Abstract

A set of unique triptycene-based and organic Schiff-base-linked polymers (TBOSBLs) are conveniently synthesized in which triptycene motifs are connected with 1,3,5-triformylphloroglucinol units via Schiff-base linkages. TBOSBLs are amorphous, thermally stable with a reasonable surface area (SABET up to 649 m2/g), and have abundant nanopores (pore size < 100 nm). TBOSBLs are good sorbents for small gas molecules (such as CO2, H2, and N2) and they can selectively capture CO2 over N2. Additionally, TBOSBLs show superior antiproliferative activity against human colorectal cancer cells relative to previously reported covalent organic frameworks (COFs). The mechanism of cell death is also studied elaborately.
1. Introduction
The term “cancer” is usually associated with a disease wherein there is an abnormal cell growth in a certain body organ.1 Cancer is a potentially life-threatening disease that is usually associated with a specific body part but may subsequently spread to other parts of the body. According to the World Health Organization (WHO), “Cancer is the second leading cause of death globally, and was responsible for an estimated 9.6 million deaths in 2018”.2 Based on its origin, cancer can be classified by oncologists as carcinomas, sarcomas, leukemia, and lymphomas.3 Uncontrolled growth of cancer cells is seen most commonly in lungs, followed by breast, colon/rectum, prostate, skin, and stomach.2 As far as cancer mortality is concerned, colorectal cancer ranks second after lung cancer. With the current advancement in medical sciences, prevention of death due to cancer is possible, if the disease is diagnosed and treated at an early stage. Among others, chemotherapy is a common mode of treatment. In general, cancer treatment is expensive requiring costly medications. Moreover, carcinoma cells may become resistant to prevalent chemotherapy by a mechanism called “multi-drug resistance” (MDR). Therefore, contemporary research demands design, development, and screening of new compounds/molecules/materials with antiproliferative activity against various cancer cells.4,5
In present-day research, there is also a lot of research interest in exploring biomedical applications of porous materials.5 Such applications include, but are not limited to, chemotherapy, drug delivery, bioimaging, tissue engineering, and others.6−9 In the context of porous materials, porous organic polymers (POPs) are highly cross-linked macromolecular frameworks containing lighter elements such as hydrogen, carbon, nitrogen, and others. Thus, POPs are distinctly different from metal–organic frameworks (MOFs—another class of porous materials) that are inorganic and contain relatively heavier (metal) atoms. In the literature, there are several examples wherein metal-based porous materials, such as zeolites and MOFs, have been considered for biomedical applications.10 However, the metal-induced toxicity and chemical instability of MOFs make them undesirable candidates for such applications.11,12 On the other hand, the absence of inherent toxicity of metal atoms and higher chemical stability observed in POPs render them more suitable as candidates for biological studies. Additionally, POPs have high surface areas and tunable porosity, and are usually obtained easily from inexpensive and commercially available monomers. Therefore, the current research trend is to explore the interaction of POPs with biological species.6 In this field, the exploration of anticancer activities of POPs decorated with various organic functional groups is still in the stage of infancy. Another major application of porous materials is in the domain of carbon dioxide capture and sequestration (CCS).13,14
Triptycene is a rigid and structurally robust molecule. Its incorporation as a structural motif in polymeric frameworks has yielded POPs having excellent gas storage ability.15−18 The paddle wheel orientation of the arene rings in triptycene causes these units to pack inefficiently in the polymeric network. This also generates an “internal molecular free volume” (IFV) in the resultant material. The void in the form of IFV, in turn, gives rise to desirable characteristics, such as excellent gas storage capability and high surface areas.19
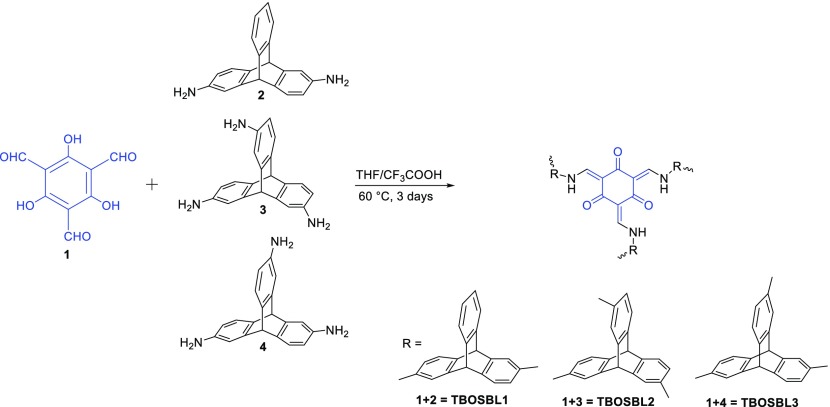
Herein, we report the synthesis of three “triptycene-based and organic Schiff-base-linked” polymers (TBOSBLs) prepared via a facile condensation reaction between di- or triaminotriptycene and 1,3,5-triformylphloroglucinol (TFP). The obtained polymeric networks were characterized using Fourier transform infrared (FTIR) and NMR spectroscopy, thermogravimetric analysis (TGA), and X-ray diffraction (XRD). It was anticipated that TBOSBLs would be porous since they bear triptycene motifs in the polymeric framework.20,21 Thus, we have explored their porous properties (surface area/pore size distribution (PSD)) and measured their ability to capture small gas molecules, such as CO2, H2, and N2.
A thorough literature search revealed that polymeric materials derived from polyphenolic units such as phloroglucinol may have considerable potential in research related to cancer therapy.22,23 This motivated us to explore the anticancer activity of the three TBOSBLs reported herein. Further, we have explored whether TBOSBLs are apoptotic inducers while proposing a mechanism of cell death. TBOSBLs are the only example of triptycene-based POPs whose cytotoxic potential against cancer cells has been studied. The mechanism of cell death was also studied.
2. Results and Discussion
2.1. Synthesis and Characterization of TBOSBL 1–3
2,6-Diaminotriptycene (DAT) and 2,6,14- and 2,7,14-triaminotriptycene were synthesized from triptycene based on a protocol reported previously by Chen and co-workers.24 1,3,5-Triformylphloroglucinol (TFP) was also synthesized, as described in a literature report.25TBOSBLs were synthesized via a facile condensation reaction between di- or triaminotriptycene and 1,3,5-triformylphloroglucinol (TFP) in a solution mixture of anhydrous tetrahydrofuran (THF) and trifluoroacetic acid (TFA, 2 mol % in THF) at 60 °C for 3 days (Scheme 1). The resulting precipitate was collected by suction filtration and washed subsequently with tetrahydrofuran, dimethylformamide (DMF), dimethyl sulfoxide (DMSO), water, methanol, acetone, and dichloromethane, separately. The precipitate obtained was washed with various solvents to ensure removal of any unreacted monomers and undesired byproducts, such as soluble small oligomers. The collected powder was then dried at 120 °C under vacuum for 24 h to yield a yellow powder with high yield and insoluble in common organic solvents.
Scheme 1. Syntheses of TBOSBL 1–3.
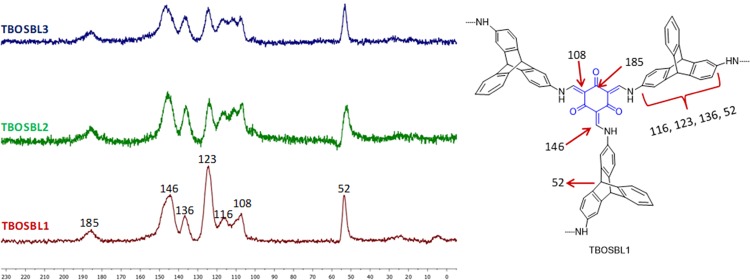
Solid-state 13C cross-polarization magic angle spinning (CP-MAS) NMR spectra were recorded to investigate the formation of TBOSBLs, as proposed in Scheme 2. The 13C CP-MAS spectra of TBOSBLs are depicted in Figure 1. Taking TBOSBL1 as a representative example, peaks with different chemical shifts appearing in its NMR spectrum are assigned appropriately in Figure 1. In all three spectra, the strong signal appearing at 52 ppm is assigned to the bridgehead carbons present in the triptycene unit, thereby indicating the incorporation of triptycene motifs in the reaction product. The peaks in the range 116–136 ppm are due to the carbons that constitute the three arene rings present in the triptycene moiety. The band centered at 185 ppm is assigned to deshielded carbonyl carbon atoms, the low-intensity signal at 146 ppm is assigned to the enamine carbon nuclei, and the peak at 108 ppm is due to sp2 hybridized carbon nuclei of the phloroglucinol unit in the final product. Also, the absence of a unique signal at 191 ppm (due to carbon present in the aldehyde group −CHO of TFP) confirmed that all three aldehyde groups present in triformylphloroglucinol (TFP) molecules reacted completely with aminotriptycenes in the Schiff-base condensation reaction shown in Scheme 1.
