Abstract
Cytomegalovirus (CMV) reactivation has been described in adults with critical illness caused by diverse etiologies, especially severe sepsis, and observational studies have linked CMV reactivation with worse clinical outcomes in this setting. In this study, we review observational clinical data linking development of CMV reactivation with worse outcomes in patients in the intensive care unit, discuss potential biologically plausible mechanisms for a causal association, and summarize results of initial interventional trials that examined the effects of CMV prevention. These data, taken together, highlight the need for a randomized, placebo-controlled efficacy trial (1) to definitively determine whether prevention of CMV reactivation improves clinical outcomes of patients with critical illness and (2) to define the underlying mechanism(s).
Keywords: acute respiratory distress syndrome, critical illness, Cytomegalovirus, sepsis
Sepsis is a major cause of critical illness worldwide, and associated acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is associated with high morbidity and mortality, despite current intensive care, and this syndrome was estimated to cost $23 billion in 2013 in the United States [1]. Despite substantial investigation, few proven interventions other than lung protective ventilation [2] and fluid restriction strategies [3] have been developed, leaving a substantial unmet clinical need for novel effective strategies.
Multiple studies have demonstrated frequent herpesvirus, and especially cytomegalovirus (CMV), reactivation in critical illness caused by bacterial sepsis and an association of CMV reactivation with worse clinical outcomes (mortality, longer lengths of intensive care unit [ICU] and hospital stay, longer duration of mechanical ventilation, increased secondary infections) [4–8]. Although multiple viruses have been shown to secondarily reactivate in patients with critical illness caused by bacterial sepsis, the largest body of evidence is for CMV [8–10]. Intense and dysregulated inflammation and a compensatory anti-inflammatory response, both described as important immune perturbations in sepsis, have been speculated to facilitate secondary CMV reactivation in this setting [11–13], but specific mediators and pathogenesis remain undefined [14–16]. Whether development of CMV reactivation in this setting represents a marker or cause for subsequent worse outcomes is currently unknown, and this can only ultimately be definitively addressed by carefully conducted studies of CMV-specific prevention/suppression and/or therapy. There are several lines of evidence compatible with the hypothesis that secondary CMV reactivation might cause worse outcomes in this setting, including the following: consistent findings across observational studies of an association of CMV reactivation with worse outcomes despite controlling for confounders; identification of biologically plausible mechanisms (extrapolated from classically immunosuppressed populations and data from an animal model of sepsis-associated CMV reactivation with lung injury); and preliminary, hypothesis-generating analyses from a small clinical trial showing improvements in lung injury-associated clinical outcomes in those randomized to prophylactic antiviral drug [17].
For the purposes of this review, we consider sepsis to be an inflammatory response to (most commonly) bacterial infection, characterized by profound immune dysregulation and life-threatening organ dysfunction [18]. We define CMV reactivation as detection of CMV replication in blood (or other sites) among CMV-seropositive patients subsequent to a diagnosis of sepsis. Although detected CMV replication could represent CMV transmitted through blood product transfusion, reactivation of endogenous latent infection is more likely for several reasons. First, in studies of hematopoietic cell transplant (HCT) recipients who received significant numbers of blood products, transmission (ie, detectable CMV replication in blood) occurred in <5% despite profound immunosuppression [19]. Second, among observational studies of CMV replication in critical illness that included only CMV-seropositive adults, the incidence of CMV replication was significantly higher than studies that included both CMV-seronegative and -seropositive adults [5]. In addition, the relatively early timing of detectable CMV replication in critically ill patients is also more compatible with reactivation of endogenous latent infection rather than primary infection through transfusion [4, 20]. The sepsis response has been described to consist of an initial hyperinflammatory phase followed by a protracted immunosuppressive phase, both of which might potentially facilitate transcriptional activation of latent CMV (ie, reactivation) [11–13]. In this review, we focus on patients who may be functionally immune suppressed as a result of sepsis, but are not receiving exogenous immune suppression, and thus are not typically considered classically immunocompromised patients (eg, recipients of organ or HCTs).
OBSERVATIONAL STUDIES OF CYTOMEGALOVIRUS REACTIVATION IN CRITICAL ILLNESS
Multiple observational studies over the past 30 years have reported development of CMV reactivation and its association with worse clinical outcomes in adults whose initial critical illness was caused by diverse etiologies including sepsis, trauma, burns, major surgery, or acute myocardial infarction [4–7, 21]. These studies have been heterogenous (diverse patient populations, different techniques and definitions of CMV reactivation, various definitions, and analytic approaches) and have had 1 or more major limitations. However, consistent key findings across studies have included frequent detection of CMV reactivation during the course of a broad range of causes of critical illness, but most frequently in sepsis (pooled incidence ~30%), and an association of CMV reactivation with a range of adverse clinical outcomes [4–7, 21].
