Abstract
 Circularly
polarized luminescence (CPL) is characterized by the differential
emission of right and left circularly polarized light by a chiral
molecule. This mini-review describes the recent developments in chiral
trivalent europium (Eu(III)) complexes with effective CPL. CPL has
many potential applications in security tags, lasers, and three-dimensional
organic electroluminescence devices, which is one of the most intensely
investigated topics in molecular luminophores. Eu(III) complexes have
attracted considerable attention as effective CPL luminophores for
the above-mentioned applications. In this review, recent studies on
the Eu(III) CPL, including the steric (dimer, tetramer, aggregates,
and coordination polymers) and electronic control (mononuclear) of
Eu(III) complexes for the construction of a luminophore with effective
CPL, are discussed. The characteristic CPL applications employing
the chiral mononuclear Eu(III) complexes are also described. Chiral
Eu(III) complexes with well-designed organic ligands can result in
the establishment of new research areas in the fields of photochemistry
and materials science.
Circularly
polarized luminescence (CPL) is characterized by the differential
emission of right and left circularly polarized light by a chiral
molecule. This mini-review describes the recent developments in chiral
trivalent europium (Eu(III)) complexes with effective CPL. CPL has
many potential applications in security tags, lasers, and three-dimensional
organic electroluminescence devices, which is one of the most intensely
investigated topics in molecular luminophores. Eu(III) complexes have
attracted considerable attention as effective CPL luminophores for
the above-mentioned applications. In this review, recent studies on
the Eu(III) CPL, including the steric (dimer, tetramer, aggregates,
and coordination polymers) and electronic control (mononuclear) of
Eu(III) complexes for the construction of a luminophore with effective
CPL, are discussed. The characteristic CPL applications employing
the chiral mononuclear Eu(III) complexes are also described. Chiral
Eu(III) complexes with well-designed organic ligands can result in
the establishment of new research areas in the fields of photochemistry
and materials science.
1. Introduction
Chiral molecules exhibit circularly polarized luminescence (CPL), which is characterized by the differential emission of right- and left-handed circularly polarized light.1,2 CPL has attracted considerable attention owing to its applications in security tags, lasers, and organic electroluminescent (EL) devices for 3D displays.3 Many studies on various chiral luminophores such as organic dyes, transition-metal complexes, and lanthanide metal complexes have been reported. Among these chiral luminophores, enantiopure lanthanide complexes exhibit the most effective CPL as chiral luminophores.4−7
Lanthanoids include La and 14 other elements (lanthanides: Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu) (Figure 1a), which form stable trivalent ions. Chiral lanthanide(III) complexes are composed of lanthanide(III) ions (i.e., a luminescent center) and organic ligands (i.e., chiral indicators) as shown in Figure 1b. The trivalent lanthanide ions are characterized by an incompletely filled 4f shell (with the exception of La(III) and Lu(III)). The 4f orbital is shielded from the surroundings by the filled 5s and 5p orbitals. On the basis of the electronic characteristics, 4f–4f transitions produce sharp emission lines (full width at half-maximum, fwhm <10 nm), which is considerably different from the emission properties of the organic dyes and transition-metal complexes.7−10 Chiral ligands induce a high CPL activity for the 4f–4f transition.
Figure 1.
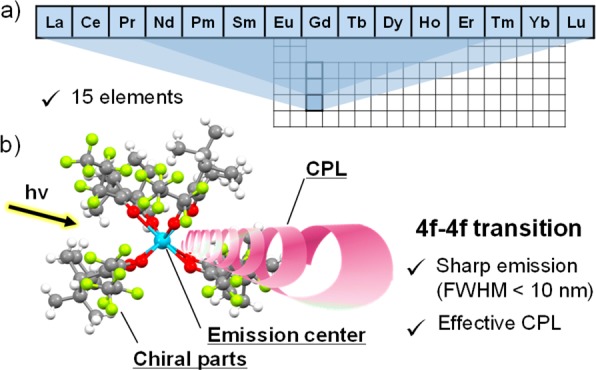
(a) Lanthanoids in the periodic table and (b) image of the chiral lanthanide luminophore ([Eu((+)-hfbc)4]−) with high CPL activity.
Herein, we introduce the history of the studies on CPL using the lanthanide complex. In 1975, Richardson et al. discovered CPL in Eu(III) and Tb(III) complexes with chiral carboxylic acid for the first time.11 Following this discovery, they also observed high CPL activity in a Eu(III) complex with chiral 3-trifluoroacetylcamphorate ligands,12 and there are several reports on CPL.13,14 During that time, the reports on the CPL were limited because of the weak effect and the lack of conventional measurement systems. In the 21st century, the CPL measurement systems became widespread, resulting in a gradual increase in the number of CPL reports. In 2011, Kaizaki and Muller reported the chiroptical properties of Cs[Eu((+)-hfbc)4] (hfbc: 3-heptafluoro butyryl camphorate).15 The emission intensity (in the 5D0 → 7F1 transition) of the left-handed light is approximately 5 times greater than that of the right-handed light. This finding further accelerated the CPL research on lanthanide(III) complexes. Various studies on lanthanide CPLs are summarized in several reviews by Muller,4 Parker,5 and Di Bari.6 The focus of this review is on the latest progress in polynuclear Eu(III) CPL and the electronic control of chiral mononuclear Eu(III) complexes to realize effective CPL. The CPL applications using the chiral mononuclear Eu(III) complexes are also described.
