Abstract
The α7 nicotinic acetylcholine receptor (α7 nAChR) is involved in various intracellular signaling pathways that mediate addiction, chronic pain, and other diseases, but its intracellular domain structures remain undetermined. The presence of seventeen native cysteines in α7 nAChR provides opportunities for extracting structural information through site-directed labeling of chemical probes in strategic locations, but it also creates uncertainties in channel function when those native cysteines must be mutated. Using site-directed mutagenesis and two-electrode voltage clamp electrophysiology measurements, we found that α7 nAChR’s function was well tolerated for mutations of all 13 cysteines as long as two pairs of disulfide-bond cysteines remained in the extracellular domain. Furthermore, surface plasmon resonance measurements showed that the cysteine mutations did not affect α7 nAChR binding to the intracellular protein PICK1. The study suggests that a high native cysteine content does not necessarily preclude the use of single cysteine labeling for acquiring structural information of functional proteins.
Keywords: Cys-loop receptors, α7 nAChR, nicotinic acetylcholine receptors, cysteine mutagenesis, intracellular domain, PICK1
Introduction
Cysteine has been used frequently for acquiring structural information because of the highly specific labeling chemistry of the cysteine thiol moiety. Site-specific labeling of cysteine is widely used in ESR, NMR, and various florescence experiments.1–4 In many applications, site specific cysteine labeling requires substitution of accessible native cysteines with other amino acids. If cysteine is in functionally important sites within proteins, its mutation can lead to loss of protein function.
Cys-loop receptors, named after a characteristic loop formed by 13 residues between two conserved disulfide-bond cysteines, play significant roles in neurological functions and disorders.5 These receptors consist of a pentameric assembly of identical or homologous subunits around a central pore and are activated upon neurotransmitter binding. Each subunit comprises the extracellular domain (ECD) that contains orthosteric agonist binding sites, the transmembrane domain (TMD) that forms the pore, and the intracellular domain (ICD) that governs interactions with intracellular proteins mediating subcellular distribution and downstream signaling events. Structures of Cys-loop receptors have been determined mostly for the ECD and TMD, only some of them contain partially resolved ICDs.6–8 The full-length ICD structures, however, remain unavailable due to the intrinsic flexibility of multiple ICD segments that challenges technical limitations of both cryo-EM and crystallography. An alternative approach is needed to overcome these limitations. NMR and ESR, in conjunction with site-directed labeling of unique cysteines in strategic locations, are promising approaches that have provided structural distance restraints for flexible and even disordered proteins.9 One of the key prerequisites of this approach is that the selected cysteine mutations must not significantly alter protein function.
α7 nAChR is one of the most abundant nAChR subtypes in the brain, has wide distribution in non-neuronal tissues, and is a therapeutic target for a wide range of disorders and diseases, including addiction, chronic pain, schizophrenia and Alzheimer’s disease.10–12 The diverse roles of α7 nAChR derive in part from the calcium permeability of the channel,13 but also from direct interactions with intracellular proteins involved in synaptic plasticity and downstream signaling pathways independent of calcium flux.14–16 Therapeutic strategies targeting the interactions of α7 nAChR with its protein partners are limited by the lack of structural information for the α7 nAChR ICD. Can site-directed labeling of unique cysteines be used to gain structural information for the ICD? Human α7 nAChR contains a total of 17 cysteines (Fig. 1), a much higher occurrence than that normally found in proteins.17 The rich content of native cysteines in α7 nAChR provides opportunities for extracting structural information through site-directed labeling with chemical probes in strategic locations, but it also presents uncertainties toward channel functions when the native cysteines are mutated. Thus, we evaluated the functional tolerance of human α7 nAChR to cysteine mutations using site-directed mutagenesis followed by measurements using two-electrode voltage clamp electrophysiology and surface plasmon resonance. The results suggest that a high native cysteine content does not preclude the use of single cysteine labeling for gathering structural information of functional proteins.
Figure 1.
