Abstract
Numerous candidate vaccines against cytomegalovirus (CMV) infection and disease are in development. Whereas the previous article [1] provides background and opinions about the issues relating to vaccination, this article provides specifics about the vaccines in active development, as reported at a National Institutes of Health-sponsored meeting in Bethesda on September 4–6, 2018. Here, vaccine developers provide synopses of their candidate vaccines to immunize women to protect against congenital CMV disease and to prevent the consequences of CMV disease in recipients of transplanted organs or hematopoietic stem calls. The projects are presented here roughly in the descending order of their stage of development in the opinion of the first author.
Keywords: cytomegalovirus, fetal infection, organ transplant, stem cell transplant
VACCINES IN ADVANCED CLINICAL DEVELOPMENT
MSD
A conditionally replication defective virus, termed V160, is currently being evaluated in a Phase II clinical trial by MSD. The vaccine was derived from the AD169 live-attenuated virus and genetically modified to restore expression of the gH/gL/pUL128-131 pentameric complex (PC) [2]. A molecular switch is also introduced to the virus genome in 2 essential genes, IE1 and UL51. The vaccine virus replication can be switched on by a synthetic compound during production; however, it is not able to complete the replication cycle after inoculation.
The V160 vaccine has shown promising safety and immunogenicity profiles in a Phase I clinical trial. Three doses of vaccine were administered at day 1 and month 1 and 6. The vaccine, in general, was well tolerated and no viral shedding was detected in vaccine recipients. In seronegative subjects, there was a significant increase in neutralizing antibody (nAb) titers against epithelial cell infection after vaccination. At 1 month after the third dose, V160-induced nAb titers were comparable to those elicited by natural infection. The antibody levels remained elevated above the baseline at month 18. Robust and durable cytomegalovirus (CMV) IE1 and pp65-specific cellular responses measured by interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assays were also observed [3].
A detailed analysis of V160 responses revealed that the IE1 and pp65-specific T cells were predominantly CD8+ with effector or effector memory phenotypes. These T cells displayed a high degree of polyfunctionality by producing multiple cytokines in response to antigen stimulation. In addition, vaccination induced as high a frequency of pentamer- and glycoprotein B (gB)-specific memory B cells as observed in long-term naturally seropositive individuals. The antibodies produced by V160 vaccination were also able to neutralize epithelial cell infection by a panel of clinical isolates [4].
Sanofi
Sanofi Pasteur has worked on CMV vaccine development since the 1990s, in particular with the gB from the Towne strain that induces neutralizing and non-nAbs [5–7]. Their original preparation of soluble gB lacking the transmembrane domain was obtained from the Chiron Corporation (now GSK) and was adjuvanted with MF59 oil in water adjuvant. The candidate gB/MF59 when evaluated in CMV-seronegative women reduced infection in the subsequent year by 50% compared with placebo. Up to 42 months after study enrollment, subjects in the vaccine group were more likely to remain free of CMV infection than were subjects in the placebo group (P = .02) [8]. In adolescent girls, overall vaccine efficacy was 43% (P = .20). Two doses were sufficient to give a vaccine efficacy of 45% (P = .08) in seronegative subjects regardless of age [9]. Finally, in both CMV-positive women and a cohort of CMV-seropositive transplant recipients, gB vaccination boosted nAb responses [10, 11]. In seronegative patients who received transplants from seropositive donors, the duration of viremia (P = .0480) and number of days of ganciclovir treatment (P = .0287) were reduced in vaccine recipients [11].
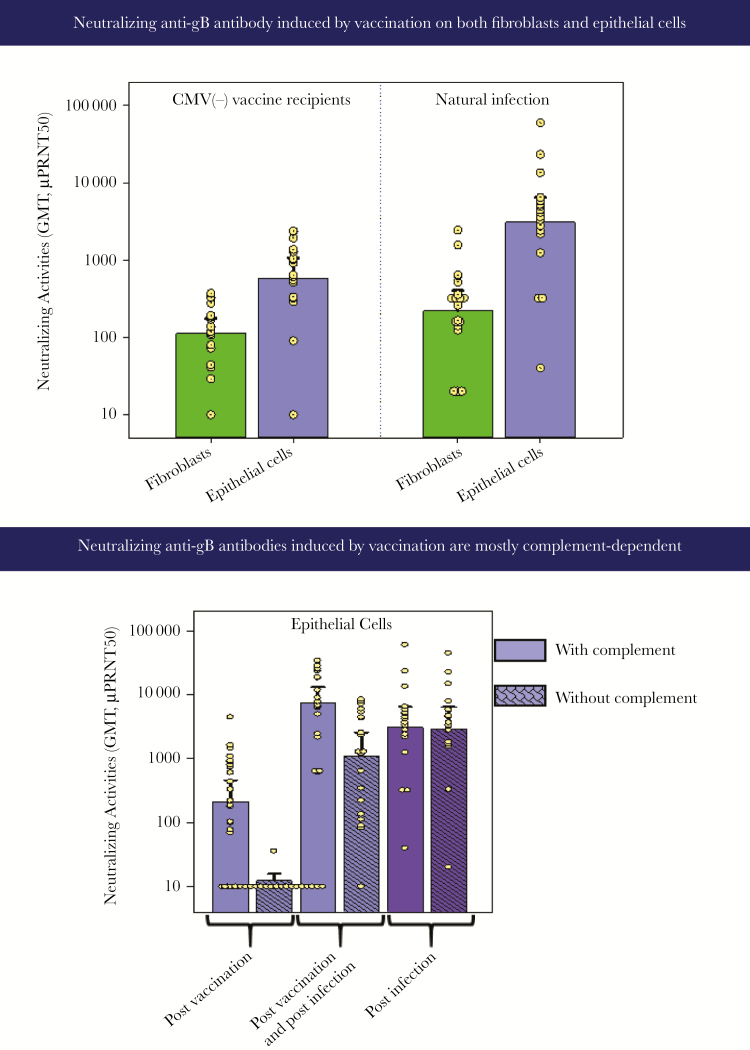
The gB/MF59 vaccine was able to induce neutralizing anti-gB antibodies that were detected using fibroblasts or epithelial cells in complement-dependent plaque reduction assays (Figure 1).