2. General Theory
CPL spectroscopy measures the difference between the intensities (ΔI) of the left (IL) and right (IR) circularly polarized emissions from the chiral luminophore, which is defined by following equation.1,2
| 1 |
The degree of dissymmetry of CPL is quantified by the difference in the relative intensities of the left and right circularly polarized emissions and is known as the luminescence dissymmetry factor (gCPL).
| 2 |
By definition, the gCPL values should be in the range of −2 to +2. gCPL is also expressed in terms of the transition electric dipole moment μ⃗ and transition magnetic dipole moment m⃗ as shown below
| 3 |
where θ is the angle between μ⃗ and m⃗. When μ⃗ = m⃗ (θ = 0, eq 3 affords the highest gCPL value (= 2) mathematically. The equation indicates that the lanthanide(III) complexes are plausible candidates for CPL with high gCPL values because the magnitude of the electric dipole moment (μ⃗) is similar to that of the magnetic dipole moment (|m⃗|). Among lanthanide(III) emissions, the 5D0 → 7F1 transition of Eu(III) complexes shows an enormous gCPL based on the allowed magnetic dipole nature.
3. Steric Structural Control by Molecular Integration
In contrast to many reports for the lanthanide CPL based on mononuclear complexes,4−6 few reports exist on the polynuclear lanthanide CPL. Polynuclear complexes show interor intramolecular interaction by integration. Herein, recent progress in the lanthanide CPL from Eu(III) tetramers,16 dimers,17 aggregates,18 and coordination polymers19,20 is reviewed.
3.1. Highly Active CPL for the Eu(III) Tetramer and Dimer
Morphology control on the molecular scale can produce effetive CPL properties. Teat demonstrated that the point chirality of a ligand decisively influenced its supramolecular assembly behavior.16 They produced a Eu(III) tetrahedral cage using 2,6-diaminoanthraquinone-based chiral ligands with different point chirality (Figure 2). Ligand 1 (Figure 2) allowed the formation of highly diastereoselective Eu tetrahedral cages, although ligands 2 and 3 (Figure 2) led to the generation of a mixture of isomers. The homochiral cages exhibit excellent CPL (|gCPL|: up to 0.16), but other cages show relatively low gCPL values. All cages show the same long emission lifetimes (1.6 ms). From these results, we consider that the cages do not exhibit the quenching states around the emitting level of the Eu(III) ion. The cage framework also shows effective energy transfer and a high emission quantum yield (Φtot = 16–18%). These results (different CPLs and similar Φtot’s) indicate that the precise steric control of the Eu(III) cage system provides the effective chiroptical change in the solution.
Figure 2.
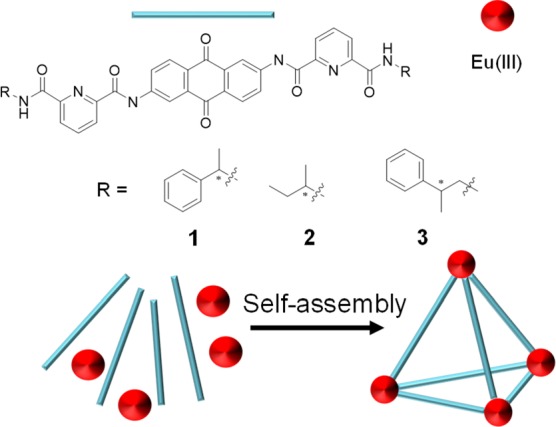
Tetranuclear chiral Eu(III) complexes.
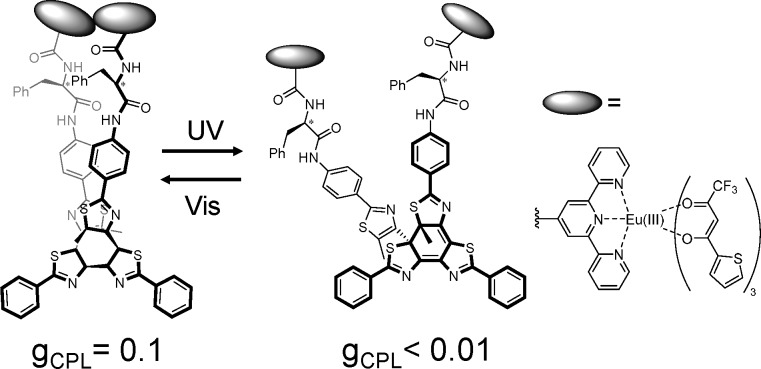
A characteristic CPL luminophore was also reported for the dynamic CPL control using a photochemical reaction. The combination of Eu(III) complexes and photochromic chiral molecule induces unique chiroptical phenomena. Nakashima and Kawai reported the photoswitching of the CPL property in the Eu(III) dimer system (Figure 3).17 The Eu(III) complex shows nine coordination structures composed of β-diketonate and terpyridine derivative with a chiral photochromic unit. The Eu(III) complexes were arranged closely in a chiral orientation by the unit. This structural modulation also induces emission intensity change based on the back-energy transfer from the Eu(III) ion to the photochromic unit. The chiral arrangement of the complex units was switched by a photoinduced structural change in the system, resulting in on–off switching of the CPL with high contrast (gCPL values of 0.1 and <0.01). Nanoscale studies on the chiroptical properties of a chiral Eu(III) complex have been recently explored, although numerous studies have been historically carried out on chiral dye oligomers based on the coupled exciton theory.3
Figure 3.
