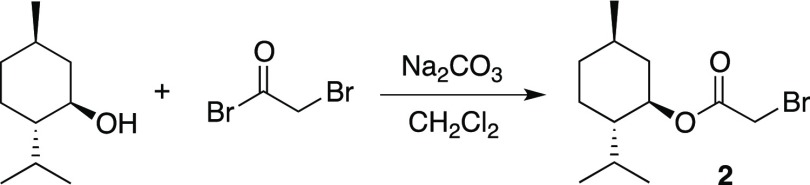Abstract
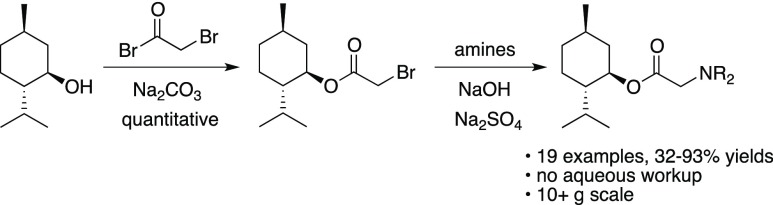
A convenient method of synthesis has been developed for a new class of potential cooling agents, menthol glycinates. These compounds are prepared in two synthetic steps, starting from bromoacetyl bromide and (−)-menthol. The resulting brominated menthol ester readily undergoes substitution reactions with NH3 and 1° or 2° amines to provide menthol glycinates. For most of the prepared compounds, the two-step synthetic procedure requires no aqueous phase extractions.
Introduction
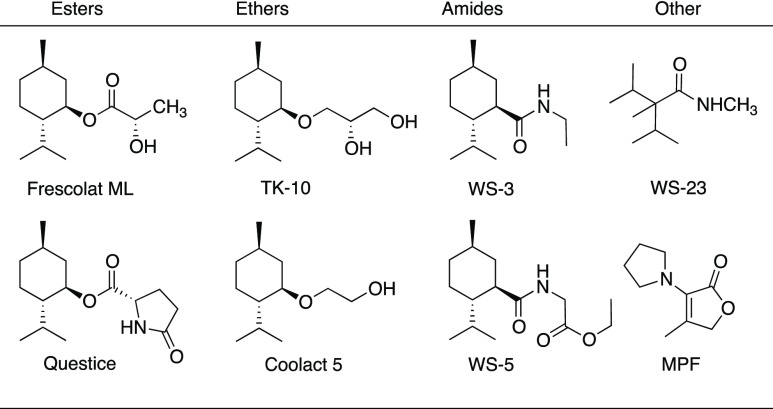
Menthol is a major commodity chemical which is derived from both natural and semisynthetic sources.1 The worldwide usage of purified menthol is estimated to be over 20,000 metric tons annually.2 Its usage includes numerous flavor applications as a cooling component and as a local anesthetic in medicinal formulations and skin products. Among the several known stereoisomers of (−)-menthol, these have been shown to be less effective cooling agents than (−)-menthol. In taste dilution tests, (−)-isomenthol exhibits a cooling threshold at 30.0 ppm, whereas (−)-menthol has a cooling threshold of just 0.8 ppm.3 Nevertheless, there has been ongoing interest in the development of agents having even more potent cooling effects. This includes (−)-menthol derivatives such as the esters of (−)-menthol, (−)-menthol ethers, menthol-related amides, and other varied structures (Figure 1).3 Depending on the assay used, WS-3 and WS-5 are both shown to have stronger cooling effects than (−)-menthol, whereas 4-methyl-3-(1-pyrrolidinyl)-2[5H]-furanone is estimated to have 35 times more cooling effects than (−)-menthol.3
Figure 1.
Known cooling agents.
No one has examined the use of menthol glycinates (1) among the derivatives of (−)-menthol that show cooling effects. Glycine itself has been used as a flavor-enhancing component;4 so, we reasoned that glycine esters with (−)-menthol could have potential as cooling agents. In the following note, we describe an efficient synthetic route to a diverse set of menthol glycinates (Scheme 1).
Scheme 1. Menthol Glycinates (1).
Results and Discussion
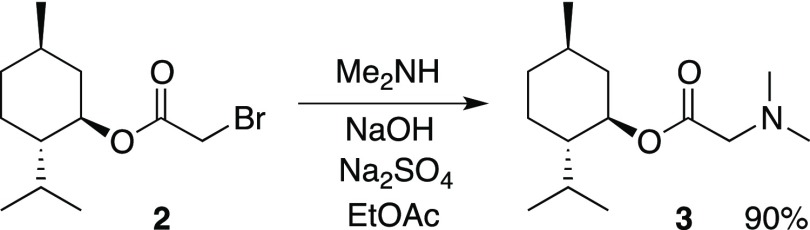
Initial efforts to prepare menthol glycinates were sought to couple glycine and related derivatives with (−)-menthol. However, these reactions were less than satisfactory; so, an alternative route was devised. We hypothesized that the menthol glycinates could be built stepwise by a sequence involving acyl transfer chemistry followed by nucleophilic substitution. Thus, an optimized procedure coupled (−)-menthol with bromoacetyl bromide in dichloromethane (DCM) with excess anhydrous sodium carbonate (Scheme 2). The conversion is quantitative, and product 2 is sufficiently pure that no purification steps are generally required. The acyl transfer chemistry is complete within 3 h, after which the product is isolated by simple filtration and removal of the DCM solvent. If nuclear magnetic resonance (NMR) or gas chromatography (GC) analysis of 2 shows any remaining (−)-menthol, product 2 is readily purified by vacuum distillation. Compound 2 has been previously synthesized by various methods, including coupling menthol with bromoacetic acid, bromoacetyl chloride, and bromoacetyl bromide.5 In addition to the quantitative yield, our procedure has the advantage of avoiding an aqueous workup of the product mixture. Similar coupling reactions are known for chloroacetyl chloride;6 however, in our hands, these conversions were not as clean as the synthesis of derivative 2. Compound 2 is the ideal scaffold for building menthol glycinates as it is expected to be highly reactive toward nucleophilic substitution.5a
Scheme 2. Preparation of Bromoacetate Substrate (2).
