Abstract
Human cytomegalovirus (HCMV) infections are among the most common complications arising in transplant patients, elevating the risk of various complications including loss of graft and death. HCMV infections are also responsible for more congenital infections worldwide than any other agent. Congenital HCMV (cCMV) infections are the leading nongenetic cause of sensorineural hearing loss and a source of significant neurological disabilities in children. While there is overlap in the clinical and laboratory approaches to diagnosis of HCMV infections in these settings, the management, follow-up, treatment, and diagnostic strategies differ considerably. As yet, no country has implemented a universal screening program for cCMV. Here, we summarize the issues, limitations, and application of diagnostic strategies for transplant recipients and congenital infection, including examples of screening programs for congenital HCMV that have been implemented at several centers in Japan, Italy, and the United States.
Keywords: human cytomegalovirus, pregnancy, congenital infection, diagnosis
It is well established that human cytomegalovirus (HCMV) causes a wide spectrum of disease, primarily in persons with some level of immune compromise, for example allograft recipients and preterm infants, as well as infection during gestation (congenital HCMV [cCMV]). Serious disease associations include pneumonitis, retinitis, hepatitis, and, among congenitally infected infants, sensorineural hearing loss (SNHL) and intellectual disability. HCMV infection in healthy children and adults is generally mild, if not asymptomatic, but the virus is responsible for about 10% of infectious mononucleosis cases. Diagnostic testing to confirm cCMV and to monitor viral loads and immune responses among solid-organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients is crucial to effective patient care.
What follows is a description of strategies for prevention, diagnosis, and monitoring of HCMV infections, based on presentations made during the session on HCMV Diagnostics at a September 2018 conference Cytomegalovirus Infection: Advancing Strategies for Prevention and Treatment, which was sponsored by the National Institutes of Allergy and Infectious Diseases and Child Health and Human Development, and held in Rockville MD.
HCMV DIAGNOSTICS IN TRANSPLANTATION
Laboratory strategies for diagnosis of HCMV infections in the context of organ or hematopoietic stem cell transplantation rely on direct detection of the virus and measurement of host immune responses. Laboratory tests that directly detect HCMV are recommended for surveillance, diagnosis, and monitoring, while assays of immune status are relied upon for HCMV risk assessment and stratification of risk factors.
Assays for Virus Detection
Direct detection of HCMV in clinical specimens is the standard method for the diagnosis of HCMV infection in transplant recipients (Table 1) [1]. An updated set of definitions for HCMV disease in transplant patients has been published, which now includes a category of “probable disease” [2]. The most common approach for direct virus detection involves detection and quantitation of the HCMV genome using commercial quantitative nucleic acid amplification tests (QNATs), which are highly sensitive and offer rapid turn-around times. Whole blood and plasma are the most common specimens for HCMV QNAT, although cerebrospinal fluid and bronchoalveolar lavage fluid (BAL) are sometimes used [3, 4]. Practical cutoff values have been suggested for BAL samples that discriminate between pneumonitis and pulmonary shedding in HSCT recipients, but these values are likely variable depending on the assay used and the population being tested [3]. HCMV QNAT of blood or plasma is also useful to support diagnosis of probable end-organ HCMV disease (eg, pneumonia or gastrointestinal disease) or when obtaining biopsy samples for histopathology is risky [3–5].
Table 1.
Characteristics, Clinical Uses, and Limitations of Cytomegalovirus Assays in Transplantation
| Assays | Test Characteristics and Examples | Clinical Uses | Limitations |
|---|---|---|---|
| Assays for virus detection | |||
| CMV QNAT | Detects and quantifies CMV nucleic acid Results reported in IU/mL Various assays including Roche (COBAS 6800 and 8800), Qiagen (artus), Abbott, and many laboratory-developed tests (home-brew assays) | Rapid and sensitive method for diagnosis of CMV infection Surveillance for preemptive therapy Monitoring of antiviral response Prognosticator of risk of CMV disease | Lack of consensus viral load threshold that is applicable across patients and clinical settings Lack of standardization among various aspects of QNAT (variable limits of detection and range of quantification, sample type, gene target and amplicon size, among others) Patients with different risk profiles will likely have different viral thresholds |
| Antigenemia | Uses monoclonal antibody to detect CMV pp65 antigen expressed in leukocytes during the early period of CMV replication Reported as number of pp65-positive cells per number of leukocytes counted | Sensitive diagnosis of CMV infection Surveillance for preemptive therapy Monitoring of antiviral response Prognosticator of risk of CMV disease | Lack of assay standardization Need for samples to have enough leukocytes (limited in HSCT and neutropenia) Labor intensive Lack of automation Subjective interpretation |
| Culture | Detects typical cytopathic effect in human fibroblasts (conventional cell culture) or viral antigen by monoclonal antibody (shell vial assay) | Highly specific for the diagnosis of CMV infection Culture can be used to assess phenotypic antiviral drug testing | Low sensitivity (compared to QNAT and antigenemia) Slow turn-around time |
| Histopathology | Detects CMV antigen and cytopathic changes in tissue specimens | Gold standard for diagnosis of most end-organ CMV diseases | Invasive procedure is needed to obtain tissue specimen for histopathology |
| Assays of CMV immune response | |||
| Serology | Detects CMV IgG or IgM antibodies Various assays including complement fixation, enzyme-linked immunosorbent assay, radioimmunoassay | Pretransplant screening of transplant candidates and potential donors for evidence of prior CMV infection Depending on the CMV serology of donor and recipient, the risk of CMV is determined (see text) | IgM is not recommended routinely due to false positivity Utility of CMV serology for diagnosis of CMV after transplant is limited |
| CMV-specific T cells | Detects INF-γ in blood or cells after stimulation ex vivo with CMV antigens Various assays including QuantiFERON-CMV, T-spot.CMV, T-track.CMV, many laboratory-developed tests | Indicator of the risk of CMV disease after transplantation Potential for guiding prevention and treatment strategies (studies are needed to support this potential indication) | Lack of standardization among assays Limited number of prospective studies and interventional trials that utilize these assays for disease management |
Abbreviations: CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplant; INF-γ, interferon-γ; QNAT, quantitative nucleic acid amplification test.
The quantitative nature of HCMV QNAT allows for assessment of the degree of HCMV replication, which is expressed as the absolute viral load value. Trends in viral loads over time (viral load kinetics) directly correlate with the likelihood of severe HCMV disease [6, 7]. Thus, higher viral loads or steep rises in viral loads correlate with a higher risk for severe HCMV disease [6–8].
QNAT is the preferred method for HCMV surveillance to guide the application of preemptive antiviral therapy. HCMV QNAT of blood is performed at weekly intervals during the high-risk period (ie, first 12 weeks after transplant). Once a defined viral load threshold is reached, preemptive therapy is initiated, with the goal of preventing progression of asymptomatic infection into clinical disease [7]. HCMV QNAT is recommended at least once weekly to monitor response to antiviral treatment [1]. Declines in viral load during antiviral therapy correlate with clinical resolution of CMV disease [9]. In contrast, a continued rise in viral load or a minimal decline suggests refractory or drug-resistant HCMV [10]. Guidelines recommend treating patients until the negative threshold is reached because persistence of viremia at the end of antiviral treatment is a risk factor for relapse [1].
