Abstract
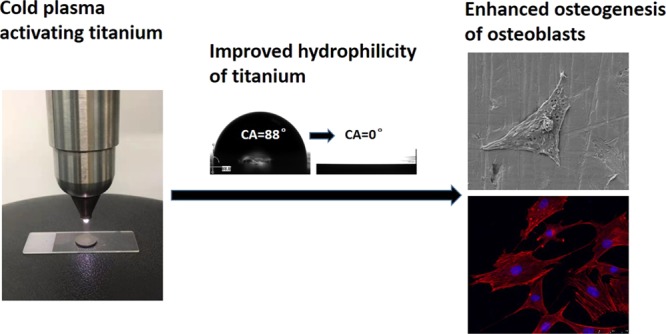
Although titanium is the most commonly used dental implant material, its biological aging directly leads to a lower rate of osseointegration. The aim of this study is to treat aged titanium disc surfaces using low-temperature argon–oxygen plasma (LTAOP) to obtain a more hydrophilic surface in order to enhance biological activities of osteoblasts on dental implant materials. In this study, smooth-machined titanium (SM Ti) and sandblasted and acid-etched titanium (SLA Ti) substrates were used. Aged titanium discs (SM and SLA Ti) were activated by LTAOP and the surface properties were analyzed. Osteoblasts were then seeded onto the aged and LTAOP-treated surfaces. Cell morphology, viability, and features of osteogenesis were examined. We showed that after the LTAOP treatment, the surfaces of both SM and SLA titanium substrates become more hydrophilic with a larger active oxygen species composition, whereas no obvious morphological changes were observed. Osteoblasts were found to be attached and stretched well on the surfaces of LTAOP treatment specimens. Moreover, the proliferation and osteocalcin secretion of osteoblasts on the plasma-activated titanium samples were superior to the untreated counterparts. LTAOP activation can enhance the attachment, proliferation, and mineralization of osteoblasts on the surfaces of the aged titanium substrates. This research provides a new strategy to modify the surface of titanium dental implants for improved biological functions.
Introduction
Titanium implants, based on the theory of osseointegration, have become favorable options for tooth rehabilitation in the dental area because of their excellent combination of biocompatibility properties.1 Osseointegration means a direct contact between reconstructed bone tissue and the implants surfaces.2 Throughout the process of osseointegration, bone regeneration around the implants is relatively slow and early mineral density of newly formed bone is relatively low, even though the implant surfaces have been modified in many ways such as physical, chemical, and biological methods.3
It was related to the biological aging of the implant surface, which reduced the biological response of the peri-implant tissues to the implant. Studies showed that although the surface of titanium, which is the most widely used material in dental implant, had high biological activities right after the (chemical or physical) modification, the biological activities of dental implant materials usually decrease along with time when exposed to air.4 This time-dependent biological aging process can affect the interactions between the implants and the proteins/cells.5 Explanations for this phenomenon have been proposed. The presence of a surface native oxide layer (TiO2; titania; passive film) of 2–5 nm thickness, which is naturally formed as titanium is exposed to air, is crucial to the biocompatibility of titanium. With high surface energy, the newly processed titania show enhanced hydrophilic properties and exhibit high affinity to osteoblasts, and thus increase cell proliferation in a short period of time.6 On the other hand, aged surfaces have a low hydrophilicity level, which prevents proteins and extracellular matrix of osteoblasts from adhering to the surface. Therefore, the cell attachment and proliferation are reduced.5a In addition, the amount of organic impurities containing hydrocarbon adsorbed on TiO2 increased after air exposure, which affects the initial affinity level for osteoblasts, consequently affecting bone morphogenesis and the degree of bone–titanium integration.5a,7 As the bioaging of a titanium surface directly influences the initial biological environment of bone formation and decreases the speed of osseointegration, one of the longstanding challenges is how to maintain the activity of titanium dental implants and/or restore the biological functions of implant materials after bioaging, thus accelerating the biological response of the peri-implant tissues to the implant.
In this study, we try to introduce low-temperature plasma to solve this problem. Atmospheric-pressure low-temperature plasma, also called cold plasma, is the partially ionized gas in the thermodynamic nonequilibrium state.8 Because the electron temperature is much higher than the ion temperature, the apparent temperature of gas is usually much lower and even down to room temperature, making it possible for chemical reaction to be carried out in the human mouth besides the dental chair. Containing high-energy electrons and a large number of excited atoms, molecules, ions, free radicals, and other active substances, low-temperature plasma can produce different surface treatment effects under different reaction conditions. (1) Surface cleaning: under suitable discharge parameters, argon plasma can remove chemical residues, adsorbed pollutants, impurities, and oxide layers from the surface of pure titanium.9 (2) Surface sterilization: low-temperature oxygen plasma can efficiently kill Gram-positive and -negative bacteria, yeasts, fungi, and spores as well as other microorganisms with active species generated in plasma.10 (3) Surface modification: surface modification by low-temperature plasma can connect a large variety of chemical functional groups to the surface of materials in a short time, resulting in significantly improved surface wettability, cell compatibility, and so forth.11 In addition, with no need for time-consuming operational procedure under atmospheric pressure, low-temperature plasma also has the advantages like high efficiency, low energy consumption, safety, and no secondary pollution. These results provide a theoretical basis for the application of cold plasma in dental implant surface processing. In this study, we use argon and oxygen mixture in a certain proportion as the input gas, and our hypothesis is that low-temperature plasma produced by the argon and oxygen mixture can produce an ideal effect on the titanium implant surface activation.
