Abstract
Background
The use of clinical data in electronic health records for machine-learning or data analytics depends on the conversion of free text into machine-readable codes. We have examined the feasibility of capturing the neurological examination as machine-readable codes based on UMLS Metathesaurus concepts.
Methods
We created a target ontology for capturing the neurological examination using 1100 concepts from the UMLS Metathesaurus. We created a dataset of 2386 test-phrases based on 419 published neurological cases. We then mapped the test-phrases to the target ontology.
Results
We were able to map all of the 2386 test-phrases to 601 unique UMLS concepts. A neurological examination ontology with 1100 concepts has sufficient breadth and depth of coverage to encode all of the neurologic concepts derived from the 419 test cases. Using only pre-coordinated concepts, component ontologies of the UMLS, such as HPO, SNOMED CT, and OMIM, do not have adequate depth and breadth of coverage to encode the complexity of the neurological examination.
Conclusion
An ontology based on a subset of UMLS has sufficient breadth and depth of coverage to convert deficits from the neurological examination into machine-readable codes using pre-coordinated concepts. The use of a small subset of UMLS concepts for a neurological examination ontology offers the advantage of improved manageability as well as the opportunity to curate the hierarchy and subsumption relationships.
Keywords: UMLS Metathesaurus, Ontology, Neurological examination, Electronic health records, SNOMED CT
Background
The aggregation of clinical data for big data projects from electronic health records poses challenges [1–5]. Much of the clinical data in electronic health records (EHRs) are represented as free text. Although progress is being made in the conversion of free text into structured data by natural language processing (NLP), these methods are not in general use [6–10]. The entry of data about neurological patients in EHRs into large databases requires a method for converting symptoms (patient complaints) and signs (examination abnormalities) into machine-readable codes.
In this paper, we will use findings to mean signs and symptoms collectively. This conversion process can be facilitated by an ontology that links neurological signs and symptoms to appropriate concepts and machine-readable codes.
The neurological examination and history
There is no standard neurological examination and history [11–13]. Depending on the examiner and patient, a neurological examination and history may take as little as three minutes or greater than 30 min to complete. Furthermore, there is no generally accepted format for recording the neurological examination and history. Some neurologists use an outline, some use a table, and others use a narrative. No agreed-upon terminology exists for recording the neurological examination and history. During an examination, a neurologist elicits symptoms such as weakness, slowness, memory loss, speech impairment, blurred vision, numbness, tingling, pain, or imbalance and abnormalities in the mental status, cranial nerves, motor system, sensory system, reflexes, coordination, and gait. Due to a lack of standard terminology, identical neurological abnormalities may be described variously. For example, a failure to abduct the eye may be variously recorded as a sixth nerve palsy, an abducens palsy, or an abducens nerve weakness. Similarly, an upgoing great toe upon stimulation of the plantar surface of the foot may be recorded variously as Babinski sign, upgoing toe, positive Babinski, or upgoing plantar response.
The need for an ontology for the neurological examination
If neurological findings are to be converted into machine-readable codes, an ontology is needed [14]. At a minimum, an ontology for the neurological examination and history that supports big data applications should do the following:
Neurological findings with the same meaning but different wording should be represented by the same concept.
A unique machine-readable code should be assigned to each concept.
The ontology should be organized hierarchically with a root concept and should support subsumption (is_a relationships).
The ontology should have a scope (breadth of coverage) and a granularity (depth of coverage) that enables it to capture findings recorded during a neurological examination faithfully.
Synonymous terms should be linked to each concept.
Some ontologies have other useful characteristics. Many ontologies attach a definition to each concept. Other ontologies, SNOMED CT in particular, allow simple concepts to be combined to form more complex concepts. For example, the concept |ankle reflex| can be combined with |absent| and is equivalent to |absent ankle reflex|. This is known as post-coordination [15]. Concept ontologies that are organized hierarchically support the calculation of inter-concept distances [16–24].
The UMLS Metathesaurus
The UMLS Metathesaurus is not an ontology per se [25, 26]. The 2019 AB release is a curated compendium of 155 distinct terminology sources with 4,258,810 concepts. Each concept is linked to a unique machine-readable code and a specified name. For example, the concept aphasia has the CUI (concept unique identifier) C0003537. Each CUI is eight characters in length and starts with the letter C, followed by seven digits. The concept aphasia is contributed 58 times to UMLS from 58 different source terminologies. Each contribution of aphasia appears in the UMLS with the same CUI but a different atom unique identifier (AUI). The UMLS maintains concept synonyms as normalized concept names. The 2019AB release has 11,882,429 normalized concept names, each with a unique LUI. For example, the concept aphasia has 22 English language synonyms in UMLS, including |loss of words|, |losing words|, |loss of power of expression or comprehension|, |aphasic syndrome|, and |difficulty finding words|. The UMLS Metathesaurus also maintains files with concept definitions, files with relationships between concepts (child-parent, etc.), and files with ontology hierarchies (paths from each concept to the root concept).