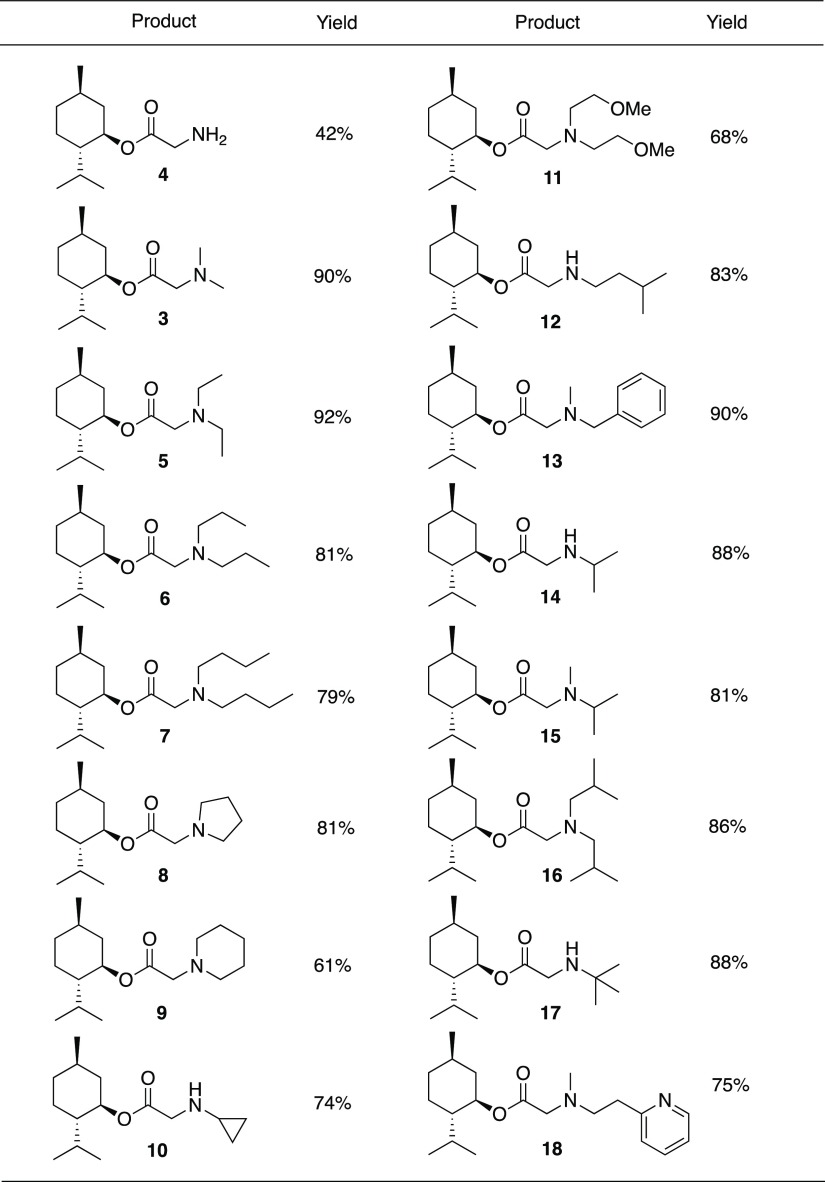
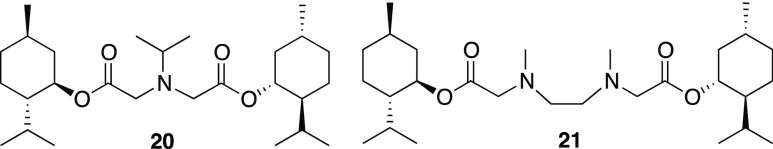
An optimized substitution reaction was developed by reacting compound 2 with amines in ethyl acetate (Table 1). For example, dimethylamine is added to a solution of bromide 2 with added NaOH and anhydrous Na2SO4 (Scheme 3). With vacuum distillation, menthol glycinate 3 is isolated in 90% yield. In order to achieve substitution with ammonia, the chemical reaction performed by adding an ethyl acetate solution of bromide 2 directly into a flask containing liquid ammonia at −78 °C. This provides the glycine ester of menthol (4) in good yield. A variety of secondary amines provided the corresponding menthol glycinates. This includes the dialkylamines to give products 5–7, 13, and 15–16. Heterocyclic systems, such as pyrrolidine and piperidine, were also found to give the substitution products (8 and 9, respectively) in good yields. Primary amines also give the expected substitution products 10, 12, 14, and 17. The synthetic method is amenable to the incorporation of structural components such as cycloalkyl groups, benzyl groups, ethers, and heterocycles. In the case of the pyridyl derivative 18, this compound is modeled after the known cooling agent, amide 19 (FEMA 4549, Scheme 4), which is estimated to be about 100 times cooler than menthol.3 In addition to monosubstitution, products may be prepared from double-substitution reactions. These types of structures could potentially be used in the design of multivalent ligands, a principle that has been used to improve the binding of substrates to receptor sites.7 Isopropyl amine reacts twice when an excess of bromide 2 is used, and compound 20 is isolated. Similarly, N,N′-dimethylethylene diamine reacts twice with bromide 2 to provide compound 21. Both 20 and 21 are purified by the removal of excess bromide 2 via distillation, followed by purification using silica gel chromatography (Scheme 5).