Photoresponsive chiral Eu(III) dimer. Adapted from ref (17).
3.2. Enhanced CPL for Eu(III) Aggregation and Coordination Polymers
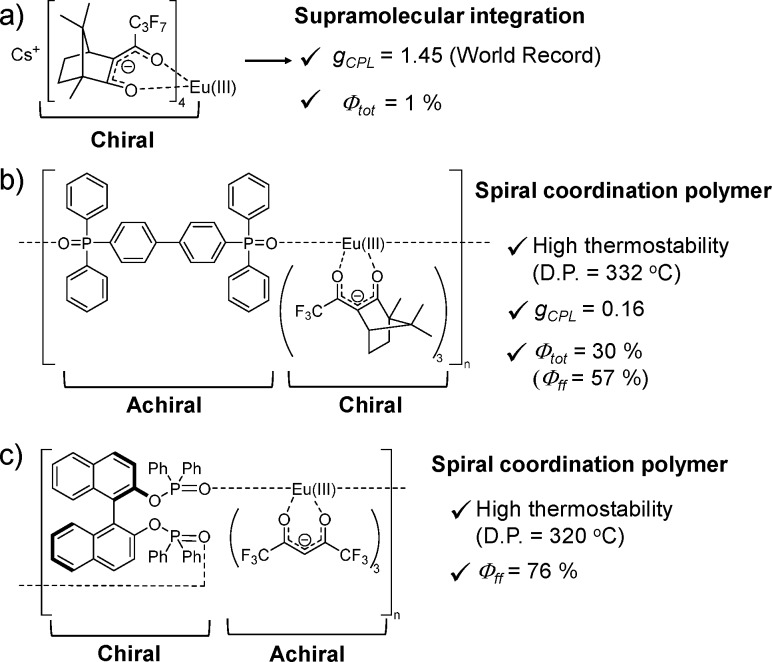
Polynuclear Eu(III) complexes show highly active circular polarized emission as described in the last chapter. Recently, enhanced gCPL through the aggregation of chiral units was also reported for the chiral Eu(III) complex with 3-heptafluorobutyryl camphorate (hfbc) and the Cs(I) ion (Figure 4a).18 The helical 1D aggregation of Cs[Eu((+)–hfbc)4] resulted in the highest gCPL value (1.45: 5D0 → 7F1 transition), although gCD (π–π* transition) of Cs[Eu((+)–hfbc)4] was the same as the aggregates. The emission intensity of the left-handed light is approximately 6 times larger than that of the right-handed light, which allowed the visualization of CPL with the naked eye using circularly polarized filters.
Figure 4.
(a) Supramolecular and (b, c) coordination-polymer-type chiral Eu(III) complexes.18−20
Compared to the large gCPL of the Eu(III) complexes with chiral β-diketonate ligands that contain a camphorate framework, the emission quantum yields are extremely low because of the existence of an energy quenching state for the emissive Eu(III) ion (1D aggregation of Cs[Eu((+)–hfbc)4]; emission quantum yield, Φtot = 1%).18 Our research group determined that the spiral polymerization of the Eu(III) complex with 3-trifluoroacetyl camphorate significantly increased the emission quantum yield.19 Herein, the Eu(III) coordination polymers were composed of chiral Eu(tfc)3 [tfc = 3-trifluoroacetylcamphorate) and dpbp joint ligand, [Eu(±tfc)3(dpbp)]n (dpbp = 4,4′-bis(diphenylphosphoryl)biphenyl), Figure 4b]. The tight packing of the Eu(III) coordination polymer afforded enhanced thermostability (332 °C). The rigid spiral-type chiral Eu(III) coordination polymer exhibited strong luminescence (Φtot = 30%) and high CPL activity (gCPL = 0.16) in comparison to those of the mononuclear chiral Eu(III) complex [Eu(tfc)3(tppo)2, tppo = triphenylphosphine oxide] in the solid state. The high Φtot is due to an effective photosensitized efficiency (ηsens = 53%) and internal emission quantum yield obtained by excitation of the lanthanide ion (Φff = 57%). The modulation of the ligand electronic structure through molecular integration is also presumed to suppress the energy quenching from the emissive Eu(III) ion, which results in a high emission quantum yield. The spiral coordinated Eu(III) polymer with the chiral phosphine oxide ligands and achiral β-diketonate ligands was also successfully synthesized by Hasegawa and co-workers (Figure 4c).20 The Eu(III) coordination polymer had a helical polymer structure with the characteristic hydrogen–fluorine/π interactions in the crystal. This polymer also exhibited high thermostability (320 °C) and an extremely high emission quantum yield obtained by the excitation of the lanthanide ion (Φff = 76%) in the solid state. The Eu(III) polymer shows an effective transformation from a polymer to a monomer structure in solution. This paper shows the gCPL (= 0.013) of the mononuclear structure in solution.
