Abstract
Background & Aims
Anemia is common in patients with celiac disease and a frequent presentation. Guidelines recommend screening iron-deficient patients with anemia for celiac disease. However, the reported prevalence of celiac disease among patients with iron-deficiency anemia (IDA) varies. We performed a systematic review to determine the prevalence of biopsy-verified celiac disease in patients with IDA.
Methods
We performed a systematic review of manuscripts published in PubMed Medline or EMBASE through July 2017 for the term celiac disease combined with anemia or iron-deficiency. We used fixed-effects inverse variance-weighted models to measure the pooled prevalence of celiac disease. Meta-regression was used to assess subgroup heterogeneity.
Results
We identified 18 studies comprising 2998 patients with IDA for inclusion in our analysis. Studies originated from the United Kingdom, United States, Italy, Turkey, Iran, and Israel. The crude unweighted prevalence of celiac disease was 4.8% (n=143). Using a weighted pooled analysis, we demonstrated a prevalence of biopsy-confirmed celiac disease of 3.2% (95% CI, 2.6%–3.9%) in patients with IDA. However, heterogeneity was high (I2 = 67.7%). The prevalence of celiac disease was not significantly higher in studies with a mean participant age older or younger than 18 years, nor in studies with a mixed-sex vs female-predominant (≥60%) population. On meta-regression, year of publication, the proportion of females, age at celiac disease testing, and the prevalence of in the general population were not associated with the prevalence of celiac disease in patients with IDA. In the 8 studies fulfilling all our quality criteria, the pooled prevalence of celiac disease was 5.5% (95% CI, 4.1%–6.9%).
Conclusions
In a systematic review and meta-analysis, we found that approximately 1 in 31 patients with IDA have histologic evidence of celiac disease. This prevalence value justifies the practice of testing patients with IDA for celiac disease.
Keywords: celiac, coeliac, iron deficiency, meta-analysis
Key terms: celiac (American spelling), coeliac (British spelling), fracture, gluten, anemia
INTRODUCTION
Celiac disease (CD) occurs in about 1 in 100 patients in the Western world1. It is an immune-mediated disease triggered by the exposure to gluten. While environmental factors are important, recent data highlight the importance of genetic factors2, 3. Patients with CD are at increased risk of a number of disorders including lymphoma4 and autoimmunity5–7.
Typically, patients with CD demonstrate small intestinal inflammation and villous atrophy8, and this may result in malabsorption of both calories and micronutrients including iron. Major guidelines for both management of CD9–11 and iron-deficiency anemia (IDA)12 point out the association between these two diseases and the need to test patients with unexplained IDA for CD. The highly-cited systematic review by Dube et al in 200513 reported a CD prevalence of between 2.9% and 6% in patients with asymptomatic IDA, increasing to 10–15% when patients with IDA and concomitant gastrointestinal symptoms were screened13. However, since the publication of the paper by Dube et al13, a large number of publications on IDA and CD have appeared, with CD prevalence varying between 1.8%14 and ≥20%15, 16. Despite this we are unaware of any up-to-date systematic review aiming to quantitatively combine prevalence data for CD in IDA.
The aim of this study was to investigate the prevalence of CD in patients with IDA, and to determine if CD prevalence differed by IDA subgroups.
METHODS
We used the PRISMA guidelines17 when planning and executing this paper. We did not publish any pre-specified protocol prior to the study.
Search
The library of Karolinska Institutet searched PubMed and EMBASE for CD (celiac disease or coeliac disease or gluten or non-tropical sprue) combined with “anemia” or “anaemia” or “iron-deficiency”, up until May 2016. We then updated our search in July 2017. The search was limited to English-language publications. SM and ML conducted the review of all search hits with assistance from JFL.
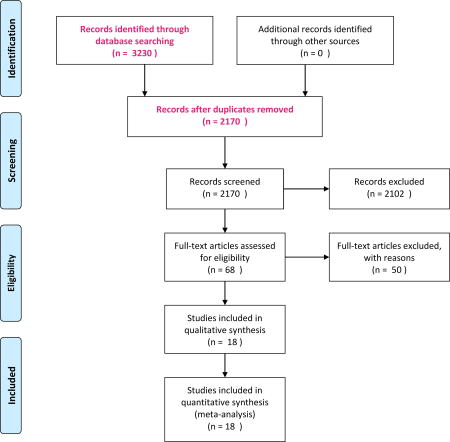
After initial review of abstracts and titles, 68 papers were read in detail. Eighteen papers were then included in the final analysis ((Table 1) and Appendix 1 (flowchart for study inclusion))14–16, 18–32. For case-control studies we restricted the extracted information to those patients with IDA.
Table 1.
Papers included in the systematic review on celiac disease prevalence in iron-deficiency anemia
| Study and Year | Country | Percentage | Celiac patients, N | Patients with IDA, N |
|---|---|---|---|---|
| McIntyre, 199318 | UK | 2.7 | 3 | 111 |
| Corazza, 199519 | Italy | 5.0 | 10 | 200 |
| Ackerman, 199620 | Israel | 12.1 | 11 | 91 |
| Carroccio, 199821 | Italy | 5.9 | 5 | 85 |
| Annibale, 200122 | Italy | 5.6 | 4 | 71 |
| Ransford, 200223 | UK | 2.3 | 11 | 484 |
| Annibale, 200324 | Italy | 8.5 | 5 | 59 |
| Mandal, 200414 | UK | 1.8 | 9 | 504 |
| Karnam, 200426 | USA | 2.9 | 3 | 105 |
| Grisolano, 200425 | USA | 8.7 | 9 | 103 |
| Kalayci, 200527 | Turkey | 2.2 | 3 | 135 |
| Ferguson, 200716 | Northern Ireland | 20.0 | 6 | 30 |
| Gonen, 200728 | Turkey | 3.0 | 3 | 100 |
| Ucardag, 200929 | Turkey | 7.8 | 6 | 77 |
| Emami, 201230 | Iran | 5.4 | 7 | 130 |
| Ertekin, 201315 | Turkey | 21.3 | 13 | 61 |
| Baghbanian, 201531 | Iran | 6.0 | 24 | 402 |
| Karaman, 201632 | Turkey | 4.4 | 11 | 250 |
Celiac disease
Small intestinal biopsy was required for the CD diagnosis. Most biopsy-confirmed patients with available serology data had a positive CD serology but this was not a requirement for diagnosis in our study. Where authors did not report the prevalence of Marsh I–III, we assumed that “biopsy-verified CD” required at least Marsh II–III; where Marsh categories were presented, grade I was not accepted as CD. It is well-known from other studies that the prevalence of CD will increase when Marsh I is accepted as proof of CD or even when biopsy-negative patients are regarded as CD positive. In a sub-analysis, we examined the risk of CD in IDA where it was explicitly stated that Marsh III was required.
