in patients with chronic heart failure (4,12–15). Because measures of systemic protein carbamylation burden may be associated with cardiovascular and mortality outcomes, it has been suggested that select carbamylated proteins can serve as useful biomarkers of disease and the presence of kidney impairment is not necessarily a prerequisite (4,5). Thus far, all of the studies have evaluated the role of carbamylated proteins on cardiovascular end points and mortality, but there are no available data on the relationship between carbamylated lipoproteins and the progression of diabetic kidney disease. Our study is the first prospective study to investigate the association between plasma carbamylated lipoproteins and CKD in patients with type 2 diabetes. First of all, we have shown that both plasma carbamylated LDL and carbamylated HDL was higher even in individuals with type 2 diabetes without CKD (eGFR >60 ml/min per 1.73 m2). This is in keeping with our previous study showing that increased carbamylation of LDL in patients with type 2 diabetes with normal kidney function was driven by myeloperoxidase activity owing to their elevated levels of myeloperoxidase (10). As expected, the highest plasma levels of carbamylated lipoproteins were seen in individuals with diabetes with advanced CKD, because protein carbamylation was predominantly driven by urea in these individuals.
We have further demonstrated that plasma carbamylated lipoproteins were associated with loss of kidney function in our cohort of patients with type 2 diabetes. The majority of previous studies have focused on the vascular effects of carbamylated lipoproteins and the process of carbamylation increases the atherogenic properties of LDL and impairs the protective function of HDL (8,16,17). Our data show that mainly carbamylated HDL level was associated with the progression of CKD, as plasma carbamylated HDL but not carbamylated LDL remained an independent determinant of kidney decline even after adjustment for baseline eGFR, albuminuria status, and other potential confounding factors. This would suggest that plasma carbamylated LDL only reflected kidney function, whereas plasma carbamylated HDL might probably have a pathogenic role. This is in keeping with the results of a recent Mendelian randomization study showing that only HDL was associated with kidney function. Analysis of data from the largest lipid and CKD cohorts demonstrated that genetically higher HDL cholesterol concentration was causally associated with better kidney function, but there was no association between genetically altered LDL cholesterol or triglyceride concentration and kidney function (18).
There is substantial evidence suggesting that altered HDL metabolism may contribute to the pathogenesis and progression of kidney disease. Recent studies have demonstrated a significant association between low HDL cholesterol levels and risks of incident CKD and CKD progression (19,20). In patients with type 2 diabetes from the Action in Diabetes and Vascular Disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study, low HDL cholesterol level was shown to be an independent risk factor for the development and progression of diabetic nephropathy (21). A global case-control study also showed an association between HDL cholesterol with kidney disease in type 2 diabetes (22). We have found that carbamylated HDL was associated with the progression of CKD independent of HDL cholesterol in our study. It has been shown that carbamylated HDL is a better metric of HDL function than HDL cholesterol. Carbamylation of HDL leads to HDL dysfunction and results in a loss of anti-inflammatory and antioxidative properties of HDL (7,23). Carbamylated HDL is also defective in promoting reverse cholesterol transport and facilitates cholesterol accumulation in cells and tissues (24,25). Although the pathophysiologic process linking dyslipidemia to diabetic kidney disease is still unclear, one hypothesis is that dysfunctional HDL particles impair reverse cholesterol transport in the kidney and contribute to intrarenal lipid accumulation, leading to glomerulosclerosis and tubulointerstitial damage (9,26). Hence, the production of nonfunctioning HDL particles by carbamylation may therefore also be a cause and a consequence of CKD. Whether carbamylated lipoproteins (especially carbamylated HDL) are involved in the pathogenesis and progression of diabetic kidney disease or are just markers of CKD warrants further investigation.
Our study has several limitations. The main limitation is that we have not performed any functional measurement of HDL particles, like cholesterol efflux capacity. Experimental studies have shown that carbamylation of HDL affects multiple functions of HDL (7,17,23–25). We do not have serial measurement of carbamylated lipoproteins over time, and changes in treatment like the subsequent initiation of lipid-lowering therapy and progression of disease were not taken into account. Furthermore, kidney biopsy was only performed in a small number of participants to ascertain the definitive cause of diabetic kidney disease. Another limitation is that our cohort of patients is likely to be more complex than those in primary care or in the general population because our unit is a secondary/tertiary referral center, and we cannot completely exclude selection bias in the recruitment process. Hence, our results may not be generalized to other patient populations. Missing baseline samples and follow-up data reduced the sample size for analysis and may also bias our results, but there were no significant differences between those with complete data and those with missing data.
In conclusion, both plasma carbamylated LDL and carbamylated HDL were significantlyhigher in individuals with type 2 diabetes with and without kidney impairment. Only plasma carbamylated HDL but not carbamylated LDL was independently associated with progression of kidney disease in type 2 diabetes.
Disclosures
Dr. Cheung, Dr. Lam, Dr. Lee, Dr. Shiu, Prof. Tan, and Dr. Wong have nothing to disclose.
Funding
This study is supported by an Endowment Fund established for the Sir David Todd Professorship in Medicine, University of Hong Kong, awarded to Prof. Tan.
