Abstract
Background
The objective of this systematic review and meta-analysis was to determine the prognostic value of total tumor-infiltrating lymphocytes (TILs) and subtypes of TILs (CD4+, CD8+, and FOXP3+) in triple-negative breast cancer (TNBC).
Methods
A systematic search of the MEDLINE, EMBASE, and Web of Science databases was conducted to identified eligible articles published before August 2019. Study screening, data extraction, and risk of bias assessment were performed by two independent reviewers. Risk of bias on the study level was assessed using the ROBINS I tool and Quality in Prognosis Studies (QUIPS) tool. We performed a meta-analysis to obtain a pooled estimate of the prognostic role of TILs using Review Manager 5.3.
Results
In total, 37 studies were included in the final analysis. Compared to TNBC patients with low TIL levels, TNBC patients with high TIL levels showed a higher rate of pathological complete response (pCR) to treatment (odds ratio [OR] 2.14, 95% confidence interval [CI] 1.43–3.19). With each 10% increase in percentage of TILs, patients with TNBC had an increased pCR (OR 1.09, 95% CI 1.02–1.16). Compared to TNBC patients with low TIL levels, patients with high TIL levels had better overall survival (OS; hazard ratio [HR] 0.58, 95% CI 0.48–0.71) and disease-free survival (DFS; HR 0.66, 95% CI 0.57–0.76). Additionally, with a continuous increase in TIL levels, patients with TNBC had improved OS (HR 0.90, 95% CI 0.87–0.93) and DFS (HR 0.92, 95% CI 0.90–0.95). A high CD4+ TIL level was associated with better OS (HR 0.49, 95% CI 0.32–0.76) and DFS (HR 0.54, 95% CI 0.36–0.80). A high CD8+ TIL level was associated better DFS only (HR 0.55, 95% CI 0.38–0.81), as no statistical association was found with OS (HR 0.70, 95% CI 0.46–1.06). A high FOXP3+ TIL level also was associated with only DFS (HR 0.50, 95% CI 0.33–0.75) and not OS (HR 1.28, 95% CI 0.24–6.88).
Conclusions
TNBC with a high level of TILs showed better short-term and long-term prognoses. High levels of specific phenotypes of TILs (CD4+, CD8+, and FOXP3+) were predictive of a positive long-term prognosis for TNBC.
Keywords: Triple-negative breast cancer, Tumor-infiltrating lymphocytes, Prognosis, Meta-analysis
Background
Triple-negative breast cancer (TNBC) is the term used to describe breast cancer cases that lack expression of estrogen receptor (ER), human epidermal growth factor receptor-2 (HER2), and progesterone receptor (PR) [1]. TNBC is characterized by a poor prognosis, and accordingly, the 5-year survival rate is only around 60% [2]. As the malignancy of breast cancer depends not only on its genetic abnormalities and biological characteristics but also on interactions between the cancer cells and their microenvironment, it is vital to understand the tumor microenvironment [3].
The microenvironment of breast cancer contains a variety of cell types, including tumor-infiltrating lymphocytes (TILs). Accumulating evidence indicates that TILs play essential roles in carcinogenesis and cancer progression [4]. Furthermore, interleukin (IL)-6 and IL-8 secreted by some subtypes of lymphocytes can generate a positive feedback loop between the immune microenvironment and tumor cells [5]. According to the results of a meta-analysis in 2014, the level of TILs was positively associated with a the prognosis of TNBC [6]. However, various subtypes of TILs have both inhibitory and stimulatory effects on the prognosis and progression of breast cancer. The CD4+ T cells and CD8+ T cells (primary effector TIL subtypes) have been linked to a better response to systemic treatment in breast cancer [7, 8]. On the contrary, FOXP3+ T-cell infiltration was found to predict a worse prognosis via the mediation of tumor immune escape [9, 10]. Because TNBC has unique clinicopathological and immunohistochemical features, determining the clinical associations of the total TIL count or the levels of specific subtypes of TILs in TNBC can improve our ability to predict the prognostic pattern and treatment response for TNBC.
The objective of the present systematic review and meta-analysis was to determine the prognostic roles of the total TILs or the levels of subtypes of TILs (CD4+, CD8+, and FOXP3+) in TNBC.
Methods
The present systematic review and meta-analysis were conducted following the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11].
Search strategy and study selection
A systematic literature search was conducted using the MEDLINE, EMBASE, and Web of Science databases to identify eligible articles published before August 2019. The keywords used for the literature search included triple-negative breast cancer (TNBC), tumor-infiltrating lymphocytes (TILs), prognosis, and survival. Review and meta-analysis articles were scanned for additional relevant studies. The literature search strategies are outlined in Additional file 1.
Outcome definitions
Pathological complete response (pCR) was defined as the absence of all invasive disease cells and lymph node metastasis [12]. Overall survival (OS) was defined as the period from the date of TNBC diagnosis to the time of death with any cause [13]. Disease-free survival (DFS) was defined as the period from the start of treatment to the first recurrence, or to death without any type of relapse [13].
Inclusion and exclusion criteria
The inclusion criteria were the following: (1) paper written in English, (2) study population or study sub-group consisted of patients with TNBC, (3) the relationships between TIL levels and short-term prognosis (i.e., pCR) and long-term prognosis (i.e., OS and DFS) were investigated, (4) original studies without restriction in study design, (5) studies containing enough data to estimate the effects (i.e., hazard ratios [HRs] and corresponding 95% confidence intervals [CIs] for OS or DFS, and odds ratios [ORs] and corresponding 95% CIs for pCR). The exclusion criteria were the following: (1) reviews, commentaries, editorials, protocols, case reports, qualitative research, or letters; (2) duplicate publications; and (3) full text not published in English, and (4) studies without usable data.
Study selection and quality assessment
Title–abstract screening was performed first to determine eligibility by two independent reviewers. Full-text articles that passed the first stage screening were downloaded for further review according to the inclusion and exclusion criteria. Disagreements were resolved by consultation with a third author or by joint discussion.
As no randomized controlled trial was found, we assessed the risk of bias using an approach based on the ROBINS I tool [14] and the Quality In Prognosis Studies (QUIPS) tool [15]. The risk of bias assessment was conducted by two reviewers independently.
Data extraction
We extracted data from the included studies using a pilot-tested data extraction form. We extracted the following data for this review: (1) first author and publication year, (2) country in which study was conducted, (3) study design, (4) participant details, (5) duration of follow-up, (6) choice of cut-off scores for defining positive TILs, (7) TIL category, (8) TIL measurement details (category or continuous). The definition of high/low TIL level were attributed to the original papers. (9) adjusted HRs with 95% CIs for OS and/or DFS (univariable HRs were recorded only if adjusted HRs were not available), and (10) adjusted ORs with 95% CIs (or accurate event numbers) for pCR (univariable ORs were recorded only if adjusted ORs were not available).
