Abstract
Background
The preservation of harvested organs plays an essential role in transplantation. Cold hypothermia is frequently applied but may lead to graft compromise resulting from reperfusion and rewarming injury. This study investigates the effect of deep hypothermia and posterior rewarming on leukocyte-endothelial interactions and junctional adhesion molecules.
Material/Methods
We established an in vitro model to investigate the transendothelial migration of leukocytes (TEM) during deep hypothermia (4°C) as well as during the post-hypothermic rewarming process. Additionally, leukocyte-endothelial interactions were analyzed by quantifying surface expression of the junctional adhesion molecules A (JAMA-A and JAM-B).
Results
While deep hypothermia at 4°C was associated with reduced leukocyte infiltration, rewarming after hypothermic preservation resulted in a significant increase in TEM. This process is mainly triggered by activation of endothelial cells. Post-hypothermic rewarming caused a significant downregulation of JAM-A, whereas JAM-B was not altered through temperature modulation.
Conclusions
Hypothermia exerts a protective effect consisting of reduced leukocyte-endothelial interaction. Rewarming after hypothermic preservation, however, causes considerable upregulation of leukocyte infiltration. Downregulation of JAM-A may play a role in modulating TEM during hypothermia and rewarming. We conclude that the rewarming process is an essential but underestimated aspect during transplantation.
MeSH Keywords: Cell Adhesion Molecules, Hypothermia, Organ Preservation, Reperfusion Injury
Background
Optimal graft preservation and preconditioning are mandatory to ensure proper function of transplanted organs. The protective effects resulting from hypothermia reducing cell metabolism are well established and have long been used, either in the form of static cold storage (deep hypothermia; 0°C to 5°C) or as part of more recent techniques such as hypothermic machine perfusion [1], to keep organs from deteriorating.
But reducing the temperature of harvested organs may also cause unwanted effects. Prolonged cold preservation may result in increased vascular damage [2] and augment reperfusion injury. Recent evidence moreover suggests that injury may result not only from reperfusion but also from rewarming [3]. Rewarming was found to trigger mitochondrial dysfunction [4] and can induce mitochondrial apoptotic pathways [5], thus compromising graft recovery. The speed of rewarming was reported to play an essential role in that gradual rewarming turned out to diminish the severity of the rewarming injury [6] and controlled rewarming exerted a preconditioning effect on grafts [7].
Beyond this, rewarming was shown to be associated with the induction of pro-inflammatory processes such as enhanced leukocyte infiltration [8,9], which may also impair graft function [10]. The interactions between leukocytes and the endothelium are strongly associated with the development of acute cellular rejection [11,12], which is important because attenuating early graft rejection helps preventing late graft loss and/or dysfunction [13,14]. Junctional adhesion molecules (JAM) are cell-cell adhesion molecules responsible, among other functions, for the endothelial barrier and permeability, and are crucial for transendothelial migration.
The present study was designed to examine the effects of deep hypothermia (4°C) and rewarming on the transendothelial migration of leukocytes (TEM) and the endothelial expression of JAM-A and JAM-B.
Material and Methods
Our study was reviewed and approved by the Ethics Committee of the University Hospital, JW Goethe University of Frankfurt, Germany (GN.: 227/13).
Peripheral blood leukocytes (PBL)
Blood obtained from healthy volunteers (n=5) was subjected to density gradient centrifugation (Polymorphprep®; Axis-Shield) to isolate peripheral blood leukocytes (PBL), which comprised peripheral blood mononuclear cells (PBMC) and polymorph nuclear cells (PMNC). Isolated PBL contain 20.9±6.36% CD177+ granulocytes, 5.7±3.8% CD19+ B-cells, 1.33±0.68% CD3+ T-cells, 1.36±0.34% CD14+ monocytes and 37.44±0.69% CD14+/CD16+ monocytes, analyzed by FACS and calculated as percentage±standard error of the mean (SEM) in respect to total cell count as previously described [15]. The remaining percentage fractions represent cells which are not positively labeled for the aforementioned surface markers. The PBL thus obtained were stored in RPMI 1640 (Life Technologies) at a temperature of 37°C. One part of the isolated PBL was activated by exposing them to 10 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich) at RPMI 1640 with 10% fetal bovine serum (Gibco) for 60 minutes, whereas the other part remains in a non-activated condition. LPS was removed after incubation by washing the cells with phosphate-buffered saline (PBS).