Scheme 2. Structures of TBOSBLs.
Figure 1.
Solid-state 13C CP-MAS NMR spectra of TBOSBL 1–3.
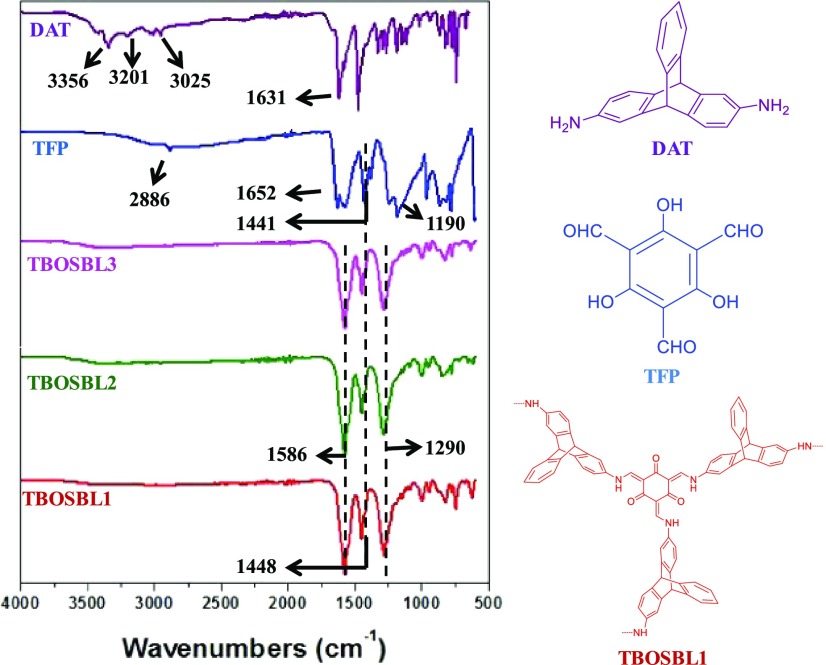
FTIR spectra of TBOSBLs are shown in Figure 2. The FTIR spectrum of TBOSBL1 has been described as a representative example. For comparison, the FTIR spectra of the corresponding triptycene-based amine monomer (DAT) and TFP are also depicted in Figure 2. Upon comparing the FTIR spectra of monomers DAT and TFP, and their polymerized product (TBOSBL1), it can be concluded that both monomers have reacted completely. In the spectrum of TBOSBL1, the absence of the carbonyl (C=O) stretching band characteristic of TFP (1652 cm–1) indicates its consumption in the polymerization reaction and its incorporation in TBOSBL1. Formation of enamine linkages in the products is confirmed from the appearance of a high-intensity band at 1290 cm–1 due to C–N stretching vibrations. The high-intensity signal at 1448 cm–1 is assigned to C–H bending vibrations. The existence of TFP in its keto form (as depicted in Scheme 1) is evident from the appearance of a high-intensity and sharp band at 1586 cm–1 in TBOSBL1 since this is characteristic of C=C bond stretching vibrations observed in the keto form of TFP.26,27 This is also supported by the fact that stretching bands of hydroxyl (in enol form of TFP) are absent in the FTIR spectrum of TBOSBL1.
Figure 2.
FTIR spectra of 2,6-diaminotriptycene (DAT), TFP, and TBOSBLs.
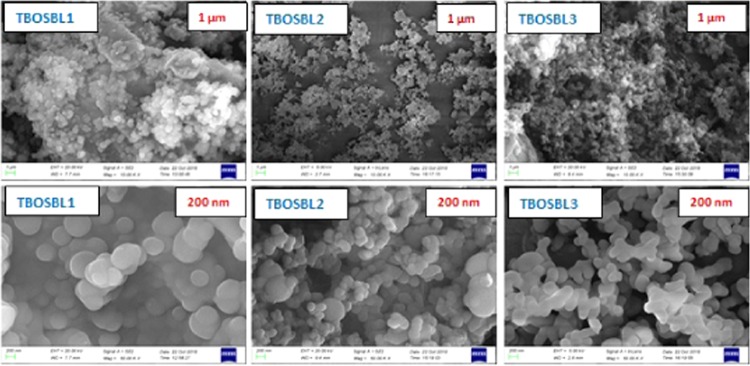
To investigate the structural morphology of TBOSBLs, field emission scanning electron microscopy (FE-SEM) was employed. FE-SEM images of TBOSBLs at two different magnifications (10.00k× and 50.00k×) are shown in Figure 3. Higher resolution images indicate the presence of uniform spherical particles that have self-assembled to form large aggregated particles. From inspection, in the case of TBOSBL1, the spherical particles are dimensionally bigger than those of TBOSBL2 and TBOSBL3. As depicted in Scheme 2, TBOSBL1 is obtained from diaminotriptycene, while the other two TBOSBLs are obtained from triaminotriptycenes. Therefore, in the former case (TBOSBL1), the extent of polymerization is expected to be lower and the chance of aggregation via H-bonding is more. This might have led to the formation of bigger aggregates in the case of TBOSBL1. The grain size of the polymers was estimated from the FE-SEM images, and the average diameter of grains is 400 ± 20, 200 ± 20, and 200 ± 20 nm for TBOSBL1, TBOSBL2, and TBOSBL3, respectively.
Figure 3.
FE-SEM images of TBOSBLs.
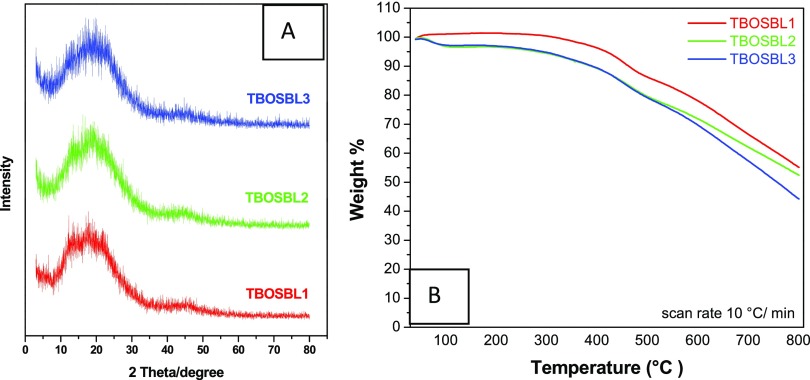
The wide-angle X-ray diffraction (WAXD) plots of TBOSBLs are shown in Figure 4A. Here, the appearance of a hump (2θ value = 18°) suggests that these polymeric materials are amorphous in nature. The Schiff-base condensation reaction is fast and irreversible, and this leads to the incorporation of bulky and robust three-dimensional (3D) rigid triptycene units. The rigidity of triptycene units prevents efficient packing that leads to materials with loss of crystallinity, as observed in TBOSBLs and other previously reported triptycene-based POPs.20,28 Samples of TBOSBL 1–3 are subjected to TGA analysis to record the thermal stability of these polymeric materials. TGA experiments were performed under a constant flow of nitrogen gas in the temperature region of 40–800 °C by heating samples at a rate of 10 °C/min. The TGA plot of each TBOSBL is shown in Figure 4B. The observed initial weight loss in the temperature range of 325–500 °C is attributed to the thermal degradation of organic functional groups. Overall, TBOSBLs may be considered as materials with moderate thermal stability, as observed from the reasonably high thermal degradation temperature (Td = 10% weight loss under a N2 atmosphere) that varies in the range 388–462 °C. We attribute this desirable feature to the inclusion of robust 3D triptycene units in the polymer framework.29,30
Figure 4.
