Abstract

An amino curing agent containing silicon/titanium flame-retardant elements (STCA) based on (3-aminopropyl)triethoxysilane (APTES) and tetrabutyl titanate was successfully prepared. The thermal decomposition and flame-retardant properties of a STCA-cured trifunctional epoxy resin, which was facilely synthesized by 1,1,1-tris(4-hydroxyphenyl)ethane and epichlorohydrin via a two-step method, were compared with those of another amino curing agent containing silicon (SCA) based on APTES and methyltrimethoxysilane. The structures of STCA and SCA were characterized by Fourier transform infrared (FT-IR), 29Si NMR, and Raman spectroscopies. The STCA-cured thermoset not only had good thermal stability with an initial decomposition temperature of 344.8 °C and a char yield of 52.7% at 800 °C but also exhibited the overall improvement of flame-retardant properties. V-0 rating was achieved using the UL-94 test, and the value of limiting oxygen index reached 33.8%. From the thermogravimetry–infrared test, the yield of pyrolysis products of the STCA-cured thermoset was significantly decreased, indicating the lower toxicity in contrast to the SCA-cured thermoset. Flame-retardant performances were also investigated using the cone calorimetry test, and the flame retardancy mechanism was studied using scanning electron microscopy, FT-IR, and energy-dispersive spectrometry. The results indicate that the introduction of silicon/titanium to the system reveals the synergistic effects to promote the formation of an intumescent, sufficient, and compact char layer during combustion, which could effectively prevent heat, oxygen, and flame from penetrating into the interior structure, and lead to the retardance of further combustion.
Introduction
Epoxy resins (EP) exhibit many excellent comprehensive performances such as good physical and mechanical properties, excellent electrical insulation, high adhesive strength to many substrates, outstanding chemical and corrosion resistance, low shrinkage while cured, and remarkable low price.1,2 They have been widely used as various advanced composite matrices in many fields ranging from surface coating, adhesives, laminates, electronic/electrical industries to automobile, shipbuilding, and space vehicles.3−6 Although EP exhibit many great properties in various fields, their application in a heated environment is strictly limited for a limiting oxygen index (LOI) of only 19.8%.7 Therefore, intrinsic flame retardancy still remains an obvious disadvantage for most of the EP. Accordingly, improving the thermal stability of EP is necessary to extend the scope of applications in ablative materials and fire-retardant materials. Currently, there are primarily two methods8 to modify EP to obtain flame-retardant materials: one is to add flame retardants into the substrate physically and the other is to introduce inorganic flame-retardant elements to molecular chains. Compared to the former, the latter displays favorable advantages because of the homogeneous dispersion excluding interfacial problem between the matrix and flame retardants.9
Traditionally, introducing a halogenated compound into EP is an effective method to improve resistance to flame.10 However, many of them will lead to various issues toward environment and human health because of the toxic and corrosive smoke released during combustion or thermolysis.11,12 In spite of severe drawback and environment-friendly development, halogenated flame retardants are not applicable in many fields. In recent years, considering environmental problems, halogen-free flame retardants containing nitrogen,13 silicon,14 phosphorus,9 boron,7,15 titanium,16 and zirconium17,18 have gradually been applied in the preparation of flame-retardant materials. In fact, the inorganic elements doped to the polymer play an effective role in forming a thermally stable phase and improving the thermal stability.19,20 Among them, silicon-containing compounds are typical flame retardants that can significantly exert thermal stability and flame retardancy for modifying EP.11,21 Polyhedral oligomeric silsesquioxane with three-dimensional and cage-shaped structure has the advantages of environmental innocuousness, good thermal and oxidative stability, and flame retardancy.22,23 Another common silicon-containing compound that has been widely used to improve the thermal, mechanical, and insulating performances of modifying resins is γ-aminopropyltriethoxysilane, which contains the reactive group −NH2 that can be used as a curing agent for EP.24−26 However, a single inorganic flame-retardant element cannot meet the increasing requirements for advanced applications, so the synergistic flame-retardant systems, which comprised multiple flame-retardant elements, have attracted researchers’ attention. The flame retardancy of materials could be significantly enhanced by synergistic effects of multiple elements than the flame-retardant element used alone, such as the silicon/phosphorus,27 nitrogen/phosphorus,1,28,29 silicon/boron,30,31 silicon/nitrogen, silicon/nitrogen/phosphorus,32 and silicone/boron/phosphorus33 system. Xu et al.34 synthesized a novel flame retardant containing a P–C–N bond and exhibiting excellent flame retardancy for epoxy thermosets. Therefore, synthesis of flame retardants containing diverse inorganic elements is an effective means to endow materials with great thermal stability.
In the last few years, specific curing agents have been investigated to modify EP to enhance the properties of flame retardancy. Many of them containing reactive groups, such as −NH2, −CONH–, Ar-OH, anhydride, imidazole-type, and so forth, can be employed in functional structure designing for EP such that it can endow EP with special performances. Functionalizing a curing agent with a reactive group is a remarkable method to cross-link into epoxy networks and obviously improve the compatibility. Deng et al.7 held the opinion that developing novel curing agents may be more imperative than producing new kinds of EP in some respects. Researchers have paid much attention on curing agents with flame retardancy for facilitating curing and introducing heat resistance. Liu et al.35 performed a novel inorganic–organic amination of the hybrid charged membrane through 3-glycidoxypropyltrimethoxysilane, tetrabutyl titanate (TBT), and trimethyl amine, and the new material doped with silicon and titanium exhibited much higher thermal stability. Tan et al.36 designed and synthesized a novel flame-retarding curing agent based on ammonium polyphosphate, which was used in cation exchange with diethylenetriamine.
It is well known that the existence of the metal in the polymer shows an efficient effect on forming a thermally stable phase and even facilitates the cross-linking of partial bonds, thus enhancing the thermal stability of the material.37 Therefore, it is a common approach to dope metals into polymers in order to endow the material with better flame retardancy. There are usually two means: (1) a certain reaction by a covalent bond and (2) dispersion as inorganic clusters.38 Zhang et al.39 reported that the introduction of titanium to the polymer strongly increases the decomposition temperature and enhances the char yield.
In our work, 1,1,1-tris(4-hydroxyphenyl)ethane (THPE), epichlorohydrin (ECH), and NaOH were used to synthesize the epoxy resin called THPE triglycidyl ether (THPE-TE), which contains three units of benzene ring with the characteristic of thermal endurance and three units of epoxy group with the effect of reactive ability, and it can be used for blending with solid curing agents at room temperature. Besides, we constructed two curing agents containing silicon/titanium flame-retardant elements (STCA) and silicon only (SCA), respectively, and the chemical structures of synthetic compounds were characterized. The thermal analysis, flame retardancy, and flame-retardant mechanism of STCA-cured THPE-TE were evaluated and compared with SCA-cured THPE-TE by some characterization techniques, including LOI, UL-94, dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), thermogravimetry-infrared (TG-IR), cone calorimetry (CC) test, scanning electron microscopy (SEM), and energy-dispersive spectrometry (EDS).
