Abstract
Although cytomegaloviruses (CMVs) are species-specific, the study of nonhuman CMVs in animal models can help to inform and direct research aimed at developing a human CMV (HCMV) vaccine. Because the driving force behind the development of HCMV vaccines is to prevent congenital infection, the animal model in question must be one in which vertical transmission of virus occurs to the fetus. Fortunately, two such animal models—the rhesus macaque CMV and guinea pig CMV—are characterized by congenital infection. Hence, each model can be evaluated in “proof-of-concept” studies of preconception vaccination aimed at blocking transplacental transmission. This review focuses on similarities and differences in the respective model systems, and it discusses key insights from each model germane to the study of HCMV vaccines.
Keywords: Congenital cytomegalovirus, cytomegalovirus glycoproteins, cytomegalovirus vaccines, guinea pig cytomegalovirus, rhesus macaque cytomegalovirus
Human cytomegalovirus (HCMV) is the most common congenital virus worldwide and is a prominent cause of disease in susceptible adults. Seroprevalence of HCMV varies widely by region, and approximately half of the population in Western countries have a history of exposure compared with greater than 90% of adults in developing nations [1, 2]. Although infection is generally subclinical in healthy children and adults, HCMV infection can cause severe complications in immunocompromised patients, including hepatitis, gastroenteritis, and pneumonitis [3]. Congenital HCMV (cCMV) infection is a major cause of neurodevelopmental delays and infant brain damage and is the leading cause of nongenetic sensorineural hearing loss (SNHL) worldwide [4, 5]. In fact, HCMV accounts for more congenital disease than all 29 newborn conditions currently screened for in the United States combined [6], yet public awareness and effective interventions are severely lacking—both for women with primary HCMV infections during pregnancy and for seropositive women with recurrent infections [7, 8].
Our understanding of HCMV infection and immunity has benefited greatly from animal model studies. It has been noted that, due to millennia of coevolution and adaptation, CMV infections are highly species-specific, and there have been no cross-species infections of primates caused by related but non-native (heterologous) strains reported to date [9]. Cytomegaloviruses are also found in a broad range of nonprimate species, including guinea pigs, mice, and rats [10]. Human CMV is also closely related to the CMVs of nonhuman primates, making these primate CMVs ideal models for studies of HCMV infection and immunity. Cytomegaloviruses of multiple nonhuman primate species have been described, including rhesus macaques (Macaca mulatta), cynomolgus macaques [11, 12], and chimpanzees [13]. A natural pathogen of rhesus macaques, rhesus macaque CMV (RhCMV) has emerged as a highly relevant, accessible experimental model. Among the nonprimate CMVs, the guinea pig CMV (GPCMV) is recognized as a highly tractable model, well suited to the study of vaccines and pathogenesis [14–17]. This review discusses the similarities and differences between non-HCMVs and HCMV in the context of species-specific viral genetics, viral protein regulation and function, transmission, and immune responses to infection, with a particular emphasis on the relative strengths of the RhCMV and GPCMV models.
SPECIES-SPECIFIC VIRAL GENETICS
Rhesus macaque CMV and HCMV have high sequence homology, with approximately 97% shared nucleotide identity for full-length RhCMV strains that have been sequenced [18, 19]. The RhCMV genome (strain 68-1) is 221 459 base pairs in length, which is slightly smaller than the 229 354 bp of HCMV [18]. Both genomes are collinear and share a similar structure, although RhCMV does not appear to isomerize as HCMV does [18]. Approximately 80% of RhCMV open reading frames (ORFs) have orthologs in HCMV, and greater than 90% of RhCMV ORFs have orthologs at the level of protein families, which strongly suggests a common ancestor [18–21]. A region at the end of the HCMV unique long (UL) genome component (UL/b’) contains multiple ORFs encoding cell tropism and immune modulating functions that have been deleted or truncated in serially passaged laboratory strains [22, 23]. Similar to the HCMV UL/b’ region, RhCMV UL/b’ is highly unstable and can accumulate mutations and deletions even after brief passages in vitro [20]. The basis for the genomic instability within RhCMV ULb’ remains unknown [20]. In addition to the ULb’ region, RhCMV-specific gene loci are located in rh165-180 ORFs [21].
The 2 most well characterized RhCMV strains are 68-1 and 180.92. Rhesus macaque CMV 68-1 is 5776 nucleotides larger than strain 180.92 [19]. Rhesus macaque CMV 68-1 has been annotated as containing 230 ORFs of >99 amino acid residues [18], compared with 180.92 that contains 258 ORFs ranging in size from 21 to 2178 amino acids [19], although each study used a different analytical technique.
In contrast to RhCMV, the GPCMV genome is much less homologous to the HCMV genome. Sequence of salivary gland homogenates demonstrated that the wild-type GPCMV genome (ATCC or 22122 strain) is 233 501 in length [24]. Similar to other rodent CMVs and RhCMV, the GPCMV genome does not isomerize. Highly conserved betaherpesvirus envelope glycoproteins, including the gB, gH/gL/gO, and gM/gN complexes, have been identified and expressed for GPCMV [25]. These proteins serve as instructive vaccine targets in the guinea pig model (discussed below). Again, with similarity to RhCMV, GPCMV is highly prone to mutation and rearrangement when passaged in cell culture [26]. In a recent study, a novel “clinical isolate” of GPCMV was isolated from salivary gland homogenates of seropositive animals purchased from a commercial colony [27]. This strain, which demonstrates divergence in glycoprotein epitopes compared with the prototypical ATCC strain, can be used to model “reinfection” studies in animals seropositive to heterologous strains.
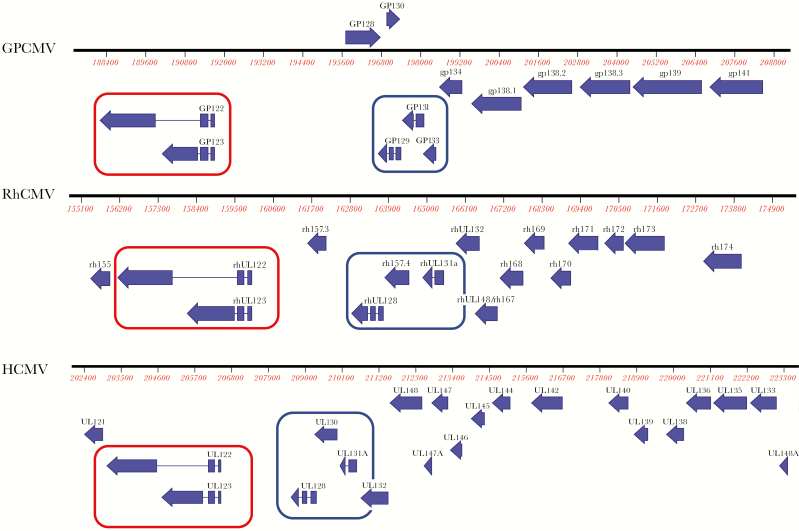
A side-by-side comparison of collinear regions of all 3 genomes is demonstrated in Figure 1, focusing on a ~20-kilobase region corresponding to the major immediate early transcription unit of each virus and the smaller subunits of the respective pentameric complex (PC) of each virus (discussed in more detail in the following section).
Figure 1.
Comparison of guinea pig cytomegalovirus (GPCMV), rhesus macaque CMV (RhCMV), and human CMV (HCMV) genomes. Side-by-side comparison of collinearity of respective CMV genomes and selected open reading frames (ORFs) in ~20-kilobase segment adjacent to major immediate early (MIE) region. (Top Panel) The GPCMV (salivary gland isolate, strain 22122; GenBank accession number NC_020231); (middle panel) RhCMV (strain 180.92, GenBank accession number DQ120516); (bottom panel) HCMV (strain TBE/40, GenBank accession number EF999921). Conserved regions circled in red represent highly conserved MIE transcripts for each virus. The ORFs circled in blue represent smaller subunits of conserved CMV pentamer. Three smaller proteins complex with gH (gpUL75) and gL (gpUL115); the nomenclature for these smaller subunits varies among the 3 CMV species as reviewed in the text. Other adjacent ORFs in this genome region have been identified as playing roles in viral pathogenesis as discussed in the text.