Wide-angle X-ray diffraction (A) and TGA (B) plots of TBOSBLs.
2.2. Porosity Measurements
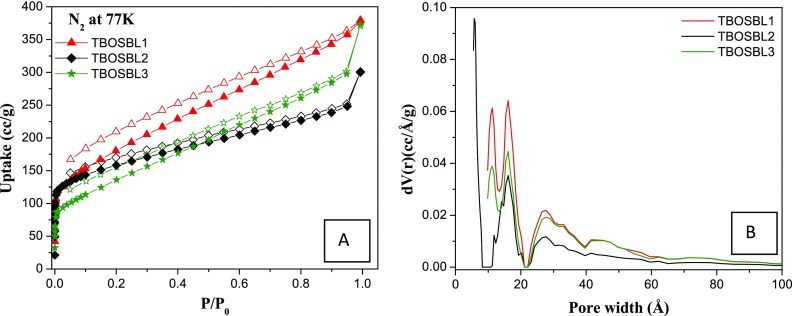
In recent literature related to POPs derived from triptycene-based monomers, it has been observed that such materials have reasonable surface areas with inherent hierarchical porosity for applications such as CO2 capture and hydrogen storage.17,31,32 This prompted us to investigate the Brunauer–Emmett–Teller (BET) surface areas and the pore size distributions of TBOSBLs. Thus, to determine the porous properties of TBOSBLs in detail, N2 adsorption–desorption isotherms were recorded. The features of N2 sorption isotherms (collected at 77 K, Figure 5A) of TBOSBLs suggest that they may be classified as type-II isotherms.33 This class of isotherms are usually given by porous materials that have a hierarchical pore structure (coexistence of narrower micropores and relatively wider mesopores). The presence of micropores was evident from the significantly high gas uptake in the very low relative pressure range (P/P0 = 0–0.01), which is depicted as a sharp increase in the corresponding adsorption isotherm.20 The microporosity present in TBOSBLs may be due to the incorporation of the 3D rigid triptycene units in the polymeric linkages.34 On the other hand, the constant increase in N2 uptake in the relatively higher partial pressure range (P/P0 = 0.1–0.99) suggested the existence of mesopores in the polymeric matrix.35 Using the Brunauer–Emmett–Teller (BET) model, the surface areas were found to be 649 m2/g (TBOSBL1), 570 m2/g (TBOSBL2), and 493 m2/g (TBOSBL3) (Table 1 and Figure S1). The corresponding Langmuir surface areas for TBOSBL1, TBOSBL2, and TBOSBL3 are 1051, 810, and 817 m2/g, respectively (Table 1 and Figure S2). These values are comparable with or better than the surface areas recorded for previously reported microporous materials with Schiff-base linkages. For example, nitrogen-rich microporous and crystalline covalent organic frameworks (COFs) bearing multiple phenolic −OH groups, synthesized via the Schiff-base condensation reaction, have relatively lower SABET (535 m2/g for TpPa-1 and 339 m2/g for TpPa-2) than TBOSBL1.36 Other examples of porous materials with a lower surface area include, but are not limited to, β-ketoenamine-linked COFs (365 m2/g for DAB-TFP COF),37 β-ketoenamine-based COFs (567 m2/g for TAPB-TFP),38 ionic covalent organic nanosheets (TpTGCl, TpTGBr, and TpTGI: 267, 305, and 298 m2/g, respectively),39 porous polymer TPDA-1 (545 m2/g),40 nitrogen-rich porous organic polymers (104–518 m2/g for N-POPs),41 COF materials bearing phloroglucinol building units (223 m2/g for EDTFP-1),22 N-rich porous organic polymer methylenedianiline-triformylphloroglucinol (283 m2/g for MDTFP-1)23 and triazine-functionalized porous covalent organic frameworks (277 m2/g for TpTt COF).25
Figure 5.
N2 adsorption–desorption isotherms at 77 K (A) and pore size distribution (B) of TBOSBLs.
Table 1. Pore Properties of TBOSBLs.
| a | b | H2 at 1 bar (mg/g) | CO2 at 1 bar (mg/g) |
selectivity CO2/N2 | ||||
|---|---|---|---|---|---|---|---|---|
| polymers | SABET (m2/g) | SALang (m2/g)a | Vtotal (cm3/g)b | 77 K | 273 K | 298 K | Qst (kJ/mol) | 273 (298) K |
| TBOSBL1 | 649 | 1051 | 0.527 | 19.5 | 175.6 | 94.4 | 35.1 | 68 (69) |
| TBOSBL2 | 570 | 810 | 0.384 | 15.2 | 149.7 | 114.4 | 32.1 | 106 (63) |
| TBOSBL3 | 493 | 817 | 0.467 | 12.9 | 124.7 | 96.8 | 32.7 | 108 (66) |
Surface area of TBOSBLs calculated based on the Langmuir model from the N2 adsorption isotherms (P/P0 = 0.05–0.35).
The total pore volume of TBOSBLs calculated at P/P0 = 0.99.
As usual, corresponding pore size distribution plots were obtained using the N2 sorption isotherms and the density functional theory (DFT) method. As predicted from the N2 isotherms, the pore size distribution (PSD) profile of TBOSBLs (Figure 5B) having maxima below and above 2 nm confirms that pores are microporous (<2 nm) as well as mesoporous (2–6 nm). The total pore volume of each TBOSBL is calculated from the volume of N2 adsorbed at P/P0 = 0.99, and this parameter is measured to be 0.527 cm3/g for TBOSBL1, 0.384 cm3/g for TBOSBL2, and 0.467 cm3/g for TBOSBL3 (Table 1). These data indicate that both surface area and total pore volume are relatively lower for the POPs (TBOSBL2 and TBOSBL3) derived from the trifunctional triptycenes in comparison with the one (TBOSBL1) derived from the bifunctional triptycene monomer. Thus, the porosity and surface area of TBOSBLs may be tailored easily by changing the number of reactive sites in the monomers.
2.3. Gas Storage and Selective CO2 Capture over N2
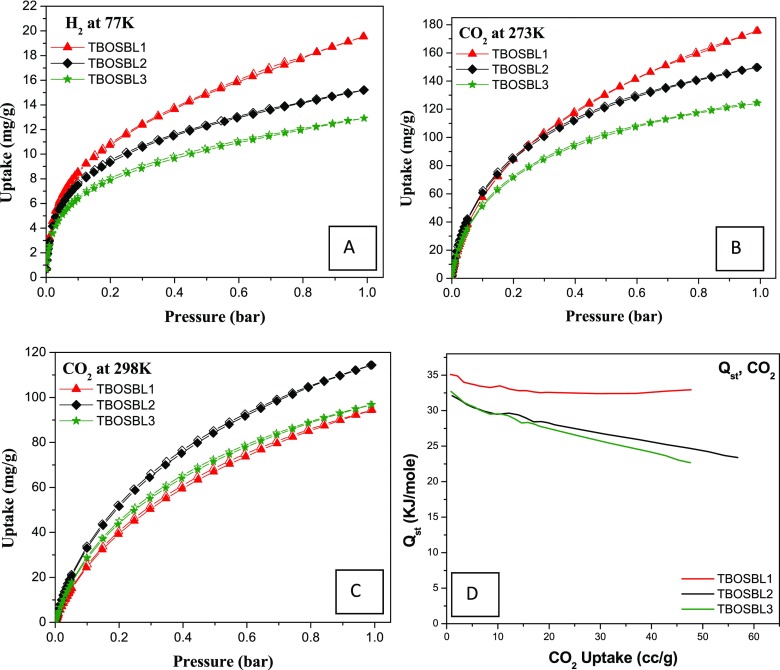
Considering the nanoporous nature of TBOSBLs, we were interested in studying their ability to act as an adsorbent for small gaseous molecules, such H2 and CO2. Therefore, adsorption isotherms of H2 (at 77 K), CO2 (at 273, 298 K), and N2 (at 273, 298 K) were collected at pressures up to 1 bar. The interest in exploring the ability of porous materials to store H2 gas has assumed importance since hydrogen gas is projected to replace fossil fuel for the global energy requirements, especially in the transportation sector. The H2 uptake by TBOSBLs was found to be in the range of 12.9–19.5 mg/g (Table 1 and Figure 6A). The highest value was observed for TBOSBL1 (19.5 mg/g or 1.95 wt % at 77 K), and this value was better than those of various microporous organic polymers and COFs—representative examples are triptycene-derived azo polymers (TAP3, 1.44 wt %),20 azo-Trip (1.46 wt %),42 3D ultramicroporous triptycene-based polyimide frameworks (1.41 wt %),43 carbazole-based porous organic polymers (CPOPs 1.19–1.29 wt %),44 solvothermally synthesized TpBD (0.7 wt %),45 TpPa-1, TpPa-2 (1.1, 0.89 wt %),45 TAPB-TFP (1.08 wt %),38 and porous porphyrin organic polymers (TpTph, 1.25 wt %).46 From the above comparison, it is clear that TBOSBLs reported herein have improved H2 uptake capabilities than several microporous polymers and imine-based COFs.