Anemia
The definition of anemia varied among the studies, with most studies requiring a hemoglobin (Hb) below 135 g/L in males and 120 in females, but both stricter20, 27 and looser22 inclusion criteria were applied (see Table 1 for details on anemia definition in individual studies). A “low Hb cut-off level” for anemia is sometimes relevant (eight of the studies included patients <18 years and some focused on younger children where Hb levels are generally lower than in an adult population and the mean/median ages in the different studies ranged from 5.3 years15 to 63 years18). The majority of studies drew patients from tertiary referral hospitals, although one study looked at screening of blood donors16, and another at blood specimens obtained from primary care providers23. Characteristics of the included studies are given in Table 1, with definitions of iron-deficiency anemia summarized in Appendix 2.
Upper and lower 95% confidence intervals (Cis) and standard errors based on the proportions reported in each paper were calculated.
Data items and risk of bias
Data on the following variables were extracted: (i) year of publication, (ii) age at screening (child<18 years, adult, mixed), (iii) country, and (iv) Marsh stage33. The prevalence of CD in the general population differs34. For this reason we examined the prevalence of CD in IDA in relationship to the underlying CD prevalence in each country (Italy35; US36; UK35; Iran37; Northern Ireland (we used UK data35); Israel38, and Turkey39).
We used the Munn, et al critical appraisal tool to grade the quality of our prevalence studies40 (Appendix 3). Since a funnel plot (eFigure 1) indicated that publication bias could not be ruled out, we carried out a separate analysis restricted to studies with a standard error ≤0.02. While random-effects models tend to give greater relative weight to imprecise results,41 especially where heterogeneity is present, we carried out such an analysis restricted also to studies with a standard error ≤0.02.
Summary measures, analysis method and heterogeneity
To calculate a weighted prevalence of CD among patients with IDA we used a fixed-effect model. This prevents smaller and imprecise studies from impacting the summary estimate unduly42. We report all estimates with 95% CIs. We also calculated the heterogeneity between studies as I2. Given the high heterogeneity observed in this study (67.7%) we explored the prevalence of CD in subgroups based on geographic region, study size, age and gender. Studies were grouped according to country of origin, classified as North America, Europe, Turkey, or Asia (Iran and Israel). We defined subgroups of study size (≤199 vs. ≥200) and gender (<60 vs. ≥60% females; the average proportion of females in the 16 studies with available data was 64%). In addition, we carried out four meta-regression analyses to examine the association of CD prevalence in IDA with: (i) study size, (ii) publication year, (iii) proportion of females and (iv) underlying CD prevalence. All these factors can potentially explain the variance of CD prevalence.
Statistics software
Stata 13 was used for all analyses.
RESULTS
Titles and abstracts were read for 2057 papers published up until May 2016 (Flowchart). In July 2017 the search was updated. Since the Karolinska Institutet Library was only able to perform searches for full years, the second search started from Jan 2016 up until July 2017 (167 hits) and therefore overlapped with the first search but yielded no additional relevant papers.
Sixty-eight papers were identified as potentially relevant for our meta-analysis, and were read in detail. Any disagreement between SM and ML was solved through consensus, or through mediation with JFL. Reasons for exclusion of the 50 excluded papers were: unclear inclusion criteria (definition of CD or IDA, n=1543–57); looked at refractory IDA or anemia of obscure origin (n=1058–67); full-text unavailable or not available in English (n=768–74); high risk of selection bias (n=632, 75–79); looked at iron deficiency without anemia, or anemia not explicitly part of criteria (n=480–83); looked at anemia in general, not IDA (n=384–86); or other reason (small intestinal biopsy not performed (n=187), case report (n=188), examined prevalence of IDA in patients with CD (n=189), not original research (n=190), examined CD in general population not IDA (n=191)).
In the quantitative part of the analysis, we hence included 18 relevant studies with 2998 patients with IDA (Table 1)14–16, 18–32. Of these 2998 patients, 143 had CD, yielding an unweighted proportion of 4.8%. The median size of the 18 studies was 104 patients. Three studies contributed more than 400 patients each14, 23, 31. The median prevalence of CD was 5.0%. Eight studies had taken place in Europe.
Prevalence of CD in anemia
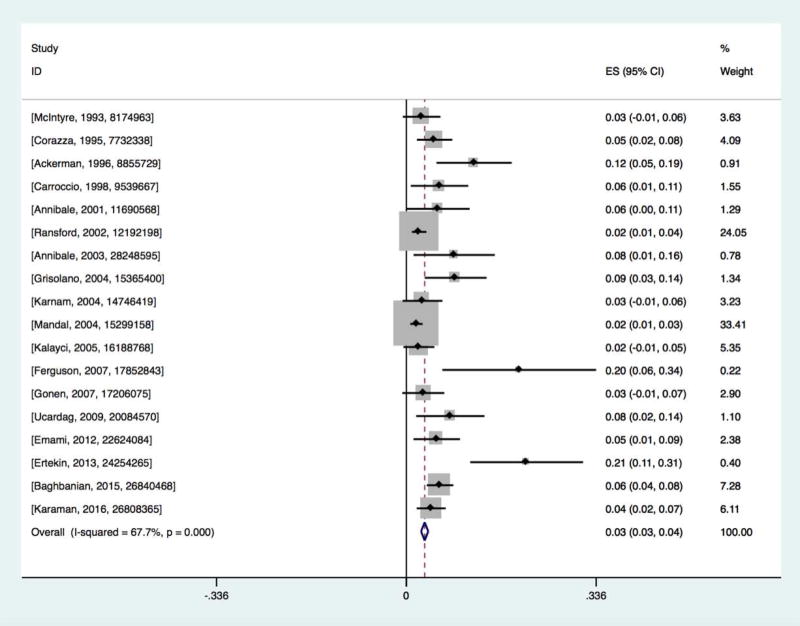
The pooled prevalence of CD in patients with IDA was 3.2% (95% CI=2.6–3.9%)(Figure 1). The heterogeneity was high (p<0.001; I2 = 67.7%). We therefore performed subgroup analyses and meta-regressions to examine this heterogeneity.