VIRAL PROTEIN FUNCTIONS
Multiple studies have characterized proteins important for infection in RhCMV and GPCMV, and they compared sequence and function to homologous proteins in HCMV. It is interesting to note that, although there is a large amount of coding potential that seems (at the amino acid sequence level) to be unique to either RhCMV and GPCMV, many of these proteins (for each virus) have functional homologs to HCMV. Highly conserved genes, as well as species-specific viral genes important in immune modulation, have also been identified and characterized [28, 29]. Conserved functions for several gene families important in immunity and pathogenesis have been investigated in both RhCMV and GPCMV. These are summarized in Table 1. For example, the IE1 and IE2 genes have critical roles in reactivation from CMV latency and acute infection. The IE1 (UL123)/IE2 (UL122) promoter and protein coding regions are generally conserved in structure, transcript organization, and protein sequence between RhCMV and HCMV [30, 31] and (albeit to a lesser extent) in GPCMV [27].
Table 1.
RhCMV and GPCMV Gene Products Used for Generation of Subunit Protein or Vectored Vaccines or Targeted for Deletion in Generation of Live, Attenuated Vaccines for Evaluation in Respective Animal Models
| HCMV Gene Product | RhCMV | GPMCV |
|---|---|---|
| IE Gene Family | • Similar role in structure and function to HCMV IE • Role in protective immunity incompletely defined • Evaluated (with pp65 homolog and gB) in RhCMV vaccine studies [32] | • Similar role in structure and function to HCMV IE • Role in antiviral immune responses and protective immunity uncharacterized |
| Glycoprotein B (gpUL55) | • Similar role in structure and function to HCMV [33] • Anti-gB plasmablast response associated with protection against transmission in RhCMV-challenged dam [34] • Evaluated in vaccine studies using MVA vectors and DNA expression plasmids [32] and as a purified protein subunit [35] | • Similar role in structure and function as HCMV gB [36] • Plasmid and adjuvanted, purified recombinant vaccines confer protection against maternal and fetal GPCMV disease [37–40] • GPCMV gB vaccines modify disease and reduced viral load and mortality in pups but do not prevent vertical transmission |
| gH/gL/gO (gpUL75, gpUL74, gpUL115; trimer) gH/gL and UL128, UL130, UL131 (pentamer) | • Conserved in RhCMV • Rh157.5, Rh157.4, and Rh157.6: homologs of UL128, UL130, and UL131 [41] • Vaccine based on RhCMV UL128/PC in MVA vector induces neutralizing antibody [42] | • Pretreatment with lectin-column purified polyclonal antibody targeting multiple glycoproteins limits infection and improves pup outcomes [43] • Protection against congenital transmission described with immunoaffinity-purified anti-gB polyclonal antibody [44] • Improved outcomes with anti-gH monoclonal antibody [45] |
| UL83 (pp65; ppUL83) | • Multiple copies of homolog found in RhCMV genome • Functional homolog Rh112 [46] • Has been evaluated (with gB and IE homologs) in RhCMV vaccine studies [32] | • GPCMV homolog of pp65, GP83, similar in function, sequence, phosphorylation [47, 48] • Monovalent GP83 VEE-vectored vaccine elicited CD4+ and CD8+ responses and reduced maternal viral load congenital transmission [40] • LCMV-vectored vaccine elicited IFN-γ ELISPOT responses and synergized with gB to improve outcomes in congenital infection experiments [49] |
| IL-10 (UL111a) | • IL-10 homolog conserved in RhCMV genome [50, 51] • Macaques immunized with a recombinant IL-10 vaccine developed antibodies that neutralized IL-10 function and demonstrate reduced shedding in bodily fluids [52] | • No IL-10 homolog found to date in GPCMV |
| IRS1/TRS1 (PKR evasin) | • Conserved in RhCMV [18] • Functions in inhibition of PKR • Has not been investigated in context of vaccination | • GP145 encodes PKR antagonist in GPCMV genome [29] • Vaccination with an attenuated GPCMV vaccine with GP145 deletion resulted in reduced maternal viremia and improved pregnancy outcome [53] |
| MHC class I evasins MHC class I homologs | • Conserved in RhCMV • MHC class I immune evasion genes Rh178 and Rh182-189 [35] • Vaccine (RhCMVRΔgL/178/182-189) based on Rh178 and Rh182-189 deletion with restored epithelial cell tropism (via insertion of Rh128-131A homologs) induced cellular immune responses but had reduced protection compared with RhCMV subunit gB [35] | • GPCMV encodes homologs of class I genes, GP147-149 [54] • Potential NK cell evasins • Targeted deletion of GP147-149 generated attenuated vaccine that retained full immunogenicity of wild-type GPCMV; preconception immunization with deletion vaccine, 3DX, reduced maternal viral loads and pup mortality after pathogenic viral challenge during pregnancy |
Abbreviations: DNA, deoxyribonucleic acid; ELISPOT, enzyme-linked immunospot; gB, glycoprotein B; GPCMV, guinea pig cytomegalovirus; HCMV, human cytomegalovirus; IE, immediate early; IFN, interferon; IL, interleukin; LCMV, lymphocytic choriomeningitis virus; MHC, major histocompatibility complex; MVA, modified vaccinia Ankara; NK, natural killer; RhCMV, rhesus macaque cytomegalovirus; VEE, Venezuelan equine encephalitis.
The HCMV phosphoprotein 65 (pp65), also known as ppUL83, is also conserved in RhCMV and GPCMV. For HCMV, this protein is the most abundant constituent of the virion tegument and is also abundant in noninfectious particles referred to as dense bodies [46, 55, 56]. Rhesus macaque CMV encodes 2 ORFs with comparable homology (pp65a 34%; pp65b 40%) that have 40% sequence identity to one another [18, 46]. Guinea pig CMV encodes a single pp65 homolog, designated as GP83 [47]. The RhCMV pp65a and pp65b proteins combined comprise approximately 11% of the entire viral proteome [21], similar to HCMV pp65, which contributes up to 15% of the total virion mass [57]. After virus penetration into cells, pp65 localizes predominantly to the nucleus and accumulates in both the nucleus and cytoplasm as the virus matures late in infection, where it may associate with a kinase [56, 58]. Guinea pig CMV GP83 similarly encodes nuclear localization motifs and is phosphorylated by a currently uncharacterized kinase [47, 48]. In both HCMV and RhCMV, pp65 associates with other tegument proteins, and deletion of pp65 in either HCMV or RhCMV results in a decrease in total tegument protein [59, 60]. However, although HCMV pp65 deletion results in nonselective lack of specific tegument proteins [59], deletion of RhCMV pp65 causes a substantial loss of a selected set of tegument proteins [60], suggesting that there are species-specific differences in pp65 tegument-binding specificities.
Envelope glycoprotein B (gB) is another CMV protein of major interest for vaccine development, insofar as it is highly conserved across all CMV species. In HCMV, RhCMV, and GPCMV, gB is essential for viral entry into all known cell types and is a dominant target for neutralizing antibody responses [33, 34, 36, 61]. In fact, the most efficacious HCMV vaccine to date is a recombinant gB subunit vaccine with MF59 adjuvant, which demonstrated ~50% efficacy in multiple phase II clinical trials in populations of seronegative postpartum women [62], adolescent women [63], and transplant recipients [64]. Human CMV gB and RhCMV gB share 60% identity at the amino acid level [33]; the GPCMV gB demonstrates 42% identity with HCMV gB overall, with even more striking identity observed in a region corresponding to a neutralizing epitope in the carboxy-terminal moiety of the gB complex, AD-1 [36]. Human CMV gB-specific antibodies are cross-reactive with RhCMV gB protein and can cross-neutralize RhCMV gB [61]. Thus, both rhesus macaques and guinea pigs provide useful models for studying vaccines and immunoglobulin (Ig)-based therapies that target gB.
In addition to gB, other glycoproteins and glycoprotein complexes are necessary for HCMV and RhCMV entry into various cell types. Human CMV entry into fibroblasts involves glycoproteins gB, gH/gL, and/or gH/gL/gO [65–67], whereas entry into epithelial and endothelial cells requires gB, gH/gL, and the PC (consisting of gH/gL/UL128/130/UL131A) [68–72]. Subunits of the PC are conserved in RhCMV [18, 19, 42]. As in HCMV, RhCMV subunits interact in a pentameric structure and are essential for entry into epithelial cells [42, 73, 74]. Furthermore, vaccination of rhesus macaques with a bacterial artificial chromosome-derived modified vaccinia Ankara (MVA) vector coexpressing all 5 RhCMV pentameric subunits elicited potent RhCMV-specific neutralizing antibody responses against RhCMV infection of epithelial cells and fibroblasts and reduced RhCMV plasma viral loads [42]. A subsequent iteration of this MVA vector was designed to coexpress the HCMV pentameric subunits [75]. When rhesus monkeys were vaccinated with this vector encoding HCMV pentameric subunits, vaccine-stimulated antibodies neutralized HCMV infection of human epithelial cells, placental macrophages, and fibroblasts [75], supporting the premise that the functional similarities between the HCMV and RhCMV pentamer proteins may allow modeling of HCMV PC-based vaccines in the rhesus model.
