Abstract
Acute pulmonary embolism is the third most common cause of cardiovascular death. Pulmonary embolism increases right ventricular afterload, which causes right ventricular failure, circulatory collapse and death. Most treatments focus on removal of the mechanical obstruction caused by the embolism, but pulmonary vasoconstriction is a significant contributor to the increased right ventricular afterload and is often left untreated. Pulmonary thromboembolism causes mechanical obstruction of the pulmonary vasculature coupled with a complex interaction between humoral factors from the activated platelets, endothelial effects, reflexes and hypoxia to cause pulmonary vasoconstriction that worsens right ventricular afterload. Vasoconstrictors include serotonin, thromboxane, prostaglandins and endothelins, counterbalanced by vasodilators such as nitric oxide and prostacyclins. Exogenous administration of pulmonary vasodilators in acute pulmonary embolism seems attractive but all come with a risk of systemic vasodilation or worsening of pulmonary ventilation-perfusion mismatch. In animal models of acute pulmonary embolism, modulators of the nitric oxide-cyclic guanosine monophosphate-protein kinase G pathway, endothelin pathway and prostaglandin pathway have been investigated. But only a small number of clinical case reports and prospective clinical trials exist. The aim of this review is to give an overview of the causes of pulmonary embolism-induced pulmonary vasoconstriction and of experimental and human investigations of pulmonary vasodilation in acute pulmonary embolism.
Keywords: right heart failure, pulmonary circulation, animal models, right ventricular afterload
Introduction
Acute pulmonary embolism (PE) occurs in about 1 in 1000 persons per year and is associated with a high morbidity and mortality,1,2 making PE the third most common cause of cardiovascular death in Europe. Cause of death in PE is right ventricular (RV) failure caused by a combination of mechanical obstruction and pulmonary vasoconstriction, which both increases RV afterload.3,4
In PE, the thrombus lodges in the pulmonary arteries and causes immediate mechanical obstruction. The embolism activates the coagulation system, damages the endothelium, stagnate pulmonary blood flow and accordingly initiate secondary pulmonary thrombosis which worsens the mechanical obstruction.5,6
RV dysfunction is related to short-term clinical deterioration7 and prognosis.4,8–10 Mechanical obstruction alone cannot explain the increased RV afterload and consequent RV dysfunction in PE (Fig. 1). Pulmonary vascular resistance (PVR) does not increase until approximately 50% of the pulmonary vasculature is embolized,6 and thrombus mass and percentage of pulmonary vascular obstruction alone correlate poorly to the hemodynamic compromise11,12 and prognosis in PE.13–15
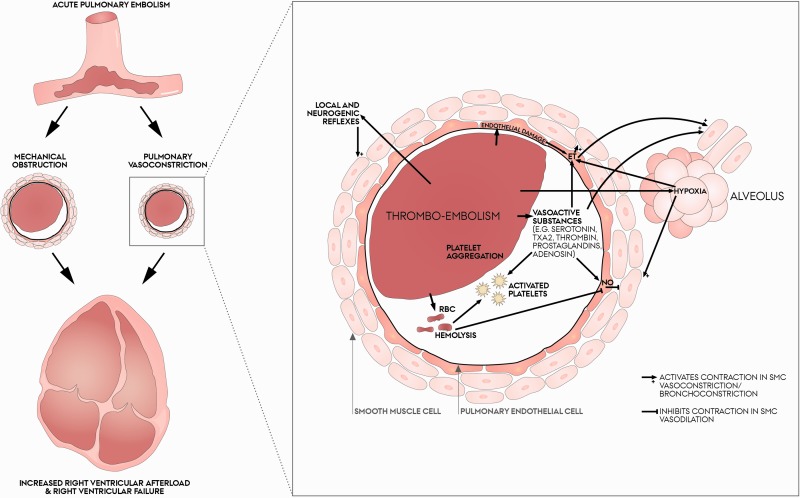
Fig. 1.
On the left, a schematic pathway showing acute pulmonary embolism (PE) to cause both mechanical obstruction of pulmonary arteries and pulmonary vasoconstriction. Both increases right ventricular (RV) afterload causing acute RV dilatation and interventricular septal shift which have been associated specifically with severe, acute PE. The RV may enter a vicious circle of right ventricular failure, circulatory collapse and death. On the right, focus on pulmonary vasoconstriction induced by a pulmonary embolism. Several mechanisms are potential underlying causes: vasoactive substances from the thrombus, hemolysis, activated platelets, endothelial damage, reflexes, and hypoxia. Please see the text for further details.
ET: endothelins; NO: nitric oxide; PEC: pulmonary endothelial cell; RBC: red blood cell; SMC: smooth muscle cell; TXA2: thromboxane A2.
This mismatch between thrombus mass and hemodynamic compromise raises the hypothesis that humoral responses and reflexes activated by the thrombus induce pulmonary vasoconstriction.
Key element in the treatment of PE is reduction of the thrombus mass. But this strategy only targets the mechanical component of the RV afterload increase. According to current guidelines, there are no recommended treatments targeting pulmonary vasoconstriction4,16 and its use is not reported in large registries,17 leaving a significant contributor to the adverse outcome in PE untreated.
Several experimental PE studies have shown a significant reduction in PVR using pulmonary vasodilators that targets a variety of pathways involved in pulmonary vascular tone.18 Despite evidence from pre-clinical studies, the clinical literature is dominated by case series and few small clinical trials using pulmonary vasodilators in PE.
We aim to provide a clinically relevant introduction to the mechanisms that induce pulmonary vasoconstriction in PE and a comprehensive review of both pre-clinical and clinical studies using pulmonary vasodilators in acute PE.
Methods
We searched MEDLINE via PubMed and Embase for relevant articles with latest update 13 September 2019 (see Appendix 1 for full search strategies).
Articles describing a medical intervention causing pulmonary vasodilation in acute PE using a clinically relevant drug were included. Both human and animal studies were included no matter the year of publication.
Exclusion criteria included especially studies on chronic thromboembolic pulmonary hypertension (CTEPH) and the other causes of pulmonary hypertension (PH) within the World Health Organization classification of PH. Please see Appendix 1 for full list of inclusion and exclusion criteria.
Pulmonary vasoconstriction in acute PE
Pulmonary vasoconstriction is a significant contributor to the increase of PVR in PE. This happens through a number of pathways which are not understood completely. The mechanisms are summarized in Fig. 1.
Hematogenous thromboembolism increases pulmonary arterial pressure (PAP) more effectively than non-hematogenous material,19 emphasizing the importance of PE-released vasoconstrictors. Evidence of these humoral or other chemicals was shown more than half a century ago.20 Activated platelets and the thrombus mass21–23 secrete thromboxane-A2, prostaglandins, adenosine, thrombin, and serotonin19,23–27 which induce platelet aggregation and pulmonary vasoconstriction.5,19,28 Platelet-activating factor is also increased with acute PE.29 Pulmonary endothelial cells inactivate serotonin and certain prostaglandins30 to maintain homeostasis.
Endothelins (ET) are produced by the pulmonary vascular endothelium when stimulated by thrombin, endothelial injury and hypoxia. ET target the ETA and ETB receptors in the smooth muscle cells, and pulmonary vasoconstriction is induced by activation of phospholipase C that increases inositol triphosphate, diacylglycerol and intracellular calcium.31,32 ET have been estimated to be in charge of 25% of the PE-induced increase in PVR,33 but findings are variable.34 ET also induce bronchoconstriction and release of TxA2 which further potentate the pulmonary vasoconstrictor effect.35
Prostaglandins cause either smooth muscle contraction or relaxation, depending on the prostaglandin subtype and receptor subtype.19 Smooth muscle contraction and subsequent vasoconstriction are mediated through receptor coupling with the phospholipase C pathway.36 In acute PE, elevated levels of prostaglandins that induce vasoconstriction have been observed,26,37 but prostaglandins may prevent the release of other vasoconstrictors.38 The clinical significance and the net pulmonary vasoconstrictor effect after vasodilation triggered by concomitant prostacyclin release are not known in acute PE.39,40
Histamine release may also play a role in acute pulmonary embolism but have only been sparsely investigated making the clinical significance unknown.41,42
Hemolysis is present in PE causing a release of arginase which converts L-arginine to L-orthinine and urea. Otherwise, L-arginine would have had potential to produce L-citrulline and nitric oxide (NO) catalyzed by nitric oxide synthase. The consequence is reduced availability of NO as vasodilator.43 Additionally, released free heme and hemoglobin (Hb) reacts fast and irreversible with NO and further limits bioavailability of NO.44,45 This is normally counteracted by heme oxygenase-145 and by haptoglobin, but haptoglobin is decreased in PE patients.46 As NO causes pulmonary vasodilation, hemolysis can be an indirect cause of vasoconstriction.47 Hemolysis-released adenosine di-phosphate and free Hb enhance platelet activation48 which may cause further obstruction of the pulmonary vessels.
Furthermore, PE-induced vasoconstriction is augmented by local and neurogenic reflexes that might be dependent on localization of the PE.19,49,50 Sympathetic activity seems to be increased in both embolized and non-embolized parts of the lung.51 PE-released substances also cause bronchoconstriction of the small airways,23,42,52 leading to hypoxia and pulmonary vasoconstriction.53 Hypoxia in the lung tissue will inhibit synthesis of vasodilating prostanoids and worsen vasoconstriction.38
For a summary, see Fig. 1. The different mechanisms of pulmonary vasoconstrictors in PE have been reviewed in details previously.6,18,19,35,54
Results
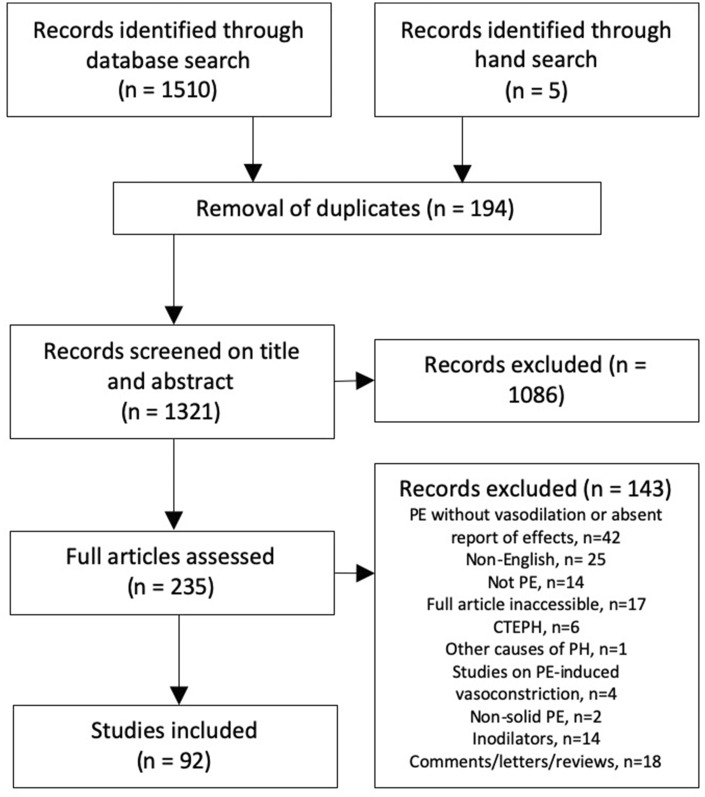
Literature search resulted in 1510 papers and additional five were found by hand search. See Fig. 2 for flow chart on the screening process. A total of 92 papers were included in this review (summarized in Tables 1 to 4).