Methods
Test-phrases
We abstracted the neurological examinations from 419 published neurological case studies [27–33]. Based on the neurological findings, we created a dataset of 2386 test-phrases for encoding as concepts by an ontology. Normal findings were ignored.
Mapping of test-phrases to UMLS concepts
Test phrases were mapped to UMLS concepts using the UMLS browser. Except for a few concepts, we ignored laterality (e.g., right ataxia, left ataxia, and bilateral ataxia were all mapped to the UMLS concept ataxia |C0004134|. We mapped the 2386 test phrases to 601 concepts. Concepts recurred a mean of 3.9 ± 5.5 times (range 1 to 47 times) in the 419 test teaching cases. The 15 most common neurology concepts are shown in Table 1.
Table 1.
Fifteen most common test concepts found in neurological teaching casesa
| Concept | CUI | Count | Proportion (%) |
|---|---|---|---|
| hyperreflexia | C0151889 | 47 | 2.0 |
| headache | C0018681 | 45 | 1.9 |
| unstable gait | C0231686 | 41 | 1.7 |
| bilateral extensor plantar responses | C0422917 | 38 | 1.6 |
| dysarthria | C0013362 | 32 | 1.3 |
| confused | C0009676 | 31 | 1.3 |
| hearing impaired | C1384666 | 31 | 1.3 |
| appendicular ataxia | C0750937 | 27 | 1.1 |
| hemiparesis | C0018989 | 24 | 1.0 |
| papilledema | C0030353 | 23 | 1.0 |
| diplopia | C0012569 | 22 | 0.9 |
| neck stiffness | C0151315 | 22 | 0.9 |
| impaired memory | C0233794 | 22 | 0.9 |
| ataxic gait | C0751837 | 20 | 0.8 |
| disorientation | C0233407 | 19 | 0.8 |
aCUI is from UMLS Metathesaurus browser. Proportion based on 2386 total concepts abstracted from 419 published neurology teaching cases
Construction of NEO (neurological examination ontology)
We reviewed the neurological history and examination as presented in three standard textbooks [11–13] and identified 1100 findings, which were either signs or symptoms. Each of the 1100 concepts was entered into the Protégé ontology editor [34] and consisted of the following:
Concept name
UMLS CUI (concept identifier)
SNOMED CT SCTID (concept identifier) if available
Parent concept
We used Protégé to organize the concepts into a mono-hierarchic ontology. The average number of children per concept was 3, and the maximum ontology depth was seven levels. The neuro-ontology is downloadable as a CSV or OWL file at the BioPortal of the National Center for Biomedical Ontology [35]. The neuro-ontology has five high-level branches to mirror the structure of the neurological examination (mental status finding, cranial nerve finding, motor finding, sensory finding, reflex finding) and four additional high-level branches (head finding, neck finding, skin finding, and neurological symptoms). Figure 1 demonstrates a partial expansion of NEO. Table 2 shows the distribution of concepts by the examination section. We had two considerations in building the concept hierarchy for NEO. First, findings related to each part of the neurological examination should be gathered under one consistent heading. Second, the concept hierarchy path distance between findings should reflect a neurologist’s thinking about which concepts are more similar while preserving subsumption. For example, a brisk biceps reflex could be subsumed under either biceps reflex or brisk reflex (see Fig. 2). We chose to subsume brisk biceps reflex under brisk reflex because a brisk biceps reflex is more similar to a brisk knee reflex than it is to an absent biceps reflex.
Fig. 1.
