Abstract
The natural history of cytomegalovirus (CMV) infection is complex. Individuals may experience primary infection, reactivation of latent infection, or reinfection with a new strain despite natural immunity. The ability of this virus to continue to replicate despite substantial immune responses is attributable to the many immune evasion genes encoded within its genome. Given this complex natural history and immunology, the design of clinical trials of CMV vaccines may require components not usually found in trials of vaccines designed to protect against viruses that cause only acute infections.
In this article, we focus on specific aspects of clinical trial design that could be adopted to address the complexities of CMV infections. We consider women of childbearing age, toddlers, recipients of solid organ transplantation, and stem cell transplant patients, emphasizing the parallels between women and solid organ transplantation that could allow vaccines to be developed in parallel in both these patient groups. We emphasize the potential for studies of passive immunity to inform the selection of immunogens as candidates for active immunization and vice versa. We also illustrate how application of whole-genomic sequencing could document whether vaccines protect against reactivation or reinfection of CMV or both.
Keywords: ante-natal, cytomegalovirus, immune responses, transplant, vaccination
The pressing need for a cytomegalovirus (CMV) vaccine to be used for universal immunization is discussed elsewhere in this supplement. In this chapter, we will build upon extensive knowledge of CMV natural history and the clinical trials that have been performed so far to suggest trial endpoints and study designs for the future. We will emphasize the similarities between solid organ transplants and women of childbearing age, before considering immunization of toddlers (defined as children 12–36 months of age). Finally, we will consider stem cell transplant patients as a distinct population.
SOLID ORGAN TRANSPLANT PATIENTS
Natural history studies show that CMV appears in the blood (viremia) of these patients in the first weeks after transplant, then it rises to the high levels necessary to cause serious end-organ disease in the lungs, liver, gastrointestinal tract, or retina [1, 2]. This adverse outcome can be routinely prevented by giving ganciclovir (or its prodrug valganciclovir) in 1 of 2 ways. For the strategy of prophylaxis, patients are given the drug for a fixed period of time, with clinical trials supporting a duration of either 100 days or 200 days posttransplant [3, 4]. This strategy is effective while the drug is being taken, but some patients return with late-onset disease once prophylaxis is stopped [5, 6]. For the strategy of preemptive therapy, no patient is given drug prophylactically, but they are all followed with regular blood tests to detect viremia [7]. Those who have a viral load above a defined threshold are then given ganciclovir or valganciclovir for a duration that is personalized for each patient by stopping therapy once 2 consecutive blood samples no longer have CMV deoxyribonucleic acid (DNA) detectable by polymerase chain reaction [7, 8].
Both prophylaxis and preemptive therapy are clinically effective strategies that are recommended in clinical guidelines for managing solid organ transplant patients, but they have different characteristics [9]. One advantage of preemptive therapy is that it defines which patients have active infection with CMV and reveals significant differences in parameters of viral load between recipients (R) depending upon the baseline immunoglobulin (Ig)G results in the donor (D). Specifically, D+R− patients may experience primary infection, D+R+ patients are at risk of both reactivation of latent virus and reinfection with a new strain, whereas D−R+ patients are at risk of reactivation only. The viral load parameters include the proportion of patients with viremia, proportion of patients with high-level viremia sufficient to trigger treatment, duration of viremia, duration of treatment, and peak viral load [7]. These viral load parameters are significantly different between the 3 groups such that high viral loads are found more frequently in D+R− patients. However, some patients in the D+R+ and D−R+ groups are at risk of developing high viral loads leading to end-organ disease. The type of end-organ disease experienced by each group is not different; only the risk of developing disease differs. These viral load parameters are sufficiently robust to be used to define the primary endpoint in phase 2 and phase 3 randomized clinical trials of antiviral drugs [10]. A second advantage of using preemptive therapy is that it allows experimental CMV vaccines to be compared with placebo for their ability to alter these posttransplant measures of viral load using a pharmacodynamic study design [11].
