Abstract
Graft‐versus‐host disease (GvHD) is an important complication that can be observed after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Acute GvHD (aGvHD) is seen after allo-HSCT and the incidence of aGvHD is around 30%-50%. aGvHD prophylaxis is essential in patients undergoing allo-HSCT. Initial therapy for aGvHD is steroids. Prognosis is poor in aGvHD patients not responding to steroids. In this article, the pathobiology, clinical findings, prophylaxis, and treatment of aGvHD will be summarized.
Keywords: Graft-versus-host disease, Acute, Chronic
Abstract
Akut graft‐versus‐host hastalığı (GvHD), allojeneik hematopoetik kök hücre nakli (alloHKHN) sonrasında görülebilen önemli bir komplikasyondur. Akut GvHD (aGvHD) insidansı yaklaşık %30-50 oranında görülmektedir. AlloHKHN yapılan hastalarda GvHD proflaksisi önemlidir. aGvHD gelişen hastalarda başlangıç tedavisi steroiddir. Steroide yanıtsız aGvHD’de prognoz kötüdür. Bu yazıda aGvHD patobiyolojisi, klinik bulguları, profilaksisi ve tedavisi özetlenecektir.
Acute Graft-Versus-Host Disease (GvHD)
Acute graft‐versus‐host disease (aGvHD) is seen after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1,2,3,4]. The incidence of aGvHD is around 30%-50% in HLA fully matched allo-HSCT [1]. aGvHD is also common in haploidentical and matched unrelated donor transplantation [1,2].
Pathobiology
In 1966, Billingham detailed the biology of GvHD development as a three-stage process: a) the graft/donor should contain immunologically competent cells, b) the recipient/host must have tissue antigens not expressed in donor cells, and c) the recipient should be unable to mount an immune response to effectively eliminate the donor cells [3,5]. Hence, during allo-HSCT, after conditioning the host, tissue antigens of the recipient are expressed to the donor T-cells, which leads to donor T-cell activation, expression, and enhanced immune response to the host; in other words, aGvHD occurs [1,2,3,4]. The mechanism underlying tissue damage in aGvHD is massive inflammatory cytokine secretion. Proinflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6] are seen, as well as the increased expression of the receptor repertoire (pattern recognition receptors) on antigen-presenting cells [6].
Risk Factors
The most important risk factor for aGvHD is HLA mismatch. Other risk factors include sex disparity between donor and recipient, the intensity of the conditioning regimen, increased age, multiparous female donors, ineffective GvHD prophylaxis, and the source of the graft. A study showed that aGvHD was significantly more common with total body irradiation involving a myeloablative regimen and peripheral stem cell transplantation from a fully matched related donor. In that study, the use of tacrolimus and methotrexate for GvHD prophylaxis was associated with a significant increase in GvHD risk compared to a cyclosporine-methotrexate combination [1].
Clinical Manifestations
GvHD can be acute or chronic based on the clinical presentation and its occurrence after or before 100 days after allo-HSCT. aGvHD may occur beyond this arbitrary cut-off of 100 days. The widely accepted National Institutes of Health consensus criteria have been used to classify GvHD. GvHD is divided into four subclasses: 1) Classic aGvHD: Diagnostic and distinctive features of chronic GvHD (cGvHD) are absent. Clinical features of aGvHD and present within 100 days of allo-HSCT or donor lymphocyte infusion (DLI). 2) Persistent and/or recurrent late-onset aGvHD: Features of classic aGvHD without diagnostic manifestations of cGvHD occurring beyond 100 days after allo-HSCT or DLI. 3) Classic cGvHD: Present at any time after HSCT. Diagnostic and distinctive features of cGvHD are present without aGvHD. 4) Overlap syndrome: Features of both cGvHD and aGvHD can be seen [4,7].