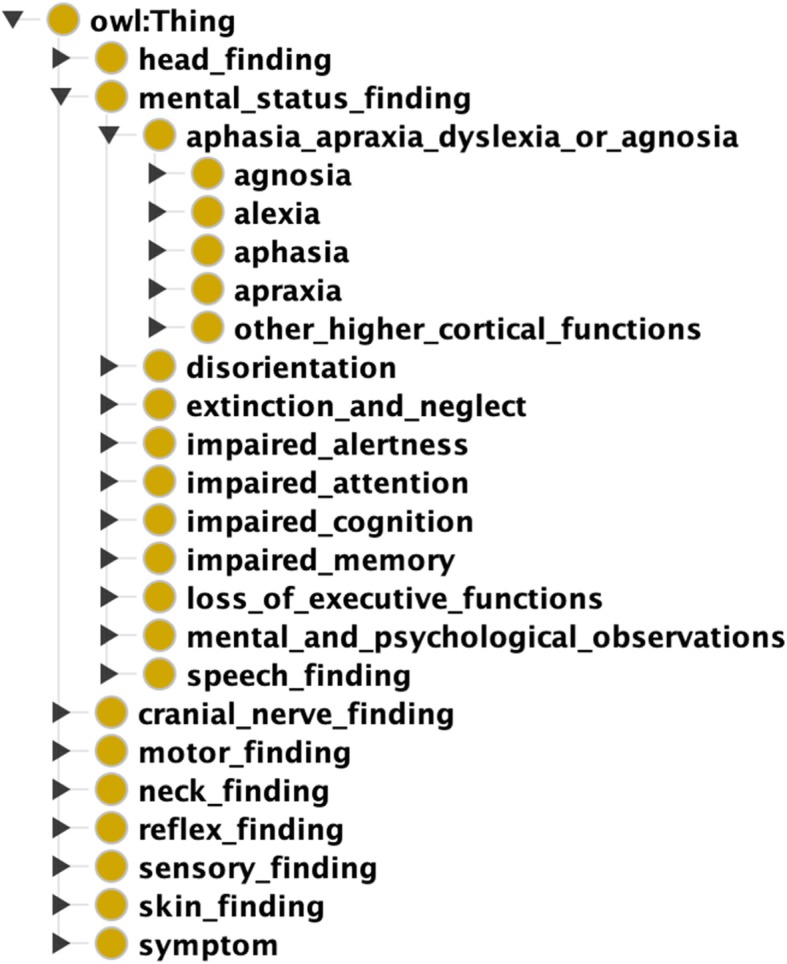
Major branches of NEO as shown in Protégé ontology editor (Image created with Protégé 5.5.0 ontology editor [34]). Mental Status Finding branch is partially expanded further
Table 2.
Composition Of Target Vocabulary By Major Branch Of Ontology
| Category | Count of Concepts |
|---|---|
| Mental Status | 191 |
| Cranial Nerve | 222 |
| Motor | 211 |
| Sensory | 133 |
| Coordination | 20 |
| Reflexes | 68 |
| Gait and Balance | 36 |
| Neck | 10 |
| Head | 7 |
| Skin findings | 6 |
| Symptoms | 196 |
| Total Concepts | 1100 |
Fig. 2.
Alternative subsumption strategies for placing brisk biceps reflex in concept hierarchy. Both strategies are semantically correct. Brisk biceps reflex can be grouped with other brisk reflexes (right panel) or with other biceps reflexes (left panel). SNOMED CT groups brisk biceps reflex with bicep reflexes, NEO groups brisk biceps reflex with other brisk reflexes (Image created with Protégé 5.5.0 ontology editor [34].)
Candidate ontologies for a neurological examination ontology
We identified five component ontologies of the UMLS Metathesaurus (Table 3) as a potential basis for a neurological examination ontology: SNOMED CT, HPO (Human Phenotype Ontology), MEDCIN, MeSH (Medical Subject Headings), and OMIM (Online Mendelian Inheritance in Man). We tested how well each of these ontologies provided concept coverage for the 601 neurology concepts identified above.
Table 3.
Candidate Ontologies For Use As A Neurological Examination Ontology
| Ontology | Concepts | |
|---|---|---|
| UMLS | Unified Medical Language System | 4,258,810 |
| SNOMED CT | SNOMED CT | 357,533 |
| MEDCIN | MEDCIN | 348,808 |
| HPO | Human Phenotype Ontology | 18,278 |
| OMIM | Online Mendelian Inheritance In Man | 109,609 |
| MeSH | Medical Subject Headings | 279,425 |
| NEO | Neurological Examination Ontology | 1100 |
Distance measures between concepts
We calculated distances between concepts for the SNOMED CT and NEO ontologies by the method of Wu and Palmer [24]. Distances between concepts were normalized with a minimum of 0.0 (closest) and a maximum of 1.0 (most distant).
Dist (a, b) is the semantic distance between concept a and concept b, LCS is the lowest common subsumer in the ontology for both a and b, depth(a) is number of levels from the root concept to concept a, depth (b) is the number of levels from the root concept to concept b, and depth(LCS) is the number of levels from the root concept to the LCS.
Results
We found 2386 neurological examination findings in 419 published neurological teaching cases. On average, each case had 5.7 ± 3.7 neurological findings. Since these cases appeared in neurology textbooks, they likely had more neurological findings per case than can be expected in general neurological practice. These 2386 findings (based on abstracted test-phrases) were mapped to 601 unique UMLS concepts. We examined how well each of the six candidate ontologies was able to cover the 601 test concepts (Table 4). None of the five candidate ontologies had sufficient scope to cover all the concepts. SNOMED CT, the largest of the candidate ontologies, covered 69% of the test concepts. The Neurology Examination Ontology (NEO) constructed from multiple terminologies in UMLS (Table 5), has enough scope to cover all test concepts.
Table 4.
Coverage Of Test Neurology Concepts By Candidate Ontologies
| Ontology | Concepts Covered | Proportion Covered |
|---|---|---|
| UMLS Metathesaurus | 601 | 100% |
| NEO | 601 | 100% |
| SNOMED CT | 412 | 69% |
| MEDCIN | 317 | 53% |
| OMIM | 278 | 46% |
| HPO | 233 | 39% |
| MeSH | 118 | 20% |
Table 5.