Fig. 2.
Flow diagram of the review selection process.
CTEPH: chronic thromboembolic pulmonary hypertension; PE: pulmonary embolism.
Table 1.
Vasodilation through the NO-sGC-cGMP pathway in acute pulmonary embolism.
| Treatment | Experimental microsphere/glass beads/air PE | Experimental autologous (blood, fat, muscle, collagen) PE | Case reports | Clinical trials | Guideline recommendation |
|---|---|---|---|---|---|
| Nitric oxide | Lowers PVR, mPAP but not MAP.66,68–71,73,80,84–86 No effect on mPAP but increase in CO.87 | Lowers PVR, mPAP, increases CO.51,65,67,72,74–76,79,81–83 Lowers cardiac damage.65,76,77 No hemodynamic effects.78 Decreased stroke volume88 or MAP/SVR79,83 | 18 PE cases with mostly positive effects92–94,96,108 Five cases in combination with thrombolysis, thrombectomy or embolectomy97,99–102 Five cases of post-operative PE98,107 Two PE cases in patients with PFO or VSD106,109 One post-partum patient with PE95 Four pediatric cases103–105 One case of autoimmune hemolytic anaemia47 One PE case with deterioration on iNO110 | Eight patients, single arm. Safe. Trend towards improvement.112 iNOPE trial, double-blinded, 78 patients with intermediate-high-risk PE randomized to iNO + O2 or placebo. Neutral on both endpoints111 | Either no recommendations16 or “Inhalation of nitric oxide may improve the hemodynamic status (…) no evidence for its clinical efficacy or safety”4 |
| sCG stimulators/ activators | Lowers mPAP and PVR. Risk of decreased MAP.84,118–120 | Lowers PVR and mPAP and increases CO75 | None | None | No recommendations4,16 |
| PDE-5 inhibitors | Lowers mPAP and PVR, no effect on MAP84,86,126–131 | Lowers mPAP and PVR75,124,125 | Three PE cases136,138,139 One case of acute-on-chronic PE137 One case with congenital VSD109 Two cases of post-operative PE98,135 One child with non-thrombotic PE140 | None | No recommendations4,16 |
Note: Summary of review of the NO-sGC-cGMP pathway to induce pulmonary vasodilation in acute pulmonary embolism. Divided by treatment option, animal or clinical data and guideline recommendation. Please see text for further details.
CO: cardiac output; iNO: inhaled nitric oxide; MAP: mean arterial pressure; mPAP: mean pulmonary arterial pressure; NO: nitric oxide; PAP: pulmonary artery pressure; PE: pulmonary embolism; PFO: persistent foramen ovale; PVR: pulmonary vascular resistance; SVR: systemic vascular resistance; VSD: ventricular septal defect.
Table 4.
Hydralazine-induced pulmonary vasodilation in acute pulmonary embolism.
| Treatment | Experimental microsphere/ glass beads/air PE | Experimental autologous (blood, fat, muscle, collagen) PE | Case reports | Clinical trials | Guideline recommendation |
|---|---|---|---|---|---|
| Hydralazine | Divergent effects on PVR.80,185 May improve CO80 | Lowers PAP and PVR, improves CI.79,82,88,184 May lower MAP79 | Six PE cases with limited effect187 One case with postoperative PE with positive effect186 | None | No recommendations4,16 |
Note: Summary of review of the hydralazine-induced pulmonary vasodilation in acute pulmonary embolism. Divided by animal or clinical data and guideline recommendation. Please see text for further details.
CI: cardiac index; CO: cardiac output; MAP: mean arterial pressure; PAP: pulmonary artery pressure; PE: pulmonary embolism; PVR: pulmonary vascular resistance.
Here we provide a detailed review of experimental and clinical studies investigating the effects of pulmonary vasodilators in PE. For clarity of presentation, we divided these into four categories based on mechanism of action which will be presented first. The included articles will be presented with the experimental research followed by case reports and clinical studies. Tables 1 to 4 summarize the effects of pulmonary vasodilation in PE according to our review of the literature divided by pathway or mechanism.
NO-sGC-cGMP pathway
The nitric oxide (NO)-soluble guanulate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway exerts its pulmonary vasodilatory effects through paracrine interaction between the pulmonary endothelial cells (PEC) and the underlying smooth muscle cells (SMC). In the PEC, either shear stress or a humoral activator (e.g. serotonin, thrombin) causes increasing cytosolic levels of Ca2+ which activates the NO synthase.55,56 The active enzyme deaminates L-arginine to L-citrulline and NO. NO diffuses to the SMC where it activates sGC that dephosphorylates guanosine tri-phosphate (GTP) to cGMP which again activates cGMP-dependent protein kinase. Subsequently, sarcoplasmatic Ca2+-pumps are activated causing decreased cytosolic Ca2+ levels57 and decreased activation of calmodulin that otherwise is essential in the activation of myosin light-chain kinase and the myosin-actin cross-bridge cycle.58 As this is interrupted, the SMC relaxes and vasodilation occurs.
Nitric oxide
Inhaled NO (iNO) acts as a selective pulmonary agent. It has been suggested to have dual effects, i.e. pulmonary vasodilation through the above-mentioned mechanism in ventilated regions and pulmonary vasoconstriction through inhibition of endogenous NO synthase, most pronounced in hypoxic regions. Therefore, iNO can attenuate ventilation-perfusion mismatch and improve oxygenation.57 iNO can dilate the non-constricted pulmonary vasculature and works in combination with inhaled prostacyclin.59 NO protects (partly) from PE-induced changes in hemodynamics and both expression of endothelial NO synthase mRNA and the fraction of expiratory NO increases in PE.60–62 NO consumption increases in both animals and humans with PE,63 and the endogenous NO production seems lifesaving in PE as antagonism of NO synthase causes death in PE-animals.62,64 Accordingly, iNO has a potential therapeutic role in PE.
In general, iNO lowered PAP and PVR in animal models65–76 and increased cardiac output (CO) in some studies.70,72 The effect is possibly caused by reduced pulmonary vascular tone in the periphery of the pulmonary artery tree, especially regions with distally localized emboli.73 The reduced RV afterload might explain less myocardial damage evident by lowered cardiac troponins.65,76,77 One study using fat-emboli in a canine model did, however, not detect any effects on pulmonary or cardiac function.78 Due to the rationale of inhaled administration, only one study noticed effect on the systemic vasculature with decreased mean arterial pressure (MAP).68 Some studies investigated dose–response relationship without consistent results,67,68 suggesting low-dose treatment to be favorable. iNO can be useful in combination with other treatment strategies where the agents showed additive effects on PAP, PVR or vessel diameter.59,72 The effects of iNO seem to be without prolonged effects.69
Other NO-donors have also been tested in animal models of PE. Both nitroglycerin, nitrite and nitroprusside lower mean PAP51,79–81 and PVR80,82–86 but not always.87 One must be aware that systemic administration of NO-donors increases the risk of systemic side effects with decrease in MAP, systemic vascular resistance (SVR) or stroke volume,79,82,83,85,88 but not consistently.51,81,84,87 Combination of NO-donor and other vasodilators may be even more efficient but increases the risk of side effects.86 NO-donor lowered sympathetic activity in the lungs, lowered ET-1 levels and increased NO levels.51
NO has additional non-hemodynamic effects. iNO lowers von Willebrand factor and glycoprotein IIb/IIIa as central parts in endothelial function and thrombosis.76 NO affects apoptotic pneumocytes,74 and both endogenous and exogenous NO inhibits platelet aggregation in animals and humans67,89–91 which might be relevant in PE.
NO has been used clinically in acute PE inspired by the experimental results and by the experience from using NO as a pulmonary vasodilator in acute respiratory distress syndrome and pulmonary hypertension.
A large variety of case reports have been published on the use of iNO, ranging from iNO administered to patients with RV failure (including PE-induced) as primary indication; as bridge to embolectomy or thrombolysis; with acute-on-chronic PE; during PE-induced cardiac arrest; on extra-corporal membrane oxygenation (ECMO) support or in case of contraindication to thrombolysis.92–98 Some cases describe the use of iNO to treat increased PAP after embolectomy of acute PE99–101 though not all with impressive hemodynamic responses.102 iNO has been used in children and even infants with PE without success.103–105 The influence of hemolysis on pulmonary vasoconstriction was evident in a case of autoimmune hemolytic anaemia, where PAP was significantly elevated despite only small clot burden, and iNO had impressive effect.47 One case described how iNO closed a persistent foramen ovale in a patient with PE and multiple cerebral infarcts.106
A number of case reports describe significant or even dramatic positive hemodynamic effects of iNO, and the effects seem to be present shortly after beginning of treatment47,93–95,99,107–109 and at low doses.97–99,108 Whether these temporal improvements represent the direct effect of iNO remain uncertain.
iNO might also have negative effects. Case report data suggest that iNO-induced pulmonary vasodilation might worsen ventilation-perfusion mismatch and decrease oxygenation. Bhorade et al.92 only saw two of four PE patients with RV failure to respond to iNO. Tulleken et al.110 reported a case with a patient in cardiogenic shock due to PE and even low-dose iNO rapidly worsened PaO2 and saturation. The side effects disappeared when iNO was withdrawn. This patient may have had undiagnosed CTEPH, but the risk must be taken into consideration. Conversely, three of four patients reported by Capellier et al.94 presented with acute PE and a history of PE, and iNO showed positive effects in all cases.
