Abstract
Kawasaki disease (KD) is an idiopathic form of acute systemic vasculitis, which clinically mimics febrile diseases. Although it has been hypothesized that immune system malfunction is associated with KD, its etiology remains unclear. The aim of the present study was to identify a KD-associated antibody. Immunoproteomic methods were used to identify KD-associated antigens that could be recognized in the sera of patients with KD. HeLa cells were used as an antigen source and KD sera were used as probe antibodies to determine the binding of the antibodies using an indirect immunofluorescence assay. Western blotting was performed to identify KD-associated antigens in HeLa whole cell lysates. Eight out of 12 serum samples obtained from patients with KD demonstrated immunoreactive bands at ~70 kDa, which was later determined to be heat shock cognate 71 kDa protein (HSP7C) by mass spectrometry. The diagnostic value of serum anti-HSP7C antibodies for KD was assessed using ELISA. Using a cut-off value of 0.267, anti-HSP7C antibodies were observed to be present in the sera of 60.00% (30/50) of patients with KD, in 21.05% (8/38) of non-KD febrile controls, and in 5.26% (2/38) of healthy controls. High serum levels of anti-HSP7C antibodies were detected in the peripheral circulation of patients with KD. To the best of our knowledge, the present study is the first to observe the high expression levels of anti-HSP7C antibodies in patients with KD. Therefore, anti-HSP7C antibodies may be used as a diagnostic marker to detect KD.
Keywords: antibodies, biomarkers, HSP7C, KD
Introduction
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an idiopathic acute vasculitis condition, which primarily occurs in infants and younger children, and clinically mimics febrile diseases (1). At present, the differential diagnosis of KD and other febrile diseases is primarily dependent on clinical symptoms, such as persistent fever for >5 days, skin rashes, purulent lymphadenitis and enlargement of lymph nodes of the neck, conjunctival hyperemia, diffuse hyperemia of the oral mucosa, palmoplantar erythema, and edema of the hands and feet (2). These clinical symptoms lack the required specificity to accurately differentiate KD from febrile diseases, often resulting in the misdiagnosis of KD. Thus, 15–25% of patients with KD are incorrectly diagnosed and receive inadequate treatment during the window of opportunity for successful treatment; consequently, the disease may progress to different degrees of coronary artery damage, and may even result in acquired heart disease (3). Notably, KD is becoming one of the primary causes of pediatric acquired heart disease (4,5). Therefore, there is an urgent requirement to identify novel differential diagnostic markers of KD.
It has previously been demonstrated that KD may be associated with certain immune factors, and the occurrence of KD may be associated with susceptibility genes and associated infections (6). Notably, these susceptibility genes may serve an important role in the onset and development of KD due to an imbalance in the immune system induced by subsequent infections (7). In the earlier phases of KD, the presence of cytokines and chemokines, which are associated with the innate immune response, are part of the defense against foreign pathogens (8), and infiltration of immunoglobulin (Ig)A antibodies and microtubule organization activate the lymphocyte immune response to specific antigens (9). In our previous study, it was demonstrated that there was a quantity of autoantibodies present in the peripheral circulation of patients with KD, including anti-phosphoglycerate kinase 1 (PGK1) antibodies in the serum of patients (10). The aim of the present study was to identify specific diagnostic markers present in the serum of patients with KD, which may be used for differentiating KD from other diseases during the earlier stages. Immunoproteomic methods were used to identify KD-associated markers and investigate their diagnostic value.
Materials and methods
Patients
The present study was approved by the Medical Ethics Committee of Beijing Children's Hospital (approval no. 2012-23), and written informed consent for treatment and clinical examinations was obtained from all guardians of the children recruited. A total of 126 subjects collected from Beijing Children's Hospital participated in the present study, including 50 patients with KD, 38 non-KD febrile controls (FCs) and 38 age-matched healthy controls (HCs). Serum samples were collected between November 2013 and June 2016 at Beijing Children's Hospital; serum samples were collected by centrifugation (8,000 × g for 5 min at 4°C) of ≥1 ml blood samples. The clinicopathological characteristics of patients with KD, and FCs and HCs are presented in Table SI, and the comparisons of clinicopathological characteristics of patients with KD, and FCs and HCs are presented in Table I. Diagnostic criteria met the fifth edition of the Research Committee of KD diagnostic criteria (11); the FC group consisted of patients with fever for >3 days that clinically mimicked KD.
Table I.
