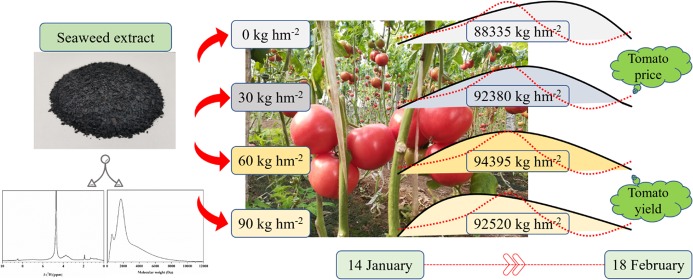
Abstract
Overuse of chemical fertilizers in the intensive greenhouse tomato cultivation system has limited the increase of plant production. Nowadays, seaweed extract has been gradually applied in agriculture as an effective way to achieve a higher yield of crops, but its effects on tomato cultivation have not been fully explored. In this study, a greenhouse experiment was conducted in Shandong province of China with a novel seaweed extract (SES) originated from Sargassum horneri, to investigate the effects of different doses of SES (0, 30, 60, and 90 kg hm–2) on yields, quality, ripening time, and net returns of tomato. The results indicated that the application of SES significantly increased tomato yield by 4.6–6.9% compared to the control, which is attributed to the improved photosynthetic capacity of tomato leaves. The yields of tomato increased first and then decreased with increasing dosage of SES, and SES applied at the dose of 60 kg hm–2 achieved the highest tomato yield. Compared to the control, SES at 60 and 90 kg hm–2 significantly increased the hardness of tomato by 10.2 and 19.8%, respectively, and this can help to reduce losses during transportation and storage. Moreover, SES shortened the ripening time of tomato, and the coincidence between tomato harvest and sale price peak achieved a high net return.
1. Introduction
Tomato, the highest-value fruit/vegetable with extensive worldwide distribution, has gradually become the preferred crop for many commercial growers in Northern China.1 Greenhouse is the most suitable facility for continuous growth of tomatoes because of its precise regulation of the condition of water, heat, and fertilizer. To achieve a higher yield, tomatoes are generally cultivated with a greater degree of management, larger input of nutrients, and more precise irrigation, especially in China, where smallholder farms dominate.2−4 Based on the principle of diminishing returns,5 continuous application of fertilizers hardly improved the yields and nutrient use efficiency of tomato, but caused a series of environmental pollution stemming from the leaching,6 runoff,7 and volatilization of excessive nutrients.8 Therefore, novel nonfertilization measures to promote tomato production as well as to ensure farmers’ income are critical for the development of sustainable agriculture.
Seaweed is an inexpensive and extensive resource along coastal agricultural areas, and it also has a great potential for commercial exploitation. Seaweed extracts isolated from seaweed, which contain a wide range of macronutrient and microelement nutrients and organic components such as growth hormones, amino acids, vitamins, betaines, cytokinins, and sterols,9,10 have played an important role in the development of the environment-friendly crops planting system.11−15 In general, seaweed extracts can induce changes in the physiological/biochemical process in agriculture associated with nutrient uptake and growth of plants. For example, seaweed extracts promoted early seed germination and establishment,11 boosted root growth, increased leaf chlorophyll,16 improved crop performance and yield of tomato,17,18 and elevated resistance to biotic/abiotic stress.19,20 In addition, seaweed extracts also affected the physical, chemical, and biological properties of soil. Seaweed extracts could combine soil metal ions to produce colloids and protect the soil aggregate structure.21 Richardson also noted that a seaweed extract originated from Ascophyllum nodosum could replace EDTA to chelate trace elements.22 Noteworthily, the benefits stemming from seaweed extracts are mainly attributed to their stimulation on plant growth but not to their provision on nutrients.11 Thus, seaweed extracts may construct a bridge between crops and fertilizer, serving a balance between agricultural input markets and economic benefit.
Economic benefit is the biggest concern for commercial growers in actual tomato production. In China, the income from tomato plantation is affected by its yield and quality, and especially its ripening time. More concretely, during the Mid-Autumn Festival and Spring Festival, the unit price of various vegetables increases with dramatic demands. Regulating the ripening time artificially during festivals can help the growers to obtain a higher price of tomato in the fierce market competition. Seaweed extracts have been confirmed to contain cytokinins,23,24 auxins,25 and ABA-like growth substances,12 which theoretically promoted early ripening of crops by their synergistic activity.26 Some papers also showed that the ability of seaweed extracts to regulate the ripening time was attributed to their effects on modulating the metabolism and catabolism of plant endogenous growth regulators.27,28 Moreover, it was reported that medium or late application of the seaweed extract could be a useful method to regulate the ripening dynamics and boost the fruit quality of grapes for production of premium red wines.29 Therefore, controlling the ripening time of tomatoes by applying seaweed extracts can be a promising measure to increase farmers’ income by the way of ensuring tomato yield. However, the effects of seaweed extracts on the ripening time of tomato and the resulting economic benefits are not yet fully explored.
In this work, a novel seaweed extract (SES) obtained from Sargassum horneri was employed to study its effects on the yield, quality, and ripening time of tomato. Before SES was applied, size exclusion chromatography (SEC) and 1H NMR spectroscopy were utilized to understand its basic structure and composition. Subsequently, a greenhouse experiment with SES at three levels (30, 60, and 90 kg hm2) was conducted to explore its effects on tomato growth in North China. The objectives of this study were to investigate: (i) the influence of the SES application rate on the yield and quality of tomato; (ii) the effect of the SES application rate on the photosynthetic capacity and soil physical/chemical properties of tomato; and (iii) the ripening time of tomato as affected by different amounts of SES.
