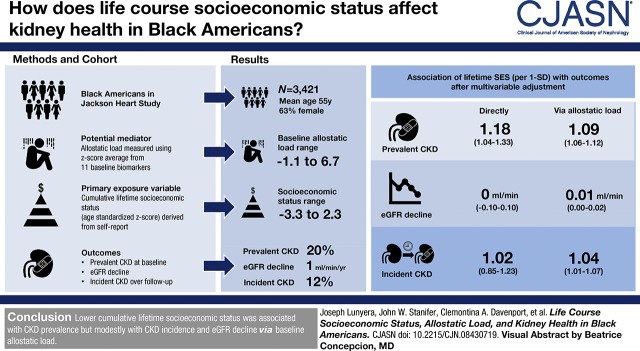
Visual Abstract
Keywords: female, humans, child, United States, incidence, allostasis, prevalence, self report, follow-up studies, social class, income, glomerular filtration rate, kidney, biomarkers, chronic renal insufficiency, longitudinal studies, socioeconomic status, life course, allostatic load, kidney diseases, kidney disease, African-Americans
Abstract
Background and objectives
Low socioeconomic status confers unfavorable health, but the degree and mechanisms by which life course socioeconomic status affects kidney health is unclear.
Design, setting, participants, & measurements
We examined the association between cumulative lifetime socioeconomic status and CKD in black Americans in the Jackson Heart Study. We used conditional process analysis to evaluate allostatic load as a potential mediator of this relation. Cumulative lifetime socioeconomic status was an age-standardized z-score, which has 1-SD units by definition, and derived from self-reported childhood socioeconomic status, education, and income at baseline. Allostatic load encompassed 11 baseline biomarkers subsuming neuroendocrine, metabolic, autonomic, and immune physiologic systems. CKD outcomes included prevalent CKD at baseline and eGFR decline and incident CKD over follow-up.
Results
Among 3421 participants at baseline (mean age 55 years [SD 13]; 63% female), cumulative lifetime socioeconomic status ranged from −3.3 to 2.3, and 673 (20%) had prevalent CKD. After multivariable adjustment, lower cumulative lifetime socioeconomic status was associated with greater prevalence of CKD both directly (odds ratio [OR], 1.18; 95% confidence interval [95% CI], 1.04 to 1.33 per 1 SD and OR, 1.45; 95% CI, 1.15 to 1.83 in lowest versus highest tertile) and via higher allostatic load (OR, 1.09; 95% CI, 1.06 to 1.12 per 1 SD and OR, 1.17; 95% CI, 1.11 to 1.24 in lowest versus highest tertile). After a median follow-up of 8 years (interquartile range, 7–8 years), mean annual eGFR decline was 1 ml/min per 1.73 m2 (SD 2), and 254 out of 2043 (12%) participants developed incident CKD. Lower cumulative lifetime socioeconomic status was only indirectly associated with greater CKD incidence (OR, 1.04; 95% CI, 1.01 to 1.07 per 1 SD and OR, 1.08; 95% CI, 1.02 to 1.14 in lowest versus highest tertile) and modestly faster annual eGFR decline, in milliliters per minute (OR, 0.01; 95% CI, 0.00 to 0.02 per 1 SD and OR, 0.02; 95% CI, 0.00 to 0.04 in lowest versus highest tertile), via higher baseline allostatic load.
Conclusions
Lower cumulative lifetime socioeconomic status was substantially associated with CKD prevalence but modestly with CKD incidence and eGFR decline via baseline allostatic load.
Introduction
Low socioeconomic status is an established risk for adverse health outcomes (1–4), including development of CKD (5). Most of the extant literature has focused on the relation of adulthood socioeconomic status with health. However, a substantial body of knowledge now recognizes childhood and adolescence as sensitive periods during which low socioeconomic status also predisposes to adverse health in adulthood through a graded, yet cumulative effect (6–11). However, few studies have leveraged a life course approach to quantify the effect of lifetime socioeconomic status on risk for CKD specifically in black Americans, who are more likely to live in low socioeconomic environments and have disproportionately greater risk for CKD than nonblack Americans (12).
The mechanisms linking socioeconomic stressors to CKD risk also remain unclear (13). Biologic stress pathways that link stressors with subsequent adverse health (14–16) through a system-wide maladaptation process may play a role (7,17). Allostatic load is a composite preclinical measure that captures this prolonged physiologic burden beyond normal homeostasis (18), and its role as a key biologic mediator of the effects of cumulative stress exposures on health has become increasingly evident (19,20). Consistent with a multisystem effect of chronic stressors on physiologic function, it predicts health better than its components that capture impairments in singular body systems. However, studies have not examined allostatic load as a mediator linking socioeconomic status with kidney health in black populations.
We hypothesized that low cumulative lifetime socioeconomic status associates with adverse kidney outcomes and that greater allostatic load burden, as a measure of physiologic ramifications of prolonged stress exposure, mediates this association. We examined these relations using data from the Jackson Heart Study (JHS).