The major limitation of HCMV QNAT is a lack of well-established viral load thresholds to guide various clinical applications. For example, there is no consensus viral load value for application to all patients for the initiation of preemptive therapy. A major reason for this is the lack of standardization among various laboratory-developed and commercially available HCMV QNAT [11]. In a study that compared 33 laboratories using different HCMV QNAT, there was wide interassay variability in viral load reporting [12]. Because the major contributor to this variability was a lack of calibrator standard [11], a World Health Organization (WHO) international standard for calibration of HCMV QNAT was developed. However, despite calibration to the WHO international standard, clinically relevant differences remain in viral load values determined by various HCMV QNAT [13]. Variability in different aspects of HCMV QNAT protocols, such as assay performance (limits of detection and quantification), sample type, method for nucleic acid extraction, gene target, and amplicon size contribute to viral load variability [13, 14]. Moreover, clinically relevant viral load values will likely differ depending on the type of patients and their risk profiles [15].
Because of the limitations outlined above, consensus guidelines recommend that transplant providers determine assay-specific viral thresholds that are tailored to their clinical practice; these viral thresholds will likely differ among various patient profiles [1].
Assays of HCMV Immune Responses in Transplant Recipients
Guidelines recommend that the risk of HCMV disease should be assessed prior to transplantation for all transplant recipients so that the appropriate HCMV prevention strategy can be implemented [1]. The most common method involves HCMV IgG screening of transplant candidates and potential donors [1]. Based on this, SOT recipients are categorized as high risk (HCMV-seropositive donor and HCMV-seronegative recipient), moderate risk (HCMV-seropositive SOT recipients), or low risk (HCMV-seronegative donor and recipient). All HCMV-seropositive HSCT recipients are at high risk. In contrast to its pretransplant value, HCMV serology has limited utility in the posttransplant setting [1].
Assays that detect HCMV-specific CD4 and CD8 T cells can help to inform the likelihood of HCMV disease after transplantation. Several methods are available, including measurement of interferon-γ (INF-γ) concentration in blood (INF-γ release assays), or the enumeration of INF-γ–producing HCMV-specific T cells by enzyme-linked immunospot (ELISPOT) or flow cytometry [16, 17]. In principle, transplant patients with undetectable or low HCMV-specific T cells are at high risk for disease following primary HCMV infection, end-organ HCMV disease, and refractory, relapsing, or antiviral-resistant HCMV [16, 17]. In a prospective study, patients who developed HCMV-specific immunity (indicated by positive HCMV INF-γ release assay) had a very low risk of HCMV relapse, while patients who did not develop HCMV-specific immunity had a high risk of relapse upon discontinuation of antiviral prophylaxis [16].
The major limitation of cell-mediated immune assays is their lack of standardization. Moreover, most studies that correlated cell-mediated immune assays with the risk of HCMV are largely descriptive. Controlled interventional studies are needed that incorporate these immune assays into the management of HCMV after transplantation. As part of such evaluations, the immunologic assays should be performed in conjunction with viral load testing to optimize the development of improved strategies for prevention, treatment, and monitoring posttransplant HCMV infections [1, 17, 18]. We anticipate that integration of viral load and host immune status information will enable development of personalized, rather than one-strategy-fits-all, approaches to HCMV management after transplantation [1].
DIAGNOSIS OF MATERNAL AND CONGENITAL HCMV INFECTIONS
cCMV infections are a leading cause of SNHL and neurologic disabilities in children [19, 20]. cCMV infection may follow maternal primary and nonprimary infection during pregnancy. The highest-risk scenario is among seronegative mothers infected during pregnancy. Transmission to the fetus occurs in around 32% of these cases and leads to disease in about 13% of congenitally infected newborns [21–23].
Among HCMV seropositive women, reactivation of an endogenous HCMV strain or reinfection with a new strain gives rise to a fetal infection in about 1% of pregnancies. This rate is much lower than for primary infections, but reactivations and reinfections occur much more frequently. Consequently, HCMV-seropositive women are a major source of congenital infections worldwide [24]. Primary and nonprimary maternal infections can result in severe sequelae [21].
In the absence of an effective vaccine, other forms of prevention are needed. Several studies have found that coaching avoidance of contact with bodily fluids of young children can reduce HCMV seroconversion in pregnant women. For example, a controlled trial provided evidence that a prevention strategy based on identifying and educating susceptible pregnant women at risk for primary infection was effective at reducing the rate of maternal HCMV infection and thereby cCMV (adjusted odds ratio, 0.14; 95% confidence interval, .05–.41) [25]. Based on data from such cohort or case-control analytical studies, the International Congenital Cytomegalovirus Recommendations Group recommended that “all pregnant women should be educated about congenital cytomegalovirus infections and preventive measures” [26]. Related to this, the US Centers for Disease Control and Prevention’s guidance to health care providers states that “Avoiding contact with saliva and urine from young children might reduce the risk of CMV infection, although research studies don’t provide a clear answer. Some examples of how to avoid contact include kissing children on the cheek or head rather than the lips and washing hands after changing diapers.” [27]. The American College of Obstetrics and Gynecology advises that “CMV can be spread by contact with an infected child’s urine or other bodily fluids. Pregnant women who work with young children, such as day care or health care workers, should take steps to prevent infection …” and “Pregnant women with young children at home also are at risk and should take these steps.” [28].
Increased recognition of cCMV and its devastating long-term effects has led to heightened exploration of strategies for the early identification of infected newborns and those at increased risk for SNHL and other sequelae. These include targeted screening of infants who fail their newborn hearing screening, infants at higher risk for cCMV (eg, preterm infants), and screening of all newborns for HCMV (universal screening). Four US states currently mandate or offer HCMV testing in newborns who fail newborn hearing screening, while several other states require education of pregnant women and public health care professionals about HCMV. Some states are considering legislation to mandate or offer HCMV screening and/or education. Globally, cCMV awareness is increasing, leading to consideration and implementation of HCMV testing of infants who fail newborn hearing screening or universal screening in Canada, Europe, Australia, and Japan. Because (1) 85%–90% of HCMV-infected newborns are asymptomatic at birth, (2) infants symptomatic at birth have diverse clinical presentations, and (3) the often-delayed appearance of cCMV-related SNHL, universal screening in the newborn period has emerged as the only viable strategy for early identification of all infants with cCMV. In one study, cost-effectiveness estimates for newborn cCMV screening predicted net societal savings for universal screening relative to targeted screening [29].