Terms Contributed To Neurological Examination Ontology (NEO) By Source Vocabulary
| Terminology | Concepts | Proportion Contributed |
|---|---|---|
| UMLS | 1100 | 100% |
| SNOMED CT | 728 | 66% |
| MEDCIN | 172 | 16% |
| OMIM | 76 | 7% |
| HPO | 52 | 5% |
| MeSH | 10 | 1% |
| Miscellaneous | 62 | 6% |
Discussion
We created a neuro-ontology of 1100 concepts derived from the UMLS Metathesaurus to encode findings from the neurological examination and history with machine-readable codes. To create an ontology with enough scope to cover common neurological findings, we drew concepts from a variety of terminologies (Table 5). We tested the completeness of the Neurological Examination Ontology (NEO) by assessing its ability to encode neurological concepts derived from 419 test teaching cases. These test teaching cases generated 2386 test phrases and 601 unique neurological concepts. NEO had adequate scope to cover 100% of the test concepts with a single concept. SNOMED CT had pre-coordinated concepts to cover 69% of the test concepts (Table 4). Coverage of test neurology concepts by MEDCIN was almost as good as SNOMED CT, while HPO, MeSH, and OMIM lack many key neurological concepts needed to cover the entire neurologic examination and history (Table 4).
Most of the concepts lacking from SNOMED CT were granular concepts needed to describe the motor and sensory examination in detail, such as which muscle groups were weak and the precise distribution and nature of sensory loss (Table 6). In this study, we did not use the post-coordination of concepts to generate more granular concepts. Post-coordination of SNOMED CT concepts would likely have increased the coverage rate. Elkin et al. [36] have shown that post-coordination of concepts can increase coverage for problems on the medical problem list from 50% to over 90%. Post-coordination of concepts is the process of joining together concepts to increase specificity and granularity of meaning. For example, the concept |weakness of right ankle dorsiflexion| can be represented by bringing together the concepts |right| with |ankle dorsiflexion| and |muscle weakness|. UMLS itself does not have a grammar for combining concepts to create post-coordinated concepts. SNOMED CT has a formal compositional grammar that specifies rules for combining concepts (post-coordination). However, the underlying grammar of post-coordination of SNOMED CT concepts is complex and requires considerable training before its successful implementation. Even professional coders may disagree on how to combine concepts to define more complex concepts [37]. Furthermore, calculating semantic distances between post-coordinated concepts and searching databases with post-coordinated concepts is more complicated than with pre-coordinated concepts.
Table 6.
Examples Of Neurological Concepts Available In UMLS And Not Found in SNOMED CT Browser
| CUI | UMLS Term |
|---|---|
| C2016536 | decreased pain and temperature sensation below T2 level |
| C2054091 | tactile sensation decreased sensory level at clavicles (T2 dermatome) |
| C2039818 | decreased tactile sensation of ulnar 1 and ½ digits of hand |
| C2230515 | weakness of ankle on dorsiflexion |
| C2230516 | weakness of ankle plantar flexion |
| C1847766 | shoulder girdle muscle atrophy |
| C2054045 | decreased tactile sensation of lateral leg and dorsum of foot (L5 dermatome) |
| C2054068 | decreased tactile sensation of middle finger only (C7 dermatome) |
| C2039817 | decreased tactile sensation of palmar aspect of radial 3 and ½ digits of hand |
For each clinical deficit, the clinical teaching cases used a variety of phrasing to express the same concept. For example, the neurological finding of bilateral extensor plantar response was expressed in 13 different ways in the clinical teaching cases (Table 7).
Table 7.
Test Phrases Mapped To |C0422917| Bilateral Extensor Plantar Response
| Test Phrase | |
| Babinski response elicited bilaterally | |
| bilateral Babinski responses | |
| bilateral Babinski signs | |
| bilateral extensor toes signs | |
| bilateral upgoing plantars | |
| bilateral upgoing toes | |
| both plantar responses were extensor | |
| both plantars were upgoing | |
| both toes upgoing | |
| plantar responses were extensor | |
| plantars extensor | |
| upgoing plantar responses bilaterally | |
| upgoing plantars | |
| UMLS Listed Synonyms | |
| Babinski reflexes bilateral | |
| Bilateral extensor plantar response (finding) |
This heterogeneity of expressions poses challenges for efforts to use natural language processing algorithms to convert free text neurological examinations into UMLS concepts [7, 8]. In a pilot study with NLM MetaMap [38, 39] in the batch mode, we were able to convert 70.3% of the 2286 test phrases to UMLS concepts. A higher conversion yield might be possible with additional post-processing and pre-processing of the longer and more complex test phrases.
