The title coordination polymers show different degrees of deprotonation of the disiloxane-dicarboxylate bridging ligands: both contain tetragonally distorted trans-NiN4O2 octahedra.
Keywords: crystal structure, macrocyclic ligand, cyclam, nickel, coordination polymers, hydrogen bonds
Abstract
The asymmetric units of the title compounds, namely, catena-poly[[(1,4,8,11-tetraazacyclotetradecane-κ4
N
1,N
4,N
8,N
11)nickel(II)]-μ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2
O:O′], [Ni(C10H24O5Si2)(C12H24N4)]n (I), and catena-poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4
N
1,N
4,N
8,N
11)nickel(II)]-μ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2
O:O′] perchlorate], {[Ni(C10H25O5Si2)(C12H24N4)]ClO4}n (II), consist of one (in I) or two crystallographically non-equivalent (in II) centrosymmetric macrocyclic cations and one centrosymmetric dianion (in I) or two centrosymmetric monoanions (in II). In each compound, the metal ion is coordinated by the four secondary N atoms of the macrocyclic ligand, which adopts the most energetically stable trans-III conformation, and the mutually trans O atoms of the carboxylate in a slightly tetragonally distorted trans-NiN4O2 octahedral coordination geometry. The crystals of both types of compounds are composed of parallel polymeric chains of the macrocyclic cations linked by the anions of the acid running along the [101] and [110] directions in I and II, respectively. In I, each polymeric chain is linked to four neighbouring ones by hydrogen bonding between the NH groups of the macrocycle and the carboxylate O atoms, thus forming a three-dimensional supramolecular network. In II, each polymeric chain contacts with only two neighbours, forming hydrogen bonds between the partially protonated carboxylic groups of the bridging ligand. As a result, a lamellar structure is formed with the layers oriented parallel to the (1 1) plane.
1) plane.
Chemical context
Transition-metal complexes of polyazamacrocyclic ligands, in particular of 1,4,8,11-tetraazacyclotetradecane (cyclam), are characterized by a number of unique properties, such as exceptionally high thermodynamic stability, kinetic inertness and unusual redox characteristics (Melson, 1979 ▸; Yatsimirskii & Lampeka, 1985 ▸), which have stimulated continuing interest in such systems for a number of decades. In conjunction with polycarboxylate ligands as spacers, macrocyclic complexes have been employed successfully for the construction of metal–organic frameworks (MOFs) (Lampeka & Tsymbal, 2004 ▸; Suh & Moon, 2007 ▸; Suh et al., 2012 ▸; Stackhouse & Ma, 2018 ▸), which are considered to be promising materials for applications in gas storage, separation, catalysis, etc. (Farrusseng, 2011 ▸; MacGillivray & Lukehart, 2014 ▸; Kaskel, 2016 ▸).
In contrast to the widespread rigid aromatic carboxylates, flexible spacers incorporating polymethylene chains have rarely been used for the design of MOFs, although this could potentially lead to frameworks possessing unusual properties, the most intriguing of which is a ‘breathing’ phenomenon (Elsaidi et al., 2018 ▸; Lee et al., 2019 ▸). A representative example of such a highly flexible ligand is 1,3-bis(3-carboxypropyl)tetramethyldisiloxane – a member of a rather restricted family of silicon-containing carboxylic acids. However, no attempt has been made so far to combine this ligand with macrocyclic complexes in MOF synthesis.
Here, we report the syntheses and crystal structures of the two coordination polymers built of the nickel(II) complex of the 14-membered macrocyclic ligand 1,4,8,11-tetraazacyclotetradecane (L) and the di- or monoanion of 1,3-bis(3-carboxypropyl)tetramethyldisiloxane (H2Cx), namely, catena-poly[[(1,4,8,11-tetraazacyclotetradecane-κ4
N
1,N
4,N
8,N
11)nickel(II)]-μ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2
O:O′], [Ni(L)(Cx)]n, (I) and catena-poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4
N
1,N
4,N
8,N
11)nickel(II)]-μ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2
O:O′] perchlorate], {[Ni(L)(HCx)]ClO4}n (II).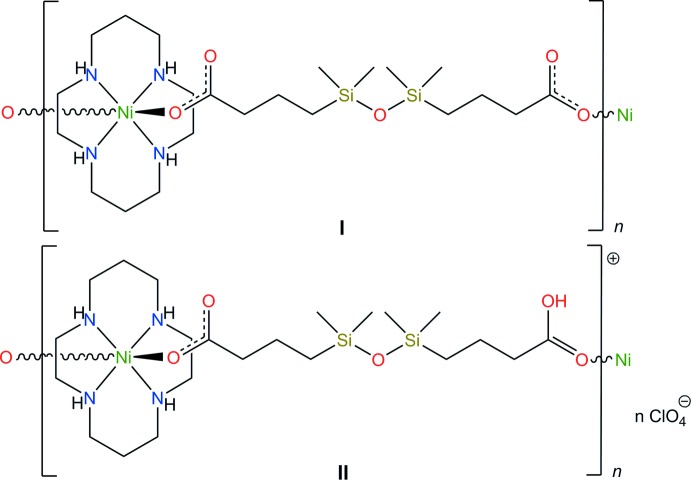
Structural commentary
The molecular structures of the title compounds are shown in Figs. 1 ▸ and 2 ▸. Both complexes are one-dimensional coordination polymers consisting of centrosymmetric macrocyclic [Ni(L)]2+ cations coordinated by the oxygen atoms of the carboxylic groups of the centrosymmetric acid, completely deprotonated (in I) and monoprotonated (in II), in the axial positions. In the latter case, there are two crystallographically independent cations and anions and the H2C and H5C acidic H atoms are distributed over two carboxylic groups with site occupancies of 50%.
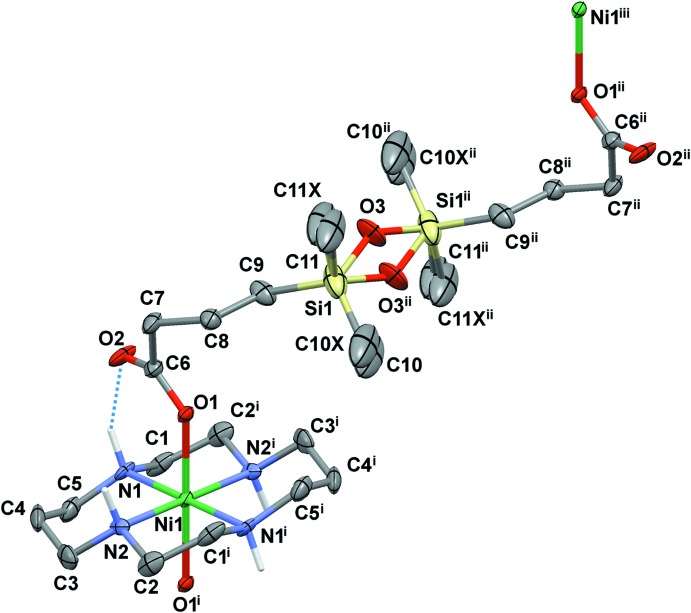
Figure 1.
View of the molecular structure of I showing atom-labelling scheme with displacement ellipsoids drawn at the 30% probability level. C-bound H atoms are omitted for clarity. Hydrogen-bonding interactions are shown as dotted lines. [Symmetry codes: (i) −x + 2, −y + 1, −z + 1; (ii) −x + 1, −y + 1, −z); (iii) x − 1, y, z − 1].
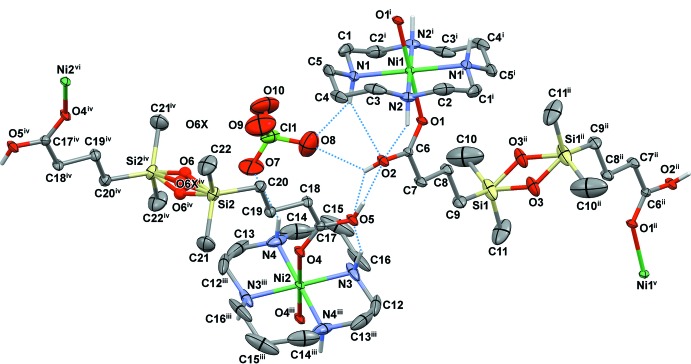
Figure 2.
View of the molecular structure of II showing atom-labelling scheme with displacement ellipsoids drawn at the 30% probability level. C-bound H atoms are omitted for clarity. Hydrogen-bonding interactions are shown as dotted lines. [Symmetry codes: (i) −x, −y, −z; (ii) −x − 1, −y − 1, −z; (iii) −x, −y − 1, −z − 1; (iv) −x + 1, −y, −z − 1; (v) x − 1, y − 1, z; (vi) x + 1, y + 1, z.]
The macrocyclic ligands in the complex cations adopt the most energetically favourable trans-III (R,R,S,S) conformation (Bosnich et al., 1965 ▸) with five-membered chelate rings in gauche and six-membered chelate rings in chair conformations. As a result of the presence of the inversion centres, all Ni(N4) fragments are strictly planar. The equatorial Ni—N bond lengths and bite angles fall in a range typical of high-spin 3d 8 nickel(II) complexes with 14-membered tetraamine ligands (Table 1 ▸). The axial Ni—O bond lengths are slightly longer than the Ni—N ones, and the geometry of the nickel(II) polyhedra can be described as tetragonally distorted trans-N4O2 octahedra.
Table 1. Selected geometrical parameters of the complex cations (Å, °).
| I | II | ||||
|---|---|---|---|---|---|
| Ni1—N1 | 2.071 (4) | Ni1—N1 | 2.058 (3) | Ni2—N3 | 2.043 (4) |
| Ni1—N2 | 2.060 (4) | Ni1—N2 | 2.060 (4) | Ni2—N4 | 2.054 (4) |
| Ni1—O1 | 2.113 (4) | Ni1—O1 | 2.125 (2) | Ni2—O4 | 2.131 (2) |
| N1—Ni1—N2i | 85.21 (19) | N1—Ni1—N2ii | 85.82 (17) | N3—Ni2—N4iii | 85.7 (2) |
| N1—Ni1—N2 | 94.79 (19) | N1—Ni1—N2 | 94.18 (17) | N3—Ni2—N4 | 94.3 (2) |
Symmetry codes: (i) −x + 2, −y + 1, −z + 1; (ii) −x, −y, −z; (iii) −x, −y − 1, −z − 1.