Based on the advantages of low-temperature plasma, the objective of this study was to instantly activate the bioaged titanium surface and restore bioactivity of the titanium surface via low-temperature argon–oxygen plasma (LTAOP) treatment in order to enhance cell attachment, proliferation, and mineralization. After the plasma treatment, surface analysis including surface morphology, hydrophilicity, and chemical composition was conducted. Moreover, Sprague-Dawley (SD) rat osteoblasts were cultured and seeded onto different specimens to examine the biological activity of the titanium materials. We hope our study can provide an experimental basis for new ways to maintain high biological activity of normal aging titanium before dental implantation and thus accelerate the process of osseointegration.
Results
Surface Characterization of LTAOP-Activated Titanium Samples
Surface Morphology
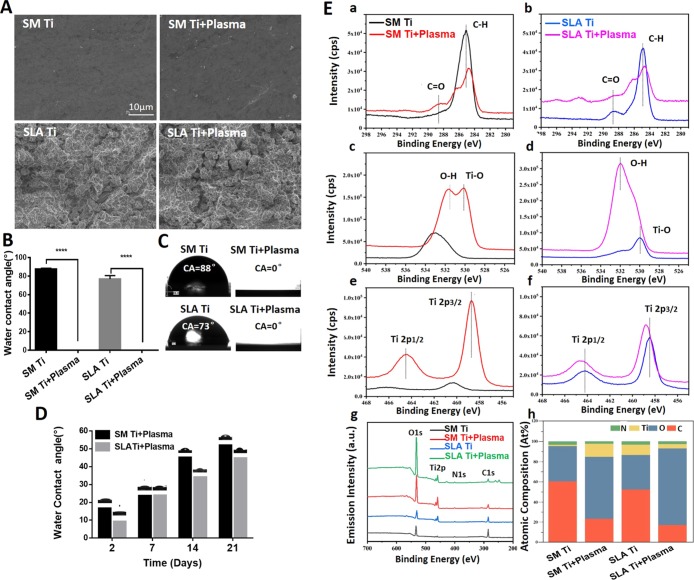
Surface morphologies of titanium discs before and after LTAOP treatment were examined by scanning electron microscopy (SEM). Smooth machined Ti (SM Ti) used in this study showed a typical surface morphology of its kind with a smooth surface at the supra-microscopic level, whereas there was a classic roughness feature at the micron level on the surface of sandblasted and acid-etched titanium (SLA Ti) disc (Figure 2A). However, there was no visible change in surface morphologies before and after low-temperature plasma treatment.
Figure 2.
Surface analysis of Ti discs before and after LTAOP treatment. (A) SEM images of samples, showing that there were no differences in surface morphologies before and after LTAOP treatment on SM Ti and SLA Ti surfaces. Scale bar represents 10 μm. (B,C) Water droplet images and water CA analysis of Ti discs before and after LTAOP treatment. Results showed the CAs of the treated groups were almost 0°, which are significantly smaller than the control groups (****, p < 0.0001). (D) Changes of CA of LTAOP-activated titanium samples. After 2, 7, 14, 21 days following LTAOP treatment, the CAs of distilled H2O on the SM Ti and SLA Ti increased gradually as time elapsed. (E) Chemical composition of the titanium samples measured by high-resolution XPS. (a,b) C 1s spectra, (c,d) O 1s spectra, and (e,f) Ti 2p spectra [(a,c,e) are for SM Ti; (b,d,f) are for SLA Ti] were determined. (g) High-resolution XPS spectra of Ti specimens without or with LTAOP treatments. (h) Atomic percentage of each element on the surface before and after LTAOP treatment.
Surface Hydrophilicity
The hydrophilicity of the control and experimental groups was measured using water contact angle (CA) analysis. There were significant differences of CAs between the control and LTAOP-treated groups (p < 0.05) (Figure 2B,C). Before LTAOP treatment, the water CA of the SM Ti and SLA Ti was 87.83 ± 0.33 and 76.80 ± 2.16°, respectively. After LTAOP treatment, the CAs were not measurable because of the superhydrophilicity with a CA of 0° (freshly processed samples; Figure 2C). However, after 2, 7, 14, 21 days following LTAOP treatment, the CAs of distilled H2O on the SM Ti and SLA Ti discs had changed to 16.13 ± 0.48 and 8.70 ± 0.51, 23.17 ± 0.34 and 23.43 ± 0.55, 44.80 ± 0.65 and 33.60 ± 0.82, 51.60 ± 0.73 and 44.30 ± 0.22°, respectively (Figure 2D). These values were significantly higher than those of freshly processed samples (p < 0.01).
Surface Chemistry
Chemical shifts and changes in the chemical composition on each Ti surface were confirmed by X-ray photoelectron spectroscopy (XPS). As shown in Figure 2E, both SM Ti and SLA Ti discs showed C 1s peaks, with the dominant peak corresponding to the hydrocarbon (−CH) at a binding energy of 285.0 eV (C1). Compared to samples without plasma treatment, the carbon composition (C–H) of LTAOP-treated discs decreased because of cleaning of carbon contamination on the titanium discs (Figure 2E(a,b,g)). Additionally, there was a small decline in the C2 peak (288.6 eV) corresponding to carbon–oxygen (C–O) bonds for both SM Ti and SLA Ti after LTAOP treatment. Similarly, the atomic content of carbon following LTAOP treatment was lower than that of those samples before LTAOP treatment, regardless of Ti surface morphology (Figure 2E(h)). In terms of the O 1s spectra, the major peak corresponding to Ti–O at a binding energy of 530.0 eV was evident for all the samples, and the peak intensity was increased after LTAOP treatment compared to specimens before LTAOP treatment, regardless of the Ti surface morphology (Figure 2E(c,d,g)). The observed changes are considered to be able to increase the hydrophilicity of the surfaces as well as the cell attachment capability.12 Additionally, the peak for the hydroxyl group (−OH) on SM Ti and SLA Ti discs at a binding energy of 532.0 eV increased after LTAOP treatment. Further analysis revealed that these changes resulted in an increase in the relative atomic content of oxygen after LTAOP exposure for both SM Ti and SLA Ti discs (Figure 2E(h)). Finally (Figure 2E(e,f,g)), shows the peaks for Ti 2p1/2 and Ti 2p3/2 (458.8 eV) components; both peaks increased in intensity regardless of the Ti surface morphologies after LTAOP exposure.