Curation of the hierarchy
One of the advantages of a small domain-specific ontology, such as NEO is that domain experts can more easily identify and correct subsumption errors. Some somewhat arbitrary subsumption decisions may influence how accurately the concept distance measures derived from a concept hierarchy align with expert opinion. Considerable effort has been devoted to finding the best distance metrics for concept hierarchies that give the closest results to expert opinion [19, 20, 22]. However, when the hierarchy itself is not aligned with expert opinion, the choice of distance metric may be less critical. For example, most neurologists would agree that an absent knee jerk is more similar to an absent biceps jerk than it is to a brisk knee jerk (Fig. 2). As a result, in NEO, we have subsumed absent ankle reflex, absent knee reflex, absent biceps reflex, and absent triceps reflex under absent reflex. SNOMED CT subsumes absent ankle reflex and brisk ankle reflex under ankle reflex finding and subsumes absent biceps reflex and brisk biceps reflex under biceps reflex finding. These are ontologically correct (Fig. 2) but yield distance measures that are not in accord with a neurologist’s opinion as to which concepts are more similar (Table 8). In a large ontology like SNOMED CT, some errors in subsumption are inevitable and need to be corrected over time based on input from domain experts [40, 41]. Two examples of likely errors in subsumption are noted in Figs. 3 and 4. Dysmetria is a form of ataxia and hence is correctly subsumed by a finding related to incoordination, but dysmetria is not a reflex finding (Fig. 3). Similarly, apraxia is a disorder of higher cortical functions akin to aphasia and agnosia and should not be subsumed under either incoordination or musculoskeletal disorders (Fig. 4). As a result, distance measures based on the SNOMED CT concept hierarchy show dysmetria as too close to absent reflexes and shows apraxia too close to ataxia (Table 8). One of the goals of SNOMED CT is to have more concepts fully defined, which is achieved by adding qualifiers to concepts to make them distinguishable from other concepts in the ontology. Fully defined concepts cannot be confused logically with any other concept in the ontology. However, adding additional subsumption relationships may yield anomalous distance measures between unrelated concepts. In Fig. 5, the subsumption of asterixis (a flapping tremor of the arm) under a finding of the upper limb yields an anomalous distance measure placing asterixis too close to hemiparesis (Table 8). Similarly, in Fig. 6, the subsumption of oral dyskinesia (adventitious spontaneous chewing and writhing movements of the lips and tongue) under oral cavity finding causes oral dyskinesia to be anomalously close to the unrelated concept of vocal cord paralysis (Table 8). Finally, in a small curated domain-specific ontology like NEO, related concepts can be grouped to provide more accurate concept distance measures. For example, by grouping manifestations of bradykinesia such as masked-facies and micrographia close to bradykinesia, distance measures calculated based on the concept hierarchy can reflect the relatedness of these concepts (Table 8).
Table 8.
Some Examples of Discordant Inter-Concept Distancesa
| First Concept | Second Concept | SNOMED CT Inter-concept distance | NEO Inter-concept distance |
|---|---|---|---|
| brisk ankle reflex | absent ankle reflex | .11 | .75 |
| brisk biceps reflex | absent biceps reflex | .17 | .75 |
| asterixis | hemiparesis | .23 | .64 |
| dysdiadochokinesis | athetosis | .27 | .69 |
| dysmetria | absent reflexes | .11 | 1.00b |
| apraxia | ataxia | .17 | 1.00b |
| bradykinesia | masked facies | .79 | .20 |
| bradykinesia | micrographia | .56 | .20 |
| oral dyskinesia | vocal cord paralysis | .33 | .67 |
aSNOMED CT and NEO. Distances are normalized with 0.0 as minimum distance, 1.0 as maximum distance. Inter-concept distances were calculated by method of Wu and Palmer based on the concept hierarcy
b When concepts are in different high-order branches of the hierarchy, the distance is 1.0
Fig. 3.
Dysmetria (an imprecision in performing pointing movements with the limbs) is subsumed by incoordination and reflex finding in SNOMED CT. Read hierarchy from right to left (Diagram from Shrimp Ontoserver [42].©Australian e-Health Research Centre)
Fig. 4.
In SNOMED CT, asterixis (a flapping tremor of the arms) is subsumed by coarse tremor and finding of upper limb. Read hierarchy from right to left. (Diagram from Shrimp Ontoserver [42].©Australian e-Health Research Centre)
Fig. 5.
In SNOMED CT, oral dyskinesia is subsumed by disease of mouth, movement disorder, and oral cavity finding. Its defining quality is movement disorder. Read hierarchy from right to left. (Diagram from Shrimp Ontoserver [42].©Australian e-Health Research Centre)
Fig. 6.