Specific baseline risk factors for subsequent CMV reactivation have not been consistently identified [4, 7, 22, 23], although several studies have found an increased risk associated with greater baseline severity of illness [5, 8]. However, not all studies have found this association [4, 6], and studies that adjusted for baseline severity of illness still reported an association of CMV reactivation with increased risk for worse clinical outcomes [22, 24, 25]. These data are consistent with the hypothesis that greater severity of illness may increase the risk for CMV reactivation, but that resultant CMV reactivation independently contributes additional risk for worse clinical outcomes. The lack of consistent baseline clinical predictive factors for CMV reactivation makes it more difficult to identify specific “enriched” patient populations for future interventional studies of CMV prevention. Development of novel approaches to identify patients at highest risk for subsequent CMV reactivation through CMV-specific immunity assessment [26] or other laboratory immune parameters is an important priority in the field that would allow for inclusion of more targeted populations in future interventional trials.
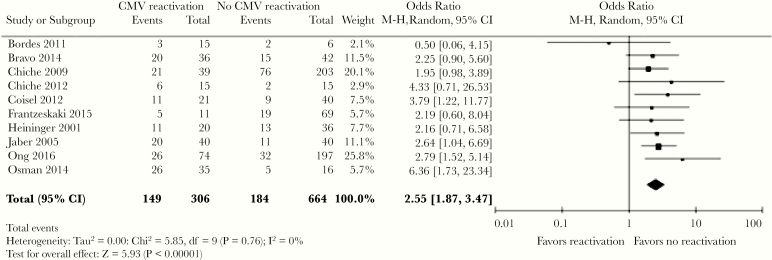
Across a number of cohort studies, CMV reactivation has consistently been associated with worse clinical outcomes that include longer duration of mechanical ventilation, higher incidence of nosocomial infections (ventilator-associated pneumonia, bacteremia, fungal infections), longer ICU and hospital length of stay (LOS), and higher overall mortality (Figure 1) [5, 6, 27]. However, many of these studies had important limitations such as small numbers of patients, study populations restricted to specific insults leading to critical illness, lack of quantitative methods for CMV detection, nonblinded assessment of endpoints, or failure to perform comprehensive statistical analyses that addressed the lack of CMV sampling in patients who died or were discharged [4].
Figure 1.
Pooled effect estimates of the association between cytomegalovirus (CMV) reactivation and intensive care unit mortality across multiple studies [6]. The odds ratio of overall mortality among patients with CMV reactivation relative to those with no reactivation was 2.02; above, improved survival was associated with no reactivation of CMV. CI, confidence interval. Reproduced from Lachance et al. Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systemic review and meta-analysis. Open Forum Infect Dis 2017;4:ofx029, by permission of Oxford University Press.
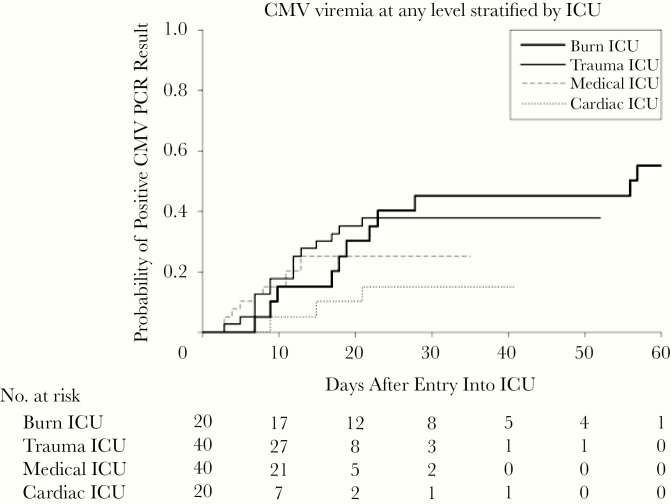
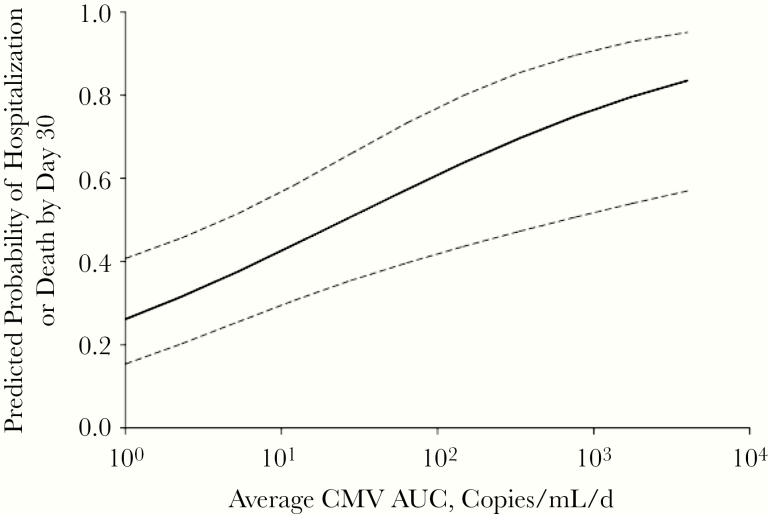
To address, at least in part, some of these limitations of prior studies, we conducted a prospective, observational study with blinded assessments of endpoints and novel statistical approaches [4] to assess the association of CMV reactivation with adverse clinical outcomes in CMV-seropositive adults with a broad range of critical illness (burns, trauma, sepsis, and cardiac comorbidities). The overall incidence of CMV reactivation at any level was 33% (39 of 120), and at >1000 copies/mL the incidence was 20% (24 of 120) (1.4 copies/IU); frequency and timing of CMV reactivation varied by cause of critical illness (Figure 2). We used novel statistical approaches that incorporated both short-term and longer-term CMV exposure (7-day moving average CMV and cumulative average CMV area under the