Materials and Methods
Study Design and Population
The JHS is a prospective cohort of black Americans in Jackson, Mississippi (21). Detailed sampling and recruitment methods have been previously published (22). In brief, JHS recruited participants aged 21–94 years between 2000 and 2004, from three counties (Hinds, Madison, and Rankin County) in the Jackson, Mississippi metropolitan area. Study participants underwent clinical examinations, provided blood and urine specimens, and completed questionnaires during three examination study visits: at baseline (examination 1: 2000–2004) and two follow-up visits (examination 2: 2005–2008; examination 3: 2009–2013). Median follow-up between examinations 1 and 3 was 8 years (interquartile range, 7–8). Institutional review boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center approved the study protocol; all participants provided informed consent.
Cumulative Lifetime Socioeconomic Status
Our primary exposure variable was cumulative lifetime socioeconomic status. We assessed this composite measure using the z-score, which quantifies the departure of a raw score from the population mean, and is typically reported in SD units (23). This composite exposure variables is on the basis of previously established definitions (24,25) and derived from participants’ self-reported childhood socioeconomic status, educational attainment, and annual household income. Because socioeconomic status varies by age in the general population, we standardized cumulative lifetime socioeconomic status to participants’ age at baseline by calculating its z-scores within three age groups: <45 years, 45–65 years, and >65 years.
Childhood socioeconomic status was a continuous measure (range, 0–9) assessed retrospectively at JHS baseline and derived by summing scores from two measures: parental education and childhood assets. The highest educational attainment achieved by either parent was categorized as 0 if less than high school, 0.5 if high school or some college/associate degree, or 1 if 4 or more years of college. Access to childhood assets ranged from 0 to 8 on the basis of number of rooms (0 if <5 or 1 if ≥5) and availability of the following (yes=1; no=0): indoor plumbing, electricity, refrigerator, telephone, television, air conditioning, and parental car ownership.
Education was scored on the basis of years of completed schooling at JHS baseline. Participants were assigned a score ranging from 1 to 12 for first to 12th grade, 13 if some college or vocational training, 14 if associate’s degree, 16 if bachelor’s degree, and 20 if more than a bachelor’s degree.
Annual household income was also a continuous score derived by assigning the median value of participants’ income category at baseline (25). We imputed income for 607 participants who did not report their income by assigning the modal value of income among participants with the same educational attainment. In instances where there were multiple modes, we used the higher mode.
Our secondary exposure variable was perceived socioeconomic status, which ranged from 1 to 10 on the basis of participants’ response to the question, “Now, think of a ladder with 10 steps representing where people stand in the United States. At step 10 are the people who are the best off. At step 1 are the people who are the worst off. Where would you place yourself on this ladder?”
Allostatic Load Assessment
A detailed account of the derivation of the definition of allostatic load used in this study and in prior JHS studies is available elsewhere (26,27). Briefly, we assessed allostatic load at baseline using four physiologic domains of neuroendocrine (cortisol), metabolic (hemoglobin A1c; total, LDL, and HDL cholesterol; waist circumference), autonomic (systolic and diastolic BP, resting heart rate), and immune function (C-reactive protein and white blood cell count) (28). We first converted values of the 11 biomarkers to z-scores and then calculated the average z-score for each of the four domains. We multiplied the z-score for HDL cholesterol by negative one because it associates with favorable health. Additionally, we accounted for sex differences in waist circumference by computing its z-score separately for men and women before recombining the within-sex scores into one score. We then derived the allostatic load score as the average z-score across all four domains for each participant. Higher allostatic load score indicated greater cumulative physiologic derangement.
Demographic, Behavioral, and Comorbid Factors
We included baseline covariates in our models, which we hypothesized would influence the association of cumulative lifetime socioeconomic status and kidney health. Covariates included age, sex, smoking status, use of routine care (receiving [versus not receiving] a routine physical examination within the past year) (29), and cardiovascular disease (self-reported history of heart attack, myocardial infarction determined by electrocardiogram or hospitalization ≥1 week for myocardial infarction, self-reported angioplasty in neck arteries, or self-reported stroke) (30). Because hemoglobin A1c (diabetes) and BP (hypertension) are components of allostatic load, we did not adjust for diabetes and hypertension in allostatic load models to avoid over adjustment (28). Incident CKD and eGFR decline models additionally included baseline eGFR, and eGFR decline models further featured baseline urine albumin-to-creatinine ratio (UACR).
In post hoc analysis, we included diet, physical activity, and sleep pattern as additional potential confounders in our models. We evaluated these behavioral factors separately because we conceptualized them as lying in the path between socioeconomic status and CKD.
Kidney Outcomes
The three primary outcomes were prevalent CKD, incident CKD, and eGFR decline. Prevalent CKD was assessed at baseline as (1) eGFR <60 ml/min per 1.73 m2 assessed using the CKD Epidemiology Collaboration equation (31) or (2) albuminuria defined by spot UACR ≥30 mg/g or, if spot urine was missing, then 24-hour urine assessment was used. Among individuals without prevalent CKD, incident CKD was defined as (1) eGFR <60 ml/min per 1.73 m2 at examination 3 accompanied by eGFR decline ≥25% over follow-up or (2) new-onset albuminuria at examination 3. eGFR decline was defined as an annualized rate of eGFR decline between examinations 1 and 3. We excluded participants who self-reported receipt of dialysis at baseline.