Results and Discussion
Synthesis and Characterization of THPE-TE
THPE-TE was facilely synthesized via ring-opening addition and ring-closing reactions, and the synthesis route of THPE-TE is shown in Scheme 1. From the Fourier transform infrared (FT-IR) spectra of THPE and THPE-TE shown in Figure 1a,b, it can be seen that THPE and THPE-TE have basically the same absorption peak positions. However, the broad absorption band at about 3300 cm–1 in Figure 1a is assigned to the phenolic hydroxyl, while it nearly disappears in Figure 1b, demonstrating that the reaction among THPE, ECH, and NaOH was nearly complete. The characteristic peak of ph–O–C– is found at around 1290 cm–1 in Figure 1b, which further confirms the existence of ph–O–CH2–. The strong absorption peak around 916 cm–1 is ascribed to the epoxy ring.40 The results indicated that we successfully incorporated the epoxy group into the molecule structure of THPE.
Scheme 1. Schematic Procedures of Synthesis for THPE-TE.
Figure 1.
FT-IR spectra of (a) THPE and (b) THPE-TE; and (c) 1H NMR spectrum of THPE-TE.
To further verify the successful synthesis of THPE-TE, 1H NMR spectroscopy was used to analyze the chemical structure of THPE-TE. As shown in Figure 1c, the chemical shifts are assigned as follows: 6.93–7.03 ppm (a, Ar-H, 6H), 6.76–6.85 ppm (b, Ar-H, 6H), 3.89–3.98 and 4.16–4.22 ppm (c, −CH2–, 6H), 3.34 ppm (d, epoxy ring, 3H), 2.74–2.75 and 2.85–2.91 ppm (e, epoxy ring, 6H), and 2.09 ppm (f, −CH3, 3H). We listed the integral areas of the resonance peaks in Figure 1c, the ratio of integral area among different hydrogens was 1.00:0.99:0.97:0.47:0.98:0.48, which was similar to the theoretical ratio of 6:6:6:3:6:3. Conclusively, the results of FT-IR and 1H NMR obtained indicated that we successfully synthesized THPE-TE.
Structural Characterization of SCA and STCA
Scheme 2 shows the synthesis process of SCA and STCA via a cohydrolytic condensation reaction. The molecular weight and molecular weight distribution of SCA and STCA are shown in Table S1. FT-IR spectroscopy, Raman spectroscopy, and solid-state 29Si NMR spectroscopy were employed to characterize the structures. From Figure 2a, it is evident that the double absorption peaks at 3450 and 3335 cm–1 are assigned to the asymmetric and symmetric stretching of −NH2, respectively, and the infrared peak at 1620 cm–1 is ascribed to its bending vibration. The characteristic absorption peak appearing at 1040 cm–1 illustrates the presence of −Si–O–Si–.41 The new vibration bands near 920 and 500 cm–1 are associated with Si–O–Ti42 and Ti–O–Ti bonds,43 respectively, which are not observed in the spectrum of SCA.
Scheme 2. Reaction Equations of (a) SCA and (b) STCA.
Figure 2.
(a) FT-IR spectra and (b) Raman spectra of SCA and STCA.
The Raman spectra also proved the presence of Si–O–Ti bond. As shown in Figure 2b, the Raman spectra of SCA and STCA are similar in most of the ranges. Nonetheless, the peaks at 491 and 1083 cm–1 are seen in the spectrum of STCA and are not observed in the spectrum of SCA, which are ascribed to the bending and asymmetric vibrations of Si–O–Ti, proving the formation of Si–O–Ti through a dehydration reaction. These structures are related to tetrahedrally coordinated silicon and titanium centers.44
The solid-state 29Si-NMR spectra of new curing agents are displayed in Figure 3. Different grafted organosiloxane units are defined as the structures of M, D, T, and Q, which represent the units of R3SiO0.5, R2Si(O0.5)2, R1Si(O0.5)3, and Si(O0.5)4, respectively, where R represents the aromatic and/or aliphatic substituents or H.45 (3-Aminopropyl)triethoxysilane (APTES) and methyltrimethoxysilane (MTMS) contain only T0 [Tm: RSi(OSi≡)m(OH)3–m] silicon after hydrolysis separately, while after the self-dehydration reaction or catalytic reaction, the final product contains T3 silicon. As shown in Figure 4, the characteristic signal at δ ≈ −68 ppm can be attributed to T3 [H3C–Si(OSi≡)3] silicon,46 suggesting that most of the MTMS was hydrolyzed and dehydrated to form Si–O–Si bonds. The sharp signal at δ ≈ −77 ppm is assigned to T3 (−H2C–Si(OSi≡)3) silicon, suggesting that Si–O–Si bonds are newly formed by the self-dehydration reaction of APTES or the condensation of APTES and MTMS. However, the intensity of the signal for T3 groups decreased in the spectrum of STCA, which is corresponded to the reduction of Si–O–Si due to the introduction of the new element of titanium to the system and replacing the part place of silicon, which was consistent with the results of FT-IR analysis.
Figure 3.

Solid-state 29Si NMR spectra of (a) SCA and (b) STCA.
Figure 4.
(a) HRR, (b) THR, (c) SPR, and (d) TSP curves of EP/STCA-4 and EP/SCA-5.
Flame-Retardant Performances
LOI and UL-94 Tests
As is well known, LOI and UL-94 vertical burning tests are generally employed to evaluate the flame-retardant properties of EP thermosets. Tables 1 and 2 show the formulas, cross-link density (νe), and flame retardancy for STCA-/SCA-cured THPE-TE. It can be seen that with the increased content of curing agents, the values of LOI are gradually improved, and the corresponding UL-94 ratings are also improved from V-2 to V-0. After the curing agent content of SCA and STCA reaches 58.3 and 56.5 wt %, respectively, the LOI value reaches 31.4 and 33.8%, respectively. Apart from that, the UL-94 ratings achieved the highest level of V-0. Then, the data of the three tests tend to be steady with the increased content of the curing agent. Under the same content of EP/SCA and EP/STCA, EP/STCA exhibits an increased LOI and νe value. The results indicate that the curing agents for EP exhibited excellent flame-retardant properties, further revealing that STCA possesses better flame retardancy than SCA for EP. Undoubtedly, there is a synergistic effect of silicon/titanium flame-retardant elements on flame retardancy for EP/STCA compared with EP/SCA.
Table 1. Formulas, Cross-link Density, and Flame Retardancy for SCA-Cured THPE-TE.
| sample mode | THPE-TE (g) | SCA (g) | SCA content (wt %) | νe (×103 mol/m3) | LOI (vol %) | UL-94 (3 mm) rating | dripping |
|---|---|---|---|---|---|---|---|
| EP/SCA-1 | 50 | 50 | 50 | 4.66 | 22.5 | NR | yes |
| EP/SCA-2 | 50 | 55 | 52.4 | 4.98 | 25.3 | V-2 | no |
| EP/SCA-3 | 50 | 60 | 54.5 | 5.27 | 27.2 | V-1 | no |
| EP/SCA-4 | 50 | 65 | 56.5 | 5.62 | 29.6 | V-1 | no |
| EP/SCA-5 | 50 | 70 | 58.3 | 5.91 | 31.4 | V-0 | no |
| EP/SCA-6 | 50 | 75 | 60 | 5.94 | 31.2 | V-0 | no |
Table 2. Formulas, Cross-link Density, and Flame Retardancy for STCA-Cured THPE-TEa.
| sample mode | THPE-TE (g) | STCA (g) | STCA content (wt %) | νe (×103 mol/m3) | LOI (vol %) | UL-94 (3 mm) rating | dripping |
|---|---|---|---|---|---|---|---|
| EP/STCA-1 | 50 | 50 | 50 | 5.03 | 23.6 | V-2 | no |
| EP/STCA-2 | 50 | 55 | 52.4 | 5.47 | 28.4 | V-1 | no |
| EP/STCA-3 | 50 | 60 | 54.5 | 5.89 | 31.2 | V-1 | no |
| EP/STCA-4 | 50 | 65 | 56.5 | 6.19 | 33.8 | V-0 | no |
| EP/STCA-5 | 50 | 70 | 58.3 | 6.23 | 33.4 | V-0 | no |
| EP/STCA-6 | 50 | 75 | 60 | 6.25 | 33.3 | V-0 | no |
CC test.