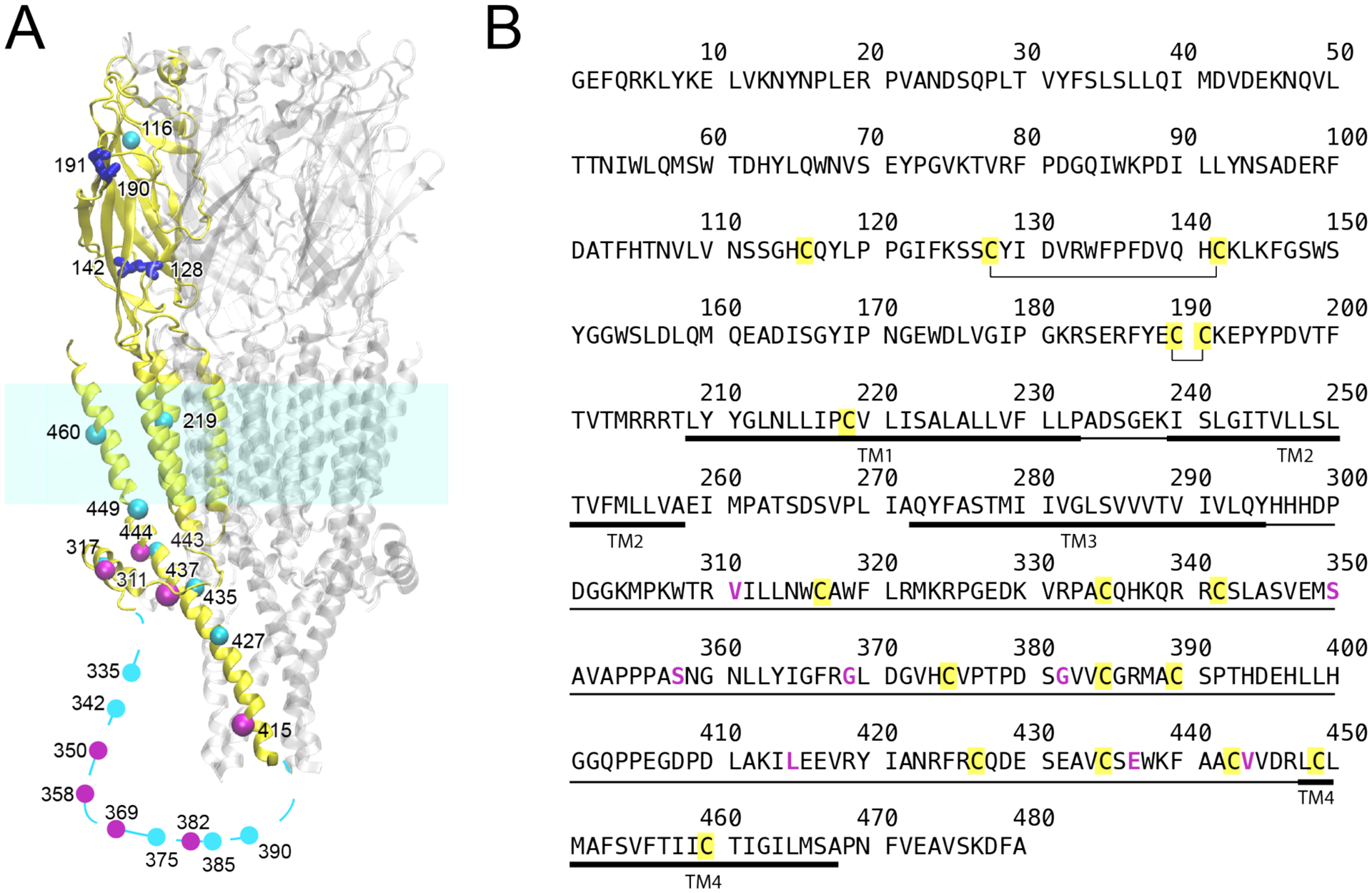
(A) Homology structure of human α7 nAChR showing cysteine positions. For clarity, cysteines are highlighted only in a single subunit (yellow). Native cysteines forming disulfide bonds are in blue; native cysteines mutated to create the Cys-min construct are in cyan; non-native cysteines introduced by mutation are in magenta. Cysteines in the transmembrane domain were mutated to alanine. All other cysteines, except four cysteines in blue, were mutated to serine. The homology model was based on 5HT3AR (PDB ID: 6BE1). The dotted line represents a region in the intracellular domain where no homology structure is available. (B) Sequence of human α7 nAChR. Disulfide bonds are annotated, native cysteines are marked in yellow, and residues mutated to cysteines are magenta. The intracellular domain and the transmembrane domain are marked in thin and thick lines, respectively.
Results and Discussion
We first created a Cys-null construct, in which all cysteines were mutated to serine except three cysteines in the TMD that were replaced by alanine (Fig. 1). Cysteine, with a pKa near 8, can have either polar or non-polar characteristics depending on the local microenvironment. Alanine was chosen to replace cysteine in the hydrophobic environment of the transmembrane domain because of its similar size. Serine, which differs from cysteine only in having a hydroxyl group instead of the sulfhydryl moiety, was used in the presumably more polar environment of the ICD. Two pairs of disulfide-bond cysteines in the ECD were previously found to affect α7 nAChR function.18, 19 One pair contributes to the cys-loop (C128-C142) and was essential for surface expression of α7 nAChR in neuroblastoma cells.18 Another pair, formed by the vicinal cysteines (C190–191 in human α7 nAChR or C189–190 in rat α7 nAChR) in loop C, was essential for agonist response.19 In line with these previous findings, our Cys-null construct expressed in Xenopus oocytes showed no activity in two electrode voltage clamp electrophysiology (TEVC) experiments (Fig. 2A). We then restored the pairs of C128-C142 and C190-C191 individually or together. Channel functions were observed only when both pairs of cysteines in the ECD were present. The α7 nAChR construct containing only the C128-C142 and C190-C191 cysteines is named Cys-min.
Figure 2.
