Abstract
Background
Vitamin D (VD) and phosphate (Pi) load are considered as contributors to cardiovascular disease in chronic kidney disease and the general population, but interactive effects of VD and Pi intake on the heart are not clearly illustrated.
Methods
We fed normal male rats with three levels of dietary VD (100, 1100 or 5000 IU/kg chow) and Pi (0.2, 0.6 or 1.6%) (3X3 design) for 8 weeks and examined renal and cardiac function and histology.
Results
High dietary Pi decreased plasma and renal Klotho and plasma 25-hydroxyvitamin D, and increased plasma Pi, fibroblast growth factor 23 and parathyroid hormone without affecting renal function, while low Pi increased plasma and renal Klotho. Both low and high VD diets enhanced high Pi-reduced Klotho expression. Low dietary VD reduced-plasma Klotho was rescued by a low Pi diet. High dietary Pi reduced-cardiac ejection fraction was not modified by a low or high VD diet, but the dietary VD effects on cardiac pathologic changes were more complex. High dietary Pi-induced cardiac hypertrophy was attenuated by a low VD and exacerbated by a high VD diet. In contrast, high dietary Pi -induced cardiac fibrosis was magnified by a low VD and attenuated by a high VD diet.
Conclusions
High Pi diet induces hypertrophy and fibrosis in left ventricles, a low VD diet accelerates high Pi-induced fibrosis, and a high VD diet exacerbated high Pi -induced hypertrophy. Therefore, cardiac phosphotoxicity is exacerbated by either high or low dietary VD in rats with normal kidney function.
Keywords: cardiac fibrosis, cardiac hypertrophy, Klotho, phosphate, vitamin D
INTRODUCTION
Extracellular phosphate (Pi) serves as an exchange pool among various Pi depots (intracellular, extracellular and calcified tissues) and Pi-regulating organs (kidney, intestine and bone) and is tightly regulated by a network of calciophosphotropic hormones—parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), 1,25-dihydroxyvitamin D [1,25(OH)2D] and Klotho [1, 2]. Disturbed Pi metabolism measured clinically as either high or low serum Pi is associated with a variety of acute and chronic diseases. Epidemiological studies have shown that high serum Pi is a risk factor for cardiovascular morbidity and mortality in both patients with chronic kidney disease (CKD) and the general population [2–4]. The nephrotoxic, cardiotoxic and vasculotoxic potentials of Pi overload have been demonstrated in animals [5–8]. In humans, Pi-lowering therapy in predialytic CKD patients did not show unequivocal clinical benefit, suggesting that the vasculopathy and progressive nature of CKD are not a simple function of dietary Pi intake or serum Pi levels [9, 10]. Therefore other factors such as vitamin D (VD) status may modulate Pi toxicity (see below). Equivalent intervention data in the general population are not available.
The active form of VD, 1,25(OH)2D, contributes to Pi homeostasis along with PTH, FGF23 and Klotho [2, 11, 12]. In addition to mineral metabolism, VD has a panoply of non-mineral effects based on both epidemiologic and laboratory evidences [13–16]. For the cardiovascular system, VD supplement may be beneficial for hypertension [17] and left ventricular hypertrophy [18, 19]. VD deficiency is prevalent in CKD patients, which coexists with Pi overload, and is associated with CKD–mineral and bone disorder [20, 21]. VD supplementation has been prescribed for CKD patients and VD-deficient patients with normal kidney function routinely for decades, but improvement of clinical outcomes and life quality are still debated [16]. Overdosage of calcitriol is associated with side effects including vascular calcification [22]. Currently VD is neither proven nor approved to treat cardiovascular disease (CVD) [21].
The effect of VD on cardiovascular complications, in particular in the context of high Pi, and the interaction between VD and Pi in normal renal function is largely unknown. It is conceivable that VD status can modulate phosphotoxicity. On the one hand, VD exerts a cardioprotective effect against phosphotoxicity based on VD’s putative effects on blood pressure and/or left ventricular hypertrophy. On the other hand, VD might aggravate phosphotoxicity by increasing the absorption of exogenous Pi load by virtue of VD’s stimulatory effect on intestinal Pi absorption [23]. To explore the combined and interactive effects of dietary VD and Pi intake on cardiac morphometry and function, we fed normal male Sprague Dawley rats with three levels of dietary VD (low, 100; normal, 1100; high, 5000 IU/kg chow) and Pi (low, 0.2; normal, 0.6; high, 1.6 g/100 g chow) (3 × 3 design; nine groups) in rodent chows for 8 weeks (Supplementary data, Figure S1 and Supplementary data, Table S1) and examined the mineral parameters and hormones and cardiac function and structure.
MATERIALS AND METHODS
Animal experiments
Experiments were performed in male Sprague Dawley rats at the age of 8–10 weeks (Charles River Laboratories, Wilmington, MA, USA). Animals were housed in a temperature-controlled room with a 12:12 h light:dark cycle with ad libitum access to tap water and standard rodent chow prior to assignment of experimental diets. After a 1-week adaptation in the University of Texas Southwestern Medical Center Animal Resource Center, rats were randomly divided into nine (3 × 3) groups of customized rodent pellet diets (Supplementary data, Figure S1 and Supplementary data, Table S1; Harlan Teklad, Indianapolis, IN, USA) for 8 weeks. Each group was composed of six rats. We chose a 0.6% inorganic Pi and 1100 IU/kg VD chow (TD120555, Harlan Teklad), identical to natural normal rodent chow (TD2016, Harlan Teklad), to be the control diet. All rats had free access to tap water and experimental rodent chows. The rats’ body weight at the beginning (0 week) and at the end of the experiment (8 weeks) was recorded (Supplementary data, Table S2).
All animal work was conducted following the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Southwestern Medical Center.
Blood, urine, kidney and heart samples collection
Prior to and at 8 weeks after dietary treatment, rats were placed individually in metabolic cages (Hatteras Instruments, Cary, NC, USA) for 24-h urine collections. The daily food intake by individual rat was also recorded (Supplementary data, Table S2). Urine samples were centrifuged (1000 g × 10 min at 20°C) and the supernatants were stored at −20°C until analysis. Rats were anesthetized with isoflurane and blood samples were collected in heparinized tubes and centrifuged (3000 g × 5 min at 4°C) and plasma was separated and stored at −80°C until analysis. At termination, rats were euthanized under anesthesia and the hearts and kidneys were isolated and sliced. One slice was fixed with 4% paraformaldehyde for histological and immunohistochemical studies; the remaining parts of the hearts and kidneys were instantly snap-frozen in liquid N2 and stored at −80°C for RNA or protein extraction and analysis.