Table 1. Products and Yields from the Substitution Reactions of Bromide 2 with Amines or Ammonia.
Scheme 3. Preparation of Menthol Glycinate (3).
Scheme 4. Structure of the Cooling Agent FEMA 4549.
Scheme 5. Double-Substitution Products Prepared.
For most of the substitution reactions described above, the optimized procedure involves using an excess of the amine nucleophile. The menthol glycinate products are isolated in pure form by removing the ethyl acetate solvent and excess amine by reduced pressure. Then, menthol glycinate is typically distilled at 150–220 °C @ 1 mm Hg. Optimization studies revealed that the excess amine allows the substitution chemistry to go to completion within a relatively short period of time. For example, dibutylamine was reacted with bromide 2 to provide compound 7, and with 1.1 equiv of the amine, the substitution reaction is only 79% complete after 3 h. If the amount of dibutylamine is increased to 1.6 equiv (0.13 M in ethyl acetate), then the substitution reaction is 100% complete in less than 3 h.
Conclusions
In summary, we have developed a simple methodology to prepare a series of menthol glycinates. Starting from bromoacetyl bromide, bromoester 2 is prepared quantitatively. This substrate undergoes substitution reactions with a series of N-nucleophiles to give menthol glycinates 3–21 in generally good yields. The procedure avoids the need for extractions or aqueous workup. The products menthol glycinates are considered potential cooling agents, similar to other derivatives of menthol. As variants of (−)-menthol, it is expected that some of these derivatives should have strong affinities for the TRMP8 receptor site which provides the physiological sensation of cooling, as well as other biological or medicinal effects.8 Preliminary screening of the new menthol glycinates has shown that several derivatives possess intense oral cooling sensations,9 including delayed onset cooling and prolonged cooling. Further testing of their potential as new cooling agents is currently in progress.
Experimental Section
Preparation of (1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-Bromoacetate (2)10
To 100 mL of CH2Cl2, (l)-menthol (7.2 g, 0.046 mmol) is dissolved and anhydrous Na2CO3 is added. The resulting solution is cooled to 0 °C and the flask is fitted with a CaCl2 drying tube. Bromoacetyl bromide (4.0 mL, 0.046 mol) is then added, the cooling bath is removed, and the solution is stirred for a minimum of 4 h. Following the reaction period, the solution is filtered through a glass wool and the solvent is removed by a vacuum. The product is isolated as a clear colorless oil (12.2 g, 0.044 mol, 96%). The analysis of the crude product by GC-flame ionization detector (FID) and NMR shows an extremely high purity of the product; however, the oil may be further refined by distillation (ca. 120 °C, 2 mm). With repeated runs, the yields of the crude product vary from 95 to 100%.
General Procedure for the Synthesis of Menthol Glycinates (1° Amines)
The amine (0.02 mmol) is dissolved in 25 mL of EtOAc, after which is added NaOH (0.6 g, 15 mmol) and anhydrous sodium sulfate (0.5 g, 3.5 mmol). To this solution, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (1.68 g, 6.06 mmol) is slowly added. The mixture is stirred for 6 h or until the GC-FID analysis shows no remaining bromoester (2). The solution is then filtered through a plug of glass wool and the solvent is removed by reduced pressure. For low-boiling amines, the excess amine is removed during this step. For less-volatile amines, fractional distillation may be necessary. The final purification of the menthol glycinate is achieved by vacuum distillation.