Clinicopathological variables of patients with KD, and FCs and HCs.
| P-value | |||||
|---|---|---|---|---|---|
| Variable | KD (n=50) | FC (n=38) | HC (n=38) | KD vs. FC | KD vs. HC |
| Sex (female/male) | 22/28 | 16/22 | 10/28 | 0.8589 | 0.0876 |
| Age (years)a | 2.5±2.2 | 2.7±1.9 | 3.3±1.8 | 0.8467 | 0.1734 |
| Coronary artery lesions (−/+) | 11/39 | ||||
| Neutrophil granulocyte %a | 52.6±23.3 | 47.5±20.4 | 0.2852 | ||
| C-reactive protein (mg/l)a | 55.8±53.8 | 27.7±28.4 | 0.0022 | ||
| Erythrocyte sedimentation rate (mm/h)a | 61.5±30.0 | 36.2±23.6 | <0.0001 | ||
| White blood cell (109/l)a | 11.1±4.8 | 10.8±4.4 | 0.7679 | ||
| Red blood cell (1012/l)a | 4.0±0.3 | 4.3±0.5 | 0.0074 | ||
| Hemoglobin (g/l)a | 104.8±10.2 | 110.4±14.0 | 0.0310 | ||
| Blood platelets (109/l)a | 397.9±144.4 | 386.4±218.2 | 0.7787 | ||
Data are presented as the mean ± SD. FC, non-KD febrile control; HC, healthy control; KD, Kawasaki disease.
Cell culture and indirect immunofluorescence assays
HeLa cells (National Infrastructure of Cell Line Resource) were plated on a sterile glass slide to generate HeLa cell chips, and were cultured in high-glucose DMEM (HyClone; GE Healthcare Life Sciences), supplemented with 10% FBS (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) in a 37°C incubator containing 5% CO2. The slides were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.2% Triton X-100 for 10 min at room temperature and blocked with 5% goat serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) for 2 h at 37°C. Subsequently, slides were incubated with serum (diluted 1:20 in PBS) obtained from patients in the KD, FC and HC groups, and incubated at 37°C for 1 h. For the positive control, serum samples were replaced with an anti-β-actin (ACTB) antibody (1:100; cat. no. 60008-1-Ig; Wuhan Sanying Biotechnology); for the negative control, serum samples were replaced with PBS. The HeLa cell chips were washed with PBS-0.3% Tween-20 (PBST) three times, then incubated with FITC-conjugated goat-anti human IgG antibodies (1:100; cat. no. bs-0297G-FITC; BIOSS) at 37°C for 1 h. Cell nuclei were counterstained with DAPI (Beyotime Institute of Biotechnology) for 5 min at room temperature. Images of cell chips were captured using an Olympus FV1000 confocal laser-scanning microscope (Olympus Corporation; magnification, ×40). Semi-quantitative analysis of the fluorescence intensity of the HeLa cell chips was conducted using ImageJ version 1.51n software (National Institutes of Health). To obtain the average fluorescence density of each cell, the fluorescence density of all the cells in the entire image were analyzed.
Western blotting of HeLa cell extracts
Total protein was extracted from HeLa cells using RIPA lysis buffer (Beyotime Institute of Biotechnology), containing 1 mM phenylmethylsulfonyl fluoride and 1% protease inhibitors. Total protein was quantified using the BCA Protein Assay kit (cat. no. C05-02001; BIOSS) and proteins (30 µg/lane) were separated by SDS-PAGE on a 12% gel. The separated proteins were transferred onto PVDF membranes and subsequently blocked with 5% skimmed milk for 1 h at 37°C. Serum samples from the KD, FC and HC groups (1:100) were used as the probe antibodies and were incubated with the PVDF membrane for 12 h at 4°C. After the incubation, the PVDF membranes were washed with 0.3% PBST three times (10 min/wash) and incubated with goat horseradish peroxidase (HRP)-conjugated anti-human IgG secondary antibody (1:10,000; cat. no. bs-0297G-HRP; BIOSS) at 37°C for 1 h. Protein bands were visualized using an enhanced chemiluminescence reagent (Applygen Technologies, Inc.) and recovered for mass spectrometry.
In-gel digestion, mass spectrometry and protein identification
In-gel digestion and mass spectrometry were performed as previously described (12). Briefly, gel pieces containing the target protein band were excised from the SDS-PAGE gels, washed with a mixture of 25 mM NH4HCO3 and 50% acetonitrile for 30 min at 25°C, dehydrated at 8,000 × g for 15 min at 4°C using vacuum centrifugation, then washed with 25 mM NH4HCO3 and 10 mM dithiothreitol for 2 h at 37°C. After cooling to room temperature, the gel pieces were washed with 25 mM NH4HCO3 containing 55 mM iodoacetamide for 45 min at 25°C in the dark. The target gel pieces were subsequently washed with 50% acetonitrile in 25 mM NH4HCO3 for 10 min at 25°C and dehydrated in a vacuum concentrator at 8,000 × g for 15 min at 4°C. The dehydrated gel pieces were digested with trypsin and 20 µl 0.05 M NH4HCO3 (Sigma-Aldrich; Merck KGaA) at 37°C for 12 h. Finally, peptide fragments of target proteins were sequenced using an LC-MALDI-TOF/TOF mass spectrometer (Applied Biosystems; Thermo Fisher Scientific, Inc.); commercial sequencing services were provided by the Institute of Microbiology, Chinese Academy of Sciences. Data were analyzed using the Mascot Server bioinformatics database search engine (Matrix Science, Inc.). Protein scores >56 were considered significant. Since HeLa cells are a human cell line, the search species was selected as Homo sapiens in the Mascot database. A further selection criterion was that the protein molecular weight was ~70 kDa.