4. Electronic Structure Control for High CPL Activity
In section 3, the recent research which describes the achievement of high CPL activity by steric control through molecular integration is discussed. The CPL magnitude and sign can also be affected by the chiral electronic structure of the Eu(III) ion surrounded by external ligands in addition to the chiral steric structure. The electronic structural modulation of chiral Eu(+tfc)3 complexes (Figure 5a, Eu(tfc)3+tppo and Eu(tfc)3+ac) provides a dramatic change in the CPL property (Figure 5b). In this paper, large negative CPL signal of Eu(tfc)3+ac at 594 nm was observed, although a small positive signal for Eu(tfc)3+tppo was observed. The electronic transitions at 583 nm of Eu(tfc)3+tppo and Eu(tfc)3+ac show the same CPL sign and similar magnitudes.
Figure 5.
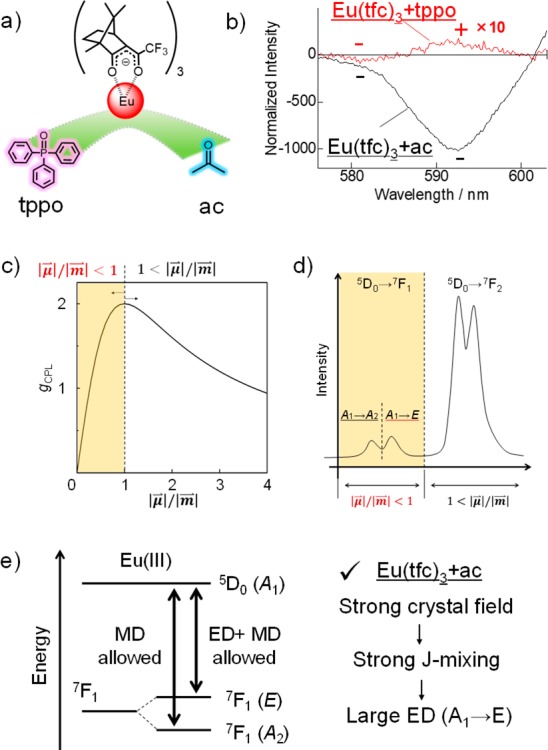
(a) Chiral Eu(III) complexes with achiral ligands. (b) Circularly polarized luminescence spectra and (c) a simulated gCPL curve as a function of the |μ⃗|/|m⃗| ratio in eq 4 with θ = 0°. (d) Photoluminescence spectrum and (e) energy diagram of the 5D0 → 7F1 transition of the Eu(III) complex (C4v or D2d) based on the electronic structure and group theory. Redrawn from ref (21).
The characteristic photophysical phenomena was analyzed using the eq 3 for gCPL. The equation can be modified as follows
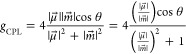 |
4 |
where θ is the angle between μ⃗ and m⃗. In the region |μ⃗|/|m⃗| < 1, the Eu(III) complex with a large |μ⃗| provides a high gCPL value (Figure 5c). In general, the |m⃗| value in the 5D0 → 7F1 transition is greater than the |μ⃗| value (|μ⃗|/|m⃗| < 1).22 Therefore, an enhanced |μ⃗| is important for the construction of the Eu(III) complex with large gCPL. The intensity of |μ⃗| in the 5D0 → 7F1 transition depends on the crystal field around the Eu(III) ion.23,24 The 7F1 energy level of the Eu(III) ion in a typical eight-coordinate structure (C4v or D2d) splits into two Stark sublevels (Figure 5d).9 The two bands at 583 and 594 nm in the CPL spectra are assigned to the A1 → A2 and A1 → E transitions, respectively, in Figure 5d,e. The observed CPL signals for the A1 → E transition are reversed for Eu(+tfc)3 with tppo compared to Eu(+tfc)3 with acetone, whereas those for the A1 → A2 transition show the same minus sign for Eu(+tfc)3 with tppo and acetone.