Figure 1.
Prevalence of biopsy-verified celiac disease in iron-deficiency anemia
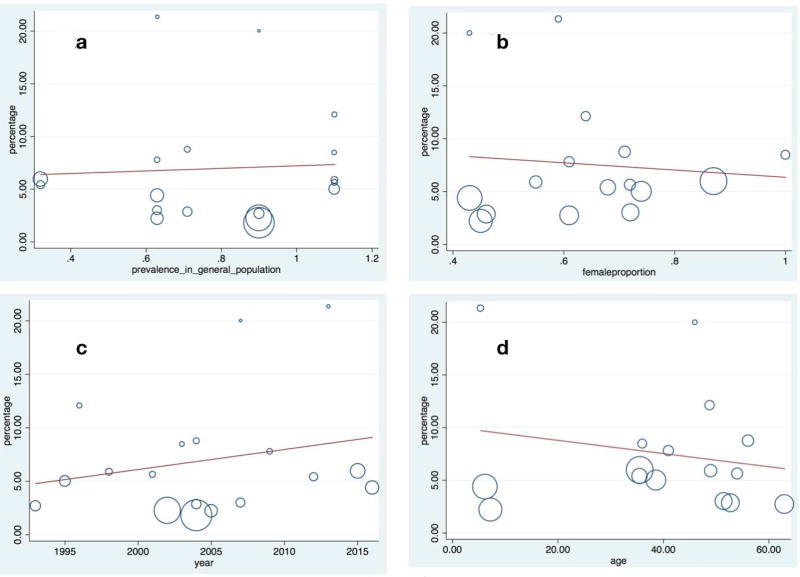
The pooled prevalence of CD in IDA was similar in studies that enrolled patients with an average age <18 years at publication (4.0%; 95%CI=2.1–5.8%)15, 27, 32, as compared to those with a mean age ≥18 years (3.1%; 95%CI=2.5–3.8%)14, 16, 18–26, 28–31. Excluding two studies14, 23 where the mean age was ≥18 but exact values were not possible to extract, we found no relationship between age at testing for CD and prevalence of CD in IDA (Figure 2a, p=0.461, see Figure legend for mean vs. median age).
Figure 2.
a. Meta-regression: Relationship between age at CD investigation and CD prevalence in iron-deficiency anemia.
Y-axis: Percentage (%) of celiac disease among patients with iron-deficiency anemia among. X-axis: Age in years when tested for CD (p=0.461). Age represents median age for the following three studies21, 22, 24 and otherwise mean age.
b. Meta-regression: Relationship between proportion of women and CD prevalence in iron-deficiency anemia.
Y-axis: Percentage (%) of celiac disease among study participants with iron-deficiency anemia.X-axis: Percentage of females in each individual study (p=0.726).
c. Meta-regression: Relationship between year of publication and CD prevalence in iron-deficiency anemia.
Y-axis: Percentage (%) of celiac disease among study participants with iron-deficiency anemia. X-axis: Year of study publication (p=0.377).
d. Meta-regression: Relationship between CD prevalence in the general population and CD prevalence in iron-deficiency anemia.
Y-axis: Percentage (%) of celiac disease among study participants with iron-deficiency anemia. X-axis: Prevalence of CD in the general population (p=0.829).
The CD prevalence in IDA varied by continent (p=0.002 for subgroup analysis). The CD prevalence was 4.6% (95%CI=1.6–7.5%) in North America25, 26. The prevalence in Europe (without Turkey)14, 16, 18, 19, 21–24 was 2.5% (95%=1.7–3.3%), with Turkey15, 27–29, 32 (4.1%; 95%CI=2.5–5.7%) and other Asian countries20, 30, 31 (6.4%; 95%CI=4.4–8.3%) showing the highest prevalence.
Two studies did not report the proportion of women14, 23. In IDA studies where at least 60% of participants were women18–20, 22, 24, 25, 28–31 the CD prevalence was 5.5% (95%C=4.2–6.7%), as opposed to 4.2% (95%CI=2.6–5.7) in studies with a more mixed population or a majority of males15, 16, 21, 26, 27, 32. The CD prevalence in the two studies without data on sex distribution was 2.0% (95%CI=1.2–2.8%). In a meta-regression, we found no association between the proportion of women and the prevalence of CD in IDA (Figure 2b, p=0.726).
Several studies have reported an increase in the prevalence of CD over time92, 93; for this reason we examined the association between CD prevalence in IDA according to year of publication. A meta-regression could not demonstrate any association with year of publication (Figure 2c, p=0.377). Fourteen studies had included consecutive patients with IDA for CD screening14, 16, 19–26, 28–30. Restricting the analysis to these studies, the CD prevalence was 2.9% (95%CI=2.2–3.6%). Seven studies explicitly reported that they required Marsh 3 for the CD diagnosis15, 16, 21, 23, 28, 31, 32. The prevalence of CD when requiring Marsh III (3.7%; 95%CI=2.1–3.8%) did not differ from that of other studies (p=0.267) where the prevalence was 2.9% (95%CI=2.3–3.9%). In eleven studies, all patients with positive antibodies had undergone biopsies14–16, 20, 22, 25, 26, 28–31. Smaller studies (≤199 participants) showed higher prevalence of CD in IDA (p=0.007)(4.8%; 95%CI=3.5–6.0%) compared to large studies (≥200 participants)(2.7%; 95%CI=2.0–3.5%). We also investigated if the underlying population prevalence of CD was associated with the CD prevalence in patients with IDA. We were unable to demonstrate any such association (Figure 2d; p=0.829). Restricting our dataset to studies with a standard error ≤0.02, the CD prevalence in IDA was 2.8% (95%CI=2.1–3.4%). Using a random-effects model applied to studies with a standard error ≤0.02, the CD prevalence in IDA was 3.3% (95%CI=2.2–4.3%).