Clinicians must remember that iNO does carry a risk of toxicity and adverse effects. NO can result in formation of methemoglobin or the oxidants NO2 or ONOO− causing oxidant damage to lung tissue.57 However, in 38 patients receiving 50 ppm iNO by nasal cannula for 24 h, methemoglobin never increased111 showing that side effects are avoidable by use of low dose iNO and close clinical surveillance. Withdrawal of iNO can result in rebound pulmonary hypertension which is why cessation must be preceded by gradual down titration of the dose.57
The experimental studies and case reports led to a more systematic testing of a protocol to administer iNO in eight patients with acute PE. The protocol was deemed safe and there was a trend toward improvement in Borg dyspnea score.112 This single arm, phase I study was followed by the phase II, iNOPE trial:113 a double-blinded, randomized, multicenter trial where 76 patients with intermediate-high risk PE were randomized 1:1 to iNO (up to 50 ppm, delivered by nasal cannula) plus oxygen or nitrogen placebo at 50 ppm.111 The composite endpoint was complete normalization of troponins and RV function on echocardiogram after 24 h. The study was neutral on its primary and secondary endpoints but did show positive effects on RV hypokinesis and dilatation.111
For summary, please see Table 1.
Soluble guanylate cyclase
The dimeric enzyme, soluble guanylate cyclase (sGC) is activated by NO and catalyzes the second messenger cGMP from GTP.114 From a pharmacological perspective, the sCG enzyme has two oxidative states that determine its ability to produce cGMP. The constitutive, non-oxidized sGC enzyme contains a prosthetic heme moiety that binds NO and allows cGMP production, and can also increase cGMP production in the presence of specifically designed organic molecules known as “sGC stimulators”. For example, the sGC stimulator Riociguat is approved for the treatment of CTEPH but not acute PE. However, when exposed to oxidant stress, the sGC enzyme discharges the NO-binding the heme moiety and therefore cannot bind NO, but can be activated by an exosite with specifically designed organic molecules known as “sGC activators”.115 One commercially available activator is cinaciguate, which has been tested in humans with heart failure, but not in humans with PE. An important hypothetical consideration is that acute PE appears to produce an oxidative state in circulating platelets116 suggesting the possibility of pulmonary arterial endothelial sGC oxidation, which may impair effectiveness of iNO. PE-induced platelet hyperactivity (evident by increased cytosolic concentration of Ca2+) was not affected by iNO, but the Ca2+ concentration was suppressed by activation, but not stimulation, of sGC (unpublished data).
Regardless of its effect on sGC in the pulmonary vascular endothelial cells, stimulation of sGC also inactivates platelets and prolongs bleeding time117 which might be salutary in patients with PE.
A few animal studies have investigated the hemodynamic effects of sGC in PE, mostly in models of non-autologous PE material. The sGC stimulator BAY 41-8543 abolished the PE-induced hemodynamic changes, lowered blood lactate and PVR and increased CO.118,119 The stimulator BAY 41-2272 lowered PAP and PVR84,120 and even showed a dose–response relationship. However, the highest dose decreased MAP and SVR, too.120 In a porcine model of autologous PE, Riociguat lowered PVR in a dose-dependent manner and increased CO at high doses.75 See Table 1 for summary.
Our review did not find any clinical reports on the use of sGC-stimulation in PE in humans.
Phosphodiesterase-5 inhibitors
Cyclic guanosine monophosphate (cGMP) is the active second messenger in relaxation of SMC and pulmonary vasodilation. cGMP is inactivated by phosphodiesterase-5 (PDE-5).121 Inhibiting PDE-5 (e.g. with sildenafil) will increase the level of cGMP and cause pulmonary vasodilation. Inhibition of PDE-5 prevents hemodynamic changes in fat-embolism,122 suggesting the pathway also to be part of PE-pathophysiology. Non-specific PDE inhibition does reduce PAP and PVR without effects on MAP in PE.123
In models of autologous PE, sildenafil improved hemodynamics in PE, though with a risk of decreased SVR.75,124,125 Sildenafil seems to prevent oxidative stress, nitric oxide consumption and pulmonary arterial endothelial apoptosis; effects that were enhanced by antioxidative N-acetylcysteine.125
In a number of animal studies with microspheres, sildenafil has shown to lower mean PAP (mPAP) and PVR index.84,86,126–131 There was, in general, no additional effect on hemodynamics when another pulmonary vasodilator was added to the sildenafil treatment which suggests the vasodilation from sildenafil to be sufficient. When added effect was noted, the combination of more vasodilators came with a risk of systemic vasodilation.86
The risk of increasing ventilation-perfusion mismatch in PE by vasodilation may also apply to sildenafil. In healthy pigs, sildenafil increases intrapulmonary shunt flow and lowers PaO2,132 and in pigs with single lung ventilation-induced pulmonary hypertension, sildenafil dose-dependently enhanced desaturation.133 This effect may be species dependent, as sildenafil actually improves oxygenation in patients with pulmonary arterial hypertension.134
Sildenafil has been used in PE in a few published case reports. Sildenafil showed promising effects in both acute post-operative PE and acute-in-chronic PE.98,135–137 In a patient with congenital heart disease and acute-in-chronic PE, sildenafil dramatically improved oxygenation.109 The use of sildenafil made withdrawal of inotropes possible shortly after treatment,135,138 whereas another case report measures the effects of sildenafil on the following day.139 This was, however, a severely ill patient with five-day history of saddle PE and in cardiogenic shock. The treatment may also be efficient in children.140
Findings are summarized in Table 1.
Prostanoid pathway
Prostaglandins are products from arachidonic acid, catalyzed by cyclooxygenase (COX). They are mostly produced in endothelial cells and act at different receptors on the SMC with different downstream effects. Some receptors activate adenylate cyclase which dephosphorylates adenosine tri-phosphate to cyclic adenosine monophosphate that lowers cytosolic Ca2+ levels and causes pulmonary vasodilation. Other receptors inhibit the adenylate cyclase or activate phospholipase C, both to increase calcium levels and cause vasoconstriction30,36 which is why the net effect of increased prostanoid release in PE is complex.
Administration of the drugs can dilate pulmonary vasculature with both normal and constricted tone.59,141 Different prostanoids are secreted in acute PE.26,37,142,143 Inhibition of the COX enzyme seems to improve hemodynamics,37,41,144–146 suggesting prostaglandin synthesis to be central in the pathology of PE. PE may even cause release of negative inotropic agents, which is synthesized through the COX-pathway147 and might represent prostanoids. In one small randomized trial, administration of diclofenac was associated with a trend toward improved right ventricular function on echocardiography in humans with PE.148
Besides hemodynamic effects, prostacyclin is one of the most potent endogenous inhibitors of platelet aggregation25,149,150 and may even enhance the effects of thrombolysis.151,152 Other prostanoids also prevent platelet aggregation,38 but platelets in acute thromboembolism may respond differently to prostanoids than normally.150 For example, on thromboelastography, platelets from patients with PE had a decreased response to adenosine diphosphate stimulation compared with platelets from healthy patients.116
Preclinical studies have shown divergent hemodynamic effects with both prostacyclin (PGI2) and prostaglandin E1 (PGE1) with either no relevant response in dogs in both synthetic and autologous emboli material66,79,88,153 or a reduction in PVR or PAP.146,151,154–156
More consistently, in porcine models of non-autologous PE, both PGE1- and PGI2-administration reduce PVR and mPAP and even better than NO-donors, hydralazine and calcium channel blockers.80,157 In mice, both a PGI and PGE1 even reduced PE-related mortality,25 maybe through protective effects on the pulmonary vasculature in PE.143,156,158 The effects of PGI2 seem to have rapid onset, but duration of effects after cessation is more uncertain146,151 (see Table 2).
Table 2.
Prostaglandin-induced pulmonary vasodilation in acute pulmonary embolism.
| Treatment | Experimental microsphere/glass beads/air PE | Experimental autologous (blood, fat, muscle, collagen) PE | Case reports | Clinical trials | Guideline recommendation |
|---|---|---|---|---|---|
| Prostaglandins | Positive effects on PAP, PVR and lung function.80,143,156–158 No effects compared to NO.66 Risk of side effects153 | Lowers mPAP and PVR.25,146,151,154,155 Risk of side effects or no pulmonary effects.79,88,152,153 | Eight PE cases160–162 One case with CO2-embolism159 One newborn with no effect105 | 14 Patients randomized to intravenous epoprostenol or placebo, no effect163 | No recommendations4,16 |
Note: Summary of review of the different prostaglandins causing pulmonary vasodilation in acute pulmonary embolism. Divided by animal or clinical data and guideline recommendation. Please see text for further details.
mPAP: mean pulmonary arterial pressure; NO: nitric oxide; PAP: pulmonary artery pressure; PE: pulmonary embolism; PVR: pulmonary vascular resistance.