Improved methods for detecting cCMV have been developed and validated in recent years. The gold standard for identification of infants with cCMV had been detection of infectious virus in saliva or urine specimens obtained within the first 2–3 weeks of life [26, 30, 31]. Development of new methods for specimen collection and application of high-throughput molecular methods such as quantitative polymerase chain reaction (qPCR) have largely supplanted culture-based methods in most laboratories, and are paving the way for widespread implementation of targeted cCMV screening programs internationally [32–35].
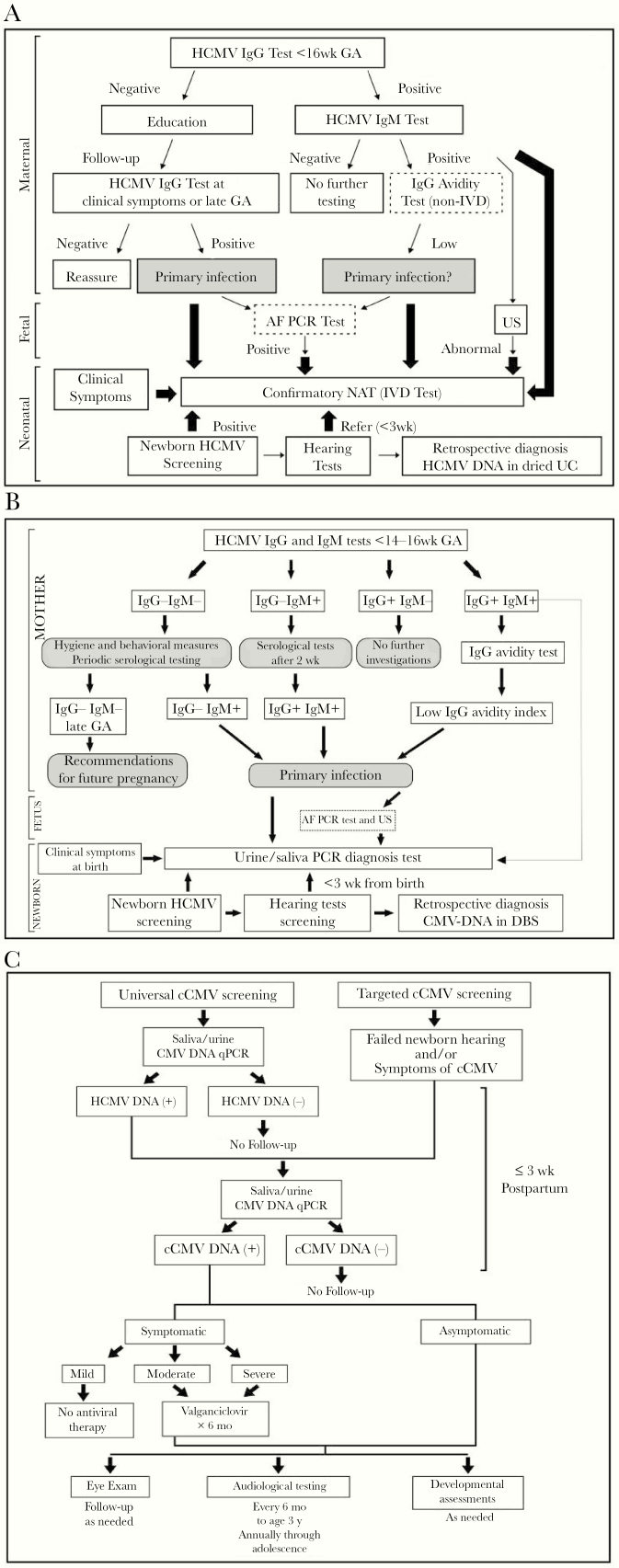
In the sections that follow, we discuss prenatal diagnosis of HCMV in pregnant women at high risk of transmitting HCMV to the fetus. Such information has been used to develop algorithms for prevention, diagnosis, and screening for cCMV that have been implemented at centers in Japan, Italy, and the United States (Figure 1). These programs provide information and models that can be used to develop perinatal screening programs elsewhere for wider societal application.
Figure 1.
Algorithms for prevention, diagnosis, and screening for cCMV, as implemented at centers in Japan, Italy, and the United States. A, cCMV screening and diagnosis as implemented in Japan [36, 37]. Optional tests are shown in dotted border boxes. Wide arrows indicate the algorithm employed since an in vitro diagnostic approved NAT became available. B, cCMV screening and diagnosis in Bologna, Italy [35]. C, cCMV screening, diagnosis, and management in Birmingham, Alabama [38].
Abbreviations: AF, amniotic fluid; cCMV, congenital cytomegalovirus; DBS, dried blood spot; GA, gestational age; HCMV, human cytomegalovirus; IVD, in vitro diagnostic; PCR, polymerase chain reaction; NAT, nucleic acid test; UC, umbilical cord; US, ultrasound.
DIAGNOSIS OF MATERNAL HCMV INFECTIONS
Clinical diagnosis of maternal HCMV infection is unreliable because most pregnant women with active infection are asymptomatic; therefore, laboratory diagnosis is the principal approach to identify women with primary HCMV infection (Table 2).
Table 2.
Outline of Diagnostics for cCMV Infection and Disease
| Prenatal | Postnatal | |
|---|---|---|
| Maternal | Fetus | |
| Infection | ||
| Serology Seroconversion HCMV IgG and IgM HCMV IgG avidity Virology HCMV DNA in maternal bodily fluids | Virology HCMV DNA in amniotic fluid | Serological screening HCMV IgM in cord or newborn blood Virology PCR/culture of virus from urine or saliva |
| Disease | ||
| Clinical presentation Fever, fatigue and headache Mild mononucleosis and flu-like symptoms | Abnormal findings Imaging by ultrasound or magnetic resonance imaging | Abnormal findings Imaging by computerized tomography or magnetic resonance Auditory brainstem response Developmental quotient |
| Others | ||
| Transmission to fetus Pentamer-specific antibodies Rapid increase of HCMV IgG antibody avidity index | Retrospective diagnosis HCMV DNA in dried blood spots and dried umbilical cords |
Abbreviations: cCMV, congenital cytomegalovirus; HCMV, human cytomegalovirus; PCR, polymerase chain reaction.
Serology
Two fundamental questions connect to serologic diagnosis of HCMV during pregnancy: (1) in what context(s) might serological testing for cCMV be offered? and (2) are serological tests for HCMV clinically meaningful in the context of cCMV diagnosis?
While various HCMV serologic methods have demonstrated value as research tools, their incorporation into clinical diagnostic algorithms requires careful evaluation and interpretation. A review of prenatal immunological diagnostics concluded that, due to the degree of individual variability of HCMV immunological parameters among pregnant women, on an individual case basis, immunologic data alone do not reliably predict the risk of fetal HCMV transmission [39]. However, as most maternal HCMV infections are asymptomatic, another review concluded that reliable detection of women at risk of transmitting the virus to their fetus is dependent on implementation of serological and virological testing early in pregnancy (before 12–14 weeks of gestation) [40]. Because serological and PCR testing performed for the first time late during gestation (ie, after 14–16 weeks of gestation) are uninformative about HCMV serostatus prior to the time of testing [41], maternal HCMV infections cannot be reliably diagnosed as primary versus nonprimary in women whose serological status is not known prior to pregnancy. In addition, while primary HCMV infection during pregnancy carries a higher risk of fetal infection, at the population level, transmission during reactivation or reinfection are more common and contribute significantly to the burden of cCMV disease [24]. Further work will be required before antenatal screening can be recommended for diagnosis of HCMV status in pregnant women.
