Abstract
Environmental exposure to lead (Pb) early in life results in a latent upregulation of genes and products associated with Alzheimer’s disease (AD), particularly the plaque forming protein amyloid beta (Aβ). Furthermore, animals exposed to Pb as infants develop cognitive decline and memory impairments in old age. Studies from our lab demonstrated that tolfenamic acid lowers the levels of the amyloid β precursor protein (APP) and its aggregative cleavage product Aβ by inducing the degradation of the transcription factor specificity protein 1 (Sp1). These changes were accompanied by cognitive improvement in transgenic APP knock-in mice. In this study, we examined the effects of tolfenamic acid on beta site APP cleaving enzyme 1 (BACE1) which is responsible for Aβ production and tested its ability to reverse Pb-induced upregulation in the amyloidogenic pathway. Mice were administered tolfenamic acid for one month and BACE1 gene expression as well as its enzymatic activity were analyzed in the cerebral cortex. Tolfenamic acid was also tested for its ability to reverse changes in Sp1, APP and Aβ that were upregulated by Pb in vitro. Differentiated SH-SY5Y neuroblastoma cells were either left unexposed, or sequentially exposed to Pb followed by tolfenamic acid. Our results show that tolfenamic acid reduced BACE1 gene expression and enzyme activity in mice. In neuroblastoma cells, Pb upregulated Sp1, APP and Aβ, while tolfenamic acid lowered their expression. These results along with previous data from our lab provide evidence that tolfenamic acid, a drug that has been used for decades for migraine, represents a candidate which can reduce the pathology of AD and may mitigate the damage of environmental risk factors associated with this disease.
Keywords: Alzheimer’s disease, Amyloid Beta, BACE1, Pb, Sp1, Tolfenamic acid
1. Introduction
A century has passed since the disease was first described by Alois Alzheimer and about 35 million patients around the world suffer today from Alzheimer’s disease (AD) without any potential cure (Anstey et al., 2013; Selkoe, 2012). The majority of AD cases are sporadic and the exact causes of the disease are unknown. As no means for prevention are available, the number of AD cases and the enormous economic costs of this devastating disease will continue to grow at an alarming rate. Knowledge on the pathophysiology of the disease continues to be gathered and reveal more possible drug targets and disease biomarkers. Two types of deposits are found in the AD brain, the amyloid plaques and the tau neurofibrillary tangles (Terry et al., 1964; Tomlinson, 1982). A lot of attention has been directed to the plaques and their main constituent amyloid beta (Aβ) as well as intermediates in Aβ production or degradation, especially after the development of the amyloid cascade hypothesis which views Aβ as a major trigger in AD pathology (Hardy and Selkoe, 2002; Hardy and Higgins, 1992). However, so far no disease-modifying drug for AD is available.
Aβ is generated following the sequential enzymatic processing of the amyloid β precursor protein (APP) by β-secretase and γ-secretase (Shoji et al., 1992). The produced Aβ is normally secreted, but also can accumulate and form insoluble aggregates (Shoji et al., 1992; Urbanc et al., 1999). The levels and activity of β-secretase are elevated in AD brains compared to control (Holsinger et al., 2002; Li et al., 2004). β-APP cleaving enzyme 1 (BACE1) is the main form of β-secretase that cleaves APP to generate Aβ (Cai et al., 2001). In an alternative pathway for processing APP, it can be cleaved by the enzyme α-secretase within the Aβ fragment resulting in non-amyloidogenic products (Selkoe, 1994). Aβ is found as 36-43 amino-acid-long peptides of which Aβ40 is the most abundant and Aβ42 is the most aggregative and is proposed to trigger plaque formation in AD (Iwatsubo et al., 1994; Nakano et al., 1999; Naslund et al., 2000).
Specificity protein 1 (Sp1) is a transcription factor that has been associated with the pathology of AD (Basha et al., 2005; Santpere et al., 2006; Zawia and Basha, 2005). Sp1 acts as a co-activator of APP transcription and regulates the expression of BACE1 (Christensen et al., 2004; Docagne et al., 2004). Sp1 regulates gene transcription by binding to GC rich promoter regions in genes like APP and BACE1 whose binding to Sp1 increases their transcription (Christensen et al., 2004; Docagne et al., 2004; Hoffman and Chernak, 1995; Pollwein et al., 1992). Overexpression of Sp1 increases BACE1 promoter activity, while the decline in Sp1 reduces BACE1 gene transcription (Christensen et al., 2004). Immunohistochemical studies from our laboratory demonstrated that Sp1 protein (SP1), APP, and Aβ co-localize in brain neurons, and that cortical and hippocampal areas with higher SP1 levels express more Aβ (Brock et al., 2008). Therefore, changes in Sp1 expression can influence APP and BACE1 transcription and consequently alter the levels of their downstream product Aβ. Sp1 represents a potential AD target, where its abnormal and elevated expression has been associated with the disease decline (Brock et al., 2008; Christensen et al., 2004; Citron et al., 2008; Hoffman and Chernak, 1995; Santpere et al., 2006; Zawia and Basha, 2005).
Exposure to the environmental toxicant lead (Pb) is considered a risk factor with detrimental effects on various organs especially the brain (White et al., 2007; Zawia and Basha, 2005; Zawia et al., 2009). Experiments conducted at our lab demonstrated that Pb exposure early in life results in AD like pathology in vitro and in vivo, in rodents and primates (Basha et al., 2005; Wu et al., 2008). Pb administration caused the upregulation of Sp1, APP, Aβ, BACE1 and other intermediates implicated in AD later in life (Basha et al., 2005; Bihaqi et al., 2013; Bihaqi et al., 2011; Bihaqi and Zawia, 2012; Huang et al., 2011; Wu et al., 2008; Zawia et al., 2009). Our most recent studies revealed that these molecular changes were accompanied by cognitive deterioration in mice administered Pb compared to controls (Bihaqi et al., 2013). On the other hand, studies in our lab also showed that promoting SP1 degradation by oral administration of the anti-migraine drug tolfenamic acid to mice reduces APP and Aβ levels as well as improves cognition (Adwan et al., 2011; Subaiea et al., 2013).