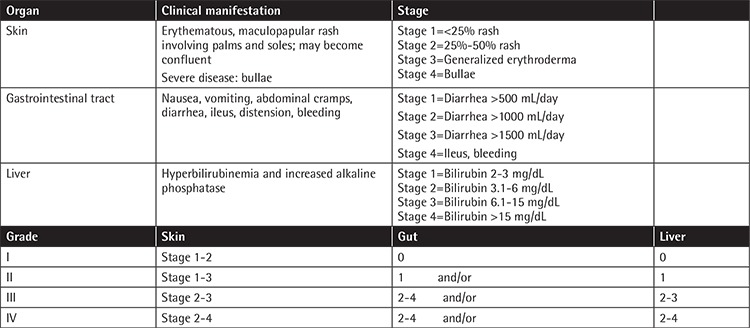
Clinically significant aGvHD may be cumbersome, affecting both morbidity and mortality [1,2,3,4]. The staging and grading of aGvHD can be seen in Table 1 [4]. The timely diagnosis of aGvHD is important. Hence, numerous novel biomarkers have been recently studied for timely diagnosis. These diagnostic and prognostic markers include systemic biomarkers (microRNAs, suppression of tumorigenicity 2), biomarkers of immune activation [TNF receptor 1, IL-7, B-cell activating factor (sBAFF)], and organ-specific biomarkers [REG3α (regenerating islet-derived 3-α], S100, TIM (T-cell immunoglobulin domain and mucin domain), cytokeratin-18, hepatocyte growth factor, and skin-derived anti-leukoproteinase, otherwise known as elafin). However, there is no specific GvHD biomarker in routine use [8].
Table 1. The clinical manifestation, staging, and grading of aGvHD.
Prevention
The most important step for the prevention of GvHD is minimizing risk factors with donor selection and a preparative regimen [2,3,4]. GvHD prophylaxis is essential for patients undergoing allo-HSCT [4]. Guidelines for GvHD prophylaxis have been proposed by the European Group for Blood and Marrow Transplantation and European LeukemiaNet [9].
The most common form of GvHD prophylaxis has been the combination of cyclosporine and a short course of methotrexate, which demonstrated improved survival compared to either drug alone. Both cyclosporine and tacrolimus decreased the proliferation of T-lymphocytes [4]. Tacrolimus plus methotrexate is better in decreasing the risk for aGvHD than the combination of cyclosporine and methotrexate, particularly in unrelated HSCT [10]. Both regimens are considered as cornerstones for most GvHD prevention strategies for patients receiving allo-HSCT [11,12]. The effects of the addition of corticosteroids to the combination of cyclosporine and a short course of methotrexate have shown conflicting results [13,14,15]. Calcineurin inhibitors and methotrexate form the main backbone of prophylactic treatment.
Treatment
The choice of initial therapy for aGvHD depends on the organs involved, the severity of symptoms, and the prophylactic regimen used. Topical steroids are the most commonly used skin-directed therapy for grade I aGvHD. Antihistamines may also be used. Bacigalupo et al. showed that steroid treatment of grade I GvHD prevents progression to grade II GvHD, but not to grade III-IV GvHD [16]. Initial therapy for grade II-IV aGvHD consists of high-dose glucocorticoid steroids. Steroid treatment is effective in approximately half of the patients; those with more severe aGvHD are less likely to respond. Treatment is usually started with the equivalent of 1-2 mg/kg/day of prednisone and then tapered after a decrease in GvHD signs or symptoms. The transplantation-related mortality rate is high in non-responders in the first 5 days of steroid use. Several agents have been added to steroids in comparative studies but no evidence supports the use of these in combination for aGvHD therapy. The best complete response rate was obtained with mycophenolate in combination with other agents (etanercept, etc.) with steroids [17]. Recently the US Food and Drug Administration approved ruxolitinib, a JAK 1/2 inhibitor, and it has been used with considerable success in the treatment of steroid-refractory aGvHD [18].
Unfortunately, there is no standard indication or timing for the initiation of second-line therapy for aGvHD. Many agents have been tested alone or in combination with corticosteroids with limited sustained efficacy [4].
There are few guidelines in the literature regarding second-line cGvHD treatment. Extracorporeal photopheresis (ECP), mycophenolate mofetil, sirolimus, everolimus, rituximab, and ibrutinib are available options. ECP is recommended in the treatment of steroid-resistant aGvHD [19] and was found to result in overall response rates of 50% to 65%.