2. Results and Discussion
2.1. Effects of Different Amounts of SES on Single Harvest and Total Yield of Tomato
Tomato yields were obviously affected by the amount of SES (Table 1). Compared to CK, SES30, SES60, and SES90 significantly increased the total yields of tomato by 4.6, 6.9, and 4.7%, respectively. Our results were consistent with the report described in previous studies that seaweed extracts improved the yield of tomato,10,17,30 but the increased yield was relatively lower than that reported by Crouch and van Staden.30 The reason is that the long history for continuous growing of tomato with high nutrients input made it hard for yield improvement and the difference in application method of seaweed extract (foliar or soil application) had different yield improvement effect.30 The increase of yield in the SES-treated plant can be associated with the hormonal substances in SES, especially cytokinin,10 which can promote the mobilization of nutrients and trigger fruit set. Crouch13 also noted that seaweed extracts increased the root length of tomato, improved the uptake of nutrients, and then boosted the yield.
Table 1. Tomato Yield with Every Single Harvest and Total Yield Under Different Amounts of SESa.
| treatment | first harvest (kg greenhouse–1) | second harvest | third harvest | fourth harvest | fifth harvest | sixth harvest | total yield | total yield increment vs CK (%) |
|---|---|---|---|---|---|---|---|---|
| CK | 506.4c | 1036.7c | 1435.8ab | 1654.1a | 1194.7a | 664.8a | 6492.6b | |
| SES30 | 922.4b | 1491.3b | 1528.4a | 1303.2b | 1037.8b | 506.8b | 6789.9a | 4.6 |
| SES60 | 1036.0b | 1658.5a | 1488.4a | 1264.2b | 1025.0b | 466.0b | 6938.0a | 6.9 |
| SES90 | 1172.7a | 1740.1a | 1326.3b | 1199.2b | 905.5b | 456.4b | 6800.2a | 4.7 |
Mean values followed by the same lowercase letter were not significantly different at the 0.05 probability level based on one-way analyses of variance (ANOVAs) followed by Duncan’s multiple range tests within the same column.
Considering the continuous harvest character of tomato, tomato yields on every single harvest were also compared. The first harvest of tomato was on 14 January 2018, and SES30, SES60, and SES90 increased the yield by 82.1, 104.6, and 131.6% compared to CK treatment, respectively (Table 1). SES30, SES60, and SES90 treatments also significantly increased the second harvest yield of tomato by 43.8, 60.0, and 67.8% compared to CK treatment, respectively. On the third harvest, no significant difference was observed between the yield of SES90 treatment and CK treatment. But on the fourth, fifth, and sixth harvests, tomato yields under SES30, SES60, and SES90 treatment were significantly reduced by 21.2–27.5, 13.1–24.2, and 23.8–31.3% compared to CK treatment, respectively. The increased yield in the early harvest stages indicated that the application of SES shortened the fruit-ripening time (days from anthesis to ripening) of tomato.31 The photos taken on 21 January 2018 further confirmed the results as more fully ripe red tomatoes existed on the plants applied with SES (Figure 1). Seaweed extracts contain cytokinins,23,24 auxins,25 and ABA-like growth substances10 and these hormonal substances have been shown to have a stimulatory effect on plant growth and fruit maturity.26−29 We speculated that the precocity of tomato affected by SES may be related to its capacity to trigger early flowing and fruit set,10 and then shortened the fruit-ripening time of tomato.
Figure 1.
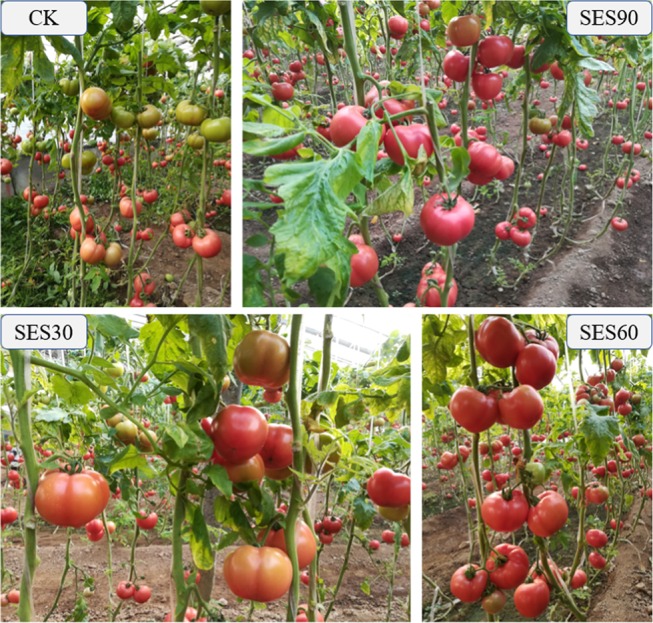
Pictures of tomatoes with different treatments on 21 January 2018.
2.2. Effects of Different Amounts of SES on the Economic Benefits of Tomato
The unit price of tomatoes is affected by market factors, and this means that tomato will have disparate profit due to the sale prices at different harvest times. In the present study, the tomato harvest period lasted 42 days and across China’s Spring Festival. In China, the harvest time that is near the Spring Festival can get a relative higher price. The price of tomato fluctuates between 0.62 and 1.11 $ kg–1 according to the local sale price (Table 2). The maximum and minimum sale price gap is 1.8 times. In addition, the sale price of tomato in the early harvest stage was higher than that in the last three harvest stages. Eventually, the total profits in SES30, SES60, and SES90 treatments were 10.1, 13.6, and 9.9 higher than that in CK treatment, which means that the application of SES can be a reliable means to achieve more benefits when cultivated in autumn and harvested during the Spring Festival.