General Procedure for the Synthesis of Menthol Glycinates (2° Amines)
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-bromoacetate (1.68 g, 6.06 mmol) is dissolved in 25 mL of EtOAc. To this solution, the amine (9 mmol) is added, followed by NaOH (0.6 g, 15 mmol) and anhydrous sodium sulfate (0.5 g, 3.5 mmol). The mixture is stirred for 6 h or until the GC-FID analysis shows no remaining bromoester (2). The solution is then filtered through a plug of glass wool, and the solvent is removed by reduced pressure. For low-boiling amines, the excess amine is removed during this step. For less-volatile amines, fractional distillation may be necessary. Final purification of the menthol glycinate is achieved by vacuum distillation.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Dimethylamino)acetate (3)11
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with dimethylamine (2.0 M solution in tetrahydrofuran) to provide methyl glycinate 3 in 86% yield as a clear oil (bp, ca. 140 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.36–0.42 (m, 2H), 0.42–0.48 (m, 2H), 0.78 (d, 3H, J = 7.0 Hz), 0.85–0.90 (m, 6H), 0.96–1.13 (m, 2H), 1.34–1.44 (m, 1H), 1.46–1.57 (m, 1H), 1.67–1.73 (m, 2H), 1.82–1.90 (m, 1H), 1.97–2.04 (m, 1H), 2.20–2.26 (m, 1H), 2.40 (br s, 1H), 3.34–3.42 (m, 2H), 4.71–4.76 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.2, 20.7, 22.0, 23.3, 26.3, 31.4, 34.2, 40.9, 45.1, 46.9, 60.5, 74.6, 169.9. Low-resolution MS (electron impact ionization): 241 (M+), 226, 138, 123, 102.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-Aminoacetate (4)12
Into a cooled (−78 °C) round-bottom flask, ammonia (ca. 2 mL, 80 mmol) is condensed and NaOH (0.5 g, 12.5 mmol) is added to the flask. To this flask, an addition funnel is connected. Menthol bromoacetate (1, 1.4 g, 5.0 mmol) is dissolved in 10 mL of ethyl acetate, and this solution is placed in the addition funnel. The solution of 1 is then added slowly to liquid ammonia. The resulting mixture is stirred at −30 °C and monitored by periodically taking samples and subjecting these samples to GC-mass spectrometry (MS) analysis. Typically, the conversion is complete within 6 h. If it is only partially complete, additional ammonia is condensed into the cooled flask. Following the completion of the reaction, the mixture is allowed to warm to room temperature and the excess ammonia boils off. To the reaction mixture, anhydrous sodium sulfate is added and the mixture is filtered through a plug of silica gel. The reaction flask is rinsed with 10 mL of ethyl acetate, and the solution is passed through silica gel. The solvent is removed by a vacuum to provide a clear oil. Further purification is accomplished by vacuum distillation at 150 °C (1 mm) and 0.467 g (2.2 mmol, 42%).
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Diethylamino)acetate (5)13
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with diethylamine to provide methyl glycinate 5 in 92% yield as a clear oil (bp, ca. 160 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.73 (d, 3H, J = 7.0 Hz), 0.82–0.91 (m, 7H), 0.92–1.2 (m, 1H), 1.05 (t, 6H, J = 7.2 Hz), 1.31–1.40 (m, 1H), 1.40–1.54 (m, 1H), 1.60–1.73 (m, 2H), 1.79–1.89 (m, 1H), 1.94–2.04 (m, 1H), 2.66 (q, 4H, J = 7.2 Hz), 3.30 (s, 2H), 4.67–4.76 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 9.1, 11.2, 16.0, 20.7, 21.8, 23.1, 26.1, 31.4, 33.8, 40.4, 42.6, 46.5, 56.9, 58.4, 77.8, 164.4. Low-resolution MS (electron impact ionization): 269 (M+), 132, 130, 116, 102.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Dipropylamino)acetate (6)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with dipropylamine to provide methyl glycinate 6 in 81% yield as a clear oil (bp, ca. 170 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.73 (d, 3H, J = 7.0 Hz), 0.80–0.89 (m, 13H), 0.90–1.08 (m, 1H), 1.30–1.51 (m, 6H), 1.60–1.67 (m, 2H), 1.79–1.88 (m, 1H), 1.92–2.03 (m, 1H), 2.48–2.55 (m, 4H), 3.29 (s, 2H), 4.65–4.74 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 11.7, 16.2, 20.6, 20.7, 22.0, 23.3, 26.2, 31.3, 34.2, 40.9, 46.9, 55.2, 56.3, 74.1, 171.1. Low-resolution MS (electron impact ionization): 297 (M+), 268, 158, 130, 114, 102. High-resolution MS (ESI): calcd for C18H36NO2, 298.2746; found, 298.2731.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Dibutylamino)acetate (7)