Statistical Analyses
Baseline characteristics are presented by tertiles of cumulative lifetime socioeconomic status using mean (SD) or median (interquartile range) for continuous variables and count (percent) for categorical variables. We used methods described by VanderWeele (32) and Hayes and Preacher (33) to examine the potential mediation via allostatic load of the association between cumulative lifetime socioeconomic status and kidney outcomes, conceptualizing cumulative lifetime socioeconomic status as predating derangements in biologic pathways subsumed in allostatic load temporally. This conditional process analysis entailed fitting two multivariable models using generalized linear regression models with canonical link functions. We assumed a binomial distribution for the prevalent and incident CKD outcomes and a normal distribution for eGFR decline. The first model regressed allostatic load, scaled in SD units, on cumulative lifetime socioeconomic status, whereas the second regressed the respective kidney outcome simultaneously on both allostatic load and cumulative lifetime socioeconomic status. Hereafter, we refer to the association of cumulative lifetime socioeconomic status with kidney outcomes via baseline allostatic load as indirect association and its association with kidney outcomes independently of allostatic load as direct association. We used both theoretical and bootstrapping (with 500 replications) techniques for hypothesis tests but reported only the bootstrapped results because estimates and confidence intervals were nearly identical between the two techniques.
In sensitivity analyses, we used other socioeconomic status measures as our exposure variables: perceived socioeconomic status, childhood socioeconomic status, education, and income. All hypothesis tests were two-sided using a significance level of α=0.05. We did not adjust for multiple testing because the three kidney outcomes assessed different aspects (CKD prevalence and incidence and kidney function decline) of the same underlying hypothesis that low socioeconomic status is associated with adverse kidney outcomes. We checked and confirmed model assumptions. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.0 (R Core Team, Vienna, Austria).
Results
Characteristics of Participants at Baseline
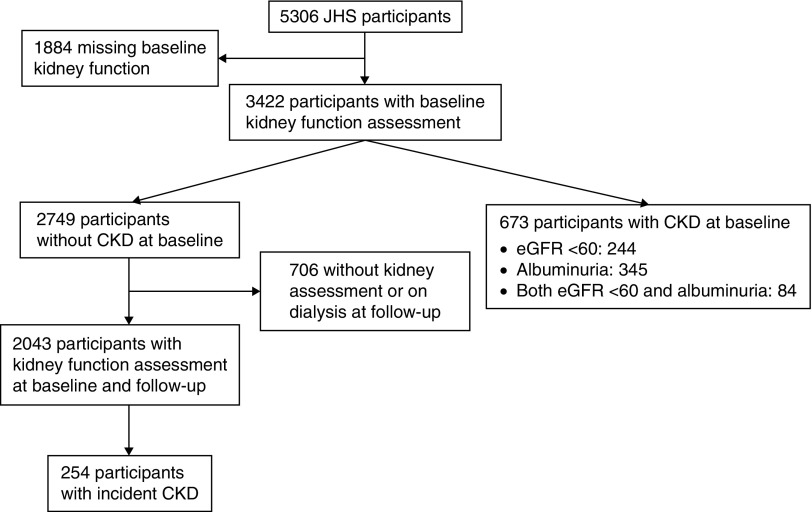
Of 5306 JHS participants, 3422 had complete data for CKD assessment (Figure 1). Compared with participants who had complete data, those without were more likely to be in the lowest tertile of cumulative lifetime socioeconomic status (39% versus 30%). Otherwise, baseline characteristics were generally similar between these two subgroups (Supplemental Table 1).
Figure 1.
Flow diagram of Jackson Heart Study (JHS) participants included in the analysis.
One of the 3422 participants did not have data on cumulative lifetime socioeconomic status. Among the remaining 3421 participants, cumulative lifetime socioeconomic status ranged from −3.27 to 2.34. Mean age was 55 (SD 13) years, and the majority were female (63%) and used routine care (72%). Participants in the lowest (versus highest) tertile of cumulative lifetime socioeconomic status were older (mean age 58 [SD 13] versus 53 [ SD 12] years), less likely to be men (33% versus 43%), more likely to be smokers (37% versus 23%), and had higher prevalence of diabetes (26% versus 19%), hypertension (64% versus 51%) and cardiovascular disease (15% versus 7%); use of routine care was comparable (Table 1).
Table 1.