All of the serologic endpoints for primary HCMV infection (seroconversion, positive IgM, and low avidity index [AI]) have limitations. Seroconversion requires pre- and postinfection specimens, which would need to be collected at intervals of several weeks, making this impractical. IgM assays have well-established limitations such as a narrow detection window, long-term persistence of HCMV IgM among some individuals, reappearance of HCMV IgM upon reactivation or reinfection, and the generally poor specificity of IgM antibodies (false positives). Consistent with this, Sonoyama et al [42] demonstrated that among 50 women with positive or equivocal IgM, only 9 delivered cCMV-infected newborns; the combination of low HCMV AI and detection of fetal abnormalities by ultrasound substantially improved the identification of cCMV cases.
The detection of anti-HCMV IgM in pregnant women is not a reliable marker for primary infection; a complementary test is needed, such as HCMV IgG avidity testing. Assessment of anti-HCMV antibody avidity prior to 12–16 weeks of gestation can identify most women with primary infections who are more likely to give birth to an infected newborn. A recently published study found that measurements of HCMV avidity during the first trimester of pregnancy have potential prognostic value for determining risk of newborn SNHL [43]. Interpretation of these results is very important because avidity assays vary from one laboratory to another; reference values and controls must be carefully assessed. Two very important rules must be considered to correctly interpret an avidity test result: (1) in samples with very low levels of anti-HCMV IgG, an accurate evaluation HCMV IgG-avidity is not possible; and (2) HCMV IgG avidity testing should only be performed on serum samples dually positive for HCMV-specific IgG and IgM antibodies. Interpretation of intermediate/moderate/grey zone results is also challenging. Because the available data are from small clinical studies, moderate HCMV IgG avidity indices detected within 12 weeks of gestation must be interpreted with caution. An international standard serum panel with well-defined HCMV AIs is needed.
Maternal Nucleic Acid Testing
Very little data are available on the natural history of HCMV shedding in body fluids during primary infection in immunocompetent individuals and the clearance of HCMV DNA after primary infection varies greatly among different compartments of shedding. Therefore, the detection of HCMV-DNA plays a secondary role in the diagnosis of primary HCMV infection in pregnant women and can only support serological diagnosis.
Data suggest that detection and, possibly, quantification of HCMV-DNA in maternal bodily fluids can be useful for prediction of intrauterine transmission in women with primary infection. Women who transmitted the virus to their fetus were approximately 2-fold more likely to be shedding CMV DNA in their blood, cervical secretions, and urine [44, 45]. Prolonged maternal DNAemia was also associated with increased risk of fetal infection [46–49].
Diagnosis of nonprimary HCMV infection is challenging. As mentioned above, serological testing is not definitive for identifying nonprimary infections. While it is possible that laboratory testing could play a role in the diagnosis of nonprimary HCMV infection in women with known serological status before pregnancy, large prospective studies are needed to develop and validate algorithms for optimal timing of specimen collection, evaluation of serologic assays, and detection of viral DNA in different body fluids. Approaches for management of HCMV infection during pregnancy that have been implemented at centers in Japan, Italy, and the United States are outlined in Figure 1.
PRENATAL DIAGNOSIS OF CONGENITAL HCMV INFECTION
Prenatal diagnosis, both invasive (amniocentesis and/or cordocentesis) and noninvasive (ultrasonographic examination), is sometimes offered to pregnant women at high risk of transmitting HCMV to the fetus, particularly to pregnant women with documented primary infection, an undetermined type of infection during the first trimester of gestation, and when abnormal ultrasound findings suggest cCMV infection.
Invasive Prenatal Diagnosis
Quantification of viral DNA in amniotic fluid (AF) is the most appropriate method for diagnosis of fetal HCMV infection [48]. When amniocentesis is performed at least 8 weeks after the onset of maternal infection [50] and at least 20–21 weeks’ gestation [48], the sensitivity of AF qPCR ranges from 80% to 90% with a specificity of 100%. Enders and coworkers suggested that amniocentesis could be performed earlier than 21 weeks’ gestation without significant loss of sensitivity, but this must be confirmed in a larger controlled study [50]. Cases of cCMV with negative AF qPCR results have been sporadically reported (negative predictive value of approximately 95%). This may reflect either low qPCR sensitivity or because transmission occurred after amniocentesis. Although negative amniocentesis tests for HCMV do not eliminate the possibility of cCMV infection, children with cCMV whose mothers had negative AF tests generally have better long-term outcomes [51]. Collection of AF during maternal HCMV DNAemia poses no risk for iatrogenic transmission of HCMV to the fetus. Maternal DNAemia at the time of amniocentesis is associated with a 3-fold greater chance of congenital infection but is not associated with symptomatic disease [47]. The prognostic value of HCMV-DNA loads in the AF is controversial. Although high viral loads in AFs may be associated with symptomatic or asymptomatic cCMV, severe disease is more likely with high viral loads [52].
While cordocentesis might provide prognostic information about outcomes in infected infants, it poses a risk to the fetus and is not generally recommended for diagnosis of fetal HCMV infection. The prediction of perinatal outcomes in HCMV-infected fetuses was enabled via the assessment of multiple markers (β-2 microglobulin, platelet count, and CMV-DNAemia) [52, 53]. However, the prognostic utility of these markers needs to be confirmed in studies that include larger numbers of symptomatic and asymptomatic cCMV cases.
Noninvasive Prenatal Diagnosis
The sensitivity of ultrasound imaging for the detection of cCMV infection is only approximately 15% [54]. Ultrasound findings suggestive of fetal infection, presented in theoretical chronological order of their development, include placentitis (defined by a thickness of 4 cm or more and a heterogeneous appearance typically with calcifications coexisting with hypoechoic areas in the placenta), oligohydramnios, hyperechogenic bowel that usually represents meconium ileus, and hepatosplenomegaly and possibly ascites (due to cholestatic hepatitis and liver insufficiency). Less common ultrasound findings include anemia-related hydrops and calcifications of the fetal liver and spleen. Intrauterine fetal growth restriction can be detected by ultrasound, but it may develop due to either fetal infection or placental dysfunction. Damage to the fetal brain is a late finding that can be identified by variable and progressive features on prenatal imaging [40].