In SNOMED CT concept hierarchy, apraxia is subsumed by finding of praxis and musculoskeletal finding and finding of incoordination. Read hierarchy from right to left. (Diagram from Shrimp Ontoserver [42].©Australian e-Health Research Centre)
Manageability
One advantage of a small ontology like NEO is improved manageability. Both the NLM and SNOMED International recommend the use of subsets of UMLS and SNOMED for certain restricted applications [26, 43]. NEO has only 1100 concepts, and the downloadable CSV file has 1100 rows. In contrast, the UMLS 2019AB release has 4,258,810 concepts, the MRREL.RRF (relationships) file has 84,189,164 rows and the MRCONSO.RRF (concepts) file has 15,172,405 rows. Similarly, the 2019 SNOMED CT US Edition release has 357,533 concepts, the relationship snapshot file has 2,989,896 rows, and the concept snapshot file has 478,117 rows. Since each row in the NEO file has the child-parent relationship for each concept, hierarchies and subsumption tables can be generated directly from the primary file.
Limitations
A major limitation of this study is that we did not attempt to combine concepts to generate missing or more complex concepts (post-coordination). Because we did not post-coordinate concepts, we were not able to grade muscle weakness when describing the motor examination. Muscle strength is usually graded on a scale of 0 to 5 (0 = ‘no movement’ to 5 = ‘full strength’). We elected to encode the clinical expression |weak quadriceps (4/5)| as |quadriceps weakness| C0577655 and ignore the degree of weakness. The sensory examination is notoriously difficult to describe in text, and in the era of the paper medical record, neurologists often used drawings to document the sensory examination. For this study, we did not use post-coordinated concepts to describe sensory findings but used instead granular concepts from MEDCIN to describe common sensory findings such as sensory loss in median nerve distribution, sensory loss in C8 dermatome distribution, a sensory level at the T2 level, etc. Similarly, we ignored quantitative visual acuity measurements such as 20/200 and encoded any visual acuity of less than normal as |reduced visual acuity| C0234632. Although UMLS has appropriate lateralized concepts for some findings (hemiparesis, rigidity, myoclonus, chorea, tremor, ptosis), there are not separate lateralized concepts for other common neurological findings (ataxia, hyperreflexia, spasticity, hyporeflexia). We did not develop a method to capture these lateralized findings when existing concepts were not available in UMLS and reverted to using the non-lateralized concept (e.g., ataxia for left ataxia).
Another limitation is that we have not validated the phrase abstraction methods or the phrase-to-concept mapping methods with other neurology experts or tested the methodology on de-identified medical records. More work is needed on whether a concise neurological examination ontology such as NEO is useful and acceptable to neurologists.
Conclusions
With certain limitations, an ontology that is a subset of UMLS with approximately 1100 concepts has adequate breadth and granularity capture the signs and symptoms of the neurological examination and history. Additional concepts may be needed to fully capture the laterality of certain findings (ataxia, hyperreflexia, etc.) as well as the severity of other findings (weakness, spasticity, rigidity, etc.). Using a subset of UMLS concepts to convert neurological signs and symptoms to machine-readable codes offers the advantage of improved manageability and coverage when compared to larger multi-purpose ontologies such as SNOMED CT, MEDCIN, and OMIM.
Acknowledgments
This work was conducted using the Protégé resource, which is supported by grant GM10331601 from the National Institute of General Medical Sciences of the United States National Institutes of Health. Figures 3, 4, 5, and 6 are reproduced by permission of the Australian E-Health Research Centre, copyright reserved.
Abbreviations
- CSV
Comma-separated value file
- CUI
Concept Unique Identifier
- HPO
Human Phenotype Ontology
- MEDCIN
MEDCIN® is a registered name of Medicomp Corporation
- MeSH
Medical Subject Headings. MeSH® is a registered name of the NLM
- NEO
Neurological Examination Ontology
- NLM
National Library of Medicine
- NLP
Natural Language Processing
- OMIM
Online Mendelian Inheritance in Man. OMIM® is a registered name of the McKusick-Nathans Institute of Genetic Medicine
- OWL
Web Ontology Language
- SCTID
SNOMED CT identifier
- SNOMED CT
SNOMED CT® is a registered name of SNOMED International. It is no longer considered an acronym
- UMLS
Unified Medical Language System. UMLS® is a registered name of the NLM
Authors’ contributions
DBH contributed to the conception, design, acquisition of data, analysis of data, drafting of the article, and final approval. SUB contributed to the analysis of data, acquisition of data, drafting of the article, and final approval.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
This ontology is available for download as a CSV or OWL file at the NCBO BioPortal at http://bioportal.bioontology.org/ontologies/NEO. The test phrases are available on request.