Baseline characteristics of participants in the Jackson Heart Study stratified by tertiles of cumulative lifetime socioeconomic status
| Baseline Characteristicsa | Overall | Cumulative Lifetime Socioeconomic Status, Tertiles | ||
|---|---|---|---|---|
| Lowest | Middle | Highest | ||
| No. of participants | 3421 | 1139 (33) | 1135 (33) | 1147 (34) |
| Socioeconomic status z-scores | ||||
| Cumulative lifetimeb | 0±0.8 | −0.9±0.4 | 0±0.2 | 0.8±0.4 |
| Childhood | 4.9±3 | 3±2.8 | 5.3±2.7 | 6.5±2.3 |
| Perceived | 6.3±2.2 | 6±2.4 | 6.3±2.1 | 6.6±2 |
| Demographics | ||||
| Age, yr | 55±13 | 58±13 | 53±13 | 53±12 |
| Sex, male | 1276 (37) | 380 (33) | 408 (36) | 488 (43) |
| Annual incomec | 44.8±30 | 21.8±14.2 | 40.1±22.1 | 72.2±26.1 |
| Education, ≤12th grade | 1228 (36) | 929 (82) | 271 (24) | 28 (2) |
| Behavioral factors | ||||
| Current/former smoker | 1029 (30) | 423 (37) | 344 (30) | 262 (23) |
| Use of routine care | 2471 (72) | 802 (70) | 809 (71) | 860 (75) |
| Comorbidities | ||||
| Diabetes | 731 (21) | 293 (26) | 223 (20) | 215 (19) |
| Diabetes medication | 487 (14) | 199 (18) | 140 (12) | 148 (13) |
| Hypertension | 1936 (57) | 723 (64) | 629 (55) | 584 (51) |
| BP-lowering medication | 1664 (49) | 616 (54) | 537 (47) | 511 (45) |
| Cardiovascular disease | 369 (11) | 173 (15) | 111 (10) | 85 (7) |
| Baseline kidney function | ||||
| eGFR, ml/min per 1.73 m2 | 93±24 | 91±26 | 95±24 | 95±22 |
| UACR, median [IQR], mg/g | 6 [4–13] | 7 [4–17] | 6 [4–12] | 6 [4–11] |
| Prevalent CKD | 673 (20) | 302 (27) | 205 (18) | 166 (15) |
| Allostatic load | ||||
| Composite z-scored | 0.0±0.4 | 0.1±0.5 | 0±0.4 | −0.1±0.4 |
| Biologic components | ||||
| Cortisol, mmol/L | 9.6±4 | 10.1±4.2 | 9.5±4 | 9.3±3.9 |
| HBA1c, % | 6.0±1.2 | 6.1±1.4 | 5.9±1.2 | 5.8±1.1 |
| LDL cholesterol, mg/dl | 126±37 | 126±38 | 125±36 | 127±36 |
| HDL cholesterol, mg/dl | 51±14 | 51±14 | 50±14 | 51±14 |
| Total cholesterol, mg/dl | 198±40 | 199±42 | 197±39 | 198±39 |
| Waist circumference, cm | 101±16 | 102±16 | 100±17 | 99±16 |
| Systolic BP, mm Hg | 127±17 | 129±18 | 126±17 | 125±16 |
| Diastolic BP, mm Hg | 76±9 | 76±9 | 76±9 | 76±9 |
| Resting heart rate, bpm | 65±11 | 65±11 | 65±10 | 64±10 |
| CRP, median [IQR], mg/dl | 0.3 [0.1–0.6] | 0.3 [0.1–0.6] | 0.3 [0.1–0.6] | 0.2 [0.1–0.5] |
| WBC, 1000 cells/cmm | 5.6±2.2 | 5.8±2.9 | 5.6±1.8 | 5.5±1.8 |
UACR, urine albumin-to-creatinine ratio; IQR, interquartile range; HBA1C, hemoglobin A1c; bpm, beats per minute; CRP, C-reactive protein; WBC, white blood cells.
Values are n (%) or mean±SD, unless stated otherwise.
Composite of childhood socioeconomic status, education attainment, and annual family income.
Annual family income scaled in $1000.
Men and women had separate z-score for waist circumference; we multiplied the HDL z-score by −1.
Allostatic Load and Kidney Outcomes
Baseline allostatic load ranged from −1.1 to 6.7. Participants in highest (versus lowest) allostatic load tertile were more likely to be older (mean age 57 [SD 12] versus 53 [SD 13] years), men (42% versus 28%), smokers (37% versus 27%), and to have diabetes (35% versus 10%) and hypertension (72% versus 40%), but had comparable use of routine care (Supplemental Table 2).
At baseline, there were 673 of 3421 (20%) participants with prevalent CKD. Over follow-up, mean annual eGFR decline was 1 (SD 2) ml/min and 254 of 2043 (12%) eligible participants developed incident CKD (Figure 1).
After adjustment for demographics, behavior, and comorbidity, each 1-SD higher allostatic load was associated with greater odds of prevalent CKD (odds ratio [OR], 1.86; 95% confidence interval [95% CI], 1.69 to 2.04) and incident CKD (OR, 1.42; 95% CI, 1.24 to 1.62) and faster annual eGFR decline (0.15 ml/min; 95% CI, 0.07 to 0.23). Results were consistent with additional adjustment for baseline UACR and eGFR (Table 2).
Table 2.
Association of allostatic load with kidney outcomes in the Jackson Heart Study
| Models | Prevalent CKD, Odds Ratio (95% CI) | Incident CKD, Odds Ratio (95% CI) | eGFR Decline, β (95% CI)a |
|---|---|---|---|
| No. of CKD events/N or Nb | 672/3421 | 254/2038 | 2403 |
| Allostatic load | |||
| Model 1 | 1.89 (1.73 to 2.07) | 1.46 (1.29 to 1.65) | 0.20 (0.13 to 0.26) |
| Model 2 | 1.86 (1.69 to 2.04) | 1.42 (1.24 to 1.62) | 0.15 (0.07 to 0.23) |
| Model 3 | NA | 1.42 (1.24 to 1.62) | 0.09 (0.01 to 0.16) |
Model 1: unadjusted. Model 2: adjusted for age, sex, use of routine care, smoking status, and cardiovascular disease. Model 3: adjusted for baseline eGFR; the eGFR decline model additionally adjusted for baseline urine albumin-to-creatinine ratio. 95% CI, 95% confidence interval; NA, not applicable.
eGFR decline: average difference in annualized eGFR decline over follow-up per 1-SD higher baseline allostatic load. Odds ratios are per 1-SD higher allostatic load.