The situation for GPCMV, on the other hand, is less clear. Similar to RhCMV, GPCMV encodes a homolog of the HCMV PC [76–79], comprising proteins gH/gL/GP129/131/133 (homologs of HCMV gH/gL/UL128/129/131a). However, it is not clear that deletion of the GPCMV PC confers a selective inability of the virus to enter cells of epithelial and endothelial origin; rather, modifications that abrogate generation of the PC confer a generalized defect in virus entry in both epithelial/endothelial cells and in cells of fibroblast origin [77]. Although the PC may not play an analogous role in cellular tropism, it does play a role in GPCMV pathogenesis. Guinea pig CMV strains defective in each of GP129, GP131, and GP133 had modified macrophage tropism [80], and a GPCMV mutant with a frame-shift mutation in the GP129 homolog was attenuated for viral dissemination [81]. Thus, although the GPCMV PC may have different functions in the viral life cycle than the homologous HCMV and RhCMV complexes, it may nonetheless be an instructive vaccine target for study in the guinea pig congenital infection model.
It is interesting to note that, due to the ability of CMV to establish indefinitely persistent, high-frequency, antigen-specific effector memory T-cell responses, RhCMV lacking pentameric subunit expression has been used as a vaccine vector to express simian immunodeficiency virus (SIV) proteins [41]. Rhesus macaque CMV 68-1 lacks the surface glycoproteins Rh157.5 and Rh157.4, which are homologs for HCMV UL128 and UL130, respectively, and therefore does not encode proteins necessary to form a functional PC. Vaccination with the RhCMV 68-1 vector encoding SIV proteins induced unconventional CD8+ T-cell responses, namely, targeting of diverse, highly promiscuous epitopes presented by major histocompatibility complex (MHC) II molecules [41], but unconventional T-cell responses only occurred when the RhCMV vector lacked the HCMV UL128-131A homologs [82]. Upon challenge with SIV infection, approximately 50% of rhesus macaques vaccinated with RhCMV 68-1 encoding SIV proteins were protected. Thus, RhCMV vectors can be genetically programmed to achieve distinct patterns of CD8+ T-cell epitope recognition, and these in turn may become valuable tools for vaccination against other pathogens, in studies that use the recombinant CMV backbone as a “vector” for expression of heterologous gene products expressed by other infectious agents.
VIRAL TRANSMISSION
Horizontal Virus Transmission
Similar to the widespread prevalence of HCMV, RhCMV is ubiquitous in both the wild and breeding nonhuman primate colonies. Breeding colonies of rhesus monkeys in India have an RhCMV prevalence of 95% [83], and close to 100% of rhesus macaques in primate centers are RhCMV seropositive [84]. In breeding colonies, the vast majority of monkeys seroconvert by one year of age [85]. RhCMV can infect a wide range of tissues, including those of urinary tract, immune system, as well as reproductive organs. Once infected, nonhuman and human primates continue to shed their respective species-specific viruses for the rest of their lives in urine and saliva [85, 86], and horizontal transmission of RhCMV is possible through similar compartments of shedding as that of HCMV, namely, urine, saliva, feces (less prominently a source of infection for HCMV), semen, and plasma [32, 87, 88]. The situation is not well described for GPCMV, but the apparently ubiquitous nature of the infection in guinea pigs maintained in captivity suggests that horizontal transmission is likely very common [89].
Vertical Virus Transmission
The key strengths of the RhCMV and GPCMV models for the study of HCMV vaccines derive from the ability to model fetal infection for each species. These attributes and comparisons are summarized in Table 2. Several features of rhesus macaque and guinea pig reproductive biology contribute to the usefulness of these models. Both rhesus monkey and guinea pig pregnancies are divided into trimesters, similar to that of human pregnancies, and share similar characteristics of placental [90–92] and fetal development, especially (in the case of macaques) with respect to organ and immune system maturation [93]. Vertical transmission of RhCMV in rhesus monkeys can occur via transplacental infection [94]. Furthermore, virus is shed in breast milk, leading to postnatal virus exposure, as is well-described for HCMV [95–97]. However, a study of natural postnatal RhCMV exposure and transmission could not conclusively determine whether transmission of RhCMV in rhesus breast milk would be a reliable postnatal model due to the intermittent nature of RhCMV shedding and the small number of infant infections that were confirmed via this route [98]. In both models, placental pathology is observed, and at higher doses of challenge virus during pregnancy, fetal mortality can be observed [16, 94]. A limitation of both models is that large doses of challenge virus are often used, employing parenteral routes of inoculation, to ensure efficient infection of the fetus. Efforts to utilize mucosal routes of challenge that more authentically mimic maternal human infection are high-priority areas for future work in both models.
Table 2.
Comparison of Aspects of Pathogenesis and Immunity of RhCMV and GPCMV Vertical Transmission Models That Recapitulate Congenital HCMV Infection
| Congenital CMV: Comparisons of HCMV Pathogenesis and Immunity With RhCMV and GPCMV Models | |||
|---|---|---|---|
| HCMV | RhCMV | GPCMV | |
| Placental transmission | • Primary infection with HCMV leads to vertical transmission 30%–40% of the time; yet, chronic infection results in transmission 1% of the time [2] • Placental transmission rate of 3.4% in nonprimary infection setting [99] | • Impact of T-depletion: 2 of 3 seronegative, immunocompetent dams transmitted, whereas 6 of 6 CD4+ T cell-depleted dams transmitted RhCMV [94] • In T-depleted dams that transmitted, 5 had fetal loss [94] | • Placental transmission well described in guinea pigs [16, 90–92] • Vertical transmission described in both primary and nonprimary [27] maternal infections • Tissue culture model of explanted guinea pig placenta has been described to model GPCMV placental infection [92] |
| Fetal sequelae | • Sensorineural hearing loss, microcephaly, ventricular calcification, developmental delay, neutropenia, thrombocytopenia, hepatitis, and fetal loss [100] | • Infection of spiral ganglion and damage to inner ear, ventriculomegaly, leptomeningitis, microcephaly, lower limb deformities, and fetal loss are all described in macaque fetus [94, 101–105] • Neutropenia, liver calcifications observed [94] | • Intrauterine growth retardation [16] • Maternal and pup mortality [16] • Brain and visceral involvement [106] • Labyrinthitis and sensorineural hearing loss observed [107–109] |
| Antibody protection against placental transfer | • Naturally acquired immunity reduces transmission rate and improves fetal outcome [100] • Antibody treatment may suppress HCMV dissemination to placenta and improve fetal outcome [110] | • Pre-existing maternal CMV-specific antibodies can reduce the incidence and/or severity of congenital CMV [105] • Combined PC and gB vaccine could generate higher and broader neutralizing antibody activity [42] | • Pretreatment with polyclonal antibody limits infection and improves pup outcomes [43] • Protection against congenital transmission described with immunoaffinity-purified anti-gB polyclonal antibody [44] • Improved outcomes with anti-gH monoclonal antibody [45] |
| Role of maternal CD4+ T cells in cCMV | • Congenital transmission of cCMV is inversely correlated with maternal CD4+ T-cell counts (reviewed in [157]) • Rapid containment of a primary HCMV infection in an immunocompetent host may depend on the simultaneous development of HCMV-specific CD4+ and CD8+ T-cells [111] | • T-cell dependent activation of plasmablasts is necessary for a rapid response [34] • Maternal CD4+ cells play an important role in control of maternal RhCMV viral load, promotion of virus-specific CD8+ T-cell responses, and production of autologous neutralizing antibodies [94] • CD4+ T cells are essential for prevention of in utero transmission [94] | • Maternal T-cell responses play a role in limiting maternal viremia and secondary dissemination to placenta and fetus • CD4+ ELISPOT responses generated against MVA-vectored and LCMV-vectored GP83 (GPCMV UL83 homolog) vaccines [49, 112] • CD4+ and CD8+ T-cell responses engendered by Venezuelan equine encephalitis virus-vectored vaccines [40] |
| Postnatal transmission | • CMV reaches the offspring via uterine cervical secretions during the birth process [96] • Breast milk is a source of postnatal HCMV transmission [95–97] | • Viral shedding of RhCMV occurs in breast milk; however, transmission via this route is less efficient than HCMV due to intermittent shedding [98] | • Not well studied; animal husbandry experiments suggest postnatal transmission in animal colonies [89] |
Abbreviations: cCMV, congenital human cytomegalovirus; CMV, cytomegalovirus; ELISPOT, enzyme-linked immunospot; gB, glycoprotein B; GPCMV, guinea pig CMV; HCMV, human CMV; LCMV, lymphocytic choriomeningitis virus; PC, pentameric complex; RhCMV, rhesus macaque CMV.