To investigate the combustion performances of cured EP, the CC test is usually used to obtain the concerned data, which could provide references on the real fire disaster. The characteristic parameters of CC test, including time to ignition (TTI), heat release rate (HRR), peak HRR (pHRR), total heat release (THR), time of peak HRR (tpHRR), smoke production rate (SPR), total smoke production (TSP), CO production (COP), CO2 production (CO2P), total oxygen consumption (TOC), and effective heat combustion (EHC), are obtained and employed to study the combustion behaviors of the ablative materials. By combining the data in Tables 1 and 2 and Figure S1, EP/SCA-5 and EP/CTCA-4 were chosen as a contrast group.
The related data of combustion behaviors of pure EP, EP/SCA-5, and EP/STCA-4 obtained from the CC test with an incident flux of 50 kW/m2 are given in Table 3. TTI is generally used as an indicator to measure the difficulty of flame retardancy on ignitability,47 and the value is defined as the beginning of the HRR curve. EP/STCA-4 has a slightly higher TTI than EP/SCA-5, and the result may be ascribed to the introduction of titanium to the system, which weakens the EP thermoset to ignition. HRR, pHRR, and THR are considered as the important parameters to evaluate the fire size and safety.47 Generally speaking, a good flame retardant tends to exhibit lower values of HRR, pHRR, and THR. Figure 4a,b shows the HRR and THR curves of EP/SCA-5 and EP/STCA-4, where we can see that EP/SCA-5 burns rapidly after ignition and reaches the first peak point promptly with a value of 288 kW/m2 at 60 s, much higher than that of EP/STCA-4 (218 kW/m2) at 65 s. For EP/SCA-5, the second peak appears at 170 s and attributes to the ulterior decomposition of the anteriorly formed loose char. The fast surface combustion and immediate char layer formation, as well as further decomposition, correspond to the lack of fire resistance of the material and further explain that the silicon alone does not exert effective flame retardancy.
Table 3. CC Test Results of EP/SCA-5 and EP/STCA-4.
| sample | pure EP | EP/SCA-5 | EP/STCA-4 |
|---|---|---|---|
| TTI (s) | 28 | 40 | 45 |
| p1HRR (kW/m2) | 362 | 288 | 218 |
| tp1HRR (s) | 45 | 60 | 65 |
| p2HRR (kW/m2) | 165 | 253 | |
| tp2HRR (s) | 170 | 110 | |
| THR (MJ/m2) | 52.65 | 35.84 | 32.81 |
| p1SPR (m2/s) | 0.28 | 0.19 | 0.15 |
| p2SPR (m2/s) | 0.20 | ||
| TSP (m2/kg) | 55.23 | 36.35 | 31.95 |
| av-COP (kg/kg) | 0.13 | 0.10 | 0.07 |
| av-CO2P (kg/kg) | 1.41 | 1.15 | 0.98 |
| mean SEA (m2/kg) | 2934.28 | 2154.31 | 1713.99 |
| TOC (g) | 32.38 | 23.33 | 21.11 |
| av-EHC (MJ/kg) | 25.56 | 19.05 | 16.50 |
Besides that, by comparing the THR data of EP/SCA-5 and EP/STCA-4, the former also showed a slight increase by 13.8%. On the basis of these results, EP/STCA-4 reveals better flame retardancy than EP/SCA-5, and it is attributed to the introduction of titanium to the curing agent, resulting in the formation of a Si–O–Ti bond. After combustion, the formation of a synergistic silicon/titanium-rich char shield stops the residual carbon from further rupturing. In addition, it refrains the heat and oxygen from entering into the interior structure in case of burning in a deep environment. In conclusion, EP/STCA-4 holds better thermal stability and flame retardancy than EP/SCA-5.
The production of smoke and toxic gases is also considered as a vital aspect for the application of flame-retardant materials because smoke and toxic gases are the main threats to human life in case of real fire.48 From SPR–time plots shown in Figure 4c,d, it can be seen that the SPR curve of EP/SCA-5 presents two obvious peak points and the value is far higher than that of EP/STCA-4, which exhibits lower TSR value than EP/SCA-5. The value of TSP also reduced from 36.35 m2/kg for EP/SCA-5 to 31.95 m2/kg for EP/STCA-4. From the data of COP and CO2P given in Table 3, we can conclude that the values of EP/SCA-5 are higher than that of EP/STCA-4, and the result was consistent with the TG-IR analysis. The main reason is that the introduction of titanium into the system stimulates the formation of a silicon/titanium-rich compact char layer, which can be used as a protective layer on the surface.16 Moreover, it can suppress the heat from entering the interior structure, as well as restrict the intensity of combustion and decrease the quantity of smoke release.
The EHC is defined as the ratio of the total heat to per mass loss, which reflects the combustion degree of volatile gases in the gas phase, and is useful for analyzing the flame-retardant mechanism. The lower EHC value indicates the existence of noncombustible gas in gas phase. As presented in Table 3, compared with the average value of EHC (av-EHC) for EP/SCA-5, the av-EHC for EP/STCA-4 decreased from 19.05 to 16.50 MJ/kg. The result may be due to two reasons: on the one hand, the thermoset could produce noncombustible gases during the combustion process, which can act as an efficient dilution effect on the flammable gases and oxygen concentration around the system, avoiding the rapid burning of the material; on the other hand, the flame retardant containing silicon and titanium can form a condensed phase during the combustion and reach the effect of flame inhibition. As a result, the nonflammable gases and the formation of a condensed phase play important roles during the combustion process.
The ablative behavior of the flame-retardant material is generally estimated by calculating the mass loss rate (MLR), which is defined as the mass loss per second, and the related data are summarized in Table 4. Compared to the MLR of 0.0605 g/s for EP/SCA-5, the MLR for EP/STCA-4 decreases to 30.4% (0.0421 g/s). Generally speaking, lower MLR manifests that the material has a lower erosion rate under heat and flame, so the result clearly suggests that EP/STCA-4 has a prominently improved antiflame behavior after the introduction of titanium.
Table 4. MLR Value of the Samples.
| sample | weight before ablation (g) | weight after ablation (g) | combustion time (s) | MLR (g/s) |
|---|---|---|---|---|
| EP/SCA-5 | 34.000 | 12.537 | 40–395 | 0.0605 |
| EP/STCA-4 | 33.600 | 18.036 | 45–415 | 0.0421 |
Thermal Properties
Dynamic Mechanical Analysis
DMA was used to obtain the loss tangent (tan δ) spectrum of cured epoxy resins. Glass-transition temperature (Tg) is a significant application parameter for EP thermosets, which reflects the minimum temperature of the polymer transforming from glass state to high elastic state. It could be calculated by the spectrum of tan δ, corresponding to the temperature of the location of the peak of tan δ. As can be seen in Figure 5, the Tg for EP/SCA-5 and EP/STCA-4 are 165 and 176 °C, respectively. This result is mainly attributed to the higher cross-link density of EP/STCA-4 and the introduction of Si–O–Ti bonds to the structure enhancing the rigidity,49 which leads to the rotational barrier of epoxy resin and other groups, ultimately increasing the Tg of the EP/STCA-4 thermoset. With the higher Tg, it shows that EP/STCA-4 possesses better heat resistance and extended application fields, especially in thermal environment.