Statistical analysis
We performed meta-analyses to obtain a pooled estimate of the prognostic role of TILs using RevMan 5.3. Category software, and continuous TILs were estimated separately to decrease the heterogeneity. The results were expressed as HR (95%CI) for OS and DFS and by OR (95% CI) as calculated by Review Manager 5.3 [16]. A P-value less than 0.05 was set as indicative of statistical significance. Between-study heterogeneity was measured using the Higgins I2 statistic and Cochrane’s Q test (P < 0.10 or I2 > 50% was considered indicative of statistically significant heterogeneity) [17]. A random effects model (Der Simonian and Laird method) was applied if heterogeneity was present. However, the fixed-effect model was used in the absence of between-study heterogeneity (P > 0.10 or I2 < 50%). We performed subgroup analyses according to different subtypes of TILs as a sensitivity analysis to confirm the robustness of our results. Funnel plots were drafted for each meta-analysis to assess the potential publication bias.
Results
Search results and study characteristics
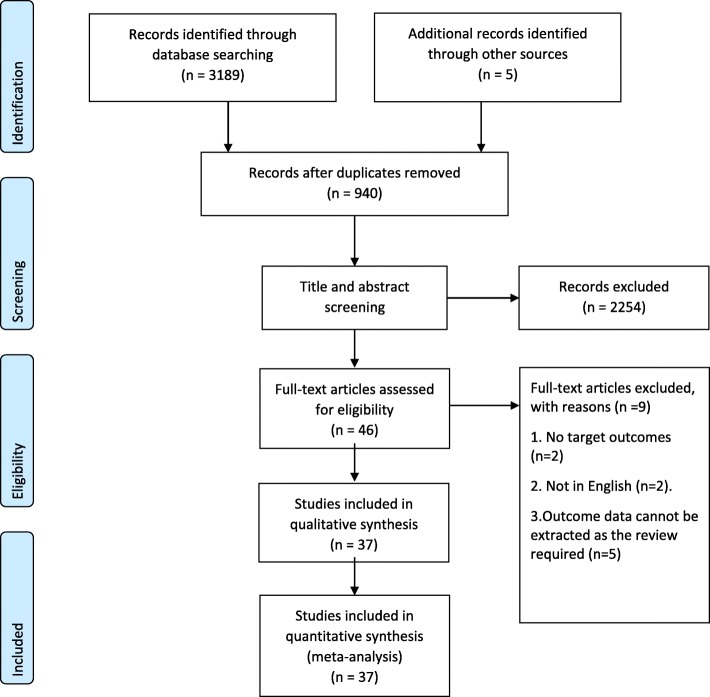
A total of 3194 articles were selected through searching the chosen electronic databases, and an additional 5 records were identified by cross-checking the bibliographies of retrieved meta-analysis or relevant reviews. After exclusion of duplicates, we screened the titles and abstracts and identified 46 articles for full-text review. We eliminated 9 papers according to the inclusion/exclusion criteria. Ultimately, 37 papers were included in the final analysis (Fig. 1) [7, 18–53].
Fig. 1.
PRISMA flow diagram detailing the search strategy and results [11]
The basic characteristics and target outcomes extracted from the included studies are listed in Table 1. All included articles (n = 37) were full-reported retrospective cohort studies. The studies were conducted in the United States (18.9%, 7/37), Japan (16.2%, 6/37), South Korea (16.2%, 6/37), China (8.1%, 3/37), France (8.1%, 3/37), Italy (3.4%, 2/37), Singapore (3.4%, 2/37), Germany (5.4%, 2/37), Australia (2.7%, 1/37), Peru (2.7%, 1/37), Spain (2.7%, 1/37), Canada (2.7%, 1/37), Ireland (2.7%, 1/37), and Switzerland (2.7%, 1/37). The population targeted was patients with TNBC. Eleven studies (29.7%, 11/37) provided evidence of the prognostic value of TILs for short-term outcomes (pCR), and five (75.7%, 28/37) provided evidence of the prognostic values of TILs for long-term outcomes (OS and/or DFS). The details of data extraction are presented in Additional file 2.
Table 1.
Clinical details of the included studies
| Author, year of publication | Country | Type of TNBC | No. of participants | TIL detection method | Location of TILs | Definition of high TIL level | TIL phenotype | Chemotherapy | Median follow-up (m) | Short-term prognosis | Target long-term prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams et al. 2014 [18] | USA | Operable TNBC | 481 | HE | Intra-epithelial and stromal | TILs involving 50% of either tumor stroma or cell nests | None specified | AT&AC | 127 | not specified |
DFS OS |
| AiErken et al. 2017 [19] | China | TNBC | 215 | HIC | Total and stromal | TILs-low (range, 0 to 10%); TILs-moderate (range, 11 to 40%); TILs-Marked (range, 41 to 100%). | PD-L1 | Anthracyclines or Anthracyclines + Taxino | 67.7 | not specified |
DFS OS |
| Althobiti et al. 2018 [20] | USA | TNBC | 230 | HE | Average stromal | Quantity of TILs was evaluated as percentage of TILs present in the stroma |
CD3+ CD8+ FOXP3+ CD20+ CD68+ |
Not specified | not specified | not specified | OS |
| Asano et al. 2018 [21] | Japan | TNBC | 61 | HE | Stromal | > 10% was considered positive for TILs | None specified | Neoadjuvant | 40.8 | pCR | DFS |
| Byun et al. 2018 [22] | South Korea | TNBC | 109 | IHC | not specified |
TILs were divided into (≥33% vs. < 33%) PD-L1 expression was categorized into two groups according to the final scores: low expression (< 100) and high expression (≥100). |
PD-L1+ TILs |
Not specified | 76 | not specified |
DFS OS |
| Cerbelli et al. 2017 [23] | Italy | TNBC received standard NACT | 54 | IHC and HE | Stromal | TILs were quantified as a percentage of the stromal area of the tumor and expressed as a continuous parameter. | PD-L1 | 4 cycles of doxorubicin + cyclophosphamide Q3W followed by 12 cycles of paclitaxel weekly | not specified | pCR | not specified |