Figure 1.
Responses to Sanofi-Pasteur glycoprotein B (gB). CMV, cytomegalovirus; GMT, geometric mean titers.
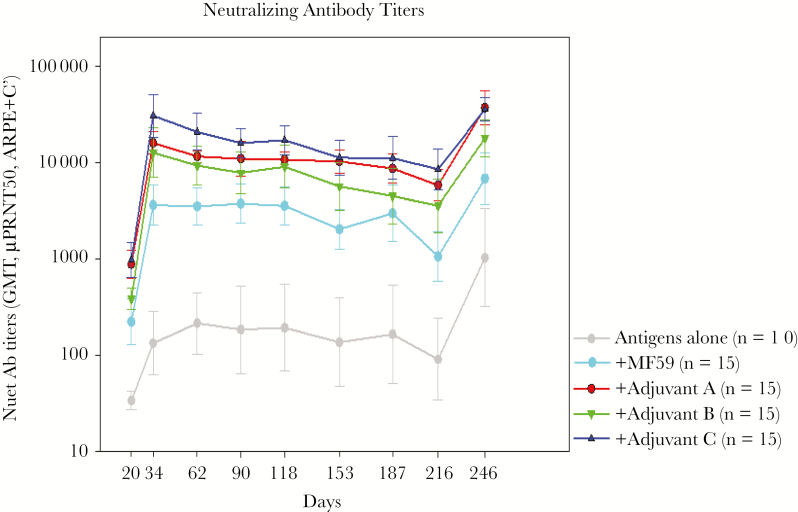
Sanofi is now working with newer unnamed adjuvants that give higher and more prolonged antibody responses, as shown in Figure 2. In addition, CD4+ T-cell responses are stimulated, which are believed to be a key correlate of protection, and Sanofi has produced a recombinant pentamer to add it to a final vaccine preparation. Based on studies of correlates of protection against maternal-fetal transmission, Sanofi will move into additional clinical trials with a combined gB/pentamer vaccine.
Figure 2.
Neutralizing antibody responses to glycoprotein B with various adjuvants. GMT, geometric mean titers.
City of Hope Triplex Vaccine
Cytomegalovirus is a major complication of allogeneic hematopoietic stem cell transplant (HCT) recipients. City of Hope selected a highly attenuated, nonproliferating viral vector referred to as modified vaccinia Ankara (MVA) to insert 3 immunodominant CMV antigens: pp65, IE-1-exon4, and IE2-exon5 [12–17]. A test of vaccine-protective efficacy has recently been completed in at-risk HCT recipients in a multicenter, randomized, and placebo-controlled Phase II clinical trial [18, 19].
Eligible patients were CMV-positive and undergoing HCT for hematologic malignancies from matched related/unrelated donors without the use of ex vivo or in vivo T-cell depletion. After randomization, patients received Triplex or placebo injections on day 28 and day 56 post-HCT and were observed for 1 year. The primary endpoint was defined as any CMV reactivation (≥500 CMV genome copies, gc/mL, or 1250 international units [IU]\mL), low-level reactivation prompting antiviral therapy, or CMV disease before day 100 post-HCT. The vaccine was well tolerated, and there were significant increases in immune response in the vaccine versus placebo arm throughout the 1-year observation period. These increases encompassed both CD4-positive and CD8-positive CMV-specific T cells. Triplex was well tolerated in seropositive HCT recipients and was significantly better than placebo in preventing viremia cases accompanied by improved CMV-specific T-cell reconstitution [18, 19].
Based on the success of the randomized Phase II trial in moderate risk HCT recipients, the City of Hope is conducting a clinical trial (NCT03560752) in which donors of CMV-positive HCT recipients will receive 1 injection of Triplex either with or without additional injections given directly to the recipient. The premise of this clinical trial is to develop a CMV-specific T-cell response within the donor that will be transferred to the HCT recipient and be competent to overcome early viremia before prophylaxis with antiviral (eg, letermovir). Further injections given to the HCT recipient will be evaluated with the goal of prolonging the period of protection beyond the risk period of 100 days through 200 days post-HCT.