Supplementary Material
Acknowledgments
Prof. Tan designed and oversaw the study, data collection, analysis and manuscript writing. Dr. Cheung contributed to study design and analyzed the data. Dr. Shiu performed the laboratory assays and analyzed the data. Dr. Lam, Dr. Lee, and Dr. Wong recruited the subjects and collected clinical data. All authors approved the final version of the manuscript. Prof. Tan is the guarantor of this work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11710919/-/DCSupplemental.
Supplemental Table 1. Clinical characteristics of individuals with diabetes according to quartiles of carbamylated LDL.
Supplemental Table 2. Clinical characteristics of individuals with diabetes according to quartiles of carbamylated HDL.
Supplemental Table 3. Association between plasma carbamylated LDL and primary kidney outcome.
References
- 1.Jaisson S, Pietrement C, Gillery P: Protein carbamylation: Chemistry, pathophysiological involvement, and biomarkers. Adv Clin Chem 84: 1–38, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson L, Lundquist P, Kågedal B, Larsson R: Plasma cyanate concentrations in chronic renal failure. Clin Chem 42: 482–483, 1996. [PubMed] [Google Scholar]
- 3.Kalim S, Karumanchi SA, Thadhani RI, Berg AH: Protein carbamylation in kidney disease: Pathogenesis and clinical implications. Am J Kidney Dis 64: 793–803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Delanghe S, Delanghe JR, Speeckaert R, Van Biesen W, Speeckaert MM: Mechanisms and consequences of carbamoylation. Nat Rev Nephrol 13: 580–593, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG: Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol 21: 1852–1857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JT, Yang K, Lu L, Zhu ZB, Zhu JZ, Ni JW, Han H, Chen N, Zhang RY: Increased carbamylation level of HDL in end-stage renal disease: Carbamylated-HDL attenuated endothelial cell function. Am J Physiol Renal Physiol 310: F511–F517, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Apostolov EO, Basnakian AG, Ok E, Shah SV: Carbamylated low-density lipoprotein: Nontraditional risk factor for cardiovascular events in patients with chronic kidney disease. J Ren Nutr 22: 134–138, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Wahl P, Ducasa GM, Fornoni A: Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol 310: F433–F445, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiu SW, Xiao SM, Wong Y, Chow WS, Lam KS, Tan KC: Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin Sci (Lond) 126: 175–181, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM: Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia 61: 1212–1219, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL: Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol 24: 853–861, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang WH, Shrestha K, Wang Z, Borowski AG, Troughton RW, Klein AL, Hazen SL: Protein carbamylation in chronic systolic heart failure: Relationship with renal impairment and adverse long-term outcomes. J Card Fail 19: 219–224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drechsler C, Kalim S, Wenger JB, Suntharalingam P, Hod T, Thadhani RI, Karumanchi SA, Wanner C, Berg AH: Protein carbamylation is associated with heart failure and mortality in diabetic patients with end-stage renal disease. Kidney Int 87: 1201–1208, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaisson S, Kerkeni M, Santos-Weiss IC, Addad F, Hammami M, Gillery P: Increased serum homocitrulline concentrations are associated with the severity of coronary artery disease. Clin Chem Lab Med 53: 103–110, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Sirpal S: Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 116: 681–695, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Santana JM, Brown CD: High-density lipoprotein carbamylation and dysfunction in vascular disease. Front Biosci 23: 2227–2234, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Lanktree MB, Thériault S, Walsh M, Paré G: HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: A Mendelian randomization study. Am J Kidney Dis 71: 166–172, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Baragetti A, Norata GD, Sarcina C, Rastelli F, Grigore L, Garlaschelli K, Uboldi P, Baragetti I, Pozzi C, Catapano AL: High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med 274: 252–262, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z: Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 89: 886–896, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Morton J, Zoungas S, Li Q, Patel AA, Chalmers J, Woodward M, Celermajer DS, Beulens JW, Stolk RP, Glasziou P, Ng MK; ADVANCE Collaborative Group : Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: Results of the ADVANCE study. Diabetes Care 35: 2201–2206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, Wanner C, Al-Rubeaan K, Aronson R, Barzon I, Bishop L, Bonora E, Bunnag P, Chuang LM, Deerochanawong C, Goldenberg R, Harshfield B, Hernández C, Herzlinger-Botein S, Itoh H, Jia W, Jiang YD, Kadowaki T, Laranjo N, Leiter L, Miwa T, Odawara M, Ohashi K, Ohno A, Pan C, Pan J, Pedro-Botet J, Reiner Z, Rotella CM, Simo R, Tanaka M, Tedeschi-Reiner E, Twum-Barima D, Zoppini G, Carey VJ: Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: A global case-control study in 13 countries. Circulation 129: 999–1008, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, Koefeler H, Beubler E, Schuligoi R, Heinemann A, Marsche G: Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid Redox Signal 14: 2337–2346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, Schuligoi R, Heinemann A, Marsche G: Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: Novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal 17: 1043–1052, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JL, Gautier T, Nijstad N, Tölle M, Schuchardt M, van der Giet M, Tietge UJ: High density lipoprotein (HDL) particles from end-stage renal disease patients are defective in promoting reverse cholesterol transport. Sci Rep 7: 41481, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaziri ND: Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J Ren Nutr 20[Suppl]: S35–S43, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.