curve [AUC], respectively) in a proportional odds model adjusted for comorbidity; both measures were significantly associated with higher odds of being hospitalized across a range of hospital LOS. More important, the cumulative average AUC was shown to be quantitatively associated with LOS after adjustment for baseline factors (Figure 3). Finally, to address, at least in part, the issue of different durations of follow-up among patients who were discharged, an analysis evaluating subsequent hospital LOS was conducted among the subset who were in the hospital and monitored for 30 days. This landmark analysis, which restricts the analysis to only those subjects who survived, were similarly monitored, and were clinically followed until the designated “landmark time,” still demonstrated an independent association of CMV reactivation with longer subsequent hospital LOS.
Figure 2.
Cumulative incidence of cytomegalovirus (CMV) viremia by intensive care unit (ICU) at 2 centers in a prospective study of CMV reactivation across multiple types of intensive care [4]. Reproduced with permission from Limaye et al. Cytomegalovirus reactivation in critically Ill immunocompetent patients. JAMA 2008;300:413–22. Copyright©(2008) American Medical Association. All rights reserved.
Figure 3.
Predicted probability of death or continued hospitalization by day 30 as a function of average cytomegalovirus (CMV) area under the curve (AUC), adjusted for unit and baseline ventilator use, from a 2-center, prospective observational study of CMV reactivation in the intensive care unit [4]. Conversion between IU/mL and copies/mL is 1.4 copies/IU. Reproduced with permission from Limaye et al. Cytomegalovirus reactivation in critically Ill immunocompetent patients. JAMA 2008;300:413–422. Copyright©(2008) American Medical Association. All rights reserved.
POTENTIAL BIOLOGICALLY PLAUSIBLE MECHANISMS FOR CYTOMEGALOVIRUS-ASSOCIATED WORSE CLINICAL OUTCOMES
The timing of CMV reactivation (ie, a lag period after the initial insult such as bacterial sepsis) and data from observational studies demonstrating an association of CMV reactivation with worse clinical outcomes are compatible with a hypothesis that critical illness (eg, bacterial sepsis) facilitates secondary CMV reactivation, and that CMV reactivation may then independently worsen the clinical course of critical illness through multiple mechanisms. There are at least 3 hypothesized general mechanisms to explain a potential causal relationship between subsequent CMV reactivation and worse clinical outcomes: direct or indirect lung injury, amplification of systemic and/or lung inflammation, and secondary immunosuppression that increases risk for secondary nosocomial infections among those who survive the initial insult. These mechanisms are not mutually exclusive, and there could be other mechanisms that contribute to overall worse clinical outcomes seen in those with secondary CMV reactivation. Consistent with this hypothesis were results of a recent randomized placebo-controlled trial done among HCT recipients. In this study, use of an antiviral drug that significantly reduced CMV reactivation improved overall survival, even though rates of CMV disease were low and similar between groups [28]. These data are consistent with a hypothesis that CMV might contribute to worse clinical outcomes through multiple mechanisms and not necessarily through a direct impact on CMV disease-associated morbidity and/or mortality.