In two cases (Ni1 in I and Ni2 in II), a monodentate coordination of the carboxylate to the complex cation is complemented by strong hydrogen bonding between the non-coordinated O atom of the carboxylic group and the NH group of the macrocycle, which is often observed in complexes of cyclam-like ligands. For the [Ni1(L)]2+ cation in II, the non-coordinated O2 atom is almost equidistant from the N1 and N2 centres [3.225 (5) and 3.143 (4) Å, respectively], so that two weak hydrogen bonds are formed in this case (Figs. 1 ▸ and 2 ▸, Tables 2 ▸ and 3 ▸).
Table 2. Hydrogen-bond geometry (Å, °) for I .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O2 | 0.98 | 1.96 | 2.845 (6) | 150 |
| N2—H2⋯O2i | 0.98 | 2.07 | 2.883 (6) | 139 |
Symmetry code: (i) 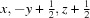 .
.
Table 3. Hydrogen-bond geometry (Å, °) for II .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O2 | 0.98 | 2.51 | 3.225 (5) | 130 |
| N1—H1⋯O8 | 0.98 | 2.45 | 3.315 (6) | 147 |
| N2—H2⋯O2 | 0.98 | 2.38 | 3.143 (4) | 134 |
| N3—H3⋯O5 | 0.98 | 2.01 | 2.901 (5) | 150 |
| N4—H4⋯O7 | 0.98 | 2.18 | 3.012 (6) | 142 |
| O2—H2C⋯O5 | 0.82 | 1.84 | 2.456 (4) | 131 |
| O2—H2C⋯O8 | 0.82 | 2.65 | 3.260 (5) | 133 |
| O5—H5C⋯O2 | 0.82 | 1.70 | 2.456 (4) | 151 |
The C—O bond lengths in the carboxylic group of the bridging ligand Cx2− in I are nearly identical [C6—O1 = 1.245 (7) and C6—O2 = 1.242 (7) Å], thus indicating essential electronic delocalization. At the same time, they differ significantly in II [C6—O1 = 1.232 (4) versus C6—O2 = 1.291 (5) Å; C17—O4 = 1.245 (4) versus C17—O5 = 1.280 (5) Å], so formally the Ni—O bonding in this compound can be treated as the interaction of the metal ion with the carbonyl oxygen atom of the carboxylic group.
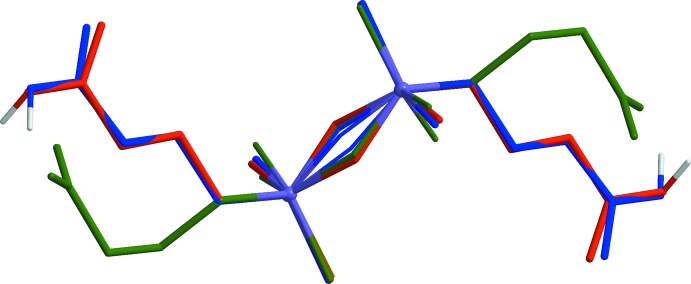
Because of the presence of flexible trimethylene fragments, the dicarboxylate ligand can adopt various conformations, both symmetric and asymmetric. In the present cases the anions possess a transoid conformation of the siloxane linkages with the disordered O3 atoms [site occupancies 50%, Si1—O3—Si1 = 141.2 (7) and 137.4 (4)° in I and II, respectively], as well as with the 25% occupancy atoms O6 and O6X in II [the corresponding Si2—O6(6X)—Si2 angles are 153.1 (17) and 167 (3)°, respectively] (Figs. 1 ▸ and 2 ▸). The geometries of the two crystallographically independent anions in complex II are actually very similar, but differ from that observed in complex I (Fig. 3 ▸).
Figure 3.
Comparison of the conformations of the dianion Cx2− in I (green) and of the monoanions HCx− in II (red and blue).
Supramolecular features
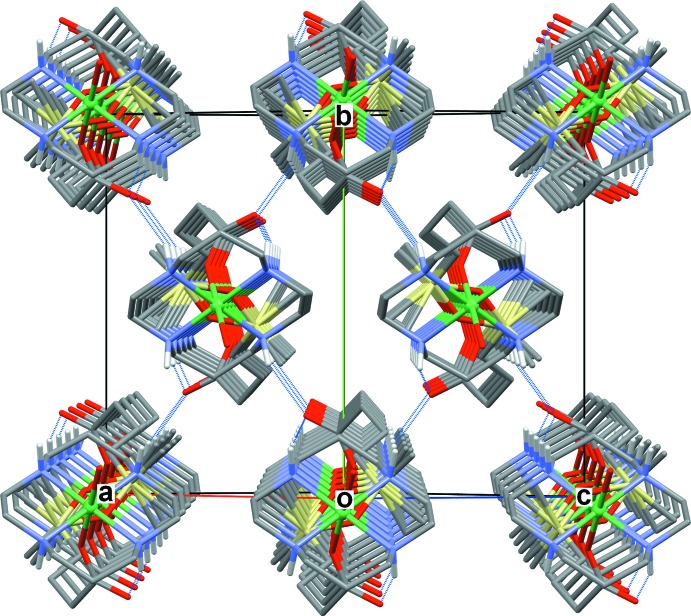
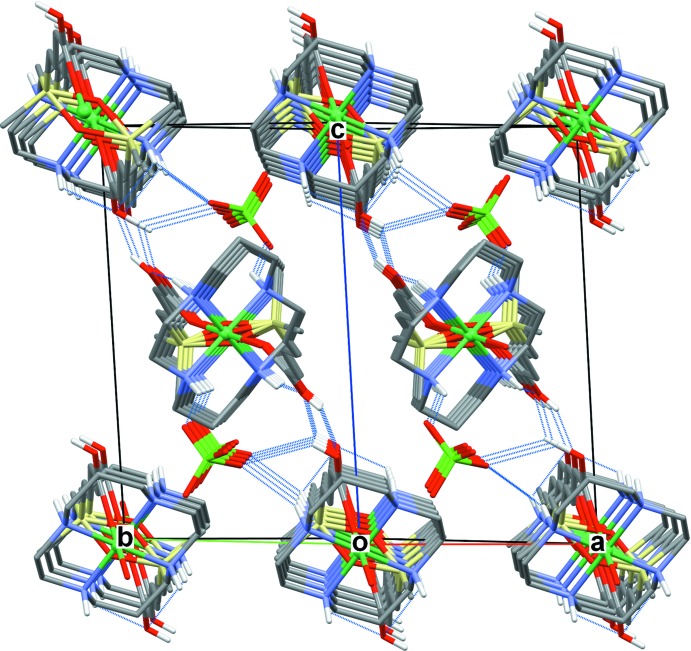
The crystals of both compounds are composed of parallel polymeric chains of [Ni(L)]2+ cations linked by carboxylate bridging ligands. The identical chains in I with an intra-chain Ni⋯Ni separation of 14.325 Å propagate along the [101] direction (Fig. 4 ▸). In II, two crystallographically independent chains formed by the Ni1 and Ni2 macrocyclic cations propagate along the [110] direction (Fig. 5 ▸) and are characterized by a slightly larger (14.684 Å) intra-chain separation between the NiII ions.
Figure 4.
The packing in I viewed down the [101] direction with polymeric chains cross-linked by N—H⋯O hydrogen bonds (dotted lines) to form a three-dimensional supramolecular network. C-bound H atoms are omitted for clarity.
Figure 5.
The packing in II viewed down the [110] direction with polymeric chains cross-linked by hydrogen bonds (dotted lines).
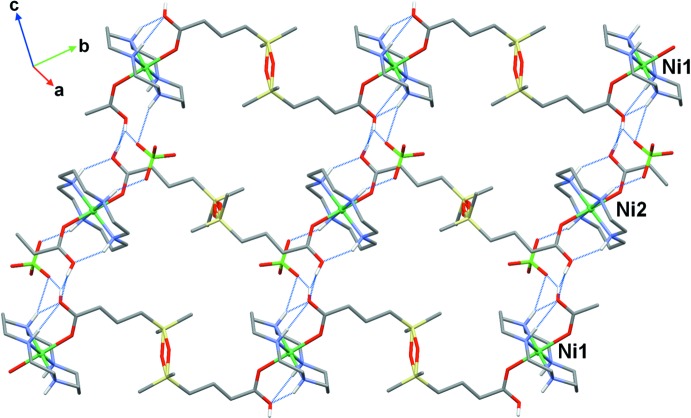
In the crystals, the interactions between the polymeric chains in I and II are characterized by markedly different features. In the first case, each chain is linked to four neighbouring ones as a result of hydrogen bonding between the N2—H2 groups of the macrocycles and carboxylate O2 atoms (Table 2 ▸), resulting in a three-dimensional supramolecular network. On the other hand, in II each polymeric chain contacts with only two neighbours via paired O2—H2C⋯O5/O2⋯H5C—O5 hydrogen bonds. The bonding is reinforced by the perchlorate anions bridging macrocyclic units: N1—H1⋯O8—Cl1—O7⋯H4—N4 (plus an additional very weak O2—H2C⋯O8 contact) (Table 3 ▸). As a result, a lamellar structure is formed with the layers lying parallel to the (1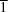 1) plane (Fig. 6 ▸).
1) plane (Fig. 6 ▸).
Figure 6.
The hydrogen-bonded sheet in II parallel to the (1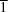 1) plane. C-bound H atoms are omitted for clarity.
1) plane. C-bound H atoms are omitted for clarity.
Database survey
A search of the Cambridge Structural Database (CSD, version 5.40, last update February 2019; Groom et al., 2016 ▸) indicated that seven compounds formed by 1,3-bis(3-carboxypropyl)tetramethyldisiloxane itself or its anions have been characterized structurally. Two of them are co-crystals of the acid with organic bases derived from pyridine [refcodes NERTOV (Vlad et al., 2013a ▸) and VIPZUR (Racles et al., 2013 ▸)]. Other complexes represent one- or two-dimensional coordination polymers formed by CuII (YIGXOD; Vlad et al., 2013b ▸), CoII (NERTIP; Vlad et al., 2013a ▸), ZnII [NERTUB (Vlad et al., 2013a ▸), GIWSAI (Vlad et al., 2014 ▸) and GAPKOA (Zaltariov et al., 2016 ▸)]. Except for the last complex, in which the secondary building unit is a hexametal oxocluster bridged by salicylaldoxime ligands, all of the other compounds contain additional heterocyclic co-ligands. No attempt was made to combine this carboxylic acid with macrocyclic cations in MOF synthesis, and thus the title compounds I and II are the first examples of such compounds described so far.
Synthesis and crystallization
All chemicals and solvents used in this work were purchased from Sigma–Aldrich and were used without further purification. The macrocyclic nickel(II) complex Ni(L)(ClO4)2 (Barefield et al., 1976 ▸) and 1,3-bis(3-carboxypropyl)tetramethyldisiloxane (H2Cx) (Mulvaney & Marvel, 1961 ▸) were prepared by the reported methods.