Three phase 2 studies of CMV vaccines have now been conducted in solid organ transplant patients. Plotkin et al [12] gave the live-attenuated Towne vaccine strain to seronegative recipients and observed that, when they proceeded to renal transplant, the severity of CMV end-organ disease was significantly reduced, although the incidence was not. This study was conducted before measures of viral load became available, but because a high viral load is required as a prerequisite for CMV end-organ disease, it is very likely that this vaccine reduced viremia [1, 13–15]. Griffiths et al [11] gave a vaccine consisting of glycoprotein B (gB) plus MF59 adjuvant to seronegative and seropositive candidates awaiting transplantation of a kidney or a liver. The vaccine induced high levels of antibody against gB in seronegative patients and boosted the gB titers of those who were already seropositive. When the patients proceeded to transplant, the parameters of viral load were reduced in those who received vaccine compared with those who received placebo, with the most likely explanation being that the effective inoculum from donor to recipient had been reduced [11]. Note that this study design has the potential to differentiate reactivation from reinfection by collecting pretransplant samples from seropositive recipients and (where available) donors for comparison with posttransplant strains by whole-genome sequencing. The correlate of protection against CMV viremia was the titer of antibodies that individuals made against gB [11]. Laboratory studies of the immune correlates of protection conferred by this vaccine are discussed in detail in the chapter by Nelson et al in this supplement. Vincenti et al [16] studied a DNA plasmid vaccine composed of 2 immunogens, pp65 (a major target of cell-mediated immunity) and gB. They did not administer vaccine pretransplant, but they gave the first dose starting at day 30 posttransplant. There was no evidence that the vaccine was immunogenic, and it did not reduce viral load parameters [16]. For future studies (Table 1), we recommend that vaccine should only be given pretransplant for 2 reasons: first, it avoids the effect of immunosuppressive drugs; and second, because natural history studies show that infection is transmitted within hours of transplantation so that 50% of D+R− patients have already developed viremia by day 30 [7, 17].
Table 1.
Issues to Consider: Vaccination of Solid Organ Transplant Patients
| Vaccine or placebo should be given pretransplant |
| No immunusuppressive drugs at that time |
| The trial cannot control who gets transplanted or when |
| Some never transplanted, so increase sample size |
| Use a pharmacodynamic readout posttransplant |
| Reduction in parameters of viral load |
| Seek an immune correlate of protection |
| Compare passive administration of correlate with placebo |
| Monoclonal antibody or T cells |
Once the correlate of protection against gB was defined as the antibody titer, P.G. proposed to Genentech that randomized controlled trials should be conducted using monoclonal antibodies specific for this protein as a way of identifying preparations with potential clinical utility and defining mechanisms of action such as neutralization or antibody-dependent cellular cytotoxicity [11]. Genentech decided to organize a multicenter, multinational, phase 2 study to compare placebo with a combination of 2 monoclonal antibodies, one reactive with glycoprotein H and another reactive with UL131, a component of the pentameric complex that is necessary and sufficient for CMV to enter endothelial and epithelial cells [18]. A total of 120 seronegative recipients destined to receive a kidney from a seropositive donor were recruited. Compared with those given placebo, significantly fewer of the patients who received the combination of monoclonal antibodies had viremia posttransplant [18]. This result confirms the proposal that humoral immunity is able to reduce transmission of CMV from donor to recipient and identifies antibody against surface proteins of CMV as a mechanistic correlate of protection [11, 19]. The result also defines quantitative and qualitative aspects of humoral immunity that should be present at the time of inoculation of virus to interrupt transmission. This information could now be adopted as a target for a series of phase 1 studies to determine whether immunogens can be prepared that are able to induce antibodies with comparable potency. If so, these immunogens could then be compared with placebo given pretransplant to determine whether posttransplant parameters of viral load can be reduced. An iterative series of paired studies with passive and active immunization can be envisaged, leading ultimately to preparations of vaccine/adjuvant and monoclonal antibodies with clinical efficacy. It is recognized that such a series of studies may require collaboration between different pharmaceutical companies.