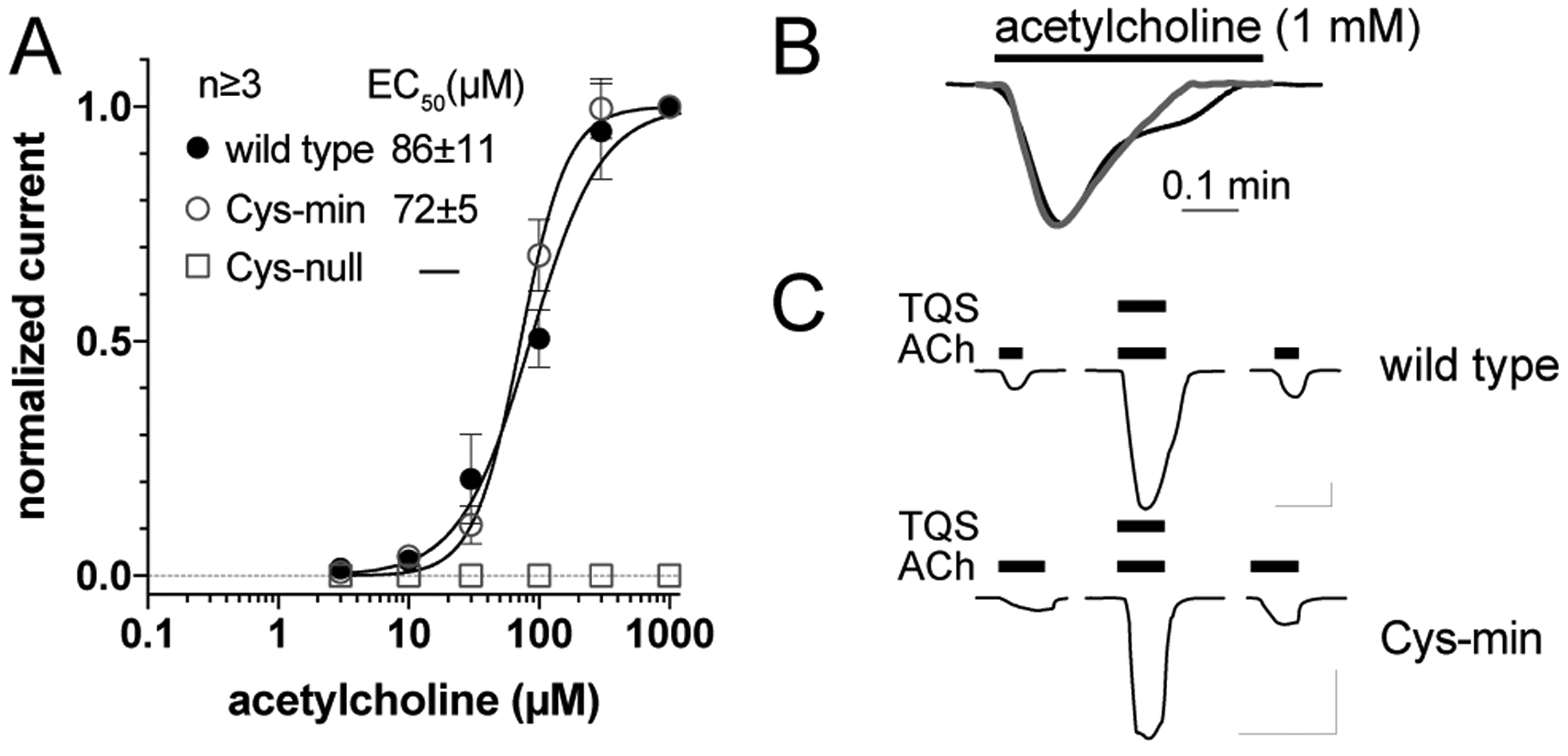
Functional validation of α7 nAChR cys-min construct. (A) Agonist concentration response curves of Xenopus oocytes expressing wild-type (wt) α7 nAChR, an α7 nAChR construct with no unpaired cysteines (Cys-min), or a construct with no cysteines (Cys-null). Error bars indicate standard error of means. (B) Representative current traces of Xenopus oocytes expressing wt α7 nAChR or the cys-min construct showing similar desensitization by 1 mM acetylcholine. (C) Representative current traces of Xenopus oocytes injected with RNA for α7 nAChR or the cys-min construct showing potentiation by positive allosteric modulator TQS. Bars indicate application of acetylcholine (20 μM) and TQS (30 μM). Scale bars are 1 μA and 30s.
Xenopus oocytes expressing Cys-min showed a response to the agonist acetylcholine with an EC50 of 72±5 μM, close to that of wild-type α7 nAChR (86±11 μM) (Fig. 2A). Cys-min also showed fast desensitization (Fig. 2B) as characterized in wild-type α7 nAChR by a rapid decay of the macroscopic current in the presence of agonists. Although TEVC experiments have a limited ability to resolve very fast kinetics, previous experiments from other groups have successfully used TEVC in Xenopus oocytes to characterize desensitization of wild-type and mutants of α7 nAChR.20, 21 These results suggest that the 13 cysteines mutated in Cys-min do not play a dominant role in the activation and desensitization processes. TQS (4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide) is a positive allosteric modulator (PAM) of α7 nAChR that prevents channel desensitization through binding to the TMD.22 Cys-min showed similar potentiation by TQS as the wild-type α7 nAChR (Fig. 2C), suggesting that the mutated cysteines have negligible influence on functional modulations by PAMs like TQS. This is consistent with results reported previously for PNU-120596 potentiation of α7 nAChR.23
The ICD of α7 nAChR contains nine native cysteines. It is known that α7 nAChR directly couples with numerous cytosolic proteins,15 including PICK1 (Protein interacting with C kinase), a widely-expressed adaptor protein implicated in numerous disorders and drugs of abuse.24 PICK1 binds to the α7 nAChR ICD via its PDZ domain.14 We measured the binding of the PDZ domain of PICK1 (PICK126) to the wild-type and Cys-min α7 nAChRs using surface plasmon resonance (Fig. 3). Equilibrium analysis shows that the apparent binding affinities for wild-type (KD = 2.5 ± 0.4 μM) and Cys-min (KD =2.6 ± 0.2 μM) α7 nAChRs are nearly identical, suggesting that the ICD structure remains intact in the Cys-min α7 nAChR.