Figure 6.
H2 uptake isotherms at 77 K (A), CO2 uptake isotherms at 273 K (B) and 298 K (C), and Qst for CO2 (D) of TBOSBLs.
The continuous increase in the concentration of atmospheric CO2 is a very serious environmental issue. The main challenges are to propose efficient means to remove atmospheric CO2 and develop technologies for effective carbon dioxide capture and sequestration (CCS). In this context, physisorption of carbon dioxide by porous materials is a widely researched CCS process that has been highlighted in several reviews. It must be mentioned that recent research achievements in this field using porous organic polymers (POPs) for CO2 capture are noteworthy. An important feature that a porous material must have to qualify for its application in CCS technology is its ability to selectively capture CO2 in the presence of N2. Thus, we were also curious to explore the ability of TBOSBLs to selectively capture CO2 over N2. CO2 sorption isotherms of TBOSBLs were measured at two temperatures 273 K (Figure 6B) and 298 K (Figure 6C) at pressures up to 1 atm. The gravimetric uptake of CO2 was the highest for TBOSBL1 (175 mg/g), while it was the lowest for TBOSBL3 (125 mg/g), as shown in Table 1. Quantitatively, the amount of CO2 captured by TBOSBL1 (175 mg/g at 273 K and 1 bar) is better than those by all previously reported imine-based COFs synthesized via the Schiff-base condensation reaction, such as TpPa-1 (153 mg/g),36 TpBD (84 mg/g),45 TpPa-F4 (69 mg/g),47 a freshly activated sample of UCBZ-1 (77 mg/g),48 and TpTph (163 mg/g).46 Moreover, this is also higher than the amounts captured by various porous organic polymers, N-rich porous polymers, and triptycene-based microporous polymers, such as N-rich POPs (109 mg/g),41 amine-based cross-linked porous polymers (66.8 mg/g),49 nanoporous organic polymers (NOPs, 86–142 mg/g),50 azo-functionalized microporous organic polymers (77.7–134.8 mg/g),51 porous covalent triazine polymers (CTPs, 6.6–29.5 mg/g),52 hexaphenylbenzene-based conjugated microporous polymers (HCMPs, 43–75 mg/g),53 3D ultramicroporous triptycene-based polyimide frameworks (149.6 mg/g),43 and triptycene-derived azo polymers bearing phloroglucinol units (TAPs, 95–150 mg/g).20 The selectivity of CO2/N2 was calculated using the Henry law and on the basis of initial slope calculations in the pressure range of 0–0.1 bar (Figures S3 and S4). The CO2/N2 selectivity results of TBOSBLs are depicted in Table 1. TBOSBLs (TBOSBL1–TBOSBL3) show a reasonably high CO2/N2 selectivity (68–108) at 273 K, and this value is better than those of various literature-reported porous materials, such as ACOF-1 (40),54 TpPa-COF (MW) (32),55 covalent triazine-based frameworks (CTFs, 20–25),56 azo-linked polymers (ALPs, 44–60),57 azine-linked covalent organic frameworks (COF-JLU2, 77),58 and triptycene-based 1,2,3-triazole-linked networks (31–48).59 Among the three triptycene-based POPs reported herein, TBOSBL3 exhibited the highest selectivity (108) for CO2 over N2 at 273 K. On the other hand, the CO2/N2 selectivity was the lowest for TBOSBL1 (68) under similar experimental conditions.
A noticeable decrease in CO2/N2 selectivity at a higher temperature (298 K) was observed for TBOSBL2 and TBOSBL3, as shown in Table 1. This trend (decreased selectivity at a higher temperature) has commonly been observed for most POPs reported to date.35 For TBOSBL1, the selectivity changes marginally at elevated temperatures (68 at 273 K to 69 at 298 K). The Qst (isosteric heats of adsorption) value of TBOSBLs for CO2 uptake was directly calculated from the experimental adsorption data collected at 273 and 298 K using the Clausius–Clapeyron relation. The Qst values for CO2 were observed to be in the range 30–35 kJ/mol at zero coverage (Table 1 and Figure 6D). Thus, TBOSBLs capture CO2 via physisorption processes because the magnitude of Qst is less than 40 kJ/mol.60 The reasonably high Qst value hints at moderate interactions between the polarizable CO2 molecule (Lewis acidic) and the abundant N–H sites (CO2-philic and Lewis basic) on the pore wall of TBOSBLs via hydrogen bonds. The Qst values of CO2 for TBOSBLs are comparable with those of previous literature-reported porous organic polymers, such as ACOF-1 (27.6 kJ/mol),54 COF-JLU2 (31 kJ/mol),58 and TpPa-COF (MW) (34.1 kJ/mol).55
2.4. Anticancer Activity of TBOSBLs
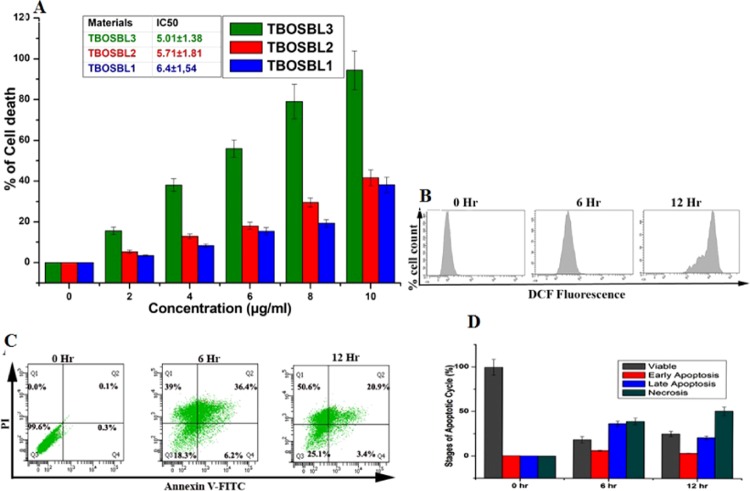
A thorough literature survey indicated that there are a handful of reports on porous COFs that demonstrated appreciable anticancer activity against cancer cells, as evident from the low magnitude of IC50 values.22,23,61 Motivated by these results, we too were curious to explore the potential of TBOSBLs as antiproliferative agents. To the best of our knowledge, such studies are not known for POPs derived from triptycene-based monomers. Thus, TBOSBLs were screened against HCT-116 (human colorectal) cancer cells. Treatment with different concentrations of TBOSBLs (0–10 μg/mL) for 6 h amplified the cell death significantly (Figure 7A), and the IC50 values were estimated to be 5.01 ± 1.38, 5.71 ± 1.81, and 6.4 ± 1.54 μg/mL for TBOSBL3, TBOSBL2, and TBOSBL1 respectively. The IC50 values of TBOSBLs are lower than that of 5-fluorouracil (a chemotherapeutic drug with IC50 = 10.24 ± 2.14 μg/mL). Furthermore, the cytotoxicity of TBOSBL3 is also superior to those of previous literature-reported porous polymeric materials, such as a triazine-based p-conjugated mesoporous two-dimensional (2D) covalent organic framework—“TrzCOF” (IC50 = 8.31 ± 1.67 μg/mL),61 a porous biodegradable nitrogen containing COF—“EDTFP-1” (IC50 = 9.89 ± 1.16 μg/mL),22 and N-rich porous organic polymer methylenedianiline-triformylphloroglucinol—“MDTFP-1” (IC50 = 5.69 ± 1.02 μg/mL).23 Thus, preliminary experimental investigations revealed that TBOSBL1–3 exhibited appreciable cytotoxicity toward the HCT-116 cell line as per 4,5-(dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay data. The cytotoxicity of TBOSBL3 is higher than that of TBOSBL2 and this may be due to a greater pore volume in the former than in the latter. The cytotoxicity of TBOSBL3 is higher than that of TBOSBL1 since the former has more functionalized groups. Based on these promising results as obtained from the MTT data, TBOSBL3 was selected for a further detailed study.