Since the transcription factor Sp1 is vital for the regulation of several genes involved in AD including BACE1, this research study was conducted to assess the effect of tolfenamic acid administration to APP yeast artificial chromosome (YAC) transgenic mice on BACE1, as a major enzyme in the production of Aβ, that is under Sp1 regulation. We also utilized an in vitro model of Pb exposure established in our lab to test the ability of tolfenamic acid to rescue proteins upregulated following Pb exposure, which induces molecular consequences that resemble pathological events observed in late onset AD (Bihaqi and Zawia, 2012; Huang et al., 2011). Following cell viability studies, differentiated SH-SY5Y cells were exposed to Pb, tolfenamic acid or both agents in chronological order and the changes on SP1, APP and Aβ were examined in comparison to control. An illustration of the mechanism of action of tolfenamic acid induced APP and BACE1 reduction can be found in Fig. 1.
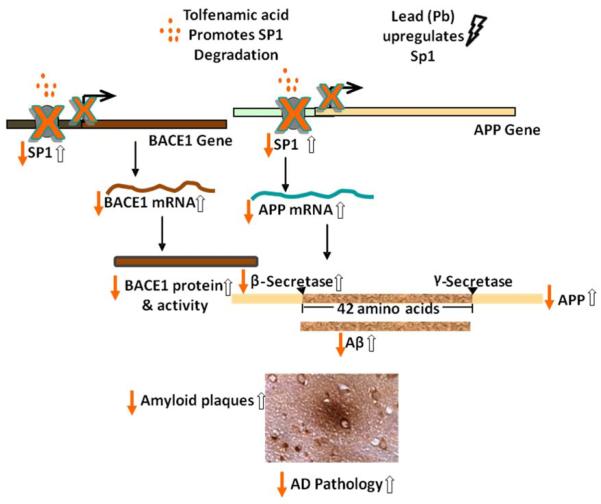
Figure 1. Downregulation of BACE1 and APP by tolfenamic acid and their upregulation by early Pb exposure.
Tolfenamic acid stimulates the degradation of the transcription factor Sp1, which reduces the transcription of APP and BACE1, consequently reducing the expression of BACE1 and APP as well as the aggregative product Aβ and the associated AD pathology. On the other hand, studies from our lab have revealed that Pb exposure early in life upregulates the expression of Sp1, APP, BACE1, Aβ and induces AD like pathology later in life. The solid arrows represent the hypothetical consequences following tolfenamic acid exposure, whereas the hollow arrows represent the latent effects after Pb exposure.
2. Materials and Methods
2.1. Animals
Female hemizygous APP YAC transgenic mice (line R1.40) were used in this study. The B6.129-Tg(APPSw)40Btla/Mmjax strain was obtained from the Jackson Laboratory, Bar Harbor, ME. Animals were bred in-house and the age of the mice used in this study was between 14-20 months. This AD animal model contains the entire human APP gene including the regulatory fragments and expresses elevated levels of Aβ especially the longer more aggregative forms Aβ42 and Aβ43 (Lamb et al., 1999; Lamb et al., 1997; Lehman et al., 2003). Animals were housed in designated rooms within the animal facility at the University of Rhode Island under standard conditions with ad libitum food and water. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available. Mice were assigned into 3 groups of similar age variations, n=6 in each group. Animals were administered 0, 5 or 50 mg/kg tolfenamic acid (Sigma-Aldrich, St. Louis, MO) in corn oil every day by oral gavage for 34 days. On day 35, mice were sacrificed and brain tissues were collected and stored at −80°C until further use. All experiments were performed in accordance with the standard guidelines and the protocol approved by the Institutional Animal Care and Use Committee of the University of Rhode Island.
2.2 Cell culture and exposure to Pb and tolfenamic acid
Human neuroblastoma SH-SY5Y cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM)/F12 (Life technologies, Grand Island, NY) with 10% fetal bovine serum (FBS) and 100 U/mL penicillin, 100 g/mL streptomycin and 2 mM L-glutamine at 5% CO2 and 37°C. Cells were subcultured at 10 5 cells/mL in flasks containing 10 mL each and were allowed to attach overnight then they were differentiated in 10 M alltrans retinoic acid (Sigma-Aldrich, St. Louis, MO) in DMEM/F12 containing 1% FBS and 100 U/mL penicillin, 100 g/mL streptomycin and 2 mM L-glutamine for 1 week following previously published methods (Huang et al., 2011; Jamsa et al., 2004). Neurite outgrowth was examined at 48, 72 h and 6 days (Jamsa et al., 2004) and the medium was changed every 48 h. Following differentiation, cells were exposed to Pb, tolfenamic acid or both sequentially. Stock solutions of 10 mM Pb acetate in sterile distilled water and 100 mM tolfenamic acid in DMSO were prepared for treatments. The stock solutions were diluted in DMEM/F12 media containing 1% FBS, 100 U/mL penicillin, 100 g/mL streptomycin and 2 mM L-glutamine for the different exposures. The concentration of DMSO in the cell culture media was maintained at 0.05% for control or treatments. Differentiated SH-SY5Y cells were treated with 0 or 25 M tolfenamic acid for 96 h with the media changed every 48 h. Cells were also exposed to 50 M Pb for 48 h after which the media was removed and replaced with media containing 0, 25 or 50 M tolfenamic acid for another 48 h. Cells exposed to media containing 0.05% DMSO, 1% FBS, 100 U/mL penicillin, 100 g/mL streptomycin and 2 mM L-glutamine with no Pb or tolfenamic acid were used as controls.