Endothelial cell culture
Human microvascular endothelial cells (HuMEC-1) are immortalized cells and were provided by Dr. Mirakaj (University Tübingen, Department of Anesthesiology and Intensive Care Medicine). HuMEC-1 enables stable experimental conditions with less heterogeneity and retains typical endothelial phenotypical characteristics in morphology and functionality. HuMEC-1 expresses microvascular endothelial surface markers like ICAM-1 and CD36 and form endothelial monolayers like primary endothelial cells [16]. Primary human umbilical vein endothelial cells (HuVEC) express ICAM-1, a typical microvascular endothelial marker, only in low-levels and only upon stimulation with interleukin (IL)-1 or tumor necrosis factor (TNF)-α. In contrast, HuMEC-1 expresses ICAM-1 constantly on a high level without the need of prior stimulation which represents more the phenotypical condition of microvascular endothelial cells and enables stable conditions for transmigration assays. Cells were processed as previously described [15]. Briefly, HuMEC-1 were cultured in MCDB-131 (Life Technologies) supplemented with 10% fetal calf serum, 1% glutamine (Gibco), 10 ng/mL Epidermal Growth Factor (Sigma Chemical), 1% penicillin/streptomycin solution (Sigma Chemical), and 1 μg/mL hydrocortisone (Sigma Chemical) at 37°C and 5% CO2 atmosphere. Following this, the HuMEC-1 were labeled with BrdU (Sigma-Aldrich), placed on fibronectin-coated 3 μm pore-size inserts (FluoroBlok®, BD) and left overnight at 37°C and 5% CO2 to form an endothelial monolayer. As previously described [15], a subset of HuMEC-1 was activated by exposing them to 100 μg/mL LPS in MCDB-131 for 60 minutes. After the treatment, the medium was removed, and the transmigration assays were started.
Subsets of experiments
At the beginning of the experiments, PBL and HuMEC-1 were kept at a temperature of 37°C. Subsequently, temperature modulations were implemented so as to yield 3 subsets of experiments in the course of which the cells were exposed to deep hypothermia at 4°C: 1) during transmigration, 2) during cell activation (treatment) as well as transmigration, and 3) during cell activation (treatment) only. In the latter case, deep hypothermia was followed by transmigration under normothermic conditions, thus simulating a clinical post-hypothermic rewarming process.
Further subgroups were created by implementing the temperature variations described in 1) non-activated PBL transmigrating non-activated HuMEC-1 [control], 2) activated PBL transmigrating non-activated HuMEC-1, 3) non-activated PBL transmigrating activated HuMEC-1, and 4) activated PBL transmigrating activated HuMEC-1.
Transendothelial migration
The PBL were stained with Calcein-AM (ABD-Bioquest), resuspended, deposed on top of the HuMEC-1 monolayer and allowed to transmigrate through the HuMEC-1 for 120 minutes under normothermic or deep hypothermic conditions (37°C or 4°C) as previously described [15]. The Calcein emission of the transmigrated PBL was measured using a Fluorometer (Berthold Technologies) at 485/535 nm, and the share of transmigrated cells was expressed as a ratio compared to the control (transmigration index, tmx). Each transmigration assay was performed in duplicates (PBL from 3 individual volunteers) or triplicates (PBL from 2 individual volunteers).
JAM-A and JAM-B surface expression during rewarming
BrdU-labeled HuMEC-1 were placed on fibronectin-coated 96-well plates and left overnight at 37°C and 5% CO2 to form an endothelial monolayer. HuMEC-1 and PBL were activated with LPS as previously described at 4°C. PBL and HuMEC-1 were co-cultured at 37°C for 120 minutes to simulate a rewarming process [9]. After removal of the PBL and washing of the HuMEC-1, the endothelial monolayer was fixed with 4% paraformaldehyde (AppliChem), followed by blocking with PBS supplemented with 10% donkey serum (Jackson ImmunoResearch).
Rabbit anti-JAM-A-IgG (1: 200; Santa Cruz Biotechnology) and goat anti-JAM-B-IgG (1: 100; Santa Cruz Biotechnology), donkey anti-rabbit-IgG-PE (1: 100; Santa Cruz Biotechnology) and donkey anti-goat-IgG-FITC (1: 100; Santa Cruz Biotechnology) in PBS containing 1% donkey serum were used for primary and secondary staining of the HuMEC-1, as previously described [9]. JAM surface protein expression was measured using a microplate reader at 485/535 nm and 530/590 nm, and the fluorescence intensity (FI) was calculated as a ratio to control. Each expression assay was performed in duplicates (PBL from 3 individual volunteers). To reduce cross-contamination of leukocytes, analysis was performed in a separate experiment independently from the transmigration assay.