WOMEN OF CHILDBEARING AGE
Natural history studies show that approximately one third of women with primary CMV infection transmit CMV across the placenta [20]. As discussed in the chapter by Nelson et al in this supplement, it has been difficult to identify laboratory measures of adaptive immunity that are able to reliably distinguish transmitting mothers from nontransmitters [21]. Therefore, the possibility exists that it is the difficult-to-measure innate immunity, acting in concert with adaptive immunity, that is responsible for protecting the fetus and that this protection can be overcome by a large inoculum of CMV. It follows that a vaccine given to women that is unable to completely protect against acquisition of primary infection in the mother may nevertheless be able to contribute to reduced transmission of virus in utero once that woman becomes pregnant and is exposed to CMV. The implication for clinical trial design is that a smaller sample size may be sufficient to demonstrate reduction in congenital CMV infection than one based on the assumption that efficacy is due entirely to prevention of maternal primary infection. We suggest that these uncertainties could be addressed by designing an adaptive phase 2 plus phase 3 study with a large overall sample size and a Data Safety Monitoring Board given clear rules for when to stop recruitment due to apparent futility and when to move from phase 2 to phase 3 (Table 2). During such a study, baseline samples could be collected from women and their children and partners to allow whole-genome sequencing to be used to prove that a vaccine provided protection against congenital CMV after maternal acquisition from both sources [22] (Figure 1).
Table 2.
Issues to Consider: Vaccination of Women of Childbearing Age
| Screen for CMV IgG antibodies |
| Recruit seronegatives in contact with children, contemplating pregnancy, including postpartum |
| Randomize to receive vaccine or placebo |
| Primary endpoint for phase 2: seroconversion |
| If phase 2 results encouraging: |
| Adaptive design to phase 3 |
| Primary endpoint for phase 3: congenital infection |
| The trial patient information sheet may empower women to avoid exposures to CMV |
| Increased sample size needed for phase 2 |
| The effect of vaccination on intrauterine transmission may be more potent than expected |
| Decreased sample size needed for phase 3 |
| Plan large sample size for phase 2 + 3 and deploy DSMB with clear rules for stopping and switching phases |
| Recruit the seropositives and randomize them to vaccine/placebo also |
Abbreviations: CMV, cytomegalovirus; DSMB, Data Safety Monitoring Board; IgG, immunoglobulin G.
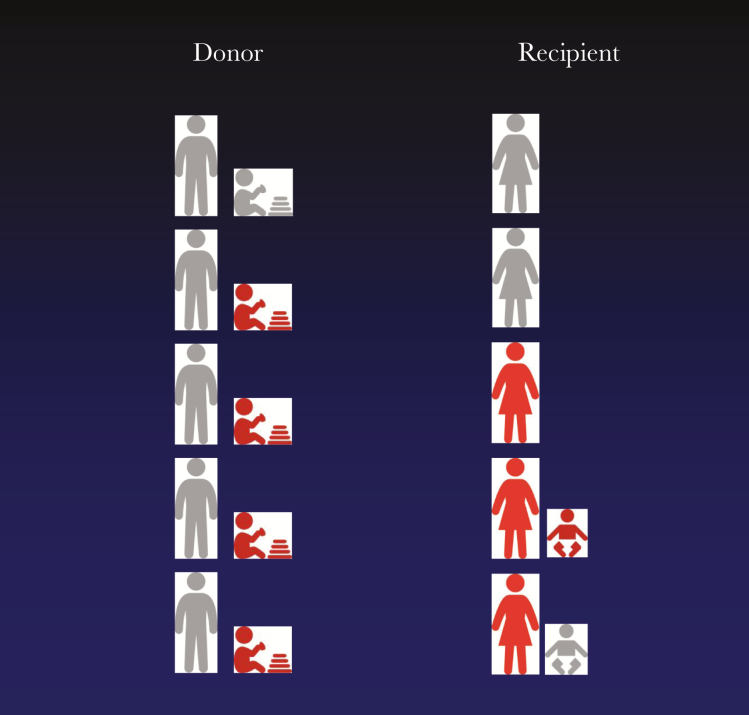
Figure 1.