Although there are differences in the pathogenesis of infection in each of the respective host species, several studies have nonetheless recapitulated many of the sequelae of cCMV infection in both GPCMV and RhCMV infection models. For example, congenital GPCMV infection has been shown to cause central nervous system infection and elicit focal neurological disease, in particular glial nodule encephalitis [106]. Vertically transmitted GPCMV is also associated with SNHL with an associated cochlear pathology [107–109]. Inner ear disease has also been observed in the RhCMV model. In a study of CMV-associated congenital hearing loss with RhCMV, inoculation of rhesus macaque fetuses with virus at a gestational age of 50 days enabled infection of cells in the spiral ganglion and structural damage to structures of the inner ear [101], similar to the pathology observed in cCMV infection. Other studies have successfully used direct fetal or intraperitoneal inoculation in rhesus macaques to demonstrate other sequelae of cCMV infection, including ventricular dilatation, leptomeningitis, microcephaly, lower limb deformities, and other systemic disease [102–104].
Although direct inoculation of the rhesus macaque fetus has provided insights into a number of CMV pathologies observed in infants, these methods were limited in their capacity to model intrauterine transmission of RhCMV. The challenge of identifying RhCMV-seronegative macaque colonies further complicates study of primary maternal infection during pregnancy, which may be associated with more severe fetal pathologies. To navigate this problem, investigators have adopted the use of specific pathogen-free breeding programs for rhesus colonies, which require separation of infants from the dam soon after birth followed by cohousing of these monkeys only with similarly treated populations [113]. In this context, the GPCMV does have the potential advantage of being amenable to studies in seronegative animals more readily accessible from commercial sources [114]. Fortunately, seronegative macaques are becoming increasingly available, and challenge studies of congenital transmission in RhCMV-seronegative dams have been conducted. These studies successfully recapitulate the pathogenesis of cCMV transmission [94]. CD4+ T-cell depletion has been used as a tool to facilitate the study of vertical transmission and to elucidate the impact of T-cell immunity on congenital infection. In one study, 2 groups of pregnant RhCMV-seronegative dams (one of which was treated with a recombinant rhesus anti-CD4+ T cell-depleting antibody 1 week before infection) were inoculated early in the second trimester of pregnancy with a mixture of RhCMV strains, 180.92, UCD52, and UCD59, to mimic cCMV infection. In this study, congenital RhCMV transmission was successfully achieved in all CD4+ T cell-depleted monkeys and in 2 of the 3 immunocompetent monkeys. Liveborn infants demonstrated detectable sequelae at birth, including a liver lesion and persistent neutropenia, at a rate similar to that of symptomatic congenitally infected human newborns [94]. Thus, both the GPCMV and RhCMV provide useful and relevant endpoints that model cCMV transmission and sequelae, allowing investigators to ask research questions that address the need to find strategies to prevent vertical virus transmission.
VIRUS-SPECIFIC IMMUNE RESPONSES
Host Cell Responses to Cytomegalovirus Infection: Antibody Responses
Elicitation of neutralizing antibodies against CMV is thought to be an essential feature for efficacious vaccine development. Because RhCMV and GPCMV both have considerable structural homology to HCMV, especially with regard to the gB and pp65 proteins, it could be possible to determine humoral immune responses critical to protection using these models [18, 24].
The ability of pre-existing, passively administered maternal antibodies to protect, or at least minimize the severity, of cCMV has been studied in both models. In the setting of CD4+ T cell-depletion, CMV-seronegative monkeys were infused with hyperimmune globulin (HIG) from plasma of RhCMV-seropositive rhesus monkey donors then challenged with RhCMV [105]. The HIG-treated monkeys transmitted RhCMV at a lower rate compared to control monkeys and had a lower rate of abortion [105]. Evaluation of the plasmablast response to primary RhCMV infection coupled with studies of RhCMV gB-specific monoclonal antibodies have suggested that targeted CMV subunit vaccines may be efficacious and can be studied in a rhesus model [34]. Likewise, passively transferred antibodies, including polyclonal sera targeting multiple glycoprotein immunogens [43], as well as anti-gB [44] and anti-gH [45] antibodies, have been shown to have an impact on vertical transmission of virus in the GPCMV model.
Both models been used to study vaccine-elicited immunity, which in turn can be used to inform and direct preclinical challenge studies. Both adjuvanted gB protein vaccines [37–39] as well as vectored vaccines based on gB and/or the pp65 homolog, GP83 [40, 49, 112, 115], have demonstrated varying degrees of protection against congenital GPCMV infection and disease. Disabled infectious single-cycle (DISC) vaccines have also been evaluated in the GPCMV model [116, 117]. Vaccine studies with RhCMV have included both gB and the PC. One study investigated the ability of rhesus macaques to develop neutralizing antibodies when exposed to a MVA virus coexpressing all 5 RhCMV proteins homologous to the HCMV UL128-C, a subset of the RhUL128C, or RhgB only, in RhCMV-seronegative animals [42]. It is interesting to note that the vaccination containing all RhUL128-C subsets stimulated high-titer neutralizing antibody responses and limited RhCMV replication in a rhesus monkey that was vaccinated after prior inoculation with RhCMV. Another study in macaques compared a CD4+ T cell-depleted dam that experienced fetal loss with an immunocompetent, nontransmitting dam; the nontransmitting dam had a more rapid and robust plasmablast response that produced a high proportion of RhCMV-reactive antibodies, including a response to membrane-associated RhCMV gB [34]. Other studies have tried to compare antibody responses between a soluble RhCMV gB vaccine and a DISC RhCMV vaccine. The DISC vaccine was deleted for glycoprotein L and a group of RhCMV-encoded MHC class I (MHC-I) immune evasion genes (Rh178 and Rh182-189), toward the goal of enhancing antibody responses [35]. DISC vaccine was superior in inducing cellular immunity to RhCMV, but it induced lower titers of neutralizing antibody and antibody to gB than the soluble gB vaccine; moreover, RhCMV challenge postvaccination was associated with a lower frequency of virus detection in the blood in the gB group, compared to the DISC group. DISC vaccines targeting deletion of virally-encoded immune modulation genes interestingly have also been evaluated in the GPCMV model, including a knock-out vaccine deleted of a GPCMV-encoded evasin of protein kinase R [53] and a vaccine with a deletion of a gene family of MHC-I homologs [54]. Both of these live, attenuated viruses were impaired for producing dissemination and disease in animals, but demonstrated protection against adverse pregnancy outcomes when used as preconception vaccines in the GPCMV congenital infection model. The recently demonstrated ability to generate viral mutants using CRISPR/Cas9 mutagenesis should enable further generation of attenuated viruses, that in turn can be used to study the impact of viral immune modulation genes on vaccine immunogenicity and efficacy [118].
Host Cell Responses to Cytomegalovirus Infection: Cellular and Innate Responses
Humoral and cellular immune responses elicited by RhCMV, HCMV, and GPCMV infection have many common features. As noted above, humoral (neutralizing antibody) responses are important in each of these models. In the guinea pig model, cell-mediated immune responses, particularly to the pp65 (ppUL83) homolog, GP83, are important in recovery from infection and in vaccine-mediated protection [40, 46, 49]. However, similar to HCMV, both GPCMV and RhCMV can establish secondary persistent infections despite CMV-specific humoral and cellular immunity by modulating host innate and adaptive immune responses.
Cytomegalovirus infection elicits effector responses including natural killer (NK) cells, which are important for the elimination of virally infected and transformed cells, and critically important for control of herpesvirus infections, including CMV [119]. Although they have traditionally been thought of as innate immune cells lacking antigen-specificity, recent studies have revealed that NK cells can possess a memory-like phenotype against multiple viral infections, most notably CMV [120]. In response to infection with HCMV as well as human immunodeficiency virus (HIV), NK cells express activating receptor NKG2C (CD159C), which is thought to delineate cells with memory and memory-like functions [121–123]. There is an increase in the NKG2C+ NK cell repertoire in response to CMV infection that persists throughout life [121], but the mechanism by which CMV infection increases NKG2C expression remains unclear. Recent studies in RhCMV have also revealed true memory NKG2C+ (KLRC2) NK cells in macaques [124], suggesting conservation of these mechanisms in primate CMVs. Understanding how to marshal these responses in the context of vaccination could be a powerful advance in devising strategies for protection against cCMV.
