Abstract
Perovskite solar cells based on multiple cations have shown excellent optoelectronic properties with high power conversion efficiency. Herein, the structural, electronic, and optical properties of mixed-cation mixed-metal perovskites MA1–xCsxPb0.25Sn0.75I3 were studied by employing the first-principles calculations for the first time. Our calculated results reveal that these perovskite materials possess direct band gaps in the range of 1.0–1.3 eV. Moreover, these compounds show excellent photovoltaic performance in terms of strong optical absorption coefficients compared with MAPbI3. Particularly, they also exhibit good structural stability and decrement of lead content. These results demonstrated that mixed-cation mixed-metal perovskites may be potential candidates for high-efficiency light-absorbing materials.
Introduction
Lead halide perovskites have emerged as highly promising materials for photovoltaic applications in the past 10 years because of their excellent properties such as suitable band gap, strong optical absorption, high carrier mobility, and long charge-carrier diffusion length.1−4 The certified power conversion efficiency (PCE) was rapidly increased from 3.8% in 20095 to 25.2% in 2019,6 which is comparable to conventional silicon solar cells. The perovskite structure has a general chemical formula of ABX3, where A is usually occupied by monovalent cations such as Cs+, CH3NH3+ (MA+), or CH(NH2)2+ (FA+), B is occupied by divalent metal cations such as Pb2+, Sn2+, or Ge2+, and X is occupied by halide anions such as Cl–, Br–, or I–. Unfortunately, the perovskite solar cells with high PCE usually contain the toxic lead and the poor stability, which seriously restricts their commercial applications. The development of stable lead-free hybrid perovskites is still a big challenge to obtain high photovoltaic performance.
With regard to the all-inorganic lead perovskite CsPbX3, CsPbI3 is the best candidate because of the improved stability and suitable band gap (1.73 eV).7 However, α-CsPbI3 is unstable below 634 K, and it can easily transform to a yellow nonperovskite δ-phase under room temperature.8 A theoretical study showed that a two-dimensional layered Ruddlesden–Popper perovskite Cs2PbI4 may not be a good material for photovoltaic applications owing to its low carrier mobility and poor optical absorption.9 CsPbBr3 was shown to have good charge transport properties,10 and it has significant potential in ferroelectric applications.11 Moreover, the Mos2/CsPbBr3 heterostructure can effectively retain some of the excellent properties of individual MoS2 and CsPbBr3.12
A series of Ge-based halide perovskites have been synthesized, and the theoretical results indicate that Ge is a suitable substitution to replace the toxic Pb.13 MAPb0.25 Ge0.75I3 can become a promising candidate for perovskite solar cells because of the proper band gap, lower effective masses, and better optical absorption.14 The photovoltaic performance of the mixed halide perovskite MAGeI3–xClx was investigated, and the transport and optical properties can be improved by increasing the concentration of I.15 The geometrical, electronic, and optical properties of the mixed metal perovskite RbGe1–xSnxI3 were systematically studied.16 The results found that RbGe0.50Sn0.50I3 has superior transport and optical properties.16
Some investigations have been carried out to explore alternative lead-based hybrid perovskites, involving analogous Sn-based perovskites.17−19 Sn is an ideal candidate element to replace lead, which is located in the same periodic table. Moreover, they have similar ionic radius. However, the Sn2+ ion is highly unstable and can be easily oxidized to Sn4+,17 which leads to a great reduction in photovoltaic performance. The PCE of Sn-based perovskite solar cells is reported to be about 6%.17−19 Recently, the use of mixed MAPb1–xSnxI3 perovskites for photovoltaic applications has been reported by experimental and theoretical works.20−25 The results have revealed that partial replacement of Pb with Sn can reduce the band gap, extending the absorption spectra toward the midinfrared region of the solar spectrum.20 The higher PCE of 9.8% has been reported for such perovskite materials, which leads to the consideration of their application to reduce the use of toxic Pb in perovskite solar cell devices.25
In light of the potential applications of mixed Sn–Pb hybrid perovskites in replacing lead-based materials, it is valuable to explore good stability and lead-less perovskite materials and to improve the photovoltaic performance. In this work, we employed first-principles calculations to investigate the electronic and optical properties of mixed-cation mixed-metal halide perovskites. The choice of mixed Pb–Sn perovskites is motivated by the fact that these materials have broader optical absorption in the visible light region. Moreover, the amount of toxic Pb would be greatly reduced in these materials. Interestingly, our calculated results indicate that the band gaps of the mixed perovskites are slightly decreased by doping the Cs cation. The optical absorption can be further enhanced in the long-wavelength region of 600–1000 nm. In addition, these mixed perovskite materials possess better structural stability.