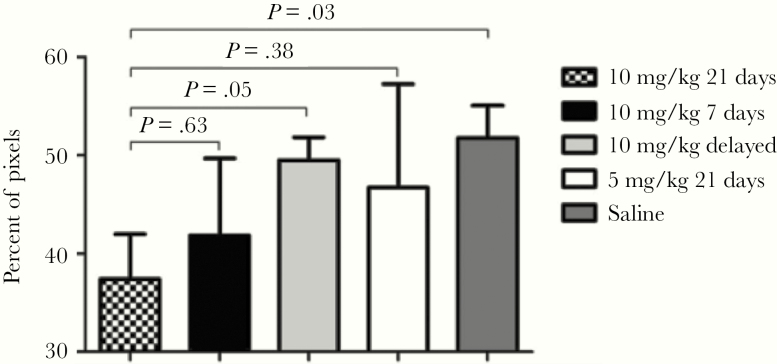
Several lines of evidence from observational data in transplant recipients, experimental human and animal models, and histologic findings support a CMV-mediated lung injury hypothesis. First, several cohort studies have reported an association between CMV reactivation and worse lung-related clinical outcomes such as longer durations of mechanical ventilation [4–6, 27]. The lungs are thought to be a major site of CMV latency and reactivation and target organ for disease [29, 30], as exemplified by the high rates of CMV pneumonia after lung transplantation from a seropositive donor into a seronegative recipient, and recognition of CMV pneumonia as a major manifestation of CMV disease after HCT [31–33]. Local lung CMV reactivation could amplify and perpetuate lung injury through direct viral toxicity [7, 34–36] or by altering the balance of matrix metalloproteinase and metalloproteinase inhibitor expression, which have been linked to lung injury repair mechanisms in patients with ALI/ARDS [37–40]. Cytomegalovirus could additionally cause lung injury by stimulating a lung-specific inflammatory response, as has been described in rodents [41, 42]. In a mouse model of sepsis, cecal ligation and puncture (CLP) leads to efficient lung reactivation of latent murine CMV (MCMV) and lung injury [27, 43]. In this model, in response to CLP-induced sepsis, MCMV+ mice had abnormally elevated levels of lung tumor necrosis factor (TNF)α, interleukin (IL)-1β, neutrophil chemokine KC, and macrophage-inflammatory protein-2 messenger ribonucleic acid and increased pulmonary injury markers compared with MCMV− mice. More important, ganciclovir treatment shortly after CLP-induced sepsis prevented MCMV reactivation, reduced abnormal TNFα expression within the lung, and reduced quantitative measures of lung injury (Figure 4). Although there are important differences between MCMV and human CMV models, studies in rodents may provide insights into sepsis-induced CMV reactivation and associated lung injury in humans.
Figure 4.
In a murine model of sepsis caused by cecal ligation and puncture, murine cytomegalovirus-seropositive mice were given ganciclovir at varying doses and evaluated for pulmonary fibrosis. Lungs were fixed, embedded, sectioned, and stained, and images were acquired and color segmented, and fibrosis was quantitated as percentage of pixels. Each bar represents mean ± standard error for 5–7 mice [27]. Reprinted from Forster et al. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antivir Res 2010;85:496–503, with permission from Elsevier.
Cytomegalovirus has also been linked to dysregulated systemic cytokine responses, including IL-6, IL-8, TNFα, and IL-10, as well as responses by CD8+ and gamma-delta T cells and macrophages [44–54]. Many of these cytokines are (1) elevated in patients with critical illness, (2) associated with an increased risk for mortality, and (3) impacted by interventions that have been shown to improve outcomes [55]. In the few trials of CMV prevention in ICU patients conducted to date, levels of IL-6, IL-8, and TNFα were not reduced by antiviral prophylaxis, despite effective CMV suppression, diminishing the likelihood of this as a potential mechanism through which CMV mediates adverse outcomes [17, 56, 57]. However, measurement of systemic (blood) cytokines might not necessarily reflect local inflammation at relevant sites such as lung, as found in animal models.
Finally, several immune-modulating mechanisms have been described for CMV, including expression of a virally encoded immune suppressive cytokine IL-10, modulation of human leukocyte antigen expression, and altered subsets of memory T cells, that could result in a net state of immune suppression and contribute to an increased risk of secondary infections among survivors of the initial injury [58–60]. This hypothesis is supported by studies in solid organ and HCT recipients that reported an increased risk for bacterial, fungal, or opportunistic infections in patients with CMV infection or disease. In addition, randomized trials of CMV prevention strategies have been associated with a decreased incidence of fungal or other viral infections [61–65]. In addition, recent data have linked CMV reactivation with an increased risk of nosocomial infections in ICU patients, including ventilator-acquired pneumonia, bacteremia, and fungal infections [6].
Thus, multiple biologically plausible mechanisms could explain a causal link between CMV reactivation and worse outcomes in the ICU setting (Figure 5). Among these, lung injury due to direct and/or indirect mechanisms seems most likely, based on available data extrapolated from other clinical settings and animal studies. These mechanisms provide support for a causal role for CMV-associated adverse clinical outcomes in ICU patients mediated through lung injury, and when combined with epidemiologic studies linking CMV reactivation with worse outcomes, they provide rationale and justification for interventional trials of CMV prevention in human patients.