The adaptive immune response against CMV infection has been well characterized in humans and rhesus macaques, but it is less so in the guinea pig model. It is remarkable that, in HCMV-seropositive individuals, CMV-specific CD4+ and CD8+ populations dominate the memory T-cell compartment [125–128]. In HCMV, CD4+ responses are particularly important, because it is well understood that the loss of CMV-specific CD4+ T cells in the setting of HIV-infection has been correlated with the presence of CMV-associated complications [129]. Acute RhCMV infection induces virus-specific CD4+ T cells that are polyclonal and undergo substantial evolution during progression of infection, and at the point of chronic infection, RhCMV-specific CD4+ T cells are relatively stable [130]. Previous rhesus macaque models of placental RhCMV transmission have also implicated CD4+ T cells as essential for prevention of in utero transmission [94]. Specifically, maternal CD4+ cells play an important role in control of maternal RhCMV viral load, promotion of virus-specific CD8+ T-cell responses, and production of autologous neutralizing antibodies. Depletion of CD4+ cells from seronegatives before RhCMV inoculation resulted in a higher rate of fetal loss than in immunocompetent monkeys [94], further underscoring the importance of these cells in the context of maternal primary infection and for cCMV transmission.
Immune Evasion
Rhesus macaque CMV, GPCMV, and HCMV all encode multiple proteins that enable immune evasion and maintenance and/or persistence of virus. Studies of HCMV and RhCMV have implicated host MHC-I antigen presentation as critical for protection from secondary infection, and have demonstrated that virally encoded inhibitors of MHC-I promote evasion of CD8+ T-cell responses [131]. Specifically, HCMV encodes (1) US2 and US11, which mediate retrograde translocation of MHC-I into the cytosol for proteasomal destruction, (2) US3, which retains MHC-I in the endoplasmic reticulum by interfering with loading of peptides by chaperone proteins, and (3) US6, which inhibits the translocation of viral and host peptides across the endoplasmic reticulum membrane by the dedicated peptide transporter. It is notable that RhCMV also encodes homologs of these proteins that inhibit MHC-I antigen presentation [23]. It is interesting to note that reinfection of RhCMV-seropositive rhesus macaques requires expression of these gene products. However, MHC-I interference is not required for primary RhCMV infection, nor is it required for establishment of a persistent secondary infection in RhCMV-seropositive macaques transiently depleted of CD8+ lymphocytes [132].
Both HCMV and RhCMV encode viral molecules that enable evasion of immune detection. Human CMV-encoded UL141 downregulates cell expression of CD155, which is a ligand for activating NK receptor DNAM-1 [133, 134]. Likewise, NKG2D, a C-type lectin-like homodimeric receptor, is expressed on NK cells and transmits stimulatory signals via the associated adaptors called NKG2DLs. Human CMV-encoded proteins UL16 and UL142 retain NKG2DLs, preventing expression at the cell surface and thus counteracting NK cell activation [135–138]. It is notable that RhCMV lacks homologs of UL16 and UL142 but instead uses Rh159, the homolog of UL148, to prevent NKG2DL surface expression [139]. Similar to HCMV UL16, Rh159 prevents surface expression of multiple NKG2DLs, and deletion of the rhesus homolog Rh159 enabled primary infection by downregulation of NKG2DL and subsequent NK cell activation [139].
Human CMV and RhCMV also attenuate host innate immunity by exploitation of the cellular interleukin (IL)-10 pathway, which is involved in suppressing cell-mediated immune responses while enhancing humoral immune responses [140]. Primate CMVs utilize 2 main mechanisms for IL-10 modulation: encoding an IL-10 homolog and manipulating the host’s cellular IL-10 signaling cascade. The viral IL-10 ortholog is encoded by the UL111A ORF in primate CMVs, including both HCMV and RhCMV [50]. Although there is considerable genetic drift between viral and cellular IL-10 proteins for both HCMV and RhCMV, HCMV IL-10 and RhCMV IL-10 show exceedingly conserved functionality with cellular IL-10 [51, 141–146]. In a rhesus macaque infection challenge model, vaccine-mediated disruption of RhCMV IL-10 signaling restricted the frequency and magnitude of RhCMV detection in the saliva and urine [52]. Likewise, deletion of RhCMV IL-10 from RhCMV 68-1 revealed quantitatively enhanced innate immune responses and more robust antiviral adaptive responses after challenge, compared with the parental 68-1 virus [146]. Thus, virally encoded IL-10 in both HCMV and RhCMV appears to contribute to establishment and/or viral persistence in an immune-competent host. Guinea pig CMV does not encode an IL-10 ortholog, but it encodes a homolog of the CC chemokine, MIP1α [147]. Mutagenesis of the gp1 gene results in a virus that is attenuated in its ability to elicit labyrinthitis and SNHL in a cochlear challenge model [148]. To date, this gene product has not been targeted in a subunit vaccine study.
In addition, similar to other Herpesviridae, CMVs can produce viral Fcγ-binding glycoproteins that compete with host FcγRs to prevent IgG-mediated immune responses. Human CMV encodes 4 viral FcγRs with distinct IgG subtype specificity that impact on antibody-mediated immune function in vitro: gp34 [149, 150], gp68 [151], gpRL13 [152, 153], and gp95 (E. Mercé-Maldonado and H. Hengel, 2019, unpublished observations). The first and thus far only FcγR gene was recently identified in RhCMV: Rh05, which encodes an IgG-Fc binding glycoprotein that antagonizes RhCMV FcγR activation [154]. Similar ORFs are noted in the GPCMV genome [24, 27], but their function has not yet been analyzed.
CONCLUSIONS
Nonhuman primate models of CMV, in particular the rhesus macaque model, are probably of the greatest value in assessing the relative merits of vaccines and immune-based therapies targeting cCMV infection (Table 2). This derives from (1) the similarities in biology and transmission for the respective viruses and (2) cross-reactivity of many immunologic reagents developed for humans, but which can be meaningfully examined in the rhesus model. The substantial degree of genetic relatedness between the two viruses, HCMV and RhCMV, in particular for envelope glycoproteins (including the PC), is highly compelling. Additional efforts should be focused on studying both the mechanisms of injury and the potential for therapies capable of ameliorating the neuropathogenesis, SNHL, and placental injury in both the RhCMV and GPCMV models, and in drawing comparisons to HCMV [88, 90, 100–104, 106–110]. In addition, the impact of preconception immunity on the relative rates of transplacental transmission in seronegative and seropositive dams should be further explored and compared with the HCMV experience [99, 155]: such comparisons will help inform and direct decisions about how HCMV vaccines will be used in clinical practice. However, studies in rhesus macaques are still limited by the high costs and relative lack of availability of RhCMV-seronegative monkeys. Thus, some studies of primary infection and congenital infection currently require the use of pathogen-free facilities and breeding specifically of RhCMV-seronegative animals. Guinea pigs have the advantage of reduced expense, while retaining similarities in experimental congenital infection endpoints (placental pathology, SNHL, brain pathology) that make the use of this model compelling for “proof-of-concept” studies of subunit vaccination. Ultimately, neither model allows testing of HCMV vaccines for efficacy against congenital infection. A combined strategy, in which subunit vaccine strategies can first be screened in the guinea pig model, before detailed protection comparisons in macaques, seems optimal. Future evaluation should focus on efforts to identify a key correlate of protective immunity for the developing fetus, because considerable uncertainty remains regarding the relative importance of T cells [111, 156] and antibody [157] in protection against cCMV infection. Both models also allow examination of the impact of targeting viral immune modulation genes in attenuated vaccine design. Side-by-side efficacy comparisons of soluble protein subunit vaccines with disabled/attenuated vaccines in these animal models can be particularly instructive for HCMV vaccine development [35]. Ultimately, both models should continue to be developed and used strategically to meet the urgent need to develop a vaccine against cCMV infection.
Notes
Financial support. This work was funded by National Institutes of Health Grants 1DP2HD075699 and 1P01-AI129859 (to S. R. P.) and HD079918 and HD098866 (to M. R. S.).