Plasma and urine chemistry, plasma Klotho, FGF23, 25(OH)D and PTH assays
Plasma and urine chemistries of animals were analyzed by a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis, Rochester, NY, USA). Plasma and urine creatinine concentrations were measured with a P/ACE MDQ Capillary Electrophoresis System and photodiode detector (Beckman-Coulter, Fullerton, CA, USA) at 214 nm [6].
Plasma Klotho was measured with an immunoprecipitation–immunoblot assay [6, 24]. Soluble Klotho protein, synthesized in our laboratory containing the ectodomain of mouse Klotho (amino acid 31-982), was used for calibration. Briefly, 100 μL of rat plasma were subjected to immunoprecipitation with 1 μg of anti-Klotho synthetic antibody sb106 [6, 8, 24, 25] and the immune complex was captured with M2 beads and was eluted with 2.5× Laemmli sample buffer after washing. Proteins were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membranes and immunoblotted with rat anti-human Klotho monoclonal antibody (KM2076, TransGenic, Kobe, Japan). Intact PTH was quantified by enzyme-linked immunosorbent assay (ELISA) (Alpco, Salem, NH, USA), 25-hydroxyvitamin D [25-(OH)D] by enzyme immunoassay (Immunodiagnostic Systems, Scottsdale, AZ, USA) and intact FGF23 by ELISA kit (Kainos, Tokyo, Japan) following the manufacturer’s instructions.
Cardiac magnetic resonance imaging (MRI)
After 8 weeks of dietary treatment, cardiac function was evaluated by MRI using a 7T small animal magnetic resonance scanner (Varian, Palo Alto, CA, USA) with a 38-mm birdcage radiofrequency (RF) coil as described [8, 26]. Under inhalational anesthesia (1.5–2% isoflurane), MRI acquisitions were gated by both cardiac and respiratory triggering [8, 26]. Epicardial and endocardial borders were manually traced by a blinded operator for calculation of left ventricular end-systolic and end-diastolic volume using ImageJ software. Left ventricular ejection fraction and left ventricular wall thickness at systolic phase and diastolic phase were calculated as described [8, 26]. One investigator performed MRI acquisition and two analyzed the data independently; both were blinded to the experimental conditions. Average data from the two analysts were used.
Assessment of cardiomyocyte size
To measure cardiomyocyte surface areas, paraffin-embedded sections were labeled with Alexa Fluor 555–conjugated wheat germ agglutinin (WGA; Invitrogen, Carlsbad, CA, USA) as described previously [8, 26]. Immunofluorescent images were taken on a Zeiss laser scanning microscope (Carl Zeiss Micro-Imaging, Thornwood, NY, USA). ImageJ software was used to quantify the cross-sectional cell surface area along the midchamber free wall based on WGA-positive staining [8, 26].
Chemicals and antibodies
The following antibodies (Ab) were used for immunoblotting and/or immunohistochemistry: rat monoclonal anti-αKlotho antibody (KM2076, TransGenic, Kobe, Japan), mouse monoclonal anti-Flag M2 (Sigma-Aldrich, St. Louis, MO, USA), mouse monoclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich), mouse monoclonal antibody against α-smooth muscle actin (α-SMA; Sigma-Aldrich), mouse monoclonal antibody against α-actinin (α-actinin; Sigma-Aldrich), rabbit polyclonal antibody against MYH6 [α-myosin heavy chain (MHC); Santa Cruz Biotechnology, Dallas, TX, USA] and mouse monoclonal antibody against β-MHC (Sigma-Aldrich). Secondary antibodies coupled with horseradish peroxidase for immunoblotting or with fluorescein isothiocyanate, Alexa Fluor or Cy5 and Syto-61 fluorescent nuclear acid stain for immunohistochemistry was purchased from Molecular Probes/Invitrogen (Eugene, OR, USA). Rhodamine-labeled WGA was purchased from Vector Laboratories (Burlingame, CA, USA).
Immunohistochemistry and immunoblot of hearts
Four-micrometer sections of paraffin-embedded heart tissues were deparaffinized for immunofluorescence. After blocking, primary Abs were incubated at 4°C overnight followed by secondary antibodies conjugated to fluorescein isothiocyanate or Alexa red. Sections were visualized using a Zeiss LSM-510 laser scanning microscope.
Heart lysates from the left ventricles were prepared as described [8, 26]. Thirty micrograms of total protein lysate was solubilized in Laemmli’s buffer, fractionated by SDS-PAGE, transferred to PVDF membrane and blotted using specific primary antibodies and monoclonal mouse antibody for GAPDH. Primary antibodies were incubated overnight at 4°C. After washing, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase (Thermo Fisher Scientific, Piscataway, NJ, USA). Specific signal was visualized using Pierce ECL immunoblotting substrate (Thermo Fisher Scientific).
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from mouse kidneys using the RNAeasy kit (Qiagen, Germantown, MD, USA). Complimentary DNA was generated with oligo-dT primers using the SuperScript III First-Strand Synthesis System (Invitrogen). Primers used for quantitative PCR (qPCR) were rat Klotho forward-5′-AACCAGCCCCTTGAAGGGAC-3′ and Klotho reverse-5′-TGCACATCCCACAGATAGAC-3′; GAPDH forward-5′-CAGTGCCAGCCTCG TCTCAT and GAPDH reverse-5′-AGGGGCCATCCACAGTCTTC-3′, rat α-actinin forward-5′-CCTGCTGTTGGACCCGGC-3′ and α-actinin reverse-5′-GGAAGTCCTCA TCGATGTTC-3′, rat α-SMA forward-5′-GATCACCATCGGGAATGAACGC-3′ and α-SMA reverse-5′-CTTAGAAGC ATTTGCGGTGGAC-3′; rat α-MHC forward-5′-TGTGGT GCCTCGTTCCA-3′ and α-MHC reverse-5′-TTTCGGAGGTACTGGGCTG-3′; and rat β-MHC forward-5′-GCATTCT CCTGCTGTTTCCTT-3′ and β-MHC reverse-5′-TGGATT CTCAAACGTGTCTAGTGA-3′. Real-time PCR was conducted with the standard condition of SYBR Green Master Mixes (Roche Applied Science, Mannheim, Germany) [27] in triplicate for each sample. Data were expressed at an amplification number of 2−ΔΔCt normalized to GAPDH and compared with controls.