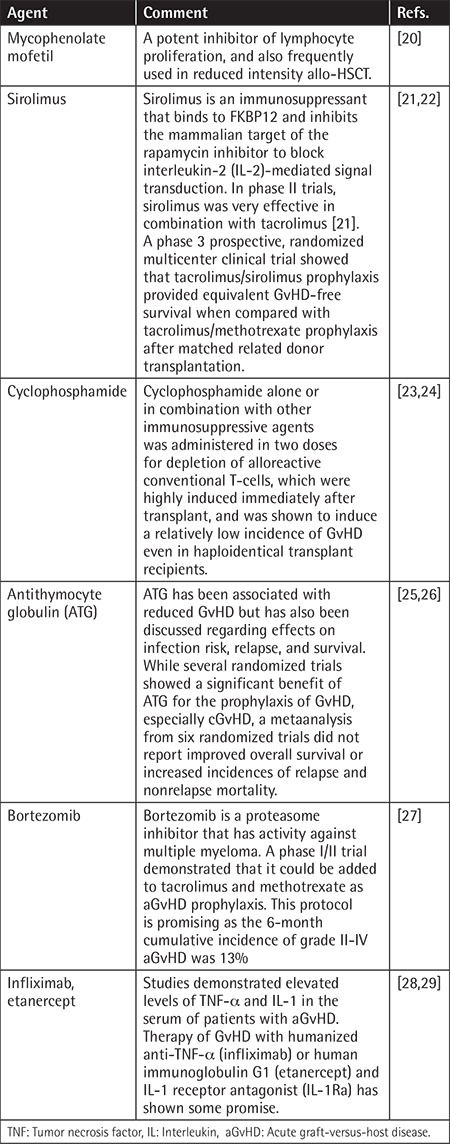
Table 2 provides a brief summary of some of the current novel second-line strategies for steroid-refractory aGvHD.
Table 2. Summary of some of the current novel second-line strategies.
Conclusion
aGvHD leads to significant morbidity and mortality. Therefore, it is crucial to prevent its development. New therapy strategies for both prevention and treatment are needed. aGvHD is a leading cause of late morbidity and mortality. The standard treatment is steroid therapy and a calcineurin inhibitor may also be added. Further treatment strategies need to be developed for the treatment of aGvHD.
Footnotes
Authorship Contributions
Concept: E.A., E.K., H.G.; Design: E.A., E.K., H.G.; Data Collection or Processing: E.A., E.K., H.G.; Analysis or Interpretation: E.A., E.K., H.G.; Literature Search: E.A., E.K., H.G., Writing: E.A., E.K., H.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ, Antin JH, Bolwell BJ, Bredeson C, Cahn JY, Cairo M, Gale RP, Gupta V, Lee SJ, Litzow M, Weisdorf DJ, Horowitz MM, Hahn T. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48:587–592. doi: 10.1038/bmt.2012.187. [DOI] [PubMed] [Google Scholar]
- 3.Chao NJ. Graft-versus-host disease: the viewpoint from the donor T cell. Biol Blood Marrow Transplant. 1997;3:1–10. [PubMed] [Google Scholar]
- 4.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 5.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- 6.Toubai T, Mathewson ND, Magenau J, Reddy P. Danger signals and graftversus- host disease: current understanding and future perspectives. Front Immunol. 2016;7:539. doi: 10.3389/fimmu.2016.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Ali AM, DiPersio JF, Schroeder MA. The role of biomarkers in the diagnosis and risk stratification of acute graft-versus-host disease: a systematic review. Biol Blood Marrow Transplant. 2016;22:1552–1564. doi: 10.1016/j.bbmt.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, Dazzi F, Dreger P, Duarte R, Finke J, Garderet L, Greinix H, Holler E, Kröger N, Lawitschka A, Mohty M, Nagler A, Passweg J, Ringdén O, Socié G, Sierra J, Sureda A, Wiktor-Jedrzejczak W, Madrigal A, Niederwieser D. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–173. doi: 10.1038/bmt.2013.107. [DOI] [PubMed] [Google Scholar]
- 10.Ram R, Storb R. Pharmacologic prophylaxis regimens for acute graft-versus-host disease: past, present and future. Leuk Lymphoma. 2013;54:1591–1601. doi: 10.3109/10428194.2012.762978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, Fay JW, Nademanee A, Antin JH, Christiansen NP, van der Jagt R, Herzig RH, Litzow MR, Wolff SN, Longo WL, Petersen FB, Karanes C, Avalos B, Storb R, Buell DN, Maher RM, Fitzsimmons WE, Wingard JR. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 12.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels P, Buell D, Fitzsimmons W, Anasetti C, Storb R, Ratanatharathorn V. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 13.Chao NJ, Schmidt GM, Niland JC, Amylon MD, Dagis AC, Long GD, Nademanee AP, Negrin RS, O’Donnell MR, Parker PM, Smith EP, Snyder DS, Stein AS, Wong RM, Blume KG, Forman SJ. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 14.Hoyt R, Ritchie DS, Roberts AW, MacGregor L, Curtis DJ, Szer J, Grigg AP. Cyclosporin, methotrexate and prednisolone for graft-versus-host disease prophylaxis in allogeneic peripheral blood progenitor cell transplants. Bone Marrow Transplant. 2008;41:651–658. doi: 10.1038/sj.bmt.1705955. [DOI] [PubMed] [Google Scholar]