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. S. R. P. reports consulting fees from Merck, Moderna, Sanofi, and Pfizer vaccines. M. R. S. reports consulting fees from Merck and GSK vaccines. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 2. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17:253–76. [DOI] [PubMed] [Google Scholar]
- 3. Emery VC. Investigation of CMV disease in immunocompromised patients. J Clin Pathol 2001; 54:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 1992; 11:93–9. [DOI] [PubMed] [Google Scholar]
- 5. Anderholm KM, Bierle CJ, Schleiss MR. Cytomegalovirus vaccines: current status and future prospects. Drugs 2016; 76:1625–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC). Impact of expanded newborn screening--United States, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1012–5. [PubMed] [Google Scholar]
- 7. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto AY, Mussi-Pinhata MM, Isaac Mde L, et al. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J 2011; 30:1043–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burwitz BJ, Malouli D, Bimber BN, et al. Cross-species rhesus cytomegalovirus infection of cynomolgus macaques. PLoS Pathog 2016; 12:e1006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 2014; 36:615–25. [DOI] [PubMed] [Google Scholar]
- 11. Marsh AK, Willer DO, Ambagala AP, et al. Genomic sequencing and characterization of cynomolgus macaque cytomegalovirus. J Virol 2011; 85:12995–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ambagala AP, Marsh A, Chan J, et al. Isolation and characterization of cynomolgus macaque (Macaca fascicularis) cytomegalovirus (CyCMV). Virology 2011; 412:125–35. [DOI] [PubMed] [Google Scholar]
- 13. Davison AJ, Dolan A, Akter P, et al. The human cytomegalovirus genome revisited: comparison with the chimpanzee cytomegalovirus genome. J Gen Virol 2003; 84:17–28. [DOI] [PubMed] [Google Scholar]
- 14. Kumar ML, Nankervis GA. Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis 1978; 138:650–4. [DOI] [PubMed] [Google Scholar]
- 15. Bia FJ, Griffith BP, Fong CK, Hsiung GD. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev Infect Dis 1983; 5:177–95. [DOI] [PubMed] [Google Scholar]
- 16. Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J 2006; 47:65–72. [DOI] [PubMed] [Google Scholar]
- 17. Schleiss MR. Developing a vaccine against congenital cytomegalovirus (CMV) infection: what have we learned from animal models? Where should we go next? Future Virol 2013; 8:1161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete sequence and genomic analysis of rhesus cytomegalovirus. J Virol 2003; 77:6620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivailler P, Kaur A, Johnson RP, Wang F. Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. J Virol 2006; 80:4179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oxford KL, Eberhardt MK, Yang KW, et al. Protein coding content of the ULb’ region of wild-type rhesus cytomegalovirus. Virology 2008; 373:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malouli D, Nakayasu ES, Viswanathan K, et al. Reevaluation of the coding potential and proteomic analysis of the BAC-derived rhesus cytomegalovirus strain 68-1. J Virol 2012; 86:8959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol 1996; 70:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dolan A, Cunningham C, Hector RD, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol 2004; 85:1301–12. [DOI] [PubMed] [Google Scholar]
- 24. Yang D, Tamburro K, Dittmer D, et al. Complete genome sequence of pathogenic guinea pig cytomegalovirus from salivary gland homogenates of infected animals. Genome Announc 2013; 1:e0005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schleiss MR, McVoy MA. Guinea pig cytomegalovirus (GPCMV): a model for the study of the prevention and treatment of maternal-fetal transmission. Future Virol 2010; 5:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nozawa N, Yamamoto Y, Fukui Y, et al. Identification of a 1.6 kb genome locus of guinea pig cytomegalovirus required for efficient viral growth in animals but not in cell culture. Virology 2008; 379:45–54. [DOI] [PubMed] [Google Scholar]
- 27. Schleiss MR, McAllister S, Armién AG, et al. Molecular and biological characterization of a new isolate of guinea pig cytomegalovirus. Viruses 2014; 6:448–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pande NT, Powers C, Ahn K, Früh K. Rhesus cytomegalovirus contains functional homologues of US2, US3, US6, and US11. J Virol 2005; 79:5786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bierle CJ, Schleiss MR, Geballe AP. Antagonism of the protein kinase R pathway by the guinea pig cytomegalovirus US22-family gene gp145. Virology 2012; 433:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alcendor DJ, Barry PA, Pratt-Lowe E, Luciw PA. Analysis of the rhesus cytomegalovirus immediate-early gene promoter. Virology 1993; 194:815–21. [DOI] [PubMed] [Google Scholar]
- 31. Barry PA, Alcendor DJ, Power MD, Kerr H, Luciw PA. Nucleotide sequence and molecular analysis of the rhesus cytomegalovirus immediate-early gene and the UL121-117 open reading frames. Virology 1996; 215:61–72. [DOI] [PubMed] [Google Scholar]
- 32. Abel K, Martinez J, Yue Y, et al. Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques. J Virol 2011; 85:2878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kravitz RH, Sciabica KS, Cho K, Luciw PA, Barry PA. Cloning and characterization of rhesus cytomegalovirus glycoprotein B. J Gen Virol 1997; 78 (Pt 8):2009–13. [DOI] [PubMed] [Google Scholar]
- 34. Fan Q, Nelson CS, Bialas KM, et al. Plasmablast response to primary rhesus cytomegalovirus (CMV) infection in a monkey model of congenital CMV transmission. Clin Vaccine Immunol 2017; 24:e00510–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valencia S, Gill RB, Dowdell KC, et al. Comparison of vaccination with rhesus CMV (RhCMV) soluble gB with a RhCMV replication-defective virus deleted for MHC class I immune evasion genes in a RhCMV challenge model. Vaccine 2019; 37:333–42. [DOI] [PubMed] [Google Scholar]
- 36. Schleiss MR. Cloning and characterization of the guinea pig cytomegalovirus glycoprotein B gene. Virology 1994; 202:173–85. [DOI] [PubMed] [Google Scholar]
- 37. Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis 2004; 189:1374–81. [DOI] [PubMed] [Google Scholar]
- 38. Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis 2003; 188:1868–74. [DOI] [PubMed] [Google Scholar]
- 39. Schleiss MR, Choi KY, Anderson J, et al. Glycoprotein B (gB) vaccines adjuvanted with AS01 or AS02 protect female guinea pigs against cytomegalovirus (CMV) viremia and offspring mortality in a CMV-challenge model. Vaccine 2014; 32:2756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schleiss MR, Lacayo JC, Belkaid Y, et al. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis 2007; 195:789–98. [DOI] [PubMed] [Google Scholar]
- 41. Hansen SG, Ford JC, Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011; 473:523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wussow F, Yue Y, Martinez J, et al. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol 2013; 87:1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bratcher DF, Bourne N, Bravo FJ, et al. Effect of passive antibody on congenital cytomegalovirus infection in guinea pigs. J Infect Dis 1995; 172:944–50. [DOI] [PubMed] [Google Scholar]
- 44. Chatterjee A, Harrison CJ, Britt WJ, Bewtra C. Modification of maternal and congenital cytomegalovirus infection by anti-glycoprotein b antibody transfer in guinea pigs. J Infect Dis 2001; 183:1547–53. [DOI] [PubMed] [Google Scholar]
- 45. Auerbach MR, Yan D, Vij R, et al. A neutralizing anti-gH/gL monoclonal antibody is protective in the guinea pig model of congenital CMV infection. PLoS Pathog 2014; 10:e1004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yue Y, Kaur A, Zhou SS, Barry PA. Characterization and immunological analysis of the rhesus cytomegalovirus homologue (Rh112) of the human cytomegalovirus UL83 lower matrix phosphoprotein (pp65). J Gen Virol 2006; 87:777–87. [DOI] [PubMed] [Google Scholar]
- 47. McGregor A, Liu F, Schleiss MR. Molecular, biological, and in vivo characterization of the guinea pig cytomegalovirus (CMV) homologs of the human CMV matrix proteins pp71 (UL82) and pp65 (UL83). J Virol 2004; 78:9872–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schleiss MR, McGregor A, Jensen NJ, Erdem G, Aktan L. Molecular characterization of the guinea pig cytomegalovirus UL83 (pp65) protein homolog. Virus Genes 1999; 19:205–21. [DOI] [PubMed] [Google Scholar]
- 49. Schleiss MR, Berka U, Watson E, et al. Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol 2017; 24:pii: e00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lockridge KM, Zhou SS, Kravitz RH, et al. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology 2000; 268:272–80. [DOI] [PubMed] [Google Scholar]