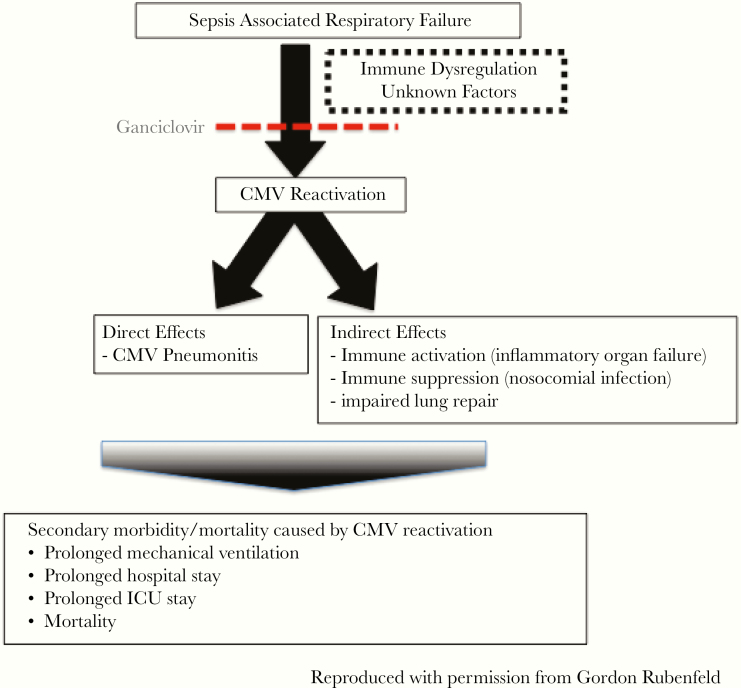
Figure 5.
Schematic of proposed mechanisms for how cytomegalovirus (CMV) reactivation in patients with sepsis could be causally associated with worse clinical outcomes. ICU, intensive care unit.
LESSONS FROM INITIAL INTERVENTIONAL STUDIES
Two relatively small interventional clinical trials that assessed the feasibility and safety of antiviral therapy to prevent CMV reactivation in seropositive adults with critical illness have been reported to date, and a third is currently in progress. Study design, inclusion criteria, and major findings of the 2 completed studies are summarized in Table 1.
Table 1.
Comparison of Two Recently Completed Interventional Trials of CMV Prevention in ICU Patients
| Study characteristics | Cowley et al [56] | Limaye et al [17] |
|---|---|---|
| Study design | Open-label randomized controlled trial | Double-blind, randomized, placebo-controlled trial |
| Major inclusion criteria | • CMV-seropositive adults • Mechanically ventilated • Non-immunosuppressed, nonneutropenic (ANC >1000) | • CMV-seropositive adults • Mechanically ventilated • Non-immunosuppressed, nonneutropenic (ANC >1000) |
| Patient population | • n = 124 • Wide array of diagnoses: 25% with trauma, 20% with an infectious diagnosis, 12% with a thromboembolic or vascular cause of critical illness | • n = 156 • Trauma (12%), severe sepsis (88%) |
| Intervention | Randomized 1:1:1 to valacyclovir, valganciclovir (450 mg daily), or no treatment | Randomized 1:1 to IV ganciclovir (5 mg/kg daily) or placebo |
| Follow-up period | 28 days or until discharge from ICU | 28 days or until discharge from hospital for majority of endpoints |
| Primary analysis | Hazard of CMV reactivation in the blood | Change in IL-6 at day 14 |
| Rate of CMV reactivation in control/placebo arm | 27% | 39% |
| Primary outcome | Significantly lower hazard of CMV reactivation in the blood in combined antiviral treatment groups vs control (HR = .1; 95% CI .04 to .5) | No difference in change of plasma IL-6 levels between day 0 and 14 |
| Other outcomes | • Higher mortality in valacyclovir group (premature discontinuation of that arm) • No differences in biomarkers between arms (IL-6, TNFα) at days 14 and 28 • No differences in renal impairment or platelet transfusions. No neutropenia or G-CSF use. | • CMV reactivation (at any level or >1000) was significantly lower in the treatment group • Key lung-specific outcomes among ganciclovir group (sepsis subset): • Higher ventilator-free days (difference in median days 3, 95% CI 0 to 4, P = .03) • Trend for fewer mechanical ventilation days (difference in median days −1, 95% CI -4 to 0, P = .06) • Higher PaO2:FiO2 ratio during the first 7 days of ventilation • No differences in mortality, ICU or hospital length of stay, or secondary bacteremia/fungemia • No differences in transfusion requirements, neutropenia, or prespecified medication-related adverse events |
Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; CMV, cytomegalovirus; G-CSF, granulocyte colony-stimulating factor; HR, hazard ratio; ICU, intensive care unit; IL, interleukin; IV, intravenous; TNF, tumor necrosis factor.