2.3. Cell viability assay
SH-SY5Y cells were loaded at 104 cells per 100 μL in each well onto 96-well plates and were allowed to attach overnight then were differentiated using 100 M all-trans retinoic acid. Differentiated cells were exposed to 0, 1, 2.5, 5, 10, 50, or 100 M tolfenamic acid for 12, 24, or 72 h with six replicates per group. Cells were incubated at 37°C with 5% CO2 and 90% humidity. Cell viability was determined using the Vybrant® MTT cell proliferation assay kit following the manufacturer’s instructions (Life technologies, Grand Island, NY). Absorbance at 570 nm was measured using Spectra Max UV/Vis Spectrometer (GMI, Ramsey, MN) and cell viability was determined in treatment groups as a percentage from control.
2.4. RNA isolation, cDNA synthesis and real time PCR
RNA was isolated from cerebral cortex tissue following the TRIzol® Reagent method (Invitrogen, Carlsbad, CA), checked for integrity by NanoDrop (Thermo Scientific, Wilmington, DE), and reverse transcribed to cDNA using iScriptTM Select cDNA Synthesis kit following manufacturer’s instructions (Bio-Rad, Hercules, CA). About 1000 ng of RNA were diluted to 19.5 μL with nuclease free water, then 3 μL Oligo (dT) mix, 6 μL 5x iScript Select reaction mix, and 1.5 μL of iScript reverse transcriptase were added. Samples were incubated at 42°C for 90 minute s then at 85°C for 5 minutes to terminate the reaction. All incubations were conducted using MJ Research MiniCyclerTM (Bio-Rad, Hercules, CA). Primer pairs for mouse BACE1 or β-actin and human APP or GAPDH were obtained from Invitrogen (Carlsbad, CA) as follows: BACE1 sense: 5′-ATG TGC ACG ATG AGT TCA GG-3′ and antisense: 5′- GCA GAG TGG CAA CAT GAA GA −3′; β-actin sense: 5′- TGT TAC CAA CTG GGA CGA CA −3′, and antisense: 5′- TCT CAG CTG TGG TGG TGA AG −3′; APP sense: 5′- GCC AAA GAG ACA TGC AGT GA −3′ and antisense: 5′- CCA GAC ATC CGA GTC ATC CT −3′; GAPDH sense: 5′- AGC TGA ACG GGA AGC TCA CT −3′, and antisense: AGG TCC ACC ACT GAC ACG TTG −3′. Each real time PCR reaction mix contained 2 μL of cDNA, 1 μL of each primer, 8.5 μL nuclease free water and 12.5 μL SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA). Real time PCR was conducted using the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) following the standard protocol: 50°C for 2 minutes followed by 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Results were analyze d using the 7500 system software with relative quantification method and β-actin as endogenous control.
2.5. Protein extraction and Western blot analyses
Cytoplasmic and nuclear extractions from neuroblastoma cells were carried out using NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer’s instructions (Thermo Scientific Pierce, Rockford, IL). Protein concentration was determined using the Micro BCA Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL). Protein extracts were stored at −80°C until further use. Samples containing 20 g nuclear protein were separated onto 4–15% precast polyacrylamide gels (Bio-Rad, Hercules, CA) at 150 V for 1-2 h and then transferred to PVDF membranes (GE-Healthcare, Piscataway, NJ). Membranes were blocked and incubated with the appropriate dilution of the specific primary antibody for 1-2 h; either 1:500 dilution of ABE135 for SP1 levels (Millipore, Billerica, MA) or 1:2000 of GAPDH T9450 (Sigma-Aldrich, St. Louis, MO) were used. Then the membranes were washed with TBST four times and incubated with the appropriate infrared dye-labeled secondary antibody (Li-Cor, Lincoln, NE) for 1 h at room temperature in the dark. Infrared signal of Western blot bands was detected and quantified using an Odyssey® Infrared Imaging System (Li-Cor, Lincoln, NE). Western blot bands were normalized against the levels of the house keeping protein GAPDH.
2.6. BACE1 activity assay
BACE1 activity within the cerebral cortices of control and treated mice was measured using SensiZyme BACE1 activity assay kit CS1060 (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s instructions. Briefly 100 μL of blank, standards, or samples containing 450 μg protein were loaded into wells pre-coated with anti-BACE1 antibody. Samples were incubated for 2 h at 4°C, after that, wells were washed 4 times then 50 μL of BACE1 substrate A was added to each well and incubated overnight at room temperature in a humidified chamber. On the next day, 50 μL of the colorimetric substrate B reagent mixture was added to the wells and incubated at room temperature for 3 h. At the end of the incubation period, absorbance was measured at 405 nm using Spectra Max UV/Vis Spectrometer (GMI, Ramsey, MN) and BACE1 activity was calculated in ng/mL using the standard curve.
2.7. ELISA Aβ40 assay
Levels of Aβ40 in cell culture media were measured using human Aβ40 kit JP27713 (IBL, Gunma, Japan). The kit is solid-phase sandwich ELISA with a highly specific antibody that is 100% reactive with human Aβ40 with a sensitivity of 5.00 pg/mL. The kit measures Aβ40 cleaved N-terminal side by any cause. The assay was conducted following manufacturer’s instructions with minor modifications. One hundred μg protein as determined by Micro BCA protein assay kit (Thermo Scientific Pierce, Rockford, IL) in 100 μL EIA buffer and assay standards were added in triplicates to 96-well plates pre-coated with anti-human Aβ mouse IgG MoAb. The plates were incubated overnight at 4°C, and washed 7 times using the 40X diluted wash buffer supplied with the kit (0.05% Tween 20 in phosphate buffer), and 100 L labeled antibody was added and incubated for 1 h at 4°C, the wells were washed again 9 times , and then 100 L of tetramethylbenzidine was added as a coloring agent, and incubated in the dark for 30 minutes at room temperature. Finally 100 L of 1N H2SO4 was added to stop the reaction, and absorbance was measured at 450 nm using Spectra Max UV/Vis spectrometer (GMI, Ramsey, MN). The concentration of Aβ40 in unknown samples was calculated as pg/mg total protein using the standard curve obtained.