Using the general procedure for 2° amines (on a larger scale), (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2, 12.97 g, 0.047 mol) is reacted with dibutylamine (16 mL, 0.095 mol) to provide methyl glycinate 7 (12.08 g, 0.036 mmol, 79% yield) as a clear oil (bp, ca. 200 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.72 (d, 3H, J = 7.0), 0.83–0.90 (m, 13H), 0.90–1.10 (m, 3H), 1.19–1.53 (m, 9H), 1.61–1.69 (m, 2H), 1.76–1.90 (m, 1H), 1.91–2.01 (m, 1H), 2.54 (t, 4H, J = 7.2), 3.27 (s, 2H), 4.65–4.74 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 14.0, 16.2, 20.7, 22.0, 23.3, 26.2, 29.6, 31.3, 34.2, 40.9, 47.0, 54.2, 55.2, 74.1, 171.2. Low-resolution MS (electron impact ionization): 325 (M+), 282, 144, 143, 142, 102, 100. High resolution MS (ESI): calcd for C20H40NO2, 326.3059; found, 326.3038.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Pyrrolidin-1-yl)acetate (8)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with pyrrolidine to provide methyl glycinate 8 in 81% yield as a clear oil (bp, ca. 160 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.75 (d, 3H, J = 7.0), 0.82–0.93 (m, 7H), 0.95–1.11 (m, 2H), 1.33–1.40 (m, 1H), 1.41–1.56 (m, 1H), 1.64–1.72 (m, 2H), 1.76–1.92 (m, 5H), 1.95–2.07 (m, 1H), 2.60–2.70 (m, 4H), 3.25–3.38 (m, 2H), 4.71–4.80 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.7, 22.0, 23.4, 23.8, 26.3, 31.4, 34.2, 40.9, 46.9, 53.9, 57.1, 74.3, 170.4. Low-resolution MS (electron impact ionization): 267 (M+), 224, 130, 128, 100. High-resolution MS (ESI): calcd for C16H33NO2, 268.2277; found, 268.2238.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Piperidin-1-yl)acetate (9)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with piperidine to provide methyl glycinate 9 in 61% yield as a clear oil (bp, ca. 180 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.70 (d, 3H, J = 4.2 Hz), 0.72–0.80 (m, 1H), 0.84 (d, 3H, J = 3.6 Hz), 0.86 (d, 3H, J = 3.0 Hz), 0.87–1.0 (m, 3H), 1.34–1.42 (m, 3H), 1.54–1.65 (m, 6H), 1.72–1.86 (m, 1H), 1.90–1.96 (m, 1H), 2.37–2.57 (m, 4H), 3.07 and 3.14 (ABq, 2H, J = 16.5 Hz), 4.61–4.75 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.7, 22.0, 23.4, 26.3, 31.4, 34.2, 41.0, 42.2, 46.9, 53.9, 58.2, 74.6, 170.0. Low-resolution MS (electron impact ionization): 281 (M+), 266, 144, 142. High-resolution MS (ESI): calcd for C17H32NO2, 282.2433; found, 282.2404.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Cyclopropylamino)acetate (10)
Using the general procedure for 1° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with cyclopropylamine to provide methyl glycinate 10 in 74% yield as a clear oil (bp, ca. 140 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.36–0.42 (m, 2H), 0.42–0.48 (m, 2H), 0.78 (d, 3H, J = 7.0 Hz), 0.85–0.90 (m, 6H), 0.96–1.13 (m, 2H), 1.34–1.44 (m, 1H), 1.46–1.57 (m, 1H), 1.67–1.73 (m, 2H), 1.82–1.90 (m, 1H), 1.97–2.04 (m, 1H), 2.20–2.26 (m, 1H), 2.40 (br s, 1H), 3.34–3.42 (m, 2H), 4.71–4.76 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 6.2, 6.3, 16.4, 20.7, 22.0, 23.5, 26.3, 29.9, 31.4, 34.2, 40.9, 47.0, 50.8, 74.7, 172.2. Low-resolution MS (electron impact ionization): 253 (M+), 224, 138, 116, 102. High-resolution MS (ESI): calcd for C15H28NO2, 254.2120; found, 254.2083.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Bis(2-methoxyethyl)amino)acetate (11)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with bis(2-methoxyethyl)amine to provide methyl glycinate 11 in 68% yield as a clear oil (bp, ca. 200 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.69 (d, 3H, J = 7.0), 0.75–0.86 (m, 7H), 0.88–1.07 (m, 2H), 1.25–1.35 (m, 1H), 1.35–1.48 (m, 1H), 1.54–1.66 (m, 2H), 1.73–1.85 (m, 1H), 1.87–1.96 (m, 1H), 2.83–2.90 (m, 4H), 3.24–3.28 (m, 6H), 3.37–3.45 (m, 6H), 4.59–4.71 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.2, 20.7, 22.0, 23.3, 26.2, 31.3, 34.2, 41.0, 46.9, 53.9, 55.9, 58.7, 71.4, 74.1, 171.1. Low-resolution MS (electron impact ionization): 297 (M+), 284, 190, 160, 146. High-resolution MS (ESI): calcd for C18H36NO4, 330.2644; found, 330.2615.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Isopentylamino)acetate (12)
Using the general procedure for 1° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with isopentylamine to provide methyl glycinate 12 in 90% yield as a clear oil (bp, ca. 180 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.75 (d, 3H, J = 7.0 Hz), 0.87–0.92 (m, 13H), 0.95–1.01 (m, 1H), 1.03–1.13 (m, 1H), 1.35–1.43 (m, 3H), 1.43–1.59 (m, 1H), 1.61–1.74 (m, 3H), 1.77–1.89 (m, 1H), 1.95–2.03 (m, 1H), 2.10 (s, 1H), 2.59–2.64 (m, 2H), 3.38 (s, 2H), 4.70–4.79 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.7, 21.9, 22.6, 23.4, 26.0, 26.3, 31.4, 34.2, 38.9, 40.9, 47.0, 47.7, 51.1, 74.7, 171.9. Low-resolution MS (electron impact ionization): 283 (M+), 226, 145, 138, 123, 100. High-resolution MS (ESI): calcd for C17H34NO2, 284.2590; found, 284.2554.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Benzyl(methyl)amino)acetate (13)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with N-methylbenzylamine to provide methyl glycinate 13 in 90% yield as a clear oil (bp, ca. 210 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.78 (d, 3H, J = 7.0 Hz), 0.89 (d, 3H, J = 7.0 Hz), 0.91 (d, 3H, J = 7.0 Hz), 0.96–1.14 (m, 2H), 1.34–1.43 (m, 1H), 1.44–1.57 (m, 1H), 1.64–1.72 (m, 2H), 1.82–1.92 (m, 1H), 1.99–2.05 (m, 1H), 2.39 (s, 3H), 3.24 (s, 2H), 3.69 (s, 2H), 4.73–4.82 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.7, 22.0, 23.4, 26.3, 31.4, 34.2, 41.0, 47.0, 57.7, 74.2, 127.1, 128.2, 129.1, 138.4, 170.5. Low-resolution MS (electron impact ionization): 317 (M+), 180, 178, 135, 134, 120. High-resolution MS (ESI): calcd for C20H32NO2, 318.2433; found, 318.2372.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Isopropylamino)acetate (14)