No. of events/N refers to number of participants with the outcome (Prevalent CKD and Incident CKD) compared with (i.e., "/") the total number of participants. Whereas N refers to the eGFR decline outcome, which has no event counts.
Allostatic Load as a Mediator of Cumulative Lifetime Socioeconomic Status and Kidney Outcomes
There was evidence of mediation by allostatic load of the associations of cumulative lifetime socioeconomic status with kidney outcomes as described below (Supplemental Figure 1).
Prevalent CKD.
Lower cumulative lifetime socioeconomic status was associated with higher odds of prevalent CKD at baseline both independently of allostatic load (OR, 1.18; 95% CI, 1.04 to 1.33 per 1 SD and OR, 1.45; 95% CI, 1.15 to 1.83 in lowest versus highest tertile) and via its association with higher allostatic load at baseline (OR, 1.09; 95% CI, 1.06 to 1.12 per 1 SD and OR, 1.17; 95% CI, 1.11 to 1.24 in lowest versus highest tertile) (Table 3).
Table 3.
Direct and mediated (via allostatic load) associations of cumulative lifetime socioeconomic status with kidney outcomes
| Prevalent CKD | Incident CKD | eGFR Decline | |||||
|---|---|---|---|---|---|---|---|
| Direct Association, OR (95% CI) | Mediated Association, OR (95% CI) | Direct Association, OR (95% CI) | Mediated Association, OR (95% CI) | Direct Association, β (95% CI) | Mediated Association, β (95% CI) | ||
| No. of events/Nb | 666/3405 | 254/2038 | 2403 | ||||
| CLSESa | 1.18 (1.04 to 1.33) | 1.09 (1.06 to 1.12) | 1.02 (0.85 to 1.23) | 1.04 (1.01 to 1.07) | 0.003 (−0.10 to 0.10) | 0.01 (0.00 to 0.02) | |
| CLSES tertiles | |||||||
| Lowest | 1.45 (1.15 to 1.83) | 1.17 (1.11 to 1.24) | 1.06 (0.76 to 1.5) | 1.08 (1.02 to 1.14) | 0.01 (−0.18 to 0.19) | 0.02 (0.00 to 0.04) | |
| Middle | 1.21 (0.95 to 1.54) | 1.07 (1.02 to 1.13) | 0.93 (0.65 to 1.33) | 1.05 (1.01 to 1.1) | 0.02 (−0.16 to 0.2) | 0.01 (0.00 to 0.03) | |
| Highest | Reference | Reference | Reference | Reference | Reference | Reference | |
All models adjusted for age, sex, use of routine care, smoking status, and cardiovascular disease; the eGFR decline model additionally adjusted for baseline albumin-to-creatinine ratio and eGFR. OR, odds ratio; 95% CI, 95% confidence interval; CLSES, cumulative lifetime socioeconomic status.
Estimates are per 1-SD lower CLSES.
No. of events/N refers to number of participants with the outcome (Prevalent CKD and Incident CKD) compared with (i.e., "/") the total number of participants, whereas N refers to the eGFR decline outcome, which has no event counts.
Incident CKD and eGFR Decline.
Cumulative lifetime socioeconomic status was not directly associated with incident CKD (OR, 1.02; 95% CI, 0.85 to 1.23 per 1 SD and OR, 1.06; 95% CI, 0.76 to 1.5 in lowest versus highest tertile) or annual eGFR decline, in milliliters per minute (OR, 0; 95% CI, −0.10 to 0.10 per 1 SD and OR, 0.01; 95% CI, −0.18 to 0.19 in lowest versus highest tertile). However, via its association with higher baseline allostatic load, lower cumulative lifetime socioeconomic status was indirectly associated with higher odds of incident CKD (OR, 1.04; 95% CI, 1.01 to 1.07 per 1 SD and OR, 1.08; 95% CI, 1.02 to 1.14 in lowest versus highest tertile) and faster annual eGFR decline, in milliliters per minute (OR, 0.01; 95% CI, 0.00 to 0.02 per 1 SD and OR, 0.02; 95% CI, 0.00 to 0.04 in lowest versus highest tertile) (Table 3).
Post Hoc Analyses
The association of allostatic load with kidney outcomes and the mediation via allostatic load of the association between cumulative lifetime socioeconomic status and kidney outcomes persisted after additional adjustment for diet, physical activity, and sleep pattern (Supplemental Tables 3 and 4).
Sensitivity Analyses
Lower income and education were modestly associated with increased odds of prevalent CKD at baseline both directly (OR, 1.08 per $10,000 lower annual income; 95% CI, 1.04 to 1.12 and OR, 1.05 per one grade lower education level; 95% CI, 1.02 to 1.07) and indirectly via allostatic load. However, education and income were not associated with longitudinal kidney outcomes. Perceived socioeconomic status and childhood socioeconomic status were not associated with allostatic load nor CKD outcomes (Supplemental Table 5).