Ethics approval and consent to participate
This work did not involve human studies. Research Protocol # 2018–1355 was reviewed and approved by the Institutional Review Board of the University of Illinois at Chicago.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel B. Hier, Email: dhier@uic.edu
Steven U. Brint, Email: sbrint@uic.edu
References
- 1.Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2:3. Published 2014 Feb 7. 10.1186/2047-2501-2-3. [DOI] [PMC free article] [PubMed]
- 2.Brown SA. Patient Similarity: Emerging Concepts in Systems and Precision Medicine. Front Physiol. 2016;7:561. Published 2016 Nov 24. 10.3389/fphys.2016.00561. [DOI] [PMC free article] [PubMed]
- 3.Wang N, Huang Y, Liu H, Fe Xi, Wei L, Zhao X, Chen H, Measurement and application of patient similarity in personalized predictive modeling based on electronic medical records. Biomed Eng Online 2019, 18: 1–15. 10.1186/s12938-019-0718-2. [DOI] [PMC free article] [PubMed]
- 4.Parimbelli E., Marini S., Sacchi L., Bellazzi R. Patient similarity for precision medicine: A systematic review. Journal of Biomedical Informatics. 2018;83:87–96. doi: 10.1016/j.jbi.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Pai Shraddha, Bader Gary D. Patient Similarity Networks for Precision Medicine. Journal of Molecular Biology. 2018;430(18):2924–2938. doi: 10.1016/j.jmb.2018.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erhardt RA-A, Schneider R, Blaschke C. Status of text-mining techniques applied to biomedical text. Drug Discov Today. 2006;11:315–325. doi: 10.1016/j.drudis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Kreimeyer Kory, Foster Matthew, Pandey Abhishek, Arya Nina, Halford Gwendolyn, Jones Sandra F, Forshee Richard, Walderhaug Mark, Botsis Taxiarchis. Natural language processing systems for capturing and standardizing unstructured clinical information: A systematic review. Journal of Biomedical Informatics. 2017;73:14–29. doi: 10.1016/j.jbi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leaman Robert, Khare Ritu, Lu Zhiyong. Challenges in clinical natural language processing for automated disorder normalization. Journal of Biomedical Informatics. 2015;57:28–37. doi: 10.1016/j.jbi.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velupillai Sumithra, Suominen Hanna, Liakata Maria, Roberts Angus, Shah Anoop D., Morley Katherine, Osborn David, Hayes Joseph, Stewart Robert, Downs Johnny, Chapman Wendy, Dutta Rina. Using clinical Natural Language Processing for health outcomes research: Overview and actionable suggestions for future advances. Journal of Biomedical Informatics. 2018;88:11–19. doi: 10.1016/j.jbi.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Kai, Vydiswaran V.G. Vinod, Liu Yang, Wang Yue, Stubbs Amber, Uzuner Özlem, Gururaj Anupama E., Bayer Samuel, Aberdeen John, Rumshisky Anna, Pakhomov Serguei, Liu Hongfang, Xu Hua. Ease of adoption of clinical natural language processing software: An evaluation of five systems. Journal of Biomedical Informatics. 2015;58:S189–S196. doi: 10.1016/j.jbi.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biller J, Gruener G, Brazis P. DeMeyer’s The neurologic examination: A programmed text. 6. New York: McGraw Hill Medical; 2011. [Google Scholar]
- 12.Campbell WM. DeJong’s The neurologic examination. 7. Philadelphia: Wolters Kluwer Health; 2013. [Google Scholar]
- 13.Prasad K, Yavdav R, Spillane J. Bickerstaff’s neurological examination in clinical practice. New Delhi: Wiley; 2013. [Google Scholar]
- 14.Bodenreider O. Bio-ontologies: current trends and future directions. Briefings in Bioinformatics. 2006;7(3):256–274. doi: 10.1093/bib/bbl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SNOMED International . Compositional Grammar - Specification and Guide. 2019. [Google Scholar]
- 16.Caviedes Jorge E., Cimino James J. Towards the development of a conceptual distance metric for the UMLS. Journal of Biomedical Informatics. 2004;37(2):77–85. doi: 10.1016/j.jbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Girardi Dominic, Wartner Sandra, Halmerbauer Gerhard, Ehrenmüller Margit, Kosorus Hilda, Dreiseitl Stephan. Using concept hierarchies to improve calculation of patient similarity. Journal of Biomedical Informatics. 2016;63:66–73. doi: 10.1016/j.jbi.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Henry Sam, McQuilkin Alex, McInnes Bridget T. Association measures for estimating semantic similarity and relatedness between biomedical concepts. Artificial Intelligence in Medicine. 2019;93:1–10. doi: 10.1016/j.artmed.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Jia Z, Lu X, Duan H, Li H. Using the distance between sets of hierarchical taxonomic clinical concepts to measure patient similarity. BMC Med Inform Decis Mak. 2019;19(1):91. Published 2019 Apr 25. 10.1186/s12911-019-0807-y. [DOI] [PMC free article] [PubMed]