In C4v or D2d symmetry, the direct product A2 (= A1 × A2) is expressed in terms of the ED-forbidden (ED: electric dipole) and MD-allowed (MD: magnetic dipole) transitions on the character table in group theory (Figure 5e). Alternately, the direct product E (= A1 × E) produces ED- and MD-allowed transitions (Figure 5e). The CPL sign at 583 nm (for the ED-forbidden A1 → A2 transition) reflects the intrinsic Λ- or Δ-type structure because of the insensitive electronic state mixing. Considering the same CPL sign at 583 nm for Eu(+tfc)3 with tppo and acetone, the structure type (Λ or Δ) with tppo is the same as that with acetone in the experiments. In contrast, the CPL sign in the ED- and MD-allowed A1 → E transition is sensitive to the electronic state mixing even for the same chiral structure type (Λ or Δ).
The drastic change in the intensity of the CPL signal in the A1 → E transition is also considered to be caused by the change in μ⃗ based on electronic state mixing. In the 5D0 → 7F1 transition, μ⃗ is mainly altered by the J mixing of 7F2 or 7F3 sublevels into 7F1. In the photoluminescence spectra, the Stark splitting energy of Eu(+tfc)3 with acetone is greater than that of Eu(+tfc)3 with tppo. The large Stark splitting energy suggests a large amount of J mixing in the A1 → E transition of the Eu(III) complex with acetone. The J mixing increases the |μ⃗| value of the ED-allowed A1 → E transition relative to that of the ED-forbidden A1 → A2 transition. An increase in |μ⃗| results in a high gCPL value in eq 4. In the case of the previously reported mononuclear Eu(III) complexes with chiral steric structures,15 a strong A1 → E transition is also related to a large gCPL. The angle (θ) between μ⃗ and m⃗ for the Eu(tfc)3 complex with acetone is greater than 90°, whereas that for the Eu(tfc)3 complex with tppo is less than 90°, suggesting that the angle is established by the change in the μ⃗ vector due to J mixing. The significant enhancement of gCPL from +0.013 to −1.0 also indicates that J mixing promotes the antiparallel direction of μ⃗ and m⃗, leading to a large gCPL. This paper demonstrates that the CPL sign and intensity are strongly influenced by the chiral electronic structure depending on μ⃗ under J mixing in the same chiral structure type. This CPL study provides a novel aspect for the molecular design of chiral Eu(III) complexes by maximizing the gCPL value and altering its sign. In the future, a detailed description of the electronic structure based on calculation science25,26 may accelerate research on electronic structure control.
5. Recent CPL Applications
5.1. Recent CPL-Based Devices
Circularly polarized (CP) electroluminescence (CP-OLEDs) is rapidly attracting considerable interest owing to its possible applications in 3D displays. The development of more efficient CP-OLEDs is required for such applications. Di Bari and co-workers found that the chiroptical properties strongly depend on the thickness of Al as a cathode layer. The authors also demonstrated that the chiral Eu complex-based CP-OLEDs (Al thickness: 6 nm) show a remarkable CPL with a large dissymmetry factor (gCPL = −1.0).27 Furthermore, the chiral Eu(III) complexes can be applied not only to the electrical devices but also to the optical devices. Parker demonstrated a chiral image contrast using Eu(III) complexes with efficient light-absorption ability and a high emission quantum yield for CPL microscopy (Figure 6b).28
Figure 6.
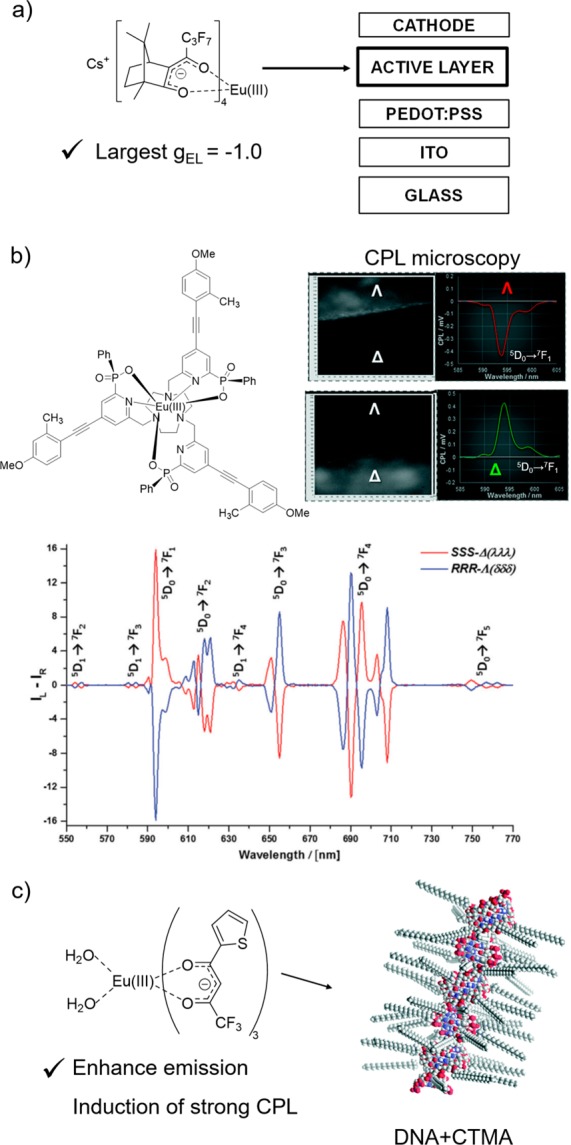
(a) CP-OLED architecture (adapted from ref (27)) and (b) a chiral Eu(III) complex with high CPL activity, CPL microscopy images, and CPL spectra. (The reproduction of material is from ref (28).) (c) DNA-based chiral Eu(III) complex. Redrawn from ref (29). Copyright (2018) The Royal Society of Chemistry.