When the HCMV status of the fetus is known, ultrasound evaluation is useful for detecting and monitoring fetal abnormalities, such as cerebral abnormalities indicative of severe disease [55]. Farkas et al evaluated the neurodevelopmental outcome of HCMV infected fetuses in whom serial neurosonographic examinations appeared normal and found that such results predicted normal early neuropsychological outcomes [56]. In several well-documented series, normal ultrasound examinations were associated with a residual risk of 1%–5% for severe infection at birth, including deafness, and 0%–5% for more severe neurodevelopmental sequelae [54, 56, 57]. A definitive prognosis for an infected fetus showing either no ultrasound features or mild anomalies is difficult to establish until about 30 weeks of gestation. Combining targeted ultrasound examination and magnetic resonance imaging in third trimester fetuses with confirmed HCMV infection provides reliable (95% sensitivity) identification of central nervous system lesions related to cCMV infection [58]. Although this is useful, the availability of accurate prognostic assessments earlier in gestation is desirable. This is especially crucial in countries where elective termination of pregnancy is not permitted after 24 weeks of gestation. Table 3 shows the interpretations of prenatal diagnosis results, performed at 20–21 weeks of gestation, for fetal HCMV infection counseling.
Table 3.
Outcome of Fetal HCMV Infections at Birth After Prenatal Testing Performed at 20–21 Weeks’ Gestation
| Amniotic Fluid qPCR | Ultrasound Findings | Outcome at Birth |
|---|---|---|
| Negative | Negative | No congenital infection (~95% of cases) Congenital infection without long-term neurological sequela (~5% of cases) |
| Low viral load | Negative | High probability of asymptomatic congenital infection |
| High viral load | Severe brain abnormalities | Symptomatic congenital infection |
| High viral load | Negative | Congenital infection; additional assessments are needed to refine the prognosis |
Abbreviations: HCMV, human cytomegalovirus; qPCR, quantitative polymerase chain reaction.
One important caveat regarding prenatal testing is that currently available techniques have limitations when considered in the context of an individual pregnancy. As mentioned above, the degree of variability of HCMV immunological parameters among pregnant women is such that these data cannot predict the risk of fetal HCMV transmission on an individual case basis [39].
IDENTIFICATION OF INFANTS WITH CONGENITAL HCMV INFECTION
Infants with cCMV shed large amounts of virus in saliva and urine; both specimens are useful for the diagnosis of cCMV in infants. Specimens for cCMV diagnosis should be collected from the infant within the first 2–3 weeks of life to distinguish congenital from postnatally acquired HCMV infection.
Virus isolation by culture from urine or saliva has long been the gold standard for identifying infants with cCMV [30, 31]. Studies have demonstrated that QNAT of newborn saliva and urine specimens has high sensitivity and specificity for screening and diagnosis of infants with cCMV [32–34, 38, 59]. PCR assays have the advantages of being less expensive, with rapid turnaround times, and no requirement for maintaining tissue culture facilities. A DNA extraction step is not required for the QNAT testing of saliva specimens [33, 38]. PCR is also less affected by specimen storage and transport conditions and can be adapted for use in high-throughput newborn screening programs.
Except for the most severely affected, most infants with cCMV are not identified at birth. This includes infants with cCMV who present with milder or nonspecific clinical findings, or are completely asymptomatic. Because a significant proportion of infants with asymptomatic cCMV (10%–15%) develop SNHL, implementation of screening programs for detection of cCMV is being considered internationally. Strategies under consideration include targeted screening of infants who fail their hearing screening, those with nonspecific clinical findings such as intrauterine growth restriction, and preterm infants. An alternative approach is to screen all newborns for cCMV (universal screening). Specimens considered for screening purposes include dried blood spots (DBS), saliva, and urine. In addition, PCR testing of dried umbilical cord specimens has been used to retrospectively diagnose cCMV [60, 61].
Dried Blood Spot Testing
In the United States, DBS are collected from all newborns for metabolic screening. Their potential for relatively easy integration into newborn HCMV screening programs has led to evaluation of their utility for cCMV newborn screening. Retrospective studies and studies of selected newborn populations suggested that testing DBS from newborns could be useful for identifying infants with cCMV [62–65]. In a study of 20 448 infants as part of the HCMV and Hearing Multicenter Screening Study (CHIMES), HCMV testing with DBS PCR was less sensitive than saliva rapid culture, suggesting limited value for cCMV screening [66]. The method employed for extraction of DNA from DBS can affect analytic sensitivity [67]. Efforts to improve methods for DNA extraction and PCR testing of DBS to achieve sensitivity sufficient for use in cCMV screening programs continue [68–71].
Testing of Urine and Saliva Specimens to Screen Newborns for cCMV
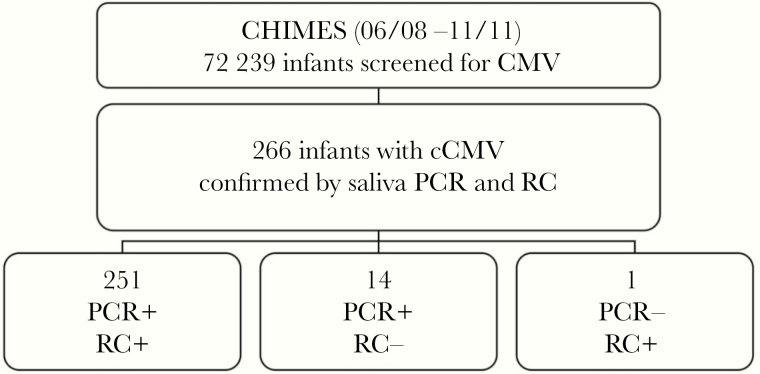
Detection of infectious virus or viral antigens in urine or saliva have long been the gold standard for identification of infants with cCMV [30, 31]. Sensitivities for PCR detection of HCMV DNA are similar with both specimen types (99.7% agreement) [32, 72]. In a newborn HCMV screening study, qPCR assays of dried saliva and liquid saliva specimens were compared to saliva rapid culture for the detection of HCMV in 34 989 newborns; the qPCR methods had 97.4%–100% sensitivity and 99.9% specificity [33]. In a follow-up study, more newborns were identified by PCR compared to culture-based testing of saliva samples, with 97.4% concordance between saliva qPCR and rapid culture (Figure 2) [38, 59].
Figure 2.
Saliva PCR identifies more newborns with congenital CMV infection than does a rapid culture assay: data from the CHIMES study [38]. Abbreviations: cCMV, congenital cytomegalovirus; PCR, polymerase chain reaction; RC, rapid culture.