Figure 7.
(A) MTT assay data of TBOSBLs for 6 h. (B) Reactive oxygen species (ROS) level with time of TBOSBL3-treated HCT-116 cells. (C) Graphical presentation of annexin V, FITC/propidium iodide (PI) positive cells with time of treatment with 5 μg/mL TBOSBL3 by flow cytometry. (D) % of apoptotic HCT-116 cells in a time-dependent manner.
It is well known that an increase in cellular levels of ROS may trigger apoptosis-induced cell death, thereby damaging proteins, nucleic acids, lipids, membranes, and organelles.62 ROS generation, in turn, may be estimated using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), which is a non-fluorescent molecule. In the presence of ROS, H2DCFDA is oxidized to 2′,7′-dichlorofluorescein (DCF), the latter being a highly fluorescent molecule. It was observed that the mean fluorescence intensity (MFI) of DCF was significantly (P < 0.05) increased in a time-dependent manner upon TBOSBL3 treatment while checking the extent of ROS generation (Figure 7B). This experiment was repeated several times to confirm the results of ROS generation. To determine whether TBOSBL3 was involved in apoptosis/necrosis, we have carried out the flow cytometric assessment using annexin V-FITC/propidium iodide (PI) staining by reading the exposed level of phosphatidylserine (PS) in the outer membrane of cells (Figure 7C). It is known that phosphatidylserine (PS) released on the outer leaflet of the plasma membrane is broadly observed during apoptosis.63 Our result showed that the percentage of apoptotic (early and late) cells was enhanced in a time-dependent manner (6.2% EA/36.4% LA/39% necrosis for 6 h, 3.4% EA/20.9% LA/50.6% necrosis for 12 h), with respect to the control cells (0.3% EA/0.1% LA/0.0% necrosis) (Figure 7D).
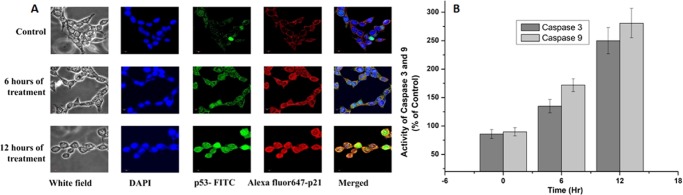
These results hint at TBOSBL3-induced cell death that is associated with cytotoxicity followed by apoptosis. We have checked the cytotoxicity of TBOSBL3 in a normal cell line, namely, human embryonic kidney cells (HEK293), where we detected that the IC50 value is 24.7 μg/mL. Thus, from the magnitude of IC50, it may be said that TBOSBL3 is almost 5 times less active against HEK293 (a normal cell line) relative to HCT-116 (a cancer cell line). Tumor proteins p53 and p21 play a major role in controlling the expression of genes involved in apoptosis.64 Therefore, after confirmation of ROS-induced apoptosis by TBOSBL3, the expression of these two apoptotic markers (p53 and p21) was estimated (Figure 8A). Experimental results suggest that apoptosis was induced in the HCT-116 cells with high levels of p53 expression. The expression of p21 is induced by tumor suppressor gene p53. Our confocal images showed a higher level of p53 and p21 in a time-dependent manner after treatment with TBOSBL3, where 4′,6-diamidino-2-phenylindole (DAPI) was used as the fluorescent stain for DNA. Activation of caspase-3 and caspase-9 is required for efficient execution of apoptosis,65 so we checked the expression of both these caspases. The increased expression of caspase-3 and caspase-9 (Figure 8B) also confirmed that a mitochondria-mediated apoptotic pathway occurred in a time-dependent manner while the cells were treated with TBOSBL3.
Figure 8.
(A) Confocal microscopy images of expression of two main apoptotic markers, p53 and p21. (B) Activity of caspase-3 and caspase-9 with time for TBOSBL3-treated HCT-116 cells.
3. Conclusions
In summary, this work reports a facile synthesis and characterization of a set of unique triptycene-based and organic Schiff-base-linked polymers (TBOSBLs) using 1,3,5-triformylphloroglucinol (TFP). The obtained polymeric networks are characterized using FTIR, NMR, TGA, and XRD. TBOSBLs are porous organic polymers (POPs) with a reasonable surface area (SABET up to 649 m2/g). TBOSBLs have the ability to capture small gas molecules, such as CO2, H2, and N2. While CO2 uptake capacity is greater than 125 mg/g at 273 K and 1 bar pressure, TBOSBLs may be considered as potential materials for molecular hydrogen storage (up to 19.5 mg/g at 77 K and 1 bar pressure). Further, TBOSBLs demonstrate very good CO2/N2 selectively (up to 108) at 273 K. Inspired by earlier reports of cytotoxicity shown by COFs (bearing phloroglucinol motifs) against cancer cells, TBOSBLs are tested to check whether they show any activity against cancer/normal cell lines. Indeed, the measured IC50 values for TBOSBLs against human colorectal cancer cells are noteworthy. The mechanism of cell death is also studied elaborately. It is explored whether TBOSBLs are apoptotic inducers while proposing a mechanism of cell death. TBOSBLs are the only example of triptycene-based POPs whose cytotoxic potential against cancer cells have been studied to date. Results of in vitro studies using TBOSBLs presented in this work suggest that this new set of POPs have immense potential as anticancer therapeutic agents.
4. Experimental Section
4.1. Materials
Triptycene, TFA, and phloroglucinol were purchased from Sigma-Aldrich, and they were used without further purification. Hexamethylene tetramine was procured from CDH. THF was also purchased from Sigma-Aldrich and was dried using common laboratory techniques before use in reactions.
4.2. Instrumentation
Solid-state 13C cross-polarization magic angle spinning (CP-MAS) NMR spectra of TBOSBL 1–3 were recorded on a Bruker 400 spectrometer equipped with an 89 mm wide bore and a 9.4 T superconducting magnet with a spinning rate of 12 kHz and CP contact time of 2 ms with a delay time of 2 s. FTIR spectra of TBOSBL 1–3 were collected using a Shimadzu IR Affinity-1 spectrometer. P-XRD analysis data were collected using a Rigaku TTRAX III X-ray diffractometer. TGA plots were recorded using a TG-DSC STA 449 F3 Jupiter (NETZSCH, Selb, Germany) at a scan rate of 10 °C/min under nitrogen flow (100 mL/min). FE-SEM images were obtained using a Carl Zeiss AG instrument (model SUPRA 55). Porosity and surface area were estimated using a Quantachrome Autosorb iQ2 analyzer. In a typical gas experimental setup, TBOSBLs (80–120 mg) were charged in a 9 mm cell and were exposed to degassing at 120 °C for 6–10 h by attaching to a degassing unit. Subsequently, the cells with degassed polymeric samples were filled up with helium gas and weighed accurately for analysis. Various temperatures of the analysis unit sample cell were maintained using a KGW isotherm bath (provided by Quantachrome), which was filled with liquid N2 (77 K), or a temperature-controlled bath (298 and 273 K).