Figure 3.
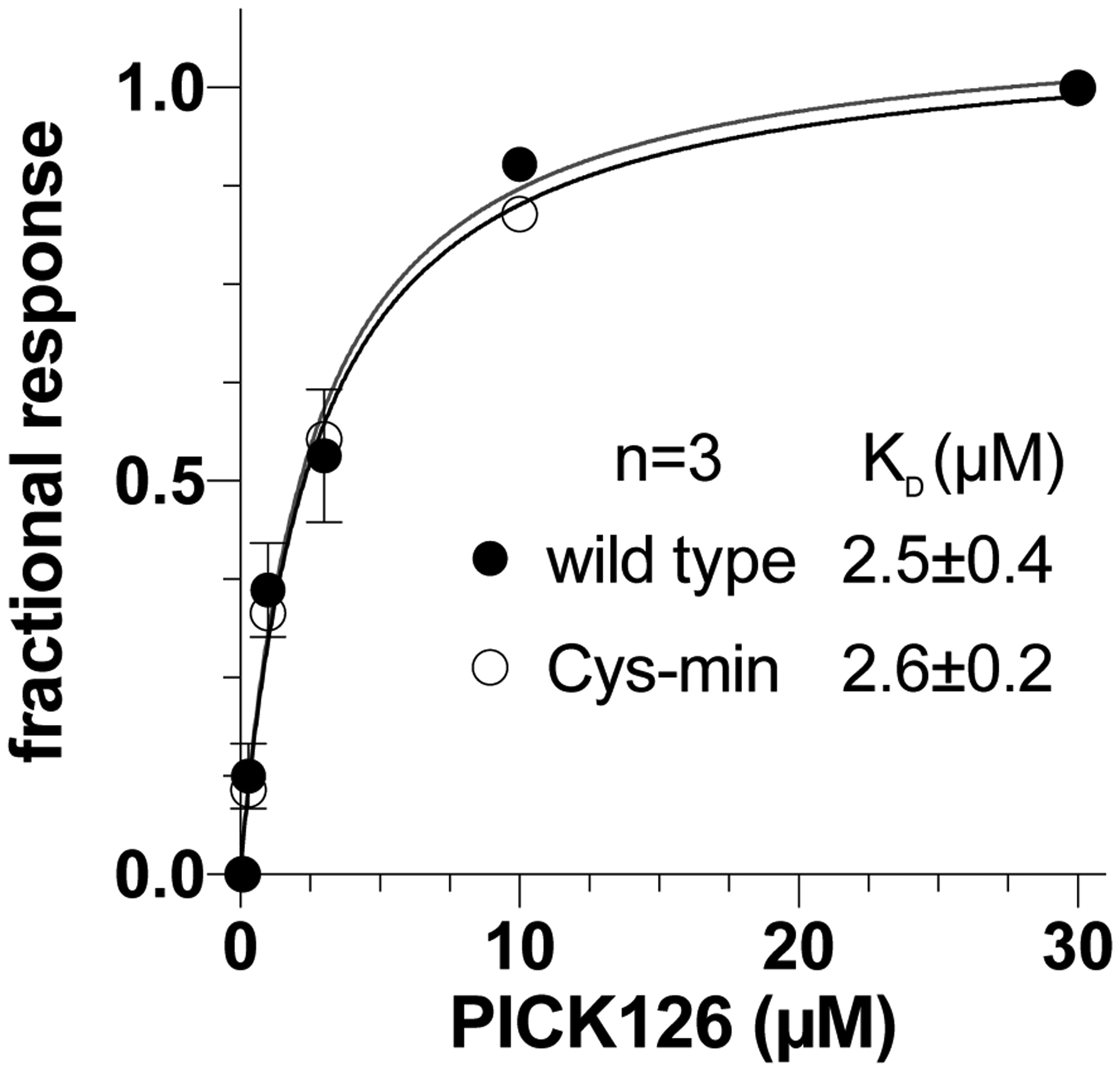
PICK1 binds α7 nAChR and the Cys-min construct similarly. For comparison, normalized data of equilibrium binding from surface plasmon resonance were fit to the Langmuir binding equation to determine apparent KD values. Error bars indicate standard error and are smaller than the symbol size when not visible.
We have further tested whether introducing additional cysteines into the ICD is functionally tolerated. On the basis of the Cys-min construct, we separately introduced eight non-native cysteines across the length of the ICD. These non-native cysteine sites were chosen to potentially provide crucial distance restraints to help define the α7 nAChR ICD structure. The majority of the chosen cysteine mutations were found to be functionally tolerated as evidenced by agonist response curves of these mutant constructs with EC50 values similar to the wild-type α7 nAChR (Fig. 4). Although the introduction of cysteines to individual sites appears to be well tolerated, one should always be cautious about potential structural perturbations introduced by a probe labeled to cysteine.1–4
Figure 4.