| Denkert et al. 2015 [24] | Germany | TNBC | 255 | IHC and HE | Stromal | TILs involving 60% of either tumor stroma or cell nests | PD1 PDL1 CD8+ FOXP3 | Not specified | not specified | pCR | not specified |
| Denkert et al. 2018 [25] | Germany | TNBC | 906 | HE | Stromal | Three predefined categories: low TILs (0–10%), intermediate TILs (11–59%), or high TILs (60–100%). | None specified | (4 cycles of doxorubicin + cyclophosphamide Q3W followed by 12 cycles of paclitaxel weekly) | for OS, 62.8 months; median follow-up for DFS, 63.3 months | pCR | DFS OS |
| Dieci et al. 2014 [26] | France | TNBC patients with residual disease | 293 | HE | Intratumoral and stromal | High-TIL if It-TIL and/or Str-TIL > 60% | None specified | Neoadjuvant chemotherapy | 75.6 | not specified | OS |
| Dieci et al. 2015 [27] | France | TNBC | 199 | HE | Intratumoral and stromal | Cases were defined as High-TIL if It-TIL and/or Str-TIL > 60% | None specified | Not specified | 152.4 | not specified | OS |
| Galvez et al. 2018 [28] | Peru | TNBC | 100 | HE | Stromal | Cases were defined as High-TIL if Str-TIL > 50% | None specified | Neoadjuvant chemotherapy | not specified | pCR | not specified |
| Goto et al. 2018 [29] | Japan | TNBC treated with neoadjuvant chemotherapy | 39 | HE and IHC | Stromal | High if TILs occupied > 10% of the stromal area | CD8+ FOXP3+ | standardised NAC protocol consisting of four courses of FEC100 (500 mg/m2 fluorouracil, 100 mg/m2 epirubicin and 500 mg/m2 cyclophosphamide) every 3 weeks, followed by 12 courses of 80 mg/m2 paclitaxel administered weekly. | not specified | not specified | OS |
| Herrero-Vicent et al. 2017 [30] | Spain | TNBC treated with neoadjuvant chemotherapy | 164 | HE | None specified | not specified | not specified | Standardised NAC protocol | not specified | pCR | not specified |
| Hida et al. 2016 [31] | Japan | TNBC | 381 | HE | None specified | Classified as high if TILs score > 50% | not specified | Neoadjuvant chemotherapy | 45 | pCR | not specified |
| Jang et al. 2018 [32] | South Korea | TNBC | 231 | HE | Stromal | Classified TILS as high (> 10%) | not specified | Anthracycline-based chemotherapy | 117 | not specified |
DFS OS |
| Kim et al. 2017 [33] | South Korea | TNBC | 40 | HE | Stromal | Classified TILS score as high (> 60%). | Glutaminase+ TILs | An adjuvant methotrexate-based regimen | 78.3 | not specified | DFS |
| Krishnamurti et al. 2017 [34] | USA | TNBC without neoadjuvant treatments | 157 | HE | Stromal | TILs estimated in intervals as < 5, 5–10%, 11–50%, and > 50% | not specified | Not specified | not specified | not specified |
DFS OS |
| Lee et al. 2016 [35] | South Korea | TNBC | 769 | HE | Stromal | TILs defined as the mean percentage of stroma of invasive carcinoma infiltrated by lymphocytes and plasma cells in 10% increments | not specified | Four cycles of adjuvant anthracycline and cyclophosphamide | not specified | not specified |
DFS OS |
| Leon-Ferre et al. 2018 [36] | USA | TNBC | 605 | HE | Stromal and intratumoral | Lymphocyte-predominant breast cancer (LPBC) was defined as having > 50% stromal or intratumoral TILs | Not specified | Anthracycline and taxane | 124.8 | not specified | DFS OS |
| Li et al. 2016 [37] | USA | TNBC | 136 | IHC and HE | not specified | TILs were evaluated as the percentage of intratumoral stroma covered by mononuclear lymphocytes. | PD-L1 PD-1 | Not specified | 49.03 | not specified | DFS OS |
| Loi et al. 2014 [38] | Australia | newly diagnosed TNBC | 145 | HE | Stromal | TILs ≥50% | not specified | Not specified | 62 | not specified | OS |
| Luen et al. 2019 [39] | France | TNBC treated with neoadjuvant chemotherapy | 375 | HE and IHC | Stromal | Quantification of TILs in the tumor stroma was recorded as a percentage of occupied stromal areas. | not specified | Anthracycline and taxane; Anthracycline alone; and Taxane alone | 72 | not specified | OS |
| Matsumoto et al. 2016 [40] | Singapore | Primary TNBC | 232 | HE and IHC | Stromal and intratumoral | Median TIL value as the cut-off for high vs. low | CD4+ CD8+ | Not specified | not specified | not specified | DFS OS |
| McIntire et al. 2018 [41] | USA | TNBC | 76 | HE and IHC | None specified | TILs within the entire tumor were estimated at 5% intervals | CD8+ | Not specified | 110 | not specified | DFS OS |