Table 2. Single Profit and Net Return of Tomatoes Under Different Amounts of SESa.
| treatment | first profit ($ greenhouse–1) | second profit | third profit | fourth profit | fifth profit | sixth profit | net profit | profit increment vs CK (%) |
|---|---|---|---|---|---|---|---|---|
| CK | 436.3 | 988.9 | 1590.4 | 1374.2 | 735.2 | 490.9 | 3686.5 | |
| SES30 | 794.7 | 1422.5 | 1693.0 | 1082.6 | 638.7 | 374.2 | 4059.8 | 10.1 |
| SES60 | 892.5 | 1582.0 | 1648.7 | 1050.3 | 630.7 | 344.1 | 4186.0 | 13.6 |
| SES90 | 1010.3 | 1659.8 | 1469.1 | 996.2 | 557.2 | 337.1 | 4051.1 | 9.9 |
The sale prices of tomato were $0.86, $0.95, $1.11, $0.83, $0.62, and $0.74 per kg for the first, second, third, fourth, fifth, and sixth profits, respectively. Base fertilizer, $848.1 greenhouse–1; water-soluble fertilizer, $19.8 greenhouse–1 time–1, 8 times in total; SES costs were $4.1, $8.2, and $12.3 greenhouse–1 time–1 for SES30 SES60, and SES90 treatments, 4 times in total; labor cost included plot arrangement, tomato picking, fertilization, and irrigation, $153.8 greenhouse–1; other costs included seedings, pesticides, transport, and other materials and expenses, 769.2 greenhouse–1.
2.3. Effects of SES Amounts on SPAD, Chlorophyll Content, and Photosynthetic Characteristics of Tomato Leaves
The SPAD values were considerably affected by the application of SES. Leaf SPADs in SES30, SES60, and SES90 treatment were significantly increased by 9.6, 8.5, and 9.5% compared to CK treatment, respectively (Table 3). The SPAD value has a good correlation with chlorophyll content, and the chlorophyll content in SES30, SES60, and SES90 treatments was significantly increased by 25.3, 18.9, and 16.6% compared to CK treatment, respectively. Numerous studies also confirmed that leaf chlorophyll content positively increased with the application of seaweed extract,32−34 which precisely coincided with our results. Blunden35 found that the application of seaweed extract had a higher chlorophyll content than unapplied treatments, which might be attributed to the reduction in chlorophyll degradation caused by betaines in the seaweed extract. Besides, some researchers found that the application of seaweed increased the leaf surface area.27 Nevertheless, there was no significant difference in both SPAD value and chlorophyll content between the treatments when applied with different amounts of SES.
Table 3. SPAD, Chlorophyll Content, and Photosynthetic Capacity of Tomato Leaves Under Different Amounts of SESa.
| treatment | SPAD | chlorophyll content (mg g–1) | Pn (μmol m–2 s–1) | Gs (μmol m–2 s–1) | Ci (μmol mol–1) | Tr (μmol m–2 s–1) |
|---|---|---|---|---|---|---|
| CK | 44.9b | 1.71b | 8.1b | 0.32b | 299.6a | 6.9a |
| SES30 | 49.2a | 2.14a | 9.5a | 0.39a | 277.9b | 6.9a |
| SES60 | 48.7a | 2.03a | 10.6a | 0.40a | 275.5b | 6.8a |
| SES90 | 49.1a | 1.99a | 10.7a | 0.42a | 265.3b | 7.1a |
Mean values followed by the same lowercase letter were not significantly different at the 0.05 probability level based on one-way ANOVAs followed by Duncan’s multiple range tests within the same column.
Photosynthesis is the basis for crops to capture solar energy and accumulate nutrients. The photosynthetic capacity of leaves directly determines the level of plant productivity. In the present study, Pn in SES30, SES60, and SES90 treatments was significantly increased by 17.5, 31.1, and 31.7% compared to CK treatment, respectively (Table 3). This may accelerate the accumulation of nutrients in plants, thereby promoting the maturation and increasing the yield of tomatoes. Moreover, Pn has been widely used as an indicator to estimate plant senescence, and the improvement of Pn in SES30, SES60, and SES90 indicated that the application of SES delayed the leaf senescence of tomato. Gs of SES30, SES60, and SES90 was significantly increased by 23.0, 26.2, and 31.3% compared to CK treatment, respectively, which means that SES can boost the gas exchange in tomato leaves. Moreover, Ci in CK acquired the highest since more CO2 is needed as the raw material in the treatment with high Pn. But there was no evidence that the SES application has a significant effect on Tr in leaves.
2.4. Effects of SES Amounts on Soil Physical/Chemical Properties and Nutrient Supply Intensity
In actual greenhouse production, available soil nutrient contents were extremely higher owing to the habitually excessive soluble fertilizer input for higher yield.2−4 In this work, the average content of NO3–, available P, and available K of soil also reached a very high level (more than 40, 90, and 430 mg kg–1, respectively) after all of the tomatoes were harvested (Table 4). Previous papers reported that several seaweeds contained high ratios of elements like Ca, P, and K.36 Although the K and ash contents of the SES were up to 6.6 and 30.2%, respectively, no obvious difference was observed in the NO3– content, NH4+ content, available P, and available K of soil among all of the treatments (Table 4), which is precisely consistent with the concept of biostimulants defined by European Biostimulant Industry Council (EBIC): “Biostimulants operate through different mechanisms than fertilizers, regardless of the presence of nutrients in the products”.11 Under the condition of such fertile soil, the yield of tomatoes was markedly increased by 4.6–6.9% with the application of SES, providing an environment-friendly way for crop cultivation in greenhouse. In the future, whether the amount of fertilizer can be reduced by adding SES while maintaining the gain yield is worth to be validated.