Success in demonstrating tolerability of the triplex vaccine in pediatric HCT recipients (clinicaltrials.gov pending, NCT03354728) would prompt an additional approach to vaccinating the donor similar to the adult trial that we described as a means to forestall early reactivation events before antiviral prophylaxis using letermovir. City of Hope is also in the planning stages of conducting clinical studies in a variety of solid organ transplant (SOT) recipients; however, the goal for this population is to develop primary immunity in CMV-negative SOT recipients to prevent CMV viremia in recipients of a CMV-positive organ such as a lung, liver, or kidney.
City of Hope Cytomegalovirus PepVax
Over the last 20 years, an effort involving the human leukocyte antigen (HLA) A* 0201 pp65495-503 cytotoxic T lymphocyte epitope as a vaccine was undertaken [20–24]. Preclinical studies that led to the clinical trials described here were recently reviewed. The Pfizer CpG adjuvant PF03512676 and the peptide vaccine was tested in a Phase I clinical study. Strong immunogenicity of the combination was demonstrated in humans. The chimeric peptide combined with a tetanus epitope was less reactogenic than the version with the PADRE (Pan DR epitope) peptide, as demonstrated in a study in healthy volunteers [25].
An Open-label Phase IB Trial was conducted at a single center in the United States. Eligible patients were CMV-seropositive, positive for HLA A* 0201, aged 18–75 years, and undergoing HCT from a matched related or matched unrelated donor [26].
Thirty-six patients were allocated to either the vaccine or observation arm, in blocks stratified by CMV donor serostatus. CMVPepVax was administered subcutaneously on days 28 and 56 post-HCT (NCT01588015 with clinicaltrials.gov). There was no evidence of reduced tolerability of the vaccine or unexpected adverse events (AEs). Better relapse-free survival was found in patients allocated to vaccine versus observation arms using Kaplan-Meier estimates in a median observation period of 461 days (P = .015). In addition, there was a significant reduction in CMV reactivation over a 180-day period in the vaccine arm compared with the observation arm using Kaplan-Meier estimates (P = .039). Blood specimens from the completed Phase 1B trial in HCT recipients were further analyzed using major histocompatibility complex class I pp65495-503 multimers as well as CD28 and CD45 memory markers to detect CMV-specific immune reconstitution [27]. The major finding was that by day 70, during a period of high risk for CMV reactivation, combined T-effector memory (TEM) and T-effector memory RA (TEMRA) phenotypes constituted a median of 90% of pp65-specific CD8 T cells in the vaccinated patients. Cytomegalovirus viremia was not detectable in the patients who received CMVPepVax and had strong immune reconstitution. Rapid reconstitution of CMV-specific T cells with a high frequency of an effector phenotype may have a role in the favorable outcomes of the Phase IB clinical trial.
A Phase II study is ongoing in which the protective benefits of the vaccine are being evaluated in a multicenter study of HCT recipients (NCT02396134 in clinicaltrials.gov). The clinical trial will accrue 96 HLA A* 0201 CMV-seropostive HCT recipients who will receive either CMVPepVax or a placebo. Cytomegalovirus cellular immunity using a multimer probe strategy will also be investigated over 365 days of observation. Success of this multicenter Phase II trial would prompt an end of Phase II meeting with the US Food and Drug Administration to coordinate further development and planning of a registration trial.