Statistical analyses
Data are expressed as means ± standard deviation (SD). Statistical analysis was performed with an unpaired Student's t-test or one-way analysis of variance (ANOVA) followed by a post hoc Student–Newman–Keuls test when applicable. P-values ≤ 0.05 were considered statistically significant.
Compliance with ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All animal procedures were approved by IACUC of the University of Texas Southwestern Medical Center and performed in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
RESULTS
Pi and calcium homeostasis
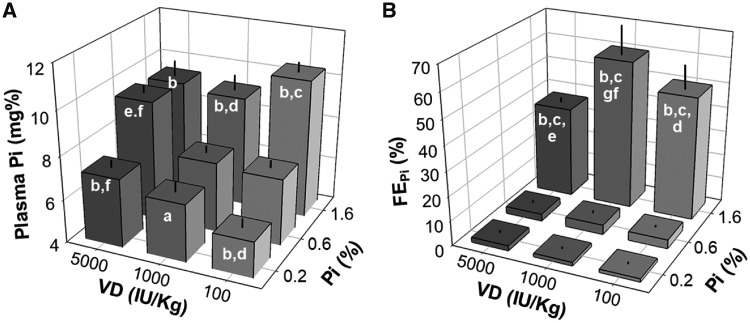
To investigate the effects of dietary Pi, VD and the interactive effects of the two on the heart, we fed normal male Sprague Dawley rats with nine dietary combinations (Supplementary data, Figure S1 and Supplementary data, Table S1). High Pi increased plasma Pi regardless of dietary VD levels (Figure 1A). A high VD diet increased plasma Pi only in the rats fed a low or normal Pi diet and not in ones on a high Pi diet who already had high plasma Pi (Figure 1B). As expected, lower fractional excretion of phosphate (FEPi) was seen in the low Pi diet, and higher FEPi in the high Pi diet rats (Figure 1B). High dietary Pi–induced elevation of FEPi was blunted by a high VD diet.
FIGURE 1.
Effects of sustained (8 weeks) variation of Pi and VD intake in normal rats on (A) plasma Pi concentrations and (B) renal FEPi. Data are the means ± SDs from six rats in each of the nine groups. Statistical significance was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. Significance tests are as follows: (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.01 versus 0.2% Pi at the same VD diet, (d) P < 0.05, (e) P < 0.01 versus 1100 IU/kg at the same Pi diet, (f) P < 0.05, (g) P < 0.01 versus 100 IU/kg at the same Pi diet.
The effect of dietary Pi and VD on plasma calcium (Ca2+) is modest compared with their effect on plasma Pi. Only in the normal VD diet group did the low and high Pi diet change plasma Ca2+ levels. Note that the low VD diet induced a statistically significant but numerically modest increase in plasma Ca2+ in rats fed with normal or high Pi diets (Supplementary data, Figure S2).
Calciophosphotropic hormones
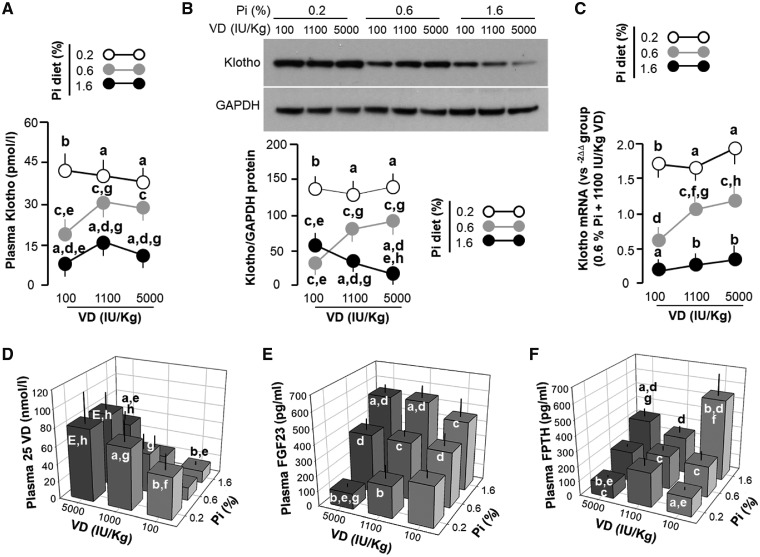
The low Pi diet, which lowered plasma Pi, increased plasma Klotho concentration irrespective of dietary VD levels, whereas the high Pi diet decreased plasma Klotho, which was further reduced by the low VD diet (Figure 2A). The reduction of plasma Klotho with the low VD diet in rats during normal or high Pi intake was fully prevented when rats were fed a low Pi diet (Figure 2A). Unexpectedly, a high VD diet did not elevate plasma Klotho in rats fed a low or high Pi diet compared with rats fed a normal VD diet (Figure 2A). Therefore a low VD and high Pi diet independently and synergistically downregulated plasma Klotho.
FIGURE 2.
Changes in (A) plasma and (B) renal Klotho, (C) renal Klotho mRNA expression and in plasma (D) 25(OH)D, (E) FGF23 and (F) PTH in rats. Data are presented as means ± SDs from six rats in each of the nine groups. Statistical significance was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. Significance tests are as follows: (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05, (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05, (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05, (h) P < 0.01 versus 100 IU/kg at the same Pi diet.
The kidney is the major organ contributing to soluble Klotho in the circulation [28, 29]. We examined Klotho protein and messenger RNA (mRNA) expression in the kidney and found that the low Pi diet–induced increase in plasma Klotho was largely congruent with the associated high renal Klotho protein (Figure 2B). Moreover, levels of renal Klotho protein (Figure 2B) and mRNA (Figure 2C) were lower in the rats fed a high Pi diet compared with rats on a normal Pi diet in the context of high or normal VD diet. Notably, the effect of the low VD diet on renal Klotho protein expression was dependent on dietary Pi. Low VD decreased renal Klotho protein under normal Pi intake, had no effect under a low Pi diet and increased renal Klotho protein under a high Pi diet (Figure 2A–C). Interestingly, we saw an inhibitory rather than stimulatory effect of high dietary VD on the Klotho expression in the kidney possibly due to a very high dose of dietary VD.
Because kidney dysfunction leads to low Klotho expression in the kidney and low plasma Klotho [6, 8, 26, 30, 31], we examined kidney function in the nine groups of rats but did not see differences in plasma creatinine, creatinine clearance and urine output (Supplementary data, Figure S3). Therefore the change in renal and soluble Klotho levels due to Pi and VD diets did not result from alterations of kidney function, that is, glomerular filtration rate.