Figure 5.

DMA curves of EP/SCA-5 and EP/STCA-4.
Thermogravimetric Analysis
Thermal stability and thermal degradation properties were evaluated by TGA under nitrogen atmosphere. The TGA and derivative thermogravimetry (DTG) curves of pure EP, EP/SCA-5, and EP/STCA-4 are shown in Figure 6. The detailed data are listed in Table S2. It can be seen that the thermal degradation behavior of the two samples has three decomposition steps. EP/SCA-5 begins to decompose at 299.6 °C and reaches the first degradation peak at 310.8 °C with a maximum weight loss rate of 7.77 wt %/min, which is attributed to the rupture of small side groups such as −CH3 and Si–CH3, and degradation as well as carbonization of the backbone including the C–C and C–O bonds. For EP/STCA-4, the temperatures of initial thermal degradation and the peak of first step increased to 348.8 and 358.4 °C, respectively. The maximum weight loss rate decreased by 26.5% to 5.71 wt %/min compared to that of EP/SCA-5 because of lack of Si–CH3; also, the bond dissociation energy (BDE) of C–C (346 kJ/mol) and C–O (345 kJ/mol) is higher compared with that of Si–C (318 kJ/mol).11,50 Next, the second degradation step of EP/SCA-5 appears at 372.9 °C, similarly for EP/STCA-4 at 402.2 °C, owing to the degradation of previously formed char layer of EP thermosets. The maximum weight loss rates of the third decomposition stage for EP/SCA-5 and EP/STCA-4 are about 0.84 wt % at 474.1 °C and 0.77 wt % at 534.2 °C, respectively. This phenomenon can be considered as the break and rearrangement of the partial Si–O–Si and Si–O–Ti bonds on the main chain and the further combustion of the remaining organic groups.51 Notably, the maximum weight loss of EP/SCA-5 is relatively higher than that of EP/STCA-4, as a result of the presence of Si–O–Ti which is of higher BDE compared with the Si–O–Si unit. Therefore, the residual yield of EP/STCA-4 is increased from 51.1% for EP/SCA-5 to 52.7% at 800 °C with a lower original ratio of the curing agent. Silicon-containing polymers are regarded as tending to form thermally stable silica when degraded at high temperature. Meanwhile, the catalytic effect of titanium makes a great contribution to enhance the formation of char layer during thermal degradation.51 Moreover, the formed silicon/titanium-rich sufficient and compact char layer is strongly capable of restraining heat from penetrating into underlying material, avoiding further decomposition. The result indicates that high residual yield plays an effective role in flame retardancy, and the interaction of silicon/titanium has a synergistic effect on the enhancement of thermal stability.
Figure 6.
(a) TGA and (b) DTG curves of EP/SCA-5 and EP/STCA-4.
In order to evaluate the thermal stability of the prepared cured resins, the statistic heat-resistant index (Ts) was put forward. It is calculated by the temperatures of 5% weight loss (T5wt%) and 30% weight loss (T30wt%) of the specimen from TGA. The Ts is defined by eq 1(52,53)
| 1 |
The Ts value of EP/STCA-4 is 191.08, higher than that of EP/SCA-5 (168.97), explaining that the STCA-cured EP has a better heat resistance. The lower heat degradation of EP/STCA-4 is due to the abound presence of Si–O–Ti and Ti–O–Ti bonds, which leads to stronger rigidity and improved heat resistance. Moreover, the comparison of thermal properties and combustion performances between different thermosets is summarized in Figure S2, Tables S3 and S4.
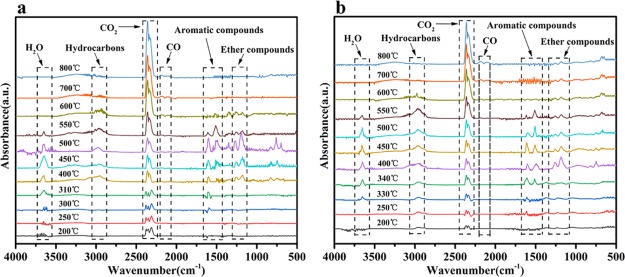
Gas-Phase Analysis
TG-IR is usually conducted to detect the gaseous phase so as to further analyze the decomposition process. In order to further clarify the important effect on flame retardancy of the synergy of silicon and titanium, the TG-IR spectra of volatile products of EP/SCA-5 and EP/STCA-4 at different degradation temperatures are obtained and presented in Figure 7. The intensive characteristic signals of H2O (3644 cm–1), hydrocarbons (2972 cm–1), CO2 (2360 cm–1), aromatic compounds (1610, 1510 cm–1), and ether compounds (1260, 1184 cm–1) are nearly similar between the two samples. Moreover, for EP/SCA-5, the intensive peaks of 3644 and 1600–2000 cm–1 begin to appear at 310 °C, but the obvious signals cannot be seen until the temperature rises to 340 °C for EP/STCA-4, which indicates that the occurrence of the degradation process for EP/STCA-4 is later than that of EP/SCA-5 and accords with the results of TGA discussed above. This phenomenon reveals that the introduction of titanium retards the decomposition of cured EP. Besides that, the absorption intensity of the main gaseous products decreases compared to that of EP/SCA-5.
Figure 7.
TG-IR spectra of (a) EP/SCA-5 and (b) EP/STCA-4 at different temperatures.
To further compare the thermal decomposition of the two cured EP, Figure 8 exhibits the curves of some characteristic absorbance intensity of volatile products versus temperature. After the incorporation of titanium to the thermoset, it can be seen that the characteristic absorbance intensity of carbon dioxide (2360 cm–1), carbon monoxide (2190 cm–1), and aromatic compounds (1510, 1610 cm–1) as well as that of total pyrolysis products decrease significantly to lower values. The result is due to the strong molecular structure and the formed compact and continual residual char after decomposition, which blocks the spread of pyrolysis products. The decrease of aromatic compounds attributed to the smoke suppression and the decrease of CO could reduce the toxicity of smoke. In summary, with the introduction of silicon and titanium to the thermoset, the fire safety has improved a lot compared to the thermoset only containing silicon.
Figure 8.
Absorbance intensity of pyrolysis products for EP/SCA-5 and EP/STCA-4: (a) total pyrolysis products, (b) CO2, (c) CO, and (d) aromatic compound.