Hookipa Pharma
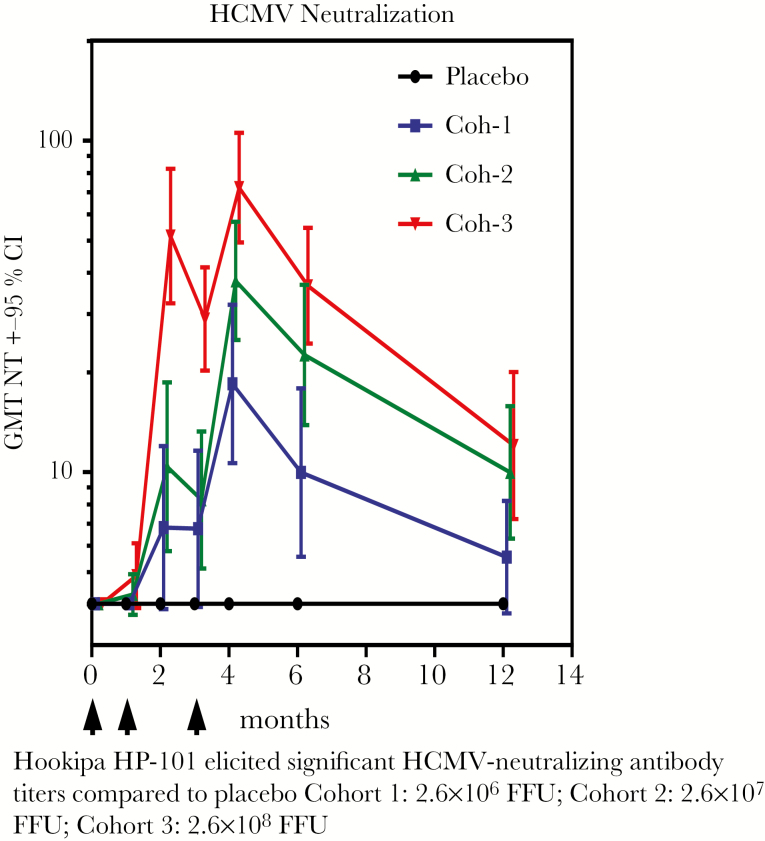
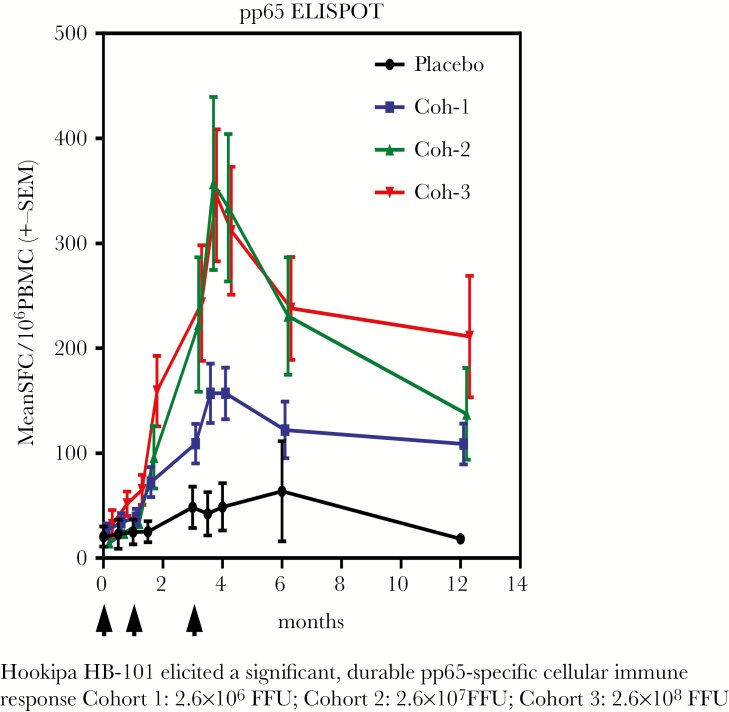
Lymphocytic choriomeningitis virus (LCMV) is an agent that is primarily a pathogen for rodents, but which when rendered noninfectious can be used as a vector that is capable of inducing both antibody and strong cellular responses, including CD8+ T cells [28]. The genes for CMV gB and pp65 antigens were inserted into the LCMV vector. Preclinical studies in the guinea pig CMV system showed good protection [29]. A placebo-controlled, double-blind Phase I dose-escalating trial was performed in 54 healthy adult volunteers (ClinicalTrials.gov Identifier NCT02798692). More important, LCMV vector nAbs were not induced. However, doses of 2.6 × 10–7 and 2.6 × 10–8 induced high levels of CMV-nAbs, and flow cytometry showed induction of a high proportion of polyfunctional human CMV (hCMV)-specific CD4+ and CD8+ T cells (Figures 3 and 4). The latter were directed against the pp65 and expressed IFN-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α. A Phase II trial has begun in CMV-seronegative kidney transplant patients who will receive organs from seropositive donors (ClinicalTrials.gov Identifier NCT03629080), to study immunogenicity and with the secondary objectives of reducing CMV viremia and use of antivirals.
Figure 3.
Hookipa HP-101 elicited significant human cytomegalovirus (HCMV)-neutralizing antibody titers compared with placebo: Cohort (Coh) 1, 2.6 × 106 focus-forming units (FFU); Coh 2, 2.6 × 107 FFU; Coh 3, 2.6 × 108 FFU. CI, confidence interval; GMT, geometric mean titers.
Figure 4.
Hookipa HB-101 elicited a significant, durable pp65-specific cellular immune response: Cohort (Coh) 1, 2.6 × 106 focus-forming units (FFU); Coh 2, 2.6 × 107 FFU; Coh 3, 2.6 × 108 FFU. ELISPOT, enzyme-linked immunospot; PBMC, peripheral blood mononuclear cells; SEM, standard error of the mean; SFC, spot-forming cells.