2.8. Statistical analysis
Data was represented as the mean ± the standard error of the mean (SEM). Statistical analysis was performed using GraphPad Instat software (GraphPad software, San Diego, CA) and statistical significance was determined by one-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons post-test. Results with p-values <0.05 were considered significantly different from the group in comparison and were marked accordingly.
3. Results
Tolfenamic acid has been used for years in humans for migraine headaches and rheumatoid arthritis. In our experiments, tolfenamic acid was well tolerated by the animals and no side effects were observed. Data obtained by our collaborators at M. D. Anderson Cancer Center also found that chronic administration of tolfenamic acid was not toxic and had no adverse effects on animals’ weight, hematocrit, stomach or intestinal lining integrity compared to control (Sankpal et al., 2013).
3.1. Tolfenamic acid lowers BACE1 gene expression in vivo
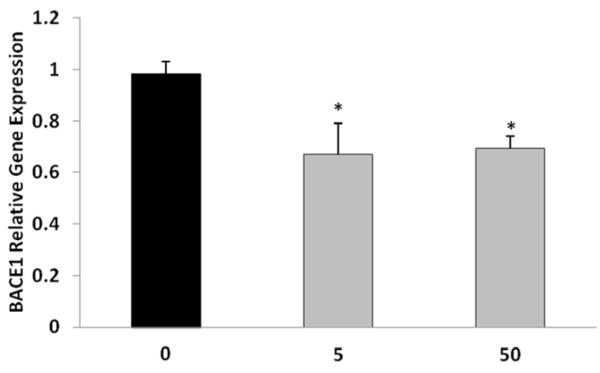
Following the administration of tolfenamic acid to APP YAC transgenic mice daily for 34 days, BACE1 gene expression within the cerebral cortex was lowered by 30% with both the 5 and 50 mg/kg doses as determined by real time PCR (Fig. 2). One-way ANOVA F(2,12)=6.614, p=0.0116, Tukey-Kramer multiple comparisons post-test p<0.05 for the control vs 5 mg/kg group and for control vs 50 mg/kg group.
Figure 2. BACE1 relative gene expression within the cerebral cortices of R1.40 transgenic mice following tolfenamic acid exposure.
Hemizygous transgenic APP YAC mice were administered 0, 5 or 50 mg/kg/day tolfenamic acid for 34 days. BACE1 mRNA levels were measured in the cerebral cortex by real time PCR with β-actin as endogenous control as illustrated in the methods section. Values shown are the mean ± SEM, n=5 in each group, p=0.0116 as determined by one-way ANOVA with Tukey-Kramer post-test *p<0.05.
3.2. Tolfenamic acid reduces BACE1 activity
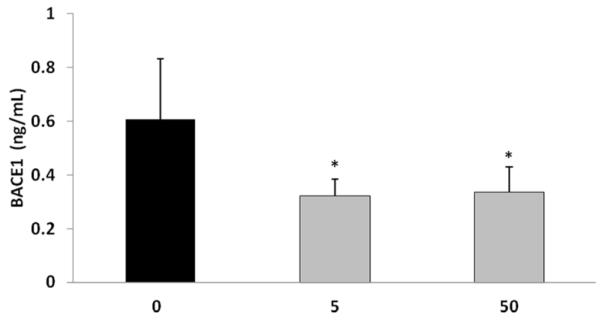
The activity of the enzyme BACE1 in the cerebral cortex was examined following the exposure of APP YAC transgenic mice to tolfenamic acid for 34 days. BACE1 enzyme activity was reduced by 45% with the 5 and 50 mg/kg/day doses as determined by One-way ANOVA (F(2,12)=5.547, p=0.0197), Tukey-Kramer multiple comparisons post-test p<0.05 for the control vs 5 mg/kg and for C vs 50 mg/kg group (Fig. 3).
Figure 3. BACE1 enzyme activity in the cerebral cortex of APP YAC transgenic mice following tolfenamic acid exposure.
BACE1 activity was measured using SensiZyme BACE1 activity assay kit from Sigma-Aldrich as illustrated in the methods section. Values shown are the mean ± SEM, n=5. One-way ANOVA p= 0.0197 *p<0.05 as determined by Tukey-Kramer post-test.
3.3. Tolfenamic acid cell viability studies in differentiated neuroblastoma cells
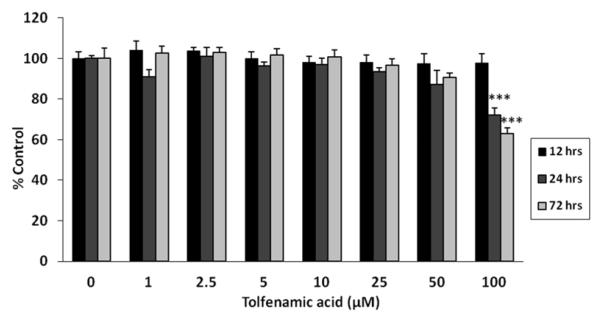
The viability of SH-SY5Y cells differentiated using all-trans retinoic acid was examined following treatments with 0-100 μM tolfenamic acid. The results show that tolfenamic acid did not cause any cytotoxicity until the highest dose of 100 μM after 24 h and 72 h of exposure (p<0.001) (Fig. 4). This suggests that the effects of tolfenamic acid on cell viability are time and dose-dependent. Overall one-way ANOVA reported a p-value less than 0.0001; one-way ANOVA p=0.8164 for groups in the 12 h exposure; p<0.0001 for the 24 h treatment groups; and p<0.0001 for groups in the 72 h exposure. Based on these results, we chose the doses of 25 and 50 μM of tolfenamic acid for the following exposure experiments in neuroblastoma cells.
Figure 4. Cell viability of differentiated SH-SY5Y cells following tolfenamic acid exposure.