Table 4. Soil pH, EC, and Available Nutrient Content Under Different Amounts of SESa.
| treatment | pH | EC (μs cm–1) | NO3– content (mg kg–1) | NH4+ content (mg kg–1) | available P (mg kg–1) | available K (mg kg–1) |
|---|---|---|---|---|---|---|
| CK | 7.20a | 702.6a | 40.8a | 11.6a | 95.5a | 427.0a |
| SES30 | 7.19a | 588.3b | 39.5a | 12.7a | 83.9a | 409.7a |
| SES60 | 7.18a | 524.9b | 41.9a | 13.7a | 104.3a | 455.9a |
| SES90 | 7.17a | 520.1b | 43.5a | 11.9a | 91.6a | 443.1a |
Mean values followed by the same lowercase letter were not significantly different at the 0.05 probability level based on one-way ANOVAs followed by Duncan’s multiple range tests within the same column.
Soil EC values in SES30, SES60, and SES90 treatments were significantly decreased by 16.3, 25.3, and 26.0% compared to CK treatment (Table 4). The greater the EC value, the higher the content of soluble salts.37,38 The decreased EC value in SES30, SES60, and SES90 treatments indicated that SES can reduce the leaching or runoff of nutrients into the environment. The decreased EC value may be attributed to the precipitate/chelate character of SES with some alkaline ions in the soil and the existence of polyuronides in SES with gelling, chelating, and hydrophilic properties such as alginates and fucoidans.15
2.5. Effects of the Amount of SES on Tomato Quality
Quality is an important factor for sustaining higher unit price of tomato. Tomato quality includes appearance character such as single fruit weight and hardness, as well as intrinsic aspect represented by nutrient content and mouthfeel. In the present study, although single tomato weight, soluble solids, titratable acid, and vitamin C content of tomato showed no significant difference in all of the treatments, the soluble sugar content in tomato was markedly increased with the application of SES (Table 5), thereby increasing the sugar/acid ratio and achieving a better flavor and taste. But there was no significant difference among the treatments with different amounts of SES.
Table 5. Tomato Quality Under Different Amounts of SESa.
| treatment | single weight (g) | hardness (kg cm–2) | soluble solids (%, FW) | titratable acid (%, FW) | soluble sugar (%, FW) | sugar acid ratio | vitamin C content (mg g–1) |
|---|---|---|---|---|---|---|---|
| CK | 211.2a | 4.20c | 4.90a | 0.33a | 3.09b | 9.36b | 2.92a |
| SES30 | 214.7a | 4.37bc | 5.00a | 0.34a | 3.40a | 10.00a | 3.20a |
| SES60 | 219.0a | 4.63b | 5.37a | 0.35a | 3.59a | 10.26a | 2.76a |
| SES90 | 217.3a | 5.03a | 5.07a | 0.35a | 3.51a | 10.03a | 2.88a |
Mean values followed by the same lowercase letter were not significantly different at the 0.05 probability level based on one-way ANOVAs followed by Duncan’s multiple range tests within the same column. FW, fresh weight.
Moreover, the hardness of tomato was markedly increased with the increasing dose of SES (Table 5). The hardness of tomato in SES60 and SES90 was 10.2 and 19.8% higher than that in CK treatment, respectively. Therefore, the application of SES can reduce the loss during transportation and storage by increasing the hardness of the tomato. Stasio38 noted that seaweed extracts increased the Ca2+ content of tomato by more than 20%, which helps to explain the mechanism of SES for the increase of hardness.
2.6. Chemical Structure and Composition of SES
The effectiveness of seaweed extracts was markedly influenced by their sources, structure, and composition.39 Therefore, a thorough understanding of SES by characterization is necessary for their efficient utilization. For instance, Stasio38 confirmed via the gas chromatography-mass spectrometry analysis that the seaweed extract was rich in bioactive compounds, which was possibly beneficial to the growth and stress adaptation of tomato. Khan10 summarized several chemical analyses of seaweeds and seaweed extracts and revealed the presence of a wide variety of plant growth-promoting substances such as auxins, cytokinins, and betaines. Despite evidence from the literature for the role/effects of these substances as single molecules on plant growth and stress protection, the diverse and complex nature of seaweed extracts makes it difficult to establish a univocal cause of the various biostimulant effects observed. Therefore, there is a need for an in-depth analysis of the functional specificities.