Using the general procedure for 1° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with isopropylamine to provide methyl glycinate 14 in 88% yield as a clear oil (bp, ca. 140 °C at 1 mm). 1H NMR (500 MHz, CDCl3): δ 0.75 (d, 3H, J = 7.0 Hz), 0.84–0.91 (m, 7H), 0.92–1.0 (m, 1H), 1.05 (d, 6H, J = 6.3 Hz), 1.34–1.40 (m, 1H), 1.45–1.53 (m, 1H), 1.64–1.69 (m, 2H), 1.80–1.86 (m, 1H), 1.96–2.00 (m, 1H), 2.79 (sep, 1H, J = 6.2 Hz), 2.85 (br s, 1H), 3.35–3.39 (m, 2H), 4.70–4.76 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 16.3, 20.7, 22.0, 22.6, 23.4, 26.3, 31.3, 34.2, 40.9, 47.0, 48.3, 48.8, 48.8, 74.7, 172.2. Low-resolution MS (electron impact ionization): 255 (M+), 240, 138, 116, 102. High-resolution MS (ESI): calcd for C15H30NO2, 256.2277; found, 256.2262.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Isopropyl(methyl)amino)acetate (15)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with methyl-iso-propylamine to provide methyl glycinate 15 in 88% yield as a clear oil (bp, ca. 160 °C at 1 mm). 13C NMR (75 MHz, CDCl3): δ 16.2, 18.4, 18.4, 20.7, 22.0, 23.4, 26.3, 31.3, 34.2, 38.3, 40.9, 46.9, 53.4, 55.1, 74.3, 171.0. Low-resolution MS (electron impact ionization): 269 (M+), 254, 138, 132, 130, 116. High-resolution MS (ESI): calcd for C16H32NO2, 270.2433; found, 270.2405.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Diisobutylamino)acetate (16)
Using the general procedure for 2° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with diisobutylamine to provide methyl glycinate 16 in 86% yield as a clear oil (bp, ca. 180 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.74 (d, 3H, J = 7.0), 0.82–0.91 (m, 19H), 0.92–1.11 (m, 2H), 1.30–1.38 (m, 1H), 1.40–1.54 (m, 1H), 1.60–1.71 (m, 4H), 1.81–1.91 (m, 1H), 1.94–2.01 (m, 1H), 2.31 (d, 4H, J = 7.3), 3.26 (s, 2H), 4.65–4.74 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.2, 20.6, 20.7, 22.0, 23.3, 26.2, 26.7, 31.4, 34.2, 41.0, 47.0, 56.0, 63.4, 73.9, 171.6. Low-resolution MS (electron impact ionization): 325 (M+), 283, 282, 144, 142, 100. High-resolution MS (ESI): calcd for C20H40NO2, 326.3059; found, 326.2999.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(tert-Butylamino)acetate (17)
Using the general procedure for 1° amines, (1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2) is reacted with tert-butylamine to provide methyl glycinate 17 in 78% yield as a clear oil (bp, ca. 180 °C at 1 mm). 1H NMR (300 MHz, CDCl3): δ 0.73 (d, 3H, J = 7.0 Hz), 0.86–0.90 (m, 7H), 0.90–1.04 (m, 1H), 1.09 (s, 9H), 1.29–1.37 (m, 1H), 1.38–1.54 (m, 1H), 1.57–1.72 (m, 3H), 1.79–1.92 (m, 1H), 1.94–2.07 (m, 1H), 3.35 (s, 2H), 4.68–4.77 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.7, 21.9, 23.4, 26.2, 28.7, 31.3, 34.2, 40.8, 44.9, 47.0, 50.2, 74.6, 172.7. Low-resolution MS (electron impact ionization): 269 (M+), 254, 130, 116. High-resolution MS (ESI): calcd for C16H32NO2, 270.2433; found, 270.2416.
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-(Methyl(2-(pyridin-2-yl)ethyl)amino)acetate (18)
(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-bromoacetate (2, 1.3 g, 4.69 mmol) is dissolved in 25 mL of EtOAc, and to this solution is added NaOH (1.2 g, 0.03 mol) and anhydrous Na2SO4 (0.5 g). 2-(2-Methylaminoethyl)pyridine (1.0 mL, 7.22 mmol) is added to the solution, and the mixture is stirred at room temperature for 4 h or until the GC-FID analysis shows no remaining bromoester 2. The solution is then filtered through a glass wool and the solvent is removed under reduced pressure. The resulting oil is subjected to vacuum distillation (120 °C at 1 mm for 2 h) to remove excess 2-(2-methylaminoethyl)pyridine. The residue oil is taken up in 20 mL of EtOAc and passed through a plug of SiO2. Following the removal of the solvent, an oil is obtained which is primarily methyl glycinate 18 (1.54 g, 4.63 mmol, 99%). The product is further refined by distillation (bp, ca. 230 °C at 1 mm) to provide pure methyl glycinate 18 in 75% yield as a clear oil (1H NMR (300 MHz, CDCl3): δ 0.73 (d, 3H, J = 7.0 Hz), 0.80–0.92 (m, 7H), 0.89–1.10 (m, 2H), 1.31–1.43 (m, 1H), 1.43–1.54 (m, 1H), 1.61–1.70 (m, 2H), 1.77–1.89 (m, 1H), 1.94–2.05 (m, 1H), 2.44 (s, 3H), 2.89–3.01 (m, 4H), 3.29 (s, 2H), 4.69–4.78 (m, 1H), 7.06–7.11 (m, 1H), 7.18 (d, 1H, J = 7.8 Hz), 7.54–7.60 (m, 1H), 8.49–8.51 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 16.2, 20.7, 22.0, 23.4, 26.3, 31.4, 34.2, 36.3, 41.0, 46.9, 56.8, 58.7, 74.4, 121.1, 123.1, 136.3, 149.2, 160.2, 170.5. High-resolution MS (ESI): calcd for C20H33N2O2, 333.2542; found, 333.2513.