Astellas
The Vical Corporation developed a deoxyribonucleic acid (DNA) plasmid vaccine based on the genes for gB and pp65. In a Phase II trial conducted in hematogenous stem cell transplant patients, the vaccine reduced CMV viral load in a clinically meaningful way, and the immune response that correlated with protection was pp65-specific IFN-γ producing T cells [30]. The product was taken over by the Astellas Corporation, which conducted a randomized trial of a similar vaccine called ASP0113 in seronegative kidney transplant patients receiving a kidney from a seropositive donor [31]. Transplant recipients were randomized (1:1) to receive 5 doses of ASP0113 (5 mg; n = 75) or placebo (n = 74) on days 30/60/90/120/180 posttransplant, and they received prophylactic valganciclovir/ganciclovir 10–100 days posttransplant. The primary endpoint was the proportion of transplant recipients with CMV viremia ≥1000 IU/mL from day 100 to 1 year after the first study vaccine injection. There was no statistically significant difference in the primary endpoint between the ASP0113 and placebo groups (odds ratio, 0.79; 95% confidence interval, 0.43–1.47; P = .307). There were similar numbers of transplant recipients with treatment-emergent AEs between groups; however, more transplant recipients reported injection site pain in the ASP0113 group compared with placebo. ASP0113 did not demonstrate efficacy in the prevention of CMV viremia in this CMV-seronegative kidney transplant population, but it demonstrated a safety profile similar to placebo. The failure of this vaccine should be considered in light of solid evidence that antibodies are protective in this type of patient and the lack of any induction of humoral immunity in the patients receiving ASP0113.
Thus, there are numerous candidate CMV vaccines in development, both those targeted to prevention of congenital infection and those targeted to prevent posttransplant infections.
VACCINES IN EARLY CLINICAL DEVELOPMENT
GlaxoSmithKline
GSK also first developed a vaccine based on the gB. In 2008, a GSK CMV candidate vaccine was evaluated in a Phase I clinical study. The candidate included a recombinant Chinese Hamster Ovary (CHO)-produced hCMV gB protein based on AD169 strain, with transmembrane and cytoplasmic domains deleted.
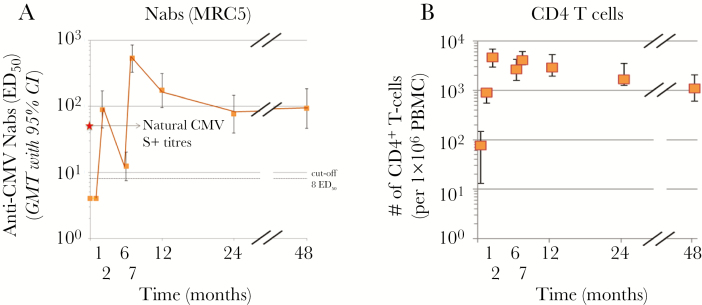
In the Phase I, open trial, healthy male CMV-seronegative subjects aged 18–40 years (n = 40) received 3 intramuscular doses of the vaccine composed of gB, 15 μg, adjuvanted with AS01E Adjuvant System (composed of 25 μg MPL, 25 μg QS21, and liposomes) at months 0, 1, and 6. Mild AEs were frequently reported, but the observed data suggested no safety concerns. The 3-dose vaccine induced high-avidity anti-gB immunoglobulin (Ig)G (enzyme-linked immunosorbent assay in the presence or absence of urea) and anti-CMV nAbs, as measured in MRC-5 fibroblasts using the pentamer-deficient AD169 strain of virus, which persisted at least until month 24 (18 months after the last vaccination) and were in the same range as those observed in natural infection (sera from 39 healthy subjects evaluated in the same assays) (Figure 5). Cell-mediated immunity was also evaluated; CMV gB-specific T cells in peripheral blood mononuclear cells were quantified by flow cytometry, using intracellular cytokine staining for production of IFN-γ, IL-2, and TNF-α and the expression of CD40L. Strong and sustained levels of gB-specific CD4+ T cells were elicited, the majority of which expressed both CD40L and IL-2. Robust levels of CMV gB-specific antibody-secreting B cells were also measured at 24 months based on ELISPOT assay. A subset of 27 vaccinated subjects were re-evaluated for fibroblast nAbs in sera at month 48 (42 months after the third vaccination), and these data confirmed good persistence of immune responses, comparable to those induced by natural infection. CD4+ T cells at this late time point were also sustained at high frequencies (Figure 6).
Figure 5.
Kinetics of cytomegalovirus (CMV)-specific humoral and B-cell responses to GSK glycoprotein B (gB) combined with AS01E adjuvant. At the indicated time points (A), anti-gB immunoglobulin (Ig)G antibodies were measured by enzyme-linked immunosorbent assay; (B) anti-CMV neutralizing antibodies (Nabs) were measured by microneutralization test using MRC5 fibroblast cells and the AD169 CMV strain; (C) anti-gB IgG avidity indexes were measured by avidity enzyme-linked immunosorbent assay using a chaotropic agent (8 M urea); and (D) gB-specific antibody-secreting B-cells were determined by enzyme-linked immunospot assay. (A) and (B) show geometric mean titer (GMT)/geometric mean concentrations (GMC) and 95% confidence interval (CI). (C) and (D) show boxplots: the box represents the upper and lower quartiles; the horizontal line within the box represents the median value; the whiskers represent the minimum and maximum values. Arrows represent time of vaccine dose administration. Natural infection levels of anti-gB IgG (and avidity index) and anti-CMV neutralizing antibodies were determined at the month 0 timepoint from 39 CMV-seropositive healthy male subjects (dotted line).