| Miyashita et al. 2014 [42] | Japan | TNBC | 110 | IHC | Stromal and intratumoral | None specified | CD8+ FOXP3+ | Not specified | not specified | pCR | not specified |
| Mori et al. 2017 [43] | Japan | TNBC | 248 | IHC | Stromal and intratumoral | PD-L1+ was defined as expression in ≥5% of TILs | PD-L1 | None specified | 68 | not specified | OS |
| O’Loughlin et al. 2018 [44] | Ireland | TNBC | 75 | HE | stromal | LPBC was defined as having > 50% stromal TILs | None specified | None specified | not specified | pCR | not specified |
| Ono et al. 2012 [45] | Japan | TNBC received NAC and subsequent surgical therapy | 102 | IHC | None specified | TIL score classified as high if the sum was 3–5 | None specified | neoadjuvant anthracycline-based regimens | not specified | pCR | not specified |
| Park et al. 2016 [46] | South Korea | Early TNBC | 133 | HE | Stromal and intratumoral | Classified TILS as high (> 10%) | not specified | None specified | None specified | not specified | DFS OS |
| Pruneri et al. 2016 [47] | USA | TNBC | 724 | Multiplexed QIF staining | Stromal | LPBC defined as > 50% stromal TILs | not specified | Anthracycline + Taxanes ± CMF Anthracycline ± CMF | 82.8 | not specified | DFS OS |
| Pruneri et al. 2016 [48] | Switzerland | TNBC | 897 | HE | Stromal | None specified | not specified | CMF CMF + AC | 98.4 | not specified | DFS OS |
| Ruan et al. 2018 [49] | China | TNBC treated with neoadjuvant chemotherapy | 166 | None specified | Stromal and intratumoral | Classified TILS as high (> 10%) | not specified | Anthracycline + paclitaxel Paclitaxel + platinum | not specified | pCR | not specified |
| Seo et al. 2013 [7] | South Korea | TNBC | 38 | IHC | None specified | Median values of TILs used as cut off, and infiltration of TILs categorized as low or high. | CD4+ CD8+ FOXP3+ | AC, AD; and ACT | not specified | pCR | not specified |
| Tian et al. 2016 [50] | China | Primary invasive TNBCs | 425 | HE | Stromal and intratumoral | LPBC was categorized as tumors involving ≥50% lymphocytic infiltration in either tumor stroma or cell nests | not specified | Anthracyclines; Anthracyclines + Taxanes | 48 | not specified | DFS OS |
| Urru et al. 2018 [51] | Italy | TNBC | 841 | IHC | Stromal | None specified | not specified | Not specified | 51.6 | not specified | DFS OS |
| West et al. 2013 [52] | Canada | TNBC | 82 | IHC | Stromal | None specified | FOXP3+ TILs | Not specified | not specified | not specified | DFS |
| Yeong et al. 2017 [53] | Singapore | TNBC | 164 | IHC | None specified | Cut-off median percentages used were also compatible to the accepted clinical pathological practices | FOXP3+ | Not specified | not specified | not specified | DFS OS |
Abbreviations: TNBC Triple negative breast cancer, HE Hematoxylin-eosin, TNP Triple-negative phenotype, AC Doxorubicin plus cyclophosphamide, AT Doxorubicin plus paclitaxel, DFS Disease-free survival, OS Overall survival, IHC Immunohistochemistry, pCR Pathological complete response, LPBC Lymphocyte-predominant breast cancer, CMF Cyclophosphamide methotrexate fluorouracil, ACT Doxorubicin plus cyclophosphamide followed by docetaxel, AD Doxorubicin plus docetaxel
TILs and pCR
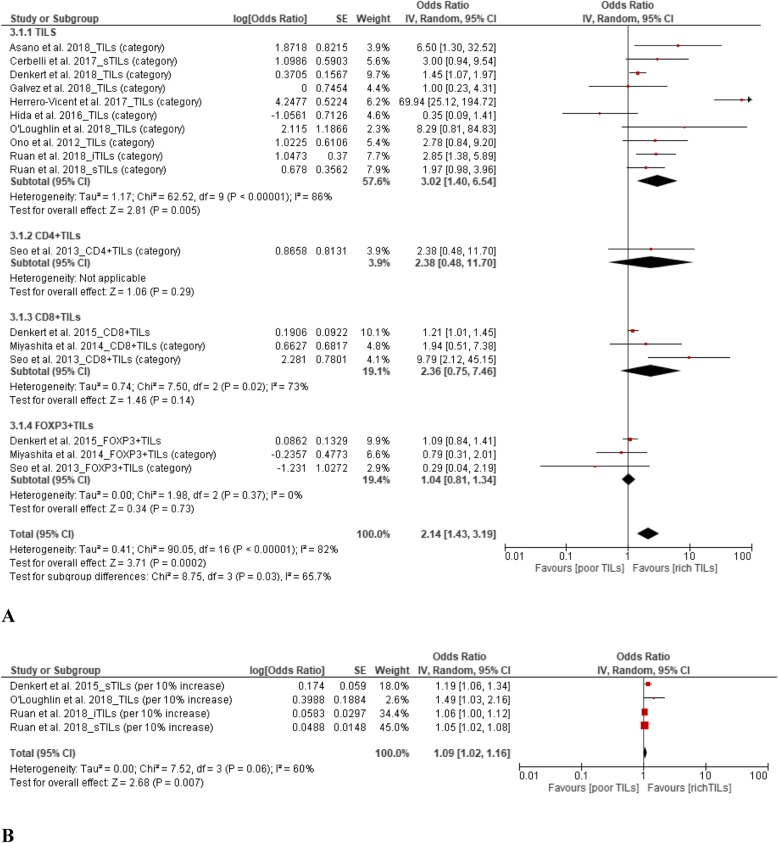
From the 11 studies demonstrating the prognostic value of TILs for pCR among TNBC patients, the results showed that upregulation of TILs predicted a higher pCR rate. The pooled ORs were 2.14 (95% CI, 1.43–3.19) for TIL level (high vs. low) and 1.09 (95% CI, 1.02–1.16) for continuous TILs (10% increase in TIL level). When stratified by the TIL phenotypes of CD4+, CD8+, and FOXP3+, no statistical differences in pCR were found in the subgroup analysis. The details pooled results are presented in Fig. 2.
Fig. 2.
Forest plots of the random-effects meta-analysis for the efficacy of tumor-infiltrating lymphocytes (TILs) for predicting pathological complete response (pCR). a Low TILs vs. high TILs stratified by TIL phenotype. b Continuous TILs (10% increase) for pCR
TILs and OS
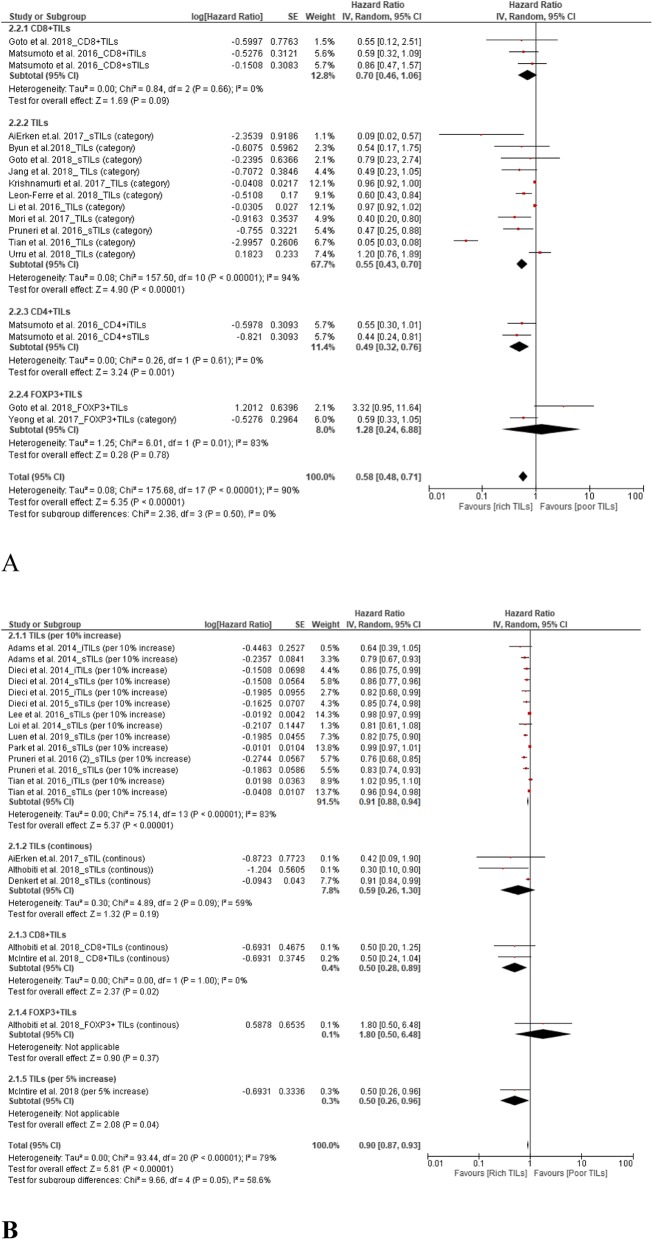
A total of 24 studies supported the prognostic value of TILs for OS in TNBC patients. The results showed upregulation of TILs predicted a better OS. The pooled HRs were 0.58 (95% CI, 0.48–0.71) for total TIL level (high vs. low) and 0.90 (95% CI, 0.87–0.93) for continuous TILs (Fig. 3).