The first [56] was open-label, enrolled a broad range of patients, included a valacyclovir arm, and used ganciclovir doses lower than typically used for CMV prophylaxis in other populations. This single-center study focused on feasibility and efficacy of CMV suppression and explored other clinical outcomes without formal statistical comparisons. It is interesting to note that there was higher mortality in the patients receiving valacyclovir, and this arm was stopped prematurely.
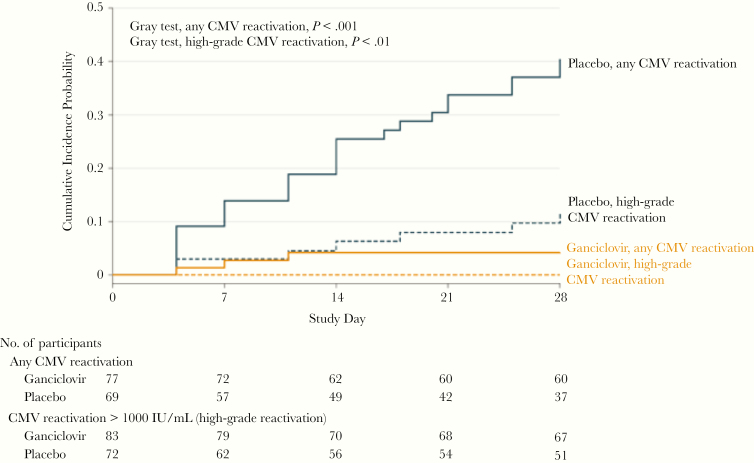
The second trial [17] was double-blind, placebo-controlled, and multicenter, and it focused on populations previously shown to have relatively high rates of CMV reactivation (sepsis, trauma). This study similarly demonstrated feasibility (ability to recruit and identify CMV-seropositive patients within a short time frame to allow for early intervention) and that the chosen ganciclovir regimen effectively suppressed CMV replication (Figure 6). The primary outcome of this trial (change in IL-6 level between day 1 and 7) was not different between groups. However, in prespecified exploratory analyses among the sepsis subset (88% of the enrolled cohorts), there were several improved outcomes in the ganciclovir arm, including the following: higher number of ventilator-free days (P = .03), shorter duration of mechanical ventilation (P = .06), and higher PaO2:FiO2 ratio among ventilated patients. In addition, a post hoc exploratory analysis among patients with sepsis who survived through day 28 showed a significantly shorter duration of mechanical ventilation in the ganciclovir arm (4 vs 6.5 median days, P = .006). These findings are compatible with CMV-mediated lung injury as potential mechanism(s) to explain worse outcomes in those with CMV reactivation, but this should be interpreted cautiously and as hypothesis-generating and needs to be confirmed in further studies. The differences between groups for these outcomes provide important background data for design of future larger trials.
Figure 6.
Decreased cytomegalovirus (CMV) reactivation after ganciclovir/valganciclovir in a phase II, randomized, placebo-controlled, clinical trial [17]. Reproduced with permission from Limaye et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA 2017;318:731–740. Copyright©(2017) American Medical Association. All rights reserved.
A third trial evaluating the efficacy of ganciclovir or acyclovir as preemptive therapy for nonexogenously immunosuppressed patients who reactivate CMV or herpes simplex virus (ClinicalTrials.gov NCT02152358) is currently ongoing.
These initial small trials have demonstrated feasibility of CMV prevention studies in the ICU setting, including serologic screening and antiviral treatment initiation in mechanically ventilated CMV-seropositive adults, and informed patient selection in this setting. Ganciclovir/valganciclovir, dosed as per the chosen regimens, appeared to be safe and effective in suppressing CMV replication in blood and other sites (lung). The results of both trials highlighted the limitations of using biomarker endpoints, but they identify alternative, clinically relevant endpoints for use in future efficacy trials of CMV prevention in the ICU setting.
THE ROLE OF CYTOMEGALOVIRUS IN CRITICAL ILLNESS
A large body of evidence from observational studies has demonstrated frequent CMV reactivation and an independent link between CMV reactivation and worse clinical outcomes in critically ill CMV-seropositive adults. Data from in vitro studies, mouse models, and classically immunosuppressed populations have identified potential biologically plausible mechanisms to explain a causal link between CMV and worse outcomes. Initial small interventional trials have demonstrated feasibility, adequate CMV suppression, and preliminary safety of selected antiviral regimens, and they have identified lung injury-related clinical endpoints for future efficacy studies. Given the major unmet clinical need, these data provide strong rationale for a randomized, placebo-controlled, efficacy trial powered to definitively determine whether prevention of CMV reactivation in critically ill adults can improve lung-associated or other clinically relevant outcomes in ICU patients.