Risk of side effects must of course be kept in mind and should not exceed the benefits of treatment. Alpert et al. showed reduction in PAP in macro-embolism but not in micro-embolism when treated with PGE1, but in both situations noted a significant decrease in SVR and MAP.153 Similar reductions in MAP is noted both by PGE1 and PGI2.79,146,151,152
Clinical case reports on the use of prostaglandins have been published. One case with CO2 gas emboli showed normalization of PAP only minutes after administration of inhaled epoprostenol.159 A total of seven cases with sub-massive PE showed positive effects of inhaled prostacyclin with a follow-up over weeks,160,161 whereas Webb et al.162 reported positive but transient effects on PAP without effects on MAP. This was in a PE patient that presented in shock and hence in a more critical condition. In one case of a newborn PE patient, epoprostenol showed no effect.105
One clinical randomized, single-blinded trial has investigated the effects of prostaglandin treatment in acute PE. Kooter et al.163 randomized 14 PE patients to receive intravenous epoprostenol or placebo on top of standard treatment. Endpoints were echocardiographic and biochemical parameters. They did not find any significant effects of epoprostenol compared to placebo.163 The patients included by Kooter et al. had preserved tricuspid annular plane systolic excursion (TAPSE) and low right ventricular to left ventricular ratio at baseline and were perhaps not affected severely enough by their acute PE for epoprostenol to show an effect. The chosen prostaglandin or the route of administration may be another explanation to the lack of positive results as inhaled and intravenous prostaglandins have shown convincing effect in both CTEPH and primary pulmonary hypertension.164–166
Endothelin pathway
ET (mostly ET-1) are produced in the lungs, especially the endothelium. Their synthesis is upregulated by shear stress, stretch, thrombin, hypoxia and pH but inhibited by NO and prostacyclin. ET exert paracrine effect on the SMC, bind to G-protein coupled ETA and ETB receptors which increases intracellular inositol triphosphate and calcium167 acting as potent vasoconstrictors and bronchoconstrictors.35,168 The effects of ET on the pulmonary vasculature might be complex, as it depends on the concentration of ET, the site of the receptor, the ongoing pathology and the tone of the pulmonary vasculature.85,168 ET also affect the release of NO and prostacyclin and play a role in the regulation of hypoxic pulmonary vasoconstriction35,167 which adds to the complexity. Whether or not ET concentrations are elevated in acute PE remains controversial.34,35
Besides hemodynamic effects, ET stimulates platelet aggregation, cell adhesion and thrombosis,167 and plasma levels of ET are increased in both humans and in animal models during acute PE.169,170
Antagonizing the ET receptors in acute PE has been investigated in a few animal studies (see Table 3). In dogs with autologous PE, ET-A antagonism lowered PAP and PVR and increased CO, also in combination with iNO.72,171,172 Han et al.171 even showed additive effects of combined ET-A antagonism and urokinase treatment. In air-embolism models, both non-selective ET-antagonism and ET-A antagonism lowered PAP and PVR,173,174 suggesting that the ET-A receptor to be responsible for most of ETs vasoconstrictive properties.85 In piglets, ET antagonism decreased PAP but lowered MAP and showed no effect on oxygenation or ventilation-perfusion mismatch.33,175 Conversely, in rodents with air-embolism, ET antagonism improved oxygenation and lowered RV systolic pressure.176,177
Table 3.
Antagonism of endothelin in acute pulmonary embolism.
| Treatment | Experimental microsphere/glass beads/air PE | Experimental autologous (blood, fat, muscle, collagen) PE | Case reports | Clinical trials | Guideline recommendation |
|---|---|---|---|---|---|
| Endothelin antagonism | Lowers mPAP and PVR85,173,174,176,177 | Lowers mPAP and PVR, increases CO33,72,171,172,175 | None | None | No recommendations4,16 |
Note: Summary of review of the endothelin antagonists to cause pulmonary vasodilation in acute pulmonary embolism. Divided by animal or clinical data and guideline recommendation. Please see text for further details.
CO: cardiac output; mPAP: mean pulmonary arterial pressure; PE: pulmonary embolism; PVR: pulmonary vascular resistance.
Clinically, ET antagonism is widely used in CTEPH patients,178 but our review did not find any reports on the clinical use of ET antagonism in acute PE.
Hydralazine
Hydralazine dilates blood vessels, lowers blood pressure and is used in hypertension and congestive heart failure. Hydralazine opens Ca2+-dependent potassium channels,179 causing hyperpolarization and closure of voltage-dependent Ca2+ channels which lowers cytosolic Ca2+ levels and causes relaxation. Mechanisms may also involve the inositol triphosphate pathway and the prostacyclin pathway.180,181 It appears that hydralazine can lower PVR in both normal and pathological conditions in animals and humans.182,183
Hydralazine has been tested in experimental PE; please see Table 4 for a summary. In dogs with autologous PE, hydralazine lowered PVR and PAP and increased cardiac output,79,82,88,184 but a reduction in MAP was also noticed.79 One study did not see a reduction in PAP but positive effect on the output pressure.185 In a porcine model of glass bead-induced PE, hydralazine lowered MAP but was unable to lower mPAP and had the smallest reduction in PVR compared to PGE-1 and NO-donors. Hydralazine was, however, the only drug to increase cardiac output.80
Besides the hemodynamic effects, hydralazine enhanced the effect of thrombolysis.184
There are only few examples of the use of hydralazine in acute PE in humans. Bates et al. reported a case on a post-operative PE patient in shock. Hydralazine lowered PVR and PAP significantly over 24 h and increased cardiac index. After withdrawal of hydralazine, the hemodynamics deteriorated, and the treatment was repeated successfully.186
McGoon et al.187 reported 26 patients with pulmonary hypertension, of which 6 had PE as the underlying cause. In the PE-subgroup, hydralazine did not affect PVR nor PAP but increased pulmonary blood flow and arteriovenous oxygen difference and lowered SVR. The time frame of treatment was not reported relative to symptom onset (see Table 4).
We did not find any prospective, clinical study on the use of hydralazine in acute PE.
Limitations
This review contains some limitations to consider. Firstly, the broad diversity of animal models of PE makes it difficult to compare results directly between them. Species, emboli material and measurements and outcomes differ significantly among the included studies. Interpretation and translation must be done with caution. Secondly, the vast majority of the clinical publications are case reports with possible publication biases and accordingly, the level of evidence is low.
Summary
Several mechanisms of PE-induced pulmonary vasoconstriction are well described and represent potential therapeutic targets for pulmonary vasodilation in PE. Many of those were tested in animal models, which differ substantially in the choice of species and embolic material. Only a small number of case reports and clinical trials exist despite the treatment options have been available for decades.
Further research in pulmonary vasodilation as an adjunct to anticoagulation in acute PE is warranted but needs to be in pathophysiological relevant models and prospective clinical trials.
Supplemental Material
Supplemental material, PUL899775 Supplemental Material1 for Pulmonary vasodilation in acute pulmonary embolism – a systematic review by Mads Dam Lyhne, Jeffrey Allen Kline, Jens Erik Nielsen-Kudsk and Asger Andersen in Pulmonary Circulation
Supplemental material, PUL899775 Supplemental Material2 for Pulmonary vasodilation in acute pulmonary embolism – a systematic review by Mads Dam Lyhne, Jeffrey Allen Kline, Jens Erik Nielsen-Kudsk and Asger Andersen in Pulmonary Circulation
Appendix 1. Search strategy
We searched MEDLINE via PubMed and Embase for relevant articles with latest update September 13th 2019. We used the following search strategies for PubMed: (acute pulmonary embolism OR “Pulmonary Embolism”[Mesh]) AND (“Vasodilation”[Mesh] OR “Vasodilator Agents”[Mesh] OR “Hydralazine”[Mesh] OR “Phosphodiesterase Inhibitors”[Mesh] OR “Sildenafil Citrate”[Mesh] OR “Soluble Guanylyl Cyclase”[Mesh] OR “Guanylate Cyclase”[Mesh] OR “riociguat” [Supplementary Concept] OR “Endothelin Receptor Antagonists”[Mesh] OR “Nitric Oxide”[Mesh] OR “Nitroprusside”[Mesh] OR “Nitroglycerin”[Mesh] OR “Prostaglandins”[Mesh] OR “Epoprostenol”[Mesh] OR hydralazine OR endothelin receptor antagonist OR bosentan OR tezosentan OR macitentan OR prostacyclin OR epoprostenol OR prostaglandins OR sildenafil OR tadalafil OR phosphodiesterase-5 inhibitor OR riociguat OR soluble guanylyl cyclase OR inhaled nitric oxide) and for Embase: (‘hydralazine’/exp OR ‘phosphodiesterase inhibitor’/exp OR ‘sildenafil’/exp OR ‘tadalafil’/exp OR ‘riociguat’/exp OR ‘guanylate cyclase activator’/exp OR ‘guanylate cyclase’/exp OR ‘endothelin receptor antagonist’/exp OR ‘bosentan’/exp OR ‘tezosentan’/exp OR ‘macitentan’/exp OR ‘nitric oxide’/exp OR ‘inhaled nitric oxide’/exp OR ‘nitroprusside sodium’/exp OR ‘glyceryl trinitrate’/exp OR ‘prostaglandin’/exp OR ‘prostacyclin derivate’/exp OR ‘iloprost’/exp OR ‘prostaglandin h2’/exp OR ‘prostaglandin e2’/exp OR ‘prostaglandin e1’/exp OR ‘prostaglandin derivate’/exp) AND (‘acute pulmonary embolism’/exp OR ‘lung embolism’/exp) AND (‘article’/it OR ‘article in press’/it).
Titles and abstracts were screened for relevance by MDL. If eligible, the article was read and deemed for inclusion or not. References were reviewed for further hits.
Articles were included if they described a medical intervention causing pulmonary vasodilation in acute PE including air embolism using a clinically relevant drug. No specific needs for comparison were required (either control group or repeated measurements). Any hemodynamic outcome was accepted. Both human and animal studies were included no matter the year of publication. Only English papers were included.
Exclusion criteria included studies on chronic thromboembolic pulmonary hypertension (CTEPH) and the other causes of pulmonary hypertension (PH) within the World Health Organization classification of PH; studies that investigated causes in PE-induced pulmonary vasoconstriction but did not intervene; and animal models that did not have an actual embolism (toxins or pharmacologically induced acute PH, e.g. by a thromboxane analog), and studies on isolated perfused lungs. We did not include studies on inodilators in this review. Case reports without sufficient description of hemodynamic effects of the vasodilatory agent were excluded. We excluded abstracts, conference papers, comments, editorials, and reviews.
Due to the broad variety of PE-models and outcome measures, no specific synthesis of outcome or meta-analysis was possible. We sum up hemodynamic findings in Tables 1 to 4.
Authors' contribution
All authors participated in design and aim. MDL did the screening of papers and drafted the article. AA is the guarantor. All authors contributed substantially to and approved the final version of the article.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
MDL receives PhD scholarship from Aarhus University. The study was funded by the Novo Nordisk Foundation (NFF17CO0024868).
ORCID iD
Mads Dam Lyhne https://orcid.org/0000-0001-5279-260X
Supplemental material
Supplemental material for this article is available online.