ELISA
Heat shock cognate 71 kDa protein (HSP7C; cat. no. 11329-H07E; Sino Biological, Inc.) was diluted in carbonate-bicarbonate buffer (0.05 M; pH 9.6) to a final concentration of 500 ng/ml and used to coat the wells of a 96-well microplate at 4°C overnight. Subsequently, the wells were blocked with 10% goat serum at 37°C for 2 h. Serum samples (100 µl) obtained from the KD (n=50), FC (n=38) and HC (n=38) groups were diluted 1:100 in 0.1% PBST and added to the microplate as probe antibodies, and incubated at 37°C for 2 h. Wells were then rinsed five times with 0.3% PBST, and subsequently a goat HRP-conjugated anti-human IgG secondary antibody (1:10,000) was added and incubated at 37°C for 1 h. Wells were rinsed, and 100 µl tetramethylbenzidine was added and incubated for 5 min at 25°C. The reaction was terminated with 50 µl 2 M H2SO4. The optical density (OD) value of each well was detected using a microplate reader at a detection wavelength of 450 nm and a reference wavelength of 620 nm.
Statistical analysis
SPSS version 17.0 software (SPSS, Chicago, IL) and GraphPad Prism version 7.0 software (GraphPad Software Inc.) were used to perform statistical analysis. Continuous data are presented as the mean ± SD and experiments were repeated two times. To compare clinicopathological variables when the variances between the two groups were equal, a Student's t-test was used; a Cox-Cochran test was used for unequal variances; and a χ2 test was used to analyze categorical data. When three groups were compared, one-way ANOVA followed by the Sidak's multiple comparison test was conducted. To determine statistical differences between the three groups analyzed by ELISA, a Kruskal-Wallis test followed by a Dunn's post hoc multiple comparisons test was used. The receiver operating characteristic (ROC) analysis was performed using MedCalc version 9.2.0.1 software (MedCalc Software) and OD values corresponding to the highest Youden index were used as the cut-off values (13). P<0.05 was considered to indicate a statistically significant difference.
Results
Antigens in HeLa cells can be recognized by KD serum
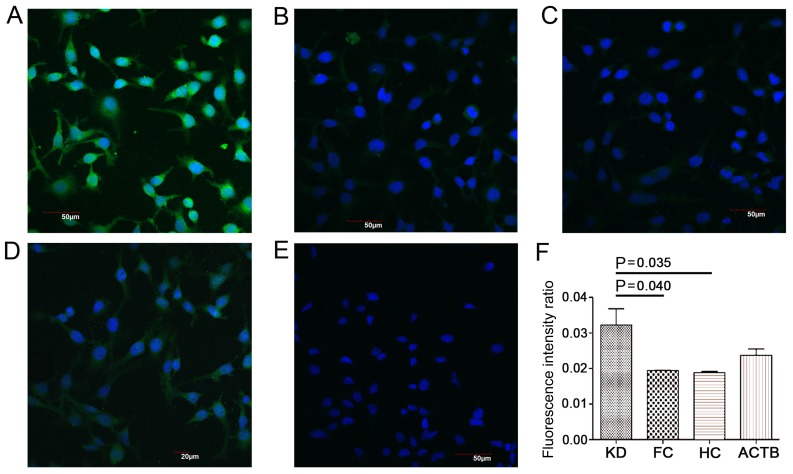
HeLa cell chips were produced and used for the indirect immunofluorescence assay, in which serum samples containing the probe antibodies were incubated with the cell chips, and anti-ACTB antibody was used as the positive control. Significantly increased fluorescence intensities were observed in the cells incubated with sera from the KD group compared with sera from the FC and HC groups (Fig. 1; P<0.05). These results suggested that certain antigens in the HeLa cells could be probed using antibodies present in the serum of patients with KD.
Figure 1.
Indirect immunofluorescence assay with HeLa cell chips. Serum samples containing the probe antibodies were incubated with the cell chips, which were visualized using an Olympus FV1000 confocal laser-scanning microscope at ×40 magnification. (A) KD serum exhibited significantly higher immunoreactivity with proteins from HeLa cells compared with (B) FC and (C) HC serum. (D) Positive control (serum samples replaced with anti-ACTB antibody) and (E) blank control (serum samples replaced with PBS) groups. (F) Semi-quantitative analysis of the fluorescence intensity of KD, FC, and HC. Positive recognition of proteins in the HeLa cells by antibodies in the serum appear green, and the nucleus is stained blue. ACTB, β-actin; FC, febrile control; HC, healthy control; KD, Kawasaki disease.