Enhanced Adhesion and Spreading of Osteoblasts on LTAOP-Activated Titanium Samples
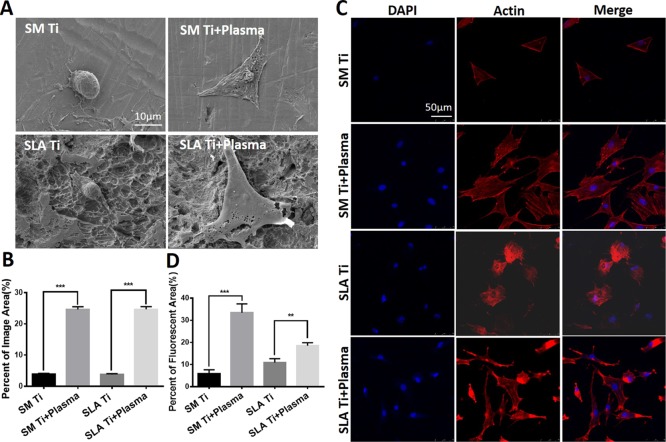
The morphology of osteoblasts on the titanium surfaces before and after LTAOP activation was observed by SEM. SEM images of osteoblasts showed that the cells were larger on LTAOP-activated titanium surfaces than on the untreated surfaces after 12 h of incubation (Figure 3A). Cells on treated surfaces demonstrated excellent adhesion and stretch, whereas the majority of cells on the untreated surfaces were shrunk and in round shape. On the surface of smoothly machined titanium discs treated by plasma, cells were obviously flat and uniformly attached to the surface of the material, and all the edges of the cells were stretched and anchored. What was different on the surface of treated SLA Ti was that cells are fibroid or polygonal in shape, extending thick and long synapses around and forming a bridge across the rough area. The quantification data show that the area of cells covering increased dramatically after plasma treatment (Figure 3B).
Figure 3.
Morphology and spreading behaviors of osteoblasts on LTAOP-activated titanium samples. (A) SEM images of osteoblasts morphologies cultured on different surfaces. Scale bar represents 10 μm. (B) Quantification of osteoblasts’ covering area in each group. The percentage area of cells covering LTAOP-treated samples increased dramatically compared to the control ones (***, p < 0.001). (C) Images of cytoskeletal actin of osteoblasts imaged by laser confocal microscopy. Scale bar represents 50 μm. (D) Quantification of osteoblasts’ fluorescent area in each group. The percentages of fluorescent area in LTAOP-treated groups were higher than those in untreated groups (**, p < 0.01, ***, p < 0.001).
Figure 3C shows the fluorescent staining of cytoskeletal actin of osteoblasts in all tested samples. After 24 h of incubation, confocal microscopic images of rhodamine phalloidin-stained osteoblasts showed that the cells were larger and much more stretched with greater numbers of cells. More intense and mature cytoskeletal development on plasma-treated surfaces than on untreated surfaces was observed (Figure 3C). For SM Ti surfaces, cells on treated surfaces showed a large area of long spindles around the nucleus, and polygonal actin bundles arranged and stretched more fully into a network with a number of cell processes, whereas the majority of cells on the untreated surfaces were a triangle and did not show enough cytoskeletal development. More interestingly, cells on treated SLA Ti surfaces were stretched with development of lamellipodia-like actin projections in multiple directions, which was specifically localized in the ends of cellular processes. The quantification results show that the areas of cells covering were significantly larger after the plasma treatment (Figure 3D). However, the percent of fluorescent area in the SLA Ti + plasma group was lower than that of the SM Ti + plasma group, which mostly likely originated from the different properties of SM and SLA Ti surfaces.
LTAOP Promotes the Proliferation of Osteoblasts on Activated Titanium Samples
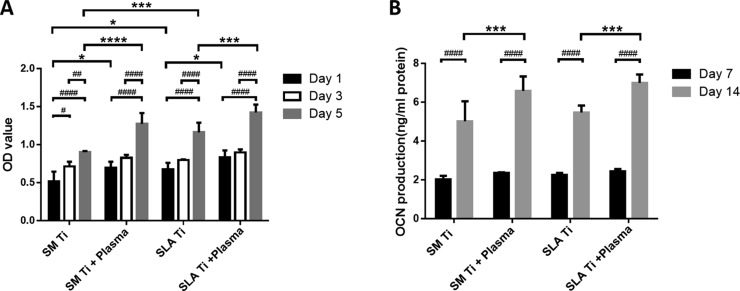
In this study, methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay was used to evaluate the viability of osteoblasts cultured on the specimens before and after plasma treatment. The results of osteoblast proliferation tests showed that the number of osteoblasts in each group increased steadily with the extension of culture duration, and there were more cells on plasma-activated surfaces than on untreated surfaces (Figure 4A). The cell proliferation rate of seeded osteoblasts on the SLA Ti group was determined to be higher than those on the SM Ti groups. However, there are no significant differences between the SM Ti + plasma group and the SLA Ti + plasma group. It was proved that the titanium surface treated by low-temperature plasma could efficiently promote the proliferation of osteoblasts.