References
- 1.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol 2007; 44: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen A, Agnelli G, Anderson F, et al. Venous thromboembolism (VTE) in Europe. Thromb Haemost 2007; 98: 756–764. [DOI] [PubMed] [Google Scholar]
- 3.Greyson C, Xu Y, Lu L, et al. Right ventricular pressure and dilation during pressure overload determine dysfunction after pressure overload. Am J Physiol Heart Circ Physiol 2000; 278: H1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2019; 39: 4208. [DOI] [PubMed] [Google Scholar]
- 5.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008; 28: 387–391. [DOI] [PubMed] [Google Scholar]
- 6.Malik AB. Pulmonary microembolism. Physiol Rev 1983; 63: 1114–1207. [DOI] [PubMed] [Google Scholar]
- 7.Kabrhel C, Okechukwu I, Hariharan P, et al. Factors associated with clinical deterioration shortly after PE. Thorax 2014; 69: 835–842. [DOI] [PubMed] [Google Scholar]
- 8.Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. CHEST 2004; 125: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, et al. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997; 134: 479–487. [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353: 1386–1389. [DOI] [PubMed] [Google Scholar]
- 11.Miller RL, Das S, Anandarangam T, et al. Association between right ventricular function and perfusion abnormalities in hemodynamically stable patients with acute pulmonary embolism. CHEST 1998; 113: 665–670. [DOI] [PubMed] [Google Scholar]
- 12.McIntyre KM, Sasahara AA. Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest 1974; 65: 534–543. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam RM, Mandrekar J, Chang C, et al. Pulmonary embolism outcome: a prospective evaluation of CT pulmonary angiographic clot burden score and ECG score. AJR Am J Roentgenol 2008; 190: 1599–1604. [DOI] [PubMed] [Google Scholar]
- 14.Vedovati MC, Germini F, Agnelli G, et al. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost 2013; 11: 2092–2102. [DOI] [PubMed] [Google Scholar]
- 15.Meinel FG, Nance JW, Schoepf UJ, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med 2015; 128: 747–59. [DOI] [PubMed] [Google Scholar]
- 16.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol 2016; 67: 162–170. [DOI] [PubMed] [Google Scholar]
- 18.Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 2000; 48: 23–33. [DOI] [PubMed] [Google Scholar]
- 19.Stratmann G, Gregory AGA. Neurogenic and humoral vasoconstriction in acute pulmonary thromboembolism. Anesthesia & Analgesia 2003; 97: 341–354. . [DOI] [PubMed] [Google Scholar]
- 20.Halmagyi DF, Starzecki B, Horner GJ. Humoral transmission of cardiorespiratory changes in experimental lung embolism. Circulation Research 1964; 14: 546–554. [DOI] [PubMed] [Google Scholar]
- 21.Gurewich V, Cohen ML, Thomas DP. Humoral factors in massive pulmonary embolism: an experimental study. Am Heart J 1968; 76: 784–794. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DP, Gurewich V, Ashford TP. Platelet adherence to thromboemboli in relation to the pathogenesis and treatment of pulmonary embolism. N Engl J Med 1966; 274: 953–956. [DOI] [PubMed] [Google Scholar]
- 23.Huval WV, Mathieson MA, Stemp LI, et al. Therapeutic benefits of 5-hydroxytryptamine inhibition following pulmonary embolism. Ann Surg 1983; 197: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith G, Smith AN. The role of serotonin in experimental pulmonary embolism. Surg Gynecol Obstet 1955; 101: 691–700. [PubMed] [Google Scholar]
- 25.Ueno Y, Kawashima A, Koike H, et al. Effect of beraprost sodium, a stable prostacyclin analogue, on pulmonary thromboembolism in mice. Thromb Res 1995; 77: 193–198. [DOI] [PubMed] [Google Scholar]
- 26.Reeves WC, Demers LM, Wood MA, et al. The release of thromboxane A2 and prostacyclin following experimental acute pulmonary embolism. Prostaglandins Leuk Med 1983; 11: 1–10. [DOI] [PubMed] [Google Scholar]
- 27.Kerbaul F, By Y, Gariboldi V, et al. Acute pulmonary embolism decreases adenosine plasma levels in anesthetized pigs. ISRN Cardiology 2011; 2011: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oates JA, FitzGerald GA, Branch RA, et al. Clinical implications of prostaglandin and thromboxane A2 formation. N Engl J Med 1988; 319: 761–767. [DOI] [PubMed] [Google Scholar]
- 29.Nakos G, Kitsiouli EI, Lekka ME. Bronchoalveolar lavage alterations in pulmonary embolism. Am J Respir Crit Care Med 1998; 158: 1504–1510. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JO, Moser KM. The current status of prostaglandins and the lungs. CHEST 1975; 68: 75–80. [DOI] [PubMed] [Google Scholar]
- 31.MacLean MR. Endothelin-1 and serotonin: mediators of primary and secondary pulmonary hypertension?. J Lab Clin Med 1999; 134: 105–114. [DOI] [PubMed] [Google Scholar]
- 32.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J 1995; 9: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 33.Tsang JYC, Lamm WJE. Estimation of endothelin-mediated vasoconstriction in acute pulmonary thromboembolism. Pulm Circ 2012; 2: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostrubiec M, Pedowska-Włoszek J, Ciurzyński M, et al. Endothelin is not elevated in acute pulmonary embolism. Thromb Res 2009; 124: 157–160. [DOI] [PubMed] [Google Scholar]
- 35.Battistini B. Modulation and roles of the endothelins in the pathophysiology of pulmonary embolism. Can J Physiol Pharmacol 2003; 81: 555–569. [DOI] [PubMed] [Google Scholar]
- 36.Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015; 24: 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todd MH, Cragg DB, Forrest JB, et al. The involvement of prostaglandins and thromboxanes in the response to pulmonary embolism in anaesthetized rabbits and isolated perfused lungs. Thromb Res 1983; 30: 81–90. [DOI] [PubMed] [Google Scholar]
- 38.Das UN. Possible role of prostaglandins in the pathogenesis of pulmonary hypertension. Prostaglandins Med 1980; 4: 163–170. [DOI] [PubMed] [Google Scholar]
- 39.Friedrich T, Lichey J, Nigam S, et al. Prostaglandin production in patients with pulmonary embolism. Biomed Biochim Acta 1984; 43: S409–12. [PubMed] [Google Scholar]
- 40.Kapsch DN, Metzler M, Silver D. Contributions of prostaglandin F2α and thromboxane A2 to the acute cardiopulmonary changes of pulmonary embolism. J Surg Res 1981; 30: 522–529. [DOI] [PubMed] [Google Scholar]
- 41.Tucker A, Weir EK, Reeves JT, et al. Pulmonary microembolism: attenuated pulmonary vasoconstriction with prostaglandin inhibitors and antihistamines. Prostaglandins 1976; 11: 31–41. [DOI] [PubMed] [Google Scholar]
- 42.Harrington MP, Silver D. Effects of serotonin, histamine, and prostaglandins on the cardiopulmonary changes of pulmonary embolism. Surg Forum 1979; 30: 183–186. [PubMed] [Google Scholar]
- 43.Watts JA, Gellar MA, Fulkerson M-BK, et al. Arginase depletes plasma l-arginine and decreases pulmonary vascular reserve during experimental pulmonary embolism. Pulmonary Pharmacol Ther 2012; 25: 48–54. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood 2009; 114: 764–771. [DOI] [PubMed] [Google Scholar]
- 45.Kline JA, Steuerwald NM, Watts JA, et al. Leukocyte expression of heme oxygenase-1 [hmox1] varies inversely with severity of tricuspid regurgitation in acute pulmonary embolism. Thromb Res 2015; 136: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Insenser M, Montes-Nieto R, Martínez-García MÁ, et al. Identification of reduced circulating haptoglobin concentration as a biomarker of the severity of pulmonary embolism: a nontargeted proteomic study. PLoS ONE 2014; 9: e100902–e1009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodson KA, Lee Y, Gopalratnam K, et al. Adding insult to injury: autoimmune haemolytic anaemia complicated by pulmonary embolism. BMJ Case Rep 2016, pp. 2016: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost 2013; 11: 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein M, Levy SE. Reflex and humoral responses to pulmonary embolism. Progr Cardiovasc Dis 1974; 17: 167–174. [DOI] [PubMed] [Google Scholar]
- 50.Kawamura Y, Hasebe N, Matsuhashi H. Experimental studies on the mechanism of reversible pressor response in pulmonary microembolism in the dog. Jpn Circ J 1991; 55: 271–280. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Yu D, Yu Y, et al. Potential role of sympathetic activity on the pathogenesis of massive pulmonary embolism with circulatory shock in rabbits. Respir Res 2019; 20: 97–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clay TP, Hughes JM. The role of prostaglandins in the bronchoconstriction induced by pulmonary micro-embolism in the guinea-pig. J Physiol (Lond) 1980; 308: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burrowes KS, Clark AR, Wilsher ML, et al. Hypoxic pulmonary vasoconstriction as a contributor to response in acute pulmonary embolism. Ann Biomed Eng 2014; 42: 1631–1643. [DOI] [PubMed] [Google Scholar]
- 54.Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax 1999; 54: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson PS, Thompson WJ, Moore TM, et al. Vasoconstriction increases pulmonary nitric oxide synthesis and circulating cyclic GMP. J Surg Res 1997; 70: 75–83. [DOI] [PubMed] [Google Scholar]
- 56.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004; 109: III27–32. [DOI] [PubMed] [Google Scholar]