Statistical analysis
The mean of the control group was calculated and set to 1. Transmigration index (tmx) and fluorescence intensity (FI) were calculated as a ratio compared to control. To illustrate the variations of the control groups, all values of the control groups were divided through the calculated mean of the control group. Data are indicated as mean±standard error of the mean (SEM) and were analyzed using Prism 6 software (Graph Pad). Group differences were determined using ANOVA and post-hoc Tukey’s multiple comparison test. Differences of P<0.05 were considered statistically significant.
Results
Transmigration assays
Hypothermic transmigration
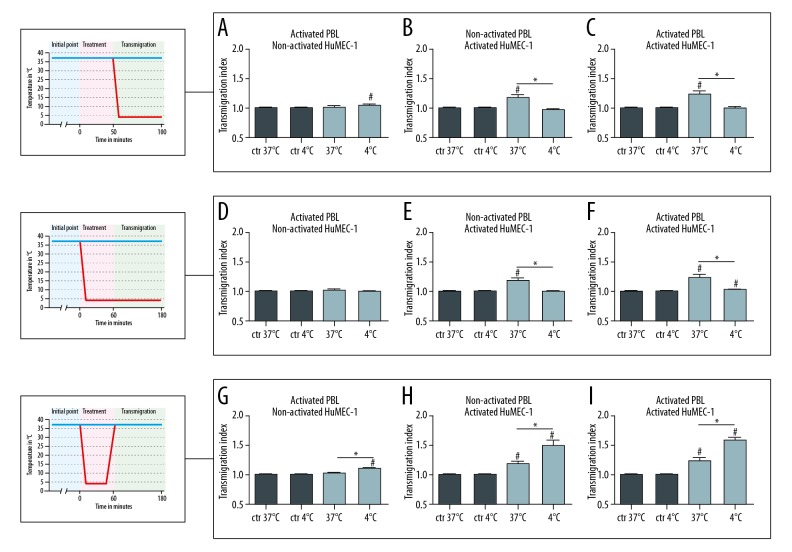
In the first subset of experiments, hypothermia (4°C) was applied during transmigration (Figure 1A–1C). Deep hypothermia applied during the transmigration of activated PBL through non-activated HuMEC-1 (37°C n=11 or 4°C n=12) caused no significant change in TEM. Temperature modulation during the transmigration of non-activated PBL through activated HuMEC-1 (37°C n=11 or 4°C n=12), in contrast, resulted in a significantly decreased TEM (P≤0.0001) at 4°C. A significant downregulation of TEM (P=0.0088) also occurred when activated PBL were allowed to transmigrate through activated HuMEC-1 (37°C n=12 or 4°C n=11) under hypothermic conditions.
Figure 1.
Influence of hypothermia and rewarming on transendothelial migration. (A–C) TEM of activated PBL through non-activated HuMEC-1 is not significantly modified (37°C n=11 or 4°C n=12), whereas TEM of non-activated PBL through activated HuMEC-1 is significantly influenced with falling temperatures (37°C n=11 or 4°C n=12). Transmigration of activated PBL through activated HuMEC-1 is significantly reduced with falling temperatures (37°C n=12 or 4°C n=11). (D–F) TEM of activated PBL through non-activated HuMEC-1 is not significantly modified (37°C n=11 or 4°C n=12) but TEM of non-activated PBL through activated HuMEC-1 is significantly reduced during hypothermia (37°C n=11 or 4°C n=10). Furthermore, TEM of activated PBL through activated HuMEC-1 is significantly downregulated with falling temperatures (37°C n=12 or 4°C n=11). (G–I) Transmigration of activated PBL through non-activated HuMEC-1 is not significantly modified (37°C n=11 or 4°C n=11), whereas TEM of non-activated PBL through activated HuMEC-1 is significantly enhanced after rewarming (37°C n=11 or 4°C n=12). Rewarming causes a significant enhanced TEM of activated PBL through activated HuMEC-1 (37°C n=12 or 4°C n=11). # P<0.05 versus control, * P<0.05. TEM – transendothelial migration of leukocytes; PBL, peripheral blood leukocytes; HuMEC-1; human microvascular endothelial cells.