Finally, we examined the prevalence of CD in IDA studies20, 22, 24–26, 28, 30, 31 fulfilling all quality criteria (except the criterion concerning the identification of subpopulations, Appendix 3)40. In these eight studies, the CD prevalence was 5.5% (95%CI=4.1–6.9%).
DISCUSSION
In this systematic review with meta-analysis of 2998 individuals, we found that biopsy-proven CD is a relatively frequent finding in patients with IDA, with a prevalence of roughly 1 in 31. The CD prevalence in IDA was not influenced by the proportion of females, the average age, or the baseline prevalence of CD in the populations studied. This is notable given that IDA is more common in certain subgroups, such as premenopausal women. Our findings suggest that IDA is an important risk factor for CD irrespective of patient demographics, and that endoscopic small bowel biopsy should be a part of the diagnostic workup for the condition, even in persons in which other etiologies may be suspected.
To our knowledge, this is the first meta-analysis to report a pooled estimate for the overall prevalence of biopsy-proven CD in patients with IDA. Prior studies have shown highly varying prevalence data for CD in IDA. Only one prior systematic review has addressed the question specifically, examining the CD prevalence in iron-deficient individuals as a subgroup of Western European populations,13 including 6 of the studies we also reviewed.18, 20, 22, 24, 94, 95 A meta-analysis was not conducted in this prior review. The prevalence of biopsy-confirmed CD in IDA was reported to be between 2.9% and 6%, which is consistent but slightly higher than our pooled estimate of 3.2% (95% CI=2.6–3.9%). It is possible that restriction to Western European individuals in Dube et al. 2005 may have accounted for the slightly higher prevalence estimates, although we did not identify an association between the baseline risk of CD in the population studied and the prevalence of CD in IDA on meta-regression.
The prevalence of IDA in the overall population varies markedly by sex and age, being most prevalent in females of reproductive age and in infancy in both sexes, likely due to higher iron requirements and poor dietary intake.96 As CD is a genetic disorder, it might be expected that the prevalence of CD might be lower in studies in which the population was more likely to have other acquired etiologies of IDA, such as in female-predominant cohorts. However, we found no relationship between the average participant age and the prevalence of CD in IDA in our meta-regression (p=0.461), or between the proportion of females in the sample and the prevalence of CD in IDA (p=0.726). We cannot, however rule out that CD may be more common in childhood IDA. The prevalence of CD in the latter population was 4.0% although only based on three studies (27 cases of CD in 446 children with IDA, non-weighted data)15, 27, 32. One reason for a higher prevalence of CD in children with IDA could be that other causes of IDA (including gastrointestinal bleeding and cancer) are less frequent in children. While IDA may occur in up to a third of CD patients, CD has also been associated with other causes of anemia such as folate and B12 deficiency as well as anemia of chronic disease97, 98. Both malnutrition and ongoing inflammation likely contribute to anemia in CD patients. In CD patients with IDA, a gluten-free diet alone has been shown to induce improvement in ferritin levels and reversal of anemia without iron supplementation, underscoring the link between the two conditions.99 Our findings do suggest that testing for CD is warranted in all patients with IDA, without exclusion of groups that have a high prevalence of other IDA etiologies.
One explanation for the significant variation in reported CD prevalence may be publication bias. Smaller studies in our meta-analysis yielded higher prevalence figures (pooled weighted estimates of 4.8% as opposed to 2.7%). Considering that small studies are more difficult to publish, publication bias is likely on the basis of our funnel plot. In this analysis, 2 of the 3 smallest studies both reported that 1 in 5 tested patients with IDA had CD, compared to distinctively lower proportions in the largest studies (1 in 37 patients with IDA in studies of ≥200 participants had CD). In order to avoid giving undue weight to smaller studies we used a fixed-effect model. Data from other fields suggest that exclusion of unpublished data (as in our study) may overestimate the pooled estimates100. We approached this potential limitation by restricting our dataset to studies with a standard error ≤0.02 in a subanalysis. This yielded a pooled prevalence of 2.8%, similar to the overall pooled prevalence of our study (3.2%) and in line with estimates from Dube et al13.
Another kind of bias that may have pushed up the CD prevalence in some earlier screenings studies is if patients with concomitant gastrointestinal symptoms and IDA were more likely to undergo endoscopy as part of their assessment than asymptomatic IDA patients. We excluded studies where IDA patients with symptoms from the digestive tract were tested, and in another attempt to eliminate selection bias specifically examined studies where patients were (explicitly) included consecutively. This did not influence the results notably (2.9%).
Significant heterogeneity was observed which could not be explained by the examined characteristics. We performed meta-regressions to examine the interaction between the age, sex, and prevalence of CD in the general population and the prevalence of CD in IDA, none of which were explanatory. We also restricted our dataset to studies fulfilling all quality criteria, with a slight increase in the pooled CD prevalence (5.5%). We found no association between baseline CD prevalence and CD prevalence in IDA (p=0.829), but when merging data from different countries, the pooled CD prevalence in IDA patients from Asia (Israel and Iran) outside Turkey was 6.4%. We urge caution when interpreting these data, however, since they were based on only three studies20, 30, 31. Of note, most previous research on biopsy-verified CD in IDA is limited to few countries (mainly Italy and Turkey), and there are little data from elsewhere. For this meta-analysis we only identified two studies from the US25, 26.
Strengths of the present study include a large pool of identified studies, drawn from two different databases (PubMed and EMBASE). This large number of studies allowed us to restrict the analysis to histologically-confirmed, rather than serologically-diagnosed CD which is frequently reported but which can overestimate the true prevalence of CD. It also afforded subgroup analysis and meta-regression with examination of important demographic variables as interacting factors.