- 57.Wang T, Kebir El D, Blaise G. Inhaled nitric oxide in 2003: a review of its mechanisms of action. Can J Anaesth 2003; 50: 839–846. [DOI] [PubMed] [Google Scholar]
- 58.Walsh MP. Calmodulin and the regulation of smooth muscle contraction. Mol Cell Biochem 1994; 135: 21–41. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda S, Shirai M, Shimouchi A, et al. Pulmonary microvascular responses to inhaled prostacyclin, nitric oxide, and their combination in anesthetized cats. Jpn J Physiol 1999; 49: 89–98. [DOI] [PubMed] [Google Scholar]
- 60.Dias-Junior CAC, Sertorio JTC, Tanus-Santos JE. Aminoguanidine produces beneficial haemodynamic effects in a canine model of acute pulmonary thromboembolism. Acta Physiol (Oxf) 2007; 191: 189–196. [DOI] [PubMed] [Google Scholar]
- 61.Miao R, Leng D, Liu M, et al. Alteration of endothelial nitric oxide synthase expression in acute pulmonary embolism: a study from bench to bioinformatics. Eur Rev Med Pharmacol Sci 2017; 21: 827–836. [PubMed] [Google Scholar]
- 62.Nilsson KF, Gustafsson LE, Adding LC, et al. Increase in exhaled nitric oxide and protective role of the nitric oxide system in experimental pulmonary embolism. Br J Pharmacol 2007; 150: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sertório JT, Neto-Neves EM, Dias-Junior CA, et al. Elevated plasma hemoglobin levels increase nitric oxide consumption in experimental and clinical acute pulmonary thromboembolism. Crit Care Med 2013; 41: e118–e124. [DOI] [PubMed] [Google Scholar]
- 64.Agvald P, Adding LC, Nilsson KF, et al. Increased expired NO and roles of CO2 and endogenous NO after venous gas embolism in rabbits. Eur J Appl Physiol 2006; 97: 210–215. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Pan K, Chen L, et al. The effect of nitric oxide inhalation on heart and pulmonary circulation in rabbits with acute massive pulmonary embolism. Exp Ther Med 2018; 16: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zwissler B, Welte M, Habler O, et al. Effects of inhaled prostacyclin as compared with inhaled nitric oxide in a canine model of pulmonary microembolism and oleic acid edema. J Cardiothorac Vasc Anesth 1995; 9: 634–640. [DOI] [PubMed] [Google Scholar]
- 67.Nong Z, Hoylaerts M, Van Pelt N, et al. Nitric oxide inhalation inhibits platelet aggregation and platelet-mediated pulmonary thrombosis in rats. Circ Res 1997; 81: 865–869. [DOI] [PubMed] [Google Scholar]
- 68.Gries A, Böttiger BW, Dörsam J, et al. Inhaled nitric oxide inhibits platelet aggregation after pulmonary embolism in pigs. Anesthesiology 1997; 86: 387–393. [DOI] [PubMed] [Google Scholar]
- 69.Böttiger BW, Motsch J, Dörsam J, et al. Inhaled nitric oxide selectively decreases pulmonary artery pressure and pulmonary vascular resistance following acute massive pulmonary microembolism in piglets. CHEST 1996; 110: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 70.Tanus-Santos JE, Moreno H, Moreno RA, et al. Inhaled nitric oxide improves hemodynamics during a venous air infusion (VAI) in dogs. Intensive Care Med 1999; 25: 983–989. [DOI] [PubMed] [Google Scholar]
- 71.Tanus-Santos JE, Moreno H, Zappellini A, et al. Small-dose inhaled nitric oxide attenuates hemodynamic changes after pulmonary air embolism in dogs. Anesth Analg 1999; 88: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 72.Lee J-H, Kim S, Park B-K, et al. The effect of a combination of inhaled nitric oxide and an endothelin A-receptor antagonist on hemodynamic dysfunction in experimental acute pulmonary thromboembolism. Lung 2005; 183: 139–149. [DOI] [PubMed] [Google Scholar]
- 73.Mélot C, Vermeulen F, Maggiorini M, et al. Site of pulmonary vasodilation by inhaled nitric oxide in microembolic lung injury. Am J Respir Crit Care Med 1997; 156: 75–85. [DOI] [PubMed] [Google Scholar]
- 74.Deng C, Yang M, Lin Q, et al. Beneficial effects of inhaled NO on apoptotic pneumocytes in pulmonary thromboembolism model. Theor Biol Med Model 2014; 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schultz J, Andersen A, Gade IL, et al. Riociguat, sildenafil and inhaled nitric oxide reduces pulmonary vascular resistance and improves right ventricular function in a porcine model of acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care Epub ahead of print 26 April 2019. DOI: 10.1177/2048872619840772. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z, Meng Z, Wang Y. Correlations of inhaled NO with the cTnI levels and the plasma clotting factor in rabbits with acute massive pulmonary embolism. Acta Cir Bras 2018; 33: 664–672. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Li Z, Chen L, et al. The effects of inhaled NO on plasma vasoactive factor and CTnI level in rabbits with acute massive pulmonary embolism1. Acta Cir Bras 2018; 33: 577–587. [DOI] [PubMed] [Google Scholar]
- 78.Byrick RJ, Mullen JB, Murphy PM, et al. Inhaled nitric oxide does not alter pulmonary or cardiac effects of fat embolism in dogs after cemented arthroplasty. Can J Anaesth 1999; 46: 605–612. [DOI] [PubMed] [Google Scholar]
- 79.Delcroix M, Mélot C, Lejeune P, et al. Effects of vasodilators on gas exchange in acute canine embolic pulmonary hypertension. Anesthesiology 1990; 72: 77–84. [DOI] [PubMed] [Google Scholar]
- 80.McLean RF, Prielipp RC, Rosenthal MH, et al. Vasodilator therapy in microembolic porcine pulmonary hypertension. Anesth Analg 1990; 71: 35–41. [DOI] [PubMed] [Google Scholar]
- 81.Yu D, Wang Y, Yu Y, et al. Acute beneficial effects of sodium nitroprusside in a rabbit model of massive pulmonary embolism associated with circulatory shock. Am J Pathol 2018; 188: 1768–1778. [DOI] [PubMed] [Google Scholar]
- 82.Lee KY, Molloy DW, Slykerman L, et al. Effects of hydralazine and nitroprusside on cardiopulmonary function when a decrease in cardiac output complicates a short-term increase in pulmonary vascular resistance. Circulation 1983; 68: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 83.Dias-Junior CAC, Gladwin MT, Tanus-Santos JE. Low-dose intravenous nitrite improves hemodynamics in a canine model of acute pulmonary thromboembolism. Free Rad Biol Med 2006; 41: 1764–1770. [DOI] [PubMed] [Google Scholar]
- 84.Dias-Junior CA, Cau SBA, Oliveira AM, et al. Nitrite or sildenafil, but not BAY 41-2272, blunt acute pulmonary embolism-induced increases in circulating matrix metalloproteinase-9 and oxidative stress. Thromb Res 2009; 124: 349–355. [DOI] [PubMed] [Google Scholar]
- 85.Fineman JR, Wong J, Mikhailov T, et al. Altered endothelial function in lambs with pulmonary hypertension and acute lung injury. Pediatr Pulmonol 1999; 27: 147–156. [DOI] [PubMed] [Google Scholar]
- 86.Dias-Junior CA, Montenegro MF, Florencio BC, et al. Sildenafil improves the beneficial haemodynamic effects of intravenous nitrite infusion during acute pulmonary embolism. Basic Clin Pharmacol Toxicol 2008; 103: 374–379. [DOI] [PubMed] [Google Scholar]
- 87.Noble WH, Kay JC. The effects of dobutamine, nitroprusside, or volume expansion on cardiac output and lung water after CPPV. Can Anaesth Soc J 1986; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 88.Priebe HJ. Efficacy of vasodilator therapy in canine model of acute pulmonary hypertension. Am J Physiol 1988; 255: H1232–H1239. [DOI] [PubMed] [Google Scholar]
- 89.Emerson M, Momi S, Paul W, et al. Endogenous nitric oxide acts as a natural antithrombotic agent in vivo by inhibiting platelet aggregation in the pulmonary vasculature. Thromb Haemost 1999; 81: 961–966. [PubMed] [Google Scholar]
- 90.Gries A, Herr A, Motsch J, et al. Randomized, placebo-controlled, blinded and cross-matched study on the antiplatelet effect of inhaled nitric oxide in healthy volunteers. Thromb Haemost 2000; 83: 309–315. [PubMed] [Google Scholar]
- 91.Dikshit M, Kumari R, Srimal RC. Pulmonary thromboembolism-induced alterations in nitric oxide release from rat circulating neutrophils. J Pharmacol Exp Ther 1993; 265: 1369–1373. [PubMed] [Google Scholar]
- 92.Bhorade S, Christenson J, O'Connor M, et al. Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med 1999; 159: 571–579. [DOI] [PubMed] [Google Scholar]
- 93.Schenk P, Mittermayer C, Ratheiser K. Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med 1999; 33: 710–714. [PubMed] [Google Scholar]
- 94.Capellier G, Jacques T, Balvay P, et al. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med 1997; 23: 1089–1092. [DOI] [PubMed] [Google Scholar]
- 95.Crerar-Gilbert A, Boots R. Use of inhaled nitric oxide in pulmonary embolism. Anaesth Intensive Care 1999; 27: 412–414. [DOI] [PubMed] [Google Scholar]
- 96.Dolmatova EV, Moazzami K, Cocke TP, et al. Extracorporeal membrane oxygenation in massive pulmonary embolism. Heart Lung 2017; 46: 106–109. [DOI] [PubMed] [Google Scholar]
- 97.Toolsie O, Gomceli U, Diaz-Fuentes G. Inhaled nitric oxide as an adjunct to thrombolytic therapy in a patient with submassive pulmonary embolism and severe hypoxemia. Case Rep Crit Care 2019, pp. 5184702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma M, Suero-Abreu GA, Neupane R, et al. Role of phosphodiesterase-5 inhibitors in acute right ventricular failure due to pulmonary embolism. Am J Case Rep 2019; 20: 1144–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faintuch S, Lang EV, Cohen RI, et al. Inhaled nitric oxide as an adjunct to suction thrombectomy for pulmonary embolism. J Vasc Intervent Radiol 2004; 15: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 100.Trummer G, Berchtold-Herz M, Martin J, et al. Successful treatment of pulmonary hypertension with inhaled nitric oxide after pulmonary embolectomy. Ann Thorac Surg 2002; 73: 1299–1301. [DOI] [PubMed] [Google Scholar]