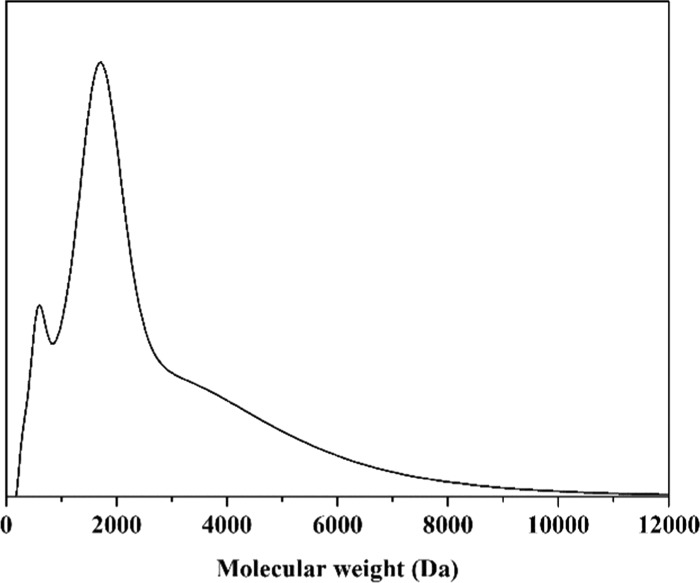
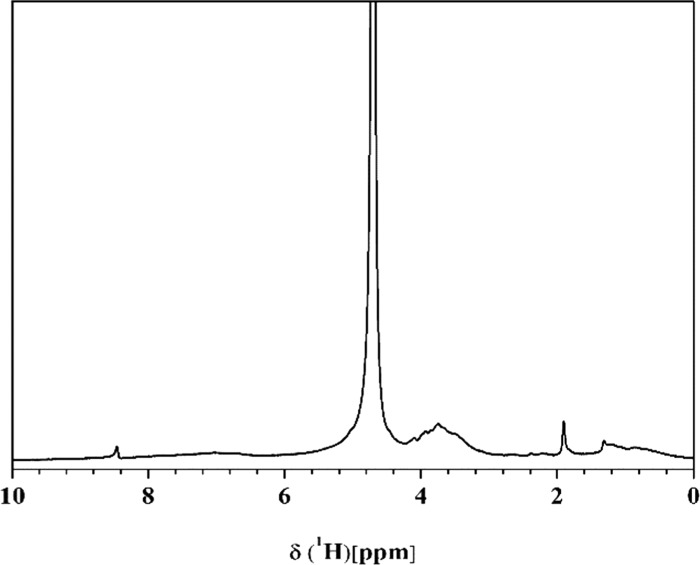
SES contains 29.2% of alginic acid, 14.1% of crude protein, 10.9% of mannitol, 30.2% of ash, 6.6% of potassium, 0.024% of iodine, as well as a variety of plant hormones (the date was provided by Worldfull Agricultural Technology Co., Ltd., Shandong, China). Furthermore, the analysis of size exclusion chromatography provided a clear insight into the molecular weight (MW) distribution of SES with a range of 179–11 949 Da (Figure 2). The proportion of the relatively low MW fraction (MW < 5 kDa) was up to 87.8%, and the higher MW (MW > 5 kDa) was only 12.2%. Therefore, SES was a biostimulant with lower MW than other seaweed extracts,40 which can be easily absorbed by the tomato root. Detailed structural information and the relative distribution of main structures were obtained by 1H NMR spectroscopy (Figure 3). SES exhibited a stronger predominance of aromatic protons (δ(1H) = 9.5–6.5 ppm) than alkyl protons (δ(1H) = 1.9–0.5 ppm). The proton content (δ(1H) = 4.9–3.1 ppm) on C atoms directly bonded to N, O, or carbohydrates is up to 57.87% (Table S1). The signals at 4.5–3.2 ppm also can be the source of H in methylene groups and/or aminomethine groups [−CH(NH−)]. The signals at 6.5–6.0 ppm can be attributed to double bonds. Although many of the various chemical components of SES and their modes of action remain unknown, it is plausible that these components exhibit synergistic activity.
Figure 2.
Size exclusion chromatography of SES.
Figure 3.
Solid-state 1H nuclear magnetic resonance spectroscopy of SES.
3. Conclusions
Results suggested that tomato yield was significantly increased by SES, which is attributed to its positive biological stimulation on the photosynthetic capacity improvement of tomato leaves, rather than providing nutrients as a fertilizer. The application of SES shortened the fruit-ripening time of tomato, and hence can be a reliable means to achieve higher benefits when cultivated in autumn and harvested during China’s Spring Festival. Moreover, SES increased the hardness of tomato. These results show that the use of seaweed extracts is a suitable method for tomato growth and development in sustainable agricultural systems.
4. Materials and Methods
4.1. Experimental Sites and Materials
The experimental site is located at Fangcun town (117°20′37″ E, 35°97′03″ N), Shandong Province, China, the largest professional production and wholesale base for tomatoes. It belongs to the warm-temperature semihumid monsoon climate, with an annual mean temperature of 13 °C and precipitation of 700 mm. The greenhouse has 12 years cropping history of continuous growing tomato two seasons per year. The rectangular greenhouses are 70 m long and 10.5 m wide, with one side wall (3.6 m high) and two end walls constructed from soil and brick (Figure S1). The main properties of top-layer soil (0–20 cm) at the experimental site in 2017 before tomato planting were: pH, 6.65 (2.5:1, the ratio of water to soil), soil total N concentration, 1.83 g kg–1; organic matter concentration, 21.50 g kg–1; NO3––N concentration, 30.73 mg kg–1; NH4+–N concentration, 10.25 mg kg–1; available P concentration; 25.71 mg kg–1; and available K concentration, 180.58 mg kg–1, respectively.
Tomato (Solanum lycopersicum Mill, Jinpeng 11), a member of the pink tomato varieties, was selected as the testing crop. The SES extracted from S. horneri was freely provided by Worldfull Agricultural Technology Co., Ltd. (Yantai, Shandong, China), and it contains 29.2% of alginic acid, 14.08% of crude protein, 10.94% of mannitol, 30.16% of ash, 6.63% of potassium, 0.024% of iodine, as well as a variety of plant hormones. The extraction and production processes of SES by the company are presented in Figure S2. The water-soluble fertilizer (N–P2O5–K2O: 20–20–20) applied in the experiment as the nutrient source of tomato was provided by Kingenta Ecological Engineering Co. Ltd. (Linshu, Shandong, China).