Discussion
In this large cohort of black Americans, lowest (versus highest) tertile of cumulative lifetime socioeconomic status was associated with 45% and 17% higher odds of CKD prevalence at baseline independently of and via allostatic load, respectively. After a median follow-up of 8 years, cumulative lifetime socioeconomic status was not associated with incident CKD or eGFR decline independently of allostatic load. However, lowest (versus highest) tertile of cumulative lifetime socioeconomic status was associated with 8% higher odds of CKD incidence and modestly faster eGFR decline via its association with higher allostatic load at baseline. Within the life course framework (10), the time scale for development of the prevalent CKD outcome spans several decades from childhood to JHS baseline. In contrast, the relatively short JHS follow-up time scale of 8 years for development of longitudinal kidney outcomes may have been insufficient to capture any meaningful direct association between cumulative lifetime socioeconomic status and changes in kidney health owing to the long latency period involved in this relationship (34). Thus, these findings underscore the need for assessments of life course exposures such as socioeconomic status when identifying social determinants of long-term kidney health and further support the emerging role of allostatic load as a target for mechanistic investigation on factors influencing these relations.
To our knowledge, this is the first study to examine the effect of life course socioeconomic status on long-term kidney health in black Americans. Prior work evaluating socioeconomic status across the lifespan has consistently linked low (versus high) socioeconomic status with subsequent unfavorable health, including higher daily physical symptom burden (35,36), three-fold increased risk for cardiovascular disease (25), and >10% higher odds of diabetes incidence (37). These associations are owing to the unfavorable effect of low socioeconomic status on three broad determinants of health: access and utilization of health care, socioenvironmental stressors, and health behavior (38). As the link between socioeconomic status and health becomes more lucid, it is now evident that socioeconomic assessments restricted to single life stages do not capture its cumulative effects on health over the lifetime (39). This is of particular relevance in kidney disease, where evidence supports the link between low socioeconomic status and poor CKD outcomes, yet few studies have examined this relation using a life course socioeconomic status measure (5). Furthermore, none have done so exclusively in black populations, where the influence of socioeconomic stressors disproportionately portends greater risk for poor kidney outcomes than in nonblack populations (40).
One important study by Shoham et al. examined measures of socioeconomic status at different adulthood stages and risk for CKD incidence in the Atherosclerosis Risk in Communities study. The authors found a stronger association between working class status and incident CKD in earlier (age 30 years) compared with later (age 50 years) adult life stages and concluded that socioeconomic status exposures experienced in early (versus later) adulthood are relatively more detrimental to kidney health (11). However, the authors did not examine the effect of socioeconomic stressors in childhood on kidney health. In this study, although adult measures of socioeconomic status were individually associated with adverse kidney outcomes (lower annual income and educational attainment were both associated with increased odds of prevalent CKD at baseline), the magnitude of association was greater with inclusion of socioeconomic status measures at other life stages, such as childhood in the composite cumulative lifetime socioeconomic status measure. Thus, our findings support the incorporation of life course socioeconomic status measures to aid in the interpretation of associations between socioeconomic status and CKD.
Our study also provides the first support for the potential role of allostatic load as a biologic mechanism underlying the adverse consequences of socioeconomic stressors on kidney health. It extends an emerging role of allostatic load as an intermediate, preclinical biologic pathway by which stressors in the social environment adversely affect health through accumulation across life stages (2). For instance, one study from the Coronary Artery Risk Development in Young Adults cohort found consistent associations between different socioeconomic stressors in early life stages (e.g., childhood adversity) and marked dysregulation in domains of allostatic load later in life (41). Subsequent studies have replicated this finding in other cohorts (42). In turn, studies have found strong associations between allostatic load and subsequent risk for mortality (43). Taken together, this growing body of work suggests higher allostatic load as a biologic pathway by which chronic exposure to life stressors unfavorably affect health later in life, including development and progression of CKD.
Our study has limitations worthy of mention. Allostatic load as an index for assessing intermediate biologic pathways between stressors and health has not been previously validated specifically in a CKD population. This is particularly noteworthy given the bidirectional relationship between some allostatic load components (e.g., BP and hemoglobin A1c) and CKD. However, as a composite measure (18), allostatic load is a multidimensional construct that captures maladaptation across multiple physiologic systems and is not weighted particularly toward one system or a subset of its constituent measures. Thus, consistent with this concept, the definition of allostatic load used in our study and prior JHS studies (26,27) averages standardized scores from constituent biomarkers both within and across physiologic domains of allostatic load. Additionally, despite the heterogeneity in the current definition of allostatic load in the literature (44,45), this definition captures physiologic pathways relevant to CKD. The observational design of the JHS also limits drawing causal inference from our data because of misclassification bias (e.g., cumulative lifetime socioeconomic status was a self-reported measure and CKD definition utilized single eGFR and UACR assessments), selection bias (owing to missing data), and residual confounding (e.g., from classes of medication that influence eGFR decline, such as angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and nonsteroidal anti-inflammatory drugs). Because we did not assess death as a competing risk, survival bias is also a concern. Finally, although our assumption of a temporal relation between cumulative lifetime socioeconomic status and allostatic load is valid conceptually, we were unable to quantify changes in allostatic load over time resulting from prolonged exposure to low socioeconomic status. Despite these limitations, our study has several key strengths. The availability of prospective kidney function data in the JHS partially fulfilled a critical mediation analysis assumption that requires a temporal relation between exposure, mediator, and outcome variables, which lends credence to our findings as evidence of mediation effects and not owing to confounding. Finally, the JHS is the largest study of cardiovascular disease in black Americans to date, and it measured a wide array of biomarkers that allowed for assessment of allostatic load in a community-dwelling population.