Figure 6.
Persistence of vaccine-induced neutralizing antibody (Nab) and CD4+ T-cell response after GSK glycoprotein B (gB) vaccine. A subset of 27 vaccinated subjects were re-evaluated for (A) anti-cytomegalovirus (CMV) neutralizing antibodies in sera at month 48 (42 months after the third vaccination) determined by microneutralization test as described in Figure 1 (natural infection levels are indicated with a star). In (B), CMV gB-specific T cells were quantified by flow cytometry, using intracellular cytokine staining of peripheral blood mononuclear cells (PBMCs), cultured in the presence of gB-derived peptides. The PBMCs were stained for CD40L, interferon-γ, interleukin-2, and tumor necrosis factor-α. The number of CD4+ T cells per 1 × 106 cells expressing at least 2 markers is shown at the indicated time points.
However, gB is no longer considered sufficient, and the scientific field in CMV virology has now turned its focus toward the hCMV PC, which has been shown to be the predominant target of nAbs in human sera [32]. It has been demonstrated that PC-specific antibodies are substantially more potent than gB antibodies at neutralizing epithelial cell entry [33, 34], and maternal antibodies against PC are correlated with lower risk of fetal infection [35]. Recent publication of the PC crystal structure provides a foundation for detailed study and design of recombinant PC by GSK for evaluation as a vaccine antigen [36]. The recently elucidated PC is likely to be a critical vaccine antigen together with gB, building on the promising data generated with recombinant gB and AS01 adjuvant.
Moderna
Modified messenger ribonucleic acid (mRNA) encapsulated in a lipid nanoparticle (LNP) has emerged as a versatile platform for rapid development of vaccines. Utilizing the mRNA platform, Moderna has developed multi-antigenic hCMV vaccines comprising multiple mRNAs encoding either for full-length hCMV gB and the gH/gL/UL128/UL130/UL131A complex (pentamer) or the dominant T-cell antigen pp65 along with gB and pentamer [37]. Both gB and pentamer were shown to be expressed on cell surface in vitro, and the correct assembly of pentamer was verified using a panel of conformation-dependent monoclonal antibodies.
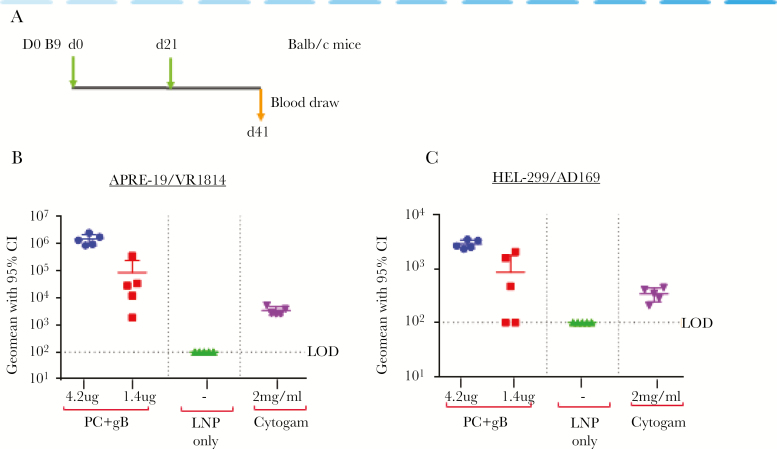
Immunization studies with these mRNA/LNP vaccines elicited potent and durable nAbs both in mice and nonhuman primates. It is worth noting that there was no interference in nAb responses by combining multiple antigens in the same LNP. CytoGam, a CMV immune globulin from pooled plasma of CMV-seropositive donors approved for prevention of CMV in transplant patients, was used as control in the neutralization assays. At the highest dose in mice, the hCMV mRNA gB+pentamer vaccine generated neutralizing responses that were between 20 and 100-fold higher than CytoGam at estimated clinical levels (Figure 7). In addition, the pentamer+gB+pp65 mRNA vaccine elicited potent T-cell responses to pentamer. However, the vaccine elicited diminished T-cell responses to pp65 when compared with a pp65 mRNA vaccine, which suggests T-cell epitope competition. The repression of T-cell responses to pp65 in presence of pentamer was overcome by a heterologous prime/boost immunization of pp65 followed by pentamer+gB+pp65 mRNA vaccine [37]. Thus, the mRNA/LNP platform provides flexibility to mix and match antigens to achieve the optimal balance of antibody and cellular responses.
Figure 7.
Moderna PC+gB messenger ribonucleic acid (mRNA)/lipid nanoparticle (LNP) vaccine elicits high neutralizing titers against human cytomegalovirus (hCMV) infection of epithelial and fibroblast cells. Neutralizing antibodies in mouse immune sera. (A) Schematic of dosing regimen. Indicated are days of dosing and blood draws. (B and C) Neutralizing titers in sera from mice immunized with PC+gB vaccine at the indicated doses or empty LNP (LNP only) against (B) VR1814 infection in ARPE-19 epithelial cells and against (C) AD169 infection in HEL299 fibroblast cells. Shown also is the neutralizing activity in Cytogam. Titers in Cytogam are shown for an approximate maximum concentration (2 mg/mL) in human sera after dosing, which was calculated based on an average body weight of 70 kg (assuming 5L of blood). CI, confidence interval; gB, glycoprotein B; LOD, limit of detection; PC, pentameric complex.