{Ni( L )(Cx)} n, (I). To a solution of 48 mg (0.24 mmol) of the ligand L in 4 ml of water, 30 mg of nickel(II) hydroxide (0.32 mmol) were added and the suspension stirred for 4 d at room temperature to give a yellow-coloured solution. The excess of Ni(OH)2 was filtered off and the filtrate was treated with the solution of 75 mg (0.24 mmol) of H2Cx in 2 ml of MeOH. This solution was rotary evaporated to give an oily material. The residue was dissolved in 2 ml of MeOH, and the product precipitated with acetonitrile. It was recrystallized in a similar fashion from a MeOH/MeCN (1:15 v/v) solvent mixture. Yield 54 mg (40%). Analysis calculated for C22H48N4NiO5Si2: C, 46.89; H, 8.59; N, 9.94%. Found: C, 46.76; H, 8.64; N, 9.85%.
Single crystals of I suitable for X-ray diffraction analysis were obtained analogously, except that precipitation was carried out using a diffusion regime (a methanolic solution of complex was layered with MeCN).
{[Ni( L )(HCx)]ClO4} n (II). A solution of 100 mg (0.26 mmol) of K2Cx in 1 ml of water was added to a solution of 130 mg (0.28 mmol) of [Ni(L)](ClO4)2 in 3 ml of water and the mixture was left at room temperature. Potassium perchlorate crystals, which formed after ca two weeks, were removed by filtration and the filtrate was allowed to evaporate slowly at room temperature. The crystals of the product formed after about one month. Yield 59 mg (34%). Analysis calculated for C22H49N4ClNiO9Si2: C, 39.80; H, 7.44; N, 8.44%. Found: C, 39.67; H, 7.51; N, 8.36%.
Single crystals of II suitable for X-ray diffraction analysis were selected from the sample resulting from the synthesis.
Safety note: Perchlorate salts of metal complexes are potentially explosive and should be handled with care.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 4 ▸. All H atoms in I and II were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H = 0.97 Å, N—H = 0.98 Å and carboxylate O—H = 0.82 Å, with U iso(H) values of 1.2 or 1.5U eq of the parent atoms.
Table 4. Experimental details.
| I | II | |
|---|---|---|
| Crystal data | ||
| Chemical formula | [Ni(C10H24O5Si2)(C12H24N4)] | [Ni(C10H25O5Si2)(C12H24N4)]ClO4 |
| M r | 563.53 | 663.99 |
| Crystal system, space group | Monoclinic, P21/c | Triclinic, P
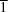
|
| Temperature (K) | 173 | 200 |
| a, b, c (Å) | 13.033 (5), 12.877 (10), 9.028 (3) | 9.3815 (7), 12.9009 (8), 14.7604 (10) |
| α, β, γ (°) | 90, 101.31 (3), 90 | 99.309 (5), 100.343 (6), 99.232 (6) |
| V (Å3) | 1485.7 (13) | 1700.9 (2) |
| Z | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.77 | 0.77 |
| Crystal size (mm) | 0.25 × 0.25 × 0.05 | 0.45 × 0.35 × 0.30 |
| Data collection | ||
| Diffractometer | Agilent Xcalibur, Eos | Agilent Xcalibur, Eos |
| Absorption correction | Multi-scan (CrysAlis PRO; Agilent, 2014 ▸) | Multi-scan (CrysAlis PRO; Agilent, 2014 ▸) |
| T min, T max | 0.694, 1.000 | 0.889, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 3957, 3957, 2499 | 9606, 9606, 5769 |
| R int | 0.040 | 0.063 |
| (sin θ/λ)max (Å−1) | 0.595 | 0.595 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.065, 0.143, 1.01 | 0.050, 0.115, 1.01 |
| No. of reflections | 3957 | 9606 |
| No. of parameters | 165 | 367 |
| No. of restraints | 6 | 7 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.56, −0.61 | 0.51, −0.44 |
Supplementary Material
Crystal structure: contains datablock(s) I, II. DOI: 10.1107/S2056989020002327/hb7892sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020002327/hb7892Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989020002327/hb7892IIsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Crystal data
| [Ni(C10H24O5Si2)(C12H24N4)] | F(000) = 608 |
| Mr = 563.53 | Dx = 1.260 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.033 (5) Å | Cell parameters from 468 reflections |
| b = 12.877 (10) Å | θ = 2.2–23.0° |
| c = 9.028 (3) Å | µ = 0.77 mm−1 |
| β = 101.31 (3)° | T = 173 K |
| V = 1485.7 (13) Å3 | Plate, clear light colourless |
| Z = 2 | 0.25 × 0.25 × 0.05 mm |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Data collection
| Agilent Xcalibur, Eos diffractometer | 3957 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2499 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.040 |
| Detector resolution: 16.1593 pixels mm-1 | θmax = 25.0°, θmin = 2.3° |
| ω scans | h = −15→15 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2014) | k = −15→15 |
| Tmin = 0.694, Tmax = 1.000 | l = −10→9 |
| 3957 measured reflections |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.065 | H-atom parameters constrained |
| wR(F2) = 0.143 | w = 1/[σ2(Fo2) + (0.0618P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max < 0.001 |
| 3957 reflections | Δρmax = 0.56 e Å−3 |
| 165 parameters | Δρmin = −0.61 e Å−3 |
| 6 restraints |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component twin. |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ni1 | 1.000000 | 0.500000 | 0.500000 | 0.0224 (3) | |
| Si1 | 0.51530 (16) | 0.4465 (2) | 0.1590 (4) | 0.0810 (8) | |
| O1 | 0.8723 (3) | 0.4020 (3) | 0.4131 (4) | 0.0278 (10) | |
| O2 | 0.9375 (4) | 0.2757 (3) | 0.2920 (5) | 0.0546 (13) | |
| O3 | 0.4991 (12) | 0.4662 (10) | −0.0400 (11) | 0.090 (4) | 0.5 |
| N1 | 1.1059 (4) | 0.4111 (4) | 0.4113 (5) | 0.0313 (13) | |
| H1 | 1.068906 | 0.348403 | 0.368383 | 0.038* | |
| N2 | 1.0173 (4) | 0.4173 (4) | 0.6987 (5) | 0.0302 (12) | |
| H2 | 0.974805 | 0.354292 | 0.677540 | 0.036* | |
| C1 | 1.1301 (5) | 0.4718 (5) | 0.2850 (7) | 0.047 (2) | |
| H1A | 1.161336 | 0.427243 | 0.219319 | 0.057* | |
| H1B | 1.179620 | 0.526309 | 0.323016 | 0.057* | |
| C2 | 0.9705 (5) | 0.4810 (5) | 0.8028 (7) | 0.050 (2) | |
| H2A | 1.018601 | 0.535473 | 0.845711 | 0.060* | |
| H2B | 0.955552 | 0.438364 | 0.884604 | 0.060* | |
| C3 | 1.1239 (5) | 0.3849 (5) | 0.7630 (7) | 0.051 (2) | |
| H3A | 1.123929 | 0.344748 | 0.853931 | 0.061* | |
| H3B | 1.167199 | 0.445811 | 0.790758 | 0.061* | |
| C4 | 1.1691 (6) | 0.3211 (6) | 0.6537 (9) | 0.056 (2) | |
| H4A | 1.118692 | 0.267578 | 0.614158 | 0.067* | |
| H4B | 1.230881 | 0.286356 | 0.709093 | 0.067* | |
| C5 | 1.1991 (5) | 0.3767 (5) | 0.5216 (8) | 0.050 (2) | |
| H5A | 1.241788 | 0.436626 | 0.557967 | 0.061* | |
| H5B | 1.240623 | 0.330662 | 0.471842 | 0.061* | |
| C6 | 0.8685 (5) | 0.3154 (5) | 0.3510 (7) | 0.0354 (16) | |
| C7 | 0.7696 (5) | 0.2514 (5) | 0.3470 (8) | 0.0449 (18) | |
| H7A | 0.778986 | 0.208718 | 0.437187 | 0.054* | |
| H7B | 0.760544 | 0.205156 | 0.260604 | 0.054* | |
| C8 | 0.6702 (5) | 0.3156 (5) | 0.3376 (7) | 0.0403 (18) | |
| H8A | 0.613522 | 0.269978 | 0.350886 | 0.048* | |
| H8B | 0.680861 | 0.365496 | 0.419680 | 0.048* | |
| C9 | 0.6386 (4) | 0.3731 (5) | 0.1896 (8) | 0.0517 (19) | |
| H9A | 0.634154 | 0.323019 | 0.108349 | 0.062* | |
| H9B | 0.694402 | 0.421166 | 0.180578 | 0.062* | |
| C10X | 0.4972 (14) | 0.5342 (14) | 0.313 (2) | 0.139 (5) | 0.5 |
| H10A | 0.504595 | 0.495602 | 0.405513 | 0.209* | 0.5 |
| H10B | 0.428590 | 0.564443 | 0.289455 | 0.209* | 0.5 |
| H10C | 0.548903 | 0.588187 | 0.324362 | 0.209* | 0.5 |
| C11 | 0.3986 (16) | 0.369 (2) | 0.171 (7) | 0.139 (5) | 0.5 |
| H11A | 0.340588 | 0.414111 | 0.173486 | 0.209* | 0.5 |
| H11B | 0.412287 | 0.327371 | 0.261206 | 0.209* | 0.5 |
| H11C | 0.382182 | 0.324019 | 0.084404 | 0.209* | 0.5 |
| C10 | 0.5355 (15) | 0.5612 (13) | 0.283 (2) | 0.139 (5) | 0.5 |