Notes
Acknowledgments. We gratefully acknowledge Robert Rakita for helpful comments regarding the manuscript.
Financial support. This work was funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (T32 AI118690-02 [to H. I.] and HHSN272201600016C, HHSN272201100041C, and HHSN272201600019C [to A. P. L.]).
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. A. P. L. reports support in the form of research funding and as site investigator and consultancy for Merck, as site investigator for Astellas, as consultant and site investigator for Gilead, as site investigator for Roche Diagnostics, and as site investigator for Oxford Immunotech. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Torio CM, Moore BJ.. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. 2016 May. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK368492/. [PubMed] [Google Scholar]
- 2. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Eng J Med 2000; 342:1301–8. [DOI] [PubMed] [Google Scholar]
- 3. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. New Eng J Med 2006; 354:2564–75. [DOI] [PubMed] [Google Scholar]
- 4. Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008; 300:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med 2009; 37:2350–8. [DOI] [PubMed] [Google Scholar]
- 6. Lachance P, Chen J, Featherstone R, Sligl WI. Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systematic review and meta-analysis. Open Forum Infect Dis 2017; 4:ofx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care 2009; 13:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One 2014; 9:e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ong DS, Bonten MJ, Spitoni C, et al. ; Molecular Diagnosis and Risk Stratification of Sepsis Consortium Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis 2017; 64:1204–10. [DOI] [PubMed] [Google Scholar]
- 10. Lopez Roa P, Hill JA, Kirby KA, et al. Coreactivation of human herpesvirus 6 and cytomegalovirus is associated with worse clinical outcome in critically ill adults. Crit Care Med 2015; 43:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev 2016; 274:330–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danahy DB, Strother RK, Badovinac VP, Griffith TS. Clinical and experimental sepsis impairs CD8 T-cell-mediated immunity. Crit Rev Immunol 2016; 36:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J Intensive Care 2014; 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377:562–72. [DOI] [PubMed] [Google Scholar]
- 16. Papazian L, Hraiech S, Lehingue S, et al. Cytomegalovirus reactivation in ICU patients. Intensive Care Med 2016; 42:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Limaye AP, Stapleton RD, Peng L, et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA 2017; 318:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowden RA, Slichter SJ, Sayers M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood 1995; 86:3598–603. [PubMed] [Google Scholar]
- 20. Falagas ME, Snydman DR, Ruthazer R, Griffith J, Werner BG. Primary cytomegalovirus infection in liver transplant recipients: comparison of infections transmitted via donor organs and via transfusions. Boston Center for Liver Transplantation CMVIG Study Group. Clin Infect Dis 1996; 23:292–7. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Huang Y, Xu Z, et al. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: a systematic review and meta-analysis. BMC Infect Dis 2018; 18:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cook CH, Martin LC, Yenchar JK, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med 2003; 31:1923–9. [DOI] [PubMed] [Google Scholar]
- 23. Chiche L, Forel JM, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med 2009; 37:1850–7. [DOI] [PubMed] [Google Scholar]
- 24. Heininger A, Haeberle H, Fischer I, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 2011; 15:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ong DS, Spitoni C, Klein Klouwenberg PM, et al. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Med 2016; 42:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 2015; 61:1228–34. [DOI] [PubMed] [Google Scholar]
- 27. Forster MR, Trgovcich J, Zimmerman P, et al. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res 2010; 85:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic-cell transplantation. Clin Infect Dis 2019; pii:ciz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanquer J, Chilet M, Benet I, et al. Immunological insights into the pathogenesis of active CMV infection in non-immunosuppressed critically ill patients. J Med Virol 2011; 83:1966–71. [DOI] [PubMed] [Google Scholar]
- 30. Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 1993; 67:5360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan AJ, Dummer JS, Paradis IL, et al. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant 1991; 10:638–44; discussion 45–6. [PubMed] [Google Scholar]
- 32. Ettinger NA, Bailey TC, Trulock EP, et al. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Washington University Lung Transplant Group. Am Rev Respir Dis 1993; 147:1017–23. [DOI] [PubMed] [Google Scholar]
- 33. Travi G, Pergam SA. Cytomegalovirus pneumonia in hematopoietic stem cell recipients. J Intensive Care Med 2014; 29:200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 2007; 35:755–62. [DOI] [PubMed] [Google Scholar]