The observed heterogeneity without explanatory factors is a significant limitation of our study, and impacts on interpretation of the pooled estimate. We had limited data on patients from many parts of the world, including Asia and Africa, and no information on the ethnicity of study participants. While our funnel plot indicated a degree of publication bias that may have overestimated CD prevalence, critical appraisal of study quality suggested that in high-quality studies, more than 1 in 20 IDA patients may have CD. We were unable to examine the linear relationship between hemoglobin levels and prevalence of CD. We also did not have complete antibody data, and were unable to examine the prevalence of biopsy-negative CD in IDA. Recently the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) developed a non-biopsy pathway to CD diagnosis in patients with repeatedly positive celiac-specific serology, positive HLA-DQ2/DQ8 and symptoms consistent with CD101, however within these guidelines patients evaluated with anemia would require biopsy.
In conclusion, this meta-analysis found that approximately 1 in 31 patients with IDA had biopsy-verified CD, and that the prevalence of CD in IDA did not vary by the average age, proportion of females, or baseline CD prevalence. While no major gastroenterology society advocates for screening the general population for CD, there is a consensus that testing should be performed in patients with IDA9, 10, 12. Our findings strongly support this recommendation, and further highlight the importance of endoscopy with histologic evaluation for CD in the diagnostic workup for patients with this condition, even in populations where other etiologies of IDA are common.
Supplementary Material
Acknowledgments
Karolinska Institutet Library for carrying out the initial search for relevant research papers.
Grant Support (Funding):
SM was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (T32 DK083256)
Independence (role of the sponsors): None of the funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations
- CD
Celiac disease
- GFD
Gluten-free diet
- IDA
iron-deficiency anemia
- VA
Villous atrophy
PRISMA 2009 Flow Diagram1
1From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
1. Medline (Ovid)

2. Embase (embase.com)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
*Appendix 4 is the PRISMA checklist and is not cited in the paper.
Disclosure statement: The authors have nothing to disclose.
Authors’ contributions:
ICMJE criteria for authorship read and met: SM, ML, JS, MB, BL, PG, JFL
Agree with the manuscript’s results and conclusions: SM, ML, JS, MB, BL, PG, JFL
Designed the experiments/the study: SM, ML, JS, MB, BL, PG, JFL
Collected data: SM, ML, with contribution from JFL
Analyzed the data: JFL
Wrote the first draft of the paper: JFL with contribution from SM and ML
Contributed to study design, interpretation of data and writing: SM, ML, JS, MB, BL, PG, JFL
Interpretation of data; approved the final version of the manuscript: SM, ML, JS, MB, BL, PG, JFL
Obtained funding: JFL
References
- 1.Lebwohl B, Ludvigsson JF, Green PH. Celiac disease and non-celiac gluten sensitivity. BMJ. 2015;351:h4347. doi: 10.1136/bmj.h4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E, Lee HS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–9. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuja-Halkola R, Lebwohl B, Halfvarson J, et al. Heritability of non-HLA genetics in coeliac disease: a population-based study in 107 000 twins. Gut. 2016;65:1793–1798. doi: 10.1136/gutjnl-2016-311713. [DOI] [PubMed] [Google Scholar]
- 4.Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35:540–51. doi: 10.1111/j.1365-2036.2011.04972.x. [DOI] [PubMed] [Google Scholar]
- 5.Elfstrom P, Sundstrom J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40:1123–32. doi: 10.1111/apt.12973. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Lindelof B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010–6. doi: 10.1038/jid.2011.162. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Kristinsson SY, Goldin LR, et al. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136:91–8. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–28. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76. doi: 10.1038/ajg.2013.79. quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–16. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 13.Dube C, Rostom A, Sy R, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Mandal AK, Mehdi I, Munshi SK, et al. Value of routine duodenal biopsy in diagnosing coeliac disease in patients with iron deficiency anaemia. Postgraduate Medical Journal. 2004;80:475–7. doi: 10.1136/pgmj.2003.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertekin V, Tozun MS, Kucuk N. The prevalence of celiac disease in children with iron-deficiency anemia. Turkish Journal of Gastroenterology. 2013;24:334–8. doi: 10.4318/tjg.2013.0529. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson C, Dickey W. Gastrointestinal investigation of iron deficiency anaemia detected by pre-blood donation screening. Scandinavian Journal of Gastroenterology. 2007;42:1386–1387. doi: 10.1080/00365520701408847. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntyre AS, Long RG. Prospective survey of investigations in outpatients referred with iron deficiency anaemia. Gut. 1993;34:1102–7. doi: 10.1136/gut.34.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corazza GR, Valentini RA, Andreani ML, et al. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153–6. doi: 10.3109/00365529509093254. [DOI] [PubMed] [Google Scholar]
- 20.Ackerman Z, Eliakim R, Stalnikowicz R, et al. Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anemia. American Journal of Gastroenterology. 1996;91:2099–102. [PubMed] [Google Scholar]
- 21.Carroccio A, Iannitto E, Cavataio F, et al. Sideropenic anemia and celiac disease: one study, two points of view. Digestive Diseases & Sciences. 1998;43:673–8. doi: 10.1023/a:1018896015530. [DOI] [PubMed] [Google Scholar]