Figure 4.
Proliferation and mineralization of osteoblasts on LTAOP-activated titanium samples. (A) MTT assay results showed after 5 days of culture that the rates of proliferation increased substantially in cultures seeded on plasma-treated surfaces (*, p < 0.05, ***, p < 0.001, ****, p < 0.0001, control vs treated groups, SM Ti vs SLA Ti, #, p < 0.05, ##, p < 0.01, ####, p < 0.0001, day 1 vs day 3 vs day 5). (B) Results of OCN expression of osteoblast cells on the titanium surface showed after 14 days of culture that OCN secreted by osteoblasts in the LTAOP-treated groups were significantly higher than those in the control groups (***, p < 0.001, control vs treated groups, ####, p < 0.0001, day 7 vs day 14).
LTAOP Promotes the Mineralization of Osteoblasts on Activated Titanium Samples
The results of the osteocalcin (OCN) assays are shown in Figure 4B. On days 7 and 14, the OCN secreted by osteoblasts increased gradually in both the plasma-treated and control groups. Furthermore, OCN secreted by osteoblasts on the plasma-treated groups were significantly higher than those on the control surfaces. These results indicate that plasma-activated titanium surfaces could efficiently promote the mineralization of osteoblasts.
Discussion
Interactions between the dental implants and alveolar bones are crucial for the clinical success of dental implants.13 The bioactivity nature of implants’ surfaces plays important roles in the osseointegration process.14 Titanium-based materials’ aging process directly affects their biological responses,5a leading to a lower rate in the process of osseointegration. Prevention of an unwanted aging process as well as development of activation strategies for aged materials are important. One aim of this study was to apply the recently highlighted portable LTAOP to modify the titanium surface. Another aim was to explore whether LTAOP activation can enhance the attachment, proliferation, and mineralization of osteoblasts on the titanium surfaces.
Several groups have produced promising results by modifying implants using low-temperature plasma with argon gas. Duske et al. treated different surfaces of titanium sheets with argon plasma jet, and found that the extensibility of the seeded osteoblasts was significantly improved.11 Coelho et al. embedded dental implants into the mandible of Beagle dogs after treatment with argon plasma at atmospheric pressure, and showed that the implant-bone contact rate of the treatment group was significantly higher than that of the control group at 3 weeks, indicating that argon plasma-modified implant surfaces can enhance early bone reaction of Beagle dogs.15 We have demonstrated previously that in a low-temperature plasma environment, the argon and oxygen mixture in a certain proportion can generate a large amount of energetic species such as active oxygen, by energy transfer reaction, the formation of which can make the surface energy of the treated material increase significantly.16 This in vitro study showed that LTAOP may be effective to activate the titanium implant surface for bone healing.
LTAOP activation has proven its efficacy on aged surfaces of commercially pure Ti-based materials without altering their existing morphologies. This was consistent with the results of previous studies.17 As shown in the results, there were significant changes of surface hydrophilicity and chemistry on the titanium surfaces. The CAs of experimental groups’ Ti were not measurable, proving the superhydrophilicity of the surfaces with LTAOP treatment. Plasma generated with argon as input gas has been shown to create hydrophilic surfaces,18 whereas in our study LTAOP could also produce superhydrophilic characteristics on titanium surfaces. The mechanism of superhydrophilic characteristics involves numerous kinds of chemical reactions and specific changes in chemical composition following LTAOP treatment, as XPS has revealed. In terms of chemical functional groups, the amount of carbon was decreased whereas oxygen-bonded compounds increased after the LTAOP treatment. Consistent with the other study,19 XPS results indicate that the constituents of the LTAOP are active enough to break the C–H bonds at the surface layer to form radicals that yield various reactive oxygen species on the surface. The carbon, as the result of the unavoidable attachment of carbon-containing atmospheric component on the surface, is normally identified on the surfaces. However, the adsorption of carbon on the surface could potentially reduce biological activity and it should be reduced to achieve improved activity.19b,20 The reduction of the carbon peak intensity and increase in the oxygen peak intensity were related to two aspects of changes on the surface with plasma treatment: surface cleaning by removing carbon chemical residues and simultaneous surface modification by connecting a large number of oxygen-bonded functional groups onto the surface of substrate. Moreover, the peaks for Ti 2p1/2 and Ti 2p3/2 components increased in intensity after plasma exposure. It indicated the surface changes in the electrochemical structure of titanium, which might generate charge loading via electronic transition by the energy of the plasma.