In conclusion, low cumulative lifetime socioeconomic status was associated with increased odds of prevalent CKD at baseline both directly and indirectly via allostatic load. After follow-up, low cumulative lifetime socioeconomic status was only indirectly associated with increased odds of incident CKD and faster eGFR decline via baseline allostatic load. Thus, our data link low life course socioeconomic status with kidney outcomes in black Americans and allostatic load is a potential mediator of this link. Future work should investigate the potential utility of allostatic load for predicting the beneficial effect of socioeconomic status interventions on subsequent kidney health.
Disclosures
Dr. Scialla reports receiving personal fees from an advisory board position at Tricida and research support for clinical event committees from Eli Lilly, GlaxoSmithKline, and Sanofi outside the submitted work. Dr. Bhavsar, Dr. Boulware, Dr. Davenport, Dr. Diamantidis, Dr. Lunyera, Dr. Mohottige, Dr. Pendergast, and Dr. Stanifer have nothing to disclose.
Funding
The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute and the National Institute for Minority Health and Health Disparities. This work was further supported by an award received by Dr. Davenport and through The National Center for Advancing Translational Sciences (UL1TR002553).
Supplementary Material
Acknowledgments
We thank Ashley Cabacungan for her editorial assistance during the preparation of this manuscript. The authors also wish to thank the staffs and participants of the Jackson Heart Study.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US Department of Health and Human Services.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08430719/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of participants in the Jackson Heart Study stratified by inclusion status.
Supplemental Table 2. Baseline characteristics of participants in the Jackson Heart Study stratified by tertiles of allostatic load.
Supplemental Table 3. Association of allostatic load with kidney outcomes with additional adjustment for diet, physical activity, and sleep in the Jackson Heart Study.
Supplemental Table 4. Direct and mediated (via allostatic load) associations of cumulative lifetime socioeconomic status with kidney outcomes after additional adjustment for diet, physical activity, and sleep pattern.
Supplemental Table 5. Direct and mediated (via allostatic load) associations of different measures of socioeconomic status with kidney outcomes.
Supplemental Figure 1. Direct and mediated (via allostatic load) associations of cumulative lifetime socioeconomic status with kidney outcomes.
References
- 1.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE: Association between children’s experience of socioeconomic disadvantage and adult health: A life-course study. Lancet 360: 1640–1645, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JP: The impact of socioeconomic status on health over the life-course. J Hum Resour 42: 739–764, 2007 [Google Scholar]
- 3.Lynch JW, Kaplan GA, Salonen JT: Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med 44: 809–819, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P; Consortium for the European Review of Social Determinants of Health and the Health Divide : WHO European review of social determinants of health and the health divide. Lancet 380: 1011–1029, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Vart P, van Zon SKR, Gansevoort RT, Bültmann U, Reijneveld SA: SES, chronic kidney disease, and race in the U.S.: A systematic review and meta-analysis. Am J Prev Med 53: 730–739, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Slopen N, Chen Y, Priest N, Albert MA, Williams DR: Emotional and instrumental support during childhood and biological dysregulation in midlife. Prev Med 84: 90–96, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg MT, Simons RL, Barr A, Beach SRH, Philibert RA: Childhood/Adolescent stressors and allostatic load in adulthood: Support for a calibration model. Soc Sci Med 193: 130–139, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Doom JR, Mason SM, Suglia SF, Clark CJ: Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long-term cardiovascular disease risk in young adulthood. Soc Sci Med 188: 166–175, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockwood KG, John-Henderson NA, Marsland AL: Early life socioeconomic status associates with interleukin-6 responses to acute laboratory stress in adulthood. Physiol Behav 188: 212–220, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Shlomo Y, Kuh D: A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 31: 285–293, 2002. [PubMed] [Google Scholar]
- 11.Shoham DA, Vupputuri S, Diez Roux AV, Kaufman JS, Coresh J, Kshirsagar AV, Zeng D, Heiss G: Kidney disease in life-course socioeconomic context: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 49: 217–226, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Norton JM, Moxey-Mims MM, Eggers PW, Narva AS, Star RA, Kimmel PL, Rodgers GP: Social determinants of racial disparities in CKD. J Am Soc Nephrol 27: 2576–2595, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powe NR: CKD progression: Teasing out contributions of elements in the human exposome. Am J Kidney Dis 73: 581–582, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y: Early childhood investments substantially boost adult health. Science 343: 1478–1485, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umaña-Taylor AJ, Kornienko O, Douglass Bayless S, Updegraff KA: A universal intervention program increases ethnic-racial identity exploration and resolution to predict adolescent psychosocial functioning one year later. J Youth Adolesc 47: 1–15, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, Emery CF, Layman R, Mrozek EE, Glaser R: Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. J Clin Oncol 32: 1040–1049, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R: Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A 100: 9090–9095, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS: Protective and damaging effects of stress mediators. N Engl J Med 338: 171–179, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Merkin SS, Karlamangla A, Roux AV, Shrager S, Seeman TE: Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: Multi-ethnic study of atherosclerosis. Am J Public Health 104: e48–e55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE: History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med 74: 75–83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor HA, Jr: The Jackson Heart Study: An overview. Ethn Dis 15[Suppl 6]: S6-1-3, 2005 [PubMed] [Google Scholar]