Common sources of cytomegalovirus (CMV) for seronegative women and implications for sample collection and clinical trial design. By analogy with transplant patients at risk of CMV infection, family members are considered as donors of virus for the female recipient. Gray represents uninfected and red represents infected. Collection and storage of serial samples from all family members is envisaged as part of clinical trial design. This would allow the strain of CMV causing congenital infection to be formally linked with the strain in the donor.
Two relevant randomized controlled trials have been published to date. Pass et al [23] conducted a phase 2 double-blind, randomized, placebo-controlled study of gB/MF59 vaccine in seronegative postpartum women. The vaccine provided approximately 50% protection against acquiring primary infection, which approaches the value of 50%–60% calculated to be required to control CMV transmission through herd immunity [24, 25]. However, the vaccine efficacy appeared to wane with time [23]. The same vaccine gave approximately 43% protection against primary infection when given to teenagers [26]. Laboratory studies of the immune correlates of protection conferred by this vaccine on adult women are discussed in detail in the chapter by Nelson et al in this supplement and show similarities between those found in solid organ transplant patients given the same vaccine [27, 28].
There are several issues to consider when planning a phase 3 study to demonstrate protection against primary infection of women and against congenital CMV infection (Table 2). First, most women are unaware of CMV and how it is transmitted [29]. No double-blind, randomized, placebo-controlled study has been conducted to show that women can take practical actions to reduce their risk of acquiring this infection during pregnancy, but there is theoretical and practical support for this possibility [30]. This means that an information sheet given to seronegative women contemplating entry into a trial evaluating a CMV vaccine may empower them to avoid exposures to CMV, thereby decreasing the rate of primary infection and increasing the sample size required to show that the vaccine is superior to placebo.
A placebo-controlled phase 3 trial of passive immunity has also been conducted (see Revello et al [31]) in pregnant women with proven primary CMV infection early in pregnancy. The women were randomized to receive infusions of Ig monthly, and the primary endpoint was congenital CMV infection. In contrast to a previous uncontrolled study using the same preparation and dosage, this randomized, controlled trial showed no significant difference between the 2 groups despite a slightly lower absolute rate of transmission in the intervention group [31]. It should be noted that there was a trend in favor of adverse pregnancy outcomes, particularly prematurity, among the recipients of Ig [31]. It should also be noted that careful histologic examination of placentas from this study did not provide any evidence that Ig reduced the damage caused by CMV to that organ [32].
Although this study provides no evidence for the use of this preparation, the experience gained shows that pregnant women with primary infection can be diagnosed in real time and recruited into studies of potential intervention [31]. A larger study with more power to detect a difference in transmission rates recently completed enrollment and results are pending (Clinicaltrials.gov). An obvious next candidate to be evaluated is the combination of monoclonal antibodies mentioned above that has significantly reduced transmission of CMV from kidney donor to recipient [18]. In order for these antibodies to transfer success from one patient group to another, it is not necessary for every step in the process to be identical. For example, as long as 1 step is shared between transmission of primary infection from organ donor to recipient and between maternal circulation to fetal circulation, then both patient populations could potentially benefit from the same pharmaceutical preparation. In practical terms, the demonstration of safety and efficacy in one human population would address the hesitancy created by requirements to treat pregnant women as a vulnerable population. As discussed above for solid organ transplantation, clinical trials of passive immunization could proceed in tandem with those of active immunization of mothers with each informing the other.
All of these studies have addressed primary CMV infection in seronegative women as a tractable target for clinical trial design. However, it should be recognized recent data suggests that most cases of congenital infection globally are born to women with nonprimary infection [33]. We suggest that future vaccines should also be evaluated in the seropositive women identified while screening a population to identify seronegative women at risk of primary infection. If a vaccine provided evidence of safety in a placebo-controlled study of seropositive women, it would remove the need for future serologic testing once the vaccine was licensed. If the study showed reduction in congenital CMV, then that would be a bonus and investigation of the potential immune correlates of protection would be informative. Indeed, by collecting baseline samples from women and their children and partners, the study could deploy whole-genome sequencing to determine whether a vaccine protected against subsequent congenital CMV caused by both reactivation and reinfection (Figure 2).