- 22.Annibale B, Capurso G, Chistolini A, et al. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. American Journal of Medicine. 2001;111:439–45. doi: 10.1016/s0002-9343(01)00883-x. [DOI] [PubMed] [Google Scholar]
- 23.Ransford RA, Hayes M, Palmer M, et al. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. J Clin Gastroenterol. 2002;35:228–33. doi: 10.1097/00004836-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Annibale B, Lahner E, Chistolini A, et al. Endoscopic evaluation of the upper gastrointestinal tract is worthwhile in premenopausal women with iron-deficiency anaemia irrespective of menstrual flow. Scandinavian Journal of Gastroenterology. 2003;38:239–245. doi: 10.1080/00365520310000690a. [DOI] [PubMed] [Google Scholar]
- 25.Grisolano SW, Oxentenko AS, Murray JA, et al. The usefulness of routine small bowel biopsies in evaluation of iron deficiency anemia. J Clin Gastroenterol. 2004;38:756–60. doi: 10.1097/01.mcg.0000139034.38568.51. [DOI] [PubMed] [Google Scholar]
- 26.Karnam US, Felder LR, Raskin JB. Prevalence of occult celiac disease in patients with iron-deficiency anemia: a prospective study. South Med J. 2004;97:30–4. doi: 10.1097/01.SMJ.0000051059.23259.56. [DOI] [PubMed] [Google Scholar]
- 27.Kalayci AG, Kanber Y, Birinci A, et al. The prevalence of coeliac disease as detected by screening in children with iron deficiency anaemia. Acta Paediatrica. 2005;94:678–81. doi: 10.1111/j.1651-2227.2005.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonen C, Yilmaz N, Yalcin M, et al. Diagnostic yield of routine duodenal biopsies in iron deficiency anaemia: a study from Western Anatolia. European Journal of Gastroenterology & Hepatology. 2007;19:37–41. doi: 10.1097/01.meg.0000250583.07867.b7. [DOI] [PubMed] [Google Scholar]
- 29.Ucardag D, Guliter S, Ceneli O, et al. Celiac disease prevalence in patients with iron deficiency anemia of obscure origin. Turkish Journal of Gastroenterology. 2009;20:266–70. doi: 10.4318/tjg.2009.0024. [DOI] [PubMed] [Google Scholar]
- 30.Emami MH, Karimi S, Kouhestani S. Is routine duodenal biopsy necessary for the detection of celiac disease in patients presenting with iron deficiency anemia? International Journal of Preventive Medicine. 2012;3:273–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Baghbanian M, Farahat A, Vahedian HA, et al. The Prevalence of Celiac Disease in Patients with Iron-Deficiency Anemia in Center and South Area of Iran. Arquivos de Gastroenterologia. 2015;52:278–82. doi: 10.1590/S0004-28032015000400006. [DOI] [PubMed] [Google Scholar]
- 32.Karaman K, Akbayram S, Kar S, et al. Prevalence of Celiac Disease in Children With Iron Deficiency Anemia in Van Lake Region of Turkey. Journal of Pediatric Hematology/Oncology. 2016;38:143–6. doi: 10.1097/MPH.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 33.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 34.Kang JY, Kang AH, Green A, et al. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–45. doi: 10.1111/apt.12373. [DOI] [PubMed] [Google Scholar]
- 35.Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–95. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 36.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–44. doi: 10.1038/ajg.2012.219. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 37.Akbari MR, Mohammadkhani A, Fakheri H, et al. Screening of the adult population in Iran for coeliac disease: comparison of the tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. 2006;18:1181–6. doi: 10.1097/01.meg.0000224477.51428.32. [DOI] [PubMed] [Google Scholar]
- 38.Israeli E, Hershcovici T, Grotto I, et al. Prevalence of celiac disease in an adult Jewish population in Israel. Isr Med Assoc J. 2010;12:266–9. [PubMed] [Google Scholar]
- 39.Ertekin V, Selimoglu MA, Kardas F, et al. Prevalence of celiac disease in Turkish children. J Clin Gastroenterol. 2005;39:689–91. doi: 10.1097/01.mcg.0000174026.26838.56. [DOI] [PubMed] [Google Scholar]
- 40.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–8. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150:469–75. doi: 10.1093/oxfordjournals.aje.a010035. [DOI] [PubMed] [Google Scholar]
- 42.Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2017 n/a-n/a. [Google Scholar]
- 43.Brooklyn TN, Di Mambro AJ, Haslam N. Patients over 45 years with iron deficiency require investigation. European Journal of Gastroenterology & Hepatology. 2003;15:535–8. doi: 10.1097/01.meg.0000059109.41030.6a. [DOI] [PubMed] [Google Scholar]
- 44.Carter D, Levi G, Tzur D, et al. Prevalence and predictive factors for gastrointestinal pathology in young men evaluated for iron deficiency anemia. Digestive Diseases & Sciences. 2013;58:1299–305. doi: 10.1007/s10620-012-2496-6. [DOI] [PubMed] [Google Scholar]
- 45.Carter D, Maor Y, Bar-Meir S, et al. Prevalence and predictive signs for gastrointestinal lesions in premenopausal women with iron deficiency anemia. Digestive Diseases & Sciences. 2008;53:3138–44. doi: 10.1007/s10620-008-0298-7. [DOI] [PubMed] [Google Scholar]
- 46.Droogendijk J, Beukers R, Berendes PB, et al. Screening for gastrointestinal malignancy in patients with iron deficiency anemia by general practitioners: An observational study. Scandinavian Journal of Gastroenterology. 2011;46:1105–1110. doi: 10.3109/00365521.2011.594082. [DOI] [PubMed] [Google Scholar]
- 47.Gulen H, Kasirga E, Yildirim SA, et al. Diagnostic yield of upper gastrointestinal endoscopy in the evaluation of iron deficiency anemia in older children and adolescents. Pediatric Hematology & Oncology. 2011;28:694–701. doi: 10.3109/08880018.2011.572145. [DOI] [PubMed] [Google Scholar]
- 48.Kibria R, Akram S, Ali SA, et al. Screening Veterans Affairs patients with iron deficiency for celiac disease. Southern Medical Journal. 2010;103:385–6. doi: 10.1097/SMJ.0b013e3181d3236e. [DOI] [PubMed] [Google Scholar]