Osteoblasts taken from skull bone of newborn SD suckling mice were used to evaluate the biological and osteogenic effects of different titanium surfaces. Cell morphological changes demonstrated that on the surface of titanium activated by LTAOP, cells showed a flattened shape and anchored evenly to the surface, exhibiting excellent adhesion and stretch properties with great numbers of cell processes and mature cytoskeletal development. This result confirmed that LTAOP used in this study could be a very feasible way to enhance cellular responses on titanium implants’ surfaces. This could be related to the increase in the surface oxygen-bonded radicals and a decrease of carbon impurities after plasma treatment, which would further increase the surface hydrophilicity and surface energy.21 In fact, the surface energy of the material plays an important role in promoting early cell adhesion. The higher of the surface energy induces the better of the early attachment of osteoblasts. Additionally, the generation of oxygen-bonded components could absorb fibronectin to regulate the structure of proteoglycan and the cytoskeleton.22
This study also demonstrated that LTAOP activation on Ti was effective in increasing the proliferation and mineralization of osteoblasts. After 1, 3, and 5 days of culture, MTT assays showed that the surfaces submitted to the LTAOP treatment allowed a sufficient increase in the cell proliferation in comparison with control groups. To ascertain the effects of the LTAOP on mineralization, we examined the activity of OCN, a protein serving as a marker of osteoblast mineralization. Results revealed that LTAOP activation of Ti surfaces can promote OCN expression, which was expected to increase the cell mineralization. These changes were because of the introduction of hydrophilicity, the possible adsorption of adhesive proteins including fibronectin, and the consequent upregulation of integrin-mediated signaling pathways. Furthermore, free radicals like hydroxyl radicals and exposed charged particles on the Ti surface treated with argon–oxygen plasma might accelerate intracellular bioelectrical signaling transduction and stimulate osteoblast proliferation. Bioelectrical signaling, including all the changes in the resting voltage potential of the plasma membrane driven by ion channels, pumps, and gap junctions, serves as a highly efficient information-bearing pathway that regulates cell proliferation, migration, and differentiation.23
In this study, we observed that there were some different results between rough-surfaced titanium and smooth ones. Sandblast-etched titanium as commonly used implant surfaces in studies showed higher hydrophilicity and increased relative atomic content of oxygen after LTAOP exposure compared to SM Ti. In agreement with previous studies,24 osteoblasts adherent to SLA Ti have been revealed to proliferate slightly faster than those adherent to smooth-surfaced ones. However, after LTAOP activation, osteoblasts on different surfaces showed a similar growth trend and level of proliferation and mineralization, which are not affected by surface morphology.
Also, there are other ways of restoring bioactivity of aged titanium such as photo-functionalization with UV light.25 In a recent study, Canullo et al. confirmed that the effects of UV and plasma on different titanium surfaces. They found similar effects on protein adsorption and cell adhesion with plasma after 12 min of treatment and UV light after 3 h of treatment.26 Compared to UV, LTAOP could activate the surface of titanium in a shorter time by a few minutes, even seconds. Moreover, low-temperature plasma could connect many different kinds of chemical functional groups onto the surface such as reactive oxygen species, so that the surface wettability and cell compatibility of materials could be significantly improved. These results provide a new method for the application of cold plasma in dental implant surface activation.27 It was observed that the hydrophilicity of the plasma-treated surfaces decreased gradually, and the effects on osteoblasts’ attachment, proliferation, and mineralization need further investigation.
Conclusions
In conclusion, we investigated the effect of LTAOP activation of aged SM Ti and SLA Ti surfaces. The LTAOP-activated surface showed carbon-cleaned and oxygen-enriched hydrophilicity. Osteoblast cells’ adhesion, proliferation, and mineralization were all significantly improved. With further in vivo studies, the low-temperature plasma treatment could be a potential effective approach to activate titanium-based dental implants for improved performances.
Experimental Section
Titanium Sample Preparation
SM Ti and SLA Ti are two kinds of commonly studied titanium-based implant materials. Titanium samples were prepared in disc (diameter, 15 mm; thickness, 1 mm) form by machining commercially pure titanium (grade 4). All discs were kindly provided by the Biomaterials Research Center, Sichuan University. SM surfaces were used as they were, whereas roughened surfaces were prepared by blasting with 50 μm Al2O3 particles for 1 min at 3 kg/m followed by etching with 19% hydrofluoric acid (w/w) at room temperature for 30 s. Titanium samples were then stored under dark ambient conditions for 6 weeks to allow sufficient aging.7a After the aging process, the specimens were decontaminated by ultrasonic rinse using a series of acetone, ethanol, and deionized water for 10 min each, and then sterilized through autoclave.
LTAOP Activation
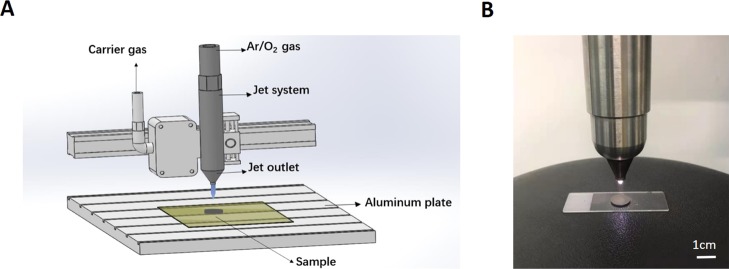
Titanium samples in the experimental groups were activated by LTAOP treatment using an atmospheric pressure glow discharge plasma system. Plasma polymerization was carried out using the Atmospheric Pressure Plasma System model AS400+PFW10, manufactured by Plasma Treat GmbH (Steinhagen, Germany). This plasma system, powered by a low-frequency power (19 kHz) supply and matched network, is a glow-discharge plasma workspace with dimensions of 33 cm by 12.5 cm.28 A schematic diagram of the system is shown in Figure 1A.
Figure 1.
LTAOP activating titanium samples. (A) Schematic diagram of the atmospheric pressure glow discharge plasma system. In this system, 95% Ar and 5% O2 were used as inputting gas to generate plasma. (B) Plasma treatment of a titanium specimen. Scale bar represents 1 cm.