Bis((1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl) 2,2′-(Isopropylazanediyl)diacetate (20)
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-bromoacetate (2, 2.33 g, 8.4 mmol) is dissolved in 25 mL of EtOAc, and to this solution is added NaOH (1.2 g, 0.03 mol) and anhydrous Na2SO4 (0.5 g). Isopropylamine (0.25 mL, 2.9 mmol) is added to the solution, and the mixture is stirred at room temperature for 5 h or until the GC-FID analysis shows no remaining bromoester 2. The solution is then filtered through a glass wool and the solvent is removed under reduced pressure. The resulting oil is subjected to vacuum distillation (160 °C at 1 mm for 20 min) to remove excess bromoester 2. The distillate is a clear oil (ca. 0.5 g) which is identified as a mixture of bromoester 2 and the desired product 20. The residue oil is taken up in 20 mL of EtOAc and passed through a plug of SiO2. Following the removal of the solvent, a clear oil is obtained, which is methyl glycinate 20 (0.425 g, 0.94 mmol, 32%). 1H NMR (300 MHz, CDCl3): δ 0.76 (d, 6H, J = 6.9 Hz), 0.85–0.95 (m, 14H), 0.94–1.04 (m, 2H), 1.04–1.10 (m, 4H), 1.33–1.42 (m, 2H), 1.42–1.55 (m, 2h), 1.64–1.73 (m, 4H), 1.80–1.93 (m, 2H), 1.96–2.04 (m, 2H), 3.04–3.13 (sep, 1H, J = 6.5 Hz), 3.54 (s, 4H), 4.68–4.77 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 16.3, 20.3, 20.7, 22.0, 23.4, 26.3, 31.4, 34.3, 40.9, 47.0, 52.7, 74.2, 171.8. High-resolution MS (ESI): calcd for C27H50NO4, 452.3740; found, 452.3701.
Bis((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl) 2,2′-(Ethane-1,2-diylbis(methylazanediyl))diacetate (21)
(1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl 2-bromoacetate (2, 2.13 g, 7.7 mmol) is dissolved in 50 mL of EtOAc, and to this solution is added NaOH (1.2 g, 0.03 mol) and anhydrous Na2SO4 (0.5 g). N,N-Dimethylethylenediamine (0.3 mL, 2.79 mmol) is added to the solution, and the mixture is stirred at room temperature for 5 h or until the GC-FID analysis shows no remaining bromoester 2. The solution is then filtered through a glass wool and the solvent is removed under reduced pressure. The resulting oil is subjected to vacuum distillation (170–190 °C at 1 mm for 30 min) to remove excess bromoester 2. The residue yellow oil is taken up in 20 mL of EtOAc and passed through a plug of SiO2. Following the removal of the solvent, a clear oil is obtained, which is methyl glycinate 21 (0.84 g, 1.75 mmol, 43%). 1H NMR (500 MHz, CDCl3): δ 0.78 (d, 6H, J = 6.7 Hz), 0.82–0.95 (m, 14H), 0.96–1.13 (m, 4H), 1.37–1.42 (m, 2H), 1.43–1.55 (br s, 2H), 1.70 (d, 4H, J = 2.4 Hz), 1.80–1.90 (m, 2H), 2.00 (d, 2H, J = 6.9 Hz), 2.48 (s, 6H), 2.82 (s, 4H), 3.42 (s, 4H), 4.74–4.78 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 16.3, 20.7, 22.0, 23.4, 26.3, 31.4, 34.2, 41.0, 42.2, 46.9, 53.9, 58.2, 74.6, 170.0. High-resolution MS (ESI): calcd for C28H53N2O4, 481.4005; found, 481.3941.
Acknowledgments
We acknowledge the generous support from the NSF MRI program (award no. CHE:1726931) for the purchase of the high-resolution mass spectrometer.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03624.
1H and 13C NMR of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Eccles R. Menthol and Related Cooling Compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Update on Menthol Production & Use. http://www.leffingwell.com/menthol1/menthol1.htm, downloaded Dec 31, 2018.
- Leffingwell J. C.Cool without Menthol & Cooler than Menthol and Cooling Compounds as Insect Repellents. http://www.leffingwell.com/cooler_than_menthol.htm, downloaded Dec 31, 2018.