Moderna's gB+pentamer mRNA vaccine is currently being evaluated in a Phase 1 study in the United States. The trial is a randomized, observer-blinded, placebo-controlled, dose-ranging study to evaluate the safety and immunogenicity of different dose levels of the vaccine in hCMV-seropositive and seronegative heathy adults. Immunogenicity assessments include antibody titers against pentamer and gB, nAb titers against hCMV infection of epithelial cells and fibroblasts, and antigen-specific T-cell responses.
VBI Vaccines
A gB vaccine has been produced based on enveloped virus-like particles (eVLP) by transfection of HEK 293 cells with a plasmid encoding murine leukemia virus Gag, which gives rise to particles. Cotransfected plasmid encoding glycoprotein(s) of interest enables particles budding from the cell surface to incorporate the glycoprotein into the lipid bilayer. The eVLPs expressing the ectodomain of the CMV gB protein fused to the transmembrane and cytoplasmic domains of the vesicular stomatitis virus G protein (gB-G) were used.
The breadth of neutralizing activity observed in past clinical trials using gB-based vaccines has been suboptimal, which may be explained by the failure to elicit antibody responses against epitopes associated with potent and broad neutralizing activity. The CMV gB protein has 5 antigenic domains [38, 39]. Almost 100% of CMV-infected individuals develop antibodies against the AD-1 domain, although these antibodies typically have no neutralizing activity [40]; in contrast, antibody responses against the AD-4 and AD-5 domains, observed in most CMV seropositive subjects, typically possess neutralizing activity. The AD-2 domain is poorly immunogenic [40, 41], but antibodies directed against 1 of 2 epitopes (Site I) possess potent and broad neutralizing activity based on the ability to block viral cell fusion but not binding [42]. Antibody responses against AD-2 are associated with a decreased incidence of viremia in CMV-seropositive recipients of SOTs [43], and AD-2 antibody responses correlate with better outcomes posttransplant [40], even in the absence of antibodies directed against the PC.
Electron microscopy analyses of eVLPs expressing native gB versus gB-G suggest different structures, and preclinical studies have demonstrated that immunization with gB-G eVLPs induces neutralizing activity against CMV infection of both fibroblast and epithelial cells substantially greater than immunization with comparable doses of gB eVLPs or recombinant gB protein. Recent epitope mapping of gB versus gB-G eVLPs has demonstrated that a monoclonal antibody against the AD-2 epitope uniquely binds to gB-G but not gB eVLPs.
A candidate CMV vaccine based on gB-G eVLPs (VBI-1501) has been evaluated in a human clinical study (clinicaltrials.gov NCT02826798). Healthy CMV-seronegative 18- to 40-year-old adults were randomized to 1 of 4 dose formulations (0.5 µg, 1 µg, or 2 µg gB-G content with alum) or 1 µg gB without alum, or placebo given on days 0, 56, and 168. Outcome measures were solicited and unsolicited AEs, severe AE (SAE), gB antibody binding titers, and nAbs against CMV infection of fibroblast and epithelial cells. Among 128 participants, the most common solicited local and general AEs were pain and headache, respectively. No SAEs or withdrawals occurred. Boosting of nAb titers was observed after doses 2 and 3, with the highest titers in the Alum-adjuvanted 2.0-µg dose recipients. Fibroblast cell nAbs were seen in 100% of 2.0-µg dose recipients, and epithelial cell nAbs were seen in 31%. Epithelial cell nAb was correlated with higher geometric mean gB binding titers, and there was a correlation between fibroblast and epithelial cell nAb titers. In summary, eVLP presentation of a novel form of CMV gB was immunogenic at very low doses in healthy seronegative adults, and no safety signals were seen. A Phase II dose-ranging study is planned for the end of 2019.
VACCINES IN PRECLINICAL DEVELOPMENT
Pfizer
Pfizer hypothesized that superior anti-PC neutralizing responses could better protect against horizontal transmission of CMV than does immunization with gB [44]. As will be described in future publications and as summarized at a high level at the National Institutes of Health Workshop, to test this hypothesis preclinically, Pfizer developed a rigorous rhesus CMV (RhCMV) oral challenge model in specific pathogen-free rhesus macaques to answer the following questions: (1) Does immunization with the PC prevent RhCMV horizontal transmission? (2) Does adding gB or a plasmid expressing pp65-2 to the PC increase efficacy?
The macaques received 3 doses of the adjuvant QS21 alone as a control, QS21 adjuvanted RhCMV PC, or QS21 adjuvanted PC with gB and a plasmid that expressed pp65-2. The immunizations were followed by 5 weekly oral inoculations of 8 × 105 plaque-forming units of RhCMV (strain UCD52, homologous to the antigens), a regimen that consistently infected most, but not all, vaccine-naive macaques.