Sample surfaces were cleaned and bombarded for 5 min with argon gas, and then underwent plasma treatment. Argon (Ar, 95%) and oxygen (O2, 5%) were used to generate plasma. Argon gas carried the precursor vapors to the jet outlet at a flow rate of 300 L/h. The precise mass flow rates were ensured with the aid of an integrated mass flow meter acting in conjunction with the control system. The substrates were fixed on an aluminum plate and the plasma jet was controlled by an X/Y/Z motion system. The plasma voltage, current, and duty ratio were 320 V, 21.4 A, and 0.6, respectively. To evaluate the plasma efficiency on Ti discs, the Ti discs were placed 2 cm in front of a nozzle (diameter = 5 mm) in the plasma system and exposed to plasma for 90 s (Figure 1B). It should be noted that the freshly processed samples were used in the surface property analysis and cell culture studies.
Surface Characterization of Titanium Samples
Surface Morphology
The surface morphology was examined before and after the LTAOP activation using SEM (VEGA TESCAN, Czech Republic).
Surface Hydrophilicity
Before and after the LTAOP treatment, changes in the hydrophilicity of SM Ti and SLA Ti discs were evaluated by water CA analysis. Distilled water (10 μL) was placed on the center of each specimen surface for 10 s, and the CA was confirmed by capturing the image at room temperature using a live video CA measurement system (Phoenix a, Meiwa-forces, Japan). Potential time-dependent change in hydrophilicity was determined for different surfaces. Freshly prepared, 2-day-old, 1-week-old, 2-week-old, and 3-week-old surfaces were all examined.
Surface Chemistry
Surface chemical composition of the control and treated groups was confirmed using X-ray photoelectron spectra (Thermo Escalab 250XI, Thermo Fisher Scientific, USA). A monochromatic Al Kα source was operated as the X-ray source (Al Kα line: 1486.6 Ev). The binding energy was referenced and calibrated to the C 1s peak at 284.8 Ev. The atomic composition and characteristics of C, O, and Ti were analyzed for each group.
Osteoblast Cell Culture and Identification
SD 1–3 day-old rats were immersed in 75% ethanol for 5 min, and the skull bones were taken under aseptic conditions with ophthalmic scissors. The attached connective tissue was removed with tweezers as much as possible. The bone tissue was soaked for 5 min in antibiotic solution (100 u/mL penicillin, 100 u/mL streptomycin). The tissue was rinsed three times in phosphate buffered solution (PBS), and then cut into small pieces of about 1 mm3 in size in a Petri dish containing an appropriate amount of Dulbecco’s modified Eagle’s medium. The shredded tissue pieces were evenly placed on the bottom of the culture flask at a distance of 5 mm between small pieces. Alpha-MEM cell culture medium (5 mL, LM008-01, Welgene, Korea) combined with 10% fetal bovine serum (Gibco, USA), 1% 100 u/mL penicillin, and 100 u/mL streptomycin were injected into the bottle. The flask was tilted after cap closure, and placed in an incubator with 5% CO2, 37 °C. After 4 h of stasis, the culture bottle was slowly turned over, so that the culture medium could completely soak the tissue block on the wall of the bottle. Then, the culture bottle was placed back in the incubator. At 80% confluency, the cells were purified three times by way of differential velocity adherences between osteoblasts and fibroblasts. After being transferred to the third generation, cells were identified as osteoblasts by methods of alkaline phosphatase staining and calcium nodule staining. Then, osteoblasts cultured to the third generation were detached with 0.25% trypsin and seeded onto the titanium discs with a density of 1 × 104 cells/mL. The culture medium was renewed every 3 days.
Morphology and Spreading Behaviors of Osteoblasts on Titanium Samples
SEM was used to observe the morphology of osteoblasts on the titanium surfaces. Cell suspension was inoculated into a 24-well plate containing titanium samples with a dilution density of 1 × 104 cells/mL; 12 h after seeding, cells underwent the following series of processes in order to be observed by SEM—fixed in 4% paraformaldehyde for 60 min, rinsed twice in PBS, fixed in 1% osmium acid for 60 min at 4 °C, dehydrated in 50–100% ethanol, and immersed in isoamyl acetate for 30 min. After critical point drying and vacuum spraying, the morphology of the osteoblasts on titanium surfaces was imaged by SEM.
The spreading behavior and cytoskeletal arrangement of osteoblasts seeded onto titanium surfaces were examined using confocal laser scanning microscopy (Leica TCS SP5 II, Leica SPE, Germany). In order to visualize the localization of actin, at 24 h after seeding, cells were fixed in 4% paraformaldehyde and stained using the fluorescent dye rhodamine phalloidin (actin filament, red color) (40734ES75, Yeasen Biotech Co., Ltd., China). 4′,6-Diamidino-2-phenylindole (cell nucleus, blue color) (40728ES03, Yeasen Biotech Co., Ltd.) was used for counterstaining. Image collection was performed in triplicate and representative images are shown. The areas of cell covering were quantified using image analysis software (ImageJ, NIH, Bethesda, ML).
Cell Proliferation Assay
MTT (thiazole blue) assay was employed to evaluate the cell proliferation. Osteoblasts cultured to the third generation were seeded onto the specimens at a density of 2 × 104 cells/mL with a volume of 200 μL and cultured for 1, 3, 5 days in the 24-well plates. MTT (20 μL, C0009, Beyotime, China) reagent (5 mg/mL) was added and incubated for another 2 h. After aspirating the supernatant in the well, 150 μL of dimethyl sulfoxide was added to each well and the plates were shaken for 10 min. Absorbance of the final solution was measured at 450 nm and cell viability was calculated according to optical density value. Experiments were performed in three replicates. Means and standard deviations are shown.