Flame-Retardant Mechanism
For the purpose of further elucidating the relationship between the flame-retardant mechanism and the morphology of the char residue, the digital pictures and SEM micrographs of EP/SCA-5 and EP/STCA-4 after CC test were obtained and shown in Figure 9. It is obvious that EP/STCA-4 presents efficient coherent intumescent char residue and gathers into a dense cluster in most areas. Moreover, the char layer of EP/STCA-4 is continuous and compact with almost no cracks. However, EP/SCA-5 apparently exhibited loose structure, including some long cracks and hole-like structures. Some pieces of char layers nearly separate from the main body without sufficient cohesion because EP/SCA-5 almost decomposes entirely; thus, it causes inadequate char formation during combustion. As shown in Figure 9b2,b3, the exterior char layer of EP/STCA-4 appears a relatively complete and homogeneous structure with a rough surface. Therefore, heat, flame, and oxygen can be prevented from entering into the interior structure. Besides, this dense char structure would effectively avert the escape of volatiles, resulting in the retardance of further combustion. Therefore, the results suggest that the introduction of a Si–O–Ti structure has a huge benefit for the enhancement of flame retardancy of EP/STCA-4. It may effectively restrain heat transfer. On the contrary, the char layer of EP/SCA-5 sample consists of multihole structure, which was probably attributed to the release of a large amount of gas.54 Thereafter, the structures extend from the surface to the interior structure; as a result, oxygen and flame have greater access to penetrate into the interior structure, leading to further burning until full combustion.
Figure 9.

Digital images of the char residues after CC test: (a1) EP/SCA-5 and (b1) EP/STCA-4; SEM micrographs of the char residues after CC test: (a2,a3) EP/SCA-5 and (b2,b3) EP/STCA-4.
In order to further investigate the flame-retardant mechanism, FT-IR and EDX were used to characterize the chemical structure of the char residue to observe the synergistic effects of silicon and titanium. The FT-IR spectrum of the char residue of EP/STCA-4 after CC test is displayed in Figure 10. The absorption peak of 1590 cm–1 is ascribed to the C=C stretching vibration of aromatic carbons, and the characteristic peaks at 1060, 920, and 460 cm–1 prove the presence of Si–O–Si, Si–O–Ti, and Ti–O–Ti bonds as ever. The results indicate that these bonds exhibit thermal stability under combustion as well as promote the formation of a solid and compact char layer containing silicon and titanium, enhancing the thermal stability of the char residue. Therefore, it could be capable of exerting the resistance to flame, resulting in good flame retardancy of EP/STCA-4.
Figure 10.

FT-IR spectra of the char residue for EP/STCA-4 after the CC test.
EDS is an effective tool to detect the elemental content of the char residue after CC test, so that we could analyze the differences between the exterior char and the interior char after combustion. As shown in Figure 11, it can be seen that the O, Si, and Ti contents of the exterior char were higher than those of the interior char because of the direct exposure to the air of the exterior char. During the combustion, silicic and titaniferous groups could generate SiO2 and TiO2 to promote char formation.11,51 Furthermore, they probably form a silicon/titanium ceramic structure to enhance the thermal stability and flame retardancy of the char. Therefore, the formation of oxygen, silicon/titanium-rich char effectively prevents the interior composition from further decomposition, reflecting the synergistic effects to improve thermal stability.
Figure 11.
EDS analysis of (a) interior and (b) exterior char residues of EP/STCA-4.
Mechanical Properties
Stress–strain curves of EP/SCA-5 and EP/STCA-4 are shown in Figure 12, and the detailed data of tensile properties and KIC of EP/SCA-5 and EP/STCA-4 are summarized in Table 5. Compared to the −Si–O–Ti– bond, the flexible −Si–O–Si– molecular chains have a significant effect to decrease the cross-link density and rigidity of the cured epoxy resin, which led to the reduction of tensile strength of EP/CSA-5. Besides, the bond angle of −Si–O–Si– is 142°, which is more open than that of the usual tetrahedral with 110°. Thanks to these structural characteristics, the −Si–O–Si– skeleton is endowed with excellent flexible performance and stress dissipating capability, which plays an effective role in the improvement of ductility, resulting in the increase of failure strain.
Figure 12.

Stress–strain curves of EP/SCA-5 and EP/STCA-4.
Table 5. Tensile Properties and KIC of the Samples.
| sample | tensile strength (MPa) | failure strain (%) | KIC (MPa m1/2) |
|---|---|---|---|
| EP/SCA-5 | 89.65 ± 2.76 | 6.12 ± 0.31 | 2.33 ± 0.18 |
| EP/STCA-4 | 94.13 ± 3.54 | 5.64 ± 0.27 | 2.13 ± 0.22 |
As shown in Table 5, after the incorporation of titanium to the epoxy thermoset, the KIC of the EP/STCA-4 reaches 2.13 MPa m1/2, only decreased by 8.6% compared with that of EP/SCA-5. Therefore, the fracture toughness of silicon/titanium-containing epoxy resin was not deeply affected by the introduction of titanium, and EP/STCA-4 exhibits high strength and relatively good toughness. The comparison of mechanical properties between different thermosets is displayed in Figure S3 and Table S5.
Conclusions
In order to simplify the procedure to prepare a flame-retardant thermoset, the curing agents of SCA and STCA containing flame-retardant elements were successfully synthesized and characterized. EP/STCA exhibits improved flame retardancy compared to EP/SCA, especially for EP/STCA-4. DMA test showed that the introduction of titanium to the curing agent significantly improves the Tg value of the composite. The temperature of maximum decomposition rate for EP/STCA-4 was shifted to a higher value, and the char yield was also increased at 800 °C by contrast with EP/SCA, indicating that the incorporation of titanium effectively enhanced the thermal stability. With the addition of STCA reaching 56.5%, V-0 rating was achieved and its LOI value attained 33.8%, higher than that of EP/SCA. The CC test showed that the av-HRR, pHRR, THR, pSPR, and TSP values of EP/STCA-4 were decreased. The yield of the gas products detected by the TG-IR technique reduced after the introduction of titanium. Moreover, the SEM results indicated that EP/STCA-4 had a more uniform and denser char layer than EP/SCA-5 because of the presence of abundant aromatic structure, Si–O–Ti, Ti–O–Ti bonds. The continual compact char layer was helpful to form a physical barrier to isolate oxygen, heat, and volatile gas from spreading to the interior structure. Because of the introduction of titanium to the curing agent, the synergistic effects of silicon and titanium precipitated the formation of sufficient and compact char layer during combustion, which led to better flame retardancy and thermal stability. Besides, EP/STCA-4 exhibits high strength and relatively good toughness.
Materials and Methods
Materials
THPE (99%) was purchased from Beijing HWRK Chemical Co., Ltd. (Beijing, China). ECH (AR) and tetrabutyl ammonium bromide [TBAB, AR, ≥99.0%] were provided by Shanghai Dibai Biotechnology Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH, AR, ≥96%), APTES (AR), MTMS (AR), acetylacetone (Acac, AR), anhydrous ethanol (C2H5OH, ≥99.7%), hydrochloric acid (36.0–38.0%), and deionized water were obtained from Shanghai Titan Scientific Co., Ltd. (Shanghai, China). TBT (≥98.0%) was supplied by Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China).
Synthesis of THPE-TE
The synthesis procedure for THPE-TE is shown in Scheme 1. THPE (0.1 mol), ECH (3.6 mol), and TBAB (0.5 mol % of THPE) were mixed proportionally in a 1000 mL four-necked flask under nitrogen atmosphere, equipped with a heating device, stirrer, thermometer. The resulting mixture was stirred, and the temperature was maintained at 105 °C for 2 h. After the completion of the first step of ring-opening addition reaction, the reactive system was used to cool the solution to 70 °C. Then, NaOH (0.35 mol) was evenly added to the system to catalyze the ring-closing reaction, and the second step reaction was continued for 2 h. After that, the solution was eluted with distilled water at 70 °C several times until the system turned to a neutral solution. At last, excess ECH was removed under reduced pressure and THPE-TE was obtained.