- Bauer K.; Garbe D.; Surburg H.. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 4th ed.; Wiley-VHC: Weinheim, Germany, 2001. [Google Scholar]
- a Hettstedt C.; Betzl W.; Karaghiosoff K. Synthesis and Characterization of (-)-Menthyl Containing N-Alkyl Cycloimmonium Salts. Z. Anorg. Allg. Chem. 2012, 638, 377–382. 10.1002/zaac.201100451. [DOI] [Google Scholar]; . For alternative routes leading to compound 2, see:; b Shankar S. P.; Jagodzinska M.; Malpezzi L.; Lazzari P.; Manca I.; Greig I. R.; Sani M.; Zanda M. Synthesis and structure-activity relationship studies of novel tubulysin U analogues - effect on cytotoxicity of structural variations in the tubuvaline fragment. Org. Biomol. Chem. 2013, 11, 2273–2287. 10.1039/c3ob27111k. [DOI] [PubMed] [Google Scholar]; c Singh R.; Ghosh S. K. Diastereoselective synthesis of a highly functionalized cyclohexene embedded with a quaternary stereogenic centre by self Diels–Alder dimerization of a [3]dendralene attached to a (−)-menthol auxiliary. Tetrahedron: Asymmetry 2014, 25, 57–62. 10.1016/j.tetasy.2013.11.005. [DOI] [Google Scholar]; d Streinz L.; Koutek B.; Saman D. Solvent- and base-free dicyclohexylcarbodiimide-promoted esterifications. Synlett 2001, 0809–0811. 10.1055/s-2001-14605. [DOI] [Google Scholar]
- Dentel H.; Chataigner I.; Lohier J.-F.; Gulea M. Asymmetric diastereoselective thia-hetero-Diels-Alder reactions of dithio esters. Tetrahedron 2012, 68, 2326–2335. 10.1016/j.tet.2012.01.039. [DOI] [Google Scholar]
- a Mammen M.; Choi S.-K.; Whitesides G. M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem., Int. Ed. 1998, 37, 2754–2794. . [DOI] [PubMed] [Google Scholar]; b Jahromi A. H.; Fu Y.; Miller K. A.; Nguyen L.; Luu L. M.; Baranger A. M.; Zimmerman S. C. Developing Bivalent Ligands to Target CUG Triplet Repeats, the Causative Agent of Myotonic Dystrophy Type 1. J. Med. Chem. 2013, 56, 9471–9481. 10.1021/jm400794z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Journé A.-S.; Habib S. A. M.; Dodda B. R.; Morcos M. N. F.; Sadek M. S.; Tadros S. A. A.; Witt-Enderby P. A.; Jockers R.; Zlotos D. N1-Linked Melatonin Dimers as Bivalent Ligands Targeting Dimeric Melatonin Receptors. MedChemComm 2014, 5, 792–796. 10.1039/c4md00079j. [DOI] [Google Scholar]; d Peng X.; Neumeyer J. Kappa Receptor Bivalent Ligands. Curr. Top. Med. Chem. 2007, 7, 363–373. 10.2174/156802607779941251. [DOI] [PubMed] [Google Scholar]
- a Yin Y.; Wu M.; Zubcevic L.; Borschel W. F.; Lander G. C.; Lee S.-Y. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. 10.1126/science.aan4325. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shirai T.; Kumihashi K.; Sakasai M.; Kusuoku H.; Shibuya Y.; Ohuchi A. Identification of a Novel TRPM8 Agonist from Nutmeg: A Promising Cooling Compound. ACS Med. Chem. Lett. 2017, 8, 715–719. 10.1021/acsmedchemlett.7b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]; and reference cited therein
- The menthol glycinate compounds were subjected to preliminary safety evaluation using toxicology prediction databases. Organoleptic evaluations were then done using sip and spit testing methodology of aqueous solutions containing 100 ppm of the compounds.
- Ma X.-P.; Zhu J.-F.; Wu S.-Y.; Chen C.-H.; Zou N.; Liang C.; Su G.-F.; Mo D.-L. Cycloaddition of Fluorenone N-Aryl Nitrones with Methylenecyclopropanes and Sequential 1,3-Rearrangement: An Entry to Synthesis of Spirofluorenylpiperidin-4-ones. J. Org. Chem. 2017, 82, 502–511. 10.1021/acs.joc.6b02544. [DOI] [PubMed] [Google Scholar]
- Narayan A. R. H.; Jiménez-Osés G.; Liu P.; Negretti S.; Zhao W.; Gilbert M. M.; Ramabhadran R. O.; Yang Y.-F.; Furan L. R.; Li Z.; Podust L. M.; Montgomery J.; Houk K. N.; Sherman D. H. Enzymatic hydroxylation of an unactivated methylene C-H bond guided by molecular dynamics simulations. Nat. Chem. 2015, 7, 653–660. 10.1038/nchem.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M. C.; Bonini B. F.; Boschi F.; Fochi M.; Fini F.; Mazzanti A.; Ricci A. First 1,3-dipolar cycloaddition of azomethine ylides with (E)-ethyl 3-fluoroacrylate: regio- and stereoselective synthesis of enantiopure fluorinated prolines. Synlett 2006, 0543–0546. 10.1055/s-2006-933127. [DOI] [Google Scholar]
- Siemieniuk A.; Szalkowska-Pagowska H.; Lochynski S.; Piatkowski K.; Filipek B.; Krupinska J.; Czarnecki R.; Librowski T.; Zebala K. Synthesis and local anesthetic and circulatory actions of aminoesters and hydroxyamine monoterpene derivatives. Pol. J. Pharmacol. Pharm. 1992, 44, 407–420. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.