| H10D | 0.581626 | 0.609001 | 0.247371 | 0.209* | 0.5 |
| H10E | 0.565898 | 0.539997 | 0.384236 | 0.209* | 0.5 |
| H10F | 0.469460 | 0.594367 | 0.282739 | 0.209* | 0.5 |
| C11X | 0.4087 (17) | 0.3521 (18) | 0.156 (7) | 0.139 (5) | 0.5 |
| H11D | 0.342613 | 0.385730 | 0.121176 | 0.209* | 0.5 |
| H11E | 0.411408 | 0.325433 | 0.255871 | 0.209* | 0.5 |
| H11F | 0.416537 | 0.296006 | 0.088976 | 0.209* | 0.5 |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0285 (5) | 0.0157 (5) | 0.0236 (5) | 0.0026 (6) | 0.0068 (5) | 0.0002 (6) |
| Si1 | 0.0406 (13) | 0.0755 (19) | 0.119 (2) | −0.0021 (12) | −0.0034 (15) | 0.0400 (17) |
| O1 | 0.029 (2) | 0.019 (3) | 0.035 (3) | 0.003 (2) | 0.0052 (18) | −0.0074 (19) |
| O2 | 0.046 (3) | 0.031 (3) | 0.091 (4) | −0.006 (2) | 0.023 (3) | −0.033 (3) |
| O3 | 0.103 (8) | 0.105 (14) | 0.043 (9) | 0.010 (10) | −0.031 (9) | 0.010 (6) |
| N1 | 0.037 (3) | 0.014 (3) | 0.046 (3) | −0.002 (3) | 0.017 (3) | −0.012 (2) |
| N2 | 0.045 (3) | 0.025 (3) | 0.019 (3) | −0.013 (3) | 0.001 (2) | 0.005 (2) |
| C1 | 0.070 (5) | 0.028 (5) | 0.056 (5) | −0.014 (4) | 0.042 (4) | −0.013 (3) |
| C2 | 0.077 (6) | 0.039 (6) | 0.040 (4) | −0.013 (4) | 0.026 (4) | 0.003 (4) |
| C3 | 0.060 (5) | 0.046 (5) | 0.040 (4) | −0.002 (4) | −0.007 (4) | 0.023 (4) |
| C4 | 0.041 (4) | 0.029 (5) | 0.089 (6) | 0.009 (4) | −0.009 (4) | 0.018 (4) |
| C5 | 0.038 (4) | 0.029 (5) | 0.085 (6) | 0.006 (4) | 0.016 (4) | −0.008 (4) |
| C6 | 0.034 (4) | 0.025 (5) | 0.046 (4) | −0.002 (3) | 0.003 (3) | −0.003 (3) |
| C7 | 0.038 (4) | 0.022 (5) | 0.074 (5) | −0.001 (3) | 0.009 (3) | −0.008 (4) |
| C8 | 0.032 (4) | 0.039 (5) | 0.050 (4) | −0.002 (3) | 0.007 (3) | −0.001 (3) |
| C9 | 0.034 (4) | 0.067 (5) | 0.053 (4) | −0.009 (4) | 0.006 (4) | 0.011 (4) |
| C10X | 0.039 (6) | 0.077 (7) | 0.295 (14) | 0.005 (5) | 0.016 (7) | 0.004 (9) |
| C11 | 0.039 (6) | 0.077 (7) | 0.295 (14) | 0.005 (5) | 0.016 (7) | 0.004 (9) |
| C10 | 0.039 (6) | 0.077 (7) | 0.295 (14) | 0.005 (5) | 0.016 (7) | 0.004 (9) |
| C11X | 0.039 (6) | 0.077 (7) | 0.295 (14) | 0.005 (5) | 0.016 (7) | 0.004 (9) |
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Geometric parameters (Å, º)
| Ni1—O1 | 2.113 (4) | C3—H3B | 0.9700 |
| Ni1—O1i | 2.113 (4) | C3—C4 | 1.491 (9) |
| Ni1—N1 | 2.071 (4) | C4—H4A | 0.9700 |
| Ni1—N1i | 2.071 (4) | C4—H4B | 0.9700 |
| Ni1—N2 | 2.060 (4) | C4—C5 | 1.508 (9) |
| Ni1—N2i | 2.060 (4) | C5—H5A | 0.9700 |
| Si1—O3 | 1.785 (11) | C5—H5B | 0.9700 |
| Si1—O3ii | 1.541 (13) | C6—C7 | 1.524 (9) |
| Si1—C9 | 1.838 (6) | C7—H7A | 0.9700 |
| Si1—C10X | 1.842 (7) | C7—H7B | 0.9700 |
| Si1—C11 | 1.842 (7) | C7—C8 | 1.525 (8) |
| Si1—C10 | 1.842 (7) | C8—H8A | 0.9700 |
| Si1—C11X | 1.842 (7) | C8—H8B | 0.9700 |
| O1—C6 | 1.245 (7) | C8—C9 | 1.512 (8) |
| O2—C6 | 1.242 (7) | C9—H9A | 0.9700 |
| O3—O3ii | 1.13 (2) | C9—H9B | 0.9700 |
| N1—H1 | 0.9800 | C10X—H10A | 0.9600 |
| N1—C1 | 1.467 (7) | C10X—H10B | 0.9600 |
| N1—C5 | 1.479 (7) | C10X—H10C | 0.9600 |
| N2—H2 | 0.9800 | C11—H11A | 0.9600 |
| N2—C2 | 1.467 (7) | C11—H11B | 0.9600 |
| N2—C3 | 1.459 (7) | C11—H11C | 0.9600 |
| C1—H1A | 0.9700 | C10—H10D | 0.9600 |
| C1—H1B | 0.9700 | C10—H10E | 0.9600 |
| C1—C2i | 1.519 (8) | C10—H10F | 0.9600 |
| C2—H2A | 0.9700 | C11X—H11D | 0.9600 |
| C2—H2B | 0.9700 | C11X—H11E | 0.9600 |
| C3—H3A | 0.9700 | C11X—H11F | 0.9600 |
| O1i—Ni1—O1 | 180.0 | N2—C3—C4 | 111.3 (5) |
| N1—Ni1—O1 | 93.58 (17) | H3A—C3—H3B | 108.0 |
| N1i—Ni1—O1 | 86.42 (17) | C4—C3—H3A | 109.4 |
| N1—Ni1—O1i | 86.42 (17) | C4—C3—H3B | 109.4 |
| N1i—Ni1—O1i | 93.58 (17) | C3—C4—H4A | 108.0 |
| N1—Ni1—N1i | 180.0 | C3—C4—H4B | 108.0 |
| N2i—Ni1—O1 | 92.40 (16) | C3—C4—C5 | 117.3 (6) |
| N2—Ni1—O1i | 92.40 (16) | H4A—C4—H4B | 107.2 |
| N2—Ni1—O1 | 87.60 (16) | C5—C4—H4A | 108.0 |
| N2i—Ni1—O1i | 87.60 (16) | C5—C4—H4B | 108.0 |
| N2—Ni1—N1i | 85.21 (19) | N1—C5—C4 | 111.6 (5) |
| N2i—Ni1—N1i | 94.79 (19) | N1—C5—H5A | 109.3 |
| N2—Ni1—N1 | 94.79 (19) | N1—C5—H5B | 109.3 |
| N2i—Ni1—N1 | 85.21 (19) | C4—C5—H5A | 109.3 |
| N2i—Ni1—N2 | 180.0 (2) | C4—C5—H5B | 109.3 |
| O3ii—Si1—O3 | 38.8 (7) | H5A—C5—H5B | 108.0 |
| O3—Si1—C9 | 98.7 (5) | O1—C6—C7 | 117.0 (6) |
| O3ii—Si1—C9 | 117.6 (6) | O2—C6—O1 | 126.5 (6) |
| O3ii—Si1—C10X | 93.6 (8) | O2—C6—C7 | 116.5 (6) |
| O3—Si1—C10X | 131.7 (9) | C6—C7—H7A | 108.7 |
| O3—Si1—C11 | 102 (2) | C6—C7—H7B | 108.7 |
| O3ii—Si1—C11 | 116.7 (16) | C6—C7—C8 | 114.4 (5) |
| O3—Si1—C10 | 118.2 (9) | H7A—C7—H7B | 107.6 |
| O3ii—Si1—C10 | 79.8 (9) | C8—C7—H7A | 108.7 |
| O3—Si1—C11X | 98 (2) | C8—C7—H7B | 108.7 |
| O3ii—Si1—C11X | 118.8 (18) | C7—C8—H8A | 108.9 |
| C9—Si1—C10X | 116.1 (7) | C7—C8—H8B | 108.9 |
| C9—Si1—C11 | 114.8 (10) | H8A—C8—H8B | 107.7 |
| C9—Si1—C10 | 107.7 (7) | C9—C8—C7 | 113.3 (5) |
| C9—Si1—C11X | 107.3 (9) | C9—C8—H8A | 108.9 |
| C11—Si1—C10X | 93.4 (18) | C9—C8—H8B | 108.9 |
| C11X—Si1—C10 | 123.8 (18) | Si1—C9—H9A | 107.9 |
| C6—O1—Ni1 | 131.4 (4) | Si1—C9—H9B | 107.9 |
| Si1ii—O3—Si1 | 141.2 (7) | C8—C9—Si1 | 117.6 (4) |
| O3ii—O3—Si1 | 58.8 (11) | C8—C9—H9A | 107.9 |
| O3ii—O3—Si1ii | 82.4 (14) | C8—C9—H9B | 107.9 |
| Ni1—N1—H1 | 107.2 | H9A—C9—H9B | 107.2 |
| C1—N1—Ni1 | 105.5 (4) | Si1—C10X—H10A | 109.5 |
| C1—N1—H1 | 107.2 | Si1—C10X—H10B | 109.5 |
| C1—N1—C5 | 114.1 (5) | Si1—C10X—H10C | 109.5 |
| C5—N1—Ni1 | 115.3 (4) | H10A—C10X—H10B | 109.5 |
| C5—N1—H1 | 107.2 | H10A—C10X—H10C | 109.5 |
| Ni1—N2—H2 | 107.3 | H10B—C10X—H10C | 109.5 |
| C2—N2—Ni1 | 106.3 (4) | Si1—C11—H11A | 109.5 |
| C2—N2—H2 | 107.3 | Si1—C11—H11B | 109.5 |
| C3—N2—Ni1 | 115.3 (4) | Si1—C11—H11C | 109.5 |
| C3—N2—H2 | 107.3 | H11A—C11—H11B | 109.5 |
| C3—N2—C2 | 112.9 (5) | H11A—C11—H11C | 109.5 |
| N1—C1—H1A | 109.9 | H11B—C11—H11C | 109.5 |
| N1—C1—H1B | 109.9 | Si1—C10—H10D | 109.5 |
| N1—C1—C2i | 108.9 (5) | Si1—C10—H10E | 109.5 |
| H1A—C1—H1B | 108.3 | Si1—C10—H10F | 109.5 |
| C2i—C1—H1A | 109.9 | H10D—C10—H10E | 109.5 |
| C2i—C1—H1B | 109.9 | H10D—C10—H10F | 109.5 |
| N2—C2—C1i | 108.3 (5) | H10E—C10—H10F | 109.5 |
| N2—C2—H2A | 110.0 | Si1—C11X—H11D | 109.5 |
| N2—C2—H2B | 110.0 | Si1—C11X—H11E | 109.5 |
| C1i—C2—H2A | 110.0 | Si1—C11X—H11F | 109.5 |
| C1i—C2—H2B | 110.0 | H11D—C11X—H11E | 109.5 |
| H2A—C2—H2B | 108.4 | H11D—C11X—H11F | 109.5 |
| N2—C3—H3A | 109.4 | H11E—C11X—H11F | 109.5 |
| N2—C3—H3B | 109.4 | ||
| Ni1—O1—C6—O2 | −18.8 (10) | C6—C7—C8—C9 | 67.1 (7) |
| Ni1—O1—C6—C7 | 161.1 (4) | C7—C8—C9—Si1 | 176.0 (4) |
| Ni1—N1—C1—C2i | −41.9 (5) | C9—Si1—O3—Si1ii | −123.8 (15) |
| Ni1—N1—C5—C4 | 53.8 (7) | C9—Si1—O3—O3ii | −123.8 (15) |
| Ni1—N2—C2—C1i | 41.2 (5) | C10X—Si1—O3—Si1ii | 13 (2) |
| Ni1—N2—C3—C4 | −57.1 (7) | C10X—Si1—O3—O3ii | 13 (2) |
| O1—C6—C7—C8 | 31.8 (8) | C10X—Si1—C9—C8 | 49.5 (9) |
| O2—C6—C7—C8 | −148.4 (6) | C11—Si1—O3—Si1ii | 118.5 (18) |
| O3ii—Si1—O3—Si1ii | 0.003 (1) | C11—Si1—O3—O3ii | 118.5 (18) |
| O3—Si1—C9—C8 | −165.0 (7) | C11—Si1—C9—C8 | −58 (2) |
| O3ii—Si1—C9—C8 | 159.0 (6) | C10—Si1—O3—Si1ii | −8.3 (19) |
| N2—C3—C4—C5 | 72.9 (8) | C10—Si1—O3—O3ii | −8.3 (19) |
| C1—N1—C5—C4 | 176.2 (5) | C10—Si1—C9—C8 | 71.5 (10) |
| C2—N2—C3—C4 | −179.6 (5) | C11X—Si1—O3—Si1ii | 127.1 (18) |
| C3—N2—C2—C1i | 168.6 (5) | C11X—Si1—O3—O3ii | 127.1 (18) |
| C3—C4—C5—N1 | −71.1 (8) | C11X—Si1—C9—C8 | −64 (2) |
| C5—N1—C1—C2i | −169.5 (5) |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) −x+1, −y+1, −z.