Identification of a novel antibody present in the serum of patients with KD
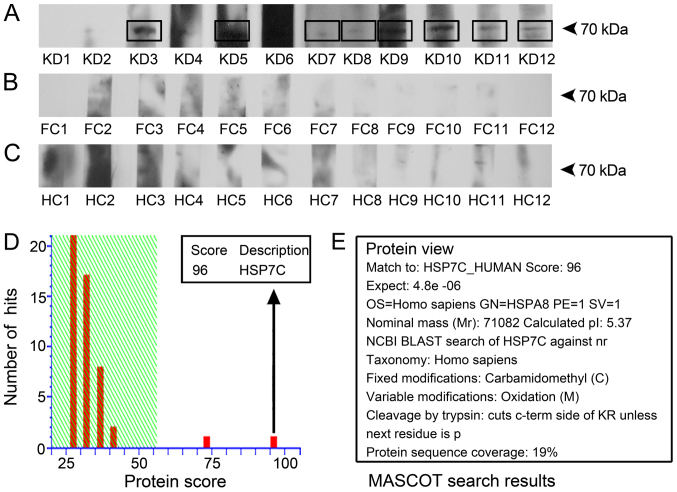
Whole extracts of HeLa cells were used as human antigen sources, and western blotting was performed to identify target antigens of antibodies present in the serum of patients with KD. As a result, eight out of 12 KD serum samples probed an antigen with a molecular weight of ~70 kDa, which was absent from cells incubated with serum from the FC and HC groups (Fig. 2A-C). The results suggested that this 70-kDa protein reacted with antibodies present in the serum samples of patients with KD. The protein band at 70 kDa was isolated from the gels and identified using mass spectrometry (Fig. 2D). The amino acid sequence of the target protein shared 19% protein sequence coverage with HSP7C, whose gene name is HSPA8 (Fig. 2D and E); and HSP7C demonstrated the highest score of 96 in the Mascot database. Therefore, further verification was performed to confirm the presence of anti-HSP7C antibodies in the serum of patients with KD.
Figure 2.
Detection and identification of HSP7C. HeLa cell lysates were used as the source of antigens, incubated with antibodies from the serum samples and analyzed using western blotting. (A) Positive ~70-kDa bands were present in 8/12 of the cell lysates treated with serum samples from patients with KD. (B and C) The 70-kDa band was not present in the (B) FC and (C) HC groups. (D and E) The amino acid sequence of the 70-kDa target band was identified as HSP7C using mass spectrometry. FC, febrile control; HC, healthy control; HSP7C, heat shock cognate 71 kDa protein; KD, Kawasaki disease.
Anti-HSP7C antibodies are present in the serum of patients with KD
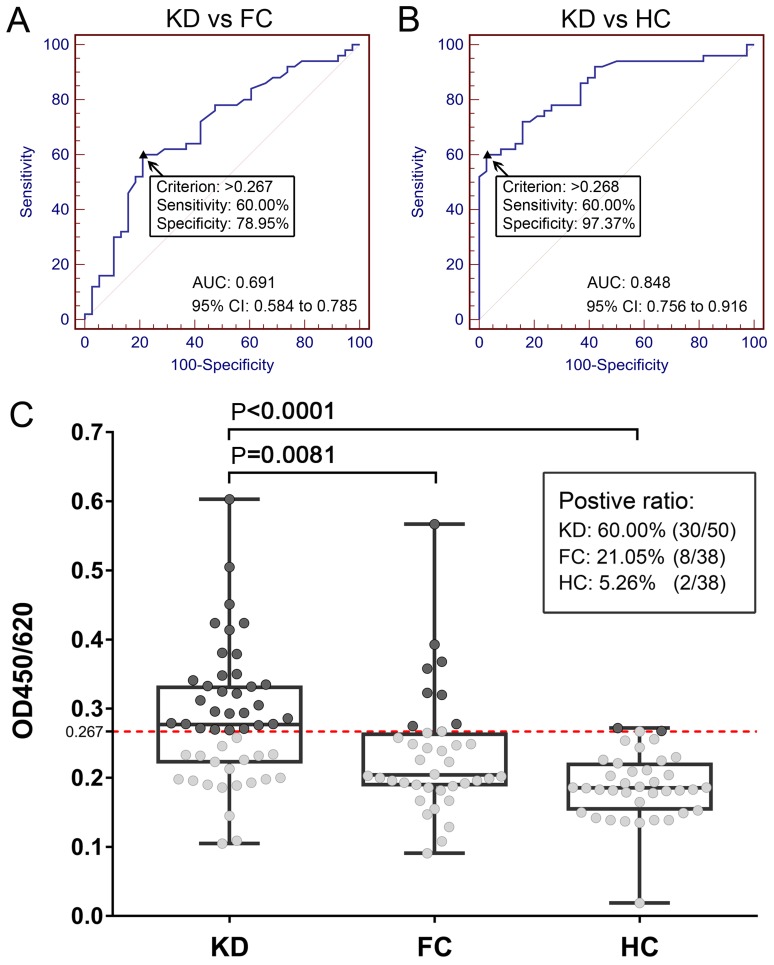
A total of 126 serum samples from the KD, FC and HC groups were used to investigate the presence of anti-HSP7C antibodies in the peripheral blood using ELISA. The OD values of the KD, FC and HC group were 0.285±0.096, 0.233±0.087 and 0.189±0.049, respectively (data not shown). The performance of the clinical differential diagnosis value of HSP7C was analyzed using a ROC curve, and the cut-off OD value was used as the corresponding maximization of the Youden index. Serum levels of anti-HSP7C antibodies could differentiate patients with KD from patients in the FC group, with an area under the curve (AUC) of 0.691 (95% CI, 0.584–0.785; P=0.0006), with 60.00% sensitivity and 78.95% specificity (Fig. 3A). When the cut-off OD value was set as 0.267, which corresponded to the highest Youden index value (KD vs. FC), the positive ratio of anti-HSP7C antibodies in the KD, FC and HC groups were 60.00 (30/50), 21.05 (8/38) and 5.26% (2/38), respectively (Fig. 3C). Serum levels of anti-HSP7C antibodies could also differentiate the KD group from the HC group with an AUC of 0.848 (95% CI, 0.756–0.916; P=0.0001), with 60.00% sensitivity and 97.37% specificity (Fig. 3B). Further analysis demonstrated that the expression levels of anti-HSP7C antibodies in the KD group were significantly increased compared with the levels in the serum from the FC and HC groups (Fig. 3C). The association between serum anti-HSP7C antibody levels and clinicopathological characteristics of patients with KD was analyzed (Table II). Patients with KD were divided into the following two subgroups: i) Lower serum expression levels of anti-HSP7C antibodies (ELISA cut-off value ≤0.267); and ii) higher expression levels of anti-HSP7C