Results and Discussion
Geometry optimization of MA1–xCsxPb0.25Sn0.75I3 compounds has been carried out using the MAPbI3 tetragonal I4/mcm experimental cell parameters.18 The optimized structures of the MA1–xCsxPb0.25Sn0.75I3 series are shown in Figure 1. The Goldschmidt tolerance factor (t) is usually used to evaluate the crystallographic stability of perovskites. The formula is as follows26
| 1 |
where RA, RB, and RX are effective ionic radii for A, B, and X, respectively. The value of the tolerance factor t should be in the range of 0.81–1.11.27 Our calculated t values are variable from 0.84 to 0.87. These results preliminarily show that these mixed halide perovskites are stable.
Figure 1.
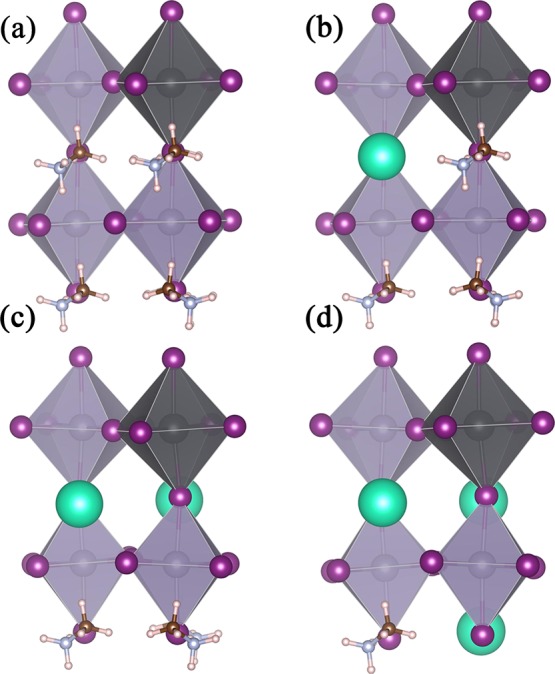
Optimized structures of (a) MAPb0.25Sn0.75I3, (b) MA0.75Cs0.25Pb0.25Sn0.75I3, (c) MA0.50Cs0.50Pb0.25Sn0.75I3, and (d) MA0.25Cs0.75Pb0.25Sn0.75I3.
To further examine the effect of Cs substitution, we have evaluated the formation energy of the mixed halide perovskites according to the following equation
| 2 |
where E(MA1–xCsxPb0.25Sn0.75I3), E(MAI), E(CsI), E(PbI2), and E(SnI2) are the energy of MA1–xCsxPb0.25Sn0.75I3, MAI, CsI, PbI2, and SnI2, respectively. x is the ratio of Cs+ doping, and we adopted the x value of 0, 0.25, 0.50, and 0.75, respectively. Our calculated (ΔH) value for MAPbI3 is 0.01 eV/f.u., which is well consistent with the previous theoretical values of −0.0128 and −0.02 eV/f.u.29 The results indicate that MAPbI3 is marginally stable and it can decompose to MAI and PbI2 under ambient condition. The calculated formation energies of the predicted perovskites are shown in Figure 2. It can be seen that all of our predicted mixed perovskites are highly stable.
Figure 2.