catena-Poly[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-1,3-bis(3-carboxylatopropyl)tetramethyldisiloxane-κ2O:O'] (I) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O2 | 0.98 | 1.96 | 2.845 (6) | 150 |
| N2—H2···O2iii | 0.98 | 2.07 | 2.883 (6) | 139 |
Symmetry code: (iii) x, −y+1/2, z+1/2.
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Crystal data
| [Ni(C10H25O5Si2)(C12H24N4)]ClO4 | Z = 2 |
| Mr = 663.99 | F(000) = 708 |
| Triclinic, P1 | Dx = 1.296 Mg m−3 |
| a = 9.3815 (7) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 12.9009 (8) Å | Cell parameters from 2033 reflections |
| c = 14.7604 (10) Å | θ = 1.7–24.6° |
| α = 99.309 (5)° | µ = 0.77 mm−1 |
| β = 100.343 (6)° | T = 200 K |
| γ = 99.232 (6)° | Block, clear light colourless |
| V = 1700.9 (2) Å3 | 0.45 × 0.35 × 0.30 mm |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Data collection
| Agilent Xcalibur, Eos diffractometer | 9606 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 5769 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.063 |
| Detector resolution: 16.1593 pixels mm-1 | θmax = 25.0°, θmin = 2.0° |
| ω scans | h = −11→11 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2014) | k = −15→15 |
| Tmin = 0.889, Tmax = 1.000 | l = −17→17 |
| 9606 measured reflections |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.050 | H-atom parameters constrained |
| wR(F2) = 0.115 | w = 1/[σ2(Fo2) + (0.0451P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max = 0.002 |
| 9606 reflections | Δρmax = 0.51 e Å−3 |
| 367 parameters | Δρmin = −0.44 e Å−3 |
| 7 restraints |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component twin. |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Ni1 | 0.000000 | 0.000000 | 0.000000 | 0.0241 (2) | |
| Si1 | −0.3977 (2) | −0.55987 (11) | −0.05469 (15) | 0.0729 (6) | |
| O1 | −0.1184 (3) | −0.1542 (2) | −0.0727 (2) | 0.0310 (8) | |
| O2 | −0.0826 (3) | −0.1583 (2) | −0.2165 (2) | 0.0339 (8) | |
| H2C | −0.034950 | −0.196653 | −0.243367 | 0.051* | 0.5 |
| O3 | −0.5535 (8) | −0.5084 (6) | −0.0332 (5) | 0.061 (3) | 0.5 |
| N1 | 0.1859 (4) | −0.0236 (3) | −0.0499 (3) | 0.0408 (11) | |
| H1 | 0.157126 | −0.087684 | −0.099864 | 0.049* | |
| N2 | −0.0809 (4) | 0.0725 (3) | −0.1075 (3) | 0.0390 (11) | |
| H2 | −0.128011 | 0.014955 | −0.161639 | 0.047* | |
| C1 | 0.2858 (6) | −0.0494 (5) | 0.0295 (4) | 0.065 (2) | |
| H1A | 0.335337 | 0.015969 | 0.073702 | 0.078* | |
| H1B | 0.360480 | −0.083840 | 0.006336 | 0.078* | |
| C2 | −0.1992 (7) | 0.1219 (4) | −0.0773 (4) | 0.0649 (19) | |
| H2A | −0.156944 | 0.189771 | −0.034619 | 0.078* | |
| H2B | −0.263674 | 0.135605 | −0.131369 | 0.078* | |
| C3 | 0.0282 (7) | 0.1448 (4) | −0.1388 (4) | 0.0634 (18) | |
| H3A | 0.071451 | 0.206181 | −0.088446 | 0.076* | |
| H3B | −0.020855 | 0.170724 | −0.191801 | 0.076* | |
| C4 | 0.1501 (7) | 0.0909 (4) | −0.1671 (4) | 0.068 (2) | |
| H4A | 0.104405 | 0.025115 | −0.211911 | 0.081* | |
| H4B | 0.205970 | 0.137198 | −0.199462 | 0.081* | |
| C5 | 0.2570 (6) | 0.0641 (4) | −0.0893 (4) | 0.0608 (18) | |
| H5A | 0.339987 | 0.043288 | −0.113487 | 0.073* | |
| H5B | 0.294711 | 0.127196 | −0.039965 | 0.073* | |
| C6 | −0.1332 (4) | −0.2035 (3) | −0.1537 (3) | 0.0254 (11) | |
| C7 | −0.2146 (5) | −0.3171 (3) | −0.1832 (3) | 0.0378 (13) | |
| H7A | −0.303379 | −0.319448 | −0.229384 | 0.045* | |
| H7B | −0.153320 | −0.358866 | −0.214036 | 0.045* | |
| C8 | −0.2580 (5) | −0.3700 (3) | −0.1066 (3) | 0.0363 (12) | |
| H8A | −0.316658 | −0.327704 | −0.073869 | 0.044* | |
| H8B | −0.169508 | −0.371782 | −0.061703 | 0.044* | |
| C9 | −0.3463 (5) | −0.4842 (3) | −0.1431 (3) | 0.0477 (14) | |
| H9A | −0.289182 | −0.524186 | −0.179202 | 0.057* | |
| H9B | −0.436249 | −0.480898 | −0.185840 | 0.057* | |
| C10 | −0.2275 (11) | −0.5710 (6) | 0.0258 (5) | 0.168 (4) | |
| H10A | −0.191865 | −0.505635 | 0.071407 | 0.252* | |
| H10B | −0.153386 | −0.583786 | −0.009580 | 0.252* | |
| H10C | −0.248872 | −0.629417 | 0.057238 | 0.252* | |
| C11 | −0.5003 (7) | −0.6957 (4) | −0.1124 (5) | 0.102 (3) | |
| H11A | −0.442542 | −0.730299 | −0.150911 | 0.153* | |
| H11B | −0.592187 | −0.690955 | −0.150829 | 0.153* | |
| H11C | −0.519460 | −0.736539 | −0.065495 | 0.153* | |
| Ni2 | 0.000000 | −0.500000 | −0.500000 | 0.0281 (2) | |
| Si2 | 0.35316 (15) | 0.04647 (10) | −0.52679 (11) | 0.0404 (4) | |
| O4 | 0.0305 (3) | −0.3297 (2) | −0.47284 (19) | 0.0333 (8) | |
| O5 | −0.0840 (3) | −0.2890 (2) | −0.3576 (2) | 0.0376 (9) | |
| H5C | −0.095950 | −0.235276 | −0.324118 | 0.056* | 0.5 |
| N3 | −0.1442 (6) | −0.5206 (3) | −0.4129 (4) | 0.0621 (15) | |
| H3 | −0.143842 | −0.449452 | −0.377416 | 0.075* | |
| N4 | 0.1844 (5) | −0.4870 (3) | −0.3968 (4) | 0.0675 (16) | |
| H4 | 0.207815 | −0.412879 | −0.361974 | 0.081* | |
| C12 | −0.2937 (7) | −0.5585 (5) | −0.4771 (6) | 0.094 (3) | |
| H12A | −0.370162 | −0.545605 | −0.442664 | 0.113* | |
| H12B | −0.309077 | −0.634809 | −0.501474 | 0.113* | |
| C13 | 0.3020 (7) | −0.5012 (5) | −0.4435 (7) | 0.111 (4) | |
| H13A | 0.295115 | −0.576820 | −0.467592 | 0.133* | |
| H13B | 0.395995 | −0.474737 | −0.399517 | 0.133* | |
| C14 | 0.1635 (10) | −0.5587 (5) | −0.3256 (6) | 0.112 (3) | |
| H14A | 0.151266 | −0.632881 | −0.356424 | 0.134* | |
| H14B | 0.251865 | −0.542354 | −0.276130 | 0.134* | |
| C15 | 0.0318 (15) | −0.5454 (6) | −0.2821 (5) | 0.136 (4) | |
| H15A | 0.035328 | −0.582999 | −0.230129 | 0.163* | |
| H15B | 0.040102 | −0.470013 | −0.256570 | 0.163* | |
| C16 | −0.1145 (11) | −0.5851 (5) | −0.3474 (6) | 0.116 (4) | |
| H16A | −0.117774 | −0.657098 | −0.380421 | 0.139* | |
| H16B | −0.191132 | −0.588632 | −0.311103 | 0.139* | |
| C17 | 0.0036 (5) | −0.2625 (3) | −0.4108 (3) | 0.0278 (11) | |
| C18 | 0.0724 (5) | −0.1463 (3) | −0.3978 (3) | 0.0287 (12) | |
| H18A | 0.132997 | −0.123812 | −0.334828 | 0.034* | |
| H18B | −0.006193 | −0.105610 | −0.401545 | 0.034* | |
| C19 | 0.1664 (5) | −0.1164 (3) | −0.4662 (3) | 0.0359 (12) | |