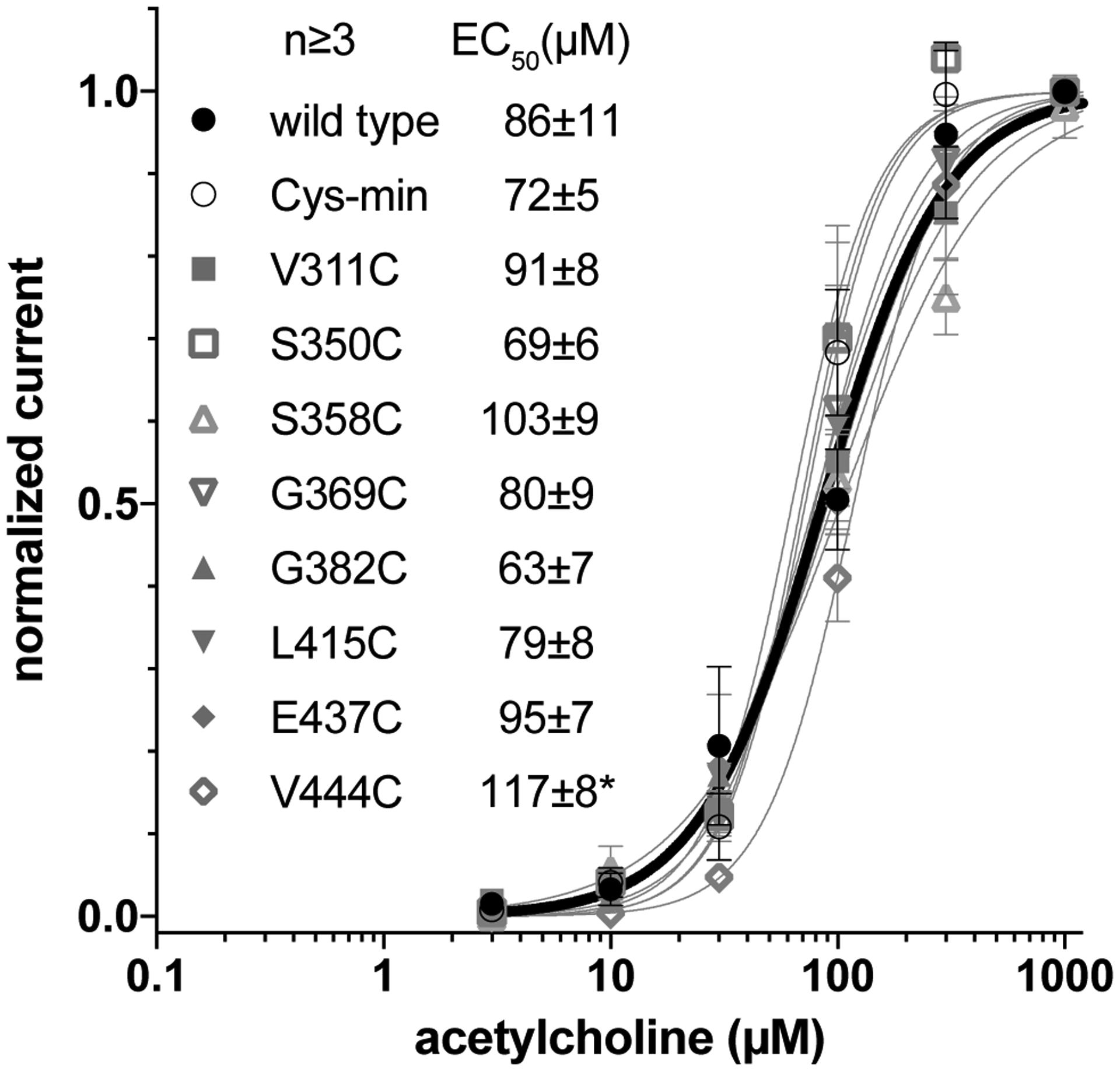
Functional validation of α7 nAChR constructs with single non-native cysteine. Response to agonist in Xenopus oocytes injected with RNA for the indicated constructs is similar to the wild-type α7 nAChR (bold line). Only V444C is significantly different from wild type (p=0.5) based on the extra sum-of-squares F test. Error bars indicate standard error.
These results demonstrate that none of the nine native cysteines in the ICD are essential for channel function or structural integrity. They also demonstrate substantial tolerance for introduction of new cysteines into the ICD for structural studies. Altogether, without significantly compromising α7 nAChR functions, one can utilize native or introduced cysteine to derive distance restraints and ultimately determine structures of the ICD through strategic placement of spin probes.
The functional tolerance of α7 nAChR to cysteine mutations is likely the result of three factors. First, protecting the conserved disulfide-bond cysteines is a prerequisite for making functional channels. This is well demonstrated in the current study (Fig. 2) and previous studies.18, 19 Secondly, since the ICD cysteines are unlikely to form disulfide bonds in the reducing environment of the cytosol,25 their mutation would not be expected to cause major disruptions of ICD structure, as evidenced by channel current measurements (Fig. 2) and PICK1 binding results (Fig. 3). Finally, choosing conservative cysteine substitutions is critically important to retain channel function. We replaced native cysteines in the hydrophilic and hydrophobic regions with serine and alanine, respectively. An excellent match between α7 nAChR and Cys-min in channel activation by the agonist acetylcholine, and potentiation by the PAM TQS, suggests that serine and alanine are ideal cysteine substitutes. However, channel functions could be severely compromised if cysteines in the same positions were mutated to other amino acids. For example, mutations of C460A, C449A and C219A in our Cys-min construct did not substantially change channel function in the absence or presence of allosteric modulators. In contrast, a previous study showed that C460Y caused a significant decrease in the level of potentiation by PAMs and C449L resulted in nonfunctional receptors.26 Similarly, the homolog to C219 in neuromuscular nAChR was found to be critical for channel gating, but could tolerate conservative substitutions, including alanine.27 These results suggest that many cysteines can be readily substituted by conservative mutations to either alanine or serine.