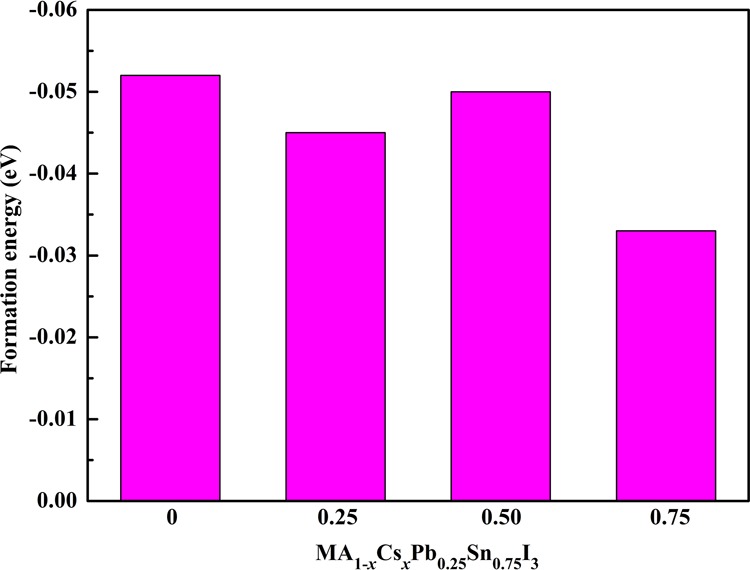
Calculated formation energies of MA1–xCsxPb0.25Sn0.75I3.
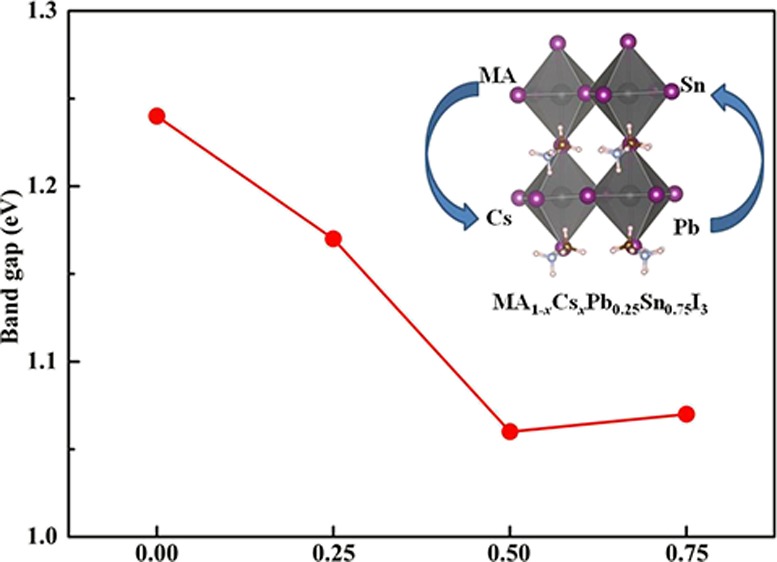
As a photovoltaic material for solar cells, the band gap is crucial to the optical absorption in the visible light region. We have calculated the band structure to explore the electronic properties of predicted perovskites by using the optB86b-vdW and HSE06 functionals. The band gaps of perovskites calculated by the two functionals are shown in Figure 3. The band gap value of MAPb0.25Sn0.75I3 with the optB86b-vdW functional is 0.71 eV, which is much lower than its experimental value. The band gap value of MAPb0.25Sn0.75I3 with the HSE06 functional is 1.24 eV, which matches well with the previous theoretical value of 1.20 eV23 and reaches the experimental values of 1.2321 and 1.17 eV.20 The results of two methods demonstrate clearly the same tendency that the values of band gaps slightly decrease when the proportion of Cs+ increases. The band gaps of mixed-cation halide perovskites are in the range of 1.0–1.3 eV. Moreover, both the valence band maximum (VBM) and conduction band minimum (CBM) of these perovskite materials are located at the same high symmetry point in the Brillouin region from optB86b-vdW calculations, indicating that these materials exhibit a direct band gap feature. MA1–xCsxPb0.25Sn0.75I3 with ideal band gaps in the visible absorption region can be potential candidates of lead-less photovoltaic materials.