Neuroblastoma cells were differentiated and exposed to media containing 0, 1, 2.5, 5, 10, 25, 50 or 100 M tolfenamic acid for 12 h, 24 h, or 72 h and cell viability was studied using MTT as illustrated in the methods section. Values shown are the mean ± SEM, n=6 in each group, overall one-way ANOVA p<0.0001, one-way ANOVA p=0.8164 for groups in the 12 h exposure; p<0.0001 for groups in the 24 h; and p<0.0001 for groups in the 72 h exposure. ***p<0.001 compared to the corresponding control group from the same time duration of exposure as determined by Tukey-Kramer post-test.
3.4. Tolfenamic acid lowers SP1 in vitro
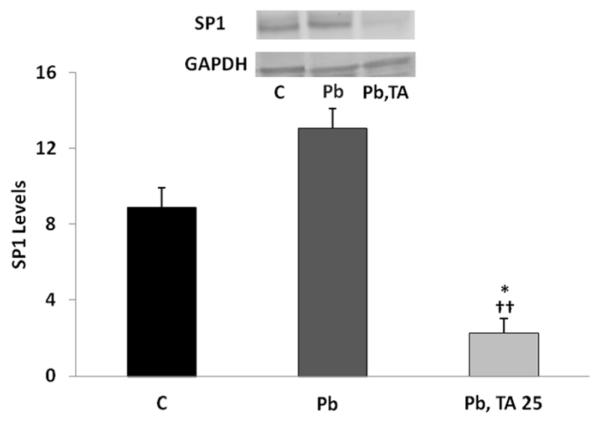
Exposure of differentiated SH-SY5Y cells to 50 M Pb for 48 h followed by Pb-free media for 48 h induced the expression of SP1 by 47% which did not reach statistical significance according to Tukey-Kramer post-test when compared to control. However, when Pb treatment for 48 h was succeeded by exposure to 25 M tolfenamic acid for 48 h, SP1 levels were decreased by 75% compared to control which was deemed statistically significant according to Tukey-Kramer post-test (p<0.05), and by 83% when compared to SP1 levels in cells exposed to Pb for 48 h followed by Pb-free media for 48 h (p<0.01). Overall one-way ANOVA between all groups reported a p-value equal to 0.003, F(2,5)=22.093 (Fig. 5).
Figure 5. SP1 levels in differentiated SH-SY5Y cells after treatment with Pb followed by Pb-free media or by tolfenamic acid.
SH-SY5Y cells were differentiated using 100 M all-trans retinoic acid and where either unexposed (control ,C) for 96 h with the media changed every 48 h, or exposed to 50 M Pb for 48 h followed 0 or 25 M tolfenamic acid (TA) for 48 h. Values shown are the mean ± SEM. Three independent experiments were performed in triplicates. SP1 levels were normalized to the levels of the house keeping protein GAPDH. One-way ANOVA p=0.003, with Tukey-Kramer post-test *p<0.05 compared to C, ††p<0.01 compared to 48 h Pb followed by 48 Pb-free media exposure group. Insert shows representative SP1 and GAPDH Western blot bands from 96 h control (C); 48 h Pb treatment followed by 48 h Pb-free media; or 48 h Pb exposure followed by 48 h tolfenamic acid (TA) treatment.
3.5. Effects of tolfenamic exposure on APP gene expression
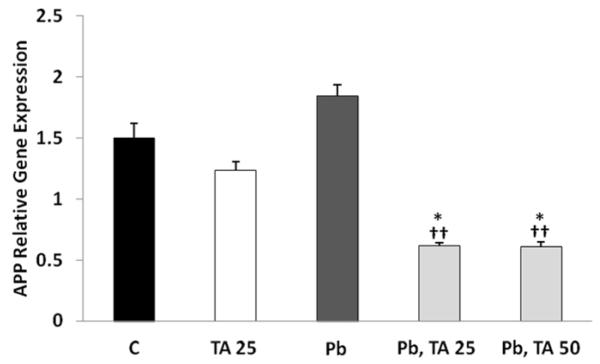
Treatment of cells with tolfenamic acid for 96 h reduced the gene expression of APP compared to control by 18% that was not statistically significant. Whereas the exposure of cells to Pb for 48 h followed by Pb-free media for 48 h increased APP gene expression by 23% which did not reach statistical significance when compared to control. Tolfenamic acid treatment after Pb lowered the Pb-induced APP gene expression in differentiated neuroblastoma cells by 60% from control (p<0.05) and by 67% from cells exposed to Pb for 48 h followed by Pb-free media for 48 h (p<0.01). Overall one-way ANOVA reported a p-value of 0.001, F(4,21)=6.905 (Fig. 6).
Figure 6. APP gene expression in differentiated SH-SY5Y cells exposed to tolfenamic acid, Pb or both.
Differentiated neuroblastoma were exposed to 25 M tolfenamic acid (TA) for 96 h with the media changed every 48 h; cells were also exposed to 50 M Pb for 48 h followed by 0, 25 or 50 M tolfenamic acid for 48 h; finally, cells were left unexposed (Control, C). APP gene expression was measured by real time PCR with GAPDH as endogenous control as illustrated in the methods section. One-way ANOVA p=0.001. Tukey-Kramer post-test *p<0.05 compared to C, ††p<0.01 compared to 48 h Pb followed by 48 C exposure group.