Hypothermic activation and transmigration
In the second subset of experiments, deep hypothermia was applied during both activation and transmigration (Figure 1D–1F). This yielded no significant change in the TEM of activated PBL through non-activated HuMEC-1 (37°C n=11 or 4°C n=12), but caused a significant downregulation of the TEM of non-activated PBL through activated HuMEC-1 (37°C n=11 or 4°C n=10) (P=0.0001) and of activated PBL through activated HuMEC-1 (37°C n=12 or 4°C n=11) (P≤0.0001).
Post-hypothermic rewarming
The third subset of experiments simulated a post-hypothermic rewarming process (Figure 1G–1I). Transmigration of activated PBL through non-activated HuMEC-1 was significantly increased (P=0.0309) after post-hypothermic rewarming (37°C n=11 or 4°C n=11). Furthermore, rewarming caused a significant increase in the TEM (P=0.0118) of non-activated PBL through activated HuMEC-1 (37°C n=11 or 4°C n=12) and a significant upregulation also of the TEM (P=0.0020) of activated PBL through activated HuMEC-1 (37°C n=12 or 4°C n=11).
JAM-A and JAM-B expression and post-hypothermic rewarming
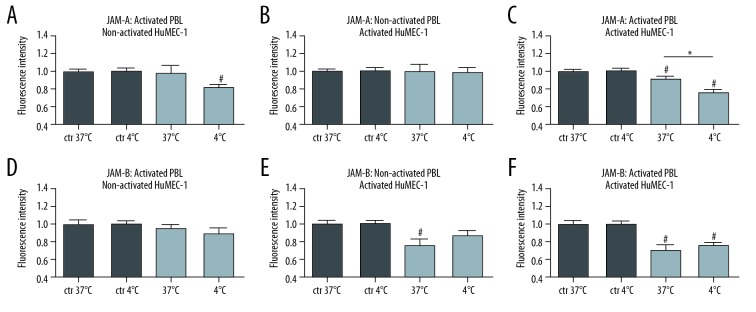
To further elucidate the significant increase in TEM during post-hypothermic rewarming, the expression of endothelial JAM-A and JAM-B was analyzed. During the post-hypothermic rewarming process, we observed an inverse correlation between the endothelial JAM-A expression and the results of the transmigration assays. Endothelial JAM-A expression was significantly reduced (P=0.0367) when activated PBL were co-cultured with activated HuMEC-1 (37°C n=8 or 4°C n=6). No significant differences in endothelial JAM-A expression were found, in contrast, when activated PBL were co-cultured with non-activated HuMEC-1 (37°C n=7 or 4°C n=6) and when non-activated PBL were co-cultured with activated HuMEC-1 (37°C n=8 or 4°C n=6).
JAM-B expression did not significantly change during post-hypothermic rewarming in activated PBL co-cultured with non-activated HuMEC-1 (37°C n=8 or 4°C n=6), non-activated PBL co-cultured with activated HuMEC-1 (37°C n=8 or 4°C n=6), and activated PBL co-cultured with activated HuMEC-1 (37°C n=8 or 4°C n=6). Figure 2 shows the results of these experiments and illustrates JAM-A and JAM-B expression as fluorescence intensity (FI).
Figure 2.
Influence of post-hypothermic rewarming on JAM-expression. JAM-A (A–C) and JAM-B (D–F) expression during rewarming. (A) JAM-A expression is not modulated in a co-culture of activated PBL with non-activated HuMEC-1 during rewarming (37°C n=8 or 4°C n=6). (B) JAM-A expression is not modulated in a co-culture of non-activated PBL with activated HuMEC-1 during temperature modulation (37°C n=7 or 4°C n=6), but (C) JAM-A expression is significantly reduced in a co-culture of activated PBL with activated HuMEC-1 during post-hypothermic rewarming (37°C n=8 or 4°C n=6). (D) JAM-B expression is neither significantly modulated in a co-culture of activated PBL with non-activated HuMEC-1 (37°C n=8 or 4°C n=6) nor (E) in a co-culture of non-activated PBL with activated HuMEC-1 during rewarming (37°C n=8 or 4°C n=6). (F) Furthermore JAM-B expression is not significantly reduced in a co-culture of activated PBL and activated HuMEC-1 during post-hypothermic rewarming (37°C n=8 or 4°C n=6). # P<0.05 versus control, * P<0.05. JAM – junctional adhesion molecules; HuMEC-1 – human microvascular endothelial cells; PBL – peripheral blood leukocytes.