4.3. Synthesis Procedure of TBOSBLs
4.3.1. Synthesis of TBOSBL1
Experiments for the synthesis of TBOSBLs (Scheme 1) are described using TBOSBL1 as a typical example. 1,3,5-Triformylphloroglucinol (1) (105 mg, 0.5 mmol) and diaminotriptycene (2) (214 mg, 0.75 mmol) were dissolved under a flow of nitrogen gas in a solution mixture of dry THF and trifluoroacetic acid (TFA, 2 mol % in THF). The reaction mixture was stirred at 60 °C for 3 days, and the resulting precipitate was collected by suction filtration. The yellow precipitate was washed with tetrahydrofuran, DMF, DMSO, water, methanol, acetone, and dichloromethane, separately. The collected powder was then dried at 120 °C under vacuum for 24 h to yield a yellow powder with 83% isolated yield.
4.3.2. Synthesis of TBOSBL2
TBOSBL2 has been synthesized following a similar scheme to that described for TBOSBL1. 1,3,5-Triformylphloroglucinol (1) (157 mg, 0.75 mmol) and 2,7,14-triaminotriptycene (3) (225 mg, 0.75 mmol) were used as monomers for the synthesis of TBOSBL2. After drying at reduced pressure at 120 °C, the final product was collected as a yellow powder (yield 81%).
4.3.3. Synthesis of TBOSBL3
Here, 1,3,5-triformylphloroglucinol (1) (157 mg, 0.75 mmol) and 2,6,14-triaminotriptycene (4) (225 mg, 0.75 mmol) were used as monomers. The final product was obtained as a yellow powder (yield 80%).
4.4. Cell Culture
Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotic cocktail at 37 °C under constant 5% CO2 in a humidified condition. After 75–80% confluence, cells were harvested with trypsin (0.25%) and ethylenediaminetetraacetic acid (EDTA, 0.52 mM) in phosphate-buffered saline (PBS) and plated at a necessary density to allow them to re-equilibrate before the experiment.
4.5. Cell Viability
(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay66 was completed to estimate cell viability. The cells were plated in 96 well plates and treated with or without different concentrations of TBOSBL1, TBOSBL2, and TBOSBL3 for 6 h. Then MTT solution was added. Four hours after the addition of MTT, formazan was solubilized with acidic isopropanol and the absorbance of the solution was measured at 595 nm using an ELISA reader.
4.6. Quantification of Apoptosis Using Flow Cytometry
Apoptotic and necrotic cell death was quantified using the annexin V-FITC/PI apoptosis detection kit (Calbiochem, CA).67 Briefly, HCT-116 cells were pretreated with 5 μg/mL of TBOSBL3 for different time durations (6 and 12 h). After an appropriate time period, the cells were washed and stained with PI and annexin V-FITC in accordance with the manufacturer’s instructions. The percentages of live, apoptotic (early and late), and necrotic cells were quantified using a flow cytometer (BD LSRFortessa TM San Jose, CA). The acquired data were analyzed.
4.7. Measurement of Intracellular Reactive Oxygen Species (ROS) Generation Using Flow Cytometry
Intracellular ROS accumulation was observed using DCFH-DA, which is a renowned ROS marker. Briefly, TBOSBL3-treated cells were incubated with 10 mM DCFH-DA at 37 °C for 25 min, and after that, the cells were analyzed using a flow cytometer (BD LSRFortessa TM San Jose, CA). The acquired data were analyzed.
4.8. Immunofluorescence
Confocal microscopy has been accomplished for observing the expression of two apoptotic markers p53 and p21. Briefly, control/treated HCT-116 cells were washed twice for 10 min each in PBS (0.01 M) and incubated for 1 h in blocking solution having 2% normal bovine serum and 0.3% Triton X-100 in PBS. After blocking, the cells were incubated overnight at 4 °C with the respective primary antibody (p53 and p21), followed by washing and incubation with respective fluorophore-conjugated secondary antibodies (anti mouse/rabbit FITC and Alexa Fluor-647) for 2 h. The slides were then counterstained with 6-diamidino-2-phenylindole (DAPI) for 10 min and mounted with the ProLong antifade reagent (Molecular Probe, Eugene, OR). Stained cells were examined using a confocal laser scanning microscope (FV 10i, Olympus, Japan).
4.9. Caspase-3 and Caspase-9 Activity Assays
The treated cells were subjected to caspase-3 and caspase-9 colorimetric assay using commercially available kits according to the manufacturer’s instructions (BioVision Research Products, Mountain View, CA) respectively.
Acknowledgments
N.D. thanks the Indian Institute of Technology Patna for providing infrastructure and instrumental facilities required for the research. A.A., A.H., and R.B. thank the Indian Institute of Technology Patna for providing their respective research fellowships. S.M. thanks ICMR for a Senior Research Fellowship. S.M. is also grateful to Tanmoy Dalui, Debalina Chakraborty, Binayak Pal, and Banasri Das of Central Instrumentation Facility, CSIR-Indian Institute of Chemical Biology (IICB), for providing flow cytometer and confocal microscope facilities. S.D. thanks the Department of Chemical Technology, University of Calcutta, for its support. K.D.S. thanks CSIR-IICB for providing research facilities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04160.
BET plot of TBOSBLs (Figure S1); Langmuir plot of TBOSBLs (Figure S2); initial gas uptake slopes of TBOSBLs at 273 K (Figure S3); initial gas uptake slopes of TBOSBLs at 298 K (Figure S4) (PDF)
Author Contributions
N.D. conceived the research and supervised the experimental work related to the synthesis and characterization of polymeric materials reported herein. A.A. synthesized all polymeric networks reported in this manuscript. A.H. assisted A.A. in the synthesis of literature-reported monomers. A.A. characterized the polymers and was assisted by R.B. K.D.S. and S.D. supervised the experimental work related to anticancer activities of polymers reported in this manuscript that was performed by S.M. All authors have contributed to the interpretation of respective results, compiled the manuscript, and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Malumbres M.; Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- https://www.who.int/news-room/fact-sheets/detail/cancer.
- https://www.cancer.net/navigating-cancer-care/cancer-basics/what-cancer.