- 20.Lee WN, Shah N, Sundlass K, Musen M. Comparison of ontology-based semantic-similarity measures. AMIA Annu Symp Proc. 2008;2008:384–388. [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes BT, Pedersen T, Pakhomov SV. UMLS-Interface and UMLS-Similarity : open source software for measuring paths and semantic similarity. AMIA Annu Symp Proc. 2009;2009:431–435. Published 2009 Nov 14. [PMC free article] [PubMed]
- 22.McInnes BT, Pedersen T. Evaluating semantic similarity and relatedness over the semantic grouping of clinical term pairs. J Biomed Inform. 2015;54:329–336. doi: 10.1016/j.jbi.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 23.McInnes Bridget T., Pedersen Ted. Evaluating semantic similarity and relatedness over the semantic grouping of clinical term pairs. Journal of Biomedical Informatics. 2015;54:329–336. doi: 10.1016/j.jbi.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z, Palmer M. Verbs semantics and lexical selection. Proceedings of the 32nd Meeting of Association of Computational Linguistics. 1994. pp. 33–138. [Google Scholar]
- 25.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Research. 2004;32(90001):267D–270. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NLM . UMLS Reference Manual. Bethesda: National Library of Medicine (US); 2009. [Google Scholar]
- 27.Blumenfeld H. Neuroanatomy through clinical cases. 2. Sunderland: Sinauer Associates; 2010. [Google Scholar]
- 28.Hauser SL, Levitt LP, Weiner HL. Case studies in neurology for the house officer. Baltimore: Williams and Wilkins; 1986. [Google Scholar]
- 29.Macleod M, Simpson M, Pal S. Clinical cases uncovered: neurology. West Sussex: Wiley; 2011. [Google Scholar]
- 30.Noseworthy JH. Fifty neurologic Cases from Mayo Clinic. Oxford: Oxford University Press; 2004. [Google Scholar]
- 31.Pendlebury ST, Anslow P, Rothwell PM. Neurological case histories. Oxford: Oxford University Press; 2007. [Google Scholar]
- 32.Toy EC, Simpson E, Mancias P, Furr-Stimming EE. Case files neurology. 3. New York: McGraw-Hill; 2018. [Google Scholar]
- 33.Waxman SG. Clinical Neuroanatomy. 28. New York: McGraw Hill Education; 2017. [Google Scholar]
- 34.Musen Mark A. The protégé project. AI Matters. 2015;1(4):4–12. doi: 10.1145/2757001.2757003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noy NF, Shah NH, Whetzel PL, Dai B, Dorf M, Griffith N, Jonquet C, Rubin DL, Storey MA, Chute CG, Musen MA. BioPortal: ontologies and integrated data resources at the click of a mouse. Nucleic Acids Res. 2009;37:W170–W173. doi: 10.1093/nar/gkp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkin Peter L., Brown Steven H., Husser Casey S., Bauer Brent A., Wahner-Roedler Dietlind, Rosenbloom S. Trent, Speroff Ted. Evaluation of the Content Coverage of SNOMED CT: Ability of SNOMED Clinical Terms to Represent Clinical Problem Lists. Mayo Clinic Proceedings. 2006;81(6):741–748. doi: 10.4065/81.6.741. [DOI] [PubMed] [Google Scholar]
- 37.Andrews J. E., Richesson R. L., Krischer J. Variation of SNOMED CT Coding of Clinical Research Concepts among Coding Experts. Journal of the American Medical Informatics Association. 2007;14(4):497–506. doi: 10.1197/jamia.M2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aronson Alan R, Lang François-Michel. An overview of MetaMap: historical perspective and recent advances. Journal of the American Medical Informatics Association. 2010;17(3):229–236. doi: 10.1136/jamia.2009.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reátegui R, Ratteé S. Comparison of MetaMap and cTAKES for entity extraction in clinical notes. BMC Med Inform Decis Mak 2018. 10.1186/s12911-018-0654-2. [DOI] [PMC free article] [PubMed]
- 40.Rector Alan L, Brandt Sam, Schneider Thomas. Getting the foot out of the pelvis: modeling problems affecting use of SNOMED CT hierarchies in practical applications. Journal of the American Medical Informatics Association. 2011;18(4):432–440. doi: 10.1136/amiajnl-2010-000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortensen Jonathan M, Minty Evan P, Januszyk Michael, Sweeney Timothy E, Rector Alan L, Noy Natalya F, Musen Mark A. Using the wisdom of the crowds to find critical errors in biomedical ontologies: a study of SNOMED CT. Journal of the American Medical Informatics Association. 2014;22(3):640–648. doi: 10.1136/amiajnl-2014-002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metke-Jimenez A, Steel J, Hansen D, Lawley M. Ontoserver: a syndicated terminology server, J Biomed Semantics 2018 9:1–10. 10.1186/s13326-018-0191-z. [DOI] [PMC free article] [PubMed]
- 43.SNOMED International . Data analytics with SNOMED CT. 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This ontology is available for download as a CSV or OWL file at the NCBO BioPortal at http://bioportal.bioontology.org/ontologies/NEO. The test phrases are available on request.