Discussion
We investigated the effects of deep hypothermia and post-hypothermic rewarming on leukocyte transmigration and endothelial integrity because graft dysfunction is associated with early infiltration of immunocompetent cells into the graft. This was described by El-Sawy et al. [17], who found that inhibition of early polymorphnuclear cell infiltration into grafts prevents cell mediated rejection.
The results of our study are in keeping with the findings of Kalia et al. [18], who observed reduced leukocyte-endothelial adhesion resulting from hypothermia in a rat model of ischemia-reperfusion injury. Our study shows that short-term application of hypothermia significantly reduces leukocyte infiltration. Long-term cold storage of grafts, in contrast, was shown to result in vascular injury with reduced expression von PECAM-1 [2], so that it may be assumed that the protective effect of hypothermia is time-dependent.
Beyond this, it is essential to keep in mind that hypothermia is followed by post-hypothermic rewarming. An ever-increasing number of studies in fact suggest that while ischemia-reperfusion injury has long been recognized as a pivotal factor influencing graft function, rewarming injury may also play an important role. Awad et al. [8] showed that post-hypothermic rewarming following short-term hypothermia results in upregulation of pro-inflammatory pathways and suggested that rewarming could be associated with enhanced leukocyte-infiltration resulting from upregulation of ICAM-1. Other studies observed similar effects consisting of an increase in leukocyte infiltration after transplantation following prolonged cold storage and described concomitant destruction of the endothelial integrity [2]. This is not only in keeping with the results of a previous study of our group, which showed a similar effect during rewarming out of mild and moderate hypothermia [9], but also with the results of our current study, showing modulation of the endothelial barrier after deep hypothermia.
Deep hypothermia, as defined for the purposes of the present study, in fact corresponds to the very temperatures occurring in cold organ ischemia before transplantation. In this respect, the significant upregulation of leukocyte infiltration we observed during post-hypothermic rewarming as well as the observation that the rewarming process significantly modulates leukocyte-endothelial interaction represent interesting findings with a view to practical aspects of organ treatment and transplantation. The used analysis of transmigrated leukocytes by fluorescence intensity leads to an indirect evaluation of transmigration which has the limitation that an absolute quantification of transmigrated leukocytes is not possible. However, the method enables a faster experimental procedure with a higher throughput and without an observer bias.
When it comes to leukocyte-endothelial interaction, the JAMs play a pivotal role not only as adhesion-molecules [19], but also in the context of the regulation of tight junctions [20]. It has been shown that JAM-A enables leukocyte-endothelial interaction through binding to LFA1 [19] and JAM-B enables a leukocyte-endothelial interaction though VLA4 [21]. Both targets are interesting factors in the regulation of leukocyte recruitment and could be modulated in post-hypothermic rewarming. Previous studies show that the used endothelial cell line HuMEC-1 expresses JAMs under different experimental conditions and that they are able to modulate TEM in vitro [22,23]. The analysis of the conducted in vitro experiments contains some limitations because JAM-expression was conducted in a separate experiment which limits the evaluation of their function in direct association to TEM. Nevertheless, in the present study, we observed a temperature-dependent modulation of JAM-A in that a significant downregulation of JAM-A occurred during post-hypothermic rewarming out of deep hypothermic conditions. This finding is of particular interest, as we demonstrated, in a previous study, that downregulation of JAM-A correlates with an enhanced leukocyte-endothelial interaction with increased TEM [9]. Considering that early leukocyte infiltration can result in higher rejection rates [17]; it may be concluded that a rewarming process resulting in augmented TEM and downregulation of JAM-A may be associated with a higher likelihood of subsequent graft dysfunction.
Conclusions
Our study showed that the application of deep hypothermia resulted in diminished leukocyte-endothelial interaction and reduced TEM. Beyond this, however, it revealed that post-hypothermic rewarming from deep hypothermic conditions enhances leukocyte-endothelial interaction and causes upregulation of TEM and suggests an association between these effects and reduced JAM-A surface expression. This study was limited to in vitro observations and the clear transfer towards in vivo effect or the impact on the clinical setting were beyond the scope of this study. Further studies investigating the rewarming process will be required to fully understand the mechanisms of rewarming injury.
Acknowledgment
We are grateful to Maryam Tabib and Falko Seyffarth for their technical support.