3.6. Tolfenamic acid lowers the levels of Aβ40 induced by Pb
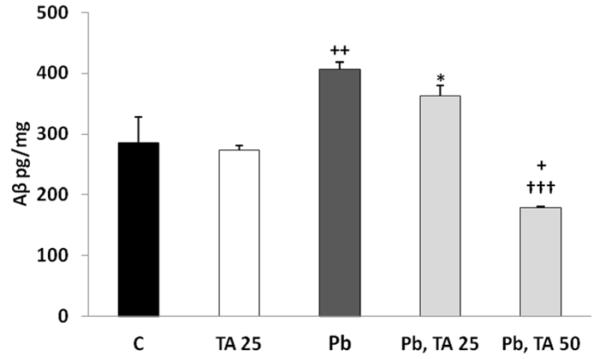
Aβ levels were increased by 42% in differentiated SH-SY5Y cells after treatment with Pb for 48 h followed by Pb-free media for additional 48 h (Fig. 7). This increase was significant when compared to cells maintained in Pb-free media for 96 h with the media changed every 48 h according to Tukey-Kramer multiple comparison test (p<0.01). When treatment of SH-SY-5Y cells by Pb for 48 h was followed by treatment with 25 μM tolfenamic acid for 48 h, there was a trend of reduction in Aβ levels in the media by 10% compared to treatment with Pb for 48 h followed by Pb-free media for 48 h. Aβ levels were decreased by 56% with the 50 μM tolfenamic acid concentration for 48 h following prior Pb exposure for 48 h which was significant compared to Aβ levels in the media of cells exposed to Pb for 48 h followed by Pb-free media for 48 h (p<0.001); and Aβ levels were reduced by 37% compared to control (p<0.05). However, treatment of cells with 25 μM tolfenamic acid for 96 h did not change Aβ levels within the media. The overall one-way ANOVA p-value between groups was p<0.0001, F(4,12)=22.537 (Fig. 7).
Figure 7. Aβ levels in differentiated SH-SY5Y cells exposed to tolfenamic acid, Pb or both.
Differentiated neuroblastoma cells were either left unexposed (control, C) or were exposed to 25 μM tolfenamic acid (TA) for 96 h with the media changed every 48 h; or 50 μM Pb for 48 h followed by either 0, 25, or 50 μM tolfenamic acid for 48 h. Aβ levels within the media were measured using ELISA as explained in the methods section. Overall one-way ANOVA revealed p<0.0001 between all groups. Tukey-Kramer post-test *p<0.05 compared to 25 μM TA group; +p<0.05, ++p<0.01 compared to C group and 25 M TA group; †††p<0.001 compared to 48 h Pb followed by 48 C group and compared to 48 h Pb followed by 48 h 25 μM TA group.
4. Discussion
Up to now, five drugs that belong to two classes have been approved for AD, the cholinesterase inhibitors and the NMDA receptor antagonist memantine. These interventions aim at improving memory functions to some extent but do not stop the dementia and the ultimate loss of daily functioning caused by AD. Many other candidates were in preclinical and clinical trials but failed to demonstrate safety or efficacy. Several AD targets under investigation are within the amyloid pathway of AD including APP, β-secretase, γ-secretase and Aβ itself. Yet, no successful candidate that can change the course of AD has been found.
Research studies including those conducted in our lab demonstrate that the transcription factor Sp1 is involved in AD pathology (Brock et al., 2008; Christensen et al., 2004; Citron et al., 2008; Docagne et al., 2004; Santpere et al., 2006). Sp1 regulates the expression of APP, BACE1 and tau (Christensen et al., 2004; Docagne et al., 2004; Heicklen-Klein and Ginzburg, 2000; Hoffman and Chernak, 1995). SP1 co-localizes with APP and Aβ in brain neurons as well as with tau in tangles (Brock et al., 2008; Santpere et al., 2006). Due to its unique role in the transcription of AD related genes, targeting Sp1 is a novel and promising approach for discovering disease-modifying drugs for AD. Aβ and other factors involved in its processing are being targeted for AD. Vaccines against Aβ are under development although several have failed in clinical trials due to life threatening adverse effects such as meningoencephalitis (Delrieu et al., 2012; Schnabel, 2011). The structural properties of the BACE active site limit the chances for development of inhibitors for this enzyme (Tamagno et al., 2012). Whereas γ-secretase inhibitors have failed due to toxicity associated with the inhibition of the Notch signaling (Mattson, 2004; Ross and Imbimbo, 2010).
Our previous work demonstrated that tolfenamic acid was able to downregulate proteins implicated in AD pathology including APP and Aβ (Adwan et al., 2011; Subaiea et al., 2013). In this study, we confirmed that tolfenamic acid also lowers BACE1, another protein that is regulated by Sp1 and takes part in the amyloidogenic pathway of AD (Christensen et al., 2004). Following tolfenamic acid daily administration for about 1 month, the gene expression and activity of BACE1 were reduced in APP YAC transgenic mice (Fig. 2, 3). In these animals, tolfenamic acid also lowered SP1, APP and Aβ as well as improved cognition as determined by behavioral tests using the Morris water maze and the Y-maze (Subaiea et al., 2013).
The toxic effects of Pb on health have been described in the literature, experiments from our lab showed that Pb induced the expression of AD related genes and proteins including Sp1, APP, Aβ, and tau (Basha et al., 2005; Bihaqi and Zawia, 2012; Huang et al., 2011; Wu et al., 2008). BACE1 gene expression and activity were also increased following early Pb exposure in mice (Bihaqi et al., 2013). Hence, tolfenamic acid and Pb represent two agents that have opposing effects when it comes to AD related pathways.
The safety of tolfenamic acid has already been established and the drug has been used for migraine headaches in Europe for decades (Hakkarainen et al., 1982; Hakkarainen et al., 1979; Myllyla et al., 1998; Tokola et al., 1984). In our studies, no signs of toxicity were observed throughout the exposure periods. In neuroblastoma cells, lower doses of tolfenamic acid did not affect cell viability (Fig. 4). A decrease in cell viability with tolfenamic acid was observed at the higher concentration of 100 μM and at the longer periods of exposure of 24 h and 72 h. The outcomes of tolfenamic acid on cell viability were dose and time dependent.