- Naksuriya O.; Okonogi S.; Schiffelers R. M.; Hennink W. E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- Li Z.; Yang Y.-W. Creation and bioapplications of porous organic polymer materials. J. Mater. Chem. B 2017, 5, 9278–9290. 10.1039/C7TB02647A. [DOI] [PubMed] [Google Scholar]
- Wu J.; Xu F.; Li S.; Ma P.; Zhang X.; Liu Q.; Fu R.; Wu D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2019, 31, 1802922 10.1002/adma.201802922. [DOI] [PubMed] [Google Scholar]
- Song Y.; Sun Q.; Aguila B.; Ma S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410 10.1002/advs.201801410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.; Zou R.; Zhao Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. 10.1002/adma.201505004. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Liu H.; Mathe S. D. R.; Dong A.; Zhang J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2018, 8, 15. 10.3390/nano8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Yang Q.; Wang C.; Luo X.; Kim J.; Wang Z.; Yamauchi Y. Porous Organic Frameworks: Advanced Materials in Analytical Chemistry. Adv. Sci. 2018, 5, 1801116 10.1002/advs.201801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity T.; Saha D.; Das S.; Koner S. Barium Carboxylate Metal–Organic Framework – Synthesis, X-ray Crystal Structure, Photoluminescence and Catalytic Study. Eur. J. Inorg. Chem. 2012, 2012, 4914–4920. 10.1002/ejic.201200417. [DOI] [Google Scholar]
- Rengaraj A.; Puthiaraj P.; Haldorai Y.; Heo N. S.; Hwang S.-K.; Han Y.-K.; Kwon S.; Ahn W.-S.; Huh Y. S. Porous Covalent Triazine Polymer as a Potential Nanocargo for Cancer Therapy and Imaging. ACS Appl. Mater. Interfaces 2016, 8, 8947–8955. 10.1021/acsami.6b00284. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Ma H.; Zhai T.-L.; Cheng G.; Xu Q.; Liu J.-M.; Yang J.; Zhang Q.-M.; Zhang Q.-P.; Zheng Y.-S.; Tan B.; Zhang C. Networked Cages for Enhanced CO2 Capture and Sensing. Adv. Sci. 2018, 5, 1800141 10.1002/advs.201800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Heasman P.; Ben T.; Qiu S. Porous Organic Materials: Strategic Design and Structure–Function Correlation. Chem. Rev. 2017, 117, 1515–1563. 10.1021/acs.chemrev.6b00439. [DOI] [PubMed] [Google Scholar]
- Chen J.-J.; Zhai T.-L.; Chen Y.-F.; Geng S.; Yu C.; Liu J.-M.; Wang L.; Tan B.; Zhang C. A triptycene-based two-dimensional porous organic polymeric nanosheet. Polym. Chem. 2017, 8, 5533–5538. 10.1039/C7PY01122A. [DOI] [Google Scholar]
- Ma H.; Chen J.-J.; Tan L.; Bu J.-H.; Zhu Y.; Tan B.; Zhang C. Nitrogen-Rich Triptycene-Based Porous Polymer for Gas Storage and Iodine Enrichment. ACS Macro Lett. 2016, 5, 1039–1043. 10.1021/acsmacrolett.6b00567. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Zhu P.-C.; Tan L.; Liu J.-M.; Tan B.; Yang X.-L.; Xu H.-B. Triptycene-Based Hyper-Cross-Linked Polymer Sponge for Gas Storage and Water Treatment. Macromolecules 2015, 48, 8509–8514. 10.1021/acs.macromol.5b02222. [DOI] [Google Scholar]
- Zhang C.; Liu Y.; Li B.; Tan B.; Chen C.-F.; Xu H.-B.; Yang X.-L. Triptycene-Based Microporous Polymers: Synthesis and Their Gas Storage Properties. ACS Macro Lett. 2012, 1, 190–193. 10.1021/mz200076c. [DOI] [PubMed] [Google Scholar]
- Swager T. M. Iptycenes in the Design of High Performance Polymers. Acc. Chem. Res. 2008, 41, 1181–1189. 10.1021/ar800107v. [DOI] [PubMed] [Google Scholar]
- Bera R.; Ansari M.; Alam A.; Das N. Triptycene, Phenolic-OH, and Azo-Functionalized Porous Organic Polymers: Efficient and Selective CO2 Capture. ACS Appl. Polym. Mater. 2019, 1, 959–968. 10.1021/acsapm.8b00264. [DOI] [Google Scholar]
- Bera R.; Ansari M.; Mondal S.; Das N. Selective CO2 capture and versatile dye adsorption using a microporous polymer with triptycene and 1,2,3-triazole motifs. Eur. Polym. J. 2018, 99, 259–267. 10.1016/j.eurpolymj.2017.12.029. [DOI] [Google Scholar]
- Bhanja P.; Mishra S.; Manna K.; Mallick A.; Das Saha K.; Bhaumik A. Covalent Organic Framework Material Bearing Phloroglucinol Building Units as a Potent Anticancer Agent. ACS Appl. Mater. Interfaces 2017, 9, 31411–31423. 10.1021/acsami.7b07343. [DOI] [PubMed] [Google Scholar]
- Bhanja P.; Mishra S.; Manna K.; Das Saha K.; Bhaumik A. Porous Polymer Bearing Polyphenolic Organic Building Units as a Chemotherapeutic Agent for Cancer Treatment. ACS Omega 2018, 3, 529–535. 10.1021/acsomega.7b01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Chen C.-F. Synthesis and Structure of 2,6,14- and 2,7,14-Trisubstituted Triptycene Derivatives. J. Org. Chem. 2006, 71, 6626–6629. 10.1021/jo061067t. [DOI] [PubMed] [Google Scholar]
- Bhadra M.; Kandambeth S.; Sahoo M. K.; Addicoat M.; Balaraman E.; Banerjee R. Triazine Functionalized Porous Covalent Organic Framework for Photo-organocatalytic E–Z Isomerization of Olefins. J. Am. Chem. Soc. 2019, 141, 6152–6156. 10.1021/jacs.9b01891. [DOI] [PubMed] [Google Scholar]
- Bhadra M.; Sasmal H. S.; Basu A.; Midya S. P.; Kandambeth S.; Pachfule P.; Balaraman E.; Banerjee R. Predesigned Metal-Anchored Building Block for In Situ Generation of Pd Nanoparticles in Porous Covalent Organic Framework: Application in Heterogeneous Tandem Catalysis. ACS Appl. Mater. Interfaces 2017, 9, 13785–13792. 10.1021/acsami.7b02355. [DOI] [PubMed] [Google Scholar]
- Shinde D. B.; Aiyappa H. B.; Bhadra M.; Biswal B. P.; Wadge P.; Kandambeth S.; Garai B.; Kundu T.; Kurungot S.; Banerjee R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 2016, 4, 2682–2690. 10.1039/C5TA10521H. [DOI] [Google Scholar]
- Bera R.; Ansari M.; Alam A.; Das N. Nanoporous azo polymers (NAPs) for selective CO2 uptake. J. CO2 Util. 2018, 28, 385–392. 10.1016/j.jcou.2018.10.016. [DOI] [Google Scholar]
- Ansari M.; Hassan A.; Alam A.; Jana A.; Das N. Triptycene based fluorescent polymers with azo motif pendants: Effect of alkyl chain on fluorescence, morphology and picric acid sensing. React. Funct. Polym. 2020, 146, 104408 10.1016/j.reactfunctpolym.2019.104408. [DOI] [Google Scholar]
- Ansari M.; Bera R.; Mondal S.; Das N. Triptycene-Derived Photoresponsive Fluorescent Azo-Polymer as Chemosensor for Picric Acid Detection. ACS Omega 2019, 4, 9383–9392. 10.1021/acsomega.9b00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A.; Bera R.; Ansari M.; Hassan A.; Das N. Triptycene Based and Amine Linked Nanoporous Networks for Efficient CO2 Capture and Separation. Front. Energy Res. 2019, 141 10.3389/fenrg.2019.00141. [DOI] [Google Scholar]
- Deng G.; Wang Z. Triptycene-Based Microporous Cyanate Resins for Adsorption/Separations of Benzene/Cyclohexane and Carbon Dioxide Gas. ACS Appl. Mater. Interfaces 2017, 9, 41618–41627. 10.1021/acsami.7b15050. [DOI] [PubMed] [Google Scholar]
- Sing K. S. W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201. 10.1351/pac198254112201. [DOI] [Google Scholar]
- Patel H. A.; Je S. H.; Park J.; Jung Y.; Coskun A.; Yavuz C. T. Directing the Structural Features of N2-Phobic Nanoporous Covalent Organic Polymers for CO2 Capture and Separation. Chem. - Eur. J. 2014, 20, 772–780. 10.1002/chem.201303493. [DOI] [PubMed] [Google Scholar]
- Arab P.; Rabbani M. G.; Sekizkardes A. K.; İslamoğlu T.; El-Kaderi H. M. Copper(I)-Catalyzed Synthesis of Nanoporous Azo-Linked Polymers: Impact of Textural Properties on Gas Storage and Selective Carbon Dioxide Capture. Chem. Mater. 2014, 26, 1385–1392. 10.1021/cm403161e. [DOI] [Google Scholar]