antibodies (ELISA cut-off value >0.267). As shown in Table II, there was a significant difference (P=0.0094) in the number of platelets (PLTs) between patients with lower antibody levels (461.6±128.0×109/l) compared with patients with higher antibody levels (355.5±140.8×109/l). In addition, serum PLT count has previously been reported to be elevated in patients with KD (14), and it has been suggested that serum PLT count in patients with KD is closely associated with coronary artery lesion (CAL) (15). In the present study, the blood PLT count in patients with KD with and without CAL was 426.8±111.7×109/l and 389.8±152.6×109/l, respectively; this finding was not significantly different (data not shown).
Figure 3.
Diagnostic value of serum anti-HSP7C antibodies for KD assessed using ELISA. (A) ROC curve analysis of anti-HSP7C antibody levels in KD and FC groups with an AUC value of 0.691 (95% CI, 0.584–0.785; P=0.0006). (B) ROC curve analysis of anti-HSP7C antibody levels in the serum samples from the KD and HC groups with an AUC value of 0.848. (95% CI, 0.756–0.916; P=0.0001). (C) Scatter plot demonstrating the difference in expression levels of anti-HSP7C antibodies between the KD, FC and HC groups. The cut-off value was set at 0.267 (red dotted line; corresponding to the highest Youden index of KD vs. FC; anti-HSP7C antibodies were detected in 30/50 KD samples (60.00%), 8/38 FC samples (21.05%) and 2/38 HC samples (5.26%). Levels of anti-HSP7C antibody in the KD serum samples were significantly increased compared with in the samples serum from the FC (P=0.0081) and HC (P<0.0001) groups. Box plots demonstrate the median and the interquartile range, and the complete range of the data. AUC, area under the curve; FC, febrile control; HC, healthy control; HSP7C, heat shock cognate 71 kDa protein; KD, Kawasaki disease; OD, optical density; ROC, receiver operating characteristic.
Table II.
Clinicopathological variables of patients with Kawasaki disease categorized according to the serum anti-HSP7C antibody expression status.
| A, Laboratory variables | |||
|---|---|---|---|
| Serum anti-HSP7C antibody level | |||
| Variable | Low (n=20) | High (n=30) | P-value |
| Sex (female/male) | 9/11 | 13/17 | 0.9074 |
| Age (years)a | 2.6±2.2 | 2.4±2.3 | 0.7165 |
| Neutrophil granulocyte %a | 52.5±24.6 | 52.7±22.9 | 0.9658 |
| C-reactive protein (mg/l)a | 53.0±60.5 | 57.7±49.8 | 0.7640 |
| Erythrocyte sedimentation rate (mm/h)a | 63.5±31.4 | 60.2±29.6 | 0.7118 |
| White blood cell (109/l)a | 11.2±4.4 | 11.0±5.1 | 0.9037 |
| Red blood cell (1012/l)a | 4.7±0.3 | 3.9±0.3 | 0.1268 |
| Hemoglobin (g/l)a | 107.3±12.8 | 103.1±7.7 | 0.2018 |
| Blood platelet (109/l)a | 461.6±128.0 | 355.5±140.8 | 0.0094 |
| B, Clinical variables | |||
| Serum anti-HSP7C antibody level | |||
| Variable | Low (n=20) | High (n=30) | P-value |
| Coronary artery lesions (−/+) | 13/7 | 26/4 | 0.0700 |
| Fever (−/+) | 1/19 | 0/30 | 0.2160 |
| Lymph nodes (−/+) | 2/18 | 3/27 | >0.9999 |
| Bilateral conjunctival hyperemia (−/+) | 1/19 | 1/29 | 0.7683 |
| Lips red and chapped (−/+) | 2/18 | 1/29 | 0.3308 |
| Hard swelling of hands and feet (−/+) | 5/15 | 8/22 | 0.8953 |
| Torsal polymorphic erythema (−/+) | 15/5 | 13/17 | 0.0271 |
Data are presented as the mean ± SD. FC, non-KD febrile control; HC, healthy control; high group, ELISA cut-off value >0.267; HSP7C, heat shock cognate 71 kDa protein; KD, Kawasaki disease; low group, ELISA cut-off value ≤0.267.