| H19A | 0.108087 | −0.140813 | −0.529665 | 0.043* | |
| H19B | 0.249045 | −0.153188 | −0.460348 | 0.043* | |
| C20 | 0.2255 (5) | 0.0035 (3) | −0.4507 (3) | 0.0373 (13) | |
| H20A | 0.276718 | 0.028112 | −0.385648 | 0.045* | |
| H20B | 0.142064 | 0.039091 | −0.460546 | 0.045* | |
| C21 | 0.2725 (6) | −0.0094 (4) | −0.6529 (4) | 0.0706 (18) | |
| H21A | 0.180766 | 0.013540 | −0.670484 | 0.106* | |
| H21B | 0.255162 | −0.086153 | −0.663382 | 0.106* | |
| H21C | 0.339876 | 0.015653 | −0.690111 | 0.106* | |
| C22 | 0.3997 (6) | 0.1939 (4) | −0.5079 (5) | 0.084 (2) | |
| H22A | 0.445438 | 0.222461 | −0.443122 | 0.126* | |
| H22B | 0.311213 | 0.221370 | −0.523912 | 0.126* | |
| H22C | 0.466709 | 0.214650 | −0.546789 | 0.126* | |
| O6X | 0.493 (3) | −0.013 (5) | −0.497 (6) | 0.037 (3)* | 0.25 |
| O6 | 0.510 (3) | 0.021 (4) | −0.4772 (12) | 0.037 (3)* | 0.25 |
| Cl1 | 0.38292 (17) | −0.21590 (12) | −0.21055 (12) | 0.0683 (5) | |
| O7 | 0.3941 (6) | −0.2883 (5) | −0.2826 (4) | 0.194 (3) | |
| O8 | 0.2362 (5) | −0.2323 (4) | −0.1955 (4) | 0.1227 (19) | |
| O9 | 0.4112 (6) | −0.1102 (4) | −0.2279 (4) | 0.147 (2) | |
| O10 | 0.4768 (6) | −0.2161 (4) | −0.1271 (4) | 0.137 (2) |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0254 (4) | 0.0202 (4) | 0.0226 (5) | −0.0021 (4) | 0.0072 (4) | −0.0040 (3) |
| Si1 | 0.1134 (15) | 0.0274 (8) | 0.0967 (15) | 0.0056 (9) | 0.0774 (14) | 0.0135 (8) |
| O1 | 0.0404 (19) | 0.0256 (16) | 0.0206 (19) | −0.0064 (14) | 0.0113 (15) | −0.0065 (14) |
| O2 | 0.051 (2) | 0.0206 (16) | 0.030 (2) | −0.0038 (14) | 0.0228 (17) | −0.0013 (14) |
| O3 | 0.085 (7) | 0.051 (4) | 0.069 (8) | 0.020 (5) | 0.056 (5) | 0.020 (5) |
| N1 | 0.033 (2) | 0.038 (2) | 0.041 (3) | −0.005 (2) | 0.016 (2) | −0.017 (2) |
| N2 | 0.052 (3) | 0.026 (2) | 0.031 (3) | 0.006 (2) | −0.001 (2) | −0.0044 (18) |
| C1 | 0.028 (3) | 0.065 (4) | 0.081 (5) | 0.019 (3) | −0.006 (3) | −0.032 (4) |
| C2 | 0.072 (4) | 0.048 (3) | 0.058 (4) | 0.024 (3) | −0.022 (4) | −0.009 (3) |
| C3 | 0.119 (5) | 0.027 (3) | 0.032 (3) | −0.010 (3) | 0.006 (4) | 0.003 (2) |
| C4 | 0.106 (5) | 0.045 (3) | 0.036 (4) | −0.041 (3) | 0.039 (4) | −0.009 (3) |
| C5 | 0.059 (4) | 0.060 (4) | 0.051 (4) | −0.023 (3) | 0.036 (3) | −0.018 (3) |
| C6 | 0.026 (3) | 0.025 (2) | 0.023 (3) | 0.000 (2) | 0.008 (2) | −0.002 (2) |
| C7 | 0.053 (3) | 0.027 (3) | 0.027 (3) | −0.010 (2) | 0.018 (3) | −0.004 (2) |
| C8 | 0.048 (3) | 0.025 (2) | 0.033 (3) | −0.001 (2) | 0.016 (3) | −0.002 (2) |
| C9 | 0.056 (3) | 0.028 (3) | 0.053 (4) | −0.010 (2) | 0.022 (3) | −0.004 (2) |
| C10 | 0.274 (12) | 0.096 (6) | 0.091 (7) | −0.042 (7) | −0.030 (7) | 0.047 (5) |
| C11 | 0.075 (5) | 0.042 (4) | 0.192 (8) | −0.006 (3) | 0.039 (5) | 0.034 (4) |
| Ni2 | 0.0285 (5) | 0.0228 (4) | 0.0292 (5) | −0.0009 (4) | 0.0125 (4) | −0.0067 (4) |
| Si2 | 0.0330 (8) | 0.0396 (8) | 0.0503 (10) | 0.0047 (7) | 0.0090 (7) | 0.0156 (7) |
| O4 | 0.0421 (19) | 0.0242 (16) | 0.0334 (19) | 0.0031 (14) | 0.0212 (16) | −0.0061 (14) |
| O5 | 0.053 (2) | 0.0263 (17) | 0.033 (2) | −0.0013 (15) | 0.0258 (18) | −0.0061 (14) |
| N3 | 0.099 (4) | 0.026 (2) | 0.073 (4) | 0.007 (3) | 0.064 (3) | −0.002 (3) |
| N4 | 0.056 (3) | 0.043 (3) | 0.080 (4) | 0.016 (3) | −0.019 (3) | −0.027 (3) |
| C12 | 0.049 (4) | 0.056 (4) | 0.167 (7) | −0.010 (3) | 0.066 (5) | −0.033 (4) |
| C13 | 0.039 (4) | 0.062 (5) | 0.188 (10) | 0.014 (4) | −0.027 (5) | −0.048 (5) |
| C14 | 0.164 (8) | 0.055 (4) | 0.088 (6) | 0.040 (5) | −0.055 (6) | 0.004 (4) |
| C15 | 0.313 (15) | 0.067 (5) | 0.049 (5) | 0.084 (8) | 0.047 (8) | 0.019 (4) |
| C16 | 0.238 (11) | 0.045 (4) | 0.088 (7) | 0.012 (6) | 0.122 (7) | 0.001 (4) |
| C17 | 0.028 (3) | 0.028 (2) | 0.024 (3) | 0.005 (2) | 0.003 (2) | 0.000 (2) |
| C18 | 0.033 (3) | 0.018 (2) | 0.036 (3) | 0.003 (2) | 0.014 (2) | 0.001 (2) |
| C19 | 0.038 (3) | 0.031 (3) | 0.041 (3) | 0.004 (2) | 0.017 (3) | 0.003 (2) |
| C20 | 0.034 (3) | 0.035 (3) | 0.041 (3) | 0.003 (2) | 0.011 (3) | 0.002 (2) |
| C21 | 0.079 (4) | 0.088 (4) | 0.045 (4) | 0.017 (4) | 0.011 (3) | 0.017 (3) |
| C22 | 0.082 (5) | 0.048 (3) | 0.116 (6) | −0.012 (3) | 0.023 (4) | 0.021 (4) |
| Cl1 | 0.0571 (10) | 0.0599 (10) | 0.0695 (12) | 0.0139 (8) | −0.0151 (10) | −0.0111 (9) |
| O7 | 0.137 (5) | 0.163 (5) | 0.202 (6) | −0.019 (4) | 0.037 (4) | −0.146 (5) |
| O8 | 0.077 (3) | 0.154 (5) | 0.144 (5) | 0.026 (3) | 0.017 (3) | 0.050 (4) |
| O9 | 0.176 (6) | 0.079 (4) | 0.158 (5) | −0.006 (4) | −0.005 (5) | 0.016 (3) |
| O10 | 0.117 (4) | 0.143 (4) | 0.115 (4) | 0.037 (3) | −0.050 (4) | −0.006 (4) |
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Geometric parameters (Å, º)
| Ni1—O1 | 2.125 (2) | Ni2—N4 | 2.054 (4) |
| Ni1—O1i | 2.125 (2) | Ni2—N4iii | 2.054 (4) |
| Ni1—N1i | 2.058 (3) | Si2—C20 | 1.864 (5) |
| Ni1—N1 | 2.058 (3) | Si2—C21 | 1.855 (5) |
| Ni1—N2i | 2.060 (4) | Si2—C22 | 1.845 (5) |
| Ni1—N2 | 2.060 (4) | Si2—O6Xiv | 1.570 (19) |
| Si1—O3 | 1.757 (8) | Si2—O6X | 1.651 (19) |
| Si1—O3ii | 1.626 (7) | Si2—O6iv | 1.66 (2) |
| Si1—C9 | 1.837 (5) | Si2—O6 | 1.632 (16) |
| Si1—C10 | 1.852 (9) | O4—C17 | 1.245 (4) |
| Si1—C11 | 1.845 (5) | O5—H5C | 0.8199 |
| O1—C6 | 1.232 (4) | O5—C17 | 1.280 (5) |
| O2—H2C | 0.8200 | N3—H3 | 0.9800 |
| O2—C6 | 1.291 (5) | N3—C12 | 1.502 (8) |
| O3—O3ii | 1.234 (13) | N3—C16 | 1.393 (9) |
| N1—H1 | 0.9800 | N4—H4 | 0.9800 |
| N1—C1 | 1.481 (6) | N4—C13 | 1.422 (8) |
| N1—C5 | 1.475 (6) | N4—C14 | 1.527 (9) |
| N2—H2 | 0.9800 | C12—H12A | 0.9700 |
| N2—C2 | 1.467 (6) | C12—H12B | 0.9700 |
| N2—C3 | 1.461 (6) | C12—C13iii | 1.502 (10) |
| C1—H1A | 0.9700 | C13—H13A | 0.9700 |
| C1—H1B | 0.9700 | C13—H13B | 0.9700 |
| C1—C2i | 1.486 (7) | C14—H14A | 0.9700 |
| C2—H2B | 0.9700 | C14—H14B | 0.9700 |
| C2—H2A | 0.9700 | C14—C15 | 1.512 (11) |
| C3—H3A | 0.9700 | C15—H15A | 0.9700 |
| C3—H3B | 0.9700 | C15—H15B | 0.9700 |
| C3—C4 | 1.516 (7) | C15—C16 | 1.488 (11) |
| C4—H4A | 0.9700 | C16—H16A | 0.9700 |