Fig. 3.
Forest plots of the random-effects meta-analysis for the efficacy of tumor-infiltrating lymphocytes (TILs) for overall survival (OS). a Low TILs vs. high TILs stratified by TIL phenotypes. b TILs stratified by continuous TILs, 5% increase in TILs, 10% increase in TILs, and phenotypes
From subgroup analyses according to TIL phenotype (high vs. low), the HRs were 0.49 (95% CI, 0.32–0.76), 0.70 (95% CI, 0.46–1.06), and 1.28 (95% CI, 0.24–6.88) for CD4+ TILs, CD8+ TILs, and FOXP3+ TILs, respectively (Fig. 3a). Subgroup analyses according to the change in TIL level (continuous) returned HRs of 0.50 (95% CI, 0.28–0.89) and 1.80 (95% CI, 0.50–6.48) for CD8+ TILs and FOXP3+ TILs, respectively (Fig. 3b).
TILs and DFS
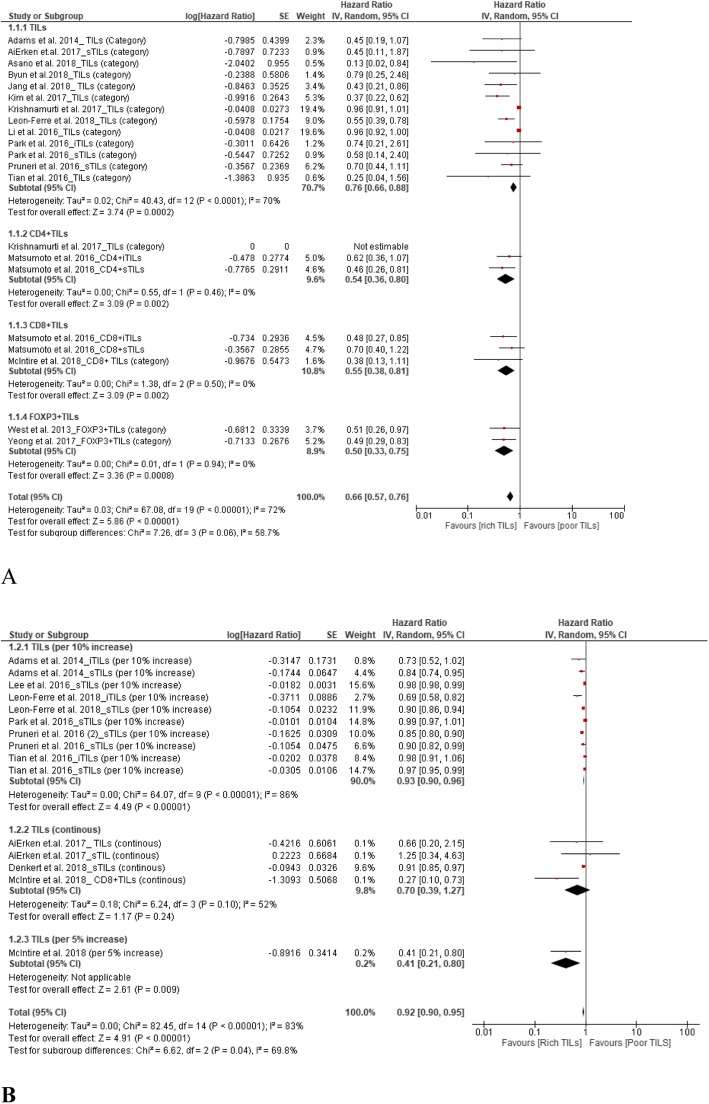
A total of 20 studies supported the prognostic value of TILs for DFS in TNBC patients. The results showed upregulation of TILs predicted better DFS, with pooled HRs of 0.66 (95% CI, 0.57–0.76) for TIL level (high vs. low) and 0.92 (95% CI, 0.90–0.95) for continuous TILs (Fig. 4).
Fig. 4.
Forest plots of the random-effects meta-analysis for the efficacy of tumor-infiltrating lymphocytes (TILs) for disease-free survival (DFS). a Low TILs vs. high TILs stratified by TIL phenotype. b TILs stratified by continuous TILs, 5% increase in TILs, and 10% increase in TILs
From subgroup analyses according to TIL phenotype (high vs. low), the HRs were 0.54 (95% CI, 0.36–0.80), 0.55 (95% CI, 0.38–0.81), and 0.50 (95% CI, 0.33–0.75) for CD4+ TILs, CD8+ TILs, and FOXP3+ TILs, respectively (Fig. 4a).
Subgroup analyses according to the change in TIL level (continuous) returned HRs of 0.93 (95% CI, 0.90–0.96), 0.70 (95% CI, 0.39–1.27), and 0.41 (95% CI, 0.21–0.80) for a 10% increase in TILs, continuous TILs, and a 5% increase in TILs of each subgroup, respectively (Fig. 4b).
Risk of bias in included studies
We evaluated the risk of bias for all included studies (n = 37). We found the main sources of bias were related to missing data, TIL measurement and confounding controls. Most of the missing data due to that not all the available patients were included in the final analysis as the information was not complete (participants were excluded due to missing data). Figure 5a shows the risk of bias assessments for each cohort. Evaluations for each domain across full reported studies are shown in Fig. 5b.
Fig. 5.