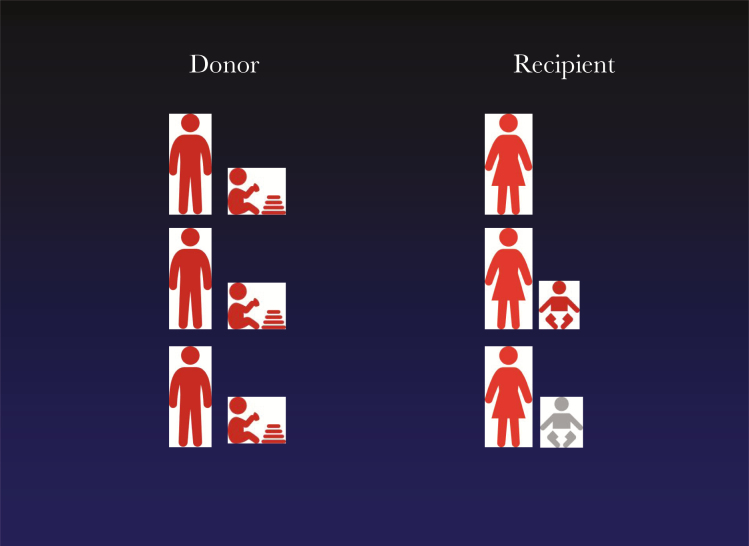
Figure 2.
Common sources of cytomegalovirus (CMV) for seropositive women and implications for sample collection and clinical trial design. By analogy with transplant patients at risk of CMV infection, family members are considered as donors of virus for the female recipient. Gray represents uninfected and red represents infected. Collection and storage of serial samples from all family members is envisaged as part of clinical trial design. This would allow the strain of CMV causing congenital infection to be formally linked with the strain in the donor. Comparison with the infection rate among people receiving placebo would prove that a vaccine could protect against either reactivation of maternal infection or reinfection from a defined donor or both.
IMMUNIZATION OF TODDLERS
As discussed elsewhere in this supplement, CMV is an important pathogen that may ultimately be controlled by universal immunization and so bring benefit to all those who receive a vaccine. However, we need to consider the possibility that any CMV vaccine may be deployed primarily to protect others, especially the mother and unborn sibling of a toddler. There is a precedent for this, in that the rubella component of measles, mumps, and rubella (MMR) vaccine is used to prevent congenital rubella in a community, whereas the recipients benefit only from prevention of rubella infection, which is generally a mild infection at that age and not worthy of prevention.
Building upon the comments made above about a high inoculum of CMV being potentially able to overcome the defense mechanisms that naturally restrict intrauterine transmission to one third of women with primary infection, we need to consider how this may affect design of clinical trials. A traditional study would give vaccine or placebo to toddlers and determine whether they were subsequently protected against primary CMV infection. Development of a vaccine preparation that failed to achieve this would normally be stopped. However, if the vaccine gave partial protection such that the quantity of CMV found in the saliva and/or urine of the toddler were significantly reduced, this could provide useful protection to the mother and unborn sibling. Therefore, a novel trial design is required in which vaccine or placebo are given to a toddler, and the endpoints of the trial are reduced primary infection in the mother and congenital infection once the sibling is born (Table 3). There are logistical challenges to organizing such a study, but these should not be insurmountable. We suggest that the parents in such a study should be asked to give consent for a vaccine “to reduce the effect that CMV may have on my family” to recognize the fact that the clinical benefit may accrue to the sibling rather than to the toddler who receives the vaccine.
Table 3.
Issues to Consider: Vaccination of Toddlers
| Vaccine is given to toddler to protect others |
| Mother |
| Unborn sibling |
| There is a precedent for this: |
| MMR vaccine to prevent congenital rubella |
| If vaccine reduces CMV viral load it may protect others without preventing infection of toddler |
| A vaccine that “fails” to protect the toddler may nevertheless be useful clinically |
| Ensure parent gives consent for vaccine “to reduce the effect CMV may have on my family” |
Abbreviations: CMV, cytomegalovirus; MMR, measles, mumps, and rubella.