Figure 3.
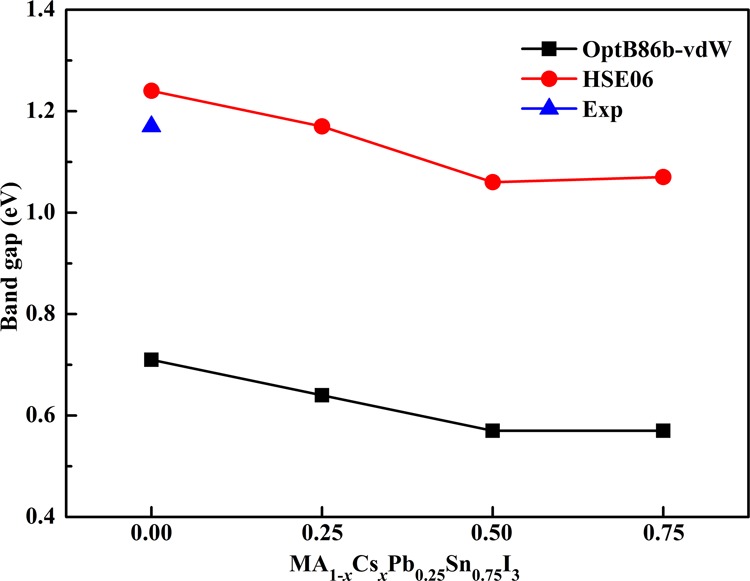
Calculated band gap trends with different methods of MA1–xCsxPb0.25Sn0.75I3 with the experimental value.
It is important to explore the intrinsic reason for the variation of band gaps in halide perovskites. The band gaps of halide perovskites are mainly affected by structural distortion, and the key factor is the Pb–I–Pb bond angle.30 The B–I–B (B = Pb or Sn) bond angles of these mixed perovskites are listed in Table 1. The larger distortion of the B–I–B bond angles is from the ab plane. For MAPb0.25Sn0.75I3 and MA0.75Cs0.25Pb0.25Sn0.75I3, the ab plane distortion may play an important role in tuning the band gap compared with MAPbI3. However, both the ab plane and the c-axis distortion should be crucial to tune the band gap for MA0.50Cs0.50Pb0.25Sn0.75I3 and MA0.25Cs0.75Pb0.25Sn0.75I3.
Table 1. B–I–B (B = Pb or Sn) Bond Angles of the Mixed MA1–xCsxPb0.25Sn0.75I3 Perovskites.
| B–I–B (B = Pb or Sn) bond angles (deg) |
||
|---|---|---|
| in the ab plane | along the c-axis | |
| MAPbI3 | 150.0 | 176.0 |
| MAPb0.25Sn0.75I3 | 155.3 | 175.7 |
| MA0.75Cs0.25Pb0.25Sn0.75I3 | 155.7 | 176.3 |
| MA0.50Cs0.50Pb0.25Sn0.75I3 | 158.5 | 172.2 |
| MA0.25Cs0.75Pb0.25Sn0.75I3 | 159.6 | 171.8 |
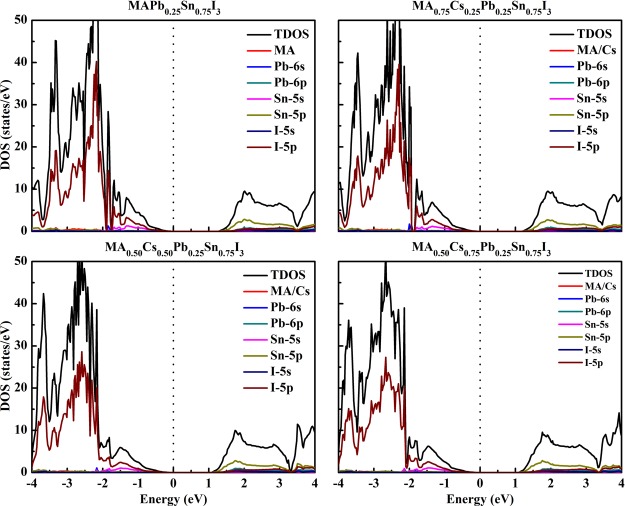
Figure 4 illustrates the density of states (DOS) and projected DOS for these mixed perovskites, calculated by the HSE06 functional. The results show that the compositions of DOS for the four materials are very similar. The VBM is mainly constituted by I-5p orbitals, while the CBM is mainly constituted by Sn-5p orbitals. The Cs/MA cations cannot affect the electronic states because they are far from the band edges.