OCN Assay
OCN is an osteoblast-specific protein and regarded as a marker of differentiated osteoblast cells. Its activity assay can provide insight into the ability of osteoblast cells to mature and mineralize. After osteoblast cells were seeded onto the specimens and cultured for 7 and 14 days, an enzyme-linked immunosorbent assay (ELISA) kit (E-EL-R0243c, Elabscience Biotechnology Co., Ltd., China) was used to detect the expression levels of OCN quantitatively. The ELISA procedure was performed according to the manufacturer’s protocol. Experiments were repeated three times. Means and standard deviations are shown.
Statistical Analysis
The number of samples was three for all in vitro culture studies (n = 3). Mean values and SDs were calculated for each outcome variable in this work. Differences before and after LTAOP treatment were analyzed via Analysis of Variance method. Statistical significance was accepted at a confidence level of 95% (p < 0.05). Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, IL).
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant no. 81500882]. L.W. and W.W. acknowledge the Biomaterials Research Center, Sichuan University, for providing the titanium samples. The authors would also like to thank Prof. Dagang Miao’s lab at Qingdao University for technical support and facilities’utilization.
Author Contributions
# L.W. and W.W. contributed equally to this study and are co-first-authors.
The authors declare no competing financial interest.
References
- Adell R.; Lekholm U.; Rockler B.; Brånemark P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Albrektsson T.; Brånemark P.-I.; Hansson H.-A.; Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- a Thevenot P.; Hu W.; Tang L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cuijpers V. M. J. I.; Jaroszewicz J.; Anil S.; Al Farraj Aldosari A.; Walboomers X. F.; Jansen J. A. Resolution, sensitivity, and in vivo application of high-resolution computed tomography for titanium-coated polymethyl methacrylate (PMMA) dental implants. Clin. Oral Implants Res. 2014, 25, 359–365. 10.1111/clr.12128. [DOI] [PubMed] [Google Scholar]; c Sjöström T.; McNamara L. E.; Yang L.; Dalby M. J.; Su B. Novel anodization technique using a block copolymer template for nanopatterning of titanium implant surfaces. ACS Appl. Mater. Interfaces 2012, 4, 6354–6361. 10.1021/am301987e. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Ogawa T. The Biological Aging of Titanium Implants. Implant Dent. 2012, 21, 415–421. 10.1097/ID.0b013e31826a51f4. [DOI] [PubMed] [Google Scholar]
- a Att W.; Hori N.; Takeuchi M.; Ouyang J.; Yang Y.; Anpo M.; Ogawa T. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. 10.1016/j.biomaterials.2009.06.040. [DOI] [PubMed] [Google Scholar]; b Hori N.; Att W.; Ueno T.; Sato N.; Yamada M.; Saruwatari L.; Suzuki T.; Ogawa T. Age-dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. 10.1177/0022034509339567. [DOI] [PubMed] [Google Scholar]
- Favero R.; Lang N. P.; Salata L. A.; Neto E. C. M.; Caroprese M.; Botticelli D. Sequential healing events of osseointegration at UnicCa and SLActive implant surfaces: an experimental study in the dog. Clin. Oral Implants Res. 2016, 27, 203–210. 10.1111/clr.12591. [DOI] [PubMed] [Google Scholar]
- a Att W.; Hori N.; Iwasa F.; Yamada M.; Ueno T.; Ogawa T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009, 30, 4268–4276. 10.1016/j.biomaterials.2009.04.048. [DOI] [PubMed] [Google Scholar]; b Att W.; Takeuchi M.; Suzuki T.; Kubo K.; Anpo M.; Ogawa T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials 2009, 30, 1273–1280. 10.1016/j.biomaterials.2008.11.024. [DOI] [PubMed] [Google Scholar]
- a Jeffries C. D. Electron-Hole Condensation in Semiconductors: Electrons and holes condense into freely moving liquid metallic droplets, a plasma phase with novel properties. Science 1975, 189, 955–964. 10.1126/science.189.4207.955. [DOI] [PubMed] [Google Scholar]; b Simionescu C.; Denes F.; Onac D.; Bloos G. Synthesis of some amino acids, sugars, and peptides in cold plasma abiotic synthesis of some high-molecular-weight proteid-like structures (V). Biopolymers 1974, 13, 943–954. 10.1002/bip.1974.360130510. [DOI] [PubMed] [Google Scholar]; c Simionescu C. I.; Totolin M. I.; Denes F. Abiotic synthesis of some polysaccharide-like and polypeptide-like structures in cold plasma. Biosystems 1976, 8, 153–158. 10.1016/0303-2647(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Aronsson B.-O.; Lausmaa J.; Kasemo B. Glow discharge plasma treatment for surface cleaning and modification of metallic biomaterials. J. Biomed. Mater. Res. 1997, 35, 49–73. . [DOI] [PubMed] [Google Scholar]
- a Annunziata M.; Canullo L.; Donnarumma G.; Caputo P.; Nastri L.; Guida L. Bacterial inactivation/sterilization by argon plasma treatment on contaminated titanium implant surfaces: In vitro study. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e118–e121. 10.4317/medoral.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Montie T. C.; Kelly-Wintenberg K.; Roth J. R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. 10.1109/27.842860. [DOI] [Google Scholar]