| C4—H4B | 0.9700 | C16—H16B | 0.9700 |
| C4—C5 | 1.507 (7) | C20—H20A | 0.9700 |
| C5—H5A | 0.9700 | C20—H20B | 0.9700 |
| C5—H5B | 0.9700 | C20—C19 | 1.521 (5) |
| C6—C7 | 1.496 (5) | C19—H19A | 0.9700 |
| C7—H7A | 0.9700 | C19—H19B | 0.9700 |
| C7—H7B | 0.9700 | C19—C18 | 1.512 (6) |
| C7—C8 | 1.496 (6) | C18—H18A | 0.9700 |
| C8—H8A | 0.9700 | C18—H18B | 0.9700 |
| C8—H8B | 0.9700 | C18—C17 | 1.501 (5) |
| C8—C9 | 1.529 (5) | C21—H21A | 0.9600 |
| C9—H9A | 0.9700 | C21—H21B | 0.9600 |
| C9—H9B | 0.9700 | C21—H21C | 0.9600 |
| C10—H10A | 0.9600 | C22—H22A | 0.9600 |
| C10—H10B | 0.9600 | C22—H22B | 0.9600 |
| C10—H10C | 0.9600 | C22—H22C | 0.9600 |
| C11—H11A | 0.9600 | O6X—O6Xiv | 0.38 (7) |
| C11—H11B | 0.9600 | O6—O6iv | 0.77 (5) |
| C11—H11C | 0.9600 | Cl1—O7 | 1.329 (4) |
| Ni2—O4iii | 2.131 (2) | Cl1—O8 | 1.421 (5) |
| Ni2—O4 | 2.131 (2) | Cl1—O9 | 1.420 (5) |
| Ni2—N3iii | 2.043 (4) | Cl1—O10 | 1.380 (5) |
| Ni2—N3 | 2.043 (4) | ||
| O1—Ni1—O1i | 180.0 | N3iii—Ni2—N4 | 85.7 (2) |
| N1i—Ni1—O1 | 88.17 (12) | N4—Ni2—O4iii | 91.33 (14) |
| N1i—Ni1—O1i | 91.83 (12) | N4—Ni2—O4 | 88.67 (14) |
| N1—Ni1—O1i | 88.17 (12) | N4iii—Ni2—O4 | 91.33 (14) |
| N1—Ni1—O1 | 91.83 (12) | N4iii—Ni2—O4iii | 88.67 (14) |
| N1i—Ni1—N1 | 180.0 | N4—Ni2—N4iii | 180.0 |
| N1i—Ni1—N2 | 85.82 (17) | C21—Si2—C20 | 111.6 (2) |
| N1—Ni1—N2i | 85.82 (17) | C22—Si2—C20 | 110.6 (2) |
| N1i—Ni1—N2i | 94.18 (17) | C22—Si2—C21 | 109.6 (3) |
| N1—Ni1—N2 | 94.18 (17) | O6Xiv—Si2—C20 | 113.4 (14) |
| N2—Ni1—O1i | 87.38 (12) | O6X—Si2—C20 | 102.5 (13) |
| N2i—Ni1—O1 | 87.38 (12) | O6X—Si2—C21 | 107 (3) |
| N2—Ni1—O1 | 92.62 (12) | O6Xiv—Si2—C21 | 107 (3) |
| N2i—Ni1—O1i | 92.62 (12) | O6X—Si2—C22 | 116 (2) |
| N2—Ni1—N2i | 180.0 | O6Xiv—Si2—C22 | 104 (2) |
| O3ii—Si1—O3 | 42.6 (4) | O6Xiv—Si2—O6X | 13 (3) |
| O3ii—Si1—C9 | 115.6 (3) | O6X—Si2—O6iv | 13 (3) |
| O3—Si1—C9 | 100.2 (3) | O6Xiv—Si2—O6iv | 17 (2) |
| O3—Si1—C10 | 131.5 (4) | O6iv—Si2—C20 | 111.2 (17) |
| O3ii—Si1—C10 | 89.4 (4) | O6—Si2—C20 | 103.5 (15) |
| O3ii—Si1—C11 | 121.3 (4) | O6—Si2—C21 | 120.4 (6) |
| O3—Si1—C11 | 95.7 (3) | O6iv—Si2—C21 | 94.3 (5) |
| C9—Si1—C10 | 109.0 (3) | O6—Si2—C22 | 100.3 (19) |
| C9—Si1—C11 | 110.1 (3) | O6iv—Si2—C22 | 118.5 (19) |
| C11—Si1—C10 | 108.8 (3) | O6—Si2—O6iv | 26.9 (17) |
| C6—O1—Ni1 | 132.9 (3) | C17—O4—Ni2 | 133.8 (3) |
| C6—O2—H2C | 109.8 | C17—O5—H5C | 109.9 |
| Si1ii—O3—Si1 | 137.4 (4) | Ni2—N3—H3 | 106.9 |
| O3ii—O3—Si1 | 63.0 (6) | C12—N3—Ni2 | 105.1 (4) |
| O3ii—O3—Si1ii | 74.4 (7) | C12—N3—H3 | 106.9 |
| Ni1—N1—H1 | 107.4 | C16—N3—Ni2 | 117.3 (4) |
| C1—N1—Ni1 | 105.0 (3) | C16—N3—H3 | 106.9 |
| C1—N1—H1 | 107.4 | C16—N3—C12 | 113.2 (6) |
| C5—N1—Ni1 | 116.2 (3) | Ni2—N4—H4 | 106.8 |
| C5—N1—H1 | 107.4 | C13—N4—Ni2 | 106.5 (4) |
| C5—N1—C1 | 113.1 (4) | C13—N4—H4 | 106.8 |
| Ni1—N2—H2 | 106.7 | C13—N4—C14 | 115.0 (6) |
| C2—N2—Ni1 | 105.7 (3) | C14—N4—Ni2 | 114.4 (4) |
| C2—N2—H2 | 106.7 | C14—N4—H4 | 106.8 |
| C3—N2—Ni1 | 116.1 (3) | N3—C12—H12A | 109.9 |
| C3—N2—H2 | 106.7 | N3—C12—H12B | 109.9 |
| C3—N2—C2 | 114.2 (4) | N3—C12—C13iii | 108.8 (5) |
| N1—C1—H1A | 109.7 | H12A—C12—H12B | 108.3 |
| N1—C1—H1B | 109.7 | C13iii—C12—H12A | 109.9 |
| N1—C1—C2i | 109.7 (4) | C13iii—C12—H12B | 109.9 |
| H1A—C1—H1B | 108.2 | N4—C13—C12iii | 109.6 (6) |
| C2i—C1—H1A | 109.7 | N4—C13—H13A | 109.8 |
| C2i—C1—H1B | 109.7 | N4—C13—H13B | 109.8 |
| N2—C2—C1i | 109.8 (4) | C12iii—C13—H13A | 109.8 |
| N2—C2—H2B | 109.7 | C12iii—C13—H13B | 109.8 |
| N2—C2—H2A | 109.7 | H13A—C13—H13B | 108.2 |
| C1i—C2—H2B | 109.7 | N4—C14—H14A | 109.0 |
| C1i—C2—H2A | 109.7 | N4—C14—H14B | 109.0 |
| H2B—C2—H2A | 108.2 | H14A—C14—H14B | 107.8 |
| N2—C3—H3A | 109.1 | C15—C14—N4 | 113.0 (6) |
| N2—C3—H3B | 109.1 | C15—C14—H14A | 109.0 |
| N2—C3—C4 | 112.3 (4) | C15—C14—H14B | 109.0 |
| H3A—C3—H3B | 107.9 | C14—C15—H15A | 108.5 |
| C4—C3—H3A | 109.1 | C14—C15—H15B | 108.5 |
| C4—C3—H3B | 109.1 | H15A—C15—H15B | 107.5 |
| C3—C4—H4A | 108.1 | C16—C15—C14 | 115.0 (6) |
| C3—C4—H4B | 108.1 | C16—C15—H15A | 108.5 |
| H4A—C4—H4B | 107.3 | C16—C15—H15B | 108.5 |
| C5—C4—C3 | 116.7 (4) | N3—C16—C15 | 113.1 (7) |
| C5—C4—H4A | 108.1 | N3—C16—H16A | 109.0 |
| C5—C4—H4B | 108.1 | N3—C16—H16B | 109.0 |
| N1—C5—C4 | 111.4 (4) | C15—C16—H16A | 109.0 |
| N1—C5—H5A | 109.4 | C15—C16—H16B | 109.0 |
| N1—C5—H5B | 109.4 | H16A—C16—H16B | 107.8 |
| C4—C5—H5A | 109.4 | Si2—C20—H20A | 108.3 |
| C4—C5—H5B | 109.4 | Si2—C20—H20B | 108.3 |
| H5A—C5—H5B | 108.0 | H20A—C20—H20B | 107.4 |
| O1—C6—O2 | 121.4 (4) | C19—C20—Si2 | 115.9 (3) |
| O1—C6—C7 | 120.8 (4) | C19—C20—H20A | 108.3 |
| O2—C6—C7 | 117.7 (4) | C19—C20—H20B | 108.3 |
| C6—C7—H7A | 108.3 | C20—C19—H19A | 109.0 |
| C6—C7—H7B | 108.3 | C20—C19—H19B | 109.0 |
| H7A—C7—H7B | 107.4 | H19A—C19—H19B | 107.8 |
| C8—C7—C6 | 116.0 (4) | C18—C19—C20 | 113.1 (3) |
| C8—C7—H7A | 108.3 | C18—C19—H19A | 109.0 |
| C8—C7—H7B | 108.3 | C18—C19—H19B | 109.0 |
| C7—C8—H8A | 109.0 | C19—C18—H18A | 108.1 |
| C7—C8—H8B | 109.0 | C19—C18—H18B | 108.1 |
| C7—C8—C9 | 112.8 (4) | H18A—C18—H18B | 107.3 |
| H8A—C8—H8B | 107.8 | C17—C18—C19 | 116.7 (3) |
| C9—C8—H8A | 109.0 | C17—C18—H18A | 108.1 |
| C9—C8—H8B | 109.0 | C17—C18—H18B | 108.1 |
| Si1—C9—H9A | 108.1 | O4—C17—O5 | 121.9 (4) |
| Si1—C9—H9B | 108.1 | O4—C17—C18 | 120.1 (4) |
| C8—C9—Si1 | 116.8 (3) | O5—C17—C18 | 118.0 (3) |
| C8—C9—H9A | 108.1 | Si2—C21—H21A | 109.5 |
| C8—C9—H9B | 108.1 | Si2—C21—H21B | 109.5 |
| H9A—C9—H9B | 107.3 | Si2—C21—H21C | 109.5 |
| Si1—C10—H10A | 109.5 | H21A—C21—H21B | 109.5 |
| Si1—C10—H10B | 109.5 | H21A—C21—H21C | 109.5 |
| Si1—C10—H10C | 109.5 | H21B—C21—H21C | 109.5 |
| H10A—C10—H10B | 109.5 | Si2—C22—H22A | 109.5 |
| H10A—C10—H10C | 109.5 | Si2—C22—H22B | 109.5 |
| H10B—C10—H10C | 109.5 | Si2—C22—H22C | 109.5 |
| Si1—C11—H11A | 109.5 | H22A—C22—H22B | 109.5 |
| Si1—C11—H11B | 109.5 | H22A—C22—H22C | 109.5 |
| Si1—C11—H11C | 109.5 | H22B—C22—H22C | 109.5 |