Figure 4.
DOS of MA1–xCsxPb0.25Sn0.75I3 at the HSE06 functional.
The optical absorption is important to photovoltaic material in applications. The optical spectra of these mixed perovskites can be calculated through the complex dielectric function by the following equation31
| 3 |
Here, ε1 and ε2 are the real and imaginary parts of dielectric function, respectively, and ω represents the frequency of light. The absorption coefficient I(ω) was given below32
| 4 |
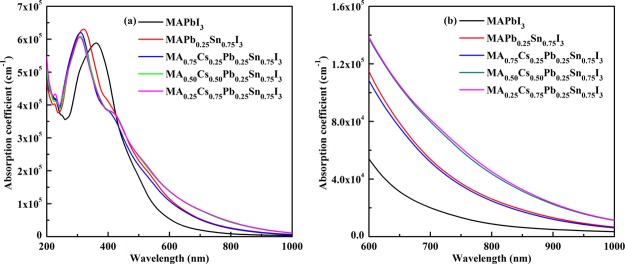
We used the optB86b-vdw functional along with the scissor correction in band gap calculation to gain as accurate as that of the HSE06 functional. The band gap is usually underestimated in Perdew–Burke–Ernzerhof (PBE) calculations and overestimated for the dielectric properties. Based on the scissor correction, we compare the optical absorption of Cs-doped MAPb0.25Sn0.75I3 with that of the pure structure MAPbI3, as shown in Figure 5a. When the wavelength is located in the range of 200–300 nm, the optical absorption of the studied perovskite materials is stronger than that of MAPbI3, and the optical absorption coefficient can reach over 10–6 cm–1. Moreover, their light absorption is also considerable in the long-wavelength region between 600 and 1000 nm, as shown in Figure 5b. These mixed perovskites exhibit good structural stability, strong optical absorption in the visible light region, and the decrement of toxic Pb. Therefore, MA1–xCsxPb0.25Sn0.75I3 has the potential to become the absorbing layer of perovskite solar cells.
Figure 5.
(a) Calculated optical absorption coefficients for MA1–xCsxPb0.25Sn0.75I3 compared with that MAPbI3. (b) Calculated optical absorption coefficients for MA1–xCsxPb0.25Sn0.75I3 in the wavelength region between 600 and 1000 nm.
Conclusions
In summary, we have systematically studied the structural stability, electronic structures, and optical properties of MA1–xCsxPb0.25Sn0.75I3 by employing the first-principles calculations to reveal their photovoltaic applications for the first time. These four compounds are direct band gap semiconductors and have good structural stability. The band gap values of these materials are in the range of 1.0–1.3 eV, which are suitable for photovoltaic devices. It should be noted that these compounds show excellent optical absorption coefficients in the visible light region. The above-mentioned performances clearly show that mixed-cation mixed-metal perovskite materials can serve as potential candidates for perovskite solar cells. Our calculations can provide theoretical guidance for the development of novel lead-less and stable perovskites for photovoltaic applications.