STEM CELL TRANSPLANT PATIENTS
Traditionally, these patients are considered along with solid organ transplant patients. We have kept them in a separate category for several reasons. First, the epidemiology is distinct from solid organ transplantation and women of childbearing age, both of whom experience primary infection, reinfection, or reactivation. Specifically, almost all cases of viremia after stem cell transplantation come from reactivation of latent virus in the recipient [34]. The high-risk groups are those in which the recipient is seropositive pretransplant and the exogenous transmission of CMV from a seropositive donor is uncommon. In fact, there is evidence that seropositive donors can adoptively transfer specific immunity into the recipient [35]. In the absence of a licensed CMV vaccine, a study was conducted in which recipients or donors or both or neither were given tetanus toxoid or hepatitis B vaccines pretransplant. The results showed that administration of vaccine to either the donor or the recipient produced significantly higher antibody titers in the recipient posttransplant [35]. When vaccine was given to both donor and recipient, the antibody titer was significantly higher than when vaccine was given to only 1 individual (Table 4).
Table 4.
Issues to Consider: Vaccination of Stem Cell Transplant Patients
| Most cases of viremia caused by reactivation from recipient |
| The donor is not irrelevant |
| Adoptive transfer of natural immunity |
| Adoptive transfer also achieved by immunization of donor |
| Vaccine given to recipient is recognized by donor cells after engraftment |
| Distinguish between studies giving vaccine to donor, or recipient, or both |
This natural history study formed the basis of the design of a phase 2, randomized, placebo-controlled trial to evaluate DNA plasmids encoding gB or pp65 [36]. The study began by immunizing stem cell donors on 4 occasions pretransplant as well as immunizing the corresponding recipients on 4 occasions posttransplant. Although the study was in progress, changes to medical practice meant that sibling donors were less likely to be chosen than were human leukocyte antigen-matched donors from international registries. This meant that it was logistically impractical to immunize donors any longer, and so the study was completed by immunizing recipients only. The results provided encouragement because the need for preemptive therapy was reduced and enzyme-linked immunospot reactions to pp65 were proposed as a correlate of immune protection [36]. Therefore, this vaccine proceeded to a phase 3 study, whose headline negative result has recently been presented orally. When the results are published in detail, it will be necessary to consider whether changes in immunogenicity between the preparations used for phase 2 and phase 3 and/or changes in study design, by omitting immunization of donors, might have been responsible for the disappointing results.
Conclusions
For future studies, we suggest that investigators consider whether it would be possible logistically to return to study of immunization of stem cell donors as a way of discovering protective immune responses against CMV. We recognize that there is a pressing need to control CMV end-organ disease in this patient group, and so studies will continue with immunization of recipients, but consider that the epidemiological and immunological differences are unlikely to allow information from this patient group to transfer readily to either solid organ transplantation or women of childbearing age.