Nakamura and Kobayashi described the DNA-based functional materials, which are of significant interest for applications in electrical and optical devices.29 They prepared novel DNA-based photofunctional materials fabricated by the association of a DNA–cetyltrimethylammonium chloride (CTMA) complex with a luminescent Eu(III) complex. The emission intensity, quantum yield, and thermal stability of the Eu(III) complex hosted by the DNA–CTMA matrix were superior to those of the complex in a conventional poly(methyl methacrylate) matrix. Furthermore, the Eu(III) complex in the DNA–CTMA film also exhibited high CPL activity through the excitation of the ligand moiety. The CPL originated from the chiral environment produced by the DNA matrix. The composite films of DNA–CTMA and luminescent lanthanide(III) complexes provide new insights for the construction of materials with high CPL activity for future applications.
5.2. CPL-Based Monitoring Methods around the Lanthanide Ion
The effective Eu(III) CPL provides information about the chiral environment around the Eu(III) ion. Researchers have developed CPL-based probes for various organic compounds. Neil and co-workers successfully demonstrated that CPL probes are useful for investigating the reversible binding of anionic chiral species such as phosphorylated amino acids and peptides.30 CPL probes are also useful for the determination of the reversible binding and competitive binding of different proteins.31−33 Recently, methods for monitoring the ADP/ATP ratio or sialic acid via the induced CPL of Eu(III) complexes have been demonstrated.34,35 These results illustrate the utility of thoughtfully designed Eu(III) probes to signal changes in the local chiral environment.
6. Conclusion
In this mini-review, the recent developments in lanthanide CPL that evaluate the steric and electronic structures to construct a luminophore with high CPL activity are described. The tetramer, dimer, aggregates, and coordination polymer-type Eu(III) complexes show high CPL activity with a large gCPL and emission quantum yields. The CPL applications based on the Eu(III) complexes are also described from the viewpoint of their practical use in devices. Further studies on the luminescent Eu(III) complexes with high CPL activity can open frontier areas in the fields of photochemistry and materials science.
Acknowledgments
This work was supported by a Grant-in-Aid for grant numbers 17K14467, 19H04556, 18H04497, and 18H02041. This work was also supported by the Institute for Chemical Reaction Design and Discovery (ICReDD), established by the World Premier International Research Initiative (WPI) of MEXT, Japan. This study was supported in part by Grants-in-Aid for the regional R&D proposal-based program from the Northern Advancement Center for Science & Technology of Hokkaido, Japan.
Biographies
Yuichi Kitagawa obtained his Ph.D. (2012) from the School of Engineering, University of Tokyo. He was a research fellow of the Japan Society for the Promotion of Science (JSPS) (2013) at Ritsumeikan University. He was an assistant professor (2014–2018) at Hokkaido University. Since 2019, he has been a lecturer at Hokkaido University. His interests are molecular photophysics and photochemistry. He is now studying the luminescence, chiroptical, and magneto-optical properties of lanthanide (III) complexes.
Makoto Tsurui is a first year master course student at Hokkaido University under the supervision of Prof. Yasuchika Hasegawa. He completed his undergraduate degree from Hokkaido University. He is now studying the luminescence and chiroptical properties of lanthanide (III) complexes.
Yasuchika Hasegawa obtained his Ph.D. (1997) at the Garaduate School of Engineering, Osaka University and was a researcher (1994–1999) at New Japan Chemical Co. Ltd. He was an assistant professor (1999–2005) at Osaka University and an associate professor (2005–2010) at Nara Institute of Science and Technology. Since 2010, he has been a professor at Hokkaido University. He received, the JPA Award (2013) and the CSJ (The Chemical Society of Japan) Award for Creative Work (2014) and an Award of the MEXT (Minister of Education, Culture, Sports, Science and Technology, Japan). His focus is the study of lanthanide photochemistry.
The authors declare no competing financial interest.