- 49.Koulaouzidis A, Yung DE, Lam JH, et al. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scandinavian Journal of Gastroenterology. 2012;47:1094–100. doi: 10.3109/00365521.2012.704938. [DOI] [PubMed] [Google Scholar]
- 50.Milano A, Balatsinou C, Filippone A, et al. A prospective evaluation of iron deficiency anemia in the GI endoscopy setting: role of standard endoscopy, videocapsule endoscopy, and CT-enteroclysis. Gastrointestinal Endoscopy. 2011;73:1002–8. doi: 10.1016/j.gie.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Monzon H, Forne M, Gonzalez C, et al. Mild enteropathy as a cause of iron-deficiency anaemia of previously unknown origin. Digestive & Liver Disease. 2011;43:448–53. doi: 10.1016/j.dld.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Muhammad A, Pitchumoni CS. Newly detected celiac disease by wireless capsule endoscopy in older adults with iron deficiency anemia. Journal of Clinical Gastroenterology. 2008;42:980–3. doi: 10.1097/MCG.0b013e3181354455. [DOI] [PubMed] [Google Scholar]
- 53.Muhammad A, Pitchumoni CS. Evaluation of iron deficiency anemia in older adults: The role of wireless capsule endoscopy. Journal of Clinical Gastroenterology. 2009;43:627–631. doi: 10.1097/mcg.0b013e318181b442. [DOI] [PubMed] [Google Scholar]
- 54.Pengelly S, Fabricius M, McMenamin D, et al. Attendance at iron deficiency anaemia clinic: audit of outcomes 5 years on. Colorectal Disease. 2013;15:423–7. doi: 10.1111/codi.12040. [DOI] [PubMed] [Google Scholar]
- 55.Robson K, Barto A, Liberman RF. The evaluation of premenopausal women with anemia: What is the yield of gastrointestinal endoscopy? Digestive Diseases and Sciences. 2009;54:1667–1671. doi: 10.1007/s10620-008-0562-x. [DOI] [PubMed] [Google Scholar]
- 56.Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. European Journal of Gastroenterology & Hepatology. 2003;15:407–13. doi: 10.1097/00042737-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Willoughby JMT, Laitner SM. Audit of the investigation of iron deficiency anaemia in a distinct general hospital, with sample guidelines for future practice. Postgraduate Medical Journal. 2000;76:218–222. doi: 10.1136/pmj.76.894.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abd El Dayem SM, Ahmed Aly A, Abd El Gafar E, et al. Screening for coeliac disease among Egyptian children. Archives of Medical Science. 2010;6:226–35. doi: 10.5114/aoms.2010.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cekin AH, Cekin Y, Sezer C. Celiac disease prevalence in patients with iron deficiency anemia. Turkish Journal of Gastroenterology. 2012;23:490–5. [PubMed] [Google Scholar]
- 60.Dickey W, Kenny BD, McMillan SA, et al. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scandinavian Journal of Gastroenterology. 1997;32:469–72. doi: 10.3109/00365529709025083. [DOI] [PubMed] [Google Scholar]
- 61.Fayed SB, Aref MI, Fathy HM, et al. Prevalence of celiac disease, Helicobacter pylori and gastroesophageal reflux in patients with refractory iron deficiency anemia. Journal of Tropical Pediatrics. 2008;54:43–53. doi: 10.1093/tropej/fmm080. [DOI] [PubMed] [Google Scholar]
- 62.Fraser JS, Woodhouse NJ, El-Shafie OT, et al. Occult celiac disease in adult Omanis with unexplained iron deficiency anemia. Saudi Medical Journal. 2003;24:791. [PubMed] [Google Scholar]
- 63.Saez LR, Alvarez DF, Martinez IP, et al. Refractory iron-deficiency anemia and gluten intolerance - Response to gluten-free diet. Revista Espanola de Enfermedades Digestivas. 2011;103:349–54. doi: 10.4321/s1130-01082011000700003. [DOI] [PubMed] [Google Scholar]
- 64.van Mook WN, Bourass-Bremers IH, Bos LP, et al. The outcome of esophagogastroduodenoscopy (EGD) in asymptomatic outpatients with iron deficiency anemia after a negative colonoscopy. European Journal of Internal Medicine. 2001;12:122–126. doi: 10.1016/s0953-6205(01)00123-6. [DOI] [PubMed] [Google Scholar]
- 65.Varma S, Malhotra P, Kochhar R, et al. Celiac disease presenting as iron-deficiency anemia in northern India. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 2001;20:234–236. [PubMed] [Google Scholar]
- 66.Zamani F, Mohamadnejad M, Shakeri R, et al. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World Journal of Gastroenterology. 2008;14:7381–5. doi: 10.3748/wjg.14.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodrigues JP, Pinho R, Silva J, et al. Appropriateness of the study of iron deficiency anemia prior to referral for small bowel evaluation at a tertiary center. World Journal of Gastroenterology. 2017;23:4444–4453. doi: 10.3748/wjg.v23.i24.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaabane NB, Youssef HB, Eljeridi N, et al. Usefulness of duodenal biopsy in the diagnosis exploration of iron deficiency anemia in the elderly. Journal Africain d'Hepato-Gastroenterologie. 2011;5:86– 89. [Google Scholar]
- 69.Garrido C, Gaya J, Liompart A, et al. Prevalence of monosymptomatic celiac disease in patients with iron deficiency anemia. Gastroenterologia y Hepatologia. 1997;20:172–4. [PubMed] [Google Scholar]
- 70.Lombardo T, Ximenes B, Ferro G. Hypochromic microcytic anemia as a clinical presentation of celiac disease. Clinical Laboratory. 2006;52:231–6. [PubMed] [Google Scholar]
- 71.Sabel'nikova EA, Parfenov AI, Krums LM, et al. Celiac disease as a cause of iron deficiency anemia. Terapevticheskii Arkhiv. 2006;78:45–8. [PubMed] [Google Scholar]
- 72.Sari R, Aydogdu I, Sevinc A, et al. Upper and lower gastrointestinal endoscopical investigation in elderly patients with iron deficiency anaemia. Haematologia. 2002;31:327–32. doi: 10.1163/15685590160141350. [DOI] [PubMed] [Google Scholar]
- 73.Vannella L, Aloe Spiriti MA, Di Giulio E, et al. Upper and lower gastrointestinal causes of iron deficiency anemia in elderly compared with adult outpatients. Minerva Gastroenterologica e Dietologica. 2010;56:397–404. [PubMed] [Google Scholar]