Abbreviations
- TEM
transendothelial migration of leukocytes
- JAM-A
junctional adhesion molecules A
- JAM-B
junctional adhesion molecules A
- PBL
peripheral blood leukocytes
- PBMC
peripheral blood mononuclear cells
- PMNC
polymorph nuclear cells
- HuMEC-1
human microvascular endothelial cells
- Tmx
transmigration index
- FI
fluorescence intensity
- PECAM-1
platelet endothelial cell adhesion molecule 1
- ICAM-1
intercellular adhesion molecule 1
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Nath J, Smith TB, Patel K, et al. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1H NMR based study. Cryobiology. 2017;74:115–20. doi: 10.1016/j.cryobiol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Dragun D, Hoff U, Park JK, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60(3):1173–81. doi: 10.1046/j.1523-1755.2001.0600031173.x. [DOI] [PubMed] [Google Scholar]
- 3.Duval M, Plin C, Elimadi A, et al. Implication of mitochondrial dysfunction and cell death in cold preservation – warm reperfusion-induced hepatocyte injury. Can J Physiol Pharmacol. 2006;84(5):547–54. doi: 10.1139/y06-014. [DOI] [PubMed] [Google Scholar]
- 4.Sammut IA, Burton K, Balogun E, et al. Time-dependent impairment of mitochondrial function after storage and transplantation of rabbit kidneys. Transplantation. 2000;69(7):1265–75. doi: 10.1097/00007890-200004150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Salahudeen AK. Cold ischemic injury of transplanted kidneys: New insights from experimental studies. Am J Physiol Renal Physiol. 2004;287(2):F181–87. doi: 10.1152/ajprenal.00098.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer DP, Mathe Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: First clinical application of a new concept. Transplantation. 2016;100(1):147–52. doi: 10.1097/TP.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 7.Minor T, Efferz P, Fox M, et al. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13(6):1450–60. doi: 10.1111/ajt.12235. [DOI] [PubMed] [Google Scholar]
- 8.Awad EM, Khan SY, Sokolikova B, et al. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 2013;11(9):1716–26. doi: 10.1111/jth.12357. [DOI] [PubMed] [Google Scholar]
- 9.Bogert NV, Werner I, Kornberger A, et al. Influence of hypothermia and subsequent rewarming upon leukocyte-endothelial interactions and expression of Junctional-Adhesion-Molecules A and B. Sci Rep. 2016;6:21996. doi: 10.1038/srep21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii D, Schenk AD, Baba S, et al. Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. Am J Transplant. 2010;10(1):59–68. doi: 10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones ND, Brook MO, Carvalho-Gaspar M, et al. Regulatory T cells can prevent memory CD8+ T-cell-mediated rejection following polymorphonuclear cell depletion. Eur J Immunol. 2010;40(11):3107–16. doi: 10.1002/eji.201040671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–82. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Saase JL, van der Woude FJ, Thorogood J, et al. The relation between acute vascular and interstitial renal allograft rejection and subsequent chronic rejection. Transplantation. 1995;59(9):1280–85. [PubMed] [Google Scholar]
- 14.Ferguson R. Acute rejection episodes – best predictor of long-term primary cadaveric renal transplant survival. Clin Transplant. 1994;8(3 Pt 2):328–31. [PubMed] [Google Scholar]
- 15.Werner I, Guo F, Bogert NV, et al. Methylene blue modulates transendothelial migration of peripheral blood cells. PLoS One. 2013;8(12):e82214. doi: 10.1371/journal.pone.0082214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99(6):683–90. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 17.El-Sawy T, Belperio JA, Strieter RM, et al. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112(3):320–31. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 18.Kalia N, Pockley AG, Wood RF, Brown NJ. Effects of hypothermia and rewarming on the mucosal villus microcirculation and survival after rat intestinal ischemia-reperfusion injury. Ann Surg. 2002;236(1):67–74. doi: 10.1097/00000658-200207000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostermann G, Weber KS, Zernecke A, et al. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3(2):151–58. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–74. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham SA, Rodriguez JM, Arrate MP, et al. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277(31):27589–92. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 22.Rabquer BJ, Amin MA, Teegala N, et al. Junctional adhesion molecule-C is a soluble mediator of angiogenesis. J Immunol. 2010;185(3):1777–85. doi: 10.4049/jimmunol.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo YL, Bai R, Chen CX, et al. Role of junctional adhesion molecule-like protein in mediating monocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2009;29(1):75–83. doi: 10.1161/ATVBAHA.108.177717. [DOI] [PubMed] [Google Scholar]