One of the concerns about testing saliva to detect CMV-infected infants is the possibility of misidentifying newborn HCMV infection due to the presence of HCMV in the mother’s genital tract and breast milk. However, in the CHIMES study, the frequency of HCMV-positive results attributable to breast milk contamination of saliva qPCR was <0.03% [33]. The frequency remained low even when adjusted for national HCMV seroprevalence and breastfeeding rates, indicating that saliva PCR results are unlikely to be significantly influenced by breastfeeding [73]. However, because saliva-based qPCR assays are not standardized, it is important that individual assays be evaluated to identify whether a cutoff level is needed to achieve the optimal balance between sensitivity and specificity. Based on results from the initial phase of the CHIMES study, a cutoff value was established of ≥5 copies/reaction to reliably identify infants with cCMV and to significantly reduce false-positive results [33]. Finally, the risk of breast milk contamination of PCR saliva samples may be further reduced by collecting screening swabs before feeding. As it is a standard practice to confirm all newborn screening results, a positive newborn saliva test for CMV should be confirmed by collecting and testing either a second saliva sample or a urine sample (Figure 1C).
The use of a filter-based diaper insert to collect urine for HCMV screening has demonstrated high sensitivity, low cost, and low contamination risk; saliva collected on such filters works as well as urine filters [34]. Using the urine filter-based assay, 71 cCMV cases were identified from 23 000 newborns [36]. CMV DNA was undetectable in 3 out of 12 DBS obtained from HCMV-positive infants, indicating that DBS testing is insufficiently sensitive for cCMV screening. The filter-based screening method was successfully introduced into several hospital diagnostic laboratories in Japan [74]. The filter for diaper-based urine collection was recently changed from the original FTA-Elute to a new ADVANTEC 4A filter, making it less expensive, free from minor skin irritations, and more durable in clinical setting.
Substantial evidence now demonstrates that saliva and urine collected from infants offer comparably excellent sensitivity, although each presents challenges when applied to routine screening. The core issue has transitioned from “saliva versus urine” to logistical and experienced-based preferences for “filter versus liquid” collection.
Implementation of Newborn CMV Screening in Japan
An approved in vitro diagnostic assay confirmatory nucleic acid amplification test was required to establish a system for routine newborn CMV in Japan. As part of the assay’s evaluation, universal screening by nucleic acid amplification was compared with data from a risk-based targeted screening approach. After assessment of newborns on the basis of 20 clinical or diagnostic indications (risks) for cCMV infection (see below), 575 were identified as having suspected cCMV. From this group, all 20 cCMV cases identified using commercial assays of liquid urine specimens were also identified by urine-filter screening conducted in parallel [37], with complete concordance between the 2 assays. Compared with universal PCR-based screening, use of the set of 20 clinical or diagnostic indicators of cCMV risk provided a substantial enrichment in cCMV cases in the set of specimens evaluated by the nucleic acid amplification assay. By reducing the number of tests, the net cost of the screening program was reduced. Based on these results, the Genelys CMV (SHINO-TEST) assay was approved as an in vitro diagnostic that is eligible for medical insurance coverage in Japan for HCMV testing of newborns with various indications associated with, but not individually specific for, cCMV. These include fetal abnormalities, tendency toward bleeding, neurologic sequelae, maternal positive IgM and low AI, fetal ultrasound abnormalities, and relevant clinical symptoms. Data from this study led to an update of the 2014 cCMV diagnostic algorithm recommended by the Japanese Study Group (Figure 1A).
FUTURE DIRECTIONS OF CCMV DIAGNOSIS
Advances in diagnostic capabilities have been essential for developing our current understanding of HCMV biology and its roles in human disease.
The use of sensitive and specific CMV qPCR has become the standard of care for solid organ and hematopoietic stem cell recipients as a means for monitoring and treating HCMV disease in transplant patients. Practical and effective approaches suitable for use in cCMV screening programs have been developed and implemented in some places. Results from screening programs of pregnant women in Europe indicate that the availability of specialized screening laboratories can lead to reductions in decisions to terminate pregnancy [75].
While significant progress has been made, it is essential to continue development of an ever-more robust and well-equipped toolbox for diagnosis of CMV infections in transplant recipients and in the context of congenital infections. This includes continued development of clinically applicable assays for cell-mediated immunity [17, 18, 76], biomarkers such as soluble HLA-G [77], peptides [78], and cytokines in accessible bodily fluids [79]. Other areas include improved resolution and image analysis algorithms for ultrasound detection of fetal abnormalities, and application of new methods for nucleic acid amplification (eg, loop-mediated isothermal amplification) and microfluidics technologies that offer the possibility of rapid results at lower cost. Wide application of the recently established international standard will help to eliminate the observed variability of DNA-based assay sensitivity [13]. Sensitive and specific markers are needed for prognostic identification of cCMV cases with potential to result in disease that can be used sufficiently early during gestation to inform pregnancy counseling.
Availability of improved therapeutics will provide additional motivation to develop improved diagnostic assays and algorithms. Large-scale international clinical and epidemiologic collaborations will be needed to realize the dream of controlling the societal burdens imposed by HCMV.
Notes
Acknowledgments. We thank all PIs and their lab members from the Japanese cCMV study group, especially S. Koyano (Asahikawa Medical College), T. Suzutani (Fukushima Medical Univ), T. Fujii and A. Oka (Tokyo University), H. Yamada and I. Morioka (Kobe University), and H. Moriuchi (Nagasaki University).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Diseases Control and Prevention.
Financial support. This work was supported by Ministry of Health, Labor, and Welfare, Japan and the Agency for Medical Research and Development, Japan (grant numbers H20-Kodomo-007, H23-Jisedai-Ippan-001, H25-Jisedai-Shitei-003, 15gk0110003h0103, 16gk0110 021h0001, 17gk0110021h0002, and 18gk0110021h0003 to N. I. for Research on Child Development and Diseases). S. G. P. and S. B. B. were supported by National Institute on Deafness and Other Communication Disorders grants to S. B. B. (N01 DC50008, HHS-N-263-2012-00010-C).
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. S. B. B. serves as a member of the CMV Vaccine Advisory Committees of Merck and Sanofi. R. R. R. reports grants from Roche and Novartis outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Cytomegalovirus Infection: Advancing Strategies for Prevention and Treatment, Rockville, MD, 4 September 2018.