- 74.Zunino F, Alessio MG, Giussani B, et al. Screening of the celiac disease (CD) among blood donors in the Bergamo province: Preliminary data on 18.498 subjects. Biochimica Clinica. 2012;36:349–357. [Google Scholar]
- 75.Javid G, Lone SN, Shoukat A, et al. Prevalence of celiac disease in adult patients with iron-deficiency anemia of obscure origin in Kashmir (India) Indian Journal of Gastroenterology. 2015;34:314–9. doi: 10.1007/s12664-015-0586-z. [DOI] [PubMed] [Google Scholar]
- 76.Kavimandan A, Sharma M, Verma AK, et al. Prevalence of celiac disease in nutritional anemia at a tertiary care center. Indian Journal of Gastroenterology. 2014;33:114–8. doi: 10.1007/s12664-013-0366-6. [DOI] [PubMed] [Google Scholar]
- 77.Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Digestive Diseases & Sciences. 1995;40:1283–9. doi: 10.1007/BF02065539. [DOI] [PubMed] [Google Scholar]
- 78.Mansoor AA, Strak SK. Prevalence of celiac disease among patients with iron deficiency anemia: Personal experience and review of literature. Pakistan Journal of Medical Sciences. 2005;21:413–416. [Google Scholar]
- 79.Lau MS, Mooney P, White W, et al. Pre-endoscopy point of care test (Simtomax- IgA/IgG-Deamidated Gliadin Peptide) for coeliac disease in iron deficiency anaemia: diagnostic accuracy and a cost saving economic model. BMC Gastroenterology. 2016;16:115. doi: 10.1186/s12876-016-0521-5. [Erratum appears in BMC Gastroenterol. 2016 Oct 5;16(1):122;]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbass R, Hopkins M, Dufour DR, et al. Celiac disease in an urban VA population with iron deficiency: the case against routine duodenal biopsy. Digestive Diseases & Sciences. 2011;56:2037–41. doi: 10.1007/s10620-010-1549-y. [DOI] [PubMed] [Google Scholar]
- 81.Alonso Cotoner C, Casellas Jorda F, Chicharro Serrano ML, et al. Iron deficiency: not always blood losses. Anales de Medicina Interna. 2003;20:227–31. [PubMed] [Google Scholar]
- 82.Cannizzaro R, Da Ponte A, Tabuso M, et al. Improving detection of celiac disease patients: a prospective study in iron-deficient blood donors without anemia in north Italy. European Journal of Gastroenterology & Hepatology. 2014;26:721–4. doi: 10.1097/MEG.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 83.Howard MR, Turnbull AJ, Morley P, et al. A prospective study of the prevalence of undiagnosed coeliac disease in laboratory defined iron and folate deficiency. Journal of Clinical Pathology. 2002;55:754–7. doi: 10.1136/jcp.55.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harewood GC, Holub JL, Lieberman DA. Variation in small bowel biopsy performance among diverse endoscopy settings: results from a national endoscopic database. American Journal of Gastroenterology. 2004;99:1790–4. doi: 10.1111/j.1572-0241.2004.40176.x. [DOI] [PubMed] [Google Scholar]
- 85.Riestra S, Dominguez F, Fernandez-Ruiz E, et al. Usefulness of duodenal biopsy during routine upper gastrointestinal endoscopy for diagnosis of celiac disease. World Journal of Gastroenterology. 2006;12:5028–32. doi: 10.3748/wjg.v12.i31.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unsworth DJ, Lock RJ, Harvey RF. Improving the diagnosis of coeliac disease in anaemic women. British Journal of Haematology. 2000;111:898–901. [PubMed] [Google Scholar]
- 87.Murray JA, McLachlan S, Adams PC, et al. Association between celiac disease and iron deficiency in Caucasians, but not non-Caucasians. Clinical Gastroenterology & Hepatology. 2013;11:808–14. doi: 10.1016/j.cgh.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kilpatrick ZM, Katz J. Occult celiac disease as a cause of iron deficiency anemia. JAMA. 1969;208:999–1001. [PubMed] [Google Scholar]
- 89.Abu Daya H, Lebwohl B, Smukalla S, et al. Utilizing HDL levels to improve detection of celiac disease in patients with iron deficiency anemia. American Journal of Gastroenterology. 2014;109:769–70. doi: 10.1038/ajg.2014.30. [DOI] [PubMed] [Google Scholar]
- 90.Pearce CB, Duncan HD, Sinclair D, et al. Small bowel biopsies in patients with iron deficiency anaemia. Gut. 2001;49:595. doi: 10.1136/gut.49.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Makharia GK, Verma AK, Amarchand R, et al. Prevalence of celiac disease in the northern part of India: a community based study. Journal of Gastroenterology & Hepatology. 2011;26:894–900. doi: 10.1111/j.1440-1746.2010.06606.x. [DOI] [PubMed] [Google Scholar]
- 92.West J, Fleming KM, Tata LJ, et al. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109:757–68. doi: 10.1038/ajg.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a north american population. Am J Gastroenterol. 2013;108:818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corazza GR, Valentini RA, Andreani ML, et al. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scandinavian Journal of Gastroenterology. 1995;30:153–6. doi: 10.3109/00365529509093254. [DOI] [PubMed] [Google Scholar]
- 95.Ransford RA, Hayes M, Palmer M, et al. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. Journal of Clinical Gastroenterology. 2002;35:228–33. doi: 10.1097/00004836-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 96.Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–6. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 97.Halfdanarson TR, Litzow MR, Murray JA. Hematological manifestations of celiac disease. Blood. 2006;109:412–21. doi: 10.1182/blood-2006-07-031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harper JW, Holleran SF, Ramakrishnan R, et al. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82:996–1000. doi: 10.1002/ajh.20996. [DOI] [PubMed] [Google Scholar]
- 99.Annibale B, Severi C, Chistolini A, et al. Efficacy of gluten-free diet alone on recovery from iron deficiency anemia in adult celiac patients. Am J Gastroenterol. 2001;96:132–7. doi: 10.1111/j.1572-0241.2001.03463.x. [DOI] [PubMed] [Google Scholar]
- 100.Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 101.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.