Discussion
KD is an idiopathic form of acute systemic vasculitis, the etiology and pathogenesis of which remain unclear. Previous studies have noted that the pathology of KD is associated with immune factors (16,17); in particular, the immune system of patients with KD appears overactive, and neutrophils, CD8+ T cells, dendritic cells and monocytes have been reported to contribute to arterial wall lesions in patients with KD. It has also been reported that the etiology of KD may be associated with bacterial infection caused by superantigens and abnormal immune responses (18). Fujieda et al (19) described peroxiredoxin 2 (PRDX2) as an immune target of KD and high serum levels of anti-PRDX2 antibodies were detected in 43.3% of patients with KD. Another study revealed that anti-4-trimethylaminobutyraldehyde dehydrogenase antibodies were also significantly increased in the serum of patients with KD (20). In our previous study, it was demonstrated that there was a marked quantity of autoantibodies present in the peripheral circulation of patients with KD; in 2018, we reported that serum anti-PGK1 antibodies were detected in 46% of serum samples obtained from patients with KD, in 13% of serum samples from the FC group and 2.6% serum samples from the HC group (10).
In the present study, a novel anti-HSP7C antibody in the serum of patients with KD was successfully identified. One interesting finding from the present study was that HeLa cells could be used as a source of antigens for immune target identification. HSP7C proteins were identified by western blotting and confirmed using mass spectrometry. The results of the present study demonstrated that the serum of patients with KD had a specific immune response to the HSP7C protein that was significantly different compared with the FC and HC groups. HSP7C is a member of a molecular chaperone family, known as heat shock proteins, which are involved in various cellular processes, including proteomic stress protection, peptide folding and transport (21–24). Studies have reported that heat shock proteins are closely associated with certain diseases (25–27); for example, HSP60 is associated with coronary heart disease (28), and HSP22 overexpression is associated with the progression and prognosis of gastric cancer (29). It has been hypothesized that the heat shock protein 65 from bacteria stimulates the heat shock protein 63 antigen of the host to participate in host immune system activation, T-cell activation, the promotion of cytokine cascade amplification reactions, the identification of host blood vessels and the induction of systemic vascular damage (30). Therefore, the production of high levels of anti-HSP7C antibodies in patients with KD may be accompanied by abnormal activity of HSP7C.
The sera of patients with KD were immunoreactive to the HSP7C protein; therefore, autoimmunity to HSP7C in patients with KD was investigated. The presence of the anti-HSP7C antibody was analyzed using ELISA, and 60% of patients with KD presented with upregulated serum levels of anti-HSP7C antibodies, whereas only 21.05 and 5.26% of patients in the FC and HC groups presented with upregulated levels of the antibody, respectively. ROC analysis was performed to determine the classification ability of serum anti-HSP7C antibody in the KD and FC groups. The AUC was 0.691, indicating that serum anti-HSP7C could differentiate the KD group from the FC group. The non-parametric analysis also demonstrated significant differences in serum levels of anti-HSP7C antibody between the KD and FC groups, with a P-value of 0.0081. On the other hand, based on the scatter plot of anti-HSP7C antibody titers, it was revealed that it was insufficient to use anti-HSP7C antibody alone as a marker to differentiate KD from FC, as some patients in the FC group also had positive antibody levels. Although the positive ratio of the KD group anti-HSP7C antibody was as high as 60.00% (30/50), whereas in the FC group, only 21.05% (8/38) individuals were positive. The onset of KD is often accompanied by a prolonged increase in body temperature. The clinical symptoms are similar to a common fever (2,5); thus, patients with fever but without KD (FC) were used as the disease control. It is reasonable to assume that the expression of the corresponding antibody may also be increased in patients with FC; however, the present study reported that the expression levels of anti-HSP7C antibody were not increased to the same degree as the patients with KD. In clinical practice, patients are generally diagnosed through a comprehensive analysis of multiple clinical indicators, such as fever for >5 days, skin rashes, purulent lymphadenitis and enlargement of lymph nodes of the neck, conjunctival hyperemia, diffuse hyperemia of the oral mucosa, palmoplantar erythema, and edema of the hands and feet (31). Therefore, the anti-HSP7C antibody may represent an additional diagnostic marker for KD, which could be used in combination with the other clinical indicators to improve the diagnosis of KD.