- 51. Eberhardt MK, Deshpande A, Fike J, et al. Exploitation of interleukin-10 (IL-10) signaling pathways: alternate roles of viral and cellular IL-10 in rhesus cytomegalovirus infection. J Virol 2016; 90:9920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eberhardt MK, Deshpande A, Chang WL, Barthold SW, Walter MR, Barry PA. Vaccination against a virus-encoded cytokine significantly restricts viral challenge. J Virol 2013; 87:11323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schleiss MR, Bierle CJ, Swanson EC, et al. Vaccination with a live attenuated cytomegalovirus devoid of a protein kinase R inhibitory gene results in reduced maternal viremia and improved pregnancy outcome in a guinea pig congenital infection model. J Virol 2015; 89:9727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crumpler MM, Choi KY, McVoy MA, Schleiss MR. A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine 2009; 27:4209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol 1986; 59:714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schmolke S, Drescher P, Jahn G, Plachter B. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol 1995; 69:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Varnum SM, Streblow DN, Monroe ME, et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol 2004; 78:10960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baldick CJ Jr, Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol 1996; 70:6097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chevillotte M, Landwehr S, Linta L, et al. Major tegument protein pp65 of human cytomegalovirus is required for the incorporation of pUL69 and pUL97 into the virus particle and for viral growth in macrophages. J Virol 2009; 83:2480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Malouli D, Hansen SG, Nakayasu ES, et al. Cytomegalovirus pp65 limits dissemination but is dispensable for persistence. J Clin Invest 2014; 124:1928–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kropff B, Mach M. Identification of the gene coding for rhesus cytomegalovirus glycoprotein B and immunological analysis of the protein. J Gen Virol 1997; 78 (Pt 8):1999–2007. [DOI] [PubMed] [Google Scholar]
- 62. Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol 2009; 46Suppl 4:S73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009; 28:484–93. [DOI] [PubMed] [Google Scholar]
- 64. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J Virol 2008; 82:11837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fouts AE, Comps-Agrar L, Stengel KF, et al. Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109. Proc Natl Acad Sci U S A 2014; 111:8209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vanarsdall AL, Chase MC, Johnson DC. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J Virol 2011; 85:11638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun SR, Ji YH, Ruan Q, et al. Structure characterization of human cytomegalovirus UL131A, UL130 and UL128 genes in clinical strains in China. Genet Mol Res 2006; 8:1191–201. [DOI] [PubMed] [Google Scholar]
- 69. Vanarsdall AL, Johnson DC. Human cytomegalovirus entry into cells. Curr Opin Virol 2012; 2:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 2004; 78:10023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ryckman BJ, Rainish BL, Chase MC, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 2008; 82:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lilleri D, Kabanova A, Revello MG, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 2013; 8:e59863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lilja AE, Shenk T. Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proc Natl Acad Sci U S A 2008; 105:19950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yue Y, Kaur A, Lilja A, Diamond DJ, Walter MR, Barry PA. The susceptibility of primary cultured rhesus macaque kidney epithelial cells to rhesus cytomegalovirus strains. J Gen Virol 2016; 97:1426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wussow F, Chiuppesi F, Martinez J, et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 2014; 10:e1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yamada S, Nozawa N, Katano H, et al. Characterization of the guinea pig cytomegalovirus genome locus that encodes homologs of human cytomegalovirus major immediate-early genes, UL128, and UL130. Virology 2009; 391:99–106. [DOI] [PubMed] [Google Scholar]
- 77. Auerbach M, Yan D, Fouts A, et al. Characterization of the guinea pig CMV gH/gL/GP129/GP131/GP133 complex in infection and spread. Virology 2013; 441:75–84. [DOI] [PubMed] [Google Scholar]
- 78. Gnanandarajah JS, Gillis PA, Hernandez-Alvarado N, et al. Identification by mass spectrometry and immune response analysis of guinea pig cytomegalovirus (GPCMV) pentameric complex proteins GP129, 131 and 133. Viruses 2014; 6:727–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Coleman S, Choi KY, Root M, McGregor A. A homolog pentameric complex dictates viral epithelial tropism, pathogenicity and congenital infection rate in guinea pig cytomegalovirus. PLoS Pathog 2016; 12:e1005755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamada S, Fukuchi S, Hashimoto K, et al. Guinea pig cytomegalovirus GP129/131/133, homologues of human cytomegalovirus UL128/130/131A, are necessary for infection of monocytes and macrophages. J Gen Virol 2014; 95:1376–82. [DOI] [PubMed] [Google Scholar]
- 81. McVoy MA, Wang JB, Dittmer DP, et al. Repair of a mutation disrupting the guinea pig cytomegalovirus pentameric complex acquired during fibroblast passage restores pathogenesis in immune-suppressed guinea pigs and in the context of congenital infection. J Virol 2016; 90:7715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hansen SG, Sacha JB, Hughes CM, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 2013; 340:1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Swack NS, Hsiung GD. Natural and experimental simian cytomegalovirus infections at a primate center. J Med Primatol 1982; 11:169–77. [PubMed] [Google Scholar]
- 84. Vogel P, Weigler BJ, Kerr H, Hendrickx AG, Barry PA. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab Anim Sci 1994; 44:25–30. [PubMed] [Google Scholar]
- 85. Huff JL, Eberle R, Capitanio J, Zhou SS, Barry PA. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J Gen Virol 2003; 84:83–92. [DOI] [PubMed] [Google Scholar]
- 86. Asher DM, Gibbs CJ Jr, Lang DJ, Gajdusek DC, Chanock RM. Persistent shedding of cytomegalovirus in the urine of healthy Rhesus monkeys. Proc Soc Exp Biol Med 1974; 145:794–801. [DOI] [PubMed] [Google Scholar]
- 87. Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CP, Barry PA. Pathogenesis of experimental rhesus cytomegalovirus infection. J Virol 1999; 73:9576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van Hoosier GL Jr, Giddens WE Jr, Gillett CS, Davis H. Disseminated cytomegalovirus disease in the guinea pig. Lab Anim Sci 1985; 35:81–4. [PubMed] [Google Scholar]
- 90. Griffith BP, McCormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol 1985; 55:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schleiss MR. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J Clin Virol 2002; 25Suppl 2:S37–49. [DOI] [PubMed] [Google Scholar]
- 92. Yamada S, Katano H, Sato Y, Fukuchi S, Hashimoto K, Inoue N. An ex vivo culture model for placental cytomegalovirus infection using slices of guinea pig placental tissue. Placenta 2016; 37:85–8. [DOI] [PubMed] [Google Scholar]
- 93. Tanimura T, Tanioka Y.. Laboratory Animal Handbook 6: Breeding Simians for Developmental Biology. Comparison of embryonic and foetal development in man and rhesus monkey. London: Laboratory Animals, 1975; pp 205–33. [Google Scholar]
- 94. Bialas KM, Tanaka T, Tran D, et al. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci U S A 2015; 112:13645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hamprecht K, Goelz R. Postnatal cytomegalovirus infection through human milk in preterm infants: transmission, clinical presentation, and prevention. Clin Perinatol 2017; 44:121–30. [DOI] [PubMed] [Google Scholar]
- 96. Diosi P. Cytomegalovirus (CMV) in cervical secretion and breast milk. A thirty years perspective. Roum Arch Microbiol Immunol 1997; 56:165–78. [PubMed] [Google Scholar]
- 97. Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 2001; 357:513–8. [DOI] [PubMed] [Google Scholar]
- 98. Kaur A, Itell HL, Ehlinger EP, Varner V, Gantt S, Permar SR. Natural history of postnatal rhesus cytomegalovirus shedding by dams and acquisition by infant rhesus monkeys. PLoS One 2018; 13:e0206330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003; 289:1008–11. [DOI] [PubMed] [Google Scholar]
- 100. Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 2009; 22:99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barry PA, Lockridge KM, Salamat S, et al. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J 2006; 47:49–64. [DOI] [PubMed] [Google Scholar]
- 102. London WT, Martinez AJ, Houff SA, et al. Experimental congenital disease with simian cytomegalovirus in rhesus monkeys. Teratology 1986; 33:323–31. [DOI] [PubMed] [Google Scholar]
- 103. Chang WL, Tarantal AF, Zhou SS, Borowsky AD, Barry PA. A recombinant rhesus cytomegalovirus expressing enhanced green fluorescent protein retains the wild-type phenotype and pathogenicity in fetal macaques. J Virol 2002; 76:9493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tarantal AF, Salamat MS, Britt WJ, Luciw PA, Hendrickx AG, Barry PA. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta). J Infect Dis 1998; 177:446–50. [DOI] [PubMed] [Google Scholar]
