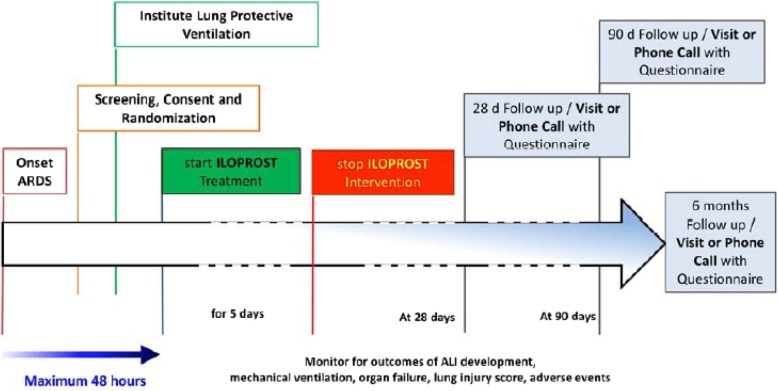
Fig. 1.
Trial protocol and intervention scheme. After screening and determination of eligibility, patients will be included after a maximum of 48 h after the onset of ARDS. Within this time period, screening, consent, and randomization will be initialized. In addition, lung-protective ventilation will be instituted. After randomization, iloprost 3 × 20 μg (intervention) or NaCl 0.9% (control) will be administered for 5 days through a standard ultrasound nebulizer. Daily recordings will be made with respect to the development of the PaO2/FiO2 ratio and the severity of ARDS, organ failure, lung injury, and potential adverse events. The treatment with iloprost or NaCl (0.9%) will be stopped after 5 days. The follow-up period will then continue up to 90 days and 6 months to determine the outcome, quality of life, and pulmonary/secondary organ function