To study the effects of tolfenamic acid on AD related genes and proteins in neuroblastoma cells, we chose the 25 and 50 μM concentrations which did not affect cell viability based on our results. The 50 M dose chosen for Pb exposure came from our previous cell viability and exposure studies with the same cell line (Bihaqi and Zawia, 2012; Huang et al., 2011). Our results show that the exposure of differentiated SH-SY5Y neuroblastoma cells to tolfenamic acid for 48 h after 48 h of Pb exposure decreased SP1 levels significantly compared to control or cells exposed to Pb followed by Pb-free media for 48 h each (Fig. 5). Furthermore, tolfenamic acid significantly reduced APP gene and Aβ expression that was induced by Pb exposure but not the basal levels of APP and Aβ (Fig. 6, 7).
The 25 M dose of tolfenamic acid was able to decrease SP1 levels and APP gene expression induced by prior Pb exposure. However, Aβ levels were only decreased significantly by the 50 M tolfenamic acid exposure after Pb. As tolfenamic acid affects transcription, time is an important factor for observing its effects and in this study, although the 25 M tolfenamic acid was very effective in lowering SP1 and APP gene expression following Pb administration, this drastic change was not translated into Aβ lowering probably due to insufficient time for effects on Aβ to show. For example, our previous studies showed that even though APP gene transcription was lowered following tolfenamic acid daily administration in mice for three days, APP protein levels were not lowered at that time (Adwan et al., 2011). Whereas the levels of both APP gene and protein were decreased after two weeks of tolfenamic acid daily administration to mice (Adwan et al., 2011).
About 90% of AD cases are sporadic and are referred to as late onset AD with age being the major risk factor (Alzheimer’s Association, 2012). Our lab has demonstrated that early Pb exposure replicates pathological events observed late in life in AD within various in vitro and in vivo models (Basha et al., 2005; Bihaqi et al., 2011; Bihaqi and Zawia, 2012; Huang et al., 2011; Wu et al., 2008). In this manuscript, we use prior Pb exposure as a model that causes upregulation of AD related intermediates including APP and Aβ by inducing the transcription factor Sp1, in a matter that resembles the environmentally inflicted late onset AD. After Pb exposure, we exposed the cells to tolfenamic acid, in order to test its ability to reverse the events caused by Pb. Our results show that tolfenamic acid was able to rescue the cells from the pathological increase in SP1, APP and Aβ.
Tolfenamic acid is a multi-target drug candidate for AD that mitigates the amyloid pathology of AD. By decreasing Sp1, tolfenamic acid was able to lower BACE1 expression and activity in vivo and to alleviate changes in APP and Aβ in vitro. The safety of tolfenamic acid use in humans has already been established as it has been approved and used for years in Europe for migraine headaches. Hence it represents a promising agent that can be repurposed for AD and was recently scheduled to be tested in AD patients.
Highlights.
The effects of tolfenamic acid exposure on amyloid pathology in vivo and in vitro.
Tolfenamic acid lowers the transcription and activity of the enzyme BACE1 in vivo.
Tolfenamic acid protects against Pb-induced increases in APP and Aβ in vitro.
Tolfenamic acid is a multi-target drug that can be repurposed for AD.
This is a novel approach for counteracting environmentally-induced AD pathogenesis.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) and by grants NIH- ES015867 and AG042695 awarded to NHZ. The research was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 8 P20 GM103430-12.
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- APP
amyloid β precursor protein
- BACE
beta-site APP cleaving enzyme
- FBS
fetal bovine serum
- Pb
lead
- SEM
standard error of the mean
- Sp1
specificity protein 1
- SP1
Sp1 protein
- YAC
yeast artificial chromosome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adwan LI, Basha R, Abdelrahim M, Subaiea GM, Zawia NH. Tolfenamic acid interrupts the de novo synthesis of the beta-amyloid precursor protein and lowers amyloid beta via a transcriptional pathway. Curr Alzheimer Res. 2011;8:385–392. doi: 10.2174/156720511795745285. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Herath PM. Development of a New Method for Assessing Global Risk of Alzheimer’s Disease for Use in Population Health Approaches to Prevention. Prev Sci. 2013 doi: 10.1007/s11121-012-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihaqi SW, Bahmani A, Subaiea GM, Zawia NH. Infantile exposure to lead and late-age cognitive decline: Relevance to AD. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihaqi SW, Huang H, Wu J, Zawia NH. Infant exposure to lead (Pb) and epigenetic modifications in the aging primate brain: implications for Alzheimer’s disease. J Alzheimers Dis. 2011;27:819–833. doi: 10.3233/JAD-2011-111013. [DOI] [PubMed] [Google Scholar]
- Bihaqi SW, Zawia NH. Alzheimer’s disease biomarkers and epigenetic intermediates following exposure to Pb in vitro. Curr Alzheimer Res. 2012;9:555–562. doi: 10.2174/156720512800617964. [DOI] [PubMed] [Google Scholar]
- Brock B, Basha R, DiPalma K, Anderson A, Harry GJ, Rice DC, Maloney B, Lahiri DK, Zawia NH. Co-localization and distribution of cerebral APP and SP1 and its relationship to amyloidogenesis. J Alzheimers Dis. 2008;13:71–80. doi: 10.3233/jad-2008-13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by. Sp1. Mol Cell Biol. 2004;24:865–874. doi: 10.1128/MCB.24.2.865-874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B, Dennis J, Zeitlin R, Echeverria V. Transcription factor Sp1 dysregulation in Alzheimer’s disease. J Neurosci Res. 2008;86:2499–2504. doi: 10.1002/jnr.21695. [DOI] [PubMed] [Google Scholar]