It is worth mentioning that when examining receptor tolerance to cysteine mutations, in general, one should also consider receptor expression. Both α4β2 and α7 nAChRs are palmitoylated at cysteine residues.28 The C273S mutation in the cytoplasmic loop TM1 and TM2 of the α4 nAChR resulted in a nonpalmitoylated nAChR, which had normal functional activity; however, the mutation led to an increase in surface expression of the receptor and a decrease in the total expression.29 α7 nAChR does not have a cysteine homologous to C273 in α4 nAChR. It is unclear which cysteine mediates palmitoylation of α7 nAChR. Deleting the segment where C317 resides in the cytoplasmic region close to TM3 abolished surface α7 nAChR expression,30 but the C317A mutation did not cause a large variation in receptor expression or channel currents.31 Similarly, C427A in the cytoplasmic region adjacent to TM4 did not show large modifications in receptor expression and currents.31 For the purpose of structural studies, variation in the receptor expression level due to cysteine mutation is less concerning, as long as one can get a sufficient amount of functional proteins.
Although human α7 nAChR was used in the current study, the conclusions obtained from this study should be applicable to other α7 nAChRs, considering that the positions of all cysteines are highly conserved across vertebrate species. In terms of other subtypes of nAChRs or other members of Cys-loop receptors, there is much less cysteine conservation besides the pair of conserved cysteines in the ECD that form a disulfide bond and stabilize the Cys-loop or additionally, a vicinal cysteine pair in the C-loop that features the agonist binding site of alpha subunits of nAChRs. Nevertheless, the functional tolerance to cysteine mutations discussed above can still be a relevant reference for Cys-loop receptors at large.
Methods
Protein constructs and expression.
Our expression constructs consist of DNA encoding the full-length human α7 nAChR. For expression in Xenopus oocytes, mutations were introduced into the oocyte expression vector pMXT-α7 nAChR32 using the QuikChange Lightning Site-directed Mutagenesis kit (Agilent) and confirmed by DNA sequencing. For expression in E. coli, mutations were introduced into the E. coli expression vector pTBSG1-α7 nAChR.33 The wild type and Cys-min α7 nAChRs were expressed in E. coli and purified as reported previously33 with the following modifications. Briefly, the α7 nAChR constructs were expressed in Rosetta 2(DE3)pLysS (Novagen) by induction with 0.2 mM IPTG at an OD600 of 0.8 for 16 h at 15 °C in LB broth containing 500 mM sorbitol and 10 mM choline. Cells from 1 liter induction medium were suspended in 150 ml of buffer containing 50 mM Tris, pH 8, 500 mM NaCl, 500 mM sucrose, 10 mM choline, and HALT protease inhibitor. All subsequent operations were at 4 °C. Cells were lysed using a Microfluidics M-110Y microfluidizer and the cell lysate was adjusted to 0.33% (w/v) dodecylphosphocholine (DPC, Anatrace) and 20 mM imidazole and incubated for 1 h. The insoluble fraction was removed by ultracentrifugation (1 h, 200,000 × g) and the supernatant incubated with 2 ml of NiNTA resin (GEHealthcare) for 1 h, mixing by inversion. The resin was washed with 100 mM imidazole pH 8, 300 mM NaCl, 0.2% DPC, 0.02 mg/ml asolectin to a flat baseline, and eluted by adjusting the imidazole concentration to 300 mM. The pentamer fraction was isolated by size exclusion chromatography (SEC) using a S200 10/300 column (GEHealthcare) equilibrated with 20 mM HEPES pH 7.4, 300 mM NaCl, 0.2% DPC, 0.02 mg/ml asolectin.
To obtain the PDZ domain of PICK1, the DNA encoding the first 126 amino acids of PICK1 (PICK126) was subcloned into the pTBGS1 vector.34 with a Twin-Strep Tag (IBA) followed by a TEV cleavage site at the N-terminus of PICK126. PICK126 was expressed in Rosetta 2(DE3)pLysS (Novagen) in LB broth containing 500 mM sorbitol. Induction was with 0.2 mm IPTG at an OD600 of 0.8 for 16 h at 15 °C. Cells were collected by centrifugation and suspended in buffer A (20 mM sodium phosphate pH 8, 300 mM sodium chloride). All subsequent operations were at 4 °C. Cells were lysed using a Microfluidics M-110Y microfluidizer and the membranes collected by ultracentrifugation (200k × g, 1 hour). After resuspension in 8 ml buffer A per gm membrane, membranes were solubilized with 2% (w/v) n-dodecyl-β-D-maltoside (DDM, Anatrace), and the clarified extract applied to a 2 ml Strep-Tactin XT Superflow column at 1 ml/min. The column was washed with buffer A containing 0.1% DDM to a flat baseline and then eluted with 5 column volumes 50 mM biotin. The eluate was concentrated and subjected to size exclusion chromatography using a S200 10/300 column (GE Healthcare) equilibrated in SPR running buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 0.05% DDM. The yield of purified PICK126 was ~0.5 mg/liter induction media.