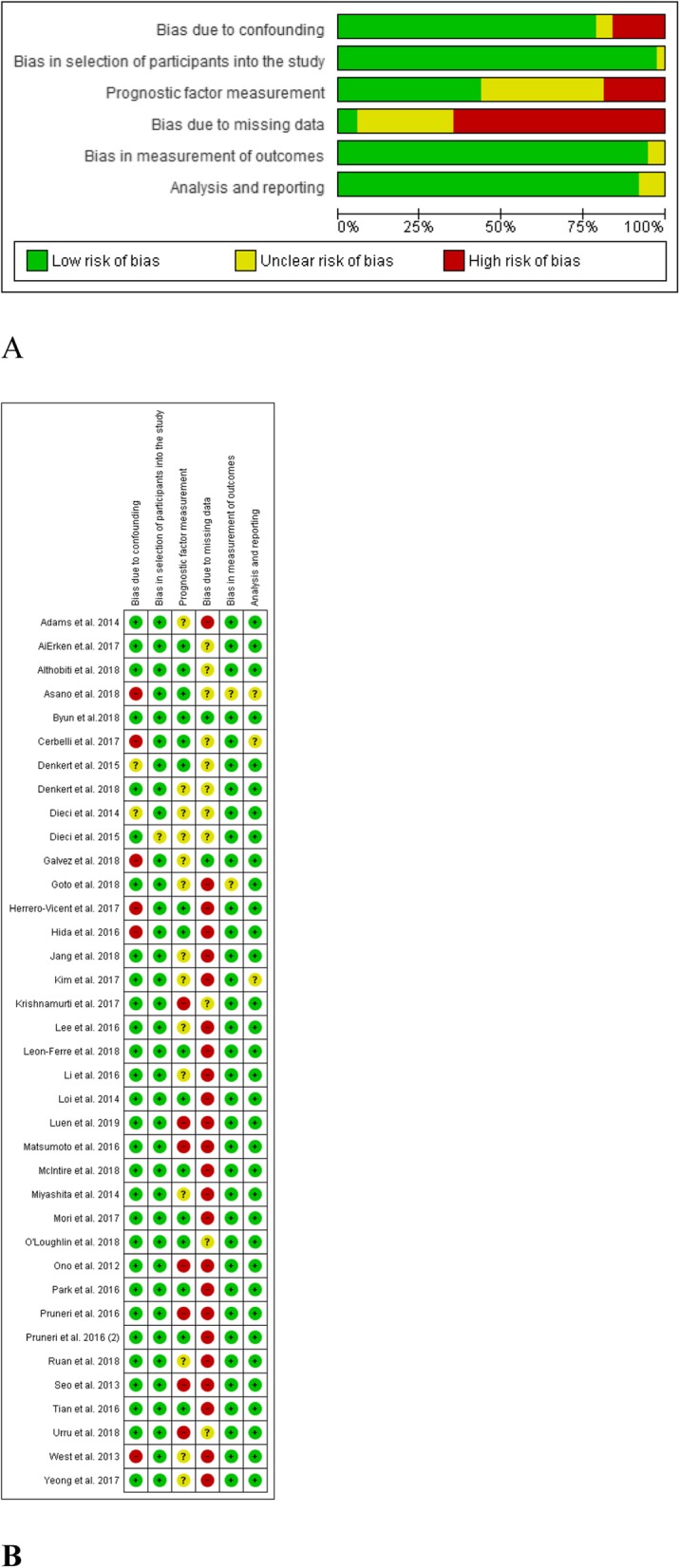
Risk of bias assessment at the study level. a Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included full reported studies (n = 37). b Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Publication bias
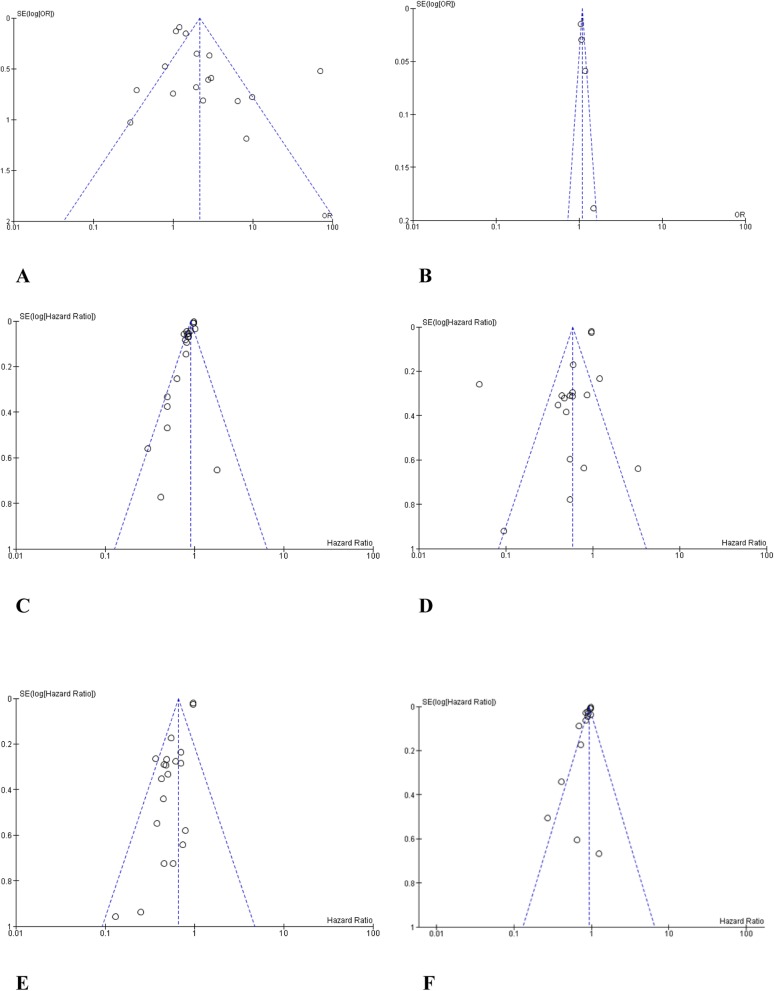
Funnel plot analysis did not indicate apparent publication bias affecting the HRs for DFS and OS or the ORs for pCR in the included studies (Fig. 6).
Fig. 6.
Funnel plot analysis of potential publication bias. a High tumor-infiltrating lymphocytes (TILs) vs. low TILs for pathological complete response (pCR). b Continuous TILs (10% increase) for pCR. c High TILs vs. low TILs for overall survival (OS). d Continuous TILs for OS. e High TILs vs. low TILs for disease-free survival (DFS). f Continuous TILs for DFS
Discussion
As TNBC is a poor prognostic subtype of breast cancer, it is important to identify biomarkers that can rigorously predict its prognosis. The present review and meta-analysis synthesized 37 studies to evaluate the association between TIL levels, both total and specific subtypes, and prognosis in TNBC patients. Our findings indicate that a high TIL level in TNBC significantly increases the likelihood of pCR and improves DFS and OS.