Clinicopathological variables were compared between patients with higher and lower expression levels of anti-HSP7C antibody. The presence of anti-HSP7C antibodies was associated with PLT counts and polymorphic erythema in patients with KD. CAL is a complication of KD, and studies have reported that the occurrence of CAL is closely associated with the duration of fever in patients, which is accompanied by a significant increase in C-reactive protein, PLT and erythrocyte sedimentation rate counts (14,32). In the present study, the PLT counts in patients with KD with and without CAL were 426.8±111.7×109/l and 389.8±152.6×109/l, respectively. The activation of platelet-derived growth factor, its receptor and the downstream pathways have previously been observed to be involved in the formation of CAL in patients with KD, whereas intravenous immunoglobulin inhibited this activation (33). In the present study, there was a significant difference in the PLT counts between patients with lower levels of HSP7C antibody (461.6±128.0×109/l) and patients with higher levels (355.5±140.8×109/l). The PLT counts in patients with KD exhibited the opposite trend compared with anti-HSP7C antibody levels.
To the best of our knowledge, the present study is the first to demonstrate a potential involvement of anti-HSP7C antibodies in patients with KD, suggesting that anti-HSP7C antibodies may be used as an auxiliary diagnostic marker for identifying and diagnosing patients with KD. However, the present study has certain limitations and investigating the presence of anti-HSP7C antibodies in patients with KD will require further studies; for example, in this study, antibody levels varied widely among patients with KD; therefore, it was hypothesized that different subtypes of KD may exist. In the future, a larger cohort and additional clinical data of patients with KD will need to be collected to further analyze the differences between patients with KD with increased levels of anti-HSP7C antibody compared with other patients with KD, in order to determine why the anti-HSP7C antibody is only upregulated in a portion of patients with KD. Furthermore, serum samples from the HC group should be collected for KD-related blood tests to help further analyze the differences in clinical indicators between patients with KD and HCs. Studies should also investigate the mechanism underlying the production of anti-HSP7C antibodies in patients with KD and determine whether patients with KD may exhibit any antibodies against antigens from exogenous pathogens, as more attention must be directed towards the antigens derived from other species related to KD.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- KD
Kawasaki disease
- FC
non-KD febrile control
- HSP7C
heat shock cognate 71 kDa protein
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81571592 and 31371203) and the Hebei Provincial Department of Science and Technology (grant nos. 17277787D and 19942410G).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HD, YW and ZD designed the study. YZ, JC and HH collected and analyzed the data. JC drafted the manuscript and YZ revised the paper. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Beijing Children's Hospital (approval no. 2012-23), and written informed consent for the treatment and clinical examinations was obtained from all guardians of the children recruited.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. (In Japanese) [PubMed] [Google Scholar]
- 2.Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.CIR.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.CIR.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 4.Benseler SM, McCrindle BW, Silverman ED, Tyrrell PN, Wong J, Yeung RS. Infections and Kawasaki disease: Implications for coronary artery outcome. Pediatrics. 2005;116:e760–e766. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin Exp Immunol. 2011;164(Suppl 1):S20–S22. doi: 10.1111/j.1365-2249.2011.04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, council on cardiovascular disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 7.Onouchi Y. Genetics of Kawasaki disease: What we know and don't know. Circ J. 2012;76:1581–1586. doi: 10.1253/circj.CJ-12-0568. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka T, Matsutani T, Iwagami S, Toyosaki-Maeda T, Yutsudo T, Tsuruta Y, Suzuki H, Uemura S, Takeuchi T, Koike M, Suzuki R. Polyclonal expansion of TCRBV2- and TCRBV6-bearing T cells in patients with Kawasaki disease. Immunology. 1999;96:465–472. doi: 10.1046/j.1365-2567.1999.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowley AH, Eckerley CA, Jäck HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159:5946–5955. [PubMed] [Google Scholar]
- 10.Cui J, Zhou Y, Hu H, Zhao L, Du Z, Du H. PGK1 as an immune target in Kawasaki disease. Clin Exp Immunol. 2018;194:371–379. doi: 10.1111/cei.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease. 2005 doi: 10.1111/j.1442-200x.2005.02149.x. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Ma H, Han G, Wu R, Zou H, Liu Y. Efficient enrichment and identification of phosphopeptides by cerium oxide using on-plate matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Rapid Commun Mass Spectrom. 2011;25:1862–1868. doi: 10.1002/rcm.5055. [DOI] [PubMed] [Google Scholar]