As expected, there was lower plasma 25(OH)D in low VD–fed rats at all levels of dietary Pi. The high Pi diet decreased and the low Pi diet increased plasma 25(OH)D levels with the exception of the 5000 IU/kg VD groups, where 25(OH)D levels were maintained high (Figure 2D). The low Pi diet reduced and the high Pi diet increased plasma FGF23 levels, whereas the high VD diet increased plasma FGF23 levels except for rats fed the low Pi diet, indicating that low Pi diet–induced suppression of FGF23 synthesis overcomes the stimulatory effect of the high VD diet (Figure 2E). When one compares plasma FGF23 levels between the high VD + low Pi versus the low VD + low Pi diet, plasma FGF23 levels were negatively correlated to VD content in rodent chow (Figure 2E). In general, the change in plasma PTH (Figure 2F) was similar to that of plasma FGF23 in rats on the low Pi and high Pi diets (Figure 2E). Dietary Pi increased PTH at all dietary VD levels while VD suppressed PTH, mainly in the background of high Pi, and it seems to have an ‘inverted U’ relationship with PTH when given a low Pi diet (Figure 2F).
Effect of dietary Pi and VD on cardiac function and morphometry
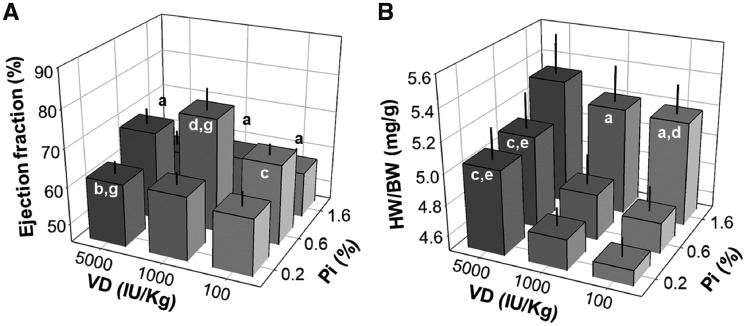
We previously showed that high dietary Pi induces pathologic cardiac remodeling in normal rats and mice and also aggravated uremic cardiomyopathy in CKD mice [8]. CKD patients are at high risk for CVD, which is frequently associated with VD deficiency. VD therapy can potentially reduce adverse cardiovascular events and increase survival in hemodialysis patients. The possible protective role of VD is postulated to extend beyond its effect on mineral metabolism [21], although there are adverse effects of VD receptor agonists. Furthermore, the effect of dietary VD on the heart in normal rodents is not known. We examined cardiac function by MRI after 8-weeks of dietary Pi and VD feeding and found that both the low Pi and high Pi diets reduced the ejection fraction and both the low VD and high VD diet reduced the ejection fraction when rats were on a normal Pi diet, with the highest ejection fraction in rats on a 0.6% Pi and 1100 IU/kg VD diet (Figure 3A). Therefore, both the low and high Pi diets decreased cardiac function, which cannot be attenuated by either low or high VD. On the other hand, left ventricular free wall thickness at either the systolic or diastolic phase was not modified by any of the diets as compared with the control diet (Supplementary data, Figure S4). The heart weight:body weight ratio was significantly increased in rats fed a high Pi diet compared with normal and low Pi diets at all levels of VD. It should be noted that daily food intake, urinary sodium and potassium excretion rate and body weight at the end of the experiment (Supplementary data, Figures S2 and S3) were similar among all nine groups, indicating that the high Pi diet did not affect dietary intake, but in fact induced cardiac hypertrophy. Interestingly, high VD also increased heart weight, and this increase was exacerbated by high Pi and alleviated by a low Pi diet (Figure 3B).
FIGURE 3.
Effects of dietary Pi and VD on cardiac function and heart weight in rats. (A) Ejection fraction, (B) heart weight per body weight (HW/BW). Data are presented as means ± SDs from six rats in each of the nine groups. Statistical significance among nine groups was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05, (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05, (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05, (h) P < 0.01 versus 100 IU/kg at the same Pi diet.
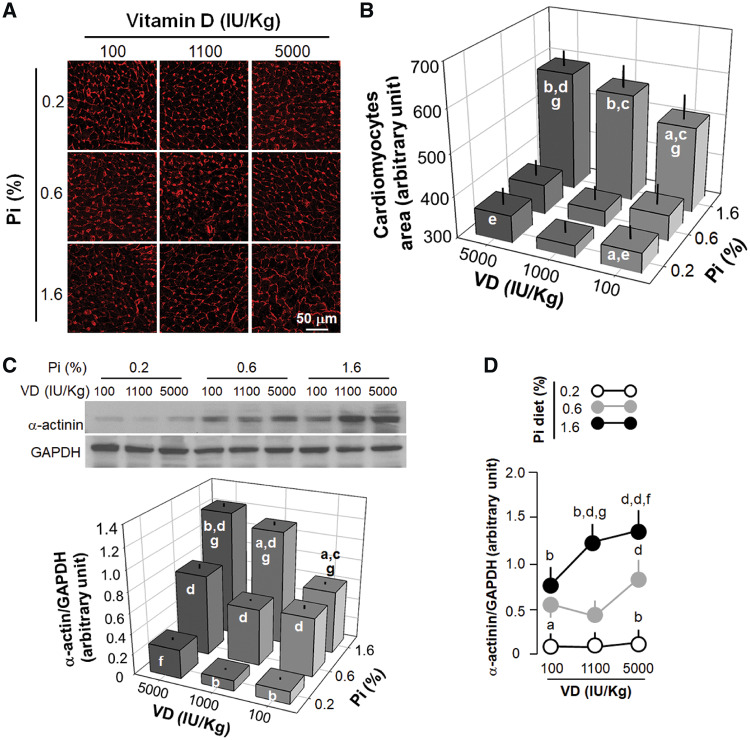
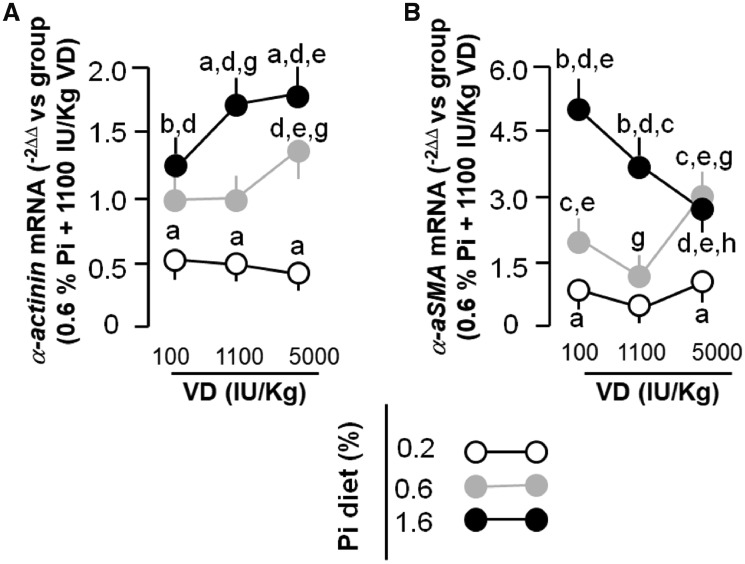
The outlining of cardiomyocytes with WGA clearly indicated that the high Pi diet induced cardiomyocyte hypertrophy, which was worsened by both low and high VD (Figure 4A and B). WGA was recently proposed to possibly label scar areas [32], but the staining pattern of scars are completely different from cardiomyocyte cell membranes. The staining pattern we observed was the boundaries of cardiomyocytes. Compared with the low and normal VD diets, the high VD diet alone induced cardiomyocyte hypertrophy irrespective of the concentration of Pi in the diet (Figure 4A and B). Quantitative analysis of α-actinin protein (Figure 4C and D) and mRNA expression (Figure 5A) were all in line with the changes in cardiomyocyte cell size based on WGA staining. Therefore both the high Pi diet and high VD diet could induce cardiac hypertrophy.