References
- Mason S. F.Molecular Optical Activity and the Chiral Discriminations; Cambridge University Press, 1982. [Google Scholar]
- Riehl J. P.; Richardson F. S. Circularly polarized luminescence spectroscopy. Chem. Rev. 1986, 86, 1–16. 10.1021/cr00071a001. [DOI] [Google Scholar]
- Sang Y. T.; Han J. L.; Zhao T. H.; Duan P. F.; Liu M. H.. Circularly Polarized Luminescence in Nanoassemblies: Generation, Amplification, and Application. Adv. Mater. 2019, 1900110, 10.1002/adma.201900110. [DOI] [PubMed] [Google Scholar]
- Muller G. Luminescent Chiral Lanthanide(iii) Complexes as Potential Molecular Probes. Dalton Trans. 2009, 9692–9707. 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R.; Evans N. H.; Parker D. Lanthanide Complexes as Chiral Probes Exploiting Circularly Polarized Luminescence. Chem. Soc. Rev. 2012, 41, 7673–7686. 10.1039/c2cs35242g. [DOI] [PubMed] [Google Scholar]
- Zinna F.; Di Bari L. Lanthanide Circularly Polarized Luminescence: Bases and Applications. Chirality 2015, 27, 1–13. 10.1002/chir.22382. [DOI] [PubMed] [Google Scholar]
- Circularly Polarised Luminescence. In Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials; de Bettencourt-Dias A., Ed.; John Wiley & Sons: Chichester, U.K., 2014; pp 77–124. [Google Scholar]
- Bünzli J.-C. G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. 10.1016/j.ccr.2014.10.013. [DOI] [Google Scholar]
- Binnemans K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. 10.1016/j.ccr.2015.02.015. [DOI] [Google Scholar]
- Hasegawa Y.; Kitagawa Y.; Nakanishi T. Effective Photosensitized, Electrosensitized, and Mechanosensitized Luminescence of Lanthanide Complexes. NPG Asia Mater. 2018, 10, 52–70. 10.1038/s41427-018-0012-y. [DOI] [Google Scholar]
- Luk C. K.; Richardson F. S. Circularly polarized luminescence and energy transfer studies on carboxylic acid complexes of europium(III) and terbium(III) in solution. J. Am. Chem. Soc. 1975, 97, 6666–6675. 10.1021/ja00856a012. [DOI] [Google Scholar]
- Brittain H. G.; Richardson F. S. Circularly Polarized Emission Studies on the Chiral Nuclear Magnetic Resonance Lanthanide Shift Reagent Tris(3-trifluoroacetyl-d-camphorato)europium(III). J. Am. Chem. Soc. 1976, 98, 5858–5863. 10.1021/ja00435a018. [DOI] [Google Scholar]
- Brittain H. G.; Richardson F. S.; Martin R. B. Terbium(III) Emission as a Probe of Calcium(II) Binding Sites in Proteins. J. Am. Chem. Soc. 1976, 98, 8255–8260. 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- Spaulding L.; Brittain H. G. Complexation of Amino-Acids by Terbium(III) Ethylenediaminetetraacetate. Inorg. Chem. 1985, 24, 3692–3698. 10.1021/ic00216a044. [DOI] [Google Scholar]
- Lunkley J. L.; Shirotani D.; Yamanari K.; Kaizaki S.; Muller G. Chiroptical Spectra of a Series of Tetrakis((+)-3-heptafluorobutylyrylcamphorato)lanthanide(III) with an Encapsulated Alkali Metal Ion: Circularly Polarized Luminescence and Absolute Chiral Structures for the Eu(III) and Sm(III) Complexes. Inorg. Chem. 2011, 50 (12), 12724–12732. 10.1021/ic201851r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C.-T.; Yim K.-H.; Wong H.-Y.; Pal R.; Lo W.-S.; Yan S.-C.; Yee-Man Wong M.; Yufit D.; Smiles D. E.; McCormick L. J.; Teat S. J.; Shuh D. K.; Wong W.-T.; Law G.-L. Chiral Transcription in Self-Assembled Tetrahedral Eu4L6 Chiral Cages Displaying Sizable Circularly Polarized Luminescence. Nat. Commun. 2017, 8, 1128. 10.1038/s41467-017-01025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y.; Nakashima T.; Yamada M.; Yuasa J.; Rapenne G.; Kawai T. Hierarchical Emergence and Dynamic Control of Chirality in a Photoresponsive Dinuclear Complex. J. Phys. Chem. Lett. 2018, 9, 2151–2157. 10.1021/acs.jpclett.8b00690. [DOI] [PubMed] [Google Scholar]
- Kumar J.; Marydasan B.; Nakashima T.; Kawai T.; Yuasa J. Chiral Supramolecular Polymerization Leading to Eye Differentiable Circular Polarization in Luminescence. Chem. Commun. 2016, 52, 9885–9888. 10.1039/C6CC05022K. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y.; Miura Y.; Kitagawa Y.; Wada S.; Nakanishi T.; Fushimi K.; Seki T.; Ito H.; Iwasa T.; Taketsuga T.; Gon M.; Tanaka K.; Chujo Y.; Hattori S.; Karasewa M.; Ishii K. Spiral Eu(iii) Coordination Polymers with Circularly Polarized Luminescence. Chem. Commun. 2018, 54, 10695–10697. 10.1039/C8CC05147J. [DOI] [PubMed] [Google Scholar]