References
- 1. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13512. [DOI] [PubMed] [Google Scholar]
- 2. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 3. Beam E, Germer JJ, Lahr B, et al. Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia. Clin Transplant 2018; 32:doi: 10.1111/ctr.13149. [DOI] [PubMed] [Google Scholar]
- 4. Boeckh M, Stevens-Ayers T, Travi G, et al. Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar lavage fluid from hematopoietic stem cell transplant recipients with CMV pneumonia. J Infect Dis 2017; 215:1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durand CM, Marr KA, Arnold CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients. Clin Infect Dis 2013; 57:1550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355:2032–6. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths PD, Rothwell E, Raza M, et al. Randomized controlled trials to define viral load thresholds for cytomegalovirus pre-emptive therapy. PLoS One 2016; 11:e0163722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natori Y, Alghamdi A, Tazari M, et al. ; CMV Consensus Forum Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis 2018; 66:617–31. [DOI] [PubMed] [Google Scholar]
- 9. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev 2013; 26:703–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chemaly RF, Chou S, Einsele H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis 2018; 68:1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayden RT, Yan X, Wick MT, et al. ; College of American Pathologists Microbiology Resource Committee Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol 2012; 50:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pang XL, Fox JD, Fenton JM, Miller GG, Caliendo AM, Preiksaitis JK; American Society of Transplantation Infectious Diseases Community of Practice; Canadian Society of Transplantation Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant 2009; 9:258–68. [DOI] [PubMed] [Google Scholar]
- 13. Preiksaitis JK, Hayden RT, Tong Y, et al. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 2016; 63:583–9. [DOI] [PubMed] [Google Scholar]
- 14. Naegele K, Lautenschlager I, Gosert R, et al. Cytomegalovirus sequence variability, amplicon length, and DNase-sensitive non-encapsidated genomes are obstacles to standardization and commutability of plasma viral load results. J Clin Virol 2018; 104:39–47. [DOI] [PubMed] [Google Scholar]
- 15. Dioverti MV, Lahr BD, Germer JJ, Yao JD, Gartner ML, Razonable RR. Comparison of standardized cytomegalovirus (CMV) viral load thresholds in whole blood and plasma of solid organ and hematopoietic stem cell transplant recipients with CMV infection and disease. Open Forum Infect Dis 2017; 4:ofx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant 2009; 9:1214–22. [DOI] [PubMed] [Google Scholar]
- 17. Meesing A, Abraham RS, Razonable RR. Clinical correlation of cytomegalovirus infection with CMV-specific CD8+ T-cell immune competence score and lymphocyte subsets in solid organ transplant recipients. Transplantation 2019; 103:832–8. [DOI] [PubMed] [Google Scholar]
- 18. Kumar D, Mian M, Singer L, Humar A. An interventional study using cell-mediated immunity to personalize therapy for cytomegalovirus infection after transplantation. Am J Transplant 2017; 17:2468–73. [DOI] [PubMed] [Google Scholar]
- 19. Morton CC, Nance WE. Newborn hearing screening–a silent revolution. N Engl J Med 2006; 354:2151–64. [DOI] [PubMed] [Google Scholar]
- 20. Britt WJ. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Nizet V, Maldonaldo Y, eds. Infectious diseases of the fetus and newborn infant. 7th ed Philadelphia: Elsevier Saunders, 2011:706–55. [Google Scholar]
- 21. Emery VC, Lazzarotto T. Cytomegalovirus in pregnancy and the neonate. F1000Res 2017; 6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 23. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 24. de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 2013; 23:241–9. [DOI] [PubMed] [Google Scholar]
- 25. Revello MG, Tibaldi C, Masuelli G, et al. ; CCPE Study Group Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine 2015; 2:1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis 2017; 17:e177–88. [DOI] [PubMed] [Google Scholar]
- 27. National Center for Immunization and Respiratory Diseases. Talking with pregnant patients about CMV: a resource for healthcare providers. https://www.cdc.gov/cmv/downloads/pregnant-patients-cmv.pdf. Accessed 16 December 2019. [Google Scholar]
- 28. American College of Obstetricians and Gynecologists. Reducing risks of birth defects. https://www.acog.org/-/media/For-Patients/faq146.pdf. Accessed 16 December 2019. [Google Scholar]
- 29. Gantt S, Dionne F, Kozak FK, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr 2016; 170:1173–80. [DOI] [PubMed] [Google Scholar]
- 30. Boppana SB, Smith RJ, Stagno S, Britt WJ. Evaluation of a microtiter plate fluorescent-antibody assay for rapid detection of human cytomegalovirus infection. J Clin Microbiol 1992; 30:721–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balcarek KB, Warren W, Smith RJ, Lyon MD, Pass RF. Neonatal screening for congenital cytomegalovirus infection by detection of virus in saliva. J Infect Dis 1993; 167:1433–6. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol 2006; 36:228–30. [DOI] [PubMed] [Google Scholar]
- 33. Boppana SB, Ross SA, Shimamura M, et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med 2011; 364:2111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nozawa N, Koyano S, Yamamoto Y, Inami Y, Kurane I, Inoue N. Real-time PCR assay using specimens on filter disks as a template for detection of cytomegalovirus in urine. J Clin Microbiol 2007; 45:1305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gruppo -Mutidisciplinare. Malattie infettive in ostetricia-ginecologia e neonatologia. Percorsi diagnostici-assistenziali in ostetricia-ginecologia e Neonatologia. Citomegalovirus. [Infectious diseases in obstetrics-gynecology and neonatology. Diagnostic-assistance pathways in obstetrics-gynecology and neonatology. Cytomegalovirus.]. 2012. Available at: www.amcli.it/wp-content/uploads/2015/10/CITOMEGALOVIRUSAprilE2012.pdf [Google Scholar]
- 36. Koyano S, Inoue N, Oka A, et al. ; Japanese Congenital Cytomegalovirus Study Group Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ Open 2011; 1:e000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujii T, Oka A, Morioka I, et al. ; Japanese Congenital Cytomegalovirus Study Group Newborn congenital cytomegalovirus screening based on clinical manifestations and evaluation of DNA-based assays for in vitro diagnostics. Pediatr Infect Dis J 2017; 36:942–6. [DOI] [PubMed] [Google Scholar]
- 38. Pinninti SG, Ross SA, Shimamura M, et al. ; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection. Pediatr Infect Dis J 2015; 34:536–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lilleri D, Gerna G. Maternal immune correlates of protection from human cytomegalovirus transmission to the fetus after primary infection in pregnancy. Rev Med Virol 2017; 27:doi: 10.1002/rmv.1921. [DOI] [PubMed] [Google Scholar]
- 40. Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol 2017; 38:97–107. [DOI] [PubMed] [Google Scholar]
- 41. Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr 2000; 137:90–5. [DOI] [PubMed] [Google Scholar]
- 42. Sonoyama A, Ebina Y, Morioka I, et al. Low IgG avidity and ultrasound fetal abnormality predict congenital cytomegalovirus infection. J Med Virol 2012; 84:1928–33. [DOI] [PubMed] [Google Scholar]