FIGURE 4.
Effects of dietary modulation (8 weeks) of Pi and VD on histologic changes and hypertrophic markers in rat cardiomyocytes. (A) Microscopic image of WGA stain in the left ventricles of rats. (B) Quantification of cardiomyocyte area. (C) α-Actinin protein expression in the left ventricular lysates. (D) Quantification of α-actinin and α-SMA protein expression levels in the left ventricular lysates. Scale bar = 50 μm. Data are presented as means ± SDs from six rats in each group. Statistical significance among the nine groups was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05, (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05, (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05.
FIGURE 5.
Cardiac hypertrophic and fibrotic markers in rats. At 8 weeks after different dietary regimens, all rats were anesthetized and their hearts were harvested. Total RNA from the left ventricles was used for quantitative analysis of (A) α-actinin and (B) α-SMA mRNA. Data are presented as means ± SDs from six rats in each group. Statistical significance among the nine groups was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05, (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05, (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05, (h) P < 0.01 versus 100 IU/kg at the same Pi diet.
We did not see any positive Von Kossa staining in the kidney, heart and aorta in all rats from nine groups (data not shown), which is different from the high dietary Pi effect on uremic rodents. The discrepant effect of high dietary Pi between normal and uremic rodents will be discussed further.
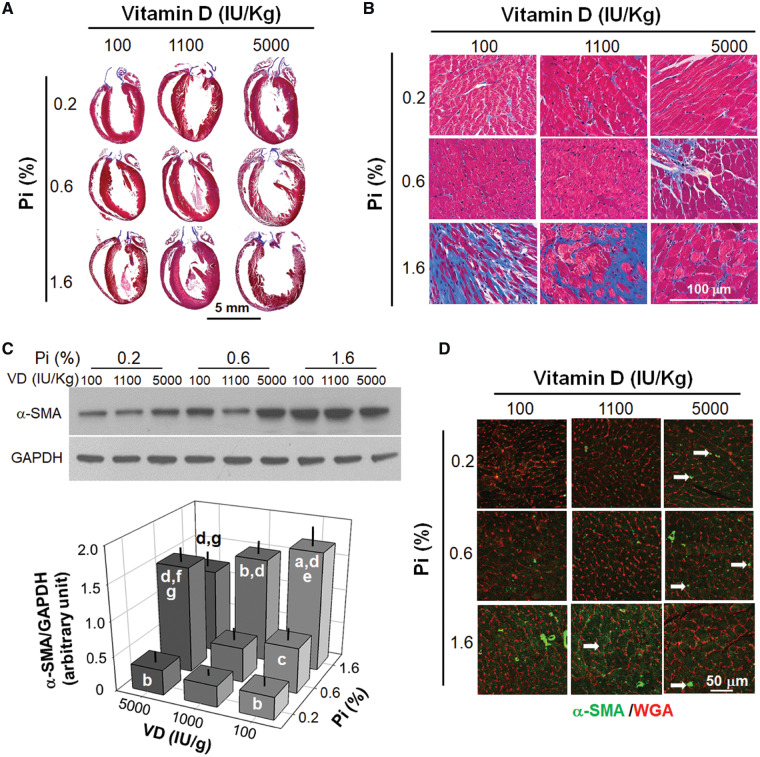
Effect of dietary Pi and VD on cardiac fibrosis
We examined if dietary Pi and VD influence cardiac fibrosis with Masson’s trichrome stain. We found more fibrosis, especially on the mitral valve leaflet (Figure 6A and B), in rats fed a high Pi diet. However, in contrast to cardiac hypertrophy, cardiac fibrosis was attenuated by high dietary VD, and this rescue was particularly pronounced in the high Pi group (Figure 6A, right column), under a normal Pi diet, a high VD diet induced ventricular valve fibrosis (Figure 6B, middle column). Of note is that rats fed a low Pi and low VD diet had only slight fibrosis in the left ventricle compared with rats fed with normal Pi and normal VD chow (Figure 6B).
FIGURE 6.
Effects of dietary (8 weeks) Pi and VD on fibrogenic markers in rat heart. (A) Gross image of trichrome stain in the heart. Scale bar = 5 mm. (B) Microscopic image of trichrome stain in the heart. Scale bar = 100 μm. (C) α-SMA protein expression (a fibroblast marker) in left ventricular lysates. Upper panel: representative blots for α-SMA and GAPDH. Bottom panel: quantification of α-SMA protein expression. (D) Microscopic image of α-SMA staining costained with WGA. Scale bar = 50 μm. Data are presented as means ± SDs from six rats in each group. Statistical significance among nine groups was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05, (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05, (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05.