Synthesis of SCA
SCA was prepared by a cohydrolytic condensation reaction. APTES (0.5 mol, 110.5 g), MTMS (0.5 mol, 68 g), and 100 mL of anhydrous ethanol were added into a 500 mL four-necked flask with a stirrer, efflux condenser, and oil bath heater under nitrogen purge. The reaction system was heated to 60 °C, and then, 36 g of H2O and dilute hydrochloric acid was added dropwise to catalyze the hydrolysis and condensation reaction until the pH value of the reaction system reached 3–4. The mixture was maintained at 60 °C for 12 h and distilled under reduced pressure at 60 °C to remove the byproducts of water, methanol, and ethanol. The synthetic route of SCA is given in Scheme 2a.
Synthesis of STCA
The synthetic process is shown in Scheme 2b. It was synthesized by sol–gel process and a cohydrolytic condensation reaction. APTES (58.5 g), TBT (34 g), Acac (3 g), and anhydrous ethanol (150 mL) as the solvent were added into a 500 mL four-necked flask equipped with a mechanical stirrer, reflux condenser, thermocouple, and a nitrogen inlet. The mixture was heated to 50 °C and maintained at that temperature for 1 h to accomplish the chelation of Acac to decrease the reactivity and stabilize the sol. Then, 36 g of deionized water was added to the mixture at a constant speed within 2 h to hydrolyze APTES and TBT. After rising at a temperature of 120 °C, 8 mL of hydrochloric acid was divided equally into four batches and added to the reaction system batchwise every half an hour. Then, the pale yellow liquid was distilled under reduced pressure at 70 °C to remove water, ethanol, and butyl alcohol. Finally, the new solid curing agent was ultimately obtained.
Preparation of Cured Epoxy Resin Specimens
The cured specimens were obtained by using a thermal curing process. The ratio of the curing agent (SCA or STCA) was calculated, and THPE-TE was stirred for 10 min at 1000 rpm in a dispersion machine and well mixed to form a homogeneous mixture. The mixture was added into the prepared molds and cured in a nitrogen convection oven. The samples were cured under the following conditions: 120 °C/2 h, 150 °C/3 h, and 180 °C/3 h. After that, the samples were cooled to room temperature slowly to avoid cracking and then used for testing. The detailed formula of cured resins is summarized in Tables 1 and 2.
Characterization
FT-IR spectra were obtained using a Nicolet 6700 infrared spectrometer at room temperature to characterize the chemical structure of THPE-TE and curing agents. The samples were thoroughly mixed with KBr and then pressed into small flakes.
The1H NMR spectrum of THPE-TE was obtained using a Bruker AVANCE III-400 MHz NMR spectrometer with deuterochloroform (CDCl3) as a solvent and tetramethylsilane as an internal standard. The 29Si NMR spectra of new curing agents were recorded on a Bruker AVANCE III-500 MHz NMR spectrometer.
Raman spectra were obtained in a Renishaw inVia Raman spectrometer equipped with a 633 nm wavelength excitation laser, recorded in the range of 3500–100 cm–1.
The LOI value measurement was performed using a JF-3 oxygen index meter (Jiangning Analysis Instrument Company, China) with bar dimensions 130 × 6.5 × 3.0 mm3 according to the ISO 4589-2:2006 standard. UL-94 tests were conducted on the NK8017A instrument (Nklsky Instrument Company, Ltd., China) with dimensions 130 × 13 × 3 mm3 according to the ASTM D3801-19 standard. The results of burning grade were denoted as NR (No Rating), V-2, V-1, V-0, and V-0, which were defined as the best flame retardancy of the refractory material. CC tests were performed on an FTT CC (West Sussex, UK) at an incident heat flux of 50 kW/m2 in accordance with the ISO 5660 standard, and the dimension of the samples was 100 × 100 × 3 mm3. Before testing, the samples were put on aluminum foil and wrapped to make only the top surfaces directly exposed to the heat source. Every sample is tested at least three times.
The cross-linking density of the corresponding cured EP was analyzed with a IIC XLDS-15 analyzer at room temperature.
DMA was performed on a TA DMA Q800 thermal analysis instrument in air atmosphere, and the sample with dimensions 30 × 10 × 4 mm3 were tested in a three-point bending configuration at a heating rate of 3 °C/min and a frequency of 1 Hz from 25 to 250 °C. The glass-transition temperature was obtained on the basis of the curve of loss tangent with the increase of temperature.
TGA was performed on a TA Instrument Q500 at a heating rate of 10 °C/min and a gas flow of 20 mL/min from ambient temperature to 800 °C under air and nitrogen atmosphere, respectively.
TG-IR spectra were recorded with a TGA Q5000 thermogravimetric analyzer, which is interfaced by a Nicolet 6700 FT-IR spectrometer. Approximately 10.0 mg of sample was tested from 25 to 800 °C at a heating rate of 10 °C/min under nitrogen atmosphere with at a flow rate of 20 mL/min.
Morphological analysis on the residual chars after CC test was conducted on a field-emission scanning electron microscope (Hitachi S-4800) at an acceleration voltage of 15 kV. The field-emission scanning electron microscope instrument was equipped with an EDS microanalyzer to perform elemental analysis.
Tensile properties were studied using a GOTECH AI-700 M testing machine (GOTECH Testing Machines Inc., China) according to ASTM D638-99 at a crosshead speed of 1 mm/min. The fracture toughness (KIC) was tested using a single-edge notched bend test according ASTM D5045-14 at a crosshead speed of 1 mm/min. KIC was calculated as follows
| 2 |
| 3 |
| 4 |
where P is the applied load; S is the span of the sample; and D, W, and a are the thickness, width, and crack length of the sample, respectively. At least 10 replicate specimens were tested.
Acknowledgments
The authors would like to acknowledge the research platform of East China University of Science and Technology and the financial support from Hubei Ruoshui New Materials Technology Co., Ltd. (Hubei, China).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04050.