4.2. Experimental Design
A randomized experiment with three replicates was conducted on 20 August 2017 in plots considering four treatments: control without SES application (CK), applied SES at the dose of 30 kg hm–2 (SES30), applied SES at the dose of 60 kg hm–2 (SES60), and applied SES at the dose of 90 kg hm–2 (SES90). Every plot was 8.5 m long and 1.8 m wide with an area of 15.3 m2. Soil ridges (30 cm height, 30 cm width) were built to ensure independent irrigation and drainage between adjacent plots. The tomato was transplanted at the four-leaf stage with a density of 30 plants plot–1 (90 cm between rows and 30 cm between plants).
Based on the local planting habits, all of the treatments were applied with organic fertilizer (70% rice hull and 30% chicken manure uniformly blended in advance, 150 t hm–2) as a base fertilizer 20 days before the transplantation of tomato seedlings. Fertilization and irrigation were carried out simultaneously during the growth of tomato. The water-soluble fertilizer (N–P2O5–K2O: 20–20–20, 195 kg hm–2 time–1) was dissolved in 400 kg of water at a pool (Figure S3) and irrigated by pipes every 15 days (achieve 90% of the field water-holding capacity, eight times in total). For the application of SES, weighed SES dependent on different treatments was dissolved in the pool and applied along with the water-soluble fertilizer on September 27, 2017, October 26, 2017, November 26, 2017, and December 25, 2017. All replications within each treatment were conducted according to the local agronomic practices, receiving identical irrigation, pruning, and control of insects and weeds.
4.3. Harvest
The harvesting process of tomatoes lasted from January 14, 2018 to February 18, 2018 for every 7 days (six times in total). Tomatoes in each treatment were individually weighed, counted, and measured. Plant samples were derived from the tomatoes harvested in the third harvest stage, and the soil samples were collected after all of the tomatoes were harvested.
4.4. Soil and Plant Sampling and Measurement
Leaf SPAD value was estimated with a chlorophyll meter (SPAD–502, Minolta Co., Japan) on 14 January 2018. The photosynthesis parameters of leaves, including net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration (Tr), were measured on 14 January 2018 between 9:00 to 11:00 h by a portable photosynthetic meter (Li-6400XT, LI-COR Co.). Subsequently, the leaves were taken off and brought back to the laboratory. The chlorophyll of leaves was dissolved in acetone (80%)41 and measured by an ultraviolet spectrophotometer (UV-2700, Shimadzu Co., Japan).
Soil EC was measured by a conductivity meter (DDS-11A, Inesa Co., China). Soil NO3––N and NH4+–N concentrations were extracted by 0.01 M CaCl242 and analyzed by an AA3 Auto-analyzer (AA3-A001-02E, Bran-Luebbe Co., Germany). Soil total N was measured by the Kjeldahl N determination method (Douglas et al., 1980).43 The available P concentration in soil was extracted by 0.5 M NaHCO3 at pH 8.544 and analyzed by a Discrete Auto-analyzer (Smart Chem 200, Alliance Co., France). The available K concentration in soil was extracted by 1 M CH3COONH4 at pH 745 and analyzed by a flame photometer (Model 410, Sherwood Co., England).
Tomato samples were homogeneously smashed with a juice machine at room temperature. Vitamin C (Vc) content was measured by the colorimetric method.46 The concentration of soluble solids was measured using a digital refractometer (RX-5000α, Atago Co., Japan).47 The titratable acidity was determined by titrating with 0.1 N NaOH of pH 8.2.48 Soluble sugar content in tomato juice was determined using the anthrone reagent method.49
Size exclusion chromatography was performed on solutions of the SES using a Sephadex G-100 medium gel (Code No. 17-0060-02 Pharmacia Biotech AB). Solid-state 1H NMR spectroscopy was performed using an Avance 600 MHz (Bruker, Karlsruhe Co., Germany) spectrometer.
4.5. Calculation of Every Single Profit and Net Return
4.6. Statistical Analyses
The response parameters were subjected to analysis of variance (ANOVA) and mean separation test using the Statistical Analysis System 9.2 (2010, SAS Institute, Cary, NC). Mean and standard error values were assessed to assemble graphs using the SigmaPlot software 10 (MMIV Systat Software, Inc., San Jose, CA).
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Grant nos. 2017YFD0200705; 2017YFD0200706).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04155.