Computational Methods
The structural optimization and electronic properties of perovskite materials were calculated using density function theory as implemented in the Vienna ab initio simulation package.33 The interaction between electron and ion was described by the projector-augmented wave method,34 while the generalized gradient approximation (GGA) of the PBE functional was used to describe the electron exchange correlation.35 The kinetic energy cutoff was 550 eV. The energy and force convergence criteria were set to be 10–5 eV and 0.01 eV/Å, respectively. The Monkhorst–Pack k points used in the structural optimization and electronic properties were 6 × 6 × 6 and 8 × 8 × 8, respectively. The optB86b-vdW functional was applied to account for dispersion interactions in all calculations.36 It is well known that GGA–PBE usually underestimates the band gaps of halide perovskites. In order to obtain more accurate band gaps, we used Heyd–Scuseria–Ernzerhof (HSE06) hybrid functional calculations.37 The precise Hartree–Fock exchange contribution for the hybrid functional was set to 30% in order to repeat the experimental value.
Acknowledgments
This work was financially supported by the Department of Fujian Science and Technology and Program for Innovative Research Team in Science and Technology in Fujian Province University (no. 2018N2001), the Key Laboratory of Green Energy and Environment Catalysis (Ningde Normal University) (no. FJ-GEEC201905), the Open Project Program of Fujian Provincial University Engineering Research Center of Green Materials and Chemical Engineering (Minjiang University) (no. MJXY-KFCH1906), and the Science and Technology Planning Project of Fuzhou City (nos 2017-G-106 and 2018-G-96). The authors thank the Supercomputer environment of Fujian Provincial Key Laboratory of Information Processing and Intelligent Control.
The authors declare no competing financial interest.
References
- Chen J.; Zhou S.; Jin S.; Li H.; Zhai T. Crystal Organometal Halide Perovskites with Promising Optoelectronic Applications. J. Mater. Chem. C 2016, 4, 11–27. 10.1039/c5tc03417e. [DOI] [Google Scholar]
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Bakr O. M.; Mohammed O. F. Powering up Perovskite Photoresponse. Science 2017, 355, 1260–1261. 10.1126/science.aam7154. [DOI] [PubMed] [Google Scholar]
- Yin W.-J.; Shi T.; Yan Y. Unique Properties of Halide Perovskites as Possible Origins of the Superior Solar Cell Performance. Adv. Mater. 2014, 26, 4653–4658. 10.1002/adma.201306281. [DOI] [PubMed] [Google Scholar]
- Kojima A.; Teshima K.; Shirai Y.; Miyasaka T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. 10.1021/ja809598r. [DOI] [PubMed] [Google Scholar]
- NREL . Best Research-Cell Efficiency Chart. https://www.nrel.gov/pv/cell-efficiency.html (accessed Aug 05, 2019).
- Eperon G. E.; Stranks S. D.; Menelaou C.; Johnston M. B.; Herz L. M.; Snaith H. J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. 10.1039/c3ee43822h. [DOI] [Google Scholar]
- Trots D. M.; Myagkota S. V. High-Temperature Structural Evolution of Caesium and Rubidium Triiodoplumbates. J. Phys. Chem. Solids 2008, 69, 2520–2526. 10.1016/j.jpcs.2008.05.007. [DOI] [Google Scholar]