To further confirm the fibrotic changes in the heart, we examined the expression of α-SMA, a myofibroblast marker, and found that α-SMA was increased in the left ventricular lysate by a high Pi diet and its increase was abolished by a high VD diet (Figure 6C). In contrast, a high VD diet upregulated α-SMA protein in the hearts of rats fed a normal Pi diet and did not have any impact on α-SMA expression in the rats fed a low Pi diet (Figure 6C). qPCR examining α-SMA mRNA expression in the left ventricle showed a comparable change to the α-SMA protein (Figure 5B). Immunofluorescent images showed that rats fed a high Pi diet had higher α-SMA expression, most evident in cardiomyocytes and less so in cardiac fibroblasts (Figure 6D), suggesting that Pi-induced cardiac fibrosis might be derived from dedifferentiated cardiomyocytes. The high VD diet rats had higher α-SMA expression in resident cardiac fibroblasts (Figure 6D), indicating that VD and Pi may have distinct mechanisms to induce cardiac fibrosis through targeting two different cell lineages in the heart. The detailed mechanisms behind the difference remain to be explored.
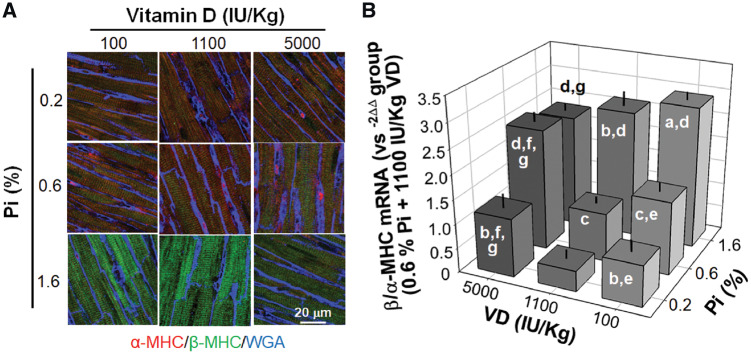
Effect of dietary Pi and VD on aberrant expression of β/α-MHC in the left ventricle
Dysregulation of β/α-MHC is associated with pathologic remodeling in rodents. We conducted quantitative and qualitative analyses of β/α-MHC expression patterns with immunofluorescence and qPCR. Rats fed a high Pi diet had a higher ratio of β/α-MHC expression, which was mildly attenuated by high dietary VD but accelerated by low dietary VD (Figure 7A and B). Interestingly and unexpectedly, both low and high dietary VD elevated the β/α-MHC expression ratio compared with normal dietary VD when rats were fed with normal and low Pi chows (Figure 7A and B). Thus a synergistic effect on upregulation of the β/α-MHC ratio in rats fed a high Pi plus low VD diet supports pathologic cardiac remodeling.
FIGURE 7.
Effects of dietary Pi and VD (8 weeks) on β/α-MHC mRNA and protein expression in rat hearts. (A) Microscopic image of β/α-MHC protein expression in left ventricles costained with WGA. (B) β/α-MHC transcripts expression. Scale bar = 20 μm. Data are presented as means ± SDs from six rats in each of the nine groups. Statistical significance among nine groups was analyzed with one-way ANOVA followed by a post hoc Student–Newman–Keuls test when applicable. (a) P < 0.05, (b) P < 0.01 versus 0.6% Pi at the same VD diet, (c) P < 0.05 (d) P < 0.01 versus 0.2% Pi at the same VD diet, (e) P < 0.05 (f) P < 0.01 versus 1100 IU/kg at the same Pi diet, (g) P < 0.05.
DISCUSSION
This study examined the role of dietary Pi and VD and their interaction in affecting cardiac morphology and function in normal rats. Eight weeks of sustained alteration in Pi and VD intake did not measurably affect renal function, allowing assessment of dietary Pi and/or VD effects on the heart and on the phosphocalcitropic endocrine axis independent of renal dysfunction. The important findings of the present study are (i) ‘effects of Pi and VD intake on phosphocalcitropic hormones’: high dietary Pi decreases plasma and renal Klotho and increases plasma FGF23 and PTH, while low dietary Pi exerts the opposite action. Decreased VD intake reduces plasma and renal Klotho in animals concomitantly fed a normal or high Pi diet. (ii) ‘Effects of dietary Pi and VD intake on cardiac function and cardiac remodeling’: a high Pi diet decreases the cardiac ejection fraction evaluated by cardiac MRI and histologically characterized by myocardial hypertrophy (worsened by high VD) and fibrosis (attenuated by high VD), whereas a low Pi diet also decreases cardiac contractility with fibrosis, but to a much lesser extent than the high Pi diet.
Phosphotoxicity is considered an independent risk for cardiovascular events in CKD patients and in the general population based on epidemiologic data [33]. These studies and animal experiments showed that high dietary Pi intake is associated with high serum Pi in both the CKD and non-CKD populations [34]. High dietary Pi intake is associated with high morbidity and mortality of CVD in the CKD population [35]. This effect is also demonstrable in the general population [36] and has raised public health concerns in view of the increases in Pi intake in large part due to increased consumption of processed food and the use of Pi as a food preservative. Dietary Pi–induced endothelial dysfunction and injury [3], vascular calcification [6] and increases in blood pressure could induce hypertrophic cardiac remodeling mainly by increasing peripheral vascular resistance. In rats with normal renal function, modestly high dietary Pi (1.2%) was demonstrated to augment cardiovascular and sympathetic responses to the muscle exercise pressor reflex and stimulate blood pressure, heart rate and renal sympathetic nerve activity [5]. In addition, chronic high dietary Pi intake in healthy young human subjects significantly and reversibly increased the mean arterial pressure and pulse rate associated with increased sympathoadrenergic activity [34]. Mice with and without CKD, albeit with an extremely high chronic dietary Pi intake (2% for 8 weeks), exhibit both vascular calcification with normal renal function and accelerated progression of preexisting CKD and uremic cardiomyopathy [6, 8]. High dietary Pi loading–induced cardiac remodeling in rodents with normal kidney function has clinical implications. The Pi additive in processed food known as nonphytate Pi can potentially have deleterious cardiovascular effects.
The absence of ectopic calcification in the heart, aorta and kidney in the rats fed with high dietary Pi appears to be contrary to the induction of vascular calcification in uremic rodents by Pi [6, 37]. The discrepancy can be for two reasons. First is that rats were loaded with modest dietary Pi (1.6%) only for a moderate period (8 weeks). Second is that experimental rats had normal renal function and histology. Therefore high Pi–induced vascular calcification might need other triggers or accelerators that were absent in our experimental rats.