Notes
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Cope AV, Sabin C, Burroughs A, Rolles K, Griffiths PD, Emery VC. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis 1997; 176:1484–90. [DOI] [PubMed] [Google Scholar]
- 2. Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med 1999; 190:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paya C, Humar A, Dominguez E, et al. ; Valganciclovir Solid Organ Transplant Study Group Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2004; 4:611–20. [DOI] [PubMed] [Google Scholar]
- 4. Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 2010; 10:1228–37. [DOI] [PubMed] [Google Scholar]
- 5. Singh N. Antiviral drugs for cytomegalovirus in transplant recipients: advantages of preemptive therapy. Rev Med Virol 2006; 16:281–7. [DOI] [PubMed] [Google Scholar]
- 6. Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 2000; 356:645–9. [DOI] [PubMed] [Google Scholar]
- 7. Atabani SF, Smith C, Atkinson C, et al. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant 2012; 12:2457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Griffiths PD, Rothwell E, Raza M, et al. Randomized controlled trials to define viral load thresholds for cytomegalovirus pre-emptive therapy. PLoS One 2016; 11:e0163722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus Guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 10. Natori Y, Alghamdi A, Tazari M, et al. ; CMV Consensus Forum Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis 2018; 66:617–31. [DOI] [PubMed] [Google Scholar]
- 11. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet 1984; 1:528–30. [DOI] [PubMed] [Google Scholar]
- 13. Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355:2032–6. [DOI] [PubMed] [Google Scholar]
- 14. Baraniak IA, Reeves MB, Griffiths PD. Criteria to define interruption of transmission of human cytomegalovirus from organ donor to recipient. Rev Med Virol 2018; 28. doi:10.1002/rmv.1958 [DOI] [PubMed] [Google Scholar]
- 15. McBride JM, Sheinson D, Jiang J, et al. Correlation of cytomegalovirus (CMV) disease severity and mortality with CMV viral burden in CMV-seropositive donor and CMV-seronegative solid organ transplant recipients. Open Forum Infect Dis 2019; 6:ofz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vincenti F, Budde K, Merville P, et al. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 2018; 18:2945–54. [DOI] [PubMed] [Google Scholar]
- 17. Lumgair HA, Rolando N, O’Beirne J, Sharma D, Griffiths PD. Transient residence of a seropositive organ is sufficient to transfer human cytomegalovirus to a seronegative recipient. Transpl Infect Dis 2014; 16:501–4. [DOI] [PubMed] [Google Scholar]
- 18. Ishida JH, Patel A, Mehta AK, et al. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 2017; 61. doi:10.1128/AAC.01794-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013; 56:1458–65. [DOI] [PubMed] [Google Scholar]
- 20. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 21. Lilleri D, Gerna G. Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy. Rev Med Virol 2017; 27. doi:10.1002/rmv.1921 [DOI] [PubMed] [Google Scholar]
- 22. Nelson CS, Vera Cruz D, Su M, et al. Intrahost dynamics of human cytomegalovirus variants acquired by seronegative glycoprotein B vaccinees. J Virol 2019; 93. doi:10.1128/JVI.01695-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffiths PD, McLean A, Emery VC. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine 2001; 19:1356–62. [DOI] [PubMed] [Google Scholar]
- 25. Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis 2007; 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 2016; 34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson CS, Huffman T, Jenks JA, et al. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc Natl Acad Sci U S A 2018; 115:6267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baraniak I, Kropff B, Ambrose L, et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc Natl Acad Sci U S A 2018; 115:6273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cannon MJ, Westbrook K, Levis D, Schleiss MR, Thackeray R, Pass RF. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med 2012; 54:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Revello MG, Tibaldi C, Masuelli G, et al. ; CCPE Study Group Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine 2015; 2:1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Revello MG, Lazzarotto T, Guerra B, et al. ; CHIP Study Group A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014; 370:1316–26. [DOI] [PubMed] [Google Scholar]
- 32. Gabrielli L, Bonasoni MP, Foschini MP, et al. Histological analysis of term placentas from hyperimmune globulin-treated and untreated mothers with primary cytomegalovirus infection. Fetal Diagn Ther 2019; 45:111–7. [DOI] [PubMed] [Google Scholar]
- 33. de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 2013; 23:241–9. [DOI] [PubMed] [Google Scholar]
- 34. Panagou E, Zakout G, Keshani J, et al. Cytomegalovirus pre-emptive therapy after hematopoietic stem cell transplantation in the era of real-time quantitative PCR: comparison with recipients of solid organ transplants. Transpl Infect Dis 2016; 18:405–14. [DOI] [PubMed] [Google Scholar]
- 35. Wimperis JZ, Brenner MK, Prentice HG, et al. Transfer of a functioning humoral immune system in transplantation of T-lymphocyte-depleted bone marrow. Lancet 1986; 1:339–43. [DOI] [PubMed] [Google Scholar]
- 36. Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:290–9. [DOI] [PubMed] [Google Scholar]