| H11A—C11—H11B | 109.5 | Si2iv—O6X—Si2 | 167 (3) |
| H11A—C11—H11C | 109.5 | O6Xiv—O6X—Si2iv | 96 (6) |
| H11B—C11—H11C | 109.5 | O6Xiv—O6X—Si2 | 71 (6) |
| O4—Ni2—O4iii | 180.00 (3) | Si2—O6—Si2iv | 153.1 (17) |
| N3iii—Ni2—O4iii | 94.96 (13) | O6iv—O6—Si2 | 78 (2) |
| N3—Ni2—O4 | 94.96 (13) | O7—Cl1—O8 | 110.3 (3) |
| N3iii—Ni2—O4 | 85.04 (13) | O7—Cl1—O9 | 112.0 (4) |
| N3—Ni2—O4iii | 85.04 (13) | O7—Cl1—O10 | 114.2 (4) |
| N3iii—Ni2—N3 | 180.0 | O9—Cl1—O8 | 105.3 (3) |
| N3iii—Ni2—N4iii | 94.3 (2) | O10—Cl1—O8 | 107.7 (4) |
| N3—Ni2—N4 | 94.3 (2) | O10—Cl1—O9 | 106.8 (3) |
| N3—Ni2—N4iii | 85.7 (2) | ||
| Ni1—O1—C6—O2 | −7.2 (6) | Ni2—N4—C14—C15 | −52.9 (7) |
| Ni1—O1—C6—C7 | 174.5 (3) | Si2—C20—C19—C18 | 176.0 (3) |
| Ni1—N1—C1—C2i | 40.9 (4) | N4—C14—C15—C16 | 68.4 (9) |
| Ni1—N1—C5—C4 | −56.2 (5) | C12—N3—C16—C15 | −178.2 (6) |
| Ni1—N2—C2—C1i | −39.8 (4) | C13—N4—C14—C15 | −176.6 (6) |
| Ni1—N2—C3—C4 | 55.3 (5) | C14—N4—C13—C12iii | 169.9 (5) |
| O1—C6—C7—C8 | −7.7 (6) | C14—C15—C16—N3 | −71.7 (8) |
| O2—C6—C7—C8 | 174.0 (4) | C16—N3—C12—C13iii | −168.2 (5) |
| O3ii—Si1—O3—Si1ii | −0.001 (2) | C20—Si2—O6X—Si2iv | −148 (28) |
| O3—Si1—C9—C8 | 81.0 (4) | C20—Si2—O6X—O6Xiv | −148 (28) |
| O3ii—Si1—C9—C8 | 39.0 (5) | C20—Si2—O6—Si2iv | 110 (7) |
| N2—C3—C4—C5 | −70.0 (6) | C20—Si2—O6—O6iv | 110 (7) |
| C1—N1—C5—C4 | −177.7 (4) | C20—C19—C18—C17 | 177.2 (4) |
| C2—N2—C3—C4 | 178.7 (4) | C19—C18—C17—O4 | 3.3 (6) |
| C3—N2—C2—C1i | −168.7 (4) | C19—C18—C17—O5 | −175.7 (4) |
| C3—C4—C5—N1 | 70.1 (5) | C21—Si2—C20—C19 | 52.0 (4) |
| C5—N1—C1—C2i | 168.6 (4) | C21—Si2—O6X—Si2iv | 95 (29) |
| C6—C7—C8—C9 | 177.5 (4) | C21—Si2—O6X—O6Xiv | 95 (29) |
| C7—C8—C9—Si1 | 176.9 (4) | C21—Si2—O6—Si2iv | −15 (9) |
| C9—Si1—O3—Si1ii | −116.9 (7) | C21—Si2—O6—O6iv | −15 (9) |
| C9—Si1—O3—O3ii | −116.9 (7) | C22—Si2—C20—C19 | 174.3 (4) |
| C10—Si1—O3—Si1ii | 10.0 (10) | C22—Si2—O6X—Si2iv | −27 (30) |
| C10—Si1—O3—O3ii | 10.0 (10) | C22—Si2—O6X—O6Xiv | −27 (30) |
| C10—Si1—C9—C8 | −59.7 (5) | C22—Si2—O6—Si2iv | −135 (8) |
| C11—Si1—O3—Si1ii | 131.5 (8) | C22—Si2—O6—O6iv | −135 (8) |
| C11—Si1—O3—O3ii | 131.5 (8) | O6Xiv—Si2—C20—C19 | −70 (4) |
| C11—Si1—C9—C8 | −179.0 (4) | O6X—Si2—C20—C19 | −62 (3) |
| Ni2—O4—C17—O5 | −16.3 (6) | O6Xiv—Si2—O6X—Si2iv | −0.01 (14) |
| Ni2—O4—C17—C18 | 164.8 (3) | O6iv—Si2—C20—C19 | −51.9 (12) |
| Ni2—N3—C12—C13iii | −39.0 (6) | O6—Si2—C20—C19 | −79.0 (15) |
| Ni2—N3—C16—C15 | 59.1 (7) | O6iv—Si2—O6—Si2iv | 0.006 (14) |
| Ni2—N4—C13—C12iii | 42.1 (6) |
Symmetry codes: (i) −x, −y, −z; (ii) −x−1, −y−1, −z; (iii) −x, −y−1, −z−1; (iv) −x+1, −y, −z−1.
catena-Poly[[[(1,4,8,11-tetraazacyclotetradecane-κ4N1,N4,N8,N11)nickel(II)]-µ-4-({[(3-carboxypropyl)dimethylsilyl]oxy}dimethylsilyl)butanoato-κ2O:O'] perchlorate] (II) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O2 | 0.98 | 2.51 | 3.225 (5) | 130 |
| N1—H1···O8 | 0.98 | 2.45 | 3.315 (6) | 147 |
| N2—H2···O2 | 0.98 | 2.38 | 3.143 (4) | 134 |
| N3—H3···O5 | 0.98 | 2.01 | 2.901 (5) | 150 |
| N4—H4···O7 | 0.98 | 2.18 | 3.012 (6) | 142 |
| O2—H2C···O5 | 0.82 | 1.84 | 2.456 (4) | 131 |
| O2—H2C···O8 | 0.82 | 2.65 | 3.260 (5) | 133 |
| O5—H5C···O2 | 0.82 | 1.70 | 2.456 (4) | 151 |
References
- Agilent (2014). CrysAlis PRO. Agilent Technologies Ltd, Yarnton, England.
- Barefield, E. K., Wagner, F., Herlinger, A. W. & Dahl, A. R. (1976). Inorg. Synth. 16, 220–224.
- Bosnich, B., Poon, C. K. & Tobe, M. C. (1965). Inorg. Chem. 4, 1102–1108.
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G., Siliqi, D. & Spagna, R. (2007). J. Appl. Cryst. 40, 609–613.
- Elsaidi, S. K., Mohamed, M. H., Banerjee, D. & Thallapally, P. K. (2018). Coord. Chem. Rev. 358, 125–152.
- Farrusseng, D. (2011). Editor. Metal-Organic Frameworks Applications from Catalysis to Gas Storage, Weinheim: Wiley-VCH.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kaskel, S. (2016). Editor. The Chemistry of Metal–Organic Frameworks: Synthesis, Characterization, and Applications. Weinheim: Wiley-VCH.
- Lampeka, Ya. D. & Tsymbal, L. V. (2004). Theor. Exp. Chem. 40, 345–371.
- Lee, J. H., Jeoung, S., Chung, Y. G. & Moon, H. R. (2019). Coord. Chem. Rev. 389, 161–188.
- MacGillivray, L. R. & Lukehart, C. M. (2014). Editors. Metal–Organic Framework Materials, Hoboken: John Wiley and Sons.
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Melson, G. A. (1979). Editor. Coordination Chemistry of Macrocyclic Compounds. New York: Plenum Press.
- Mulvaney, J. E. & Marvel, C. S. (1961). J. Polym. Sci. 50, 541–547.
- Racles, C., Shova, S., Cazacu, M. & Timpu, D. (2013). Polymer, 54, 6096–6104.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Stackhouse, C. A. & Ma, S. (2018). Polyhedron, 145, 154–165.
- Suh, M. P. & Moon, H. R. (2007). Advances in Inorganic Chemistry, Vol. 59, edited by R. van Eldik & K. Bowman-James, pp. 39–79. San Diego: Academic Press.
- Suh, M. P., Park, H. J., Prasad, T. K. & Lim, D.-W. (2012). Chem. Rev. 112, 782–835. [DOI] [PubMed]
- Vlad, A., Cazacu, M., Zaltariov, M.-F., Bargan, A., Shova, S. & Turta, C. (2014). J. Mol. Struct. 1060, 94–101.
- Vlad, A., Cazacu, M., Zaltariov, M.-F., Shova, S., Turta, C. & Airinei, A. (2013a). Polymer, 54, 43–53.
- Vlad, A., Zaltariov, M.-F., Shova, S., Novitchi, G., Varganici, C.-D., Train, C. & Cazacu, M. (2013b). CrystEngComm, 15, 5368–5375.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yatsimirskii, K. B. & Lampeka, Ya. D. (1985). Physicochemistry of Metal Complexes with Macrocyclic Ligands, Kiev: Naukova Dumka. (In Russian.)
- Zaltariov, M.-F., Cazacu, M., Sacarescu, L., Vlad, A., Novitchi, G., Train, C., Shova, S. & Arion, V. B. (2016). Macromolecules, 49, 6163–6172.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II. DOI: 10.1107/S2056989020002327/hb7892sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020002327/hb7892Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989020002327/hb7892IIsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report