- Ding Y.-F.; Zhao Q.-Q.; Yu Z.-L.; Zhao Y.-Q.; Liu B.; He P.-B.; Zhou H.; Li K.; Yin S.-F.; Cai M.-Q. Strong Thickness-Dependent Quantum Confinement in All-Inorganic Perovskite Cs2PbI4 with a Ruddlesden–Popper Structure. J. Mater. Chem. C 2019, 7, 7433–7441. 10.1039/c9tc02267h. [DOI] [Google Scholar]
- Stoumpos C. C.; Malliakas C. D.; Peters J. A.; Liu Z.; Sebastian M.; Im J.; Chasapis T. C.; Wibowo A. C.; Chung D. Y.; Freeman A. J.; Wessels B. W.; Kanatzidis M. G. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. 10.1021/cg400645t. [DOI] [Google Scholar]
- Zhao Y.-Q.; Ma Q.-R.; Liu B.; Yu Z.-L.; Cai M.-Q. Pressure-Induced Strong Ferroelectric Polarization in Tetra-Phase Perovskite CsPbBr3. Phys. Chem. Chem. Phys. 2018, 20, 14718–14724. 10.1039/c8cp01338a. [DOI] [PubMed] [Google Scholar]
- Liao C.-S.; Zhao Q.-Q.; Zhao Y.-Q.; Yu Z.-L.; Zhou H.; He P.-B.; Yang J.-L.; Cai M.-Q. First-Principles Investigations of Electronic and Optical Properties in the MoS2/CsPbBr3 Heterostructure. J. Phys. Chem. Solids 2019, 135, 109060. 10.1016/j.jpcs.2019.06.008. [DOI] [Google Scholar]
- Krishnamoorthy T.; Ding H.; Yan C.; Leong W. L.; Baikie T.; Zhang Z.; Sherburne M.; Li S.; Asta M.; Mathews N.; Mhaisalkar S. G. Lead-Free Germanium Iodide Perovskite Materials for Photovoltaic Applications. J. Mater. Chem. A 2015, 3, 23829–23832. 10.1039/c5ta05741h. [DOI] [Google Scholar]
- Sun P.-P.; Li Q.-S.; Feng S.; Li Z.-S. Mixed Ge/Pb Perovskite Light Absorbers with an Ascendant Efficiency Explored from Theoretical View. Phys. Chem. Chem. Phys. 2016, 18, 14408–14418. 10.1039/c6cp02105k. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-Q.; Wang X.; Liu B.; Yu Z.-L.; He P.-B.; Wan Q.; Cai M.-Q.; Yu H.-L. Geometric Structure and Photovoltaic Properties of Mixed Halide Germanium Perovskites from Theoretical View. Org. Electron. 2018, 53, 50–56. 10.1016/j.orgel.2017.11.005. [DOI] [Google Scholar]
- Deng X.-Z.; Zhao Q.-Q.; Zhao Y.-Q.; Cai M.-Q. Theoretical Study on Photoelectric Properties of Lead-Free Mixed Inorganic Perovskite RbGe1-xSnxI3. Curr. Appl. Phys. 2019, 19, 279–284. 10.1016/j.cap.2018.12.007. [DOI] [Google Scholar]
- Noel N. K.; Stranks S. D.; Abate A.; Wehrenfennig C.; Guarnera S.; Haghighirad A.-A.; Sadhanala A.; Eperon G. E.; Pathak S. K.; Johnston M. B.; Petrozza A.; Herz L. M.; Snaith H. J. Lead-Free Organic-Inorganic Tin Halide Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 3061–3068. 10.1039/c4ee01076k. [DOI] [Google Scholar]
- Stoumpos C. C.; Malliakas C. D.; Kanatzidis M. G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. 10.1021/ic401215x. [DOI] [PubMed] [Google Scholar]
- Hao F.; Stoumpos C. C.; Cao D. H.; Chang R. P. H.; Kanatzidis M. G. Lead-Free Solid-State Organic–Inorganic Halide Perovskite Solar Cells. Nat. Photonics 2014, 8, 489–494. 10.1038/nphoton.2014.82. [DOI] [Google Scholar]
- Hao F.; Stoumpos C. C.; Chang R. P. H.; Kanatzidis M. G. Anomalous Band Gap Behavior in Mixed Sn and Pb Perovskites Enables Broadening of Absorption Spectrum in Solar Cells. J. Am. Chem. Soc. 2014, 136, 8094–8099. 10.1021/ja5033259. [DOI] [PubMed] [Google Scholar]
- Ogomi Y.; Morita A.; Tsukamoto S.; Saitho T.; Fujikawa N.; Shen Q.; Toyoda T.; Yoshino K.; Pandey S. S.; Ma T.; Hayase S. CH3NH3SnxPb(1–x)I3 Perovskite Solar Cells Covering up to 1060 Nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. 10.1021/jz5002117. [DOI] [PubMed] [Google Scholar]