In the present study, we used pCR as the indicator of short-term prognosis for patients with TNBC. Previous studies reported that higher TIL levels predict a better response to chemotherapy in patients with breast cancer [54–56]. According to our pooled results, compared to TNBC patients with low TIL levels, TNBC patients with high TIL levels had a higher rate of pCR to treatment (OR 2.14, 95% CI 1.43–3.19). Moreover, with each 10% increase in TIL level, patients with TNBC had an increased pCR rate (OR 1.09, 95% CI 1.02–1.16). A potential explanation for these findings is the influence of TILs to tumor immunosurveillance and tumor immunosuppression [57]. In addition, the treatment used in the included articles was inconsistent. However, no significant pCR improvement was observed for high levels of the CD4+, CD8+, and FOXP3+ TIL subgroups. This may due to the limited amount of data available for these subgroups.
The indicators of long-term prognosis in this study were OS and DFS. According to our pooled results, compared to TNBC patients with low TIL levels, patients with high TIL levels showed better OS (HR 0.58, 95% CI 0.48–0.71) and DFS (HR 0.66, 95% CI 0.57–0.76). Additionally, with a continuously increasing TIL levels, patients with TNBC had improved OS (HR 0.90, 95% CI 0.87–0.93) and DFS (HR 0.92, 95% CI 0.90–0.95). This finding is consistent with previous conclusions [3, 9, 25, 58, 59]. Our results indicate that a high level of TILs is a positive predictor for the prognosis of patients with TNBC.
The CD4+ TIL subgroup (high vs. low) showed a better OS (HR 0.49, 95%CI 0.32–0.76) and DFS (HR 0.54, 95%CI 0.36–0.80), and the CD8+ TIL subgroup (high vs. low) showed a better DFS only (HR 0.55, 95% CI 0.38–0.81). Nevertheless, the pooled results indicated CD4+ TILs and CD8+ TILs were positive predictors for long-term prognosis in TNBC. This is consistent with previous meta-analysis results [6]. The FOXP3+ TIL subgroup (high vs. low) also showed only better DFS (HR 0.50, 95% CI 0.33–0.75), with no statistical association with OS (HR 1.28, 95% CI 0.24–6.88). This finding for FOXP3+ TILs is opposite to that of previous meta-analyses [3, 6], and the reason for this inconsistency is unclear. More studies of the association of FOXP3+ TILs with the prognosis of TNBC are needed.
To our best knowledge, this was the first meta-analysis to pool the prognostic results for categorical TIL level and continuous TILs separately. Therefore, from the results, we can definitively conclude that a higher density of TILs corresponds to a better prognosis for TNBC. Our study does have some limitations. First, all included studies were retrospective cohort studies, with risks of bias related to missing data, TIL measurement, and confounding controls. Next, the variation in the definition of high/low TIL level, and the timeline(s) used for PFS and OS among the included studies can affect the accuracy of the results.
Conclusions
TNBC with higher levels of TILs showed better short-term and long-term prognoses. High levels of specific phenotypes of TILs (CD4+, CD8+, and FOXP3+) could positively predict the long-term prognosis for TNBC.
Supplementary information
Additional file 1. Literature search strategy. For additional file1, the content is the search strategies of EMBASE and MEDLINE. For additional file1, the content is the data extraction details of all included articles.
Additional file 2. The data extraction details for the included articles.
Acknowledgements
The authors would like to thank all of the involved study investigators for dedicating their time and skills to the completion of this study.
Abbreviations
- CD
Cluster of differentiation
- CI
Confidence intervals
- DFS
Disease-free survival
- ER
Estrogen receptor
- FBP3
Forkhead box P3
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratios
- IL
Interleukin
- OR
Odds ratio
- OS
Overall survival
- pCR
Pathological complete response
- PR
Progesterone receptor
- PRISMA
Systematic Reviews and Meta-Analyses
- QUIPS
Quality in Prognosis Studies
- ROBIN I
Risk Of Bias In Non-randomised Studies - of Interventions
- TILs
Tumor-infiltrating lymphocytes
- TNBC
Triple-negative breast cancer
Authors’ contributions
GG carried out the initial background research and drafted the manuscript. GG and ZW acted as independent reviewers in screening literature, extracting data, and assessing the quality of each study. GG, ZZ and XQ helped in developing the manuscript or revising it critically for important intellectual content. The author(s) read and approved the final manuscript.
Funding
This study was supported by the National Key Technologies R&D Program (No. 2015BAI13B09) and the Research Foundation of Beijing Friendship Hospital, Capital Medical University (No. YYQDKT2018–11). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-6668-z.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast Cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16:53–61. doi: 10.1097/PPO.0b013e3181d24ff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18:497–506. doi: 10.1007/s12094-015-1391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdiguero EG, Geissmann F. Identifying the infiltrators. Science. 2014;344:801–802. doi: 10.1126/science.1255117. [DOI] [PubMed] [Google Scholar]
- 5.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148:467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 7.Seo A, Lee H, Kim E, Kim H, Jang M, Lee H, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–632. doi: 10.3892/mco.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 13.Ovcaricek T, Frkovic S, Matos E, Mozina B, Borstnar S. Triple negative breast cancer-prognostic factors and survival. Radiol Oncol. 2011;45:46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Manager R. Version 5.0. The Nordic Cochrane Centre. Denmark: The Cochrane Collaboration Copenhagen; 2008. [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AiErken N, Shi HJ, Zhou Y, Shao N, Zhang J, Shi Y, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast Cancer patients. Int J Biol Sci. 2017;13:1172–1179. doi: 10.7150/ijbs.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Althobiti M, Aleskandarany MA, Joseph C, Toss M, Mongan N, Diez-Rodriguez M, et al. Heterogeneity of tumour-infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology. 2018;73:887–896. doi: 10.1111/his.13695. [DOI] [PubMed] [Google Scholar]
- 21.Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of treatment response to Neoadjuvant chemotherapy in breast Cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res. 2018;38:2311–2321. doi: 10.21873/anticanres.12604. [DOI] [PubMed] [Google Scholar]