- 105. Nelson CS, Cruz DV, Tran D, et al. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2017; 2:e94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Griffith BP, Lucia HL, Hsiung GD. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr Res 1982; 16:455–9. [DOI] [PubMed] [Google Scholar]
- 107. Woolf NK. Guinea pig model of congenital CMV-induced hearing loss: a review. Transplant Proc 1991; 23:32–4, discussion 34. [PubMed] [Google Scholar]
- 108. Woolf NK, Koehrn FJ, Harris JP, Richman DD. Congenital cytomegalovirus labyrinthitis and sensorineural hearing loss in guinea pigs. J Infect Dis 1989; 160:929–37. [DOI] [PubMed] [Google Scholar]
- 109. Park AH, Gifford T, Schleiss MR, et al. Development of cytomegalovirus-mediated sensorineural hearing loss in a guinea pig model. Arch Otolaryngol Head Neck Surg 2010; 136:48–53. [DOI] [PubMed] [Google Scholar]
- 110. Maidji E, Nigro G, Tabata T, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol 2010; 177:1298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lilleri D, Zelini P, Fornara C, Comolli G, Revello MG, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T cell responses in primary infection of the immunocompetent and the immunocompromised host. Clin Immunol 2009; 131:395–403. [DOI] [PubMed] [Google Scholar]
- 112. Swanson EC, Gillis P, Hernandez-Alvarado N, et al. Comparison of monovalent glycoprotein B with bivalent gB/pp65 (GP83) vaccine for congenital cytomegalovirus infection in a guinea pig model: inclusion of GP83 reduces gB antibody response but both vaccine approaches provide equivalent protection against pup mortality. Vaccine 2015; 33:4013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barry PA, Strelow L. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp Med 2008; 58:43–6. [PMC free article] [PubMed] [Google Scholar]
- 114. Pritchett Corning KR, Mulder GB, Henderson KS. Using hysterectomy rederivation to produce guinea pigs (Cavia porcellus) free of guinea pig cytomegalovirus. J Am Assoc Lab Anim Sci 2018; doi: 10.30802/AALAS-JAALAS-18-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cardin RD, Bravo FJ, Pullum DA, et al. Replication-defective lymphocytic choriomeningitis virus vectors expressing guinea pig cytomegalovirus gB and pp65 homologs are protective against congenital guinea pig cytomegalovirus infection. Vaccine 2016; 34:1993–9. [DOI] [PubMed] [Google Scholar]
- 116. Choi KY, Root M, McGregor A. A novel non-replication-competent cytomegalovirus capsid mutant vaccine strategy is effective in reducing congenital infection. J Virol 2016; 90:7902–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Choi KY, El-Hamdi NS, McGregor A. Inclusion of the viral pentamer complex in a vaccine design greatly improves protection against congenital cytomegalovirus in the guinea pig model. J Virol 2019; doi: 10.1128/JVI.01442-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bierle CJ, Anderholm KM, Wang JB, McVoy MA, Schleiss MR. Targeted mutagenesis of guinea pig cytomegalovirus using CRISPR/Cas9-mediated gene editing. J Virol 2016; 90:6989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 1989; 320:1731–5. [DOI] [PubMed] [Google Scholar]
- 120. Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189:5082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004; 104:3664–71. [DOI] [PubMed] [Google Scholar]
- 122. Lopez-Vergès S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 2011; 108:14725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hendricks DW, Balfour HH Jr, Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol 2014; 192:4492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ram DR, Manickam C, Hueber B, et al. Tracking KLRC2 (NKG2C)+ memory-like NK cells in SIV+ and rhCMV+ rhesus macaques. PLoS Pathog 2018; 14:e1007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest 1997; 99:1739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 2001; 279:459–70. [DOI] [PubMed] [Google Scholar]
- 127. Sester M, Sester U, Gärtner B, et al. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol 2002; 76:3748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol 2004; 65:456–64. [DOI] [PubMed] [Google Scholar]
- 129. Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med 1998; 4:953–6. [DOI] [PubMed] [Google Scholar]
- 130. Price DA, Bitmansour AD, Edgar JB, et al. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J Immunol 2008; 180:269–80. [DOI] [PubMed] [Google Scholar]
- 131. Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J 2009; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hansen SG, Powers CJ, Richards R, et al. Evasion of CD8+ T cells is critical for super-infection by cytomegalovirus. Science 2010; 328:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003; 198:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tomasec P, Wang EC, Davison AJ, et al. Downregulation of natural killer cell-activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol 2005; 6:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wu J, Chalupny NJ, Manley TJ, Riddell SR, Cosman D, Spies T. Intracellular retention of the MHC class I-related chain B ligand of NKG2D by the human cytomegalovirus UL16 glycoprotein. J Immunol 2003; 170:4196–200. [DOI] [PubMed] [Google Scholar]
- 136. Dunn C, Chalupny NJ, Sutherland CL, et al. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med 2003; 197:1427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Eagle RA, Traherne JA, Hair JR, Jafferji I, Trowsdale J. ULBP6/RAET1L is an additional human NKG2D ligand. Eur J Immunol 2009; 39:3207–16. [DOI] [PubMed] [Google Scholar]
- 138. Bennett NJ, Ashiru O, Morgan FJ, et al. Intracellular sequestration of the NKG2D ligand ULBP3 by human cytomegalovirus. J Immunol 2010; 185:1093–102. [DOI] [PubMed] [Google Scholar]
- 139. Sturgill ER, Malouli D, Hansen SG, et al. Natural killer cell evasion is essential for infection by rhesus cytomegalovirus. PLoS Pathog 2016; 12:e1005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19:683–765. [DOI] [PubMed] [Google Scholar]
- 141. Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol 2002; 76:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chang WL, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol 2004; 78:8720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schönrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol 2004; 173:3383–91. [DOI] [PubMed] [Google Scholar]
- 144. Chang WL, Barry PA, Szubin R, Wang D, Baumgarth N. Human cytomegalovirus suppresses type I interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology 2009; 390:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spencer JV, Cadaoas J, Castillo PR, Saini V, Slobedman B. Stimulation of B lymphocytes by cmvIL-10 but not LAcmvIL-10. Virology 2008; 374:164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chang WL, Barry PA. Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci U S A 2010; 107:22647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Penfold M, Miao Z, Wang Y, Haggerty S, Schleiss MR. A macrophage inflammatory protein homolog encoded by guinea pig cytomegalovirus signals via CC chemokine receptor 1. Virology 2003; 316:202–12. [DOI] [PubMed] [Google Scholar]
- 148. Schraff SA, Brown DK, Schleiss MR, Meinzen-Derr J, Greinwald JH, Choo DI. The role of CMV inflammatory genes in hearing loss. Otol Neurotol 2007; 28:964–9. [DOI] [PubMed] [Google Scholar]
- 149. Atalay R, Zimmermann A, Wagner M, et al. Identification and expression of human cytomegalovirus transcription units coding for two distinct Fcgamma receptor homologs. J Virol 2002; 76:8596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Corrales-Aguilar E, Trilling M, Hunold K, et al. Human cytomegalovirus Fcγ binding proteins gp34 and gp68 antagonize Fcγ receptors I, II and III. PLoS Pathog 2014; 10:e1004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sprague ER, Reinhard H, Cheung EJ, et al. The human cytomegalovirus Fc receptor gp68 binds the Fc CH2-CH3 interface of immunoglobulin G. J Virol 2008; 82:3490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Stanton RJ, Baluchova K, Dargan DJ, et al. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J Clin Invest 2010; 120:3191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dargan DJ, Douglas E, Cunningham C, et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J Gen Virol 2010; 91:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kolb P, Sijmons S, McArdle MR, et al. Identification and functional characterization of a novel Fc gamma-binding glycoprotein in rhesus cytomegalovirus. J Virol 2019; 93:pii: e02077-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Simonazzi G, Curti A, Cervi F, et al. Perinatal outcomes of non-primary maternal cytomegalovirus infection: a 15-year experience. Fetal Diagn Ther 2018; 43:138–42. [DOI] [PubMed] [Google Scholar]
- 156. Gantt S, Leister E, Jacobsen DL, et al. Risk of congenital cytomegalovirus infection among HIV-exposed uninfected infants is not decreased by maternal nelfinavir use during pregnancy. J Med Virol 2016; 88:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Permar SR, Schleiss MR, Plotkin SA. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol 2018; 92:e00030–18. [DOI] [PMC free article] [PubMed] [Google Scholar]