Electrophysiology measurements.
TEVC measurements of α7 nAChR constructs in Xenopus oocytes have been described previously.33, 35 Briefly, capped complementary RNA (cRNA) was synthesized with the mMessage mMachine kit (Ambion), purified with the RNeasy kit (Qiagen) and 25ng α7 nAChR RNA co-injected with 25 ng RIC3 RNA into Xenopus laevis oocytes (stages 5–6). After 1–2 days, channel function was measured in a 20-μl oocyte recording chamber (Automate Scientific) clamped at −60 mV with an OC-725C Amplifier (Warner Instruments). The perfusion rate was 2.4 ml/min, providing complete buffer exchange for the 20 μl recording chamber every 0.5 sec. The recording solutions contained 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm HEPES, pH 7.0 and the indicated concentrations of the acetylcholine and TQS. Data were collected and processed using Clampex 10 software (Molecular Devices). Nonlinear regressions and statistical analysis were performed using Prism software (GraphPad). Comparisons between agonists response curves were assessed using the extra sum-of-squares F test. To measure desensitization, current was elicited by the application of 1 mM acetycholine that was continuously applied until the elicited current had returned to baseline.
Surface Plasmon Resonance
In the surface plasmon resonance (SPR) experiments, equilibrium analysis36 was used to determine the apparent KD for PICK126 binding to each immobilized construct. Purified wild type and Cys-min α7 nAChR were immobilized on an NTA sensor chip (GE Healthcare) with densities between 500 and 1200 response units (RU) using a Biacore 3000. Responses to PICK126 binding for a series of concentrations ranging from 0.1 to 30 μM were measured at a flow rate of 30 μL/min. After reference and buffer subtraction, the equilibrium values for each sensogram were fit by non-linear regression and used to plot the resonance response as a function of PICK126 concentration. Each concentration of PICK126 was measured by three separate injections. Dissociation constants were derived by non-linear regression analysis using the Langmuir isotherm equation.
Acknowledgements
The authors thank other members of the Tang laboratory for helpful discussion.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The research was supported by funding from NIH (R01DA046939)
Footnotes
Competing Interests
The authors declare no competing financial interest.
References
- 1.Klug CS, Feix JB (2008) Methods and applications of site-directed spin labeling EPR spectroscopy, Methods Cell Biol. 84, 617–658. [DOI] [PubMed] [Google Scholar]
- 2.Kinde MN, Chen Q, Lawless MJ, Mowrey DD, Xu J, Saxena S, Xu Y, and Tang P (2015) Conformational Changes Underlying Desensitization of the Pentameric Ligand-Gated Ion Channel ELIC, Structure 23, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondarenko V, Wells MM, Chen Q, Singewald KC, Saxena S, Xu Y, and Tang P (2019) (19)F Paramagnetic Relaxation-Based NMR for Quaternary Structural Restraints of Ion Channels, ACS Chem. Biol 14, 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Zhang L, Zhou Y, and Guo YL (2007) A novel pyrimidine-based stable-isotope labeling reagent and its application to quantitative analysis using matrix-assisted laser desorption/ionization mass spectrometry, J. Mass Spectrom 42, 1514–1521. [DOI] [PubMed] [Google Scholar]
- 5.Thompson AJ, Lester HA, and Lummis SC (2010) The structural basis of function in Cys-loop receptors, Q. Rev. Biophys 43, 449–499. [DOI] [PubMed] [Google Scholar]
- 6.Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, Moreau C, Li XD, Poitevin F, Vogel H, and Nury H (2014) X-ray structure of the mouse serotonin 5-HT3 receptor, Nature 512, 276–281. [DOI] [PubMed] [Google Scholar]
- 7.Basak S, Gicheru Y, Samanta A, Molugu SK, Huang W, Fuente M, Hughes T, Taylor DJ, Nieman MT, Moiseenkova-Bell V, and Chakrapani S (2018) Cryo-EM structure of 5-HT3A receptor in its resting conformation, Nat Commun 9, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr., Cabuco R, Howard RJ, Zaveri NT, Lindahl E, and Hibbs RE (2019) Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor, Neuron 104, 501–511 e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliezer D (2012) Distance information for disordered proteins from NMR and ESR measurements using paramagnetic spin labels, Methods Mol. Biol 895, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunzell DH, McIntosh JM (2012) Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia, Neuropsychopharmacology 37, 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI (2018) New Insights on Neuronal Nicotinic Acetylcholine Receptors as Targets for Pain and Inflammation: A Focus on alpha7 nAChRs, Curr. Neuropharmacol 16, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouzat C, Lasala M, Nielsen BE, Corradi J, and Esandi MDC (2018) Molecular function of alpha7 nicotinic receptors as drug targets, J. Physiol 596, 1847–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, and Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium, J. Neurosci 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baer K, Burli T, Huh KH, Wiesner A, Erb-Vogtli S, Gockeritz-Dujmovic D, Moransard M, Nishimune A, Rees MI, Henley JM, Fritschy JM, and Fuhrer C (2007) PICK1 interacts with alpha7 neuronal