- 22.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr: Recruiting African-American research participation in the Jackson Heart Study: Methods, response rates, and sample description. Ethn Dis 15[Suppl 6]: S6-18-29, 2005 [PubMed] [Google Scholar]
- 23.Munro BH: Statistical Methods for Health Care Research, 5th Ed., Philadelphia, Lippincott Williams and Wilkins, 2005 [Google Scholar]
- 24.Subramanyam MA, James SA, Diez-Roux AV, Hickson DA, Sarpong D, Sims M, Taylor HA Jr, Wyatt SB: Socioeconomic status, John Henryism and blood pressure among African-Americans in the Jackson Heart Study. Soc Sci Med 93: 139–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebreab SY, Diez Roux AV, Brenner AB, Hickson DA, Sims M, Subramanyam M, Griswold ME, Wyatt SB, James SA: The impact of lifecourse socioeconomic position on cardiovascular disease events in African Americans: The Jackson Heart Study. J Am Heart Assoc 4: e001553, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber S, Hickson DA, Kawachi I, Subramanian SV, Earls F: Neighborhood disadvantage and cumulative biological risk among a socioeconomically diverse sample of African American adults: An examination in the Jackson Heart Study. J Racial Ethn Health Disparities 3: 444–456, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickson DA, Diez Roux AV, Gebreab SY, Wyatt SB, Dubbert PM, Sarpong DF, Sims M, Taylor HA: Social patterning of cumulative biological risk by education and income among African Americans. Am J Public Health 102: 1362–1369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunyera J, Davenport CA, Jackson CL, Johnson DA, Bhavsar NA, Sims M, Scialla JJ, Stanifer JW, Pendergast J, McMullan CJ, Ricardo AC, Boulware LE, Diamantidis CJ: Evaluation of allostatic load as a mediator of sleep and kidney outcomes in black Americans. Kidney Int Rep 4: 425–433, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamantidis CJ, Davenport CA, Lunyera J, Bhavsar N, Scialla J, Hall R, Tyson C, Sims M, Strigo T, Powe NR, Boulware LE: Low use of routine medical care among African Americans with high CKD risk: The Jackson Heart Study. BMC Nephrol 20: 11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D: Cardiovascular disease event classification in the Jackson Heart Study: Methods and procedures. Ethn Dis 15[Suppl 6]: S6-62-70, 2005 [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd., Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, VanderWeele T: Explanation in Causal Inference: Methods for Mediation and Interaction, New York, NY, Oxford University Press, 2015 [Google Scholar]
- 33.Hayes AF, Preacher KJ: Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol 67: 451–470, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Pencina MJ, Larson MG, D’Agostino RB: Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med 26: 1343–1359, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Surachman A, Wardecker B, Chow SM, Almeida D: Life course socioeconomic status, daily stressors, and daily well-being: Examining chain of risk models. J Gerontol B Psychol Sci Soc Sci 74: 126–135, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres JM, Rizzo S, Wong R: Lifetime socioeconomic status and late-life health trajectories: Longitudinal results from the Mexican Health and Aging Study. J Gerontol B Psychol Sci Soc Sci 73: 349–360, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsenkova V, Pudrovska T, Karlamangla A: Childhood socioeconomic disadvantage and prediabetes and diabetes in later life: A study of biopsychosocial pathways. Psychosom Med 76: 622–628, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adler NE, Newman K: Socioeconomic disparities in health: Pathways and policies. Health Aff (Millwood) 21: 60–76, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Shavers VL: Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 99: 1013–1023, 2007. [PMC free article] [PubMed] [Google Scholar]
- 40.Johns TS, Estrella MM, Crews DC, Appel LJ, Anderson CA, Ephraim PL, Cook C, Boulware LE: Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. J Am Soc Nephrol 25: 2649–2657, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE: Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A 110: 17149–17153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickrama KA, Lee TK, O’Neal CW: Stressful life experiences in adolescence and cardiometabolic risk factors in young adulthood. J Adolesc Health 56: 456–463, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Castagné R, Garès V, Karimi M, Chadeau-Hyam M, Vineis P, Delpierre C, Kelly-Irving M; Lifepath Consortium : Allostatic load and subsequent all-cause mortality: Which biological markers drive the relationship? Findings from a UK birth cohort. Eur J Epidemiol 33: 441–458, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson SC, Cavallaro FL, Leon DA: A systematic review of allostatic load in relation to socioeconomic position: Poor fidelity and major inconsistencies in biomarkers employed. Soc Sci Med 192: 66–73, 2017. [DOI] [PubMed] [Google Scholar]
- 45.Duong MT, Bingham BA, Aldana PC, Chung ST, Sumner AE: Variation in the calculation of allostatic load score: 21 examples from NHANES. J Racial Ethn Health Disparities 4: 455–461, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.