- Koiso N.; Kitagawa Y.; Takayuki N.; Koji F.; Yasuchika H. Eu(III) Chiral Coordination Polymer with a Structural Transformation System. Inorg. Chem. 2017, 56, 5741–5747. 10.1021/acs.inorgchem.7b00337. [DOI] [PubMed] [Google Scholar]
- Wada S.; Kitagawa Y.; Nakanishi T.; Gon M.; Tanaka K.; Fushimi K.; Chujo Y.; Hasegawa Y. Electronic Chirality Inversion of Lanthanide Complex Induced by Achiral Molecules. Sci. Rep. 2018, 8, 16395. 10.1038/s41598-018-34790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson F. S.; Berry M. T.; Reid M. F. Ligand Polarization Contributions to Lanthanide 4f → 4f Magnetic Dipole Transition Moments and Rotatory Strengths. Mol. Phys. 1986, 58, 929–945. 10.1080/00268978600101681. [DOI] [Google Scholar]
- Forsberg J. H. Complexes of Lanthanide (III) Ions with Nitrogen Donor Ligands. Coord. Chem. Rev. 1973, 10, 195–226. 10.1016/S0010-8545(00)80235-0. [DOI] [Google Scholar]
- Ma C. G.; Brik M. G.; Kiisk V.; Kangur T.; Sildos I. Spectroscopic and Crystal-field Analysis of Energy Levels of Eu3+ in SnO2 in Comparison with ZrO2 and TiO2. J. Alloys Compd. 2011, 509, 3441–3451. 10.1016/j.jallcom.2010.12.071. [DOI] [Google Scholar]
- Gendron F.; Moore B.; Cador O.; Pointillart F.; Autschbach J.; Le Guennic B. Ab Initio Study of Circular Dichroism and Circularly Polarized Luminescence of Spin-Allowed and Spin-Forbidden Transitions: From Organic Ketones to Lanthanide Complexes. J. Chem. Theory Comput. 2019, 15, 4140–4155. 10.1021/acs.jctc.9b00286. [DOI] [PubMed] [Google Scholar]
- Wu T.; Prusa J.; Kessler J.; Dracinsky M.; Velenta J. Detection of Sugars via Chirality Induced in Europium (III) Compounds. Anal. Chem. 2016, 88, 8878–8885. 10.1021/acs.analchem.6b02505. [DOI] [PubMed] [Google Scholar]
- Zinna F.; Pasini M.; Galeotti F.; Botta C.; Di Bari L.; Giovanella U. Design of Lanthanide-Based OLEDs with Remarkable Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2017, 27, 1603719. 10.1002/adfm.201603719. [DOI] [Google Scholar]
- Frawley A. T.; Pal R.; Parker D. Very Bright, Enantiopure Europium(iii) Complexes Allow Time-Gated Chiral Contrast Imaging. Chem. Commun. 2016, 52, 13349–13352. 10.1039/C6CC07313A. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Minami H.; Sagara A.; Itamoto N.; Kobayashi N. Enhanced Red Emissions of Europium(iii) Chelates in DNA-CTMA Complexes. J. Mater. Chem. C 2018, 6, 4516–4522. 10.1039/C8TC00255J. [DOI] [Google Scholar]
- Neil E. R.; Fox M. A.; Pal R.; Parker D. Induced Europium CPL for the Selective Signalling of Phosphorylated Amino-Acids and O-Phosphorylated Hexapeptides. Dalton Trans. 2016, 45, 8355–8366. 10.1039/C6DT01212D. [DOI] [PubMed] [Google Scholar]
- Carr R.; Puckrin R.; McMahon B. K; Pal R.; Parker D.; Palsson L.-O. Induced Circularly Polarized Luminescence Arising from Anion or Protein Binding to Racemic Emissive Lanthanide Complexes. Methods Appl. Fluoresc. 2014, 2, 024007. 10.1088/2050-6120/2/2/024007. [DOI] [PubMed] [Google Scholar]
- Shuvaev S.; Suturina E. A.; Mason K.; Parker D. Chiral probes for [small alpha]1-AGP reporting by species-specific induced circularly polarised luminescence. Chem. Sci. 2018, 9, 2996–3003. 10.1039/C8SC00482J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L.; Waters R. S.; Pal R.; Parker D. Induced Europium Circularly Polarized Luminescence Monitors Reversible Drug Binding to Native A1-Acid Glycoprotein. ChemMedChem 2017, 12, 271–277. 10.1002/cmdc.201600571. [DOI] [PubMed] [Google Scholar]
- Shuvaev S.; Fox M. A.; Parker D. Monitoring the ADP/ATP Ratio via Induced Circularly-Polarised Europium Luminescence. Angew. Chem., Int. Ed. 2018, 57, 7488–7492. 10.1002/anie.201801248. [DOI] [PubMed] [Google Scholar]
- Neil E. R.; Parker D. Selective Signalling of Sialic Acid in Solution by Circularly Polarised Luminescence Spectroscopy Using a Dynamically Racemic Europium(iii) Complex. RSC Adv. 2017, 7, 4531–4540. 10.1039/C6RA26662B. [DOI] [Google Scholar]