THR curves of EP/SCA and EP/STCA with loading of different curing agents; TGA curves of STCA-cured different EPs; stress and strain curves of STCA-cured different EPs; molecular weight and molecular weight distribution of SCA and STCA; thermal decomposition data of pure EP, EP/SCA-5, and EP/STCA-4; results of flammability and thermal properties of the EP composites; TGA data of STCA-cured different EPs; and tensile properties and KIC of STCA-cured different EPs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xu M.-J.; Xu G.-R.; Leng Y.; Li B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. 10.1016/j.polymdegradstab.2015.11.018. [DOI] [Google Scholar]
- Luo Q.; Yuan Y.; Dong C.; Huang H.; Liu S.; Zhao J. Highly Effective Flame Retardancy of a Novel DPPA-Based Curing Agent for DGEBA Epoxy Resin. Ind. Eng. Chem. Res. 2016, 55, 10880–10888. 10.1021/acs.iecr.6b02083. [DOI] [Google Scholar]
- Aghabararpour M.; Naderi M.; Motahari S.; Najafi M. A study on resorcinol formaldehyde carbon aerogel/epoxy nanocomposites: the effect of carbon aerogel pyrolysis time. J. Polym. Res. 2019, 26, 59. 10.1007/s10965-019-1721-9. [DOI] [Google Scholar]
- Raimondo M.; Guadagno L.; Speranza V.; Bonnaud L.; Dubois P.; Lafdi K. Multifunctional graphene/POSS epoxy resin tailored for aircraft lightning strike protection. Composites, Part B 2018, 140, 44–56. 10.1016/j.compositesb.2017.12.015. [DOI] [Google Scholar]
- Wan J.; Li C.; Bu Z.-Y.; Xu C.-J.; Li B.-G.; Fan H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. 10.1016/j.cej.2012.01.134. [DOI] [Google Scholar]
- Moosburger-Will J.; Lachner E.; Löffler M.; Kunzmann C.; Greisel M.; Ruhland K.; Horn S. Adhesion of carbon fibers to amine hardened epoxy resin: Influence of ammonia plasma functionalization of carbon fibers. Appl. Surf. Sci. 2018, 453, 141–152. 10.1016/j.apsusc.2018.05.057. [DOI] [Google Scholar]
- Deng P.; Shi Y.; Liu Y.; Liu Y.; Wang Q. Solidifying process and flame retardancy of epoxy resin cured with boron-containing phenolic resin. Appl. Surf. Sci. 2018, 427, 894–904. 10.1016/j.apsusc.2017.07.278. [DOI] [Google Scholar]
- Lu S.-Y.; Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. 10.1016/s0079-6700(02)00018-7. [DOI] [Google Scholar]
- Deng P.; Liu Y.; Liu Y.; Xu C.; Wang Q. Preparation of phosphorus-containing phenolic resin and its application in epoxy resin as a curing agent and flame retardant. Polym. Adv. Technol. 2018, 29, 1294–1302. 10.1002/pat.4241. [DOI] [Google Scholar]
- Chen L.; Wang Y.-Z. A review on flame retardant technology in China. Part I: development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. 10.1002/pat.1550. [DOI] [Google Scholar]
- Li J.; Wang H.; Li S. A novel phosphorus–silicon containing epoxy resin with enhanced thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stab. 2019, 164, 36–45. 10.1016/j.polymdegradstab.2019.03.020. [DOI] [Google Scholar]
- Zhao X.; Babu H. V.; Llorca J.; Wang D.-Y. Impact of halogen-free flame retardant with varied phosphorus chemical surrounding on the properties of diglycidyl ether of bisphenol-A type epoxy resin: synthesis, fire behaviour, flame-retardant mechanism and mechanical properties. RSC Adv. 2016, 6, 59226–59236. 10.1039/c6ra13168a. [DOI] [Google Scholar]
- Liu Y.; Wang Q. The investigation on the flame retardancy mechanism of nitrogen flame retardant melamine cyanurate in polyamide 6. J. Polym. Res. 2009, 16, 583–589. 10.1007/s10965-008-9263-6. [DOI] [Google Scholar]
- Zhong H.; Wei P.; Jiang P.; Wu D.; Wang G. Synthesis and characteristics of a novel silicon-containing flame retardant and its application in poly[2,2-propane-(bisphenol)carbonate]/acrylonitrile butadiene styrene. J. Polym. Sci., Part B: Polym. Phys. 2007, 45, 1542–1551. 10.1002/polb.21151. [DOI] [Google Scholar]
- Martín C.; Ronda J. C.; Cásdiz V. Development of novel flame-retardant thermosets based on boron-modified phenol–formaldehyde resins. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 3503–3512. 10.1002/pola.21458. [DOI] [Google Scholar]
- Zeng B.; Liu Y.; Yang L.; Zheng W.; Chen T.; Chen G.; Xu Y.; Yuan C.; Dai L. Flame retardant epoxy resin based on organic titanate and polyhedral oligomeric silsesquioxane-containing additives with synergistic effects. RSC Adv. 2017, 7, 26082–26088. 10.1039/c7ra02529g. [DOI] [Google Scholar]
- Lee T.-M.; Ma C.-C. M.; Hsu C.-W.; Wu H.-L. Syntheses of epoxy-bridged polyorganosiloxanes and the effects of terminated alkoxysilanes on cured thermal properties. J. Appl. Polym. Sci. 2006, 99, 3491–3499. 10.1002/app.22973. [DOI] [Google Scholar]
- Huang Y.; Huang J.; Cao L.; Li C.; Hao W.; Zhu K. Influence of solvothermal treatment time on oxidation of carbon/carbon composites containing ZrB2 micro-particles. Ceram. Int. 2014, 40, 13529–13535. 10.1016/j.ceramint.2014.05.041. [DOI] [Google Scholar]
- Yang S.; Zhang Q.; Hu Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. 10.1016/j.polymdegradstab.2016.09.023. [DOI] [Google Scholar]
- Ramis X.; Cadenato A.; Morancho J. M.; Salla J. M. Curing of a thermosetting powder coating by means of DMTA, TMA and DSC. Polymer 2003, 44, 2067–2079. 10.1016/s0032-3861(03)00059-4. [DOI] [Google Scholar]
- Lin C. H.; Lin T. L.; Chang S. L.; Dai S. A.; Cheng R. J.; Hwang K. Y.; Tu A. P.; Su W. C. Facile preparation of novel epoxy curing agents and their high-performance thermosets. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 7898–7912. 10.1002/pola.23069. [DOI] [Google Scholar]
- Li S.; Li H.; Li Z.; Zhou H.; Guo Y.; Chen F.; Zhao T. Polysiloxane modified phenolic resin with co-continuous structure. Polymer 2017, 120, 217–222. 10.1016/j.polymer.2017.05.063. [DOI] [Google Scholar]
- Chruściel J. J.; Leśniak E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. 10.1016/j.progpolymsci.2014.08.001. [DOI] [Google Scholar]
- Kundu C. K.; Wang X.; Liu L.; Song L.; Hu Y. Few layer deposition and sol-gel finishing of organic-inorganic compounds for improved flame retardant and hydrophilic properties of polyamide 66 textiles: A hybrid approach. Prog. Org. Coat. 2019, 129, 318–326. 10.1016/j.porgcoat.2019.01.010. [DOI] [Google Scholar]
- Liu Y.; Wang Q.-Q.; Jiang Z.-M.; Zhang C.-J.; Li Z.-F.; Chen H.-Q.; Zhu P. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J. Anal. Appl. Pyrolysis 2018, 135, 289–298. 10.1016/j.jaap.2018.08.024. [DOI] [Google Scholar]
- Kundu C. K.; Wang X.; Hou Y.; Hu Y. Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol-gel process of organo-silane. Carbohydr. Polym. 2018, 181, 833–840. 10.1016/j.carbpol.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Zhi M.; Liu Q.; Chen H.; Chen X.; Feng S.; He Y. Thermal Stability and Flame Retardancy Properties of Epoxy Resin Modified with Functionalized Graphene Oxide Containing Phosphorus and Silicon Elements. ACS Omega 2019, 4, 10975–10984. 10.1021/acsomega.9b00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.-R.; Xu M.-J.; Li B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym. Degrad. Stab. 2014, 109, 240–248. 10.1016/j.polymdegradstab.2014.07.020. [DOI] [Google Scholar]