Integration area of 1H NMR spectra from SES (Table S1); photos of greenhouse and the plot (Figure S1); production process of SES (Figure S2); and schematic diagram of the irrigation system (Figure S3) (PDF)
Author Contributions
All authors contributed to reading and approved the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Agbna G. H. D.; She D.; Liu Z.; Nazar A. E.; Shao G.; Luis C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. 10.1016/j.scienta.2017.05.004. [DOI] [Google Scholar]
- He F.; Chen Q.; Jiang R.; Chen X.; Zhang F. Yield and nitrogen balance of greenhouse tomato (Lycopersicum esculentum Mill.) with conventional and site-specific nitrogen management in Northern China. Nutr. Cycling Agroecosyst. 2007, 77, 1–14. 10.1007/s10705-006-6275-7. [DOI] [Google Scholar]
- Gao J. J.; Bai X. L.; Zhou B.; Zhou J. B.; Chen Z. J. Soil nutrient content and nutrient balances in newly-built solar greenhouses in northern China. Nutr. Cycl Agroecosyst. 2012, 94, 63–72. 10.1007/s10705-012-9526-9. [DOI] [Google Scholar]
- Bai X.; Gao J.; Wang S.; Cai H.; Chen Z.; Zhou J. Excessive nutrient balance surpluses in newly built solar greenhouses over five years leads to high nutrient accumulations in soil. Agric., Ecosyst. Environ. 2020, 288, 106717 10.1016/j.agee.2019.106717. [DOI] [Google Scholar]
- Mueller N. D.; Gerber J. S.; Johnston M.; Ray D. K.; Ramankutty N.; Foley J. A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. 10.1038/nature11420. [DOI] [PubMed] [Google Scholar]
- Hartmann T. E.; Yue S. C.; Schulz R.; He X. K.; Chen X. P.; Zhang F. S.; Müller T. Yield and N use efficiency of a maize-wheat cropping system as affected by different fertilizer management strategies in a farmer’s field of the North China Plain. Field Crops Res. 2015, 174, 30–39. 10.1016/j.fcr.2015.01.006. [DOI] [Google Scholar]
- Geng J. B.; Sun Y. B.; Zhang M.; Li C. L.; Yang Y. C.; Liu Z. G.; Li S. L. Long-term effects of controlled release urea application on crop yields and soil fertility under rice-oilseed rape rotation system. Field Crops Res. 2015, 184, 65–73. 10.1016/j.fcr.2015.09.003. [DOI] [Google Scholar]
- Wang X.; Fan J.; Xing Y.; Xu G.; Wang H.; Deng J.; Wang Y.; Zhang F.; Li P.; Li Z. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. 10.1016/bs.agron.2018.08.003. [DOI] [Google Scholar]
- Sivasankari S.; Venkatesalu V.; Anantharaj M.; Chandrasekaran M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. 10.1016/j.biortech.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Khan W.; Rayirath U. P.; Subramanian S.; Jithesh M. N.; Rayorath P.; Hodges D. M.; Critchley A. T.; Craigie J. S.; Norrie J.; Prithiviraj B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. 10.1007/s00344-009-9103-x. [DOI] [Google Scholar]
- Calvo P.; Nelson L.; Kloepper J. W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. 10.1007/s11104-014-2131-8. [DOI] [Google Scholar]
- Bulgari R.; Cocetta G.; Trivellini A.; Vernieri P.; Ferrante A. Biostimulant and crop responses: a review. Biol. Agric. Hortic. 2015, 31, 1–17. 10.1080/01448765.2014.964649. [DOI] [Google Scholar]
- Crouch I. J.; Beckett R. P.; van Staden J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J. Appl. Phycol. 1990, 2, 269–272. 10.1007/BF02179784. [DOI] [Google Scholar]
- Rayorath P.; Narayanan J. M.; Farid A.; Khan W.; Palanisamy R.; Hankins S. D.; Critchley A. T.; Prithiviraj B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum(L.) Le Jol. using a model plant, Arabidopsis thaliana(L.). J. Appl. Phycol. 2008, 20, 423–429. 10.1007/s10811-007-9280-6. [DOI] [Google Scholar]
- Craigie J. S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. 10.1007/s10811-010-9560-4. [DOI] [Google Scholar]
- Jannin L.; Arkoun M.; Etienne P.; Laîné P.; Goux D.; Garnica M.; Fuentes M.; Francisco S. S.; Baigorri R.; Cruz F.; Houdusse F.; Garcia-Mina J. M.; Yvin J. C.; Ourry A. Brassica napus growth is promoted by Ascophyllum nodosum(L.) Le Jol. Seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. 10.1007/s00344-012-9273-9. [DOI] [Google Scholar]
- Zodape S. T.; Gupta A.; Bhandari S. C. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Kumari R.; Kaur I.; Bhatnagar A. K. Effect of aqueous extract of Sargassum johnstonii Setchell& Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. 10.1007/s10811-011-9651-x. [DOI] [Google Scholar]
- Spinelli F.; Fiori G.; Noferini M.; Sprocatti M.; Costa G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. 10.1016/j.scienta.2010.03.011. [DOI] [Google Scholar]
- González A.; Castro J.; Vera J.; Moenne A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. 10.1007/s00344-012-9309-1. [DOI] [Google Scholar]
- Halpern M.; Bar-Tal A.; Ofek M.; Minz D.; Muller T.; Yermiyahu U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Richardson A. E.; Barea J. M.; McNeill A. M.; Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- Stirk W. A.; van Staden J. Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratusL. J. Appl. Phycol. 1997, 9, 327–330. 10.1023/A:1007910110045. [DOI] [Google Scholar]
- Brain K. R.; Chalopin M. C.; Turner T. D.; Blunden G.; Wildgoose P. B. Cytokinin activity of commercial aqueous seaweed extract. Plant Sci. Lett. 1973, 1, 241–245. 10.1016/0304-4211(73)90026-6. [DOI] [Google Scholar]
- Crouch I. J.; van Staden J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. 10.1007/BF00207588. [DOI] [Google Scholar]