- 43. Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis 2019; 69:1526–32. [DOI] [PubMed] [Google Scholar]
- 44. Delforge ML, Costa E, Brancart F, et al. Presence of cytomegalovirus in urine and blood of pregnant women with primary infection might be associated with fetal infection. J Clin Virol 2017; 90:14–7. [DOI] [PubMed] [Google Scholar]
- 45. Tanimura K, Tairaku S, Ebina Y, et al. Prediction of congenital cytomegalovirus infection in high-risk pregnant women. Clin Infect Dis 2017; 64:159–65. [DOI] [PubMed] [Google Scholar]
- 46. Zavattoni M, Furione M, Lanzarini P, et al. Monitoring of human cytomegalovirus DNAemia during primary infection in transmitter and non-transmitter mothers. J Clin Virol 2016; 82:89–93. [DOI] [PubMed] [Google Scholar]
- 47. Simonazzi G, Cervi F, Zavatta A, et al. Congenital cytomegalovirus infection: prognostic value of maternal DNAemia at amniocentesis. Clin Infect Dis 2017; 64:207–10. [DOI] [PubMed] [Google Scholar]
- 48. Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011; 17:1285–93. [DOI] [PubMed] [Google Scholar]
- 49. Revello MG, Fabbri E, Furione M, et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: a 20-year experience. J Clin Virol 2011; 50:303–7. [DOI] [PubMed] [Google Scholar]
- 50. Enders M, Daiminger A, Exler S, Enders G. Amniocentesis for prenatal diagnosis of cytomegalovirus infection: challenging the 21 weeks’ threshold. Prenat Diagn 2017; 37:940–2. [DOI] [PubMed] [Google Scholar]
- 51. Bilavsky E, Pardo J, Attias J, et al. Clinical implications for children born with congenital cytomegalovirus infection following a negative amniocentesis. Clin Infect Dis 2016; 63:33–8. [DOI] [PubMed] [Google Scholar]
- 52. Leruez-Ville M, Stirnemann J, Sellier Y, et al. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am J Obstet Gynecol 2016; 215:342.e1–9. [DOI] [PubMed] [Google Scholar]
- 53. Fabbri E, Revello MG, Furione M, et al. Prognostic markers of symptomatic congenital human cytomegalovirus infection in fetal blood. BJOG 2011; 118:448–56. [DOI] [PubMed] [Google Scholar]
- 54. Guerra B, Simonazzi G, Puccetti C, et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 2008; 198:380.e1–7. [DOI] [PubMed] [Google Scholar]
- 55. Picone O, Vauloup-Fellous C, Cordier AG, et al. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 2013; 33:751–8. [DOI] [PubMed] [Google Scholar]
- 56. Farkas N, Hoffmann C, Ben-Sira L, et al. Does normal fetal brain ultrasound predict normal neurodevelopmental outcome in congenital cytomegalovirus infection? Prenat Diagn 2011; 31:360–6. [DOI] [PubMed] [Google Scholar]
- 57. Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol 2013; 41:508–14. [DOI] [PubMed] [Google Scholar]
- 58. Capretti MG, Lanari M, Tani G, et al. Role of cerebral ultrasound and magnetic resonance imaging in newborns with congenital cytomegalovirus infection. Brain Dev 2014; 36:203–11. [DOI] [PubMed] [Google Scholar]
- 59. Ross SA, Ahmed A, Palmer AL, et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 2014; 210:1415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ogawa H, Suzutani T, Baba Y, et al. Etiology of severe sensorineural hearing loss in children: independent impact of congenital cytomegalovirus infection and GJB2 mutations. J Infect Dis 2007; 195:782–8. [DOI] [PubMed] [Google Scholar]
- 61. Koyano S, Inoue N, Nagamori T, et al. Dried umbilical cords in the retrospective diagnosis of congenital cytomegalovirus infection as a cause of developmental delays. Clin Infect Dis 2009; 48:e93–5. [DOI] [PubMed] [Google Scholar]
- 62. Kharrazi M, Hyde T, Young S, Amin MM, Cannon MJ, Dollard SC. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. J Pediatr 2010; 157:191–7. [DOI] [PubMed] [Google Scholar]
- 63. Leruez-Ville M, Vauloup-Fellous C, Couderc S, et al. Retrospective diagnosis of congenital CMV infection in DBS from Guthrie cards: French experience. Arch Pediatr 2009; 16:1503–6. [DOI] [PubMed] [Google Scholar]
- 64. Atkinson C, Walter S, Sharland M, et al. Use of stored dried blood spots for retrospective diagnosis of congenital CMV. J Med Virol 2009; 81:1394–8. [DOI] [PubMed] [Google Scholar]
- 65. Barbi M, Binda S, Primache V, Luraschi C, Corbetta C. Diagnosis of congenital cytomegalovirus infection by detection of viral DNA in dried blood spots. Clin Diagn Virol 1996; 6:27–32. [DOI] [PubMed] [Google Scholar]
- 66. Boppana SB, Ross SA, Novak Z, et al. ; National Institute on Deafness and Other Communication Disorders CMV and Hearing Multicenter Screening (CHIMES) Study Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 2010; 303:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koontz D, Baecher K, Amin M, Nikolova S, Gallagher M, Dollard S. Evaluation of DNA extraction methods for the detection of cytomegalovirus in dried blood spots. J Clin Virol 2015; 66:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. de Vries JJC, Claas ECJ, Kroes ACM, Vossen ACTM. Evaluation of DNA extraction methods for dried blood spots in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2009; 46:S37–42. [DOI] [PubMed] [Google Scholar]
- 69. Göhring K, Dietz K, Hartleif S, Jahn G, Hamprecht K. Influence of different extraction methods and PCR techniques on the sensitivity of HCMV-DNA detection in dried blood spot (DBS) filter cards. J Clin Virol 2010; 48:278–81. [DOI] [PubMed] [Google Scholar]
- 70. Atkinson C, Emery VC, Griffiths PD. Development of a novel single tube nested PCR for enhanced detection of cytomegalovirus DNA from dried blood spots. J Virol Methods 2014; 196:40–4. [DOI] [PubMed] [Google Scholar]
- 71. Koontz D, Dollard S, Cordovado S. Evaluation of rapid and sensitive DNA extraction methods for detection of cytomegalovirus in dried blood spots. J Virol Methods 2019; 265:117–20. [DOI] [PubMed] [Google Scholar]
- 72. Ross SA, Ahmed A, Palmer AL, et al. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 2014; 210:1415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ross SA, Michaels MG, Ahmed A, et al. Contribution of breastfeeding to false-positive saliva polymerase chain reaction for newborn congenital cytomegalovirus screening. J Infect Dis 2018; 217:1612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nishida K, Morioka I, Nakamachi Y, et al. Neurological outcomes in symptomatic congenital cytomegalovirus-infected infants after introduction of newborn urine screening and antiviral treatment. Brain Dev 2016; 38:209–16. [DOI] [PubMed] [Google Scholar]
- 75. Guerra B, Simonazzi G, Banfi A, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol 2007; 196:221.e1–6. [DOI] [PubMed] [Google Scholar]
- 76. Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 2015; 61:1228–34. [DOI] [PubMed] [Google Scholar]
- 77. Rizzo R, Gabrielli L, Bortolotti D, et al. Study of soluble HLA-G in congenital human cytomegalovirus infection. J Immunol Res 2016; 2016:3890306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Desveaux C, Klein J, Leruez-Ville M, et al. Identification of symptomatic fetuses infected with cytomegalovirus using amniotic fluid peptide biomarkers. PLoS Pathog 2016; 12:e1005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eldar-Yedidia Y, Bar-Meir M, Hillel M, et al. Low interferon relative-response to cytomegalovirus is associated with low likelihood of intrauterine transmission of the virus. PLoS One 2016; 11:e0147883. [DOI] [PMC free article] [PubMed] [Google Scholar]