- Vothi H.; Nguyen C.; Pham L. H.; Hoang D.; Kim J. Novel Nitrogen-Phosphorus Flame Retardant Based on Phosphonamidate: Thermal Stability and Flame Retardancy. ACS Omega 2019, 4, 17791–17797. 10.1021/acsomega.9b02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Chen F.; Zhang B.; Luo Z.; Li H.; Zhao T. Structure and improved thermal stability of phenolic resin containing silicon and boron elements. Polym. Degrad. Stab. 2016, 133, 321–329. 10.1016/j.polymdegradstab.2016.07.020. [DOI] [Google Scholar]
- Yang H.; Wang X.; Yu B.; Song L.; Hu Y.; Yuen R. K. K. Effect of borates on thermal degradation and flame retardancy of epoxy resins using polyhedral oligomeric silsesquioxane as a curing agent. Thermochim. Acta 2012, 535, 71–78. 10.1016/j.tca.2012.02.021. [DOI] [Google Scholar]
- Liao F.; Zhou L.; Ju Y.; Yang Y.; Wang X. Synthesis of A Novel Phosphorus–Nitrogen-Silicon Polymeric Flame Retardant and Its Application in Poly(lactic acid). Ind. Eng. Chem. Res. 2014, 53, 10015–10023. 10.1021/ie5008745. [DOI] [Google Scholar]
- Wang Q.; Xiong L.; Liang H.; Chen L.; Huang S. Synthesis of a novel polysiloxane containing phosphorus, and boron and its effect on flame retardancy, mechanical, and thermal properties of epoxy resin. Polym. Compos. 2018, 39, 807–814. 10.1002/pc.24002. [DOI] [Google Scholar]
- Ma W.; Xu B.; Shao L.; Liu Y.; Chen Y.; Qian L. Synthesis of (1,4-Methylenephenylphosphinic acid) Piperazine and Its Application as a Flame Retardant in Epoxy Thermosets. Macromol. Mater. Eng. 2019, 304, 1900419. 10.1002/mame.201900419. [DOI] [Google Scholar]
- Liu J.; Xu T.; Gong M.; Fu Y. Fundamental studies of a new hybrid (inorganic–organic) positively charged membrane. II. Membrane preparation via alcoholysis reaction and amination processes of silicone and titanate coupling agents. J. Membr. Sci. 2005, 264, 87–96. 10.1016/j.memsci.2005.03.058. [DOI] [Google Scholar]
- Tan Y.; Shao Z.-B.; Chen X.-F.; Long J.-W.; Chen L.; Wang Y.-Z. Novel Multifunctional Organic-Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. 10.1021/acsami.5b04570. [DOI] [PubMed] [Google Scholar]
- Fina A.; Abbenhuis H. C. L.; Frache A.; Camino G. Polypropylene metal functionalised POSS nanocompositions: A study by thermogravimetric analysis. Polym. Degrad. Stab. 2006, 91, 1064–1070. 10.1016/j.polymdegradstab.2005.07.013. [DOI] [Google Scholar]
- Kickelbick G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. 10.1016/s0079-6700(02)00019-9. [DOI] [Google Scholar]
- Zhang Y.; Shen S.; Liu Y. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure. Polym. Degrad. Stab. 2013, 98, 514–518. 10.1016/j.polymdegradstab.2012.12.006. [DOI] [Google Scholar]
- Paluvai N. R.; Mohanty S.; Nayak S. K. Studies on thermal degradation and flame retardant behavior of the sisal fiber reinforced unsaturated polyester toughened epoxy nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42068. 10.1002/app.42068. [DOI] [Google Scholar]
- Hernandezpadron G.; Rojas F.; Garciagarduno M.; Canseco M.; Castano V. Development of hybrid materials consisting of SiO2 microparticles embedded in phenolic-formaldehydic resin polymer matrices. Mater. Sci. Eng., A 2003, 355, 338–347. 10.1016/s0921-5093(03)00101-1. [DOI] [Google Scholar]
- Carniato F.; Boccaleri E.; Marchese L.; Fina A.; Tabuani D.; Camino G. Synthesis and Characterisation of Metal Isobutylsilsesquioxanes and Their Role as Inorganic–Organic Nanoadditives for Enhancing Polymer Thermal Stability. Eur. J. Inorg. Chem. 2007, 585–591. 10.1002/ejic.200600683. [DOI] [Google Scholar]
- Ramalingam V.; Sundaramahalingam S.; Rajaram R. Size-dependent antimycobacterial activity of titanium oxide nanoparticles against Mycobacterium tuberculosis. J. Mater. Chem. B 2019, 7, 4338–4346. 10.1039/c9tb00784a. [DOI] [Google Scholar]
- Yahyaei H.; Mohseni M.; Ghanbari H.; Messori M. Synthesis and characterization of polyhedral oligomeric titanized silsesquioxane: A new biocompatible cage like molecule for biomedical application. Mater. Sci. Eng., C 2016, 61, 293–300. 10.1016/j.msec.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Engelhardt G J. H. Structure investigation of organosilicon polymers by silicon-29 NMR. Polym. Bull. 1981, 5, 577–584. 10.1007/bf00255295. [DOI] [Google Scholar]
- Protsak I. S.; Morozov Y. M.; Dong W.; Le Z.; Zhang D.; Henderson I. M. A (29)Si, (1)H, and (13)C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. 10.1186/s11671-019-2982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.; Wilkie C. A. Synergistic effect of carbon nanotubes and decabromodiphenyl oxide/Sb2O3 in improving the flame retardancy of polystyrene. Polym. Degrad. Stab. 2010, 95, 564–571. 10.1016/j.polymdegradstab.2009.12.011. [DOI] [Google Scholar]
- Chen X.; Jiang Y.; Jiao C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. 10.1016/j.jhazmat.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Maroneze C. M.; Arenas L. T.; Luz R. C. S.; Benvenutti E. V.; Landers R.; Gushikem Y. Meldola blue immobilized on a new SiO2/TiO2/graphite composite for electrocatalytic oxidation of NADH. Electrochim. Acta 2008, 53, 4167–4175. 10.1016/j.electacta.2007.12.072. [DOI] [Google Scholar]
- Florea N. M.; Lungu A.; Badica P.; Craciun L.; Enculescu M.; Ghita D. G.; Ionescu C.; Zgirian R. G.; Iovu H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Composites, Part B 2015, 75, 226–234. 10.1016/j.compositesb.2015.01.043. [DOI] [Google Scholar]
- Zhan X.; Xing Q.; Liu H.; Zhang J.; Cheng J.; Lin X. A facile method for fabrication of titanium-doped hybrid materials with high refractive index. RSC Adv. 2014, 4, 13909–13918. 10.1039/c3ra46359a. [DOI] [Google Scholar]
- Aouf C.; Nouailhas H.; Fache M.; Caillol S.; Boutevin B.; Fulcrand H. Multi-functionalization of gallic acid. Synthesis of a novel bio-based epoxy resin. Eur. Polym. J. 2013, 49, 1185–1195. 10.1016/j.eurpolymj.2012.11.025. [DOI] [Google Scholar]
- Lehrle R. S. Thermal Degradation of Bacterial Poly(hydroxybutyric acid): Mechanisms from the Dependence of Pyrolysis Yields on Sample Thickness. Macromolecules 1994, 27, 3782–3789. 10.1021/ma00092a017. [DOI] [Google Scholar]
- Sturm H.; Schartel B.; Weiß A.; Braun U. SEM/EDX: Advanced investigation of structured fire residues and residue formation. Polym. Test. 2012, 31, 606–619. 10.1016/j.polymertesting.2012.03.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.