- Vernieri P.; Borghesi E.; Ferrante A.; Magnani G. Application of biostimulants in floating system for improving rocket quality. J. Food, Agric. Environ. 2005, 3, 86–88. [Google Scholar]
- Lola-Luz T.; Hennequart F.; Gaffney M. Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleraceae) following application of commercial seaweed extracts of the brown seaweed (Ascophyllum nodosum). Agric. Food Sci. 2013, 22, 288–295. 10.23986/afsci.7676. [DOI] [Google Scholar]
- Lola-Luz T.; Hennequart F.; Gaffney M. Effect on yield, total phenolic, total flavonoid and total isothiocyanate content of two broccoli cultivars (Brassica oleraceaevar italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). Agric. Food Sci. 2014, 23, 28–37. 10.23986/afsci.8832. [DOI] [Google Scholar]
- Frioni T.; Sabbatini P.; Tombesi S.; Norrie J.; Poni S.; Gatti M.; Palliotti A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. 10.1016/j.scienta.2017.12.054. [DOI] [Google Scholar]
- Crouch I. J.; van Staden J. Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. J. Appl. Phycol. 1992, 4, 291–296. 10.1007/BF02185785. [DOI] [Google Scholar]
- Doganlar S.; Tanksley S. D.; Mutschler M. A. Identification and molecular mapping of loci controlling fruit ripening time in tomato. Theor. Appl. Genet. 2000, 100, 249–255. 10.1007/s001220050033. [DOI] [Google Scholar]
- Krajnc A. U.; Ivanuš A.; Kristl J.; Šušek A. Seaweed extract elicits the metabolic responses in leaves and enhances growth of pelagornium cuttings. Eur. J. Hortic. Sci. 2012, 77, 170–181. [Google Scholar]
- Spinelli F.; Fiori G.; Noferini M.; Sprocatti M.; Costa G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. 10.1016/j.scienta.2010.03.011. [DOI] [Google Scholar]
- Whapham C. A.; Blunden G.; Jenkins T.; Hankins S. D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231–234. 10.1007/BF00004023. [DOI] [Google Scholar]
- Blunden G.; Jenkins T.; Liu Y. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. 10.1007/BF02186333. [DOI] [Google Scholar]
- Hong D. D.; Hien H. M.; Son P. N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. 10.1007/s10811-007-9228-x. [DOI] [Google Scholar]
- Cardozo K. H. M.; Guaratini T.; Barros M. P.; Falcão V. R.; Tonon A. P.; Lopes N. P.; Campos S.; Torres M. A.; Souza A. O.; Colepicolo P.; Pinto E. Metabolites from algae with economical impact. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2007, 146, 60–78. 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Di Stasio E.; Oosten M. J. V.; Silletti S.; Raimondi D.; dell’Aversana E.; Carillo P.; Maggio A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. 10.1007/s10811-018-1439-9. [DOI] [Google Scholar]
- Olaetxea M.; David D. H.; Andrés C. A.; Fuentes M.; Baigorri R.; Mora V.; Garnica M.; Urrutia O.; Erro J.; Zamarreño A. M.; Berbara R. L.; Garcia-Mina J. M. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil. Ecol. 2018, 123, 521–537. 10.1016/j.apsoil.2017.06.007. [DOI] [Google Scholar]
- Rioux L. E.; Turgeon S. L.; Beaulieu M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. 10.1016/j.carbpol.2007.01.009. [DOI] [Google Scholar]
- Ma J. Z.; Zhang M.; Liu Z. G.; Chen H. N.; Li Y. C.; Sun Y.; Ma Q.; Zhao C. H. Effects of foliar application of the mixture of copper and chelated iron on the yield, quality, photosynthesis, and microelement concentration of table grape (Vitis viniferaL.). Sci. Hortic. 2019, 254, 106–115. 10.1016/j.scienta.2019.04.075. [DOI] [Google Scholar]
- Zheng W. K.; Zhang M.; Liu Z. G.; Zhou H. Y.; Lu H.; Zhang W. T.; Yang Y. C.; Li C. L.; Chen B. C. Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res. 2016, 197, 52–62. 10.1016/j.fcr.2016.08.004. [DOI] [Google Scholar]
- Douglas L. A.; Riazi A.; Smith C. J. A semi-micro method for determining total N in soils and plant material containing nitrite and nitrate. Soil Sci. Soc. Am. J. 1980, 44, 431–433. 10.2136/sssaj1980.03615995004400020047x. [DOI] [Google Scholar]
- Zheng W. K.; Sui C. L.; Liu Z. G.; Geng J. B.; Tian X. F.; Yang X. Y.; Li C. L.; Zhang M. Long-term effects of controlled-release urea on crop yields and soil fertility under wheat–corn double cropping systems. Agron. J. 2016, 108, 1703–1716. 10.2134/agronj2015.0581. [DOI] [Google Scholar]
- Sharma S. K.; Sharma A.; Rana S.; Kumar N. Evaluation of multi-nutrient extractants for determination of available P, K, and micronutrient cations in soil. J. Plant Nutr. 2018, 41, 782–792. 10.1080/01904167.2018.1426019. [DOI] [Google Scholar]
- Qu Z. M.; Qi X. C.; Wang J.; Chen Q.; Li C. L. Effects of nitrogen application rate and topdressing times on yield and quality of Chinese cabbage and soil nitrogen dynamics. Environ. Pollut. Bioavailability 2019, 31, 1–8. 10.1080/09542299.2018.1546555. [DOI] [Google Scholar]
- Zhu S.; Liang Y.; An X.; Kong F.; Gao D.; Yin H. Changes in sugar content and related enzyme activities in table grape (Vitis vinifera L.) in response to foliar selenium fertilizer. J. Sci. Food Agric. 2017, 97, 4094–4102. 10.1002/jsfa.8276. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Liang Y.; Chu G. Applying silicate fertilizer increases both yield and quality of table grape (Vitis vinifera L.) grown on calcareous grey desert soil. Sci. Hortic. 2017, 225, 757–763. 10.1016/j.scienta.2017.08.019. [DOI] [Google Scholar]
- Davarpanah S.; Tehranifar A.; Davarynejad G.; Abadía J.; Khorasani R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. 10.1016/j.scienta.2016.07.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.