- 22.Byun KD, Hwang HJ, Park KJ, Kim MC, Cho SH, Ju MH, et al. T-cell immunoglobulin mucin 3 expression on tumor infiltrating lymphocytes as a positive prognosticator in triple-negative breast cancer. J Breast Cancer. 2018;21:406–414. doi: 10.4048/jbc.2018.21.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerbelli B, Pernazza A, Botticelli A, Fortunato L, Monti M, Sciattella P, et al. PD-L1 Expression in TNBC: A Predictive Biomarker of Response to Neoadjuvant Chemotherapy? Biomed Res Int. 2017;2017:1750925. doi: 10.1155/2017/1750925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 25.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 26.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieci MV, Mathieu MC, Guarneri V, Conte P, Delaloge S, Andre F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galvez M, Castaneda CA, Sanchez J, Castillo M, Rebaza LP, Calderon G, et al. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy. World J Clin Oncol. 2018;9:33–41. doi: 10.5306/wjco.v9.i2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto W, Kashiwagi S, Asano Y, Takada K, Takahashi K, Hatano T, et al. Predictive value of improvement in the immune tumour microenvironment in patients with breast cancer treated with neoadjuvant chemotherapy. ESMO Open. 2018;3(6):e000305. doi: 10.1136/esmoopen-2017-000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero-Vicent C, Guerrero A, Gavila J, Gozalbo F, Hernandez A, Sandiego S, et al. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience. 2017;11:759. doi: 10.3332/ecancer.2017.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, et al. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat. 2016;158:1–9. doi: 10.1007/s10549-016-3848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang N, Kwon HJ, Park MH, Kang SH, Bae YK. Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast Cancer. Ann Surg Oncol. 2018;25:937–946. doi: 10.1245/s10434-017-6332-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Heo SH, Choi SK, Song IH, Park IA, Kim YA, et al. Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch. 2017;470:381–389. doi: 10.1007/s00428-017-2083-5. [DOI] [PubMed] [Google Scholar]
- 34.Krishnamurti U, Wetherilt CS, Yang J, Peng L, Li X. Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers. Hum Pathol. 2017;64:7–12. doi: 10.1016/j.humpath.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, et al. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016;69:422–430. doi: 10.1136/jclinpath-2015-203089. [DOI] [PubMed] [Google Scholar]
- 36.Leon-Ferre RA, Polley MY, Liu H, Gilbert JA, Cafourek V, Hillman DW, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167:89–99. doi: 10.1007/s10549-017-4499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XX, Wetherilt CS, Krishnamurti U, Yang J, Ma YM, Styblo TM, et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast Cancer. Am J Clin Pathol. 2016;146:496–502. doi: 10.1093/ajcp/aqw134. [DOI] [PubMed] [Google Scholar]
- 38.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 39.Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. 2019;30:236–242. doi: 10.1093/annonc/mdy547. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto H, Thike AA, Li HH, Yeong J, Koo SL, Dent RA, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156:237–247. doi: 10.1007/s10549-016-3743-x. [DOI] [PubMed] [Google Scholar]
- 41.McIntire PJ, Irshaid L, Liu YF, Chen ZM, Menken F, Nowak E, et al. Hot Spot and Whole-Tumor Enumeration of CD8(+) Tumor-Infiltrating Lymphocytes Utilizing Digital Image Analysis Is Prognostic in Triple-Negative Breast Cancer. Clin Breast Cancer. 2018;18:451. doi: 10.1016/j.clbc.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148:525–534. doi: 10.1007/s10549-014-3197-y. [DOI] [PubMed] [Google Scholar]
- 43.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584–15592. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Loughlin M, Andreu X, Bianchi S, Chemielik E, Cordoba A, Cserni G, et al. Reproducibility and predictive value of scoring stromal tumour infiltrating lymphocytes in triple-negative breast cancer: a multi-institutional study. Breast Cancer Res Treat. 2018;171:1–9. doi: 10.1007/s10549-018-4825-8. [DOI] [PubMed] [Google Scholar]
- 45.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 46.Park HS, Heo I, Kim JY, Kim S, Nam S, Park S, et al. No effect of tumor-infiltrating lymphocytes (TILs) on prognosis in patients with early triple-negative breast cancer: validation of recommendations by the international TILs working group 2014. J Surg Oncol. 2016;114:17–21. doi: 10.1002/jso.24275. [DOI] [PubMed] [Google Scholar]
- 47.Pruneri G, Gray KP, Vingiani A, Viale G, Curigliano G, Criscitiello C, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat. 2016;158:323–331. doi: 10.1007/s10549-016-3863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 49.Ruan M, Tian T, Rao J, Xu X, Yu B, Yang W, et al. Predictive value of tumor-infiltrating lymphocytes to pathological complete response in neoadjuvant treated triple-negative breast cancers. Diagn Pathol. 2018;13:66. doi: 10.1186/s13000-018-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian T, Ruan M, Yang W, Shui R. Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget. 2016;7:44395–44405. doi: 10.18632/oncotarget.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urru SAM, Gallus S, Bosetti C, Moi T, Medda R, Sollai E, et al. Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Cancer. 2018;18:11. doi: 10.1186/s12885-017-3969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West N, Kost S, Martin S, Milne K, Deleeuw R, Nelson B, et al. Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeong J, Thike AA, Lim JCT, Lee B, Li HH, Wong SC, et al. Higher densities of Foxp3(+) regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2017;163:21–35. doi: 10.1007/s10549-017-4161-4. [DOI] [PubMed] [Google Scholar]
- 54.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 55.Liu S, Duan X, Xu L, Xin L, Cheng Y, Liu Q, et al. Optimal threshold for stromal tumor-infiltrating lymphocytes: its predictive and prognostic value in HER2-positive breast cancer treated with trastuzumab-based neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015;154:239–249. doi: 10.1007/s10549-015-3617-7. [DOI] [PubMed] [Google Scholar]
- 56.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One. 2016;11:e0152500. doi: 10.1371/journal.pone.0152500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mao Yan, Qu Qing, Zhang Yuzi, Liu Junjun, Shen Kunwei. Tumor infiltrating lymphocytes (TIL) to predict response to neoadjuvant chemotherapy in breast cancer: A systemic review and meta-analysis. Journal of Clinical Oncology. 2014;32(26_suppl):138–138. doi: 10.1200/jco.2014.32.26_suppl.138. [DOI] [Google Scholar]
- 59.Carbognin L, Pilotto S, Nortilli R, Brunelli M, Nottegar A, Sperduti I, et al. Predictive and prognostic role of tumor-infiltrating lymphocytes for early breast Cancer according to disease subtypes: sensitivity analysis of randomized trials in adjuvant and Neoadjuvant setting. Oncologist. 2016;21:283–291. doi: 10.1634/theoncologist.2015-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Literature search strategy. For additional file1, the content is the search strategies of EMBASE and MEDLINE. For additional file1, the content is the data extraction details of all included articles.
Additional file 2. The data extraction details for the included articles.
Data Availability Statement
Not applicable.