- 101.Schenk P, Pernerstorfer T, Mittermayer C, et al. Inhalation of nitric oxide as a life-saving therapy in a patient after pulmonary embolectomy. Br J Anaesth 1999; 82: 444–447. [DOI] [PubMed] [Google Scholar]
- 102.Spöhr F, Rehmert GC, Böttiger BW, et al. Successful thrombolysis after pulmonary embolectomy for persistent massive postoperative pulmonary embolism. Resuscitation 2004; 62: 113–118. [DOI] [PubMed] [Google Scholar]
- 103.Baird JS, Killinger JS, Kalkbrenner KJ, et al. Massive pulmonary embolism in children. J Pediatr 2010; 156: 148–151. [DOI] [PubMed] [Google Scholar]
- 104.Allred L, Ruiz R, Jones D, et al. Intractable respiratory failure in a term newborn. Am J Perinatol 2008; 25: 101–103. [DOI] [PubMed] [Google Scholar]
- 105.Odackal NJ, McCulloch MA, Hainstock MR, et al. Respiratory failure secondary to congenital pulmonary arterial thrombus with lung dysplasia. BMJ Case Rep.. 2019; 12: e227925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Estagnasie P. Use of inhaled nitric oxide to reverse flow through a patent foramen ovale during pulmonary embolism. Ann Intern Med 1994; 120: 757. [DOI] [PubMed] [Google Scholar]
- 107.Szold O, Khoury W, Biderman P, et al. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: a report of four patients and review of the literature. Lung 2006; 184: 1–5. [DOI] [PubMed] [Google Scholar]
- 108.Summerfield DT, Desai H, Levitov A, et al. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care 2012; 57: 444–448. [DOI] [PubMed] [Google Scholar]
- 109.Lewis GD, Bloch KD, Semigran MJ. Pulmonary thromboembolism superimposed on a congenital ventricular septal defect in a 50-year-old man. Cardiol Rev 2004; 12: 188–190. [DOI] [PubMed] [Google Scholar]
- 110.Tulleken JE, Zijlstra JG, Evers K, et al. Oxygen desaturation after treatment with inhaled nitric oxide for obstructive shock due to massive pulmonary embolism-to the editor. CHEST 1997; 112: 296–297. [DOI] [PubMed] [Google Scholar]
- 111.Kline JA, Puskarich MA, Jones AE, et al. Inhaled nitric oxide to treat intermediate risk pulmonary embolism: a multicenter randomized controlled trial. Nitric Oxide 2019; 84: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kline JA, Hernandez J, Garrett JS, et al. Pilot study of a protocol to administer inhaled nitric oxide to treat severe acute submassive pulmonary embolism. Emerg Med J 2014; 31: 459–462. [DOI] [PubMed] [Google Scholar]
- 113.Kline JA, Hall CL, Jones AE, et al. Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: The iNOPE trial. Am Heart J 2017; 186: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghofrani H-A, Humbert M, Langleben D, et al. Riociguat: mode of action and clinical development in pulmonary hypertension. CHEST 2017; 151: 468–480. [DOI] [PubMed] [Google Scholar]
- 115.Stasch J-P, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rezania S, Puskarich MA, Petrusca DN, et al. Platelet hyperactivation, apoptosis and hypercoagulability in patients with acute pulmonary embolism. Thromb Res 2017; 155: 106–115. [DOI] [PubMed] [Google Scholar]
- 117.Teng CM, Wu CC, Ko FN, et al. YC-1, a nitric oxide-independent activator of soluble guanylate cyclase, inhibits platelet-rich thrombosis in mice. Eur J Pharmacol 1997; 320: 161–166. [DOI] [PubMed] [Google Scholar]
- 118.Watts JA, Gellar MA, Fulkerson M-BK, et al. A soluble guanylate cyclase stimulator, BAY 41-8543, preserves right ventricular function in experimental pulmonary embolism. Pulmon Pharmacol Ther 2013; 26: 205–211. [DOI] [PubMed] [Google Scholar]
- 119.Watts JA, Gellar MA, Fulkerson M-BK, et al. Pulmonary vascular reserve during experimental pulmonary embolism: effects of a soluble guanylate cyclase stimulator, BAY 41-8543*. Crit Care Med 2011; 39: 2700–2704. [DOI] [PubMed] [Google Scholar]
- 120.Cau SBA, Dias-Junior CA, Montenegro MF, et al. Dose-dependent beneficial hemodynamic effects of BAY 41-2272 in a canine model of acute pulmonary thromboembolism. Eur J Pharmacol 2008; 581: 132–137. [DOI] [PubMed] [Google Scholar]
- 121.Matot I, Gozal Y. Pulmonary responses to selective phosphodiesterase-5 and phosphodiesterase-3 inhibitors. CHEST 2004; 125: 644–651. [DOI] [PubMed] [Google Scholar]
- 122.Krebs J, Ferguson SJ, Nuss K, et al. Sildenafil prevents cardiovascular changes after bone marrow fat embolization in sheep. Anesthesiology 2007; 107: 75–81. [DOI] [PubMed] [Google Scholar]
- 123.Tajimi K, Tanaka H, Kasai T, et al. Selective pulmonary vasodilatory effect of ZSY-27 in dogs with pulmonary hypertension due to pulmonary embolism. Crit Care Med 1989; 17: 163–165. [DOI] [PubMed] [Google Scholar]
- 124.Neto-Neves EM, Dias-Junior CA, Uzuelli JA, et al. Sildenafil improves the beneficial hemodynamic effects exerted by atorvastatin during acute pulmonary thromboembolism. Eur J Pharmacol 2011; 670: 554–560. [DOI] [PubMed] [Google Scholar]
- 125.Zhang R, Wang Y, Pan L, et al. N-Acetylcysteine potentiates the haemodynamic-improving effect of sildenafil in a rabbit model of acute pulmonary thromboembolism via the p38 MAPK pathway. Clin Exp Pharmacol Physiol 2019; 46: 163–172. [DOI] [PubMed] [Google Scholar]
- 126.Dias-Junior CA, Vieira TF, Moreno H, et al. Sildenafil selectively inhibits acute pulmonary embolism-induced pulmonary hypertension. Pulmon Pharmacol Ther 2005; 18: 181–186. [DOI] [PubMed] [Google Scholar]
- 127.Souza-Silva AR, Dias-Junior CA, Uzuelli JA, et al. Hemodynamic effects of combined sildenafil and L-arginine during acute pulmonary embolism-induced pulmonary hypertension. Eur J Pharmacol 2005; 524: 126–131. [DOI] [PubMed] [Google Scholar]
- 128.Dias-Junior CA, Tanus-Santos JE. Hemodynamic effects of sildenafil interaction with a nitric oxide donor compound in a dog model of acute pulmonary embolism. Life Sci 2006; 79: 469–474. [DOI] [PubMed] [Google Scholar]
- 129.Dias-Junior CA, Souza-Costa DC, Zerbini T, et al. The effect of sildenafil on pulmonary embolism-induced oxidative stress and pulmonary hypertension. Anesth Analg 2005; 101: 115–120. [DOI] [PubMed] [Google Scholar]
- 130.Dias-Junior CA, Neto-Neves EM, Montenegro MF, et al. Hemodynamic effects of inducible nitric oxide synthase inhibition combined with sildenafil during acute pulmonary embolism. Nitric Oxide 2010; 23: 284–288. [DOI] [PubMed] [Google Scholar]
- 131.Velásquez DRB, Teixeira-Neto FJ, Lagos-Carvajal AP, et al. Effects of different inspired oxygen fractions on sildenafil-induced pulmonary anti-hypertensive effects in a sheep model of acute pulmonary embolism. Life Sci 2015; 127: 26–31. [DOI] [PubMed] [Google Scholar]
- 132.Kleinsasser A, Loeckinger A, Hoermann C, et al. Sildenafil modulates hemodynamics and pulmonary gas exchange. Am J Respir Crit Care Med 2001; 163: 339–343. [DOI] [PubMed] [Google Scholar]
- 133.Becker EM, Stasch J-P, Bechem M, et al. Effects of different pulmonary vasodilators on arterial saturation in a model of pulmonary hypertension. PLoS ONE 2013; 8: e73502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. JAC 2004; 44: 1488–1496. [DOI] [PubMed] [Google Scholar]
- 135.Bonatti HJR, Sawyer RG, Hagspiel KD. Use of sildenafil in a liver transplant recipient with acute pulmonary embolism. Eur Surg 2010; 42: 65–67. [Google Scholar]
- 136.Ganière V, Feihl F, Tagan D. Dramatic beneficial effects of sildenafil in recurrent massive pulmonary embolism. Intensive Care Med 2006; 32: 452–454. [DOI] [PubMed] [Google Scholar]
- 137.Hamilton-Craig CR, McNeil K, Dunning J, et al. Treatment options and strategies for acute severe pulmonary embolism. Intern Med J 2008; 38: 657–667. [DOI] [PubMed] [Google Scholar]
- 138.Galea M, Quiney N. Sildenafil in acute pulmonary embolism: case report and review of literature. J Intens Care Soc 2009; 10: 44–45. [Google Scholar]
- 139.Bonatti HJR, Harris T, Bauer T, et al. Transfemoral catheter thrombolysis and use of sildenafil in acute massive pulmonary embolism. J Cardiothorac Vasc Anesth 2010; 24: 980–984. [DOI] [PubMed] [Google Scholar]
- 140.Naik SS, Sudhir V, Arvinda HR, et al. Embolisation of pulmonary vasculature during endovascular therapy – a case report. Childs Nerv Syst 2015; 31: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 141.Van Obbergh LJ, Charbonneau M, Blaise G. Combination of inhaled nitric oxide with i.v. nitroglycerin or with a prostacyclin analogue in the treatment of experimental pulmonary hypertension. Br J Anaesth 1996; 77: 227–231. [DOI] [PubMed] [Google Scholar]
- 142.Lindsey HE, Wyllie JH. Release of prostaglandins from embolized lungs. Br J Surg 1970; 57: 738–741. [DOI] [PubMed] [Google Scholar]
- 143.Hirose T, Aoki E, Domae M, et al. The effect of prostacyclin infusion on increased pulmonary vascular permeability following microembolization in dogs. Prostaglandins Leukot Med 1983; 11: 51–61. [DOI] [PubMed] [Google Scholar]
- 144.Konstam MA, Hill NS, Bonin JD, et al. Prostaglandin mediation of hemodynamic responses to pulmonary microembolism in rabbits: effects of ibuprofen and meclofenamate. Exp Lung Res 1987; 12: 331–345. [DOI] [PubMed] [Google Scholar]