- 15.Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus- host disease after haploidentical transplantation. J Clin Oncol. 2016;34:1855–1863. doi: 10.1200/JCO.2015.63.8817. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Milone G, Cupri A, Severino A, Fagioli F, Berger M, Santarone S, Chiusolo P, Sica S, Mammoliti S, Sorasio R, Massi D, Van Lint MT, Raiola AM, Gualandi F, Selleri C, Sormani MP, Signori A, Risitano A, Bonifazi F; Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Steroid treatment of acute graft-versus-host disease grade I: a randomized trial. Haematologica. 2017;102:2125–2133. doi: 10.3324/haematol.2017.171157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashidi A, DiPersio JF, Sandmaier BM, Colditz GA, Weisdorf DJ. Steroids versus steroids plus additional agent in frontline treatment of acute graft-versus-host disease: a systematic review and meta-analysis of randomized trials. Biol Blood Marrow Transplant. 2016;22:1133–1137. doi: 10.1016/j.bbmt.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, Ajib S, de Fontbrune FS, Na IK, Penter L, Holtick U, Wolf D, Schuler E, Meyer E, Apostolova P, Bertz H, Marks R, Lübbert M, Wäsch R, Scheid C, Stölzel F, Ordemann R, Bug G, Kobbe G, Negrin R, Brune M, Spyridonidis A, Schmitt-Gräff A, van der Velden W, Huls G, Mielke S, Grigoleit GU, Kuball J, Flynn R, Ihorst G, Du J, Blazar BR, Arnold R, Kröger N, Passweg J, Halter J, Socié G, Beelen D, Peschel C, Neubauer A, Finke J, Duyster J, von Bubnoff N. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredeson C, Rumble RB, Varela NP, Kuruvilla J, Kouroukis CT; Stem Cell Transplant Steering Committee. Extracorporeal photopheresis in the management of graft-versus-host disease. Curr Oncol. 2014;21:e310–325. doi: 10.3747/co.21.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurray RW, Harisdangkul V. Mycophenolate mofetil: selective T cell inhibition. Am J Med Sci. 2002;323:194–196. doi: 10.1097/00000441-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, Antin JH. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:920–926. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, Hogan WJ, Pasquini M, MacMillan ML, Hsu JW, Waller EK, Grupp S, McCarthy P, Wu J, Hu ZH, Carter SL, Horowitz MM, Antin JH. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124:1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luznik L, Bolaños-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, Goodman SN, Hess A, Levitsky HI, Ambinder RF, Jones RJ, Fuchs EJ. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, Itälä-Remes M, Blaise D, Meijer E, Koc Y, Milpied N, Schouten HC, Kroeger N, Mohty M, Nagler A. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40. doi: 10.1186/s13045-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arai Y, Jo T, Matsui H, Kondo T, Takaori-Kondo A. Efficacy of antithymocyte globulin for allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Leuk Lymphoma. 2017;58:1840–1848. doi: 10.1080/10428194.2016.1266624. [DOI] [PubMed] [Google Scholar]
- 26.Theurich S, Fischmann H, Shimabukuro-Vornhagen A, Chemnitz JM, Holtick U, Scheid C, Skoetz N, von Bergwelt-Baildon M. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst Rev. 2012;CD009159. doi: 10.1002/14651858.CD009159.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, Ho VT, Cutler C, Blazar BR, Antin JH, Soiffer RJ, Ritz J, Alyea EP 3rd. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullup H, Dickinson AM, Jackson GH, Taylor PR, Cavet J, Middleton PG. Donor interleukin 1 receptor antagonist genotype associated with acute graft-versus-host disease in human leucocyte antigen-matched sibling allogeneic transplants. Br J Haematol. 2001;113:807–813. doi: 10.1046/j.1365-2141.2001.02811.x. [DOI] [PubMed] [Google Scholar]
- 29.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, Jones D, Khaled Y, Kitko CL, Bickley D, Krijanovski O, Reddy P, Yanik G, Ferrara JL. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]