- 35. Papazian L, Thomas P, Bregeon F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology 1998; 88:935–44. [DOI] [PubMed] [Google Scholar]
- 36. Papazian L, Fraisse A, Garbe L, et al. Cytomegalovirus. an unexpected cause of ventilator-associated pneumonia. Anesthesiology 1996; 84:280–7. [DOI] [PubMed] [Google Scholar]
- 37. Esteso G, Luzon E, Sarmiento E, et al. Altered microRNA expression after infection with human cytomegalovirus leads to TIMP3 downregulation and increased shedding of metalloprotease substrates, including MICA. J Immunol 2014; 193:1344–52. [DOI] [PubMed] [Google Scholar]
- 38. Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development, repair, and destruction of the lungs. Prog Mol Biol Transl Sci 2017; 148:1–29. [DOI] [PubMed] [Google Scholar]
- 39. Qiu Z, Hu J, Van den Steen PE, Opdenakker G. Targeting matrix metalloproteinases in acute inflammatory shock syndromes. Comb Chem High Throughput Screen 2012; 15:555–70. [DOI] [PubMed] [Google Scholar]
- 40. Strååt K, de Klark R, Gredmark-Russ S, Eriksson P, Söderberg-Nauclér C. Infection with human cytomegalovirus alters the MMP-9/TIMP-1 balance in human macrophages. J Virol 2009; 83:830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steinhoff G, You XM, Steinmüller C, et al. Induction of endothelial adhesion molecules by rat cytomegalovirus in allogeneic lung transplantation in the rat. Scand J Infect Dis Suppl 1995; 99:58–60. [PubMed] [Google Scholar]
- 42. Senchenkov E, Khoretonenko MV, Leskov IL, Ostanin DV, Stokes KY. P-selectin mediates the microvascular dysfunction associated with persistent cytomegalovirus infection in normocholesterolemic and hypercholesterolemic mice. Microcirculation 2011; 18:452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med 2006; 34:842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015; 160:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boeckh M, Nichols WG. Immunosuppressive effects of beta-herpesviruses. Herpes 2003; 10:12–6. [PubMed] [Google Scholar]
- 46. Carlquist JF, Edelman L, Bennion DW, Anderson JL. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-kappaB. J Infect Dis 1999; 179:1094–100. [DOI] [PubMed] [Google Scholar]
- 47. Compton T, Kurt-Jones EA, Boehme KW, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 2003; 77:4588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murayama T, Ohara Y, Obuchi M, et al. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol 1997; 71:5692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Craigen JL, Yong KL, Jordan NJ, et al. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology 1997; 92:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iwamoto GK, Konicek SA. Cytomegalovirus immediate early genes upregulate interleukin-6 gene expression. J Investig Med 1997; 45:175–82. [PubMed] [Google Scholar]
- 51. Tong CY, Bakran A, Williams H, Cuevas LE, Peiris JS, Hart CA. Association of tumour necrosis factor alpha and interleukin 6 levels with cytomegalovirus DNA detection and disease after renal transplantation. J Med Virol 2001; 64:29–34. [DOI] [PubMed] [Google Scholar]
- 52. Humar A, St Louis P, Mazzulli T, et al. Elevated serum cytokines are associated with cytomegalovirus infection and disease in bone marrow transplant recipients. J Infect Dis 1999; 179:484–8. [DOI] [PubMed] [Google Scholar]
- 53. Humbert M, Delattre RM, Fattal S, et al. In situ production of interleukin-6 within human lung allografts displaying rejection or cytomegalovirus pneumonia. Transplantation 1993; 56:623–7. [DOI] [PubMed] [Google Scholar]
- 54. Humbert M, Devergne O, Cerrina J, et al. Activation of macrophages and cytotoxic cells during cytomegalovirus pneumonia complicating lung transplantations. Am Rev Respir Dis 1992; 145:1178–84. [DOI] [PubMed] [Google Scholar]
- 55. Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33:1–6; discussion 230–2. [DOI] [PubMed] [Google Scholar]
- 56. Cowley NJ, Owen A, Shiels SC, et al. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial. JAMA Intern Med 2017; 177:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van de Groep K, Nierkens S, Cremer OL, et al. Effect of cytomegalovirus reactivation on the time course of systemic host response biomarkers in previously immunocompetent critically ill patients with sepsis: a matched cohort study. Crit Care 2018; 22:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol 2016; 7:2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 2011; 121:1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol 2007; 81:7759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med 1993; 118:173–8. [DOI] [PubMed] [Google Scholar]
- 62. Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 1997; 90:2502–8. [PubMed] [Google Scholar]
- 63. Snydman DR, Werner BG, Dougherty NN, et al. ; Boston Center for Liver Transplantation CMVIG Study Group Cytomegalovirus immune globulin prophylaxis in liver transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1993; 119:984–91. [DOI] [PubMed] [Google Scholar]
- 64. Lowance D, Neumayer HH, Legendre CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med 1999; 340:1462–70. [DOI] [PubMed] [Google Scholar]
- 65. Snydman DR, Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 1987; 317:1049–54. [DOI] [PubMed] [Google Scholar]