- 145.Jones AE, Watts JA, Debelak JP, et al. Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. Am J Physiol Lung Cell Mol Physiol 2003; 284: L1072–81. [DOI] [PubMed] [Google Scholar]
- 146.Utsunomiya T, Krausz MM, Levine L, et al. Thromboxane mediation of cardiopulmonary effects of embolism. J Clin Invest 1982; 70: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Utsunomiya T, Krausz MK, Dunham B, et al. Circulating negative inotropic agent(s) following pulmonary embolism. Surgery 1982; 91: 402–408. [PubMed] [Google Scholar]
- 148.Jimenez D, Nieto R, Corres J, et al. Diclofenac for reversal of right ventricular dysfunction in acute normotensive pulmonary embolism: a pilot study. Thromb Res 2018; 162: 1–6. [DOI] [PubMed] [Google Scholar]
- 149.Demling RH. Role of prostaglandins in acute pulmonary microvascular injury. Ann N Y Acad Sci 1982; 384: 517–534. [DOI] [PubMed] [Google Scholar]
- 150.Cooper B. Diminished platelet adenylate cyclase activation by prostaglandin D2 in acute thrombosis. Blood 1979; 54: 684–693. [PubMed] [Google Scholar]
- 151.Utsunomiya T, Krausz MM, Valeri CR, et al. Treatment of pulmonary embolism with prostacyclin. Surgery 1980; 88: 25–30. [PubMed] [Google Scholar]
- 152.Schneider J. Taprostene, a stable prostacyclin analogue, enhances the thrombolytic efficacy of saruplase (recombinant single-chain urokinase-type plasminogen activator) in rabbits with pulmonary embolized thrombi. Prostaglandins 1991; 41: 595–606. [DOI] [PubMed] [Google Scholar]
- 153.Alpert JS, Haynes FW, Dalen JE, et al. Experimental pulmonary embolism; effect on pulmonary blood volume and vascular compliance. Circulation 1974; 49: 152–157. [DOI] [PubMed] [Google Scholar]
- 154.Utsunomiya T, Krausz MM, Shepro D, et al. Prostaglandin control of plasma and platelet 5-hydroxytryptamine in normal and embolized animals. Am J Physiol 1981; 241: H766–71. [DOI] [PubMed] [Google Scholar]
- 155.Utsunomiya T, Krausz MM, Valeri CR, et al. Treatment of pulmonary embolism with positive end-expiratory pressure and prostaglandin E1. Surg Gynecol Obstet 1981; 153: 161–168. [PubMed] [Google Scholar]
- 156.Dervin G, Calvin JE. Role of prostaglandin E1 in reducing pulmonary vascular resistance in an experimental model of acute lung injury. Crit Care Med 1990; 18: 1129–1133. [DOI] [PubMed] [Google Scholar]
- 157.Prielipp RC, McLean R, Rosenthal MH, et al. Hemodynamic profiles of prostaglandin E1, isoproterenol, prostacyclin, and nifedipine in experimental porcine pulmonary hypertension. Crit Care Med 1991; 19: 60–67. [DOI] [PubMed] [Google Scholar]
- 158.Hirose T, Aoki E, Ishibashi M, et al. The effect of prostacyclin on increased hydraulic conductivity of pulmonary exchange vessels following microembolization in dogs. Microvasc Res 1983; 26: 193–204. [DOI] [PubMed] [Google Scholar]
- 159.Martineau A, Arcand GV, Couture P, et al. Transesophageal echocardiographic diagnosis of carbon dioxide embolism during minimally invasive saphenous vein harvesting and treatment with inhaled epoprostenol. Anesth Analg 2003; 96: 962–964. . [DOI] [PubMed] [Google Scholar]
- 160.Idrees MM, Batubara E, Kashour T. Novel approach for the management of sub-massive pulmonary embolism. Ann Thorac Med 2012; 7: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alsaghir AH, Alaithan SA, Alsihati B, et al. Iloprost in pulmonary hypertension due to sub-massive pulmonary embolism: report of two cases. Libyan J Med 2013; 8: 22391–22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Webb SAR, Stott S, van Heerden PV. The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med 1996; 22: 353–355. [DOI] [PubMed] [Google Scholar]
- 163.Kooter AJ, Ijzerman RG, Kamp O, et al. No effect of epoprostenol on right ventricular diameter in patients with acute pulmonary embolism: a randomized controlled trial. BMC Pulm Med 2010; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension*. CHEST 2003; 123: 338–343. [DOI] [PubMed] [Google Scholar]
- 165.Krug S, Hammerschmidt S, Pankau H, et al. Acute improved hemodynamics following inhaled iloprost in chronic thromboembolic pulmonary hypertension. Respiration 2008; 76: 154–159. [DOI] [PubMed] [Google Scholar]
- 166.Hoeper MM, Olschewski H, Ghofrani HA, et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. JACC 2000; 35: 176–182. [DOI] [PubMed]
- 167.Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000; 102: 2434–2440. [DOI] [PubMed] [Google Scholar]
- 168.Sauvageau S, Thorin E, Caron A, et al. Endothelin-1-induced pulmonary vasoreactivity is regulated by ET(A) and ET(B) receptor interactions. J Vasc Res 2007; 44: 375–381. [DOI] [PubMed] [Google Scholar]
- 169.Tsang J, Battistini B, Dussault P, et al. Biphasic release of immunoreactive endothelins following acute pulmonary thromboembolism in pigs. J Cardiovasc Pharmacol 2000; 36: S221–S224. [DOI] [PubMed] [Google Scholar]
- 170.Sofia M, Faraone S, Alifano M, et al. Endothelin abnormalities in patients with pulmonary embolism. CHEST 1997; 111: 544–549. [DOI] [PubMed] [Google Scholar]
- 171.Han L, Li Q-Y, Zhou L, et al. Effects of thrombolytic drugs and a selective endothelin-1 receptor antagonist on acute pulmonary thromboembolism in dogs. Chin Med J 2010; 123: 395–400. [PubMed] [Google Scholar]
- 172.Lee JH, Chun YG, Lee IC, et al. Pathogenic role of endothelin 1 in hemodynamic dysfunction in experimental acute pulmonary thromboembolism. Am J Respir Crit Care Med 2001; 164: 1282–1287. [DOI] [PubMed] [Google Scholar]
- 173.Tanus-Santos JE, Gordo WM, Udelsmann A, et al. Nonselective endothelin-receptor antagonism attenuates hemodynamic changes after massive pulmonary air embolism in dogs. CHEST 2000; 118: 175–179. [DOI] [PubMed] [Google Scholar]
- 174.Tanus-Santos JE, Gordo WM, Udelsmann A, et al. The hemodynamic effects of endothelin receptor antagonism during a venous air infusion in dogs. Anesth Analg 2000; 90: 102–106. [DOI] [PubMed] [Google Scholar]
- 175.Tsang JYC, Lamm WJE, Neradilek B, et al. Endothelin receptor blockade does not improve hypoxemia following acute pulmonary thromboembolism. J Appl Physiol 2007; 102: 762–771. [DOI] [PubMed] [Google Scholar]
- 176.Ayach B, Tsang J, Jeng AY, et al. Effects of a selective endothelin A receptor antagonist, ABT-627, in healthy normotensive anaesthetized rats developing acute pulmonary air embolism. Clin Sci 2002; 103(Suppl 48): 371S–375S. [DOI] [PubMed] [Google Scholar]
- 177.Battistini B, Verreault M, Ayach B, et al. Role of the endothelin system in secondary pulmonary hypertension related to air embolism: lessons learned from testing four classes of endothelin blockers in a rat model. J Cardiovasc Pharmacol 2004; 1(44 Suppl 1): S386–9. [DOI] [PubMed] [Google Scholar]
- 178.Becattini C, Manina G, Busti C, et al. Bosentan for chronic thromboembolic pulmonary hypertension: Findings from a systematic review and meta-analysis. Thromb Res 2010; 126: e51–e56. [DOI] [PubMed] [Google Scholar]
- 179.Bang L, Nielsen-Kudsk JE, Gruhn N, et al. Hydralazine-induced vasodilation involves opening of high conductance Ca2-activated K channels. Eur J Pharmacol 1998; 361: 43–49. [DOI] [PubMed] [Google Scholar]
- 180.Ellershaw DC, Gurney AM. Mechanisms of hydralazine induced vasodilation in rabbit aorta and pulmonary artery. Br J Pharmacol 2001; 134: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maille N, Gokina N, Mandalà M, et al. Mechanism of hydralazine-induced relaxation in resistance arteries during pregnancy: hydralazine induces vasodilation via a prostacyclin pathway. Vasc Pharmacol 2016; 78: 36–42. [DOI] [PubMed] [Google Scholar]
- 182.Lupi-Herrera E, Furuya ME, Sandoval J, et al. Effect of hydralazine on vascular mechanics in a canine lobar preparation of pulmonary embolism. Lung 1992; 170: 291–309. [DOI] [PubMed] [Google Scholar]
- 183.Rubin LJ, Peter RH. Oral hydralazine therapy for primary pulmonary hypertension. N Engl J Med 1980; 302: 69–73. [DOI] [PubMed] [Google Scholar]
- 184.Prewitt RM, Downes AMT, Gu S, et al. Effects of hydralazine and increased cardiac output on recombinant tissue plasminogen activator-induced thrombolysis in canine pulmonary embolism*. CHEST 1991; 99: 708–714. [DOI] [PubMed] [Google Scholar]
- 185.Ducas J, Girling L, Schick U, et al. Pulmonary vascular effects of hydralazine in a canine preparation of pulmonary thromboembolism. Circulation 1986; 73: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 186.Bates ER, Crevey BJ, Sprague FR, et al. Oral hydralazine therapy for acute pulmonary embolism and low output state. Arch Intern Med 1981; 141: 1537–1538. [PubMed] [Google Scholar]
- 187.McGoon MD, Seward JB, Vlietstra RE, et al. Haemodynamic response to intravenous hydralazine in patients with pulmonary hypertension. Br Heart J 1983; 50: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL899775 Supplemental Material1 for Pulmonary vasodilation in acute pulmonary embolism – a systematic review by Mads Dam Lyhne, Jeffrey Allen Kline, Jens Erik Nielsen-Kudsk and Asger Andersen in Pulmonary Circulation
Supplemental material, PUL899775 Supplemental Material2 for Pulmonary vasodilation in acute pulmonary embolism – a systematic review by Mads Dam Lyhne, Jeffrey Allen Kline, Jens Erik Nielsen-Kudsk and Asger Andersen in Pulmonary Circulation