- Kandambeth S.; Mallick A.; Lukose B.; Mane M. V.; Heine T.; Banerjee R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. 10.1021/ja308278w. [DOI] [PubMed] [Google Scholar]
- DeBlase C. R.; Silberstein K. E.; Truong T.-T.; Abruña H. D.; Dichtel W. R. β-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. 10.1021/ja409421d. [DOI] [PubMed] [Google Scholar]
- Kaleeswaran D.; Vishnoi P.; Murugavel R. [3+3] Imine and β-ketoenamine tethered fluorescent covalent-organic frameworks for CO2 uptake and nitroaromatic sensing. J. Mater. Chem. C 2015, 3, 7159–7171. 10.1039/C5TC00670H. [DOI] [Google Scholar]
- Mitra S.; Kandambeth S.; Biswal B. P.; Khayum M. A.; Choudhury C. K.; Mehta M.; Kaur G.; Banerjee S.; Prabhune A.; Verma S.; Roy S.; Kharul U. K.; Banerjee R. Self-Exfoliated Guanidinium-Based Ionic Covalent Organic Nanosheets (iCONs). J. Am. Chem. Soc. 2016, 138, 2823–2828. 10.1021/jacs.5b13533. [DOI] [PubMed] [Google Scholar]
- Bhanja P.; Das S. K.; Bhunia K.; Pradhan D.; Hayashi T.; Hijikata Y.; Irle S.; Bhaumik A. A New Porous Polymer for Highly Efficient Capacitive Energy Storage. ACS Sustainable Chem. Eng. 2018, 6, 202–209. 10.1021/acssuschemeng.7b02234. [DOI] [Google Scholar]
- Li J.; Cheng Z.; Zhu M.; Thomas A.; Liao Y. Facile Synthesis of Nitrogen-Rich Porous Organic Polymers for Latent Heat Energy Storage. ACS Appl. Energy Mater. 2018, 1, 6535–6540. 10.1021/acsaem.8b01444. [DOI] [Google Scholar]
- Dang Q.-Q.; Wang X.-M.; Zhan Y.-F.; Zhang X.-M. An azo-linked porous triptycene network as an absorbent for CO2 and iodine uptake. Polym. Chem. 2016, 7, 643–647. 10.1039/C5PY01671A. [DOI] [Google Scholar]
- Ghanem B.; Belmabkhout Y.; Wang Y.; Zhao Y.; Han Y.; Eddaoudi M.; Pinnau I. A unique 3D ultramicroporous triptycene-based polyimide framework for efficient gas sorption applications. RSC Adv. 2016, 6, 97560–97565. 10.1039/C6RA21388J. [DOI] [Google Scholar]
- Zhu J.-H.; Chen Q.; Sui Z.-Y.; Pan L.; Yu J.; Han B.-H. Preparation and adsorption performance of cross-linked porous polycarbazoles. J. Mater. Chem. A 2014, 2, 16181–16189. 10.1039/C4TA01537A. [DOI] [Google Scholar]
- Biswal B. P.; Chandra S.; Kandambeth S.; Lukose B.; Heine T.; Banerjee R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. 10.1021/ja4017842. [DOI] [PubMed] [Google Scholar]
- Mukherjee G.; Thote J.; Aiyappa H. B.; Kandambeth S.; Banerjee S.; Vanka K.; Banerjee R. A porous porphyrin organic polymer (PPOP) for visible light triggered hydrogen production. Chem. Commun. 2017, 53, 4461–4464. 10.1039/C7CC00879A. [DOI] [PubMed] [Google Scholar]
- Chandra S.; Kandambeth S.; Biswal B. P.; Lukose B.; Kunjir S. M.; Chaudhary M.; Babarao R.; Heine T.; Banerjee R. Chemically Stable Multilayered Covalent Organic Nanosheets from Covalent Organic Frameworks via Mechanical Delamination. J. Am. Chem. Soc. 2013, 135, 17853–17861. 10.1021/ja408121p. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Zhang W. Reversible tuning of pore size and CO2 adsorption in azobenzene functionalized porous organic polymers. Chem. Sci. 2014, 5, 4957–4961. 10.1039/C4SC02305F. [DOI] [Google Scholar]
- Abdelnaby M. M.; Alloush A. M.; Qasem N. A. A.; Al-Maythalony B. A.; Mansour R. B.; Cordova K. E.; Al Hamouz O. C. S. Carbon dioxide capture in the presence of water by an amine-based crosslinked porous polymer. J. Mater. Chem. A 2018, 6, 6455–6462. 10.1039/C8TA00012C. [DOI] [Google Scholar]
- Fan C.; Chen Z.; Pang L.; Ming S.; Dong C.; Brou Albert K.; Liu P.; Wang J.; Zhu D.; Chen H.; Li T. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. 10.1016/j.cej.2017.09.181. [DOI] [Google Scholar]
- Yang Z.; Zhang H.; Yu B.; Zhao Y.; Ma Z.; Ji G.; Han B.; Liu Z. Azo-functionalized microporous organic polymers: synthesis and applications in CO2 capture and conversion. Chem. Commun. 2015, 51, 11576–11579. 10.1039/C5CC03151F. [DOI] [PubMed] [Google Scholar]
- Lee S.-P.; Mellon N.; Shariff A. M.; Leveque J.-M. Geometry variation in porous covalent triazine polymer (CTP) for CO2 adsorption. New J. Chem. 2018, 42, 15488–15496. 10.1039/C8NJ00638E. [DOI] [Google Scholar]
- Liao Y.; Weber J.; Mills B. M.; Ren Z.; Faul C. F. J. Highly Efficient and Reversible Iodine Capture in Hexaphenylbenzene-Based Conjugated Microporous Polymers. Macromolecules 2016, 49, 6322–6333. 10.1021/acs.macromol.6b00901. [DOI] [Google Scholar]
- Li Z.; Feng X.; Zou Y.; Zhang Y.; Xia H.; Liu X.; Mu Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828. 10.1039/C4CC05665E. [DOI] [PubMed] [Google Scholar]
- Wei H.; Chai S.; Hu N.; Yang Z.; Wei L.; Wang L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. 10.1039/C5CC04680G. [DOI] [PubMed] [Google Scholar]
- Dey S.; Bhunia A.; Esquivel D.; Janiak C. Covalent triazine-based frameworks (CTFs) from triptycene and fluorene motifs for CO2 adsorption. J. Mater. Chem. A 2016, 4, 6259–6263. 10.1039/C6TA00638H. [DOI] [Google Scholar]
- Arab P.; Parrish E.; İslamoğlu T.; El-Kaderi H. M. Synthesis and evaluation of porous azo-linked polymers for carbon dioxide capture and separation. J. Mater. Chem. A 2015, 3, 20586–20594. 10.1039/C5TA04308E. [DOI] [Google Scholar]
- Li Z.; Zhi Y.; Feng X.; Ding X.; Zou Y.; Liu X.; Mu Y. An Azine-Linked Covalent Organic Framework: Synthesis, Characterization and Efficient Gas Storage. Chem. - Eur. J. 2015, 21, 12079–12084. 10.1002/chem.201501206. [DOI] [PubMed] [Google Scholar]
- Mondal S.; Das N. Triptycene based 1,2,3-triazole linked network polymers (TNPs): small gas storage and selective CO2 capture. J. Mater. Chem. A 2015, 3, 23577–23586. 10.1039/C5TA06939D. [DOI] [Google Scholar]
- Patel H. A.; Hyun Je S.; Park J.; Chen D. P.; Jung Y.; Yavuz C. T.; Coskun A. Unprecedented high-temperature CO2 selectivity in N2-phobic nanoporous covalent organic polymers. Nat. Commun. 2013, 4, 1357 10.1038/ncomms2359. [DOI] [PubMed] [Google Scholar]
- Kanti Das S.; Mishra S.; Manna K.; Kayal U.; Mahapatra S.; Das Saha K.; Dalapati S.; Das G. P.; Mostafa A. A.; Bhaumik A. A new triazine based π-conjugated mesoporous 2D covalent organic framework: its in vitro anticancer activities. Chem. Commun. 2018, 54, 11475–11478. 10.1039/C8CC07289B. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M.; Averill-Bates D. A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta, Mol. Cell Res. 2016, 1863, 2977–2992. 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Meng X. W.; Flatten K. S.; Loegering D. A.; Kaufmann S. H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2013, 20, 64–76. 10.1038/cdd.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Kon N.; Jiang L.; Tan M.; Ludwig T.; Zhao Y.; Baer R.; Gu W. Tumor Suppression in the Absence of p53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell 2012, 149, 1269–1283. 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentnall M.; Rodriguez-Menocal L.; De Guevara R. L.; Cepero E.; Boise L. H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Dey S. K.; Bose D.; Hazra A.; Naskar S.; Nandy A.; Munda R. N.; Das S.; Chatterjee N.; Mondal N. B.; Banerjee S.; Saha K. D. Cytotoxic Activity and Apoptosis-Inducing Potential of Di-spiropyrrolidino and Di-spiropyrrolizidino Oxindole Andrographolide Derivatives. PLoS One 2013, 8, e58055 10.1371/journal.pone.0058055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.