- Delrieu J, Ousset PJ, Caillaud C, Vellas B. ’Clinical trials in Alzheimer’s disease’: immunotherapy approaches. J Neurochem. 2012;120(Suppl 1):186–193. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- Docagne F, Gabriel C, Lebeurrier N, Lesne S, Hommet Y, Plawinski L, Mackenzie ET, Vivien D. Sp1 and Smad transcription factors co-operate to mediate TGF-beta-dependent activation of amyloid-beta precursor protein gene transcription. Biochem J. 2004;383:393–399. doi: 10.1042/BJ20040682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkarainen H, Parantainen J, Gothoni G, Vapaatalo H. Tolfenamic acid and caffeine: a useful combination in migraine. Cephalalgia : an international journal of headache. 1982;2:173–177. doi: 10.1046/j.1468-2982.1982.0204173.x. [DOI] [PubMed] [Google Scholar]
- Hakkarainen H, Vapaatalo H, Gothoni G, Parantainen J. Tolfenamic acid is as effective as ergotamine during migraine attacks. Lancet. 1979;2:326–328. doi: 10.1016/s0140-6736(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Heicklen-Klein A, Ginzburg I. Tau promoter confers neuronal specificity and binds Sp1 and AP-2. J Neurochem. 2000;75:1408–1418. doi: 10.1046/j.1471-4159.2000.0751408.x. [DOI] [PubMed] [Google Scholar]
- Hoffman PW, Chernak JM. DNA binding and regulatory effects of transcription factors SP1 and USF at the rat amyloid precursor protein gene promoter. Nucleic Acids Res. 1995;23:2229–2235. doi: 10.1093/nar/23.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Huang H, Bihaqi SW, Cui L, Zawia NH. In vitro Pb exposure disturbs the balance between Abeta production and elimination: the role of AbetaPP and neprilysin. Neurotoxicology. 2011;32:300–306. doi: 10.1016/j.neuro.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Jamsa A, Hasslund K, Cowburn RF, Backstrom A, Vasange M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun. 2004;319:993–1000. doi: 10.1016/j.bbrc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- Lamb BT, Bardel KA, Kulnane LS, Anderson JJ, Holtz G, Wagner SL, Sisodia SS, Hoeger EJ. Amyloid production and deposition in mutant amyloid precursor protein and presenilin-1 yeast artificial chromosome transgenic mice. Nat Neurosci. 1999;2:695–697. doi: 10.1038/11154. [DOI] [PubMed] [Google Scholar]
- Lamb BT, Call LM, Slunt HH, Bardel KA, Lawler AM, Eckman CB, Younkin SG, Holtz G, Wagner SL, Price DL, Sisodia SS, Gearhart JD. Altered metabolism of familial Alzheimer’s disease-linked amyloid precursor protein variants in yeast artificial chromosome transgenic mice. Hum Mol Genet. 1997;6:1535–1541. doi: 10.1093/hmg/6.9.1535. [DOI] [PubMed] [Google Scholar]
- Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyla VV, Havanka H, Herrala L, Kangasniemi P, Rautakorpi I, Turkka J, Vapaatalo H, Eskerod O. Tolfenamic acid rapid release versus sumatriptan in the acute treatment of migraine: comparable effect in a double-blind, randomized, controlled, parallel-group study. Headache. 1998;38:201–207. doi: 10.1046/j.1526-4610.1998.3803201.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Kondoh G, Kudo T, Imaizumi K, Kato M, Miyazaki JI, Tohyama M, Takeda J, Takeda M. Accumulation of murine amyloidbeta42 in a gene-dosage-dependent manner in PS1 ‘knock-in’ mice. Eur J Neurosci. 1999;11:2577–2581. doi: 10.1046/j.1460-9568.1999.00698.x. [DOI] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Pollwein P, Masters C, Beyreuther K. The expression of the amyloid precursor protein (APP) is regulated by two GC-elements in the promoter. Nucleic Acids Res. 1992;20:63–68. doi: 10.1093/nar/20.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, Imbimbo BP. Are gamma-secretase inhibitors detrimental for Alzheimer’s disease patients? J Alzheimers Dis. 2010;22:401–404. doi: 10.3233/JAD-2010-101548. [DOI] [PubMed] [Google Scholar]
- Sankpal UT, Lee CM, Connelly SF, Kayaleh O, Eslin D, Sutphin R, Goodison S, Adwan L, Zawia NH, Lichtenberger LM, Basha R. Cellular and Organismal Toxicity of the Anti-Cancer Small Molecule, Tolfenamic Acid: a Pre-Clinical Evaluation. Cell Physiol Biochem. 2013;32:675–686. doi: 10.1159/000354471. [DOI] [PubMed] [Google Scholar]
- Santpere G, Nieto M, Puig B, Ferrer I. Abnormal Sp1 transcription factor expression in Alzheimer disease and tauopathies. Neurosci Lett. 2006;397:30–34. doi: 10.1016/j.neulet.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Schnabel J. Vaccines: chasing the dream. Nature. 2011;475:S18–19. doi: 10.1038/475S18a. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Preventing Alzheimer’s disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Subaiea GM, Adwan LI, Ahmed AH, Stevens KE, Zawia NH. Short-term treatment with tolfenamic acid improves cognitive functions in Alzheimer’s disease mice. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Monteleone D, Vercelli A, Tabaton M. Transcriptional and post-transcriptional regulation of beta-secretase. IUBMB Life. 2012;64:943–950. doi: 10.1002/iub.1099. [DOI] [PubMed] [Google Scholar]
- Terry RD, Gonatas NK, Weiss M. Ultrastructural Studies in Alzheimer’s Presenile Dementia. Am J Pathol. 1964;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Tokola RA, Kangasniemi P, Neuvonen PJ, Tokola O. Tolfenamic acid, metoclopramide, caffeine and their combinations in the treatment of migraine attacks. Cephalalgia. 1984;4:253–263. doi: 10.1046/j.1468-2982.1984.0404253.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE. Plaques, tangles and Alzheimer’s disease. Psychol Med. 1982;12:449–459. doi: 10.1017/s0033291700055549. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Buldyrev SV, Havlin S, Irizarry MC, Stanley HE, Hyman BT. Dynamics of plaque formation in Alzheimer’s disease. Biophys J. 1999;76:1330–1334. doi: 10.1016/S0006-3495(99)77295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med. 2009;46:1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]