Low plasma Klotho associates with high morbidity and high mortality of cvd in CKD patients and in the general population [6, 24, 38]. This study shows that low renal and plasma Klotho is a consequence of a high Pi diet, while increases in Klotho result from a diet low in Pi, which confirmed the effects of dietary Pi on the modulation of renal Klotho [8, 26]. It is conceivable that cardiac phosphotoxicity may be attributable, at least in part, to the Klotho-deficient state. However, one recent study in normal human subjects showed small increases in plasma and urine Klotho levels within the normal range after 6 weeks of a modest increase in plasma Pi [34]. The mechanisms of this apparent species difference need clarification in the future. On the other hand, there was lower cardiac ejection and fibrosis in rats fed a low Pi diet, which is similar to clinical observations, in contrast to the condition of CKD in which lowering Pi intake has been repeatedly shown to exert beneficial effects [39]. Moreover, hypophosphatemia resulting from any genetic or acquired diseases is associated with low energy metabolism in skeletal muscles [40] and in cardiac muscles, as shown in this study. Therefore the deleterious effect of 8 weeks of low dietary Pi on the heart in rats with normal renal function is of concern.
Pi-induced elevation of circulating FGF23 is well established [5, 8, 41, 42]. High FGF23 may induce cardiomyopathy both directly and indirectly [25, 43]. The present in vivo Pi-modulating experiments cannot rule out a pathogenic role of high FGF23 in cardiac remodeling induced by high Pi. PTH is another uremic toxin and hyperparathyroidism has been shown to be associated with left ventricular hypertrophy in renal patients and the general population [44]. Although this study cannot confirm or refute a direct effect of high PTH induced by chronic Pi loading on cardiac remodeling, it at least provides in vivo evidence about the association of dietary Pi–upregulated PTH with cardiac remodeling.
VD deficiency is linked to increased mortality and may be a detrimental factor in left ventricular hypertrophy and cardiac dysfunction in CKD patients [18, 19]. We found that low VD in combination with a low Pi diet stimulates FGF23, providing at least one potential mechanism for the high cardiac morbidity and ventricular hypertrophy in VD deficiency. In further support, higher 25(OH)D levels were associated with significantly improved survival in CKD patients [45] and VD treatment was associated with a reduction in cardiovascular death and an improvement in left ventricular hypertrophy in dialysis patients [20, 46] in most, but not all, studies [47]. This study does not confirm the beneficial effect of high dietary VD on high Pi diet–induced cardiovascular toxicity. In contrast, a high VD diet appears to exacerbate Pi toxicity on the cardiovascular system. Therefore extremely high VD loading (5000 IU/kg) is harmful.
On the basis of high Pi–induced cardiac hypertrophy, both low and high dietary VD reduce the ejection fraction with a dilated left ventricle, resembling dilated cardiomyopathy in CKD patients [48]. Unfortunately our MRI technique without contrast medium did not allow us to provide precise left ventricular volume. Furthermore, high VD induces cardiac hypertrophy and fibrosis while low dietary VD induces modest cardiac atrophy in rats with a normal Pi diet. Therefore either extremely low or high dietary VD is harmful to the rat heart when renal function is not impaired. These observations might also explain, at least in part, the observation in humans that excessively high VD supplementation augmented the risk of hypercalcemia, vascular calcification and reduced survival in CKD patients [19].
Left ventricular fibrosis is a striking feature of pathologic cardiac remodeling, impairs cardiac function and induces arrhythmia [49]. Cardiac fibrosis can be derived from endothelial dedifferentiation [50], resident cardiac fibroblast proliferation [51] or cardiomyocyte dedifferentiation [52]. Because high Pi diet induced massive fibrotic changes mainly in cardiomyocytes, whereas high VD diet induced fibrosis in cardiac fibroblasts, there are distinct cellular targets for a fibrotic process in the heart for high Pi as compared with low VD. The molecular mechanism and signaling pathway of cardiac fibrosis induced by Pi or VD remain to be illustrated.
There are several limitations in this in vivo experiment. First, we were unable to measure plasma 1,25(OH)2D because of limited plasma samples. Although we already confirmed that both low dietary VD and high dietary Pi induced VD deficiency in the rats, we are still unable to determine if the changes in plasma 1,25(OH)2D are similar to those of plasma 25(OH)D. Second, we did not find a stimulatory effect of high dietary VD on renal and plasma Klotho, probably due to an extremely high dose of 25(OH)D, which may reach toxic levels. Third, although we found that rats treated with a high Pi diet had plasma PTH, this study does not allow us to conclude the detrimental effect of high PTH on pathologic cardiac remodeling in rats fed a high dietary Pi.
In conclusion, a high Pi diet induces cardiac dysfunction and pathologic remodeling consisting of cardiac hypertrophy and fibrosis and reduces renal and circulating Klotho, which can synergistically enhance Pi cardiotoxicity. High FGF23 may also participate in pathologic cardiac remodeling in the rats with high Pi. A low VD diet accelerates high Pi–induced cardiotoxicity, whereas a high VD diet attenuates cardiac fibrosis but exacerbates cardiac hypertrophy. Therefore this study provides experimental evidence to demonstrate a complex interactive relationship between Pi and VD and shows that the overall effect of either extremely high or low dietary VD intake is harmful to the heart when animals are chronically Pi loaded.
Supplementary Material
ACKNOWLEDGEMENTS
The authors particularly thank John Poindexter for professional assistance in the graphics preparation, Dr Shanrong Zhang for conducting MRI studies at Mouse MRI Core in George O'Brien Kidney Research Center. UT Southwestern Medical Center and Dr. Aktar Ali for expert technical assistance.
FUNDING
The study was supported in part by a grant from the Swiss National Science Foundation (National Center of Competence in Research Kidney), the National Institutes of Health (R01-DK091392, R01-DK092461), the George O’Brien Kidney Research Center at the University of Texas Southwestern Medical Center (P30-DK079328), Charles and Jane Pak Center Innovative Research Support and the Pak-Seldin Center for Metabolic and Clinical Research. The authors also thank NIH for a shared instrumental grant to upgrade the animal MRI scanner (1S10OD023552-01).