- 13.Rucker G, Schumacher M. Summary ROC curve based on a weighted Youden index for selecting an optimal cutpoint in meta-analysis of diagnostic accuracy. Stat Med. 2010;29:3069–3078. doi: 10.1002/sim.3937. [DOI] [PubMed] [Google Scholar]
- 14.Ruan Y, Ye B, Zhao X. Clinical characteristics of Kawasaki syndrome and the risk factors for coronary artery lesions in China. Pediatr Infect Dis J. 2013;32:e397–e402. doi: 10.1097/INF.0b013e31829dd45e. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Gao F, Huo J, Yi Q. Study on the relationship between mean platelet volume and platelet distribution width with coronary artery lesion in children with Kawasaki disease. Platelets. 2012;23:11–16. doi: 10.3109/09537104.2011.586073. [DOI] [PubMed] [Google Scholar]
- 16.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: Vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67:1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Meissner HC, Leung DY. Superantigens, conventional antigens and the etiology of Kawasaki syndrome. Pediatr Infect Dis J. 2000;19:91–94. doi: 10.1097/00006454-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fujieda M, Karasawa R, Takasugi H, Yamamoto M, Kataoka K, Yudoh K, Kato T, Ozaki S, Wakiguchi H. A novel anti-peroxiredoxin autoantibody in patients with Kawasaki disease. Microbiol Immunol. 2012;56:56–61. doi: 10.1111/j.1348-0421.2011.00393.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga A, Harita Y, Shibagaki Y, Shimizu N, Shibuya K, Ono H, Kato H, Sekine T, Sakamoto N, Igarashi T, Hattori S. Identification of 4-Trimethylaminobutyraldehyde dehydrogenase (TMABA-DH) as a candidate serum autoantibody target for Kawasaki disease. PLoS One. 2015;10:e0128189. doi: 10.1371/journal.pone.0128189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto YH, Kimura T, Momohara S, Takeuchi M, Tani T, Kimata Y, Kadokura H, Kohno K. A novel ER J-protein DNAJB12 accelerates ER-associated degradation of membrane proteins including CFTR. Cell Struct Funct. 2010;35:107–116. doi: 10.1247/csf.10023. [DOI] [PubMed] [Google Scholar]
- 22.Sopha P, Kadokura H, Yamamoto YH, Takeuchi M, Saito M, Tsuru A, Kohno K. A novel mammalian ER-located J-protein, DNAJB14, can accelerate ERAD of misfolded membrane proteins. Cell Struct Funct. 2012;37:177–187. doi: 10.1247/csf.12017. [DOI] [PubMed] [Google Scholar]
- 23.Kim CP, Hantouche C, Wong M, Matthes E, Robert R, Hanrahan JW, Shrier A, Young JC. Hsp70 and DNAJA2 limit CFTR levels through degradation. PLoS One. 2019;14:e220984. doi: 10.1371/journal.pone.0220984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K, Jiang Q, Bai X, Yang YF, Ruan MY, Cai SQ. Tetrameric assembly of K+ channels requires ER-located chaperone proteins. Mol Cell. 2017;65:52–65. doi: 10.1016/j.molcel.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: Possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171:5736–5742. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 26.Lenzi C, Palazzuoli A, Giordano N, Alegente G, Gonnelli C, Campagna MS, Santucci A, Sozzi M, Papakostas P, Rollo F, et al. H pylori infection and systemic antibodies to CagA and heat shock protein 60 in patients with coronary heart disease. World J Gastroenterol. 2006;12:7815–7820. doi: 10.3748/wjg.v12.i48.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XS, Xu Q, Fu XY, Luo WS. Heat shock protein 22 overexpression is associated with the progression and prognosis in gastric cancer. J Cancer Res Clin Oncol. 2014;140:1305–1313. doi: 10.1007/s00432-014-1698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothenbacher D, Hoffmeister A, Bode G, Miller M, Koenig W, Brenner H. Helicobacter pylori heat shock protein 60 and risk of coronary heart disease: A case control study with focus on markers of systemic inflammation and lipids. Atherosclerosis. 2001;156:193–199. doi: 10.1016/S0021-9150(00)00632-8. [DOI] [PubMed] [Google Scholar]
- 29.Li XS, Xu Q, Fu XY, Luo WS. Heat shock protein 22 overexpression is associated with the progression and prognosis in gastric cancer. J Cancer Res Clin Oncol. 2014;140:1305–1313. doi: 10.1007/s00432-014-1698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Zhai S. Development of Kawasaki disease pathogenesis. J App Clin Pediatrics. 2007;22:1037. [Google Scholar]
- 31.Guidelines for diagnosis and management of cardiovascular Sequelae in Kawasaki disease (JCS 2003) J Cardiol. 2004;43:263–283. (In Japanese) [PubMed] [Google Scholar]
- 32.Zhang W, Li Q, Zhao XD, Tang XM, Wang XG, Wang M, Wu DQ, Ou Q, Yang XQ. Clinical analysis of 942 cases of Kawasaki disease. Zhonghua Er Ke Za Zhi. 2006;44:324–328. (In Chinese) [PubMed] [Google Scholar]
- 33.Ueno K, Nomura Y, Hashiguchi T, Masuda K, Morita Y, Hazeki D, Eguchi T, Maruyama I, Kawano Y. Platelet vascular endothelial growth factor is a useful predictor for prognosis in Kawasaki syndrome. Br J Haematol. 2010;148:285–292. doi: 10.1111/j.1365-2141.2009.07922.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.