- Ju M.-G.; Sun G.; Zhao Y.; Liang W. A Computational View of the Change in the Geometric and Electronic Properties of Perovskites Caused by the Partial Substitution of Pb by Sn. Phys. Chem. Chem. Phys. 2015, 17, 17679–17687. 10.1039/c5cp01991e. [DOI] [PubMed] [Google Scholar]
- Mosconi E.; Umari P.; De Angelis F. Electronic and Optical Properties of Mixed Sn–Pb Organohalide Perovskites: A First Principles Investigation. J. Mater. Chem. A 2015, 3, 9208–9215. 10.1039/c4ta06230b. [DOI] [Google Scholar]
- Chang J.; Wang G.; Huang Y.; Luo X.; Chen H. New Insights into the Electronic Structures and Optical Properties in the Orthorhombic Perovskite MAPbI3: A Mixture of Pb and Ge/Sn. New J. Chem. 2017, 41, 11413–11421. 10.1039/C7NJ01442B. [DOI] [Google Scholar]
- Zuo F.; Williams S. T.; Liang P.-W.; Chueh C.-C.; Liao C.-Y.; Jen A. K.-Y. Binary-Metal Perovskites toward High-Performance Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 6454–6460. 10.1002/adma.201401641. [DOI] [PubMed] [Google Scholar]
- Snaith H. J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. 10.1021/jz4020162. [DOI] [Google Scholar]
- Zhao Y.; Zhu K. Organic-Inorganic Hybrid Lead Halide Perovskites for Optoelectronic and Electronic Applications. Chem. Soc. Rev. 2016, 45, 655–689. 10.1039/c4cs00458b. [DOI] [PubMed] [Google Scholar]
- Ali R.; Hou G.-J.; Zhu Z.-G.; Yan Q.-B.; Zheng Q.-R.; Su G. Predicted Lead-Free Perovskites for Solar Cells. Chem. Mater. 2018, 30, 718–728. 10.1021/acs.chemmater.7b04036. [DOI] [Google Scholar]
- Yang D.; Lv J.; Zhao X.; Xu Q.; Fu Y.; Zhan Y.; Zunger A.; Zhang L. Functionality-Directed Screening of Pb-Free Hybrid Organic–Inorganic Perovskites with Desired Intrinsic Photovoltaic Functionalities. Chem. Mater. 2017, 29, 524–538. 10.1021/acs.chemmater.6b03221. [DOI] [Google Scholar]
- Xiao Z.; Meng W.; Wang J.; Mitzi D. B.; Yan Y. Searching for Promising New Perovskite-Based Photovoltaic Absorbers: The Importance of Electronic Dimensionality. Mater. Horiz. 2017, 4, 206–216. 10.1039/c6mh00519e. [DOI] [Google Scholar]
- Fried M.; Lohner T.; Aarnink W. A. M.; Hanekamp L. J.; van Silfhout A. Determination of Complex Dielectric Functions of Ion Implanted and Implanted-Annealed Amorphous Silicon by Spectroscopic Ellipsometry. J. Appl. Phys. 1992, 71, 5260–5262. 10.1063/1.350587. [DOI] [Google Scholar]
- Saha S.; Sinha T. P.; Mookerjee A. Electronic Structure, Chemical Bonding, and Optical Properties of Paraelectric BaTiO3. Phys. Rev. B: Condens. Matter Mater. Phys. 2000, 62, 8828–8834. 10.1103/physrevb.62.8828. [DOI] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector Augmented-Wave Method. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 50, 17953–17979. 10.1103/physrevb.50.17953. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/physrevlett.77.3865. [DOI] [PubMed] [Google Scholar]
- Thonhauser T.; Cooper V. R.; Li S.; Puzder A.; Hyldgaard P.; Langreth D. C. Van Der Waals Density Functional: Self-Consistent Potential and the Nature of the Van Der Waals Bond. Phys. Rev. B: Condens. Matter Mater. Phys. 2007, 76, 125112. 10.1103/physrevb.76.125112. [DOI] [Google Scholar]
- Heyd J.; Scuseria G. E.; Ernzerhof M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. 10.1063/1.1564060. [DOI] [Google Scholar]