AUTHORS’ CONTRIBUTIONS
M.C.H., O.W.M. and R.K. conceived and designed the research, analyzed data, edited and revised the manuscript and approved the final version of the manuscript. R.S., J.Y., J.Z., M.S., J.M. and B.F. performed the experiments. M.C.H., R.S., O.W.M. and R.K. interpreted the results of the experiments. M.C.H., J.Y., J.Z., M.S., J.M. and B.F. prepared the figures. M.C.H. drafted the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Hu MC, Shiizaki K, Kuro-O M et al. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 2013; 75: 503–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bian A, Xing C, Hu MC. Alpha Klotho and phosphate homeostasis. J Endocrinol Invest 2014; 37: 1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevens KK, Patel RK, Mark PB et al. Phosphate as a cardiovascular risk factor: effects on vascular and endothelial function. Lancet 2015; 385(Suppl 1): S10. [DOI] [PubMed] [Google Scholar]
- 4. Mathew S, Tustison KS, Sugatani T et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 2008; 19: 1092–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mizuno M, Mitchell JH, Crawford S et al. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 2016; 311: R39–R48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu MC, Shi M, Zhang J et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 2010; 24: 3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu MC, Shi M, Cho HJ et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015; 26: 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zoccali C, Mallamaci F. Moderator’s view: phosphate binders in chronic kidney disease patients: a clear ‘no’ at the moment, but stay tuned. Nephrol Dial Transplant 2016; 31: 196–199 [DOI] [PubMed] [Google Scholar]
- 10. Selamet U, Tighiouart H, Sarnak MJ et al. Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3-5: the Modification of Diet in Renal Disease Study. Kidney Int 2016; 89: 176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract 2014; 128: 1–10 [DOI] [PubMed] [Google Scholar]
- 12. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007; 117: 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Querfeld U. Vitamin D and inflammation. Pediatr Nephrol 2013; 28: 605–610 [DOI] [PubMed] [Google Scholar]
- 14. D'Arrigo G, Pizzini P, Cutrupi S et al. Vitamin D receptor activation raises soluble thrombomodulin levels in chronic kidney disease patients: a double blind, randomized trial. Nephrol Dial Transplant 2019; 34: 819–824 [DOI] [PubMed] [Google Scholar]
- 15. Abdel-Mohsen MA, El-Braky AA, Ghazal AAE et al. Autophagy, apoptosis, vitamin D, and vitamin D receptor in hepatocellular carcinoma associated with hepatitis C virus. Medicine (Baltimore) 2018; 97: e0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nwaohiri NK. Studying the effect of vitamin D supplementation on vascular function in CKD: a work in progress. J Am Soc Nephrol 2018; 29: 1578–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Legarth C, Grimm D, Wehland M et al. The impact of vitamin D in the treatment of essential hypertension. Int J Mol Sci 2018; 19:455–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Panizo S, Barrio-Vazquez S, Naves-Diaz M et al. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant 2013; 28: 2735–2744 [DOI] [PubMed] [Google Scholar]
- 19. Covic A, Voroneanu L, Goldsmith D. The effects of vitamin D therapy on left ventricular structure and function – are these the underlying explanations for improved CKD patient survival? Nephron Clin Pract 2010; 116: c187–c195 [DOI] [PubMed] [Google Scholar]
- 20. Moe SM. Vitamin D, cardiovascular disease, and survival in dialysis patients. J Bone Miner Res 2007; 22(Suppl 2): V95–V99 [DOI] [PubMed] [Google Scholar]
- 21. Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 2005; 68: 1973–1981 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Parra E, Rojas-Rivera J, Tunon J et al. Vitamin D receptor activation and cardiovascular disease. Nephrol Dial Transplant 2012; 27(Suppl 4): iv17–iv21 [DOI] [PubMed] [Google Scholar]
- 23. Rizzoli R, Fleisch H, Bonjour JP. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest 1977; 60: 639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker SL, Pastor J, Carranza D et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 2015; 30: 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faul C, Amaral AP, Oskouei B et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu MC, Shi M, Gillings N et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 2017; 91: 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Q, Moe OW, Garcia JA et al. Regulated expression of hypoxia-inducible factors during postnatal and postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 2006; 290: L880–L889 [DOI] [PubMed] [Google Scholar]
- 28. Lindberg K, Amin R, Moe OW et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 2014; 25: 2169–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu MC, Shi M, Zhang J et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 2016; 27: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu MC, Shi M, Zhang J et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 2010; 78: 1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panesso MC, Shi M, Cho HJ et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 2014; 85: 855–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emde B, Heinen A, Godecke A et al. Wheat germ agglutinin staining as a suitable method for detection and quantification of fibrosis in cardiac tissue after myocardial infarction. Eur J Histochem 2014; 58: 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol 2009; 4: 1136–1139 [DOI] [PubMed] [Google Scholar]
- 34. Mohammad J, Scanni R, Bestmann L et al. A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol 2018; 29: 2089–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarty MF, DiNicolantonio JJ. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutrition 2014; 30: 739–747 [DOI] [PubMed] [Google Scholar]
- 36. de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53: 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lau WL, Leaf EM, Hu MC et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 2012; 82: 1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Semba RD, Cappola AR, Sun K et al. Plasma Klotho and cardiovascular disease in adults. J Am Geriatr Soc 2011; 59: 1596–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ariyoshi N, Nogi M, Ando A et al. Hypophosphatemia-induced cardiomyopathy. Am J Med Sci 2016; 352: 317–323 [DOI] [PubMed] [Google Scholar]
- 40. Beck L, Karaplis AC, Amizuka N et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 1998; 95: 5372–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scanni R, vonRotz M, Jehle S et al. The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol 2014; 25: 2730–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagano N, Miyata S, Abe M et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int 2006; 69: 531–537 [DOI] [PubMed] [Google Scholar]
- 43. Grabner A, Amaral AP, Schramm K et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 2015; 22: 1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piovesan A, Molineri N, Casasso F et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999; 50: 321–328 [DOI] [PubMed] [Google Scholar]
- 45. Pilz S, Iodice S, Zittermann A et al. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 2011; 58: 374–382 [DOI] [PubMed] [Google Scholar]
- 46. Achinger SG, Ayus JC. The role of vitamin D in left ventricular hypertrophy and cardiac function. Kidney Int 2005; 67(Suppl 95): S37–S42 [DOI] [PubMed] [Google Scholar]
- 47. Pittas AG, Chung M, Trikalinos T et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010; 152: 307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Di Lullo L, Gorini A, Russo D et al. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med 2015; 5: 254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 2011; 89: 744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zeisberg EM, Tarnavski O, Zeisberg M et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007; 13: 952–961 [DOI] [PubMed] [Google Scholar]
- 51. Herum KM, Choppe J, Kumar A et al. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell 2017; 28: 1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takeshita K, Hayashi M, Iino S et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol 2004; 164: 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.