- Duske K.; Koban I.; Kindel E.; Schröder K.; Nebe B.; Holtfreter B.; Jablonowski L.; Weltmann K. D.; Kocher T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. 10.1111/j.1600-051x.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- Iwasa F.; Hori N.; Ueno T.; Minamikawa H.; Yamada M.; Ogawa T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. 10.1016/j.biomaterials.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Guglielmotti M. B.; Olmedo D. G.; Cabrini R. L. Research on implants and osseointegration. Periodontology 2019, 79, 178–189. 10.1111/prd.12254. [DOI] [PubMed] [Google Scholar]
- Barfeie A.; Wilson J.; Rees J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. 10.1038/sj.bdj.2015.171. [DOI] [PubMed] [Google Scholar]
- Coelho P. G.; Giro G.; Teixeira H. S.; Marin C.; Witek L.; Thompson V. P.; Tovar N.; Silva N. R. F. A. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res., Part A 2012, 100, 1901–1906. 10.1002/jbm.a.34127. [DOI] [PubMed] [Google Scholar]
- a Liu F.; Sun P.; Bai N.; Tian Y.; Zhou H.; Wei S.; Zhou Y.; Zhang J.; Zhu W.; Becker K.; Fang J. Inactivation of Bacteria in an Aqueous Environment by a Direct-Current, Cold-Atmospheric-Pressure Air Plasma Microjet. Plasma Process. Polym. 2010, 7, 231–236. 10.1002/ppap.200900070. [DOI] [Google Scholar]; b Bai N.; Sun P.; Zhou H.; Wu H.; Wang R.; Liu F.; Zhu W.; Lopez J. L.; Zhang J.; Fang J. Inactivation of Staphylococcus aureus in Water by a Cold, He/O2 Atmospheric Pressure Plasma Microjet. Plasma Process. Polym. 2011, 8, 424–431. 10.1002/ppap.201000078. [DOI] [Google Scholar]
- Jeong W.-S.; Kwon J.-S.; Choi E.-H.; Kim K.-M. The Effects of Non-Thermal Atmospheric Pressure Plasma treated Titanium Surface on Behaviors of Oral Soft Tissue Cells. Sci. Rep. 2018, 8, 15963. 10.1038/s41598-018-34402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.; Shah S.; Mani G.; Wenke J.; Agrawal M. Endothelial cell behaviour on gas-plasma-treated PLA surfaces: The roles of surface chemistry and roughness. J. Regen. Med. Tissue Eng. 2011, 5, 301–312. 10.1002/term.316. [DOI] [PubMed] [Google Scholar]
- a Seo H. Y.; Kwon J.-S.; Choi Y.-R.; Kim K.-M.; Choi E. H.; Kim K.-N. Cellular Attachment and Differentiation on Titania Nanotubes Exposed to Air- or Nitrogen-Based Non-Thermal Atmospheric Pressure Plasma. PloS One 2014, 9, e113477. 10.1371/journal.pone.0113477. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Choi S.-H.; Jeong W.-S.; Cha J.-Y.; Lee J.-H.; Yu H.-S.; Choi E.-H.; Kim K.-M.; Hwang C.-J. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci. Rep. 2016, 6, 36430. 10.1038/srep36430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R.; Ueno T.; Migita S.; Tsutsumi Y.; Doi H.; Ogawa T.; Hanawa T.; Wakabayashi N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698. 10.1177/0022034514536578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shen H.; Hu X.; Yang F.; Bei J.; Wang S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide). Biomaterials 2007, 28, 4219–4230. 10.1016/j.biomaterials.2007.06.004. [DOI] [PubMed] [Google Scholar]; b Wang H.; Kwok D. T. K.; Wang W.; Wu Z.; Tong L.; Zhang Y.; Chu P. K. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 2010, 31, 413–419. 10.1016/j.biomaterials.2009.09.066. [DOI] [PubMed] [Google Scholar]
- Salido M.; Vilches J. I.; Gutiérrez J. L. Actin cytoskeletal organization in human osteoblasts grown on different dental titanium implant surfaces. Histol. Histopathol. 2008, 22, 1355–1364. 10.14670/HH-22.1355. [DOI] [PubMed] [Google Scholar]
- Adams D. S.; Levin M. Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013, 352, 95–122. 10.1007/s00441-012-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.-X. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J. Biomed. Mater. Res., Part A 2013, 101, 748. 10.1002/jbm.a.34377. [DOI] [PubMed] [Google Scholar]
- Att W.; Ogawa T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: a novel understanding of osseointegration. Int. J. Oral Maxillofac. Implants 2012, 27, 753–761. 10.1016/s0022-3913(12)60182-6. [DOI] [PubMed] [Google Scholar]
- Canullo L.; Genova T.; Tallarico M.; Gautier G.; Mussano F.; Botticelli D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces. J. Dent. Res. 2016, 95, 566–573. 10.1177/0022034516629119. [DOI] [PubMed] [Google Scholar]
- Park C.; Park S. W.; Yun K. D.; Ji M. K.; Kim S.; Yang Y. P.; Lim H. P. Effect of Plasma Treatment and Its Post Process Duration on Shear Bonding Strength and Antibacterial Effect of Dental Zirconia. Materials 2018, 11, 2233. 10.3390/ma11112233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Pu Y.; Miao D.; Ning X. Fabrication of Durably Superhydrophobic Cotton Fabrics by Atmospheric Pressure Plasma Treatment with a Siloxane Precursor. Polymers 2018, 10, 460. 10.3390/polym10040460. [DOI] [PMC free article] [PubMed] [Google Scholar]