nicotinic acetylcholine receptors and controls their clustering, Mol. Cell. Neurosci 35, 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulo JA, Brucker WJ, and Hawrot E (2009) Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome, J. Proteome Res 8, 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King JR, Ullah A, Bak E, Jafri MS, and Kabbani N (2018) Ionotropic and Metabotropic Mechanisms of Allosteric Modulation of alpha7 Nicotinic Receptor Intracellular Calcium, Mol. Pharmacol 93, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miseta A, and Csutora P (2000) Relationship between the occurrence of cysteine in proteins and the complexity of organisms, Mol. Biol. Evol 17, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 18.Dunckley T, Wu J, Zhao L, and Lukas RJ (2003) Mutational analysis of roles for extracellular cysteine residues in the assembly and function of human alpha 7-nicotinic acetylcholine receptors, Biochemistry 42, 870–876. [DOI] [PubMed] [Google Scholar]
- 19.Blum AP, Gleitsman KR, Lester HA, and Dougherty DA (2011) Evidence for an extended hydrogen bond network in the binding site of the nicotinic receptor: role of the vicinal disulfide of the alpha1 subunit, J. Biol. Chem 286, 32251–32258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, and Changeux JP (1991) Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor, Nature 353, 846–849. [DOI] [PubMed] [Google Scholar]
- 21.Placzek AN, Grassi F, Papke T, Meyer EM, and Papke RL (2004) A single point mutation confers properties of the muscle-type nicotinic acetylcholine receptor to homomeric alpha7 receptors, Mol. Pharmacol 66, 169–177. [DOI] [PubMed] [Google Scholar]
- 22.Gill JK, Savolainen M, Young GT, Zwart R, Sher E, and Millar NS (2011) Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site, Proc. Natl. Acad. Sci. U. S. A 108, 5867–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.daCosta CJ, Free CR, Corradi J, Bouzat C, and Sine SM (2011) Single-channel and structural foundations of neuronal alpha7 acetylcholine receptor potentiation, J. Neurosci 31, 13870–13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YH, Zhang N, Wang YN, Shen Y, and Wang Y (2016) Multiple faces of protein interacting with C kinase 1 (PICK1): Structure, function, and diseases, Neurochem. Int 98, 115–121. [DOI] [PubMed] [Google Scholar]
- 25.Derman AI, Prinz WA, Belin D, and Beckwith J (1993) Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli, Science 262, 1744–1747. [DOI] [PubMed] [Google Scholar]
- 26.Young GT, Zwart R, Walker AS, Sher E, and Millar NS (2008) Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site, Proc. Natl. Acad. Sci. U. S. A 105, 14686–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo DC, Pinkham JL, and Stevens CF (1991) Role of a key cysteine residue in the gating of the acetylcholine receptor, Neuron 6, 31–40. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JK, Govind AP, Drisdel RC, Blanton MP, Vallejo Y, Lam TT, and Green WN (2010) Palmitoylation of nicotinic acetylcholine receptors, J. Mol. Neurosci 40, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amici SA, McKay SB, Wells GB, Robson JI, Nasir M, Ponath G, and Anand R (2012) A highly conserved cytoplasmic cysteine residue in the alpha4 nicotinic acetylcholine receptor is palmitoylated and regulates protein expression, J. Biol. Chem 287, 23119–23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valor LM, Mulet J, Sala F, Sala S, Ballesta JJ, and Criado M (2002) Role of the large cytoplasmic loop of the alpha 7 neuronal nicotinic acetylcholine receptor subunit in receptor expression and function, Biochemistry 41, 7931–7938. [DOI] [PubMed] [Google Scholar]
- 31.Castelan F, Mulet J, Aldea M, Sala S, Sala F, and Criado M (2007) Cytoplasmic regions adjacent to the M3 and M4 transmembrane segments influence expression and function of alpha7 nicotinic acetylcholine receptors. A study with single amino acid mutants, J. Neurochem 100, 406–415. [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Katz M, Gerzanich V, Anand R, and Lindstrom J (1994) Human alpha 7 acetylcholine receptor: cloning of the alpha 7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha 7 homomers expressed in Xenopus oocytes, Mol. Pharmacol 45, 546–554. [PubMed] [Google Scholar]
- 33.Tillman TS, Alvarez FJ, Reinert NJ, Liu C, Wang D, Xu Y, Xiao K, Zhang P, and Tang P (2016) Functional Human alpha7 Nicotinic Acetylcholine Receptor (nAChR) Generated from Escherichia coli, J. Biol. Chem 291, 18276–18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin H, Hu J, Hua Y, Challa SV, Cross TA, and Gao FP (2008) Construction of a series of vectors for high throughput cloning and expression screening of membrane proteins from Mycobacterium tuberculosis, BMC Biotechnol. 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillman TS, Seyoum E, Mowrey DD, Xu Y, and Tang P (2014) ELIC-alpha7 Nicotinic acetylcholine receptor (alpha7nAChR) chimeras reveal a prominent role of the extracellular-transmembrane domain interface in allosteric modulation, J. Biol. Chem 289, 13851–13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myszka DG, Jonsen MD, Graves BJ (1998) Equilibrium analysis of high affinity interactions using BIACORE, Anal. Biochem 265, 326–330. [DOI] [PubMed] [Google Scholar]