The vaccine elicited RhCMV nAb titers similar to, and pp65-specific IFN-γ-positive ELISPOT responses greater than, those elicited by natural RhCMV infection. Yet, the vaccine did not prevent infection with the orally inoculated virus or block virus spread to the urinary tract. Thus, high-titer nAbs and T-cell responses against an adjuvanted combination of the PC, gB, and pp65 in a subunit/DNA vaccine did not protect against oral RhCMV infection or subsequent virus dissemination. Immune evasion by RhCMV, through mechanisms analogous to those that allow RhCMV to reinfect after primary infection, may also have allowed RhCMV to infect despite immunization of the macaques.
In these studies, potential protection against vertical transmission was not tested. Intravenous (IV) infusion of potent RhCMV hyperimmune globulin inhibits vertical transmission of IV-inoculated RHCMV in RhCMV seronegative, pregnant, CD4+ T cell-depleted rhesus monkeys and prevents fetal loss [6]. Neutralizing titers on rhesus epithelial cells elicited by the PC-containing vaccine are equivalent to serum-neutralizing titers reached after infusion of high-potency HIG. Therefore, future studies to test whether PC-containing formulations can prevent vertical transmission could be warranted.
Serum Institute of India
Pepperl et al [45] have taken advantage of the production by CMV in cell culture of what are called dense bodies: noninfectious enveloped particles that contain many of the important CMV antigens. This approach is being developed by one of the largest vaccine manufacturers in the world. The dense bodies have been shown to induce both antibodies and cytotoxic T cells in mice transgenic for the human HLA-A2 molecule without the use of adjuvant [45]. Considering that congenital infections with CMV are also common in the developing world, the involvement of the Serum Institute is important [46].
CONCLUSIONS
Thus, there are numerous candidate CMV vaccines in development, both those targeted to prevent congenital infection and those targeted to prevent posttransplant infections. There is early evidence from Phase II trials that vaccination can prevent acquisition of CMV by seronegative women exposed to CMV in nature, and there is solid evidence that CMV disease in seronegative solid organ recipients and in hematogenous stem cell recipients can be prevented. There are as yet no Phase III data, but numerous candidate vaccines are moving forward, including several aimed at the goal of preventing congenital CMV disease.
Notes
Supplement sponsorship. This supplement was sponsored by NIAID and NICHD.
Potential conflicts of interest. Where indicated the author is an employee of the company developing the candidate vaccine. Dr S. A. P. is a paid consultant to many of the companies developing CMV vaccines. D. W. is an employee and shareholder of MSD. D. W. also received royalties from patents on CMV pentameric complex owned by Princeton University. A. O. an employee of Sanofi Pasteur and shareholder of Sanofi. A. C. is an employee of Moderna. S. M. is an employee and shareholder of the GSK group of companies. D. A. is an employee and shareholder of VBI Vaccines. P. R. D. is an employee and shareholder of Pfizer. Dr C. N. K. reports consulting from Hookipa, consulting/adjudication from Merck, consulting from GSK, consulting/adjudication from Shire, consulting from Oxford Immunotec, consulting from QIAGEN, adjudication from Astellas, during the conduct of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Plotkin SA. Can Infection by the human Cytomegalovirus be Prevented? J Infect Dis 2019. [Google Scholar]
- 2. Wang D, Freed DC, He X, et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med 2016; 8:362ra145. [DOI] [PubMed] [Google Scholar]
- 3. Adler SP, Lewis N, Conlon A, et al. Phase 1 clinical trial of a conditionally replication-defective human cytomegalovirus (CMV) vaccine in CMV-seronegative subjects. J Infect Dis 2019; 220:411–9. [DOI] [PubMed] [Google Scholar]
- 4. Ha S, Li F, Troutman MC, et al. Neutralization of diverse human cytomegalovirus strains conferred by antibodies targeting viral gH/gL/pUL128-131 pentameric complex. J Virol 2017; 91. doi: 10.1128/jvi.02033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonczol E, Ianacone J, Ho WZ, Starr S, Meignier B, Plotkin S. Isolated gA/gB glycoprotein complex of human cytomegalovirus envelope induces humoral and cellular immune-responses in human volunteers. Vaccine 1990; 8:130–6. [DOI] [PubMed] [Google Scholar]
- 6. Pass RF, Duliegè AM, Boppana S, et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis 1999; 180:970–5. [DOI] [PubMed] [Google Scholar]
- 7. Nelson CS, Cruz DV, Tran D, et al. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2017; 2. doi: 10.1172/jci.insight.94002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 2016; 34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. La Rosa C, Longmate J, Martinez J, et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 2017; 129:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Zhou W, Srivastava T, et al. A fusion protein of HCMV IE1 exon4 and IE2 exon5 stimulates potent cellular immunity in an MVA vaccine vector. Virology 2008; 377:379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Martinez J, Zhou W, et al. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 2010; 28:1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z, La Rosa C, Mekhoubad S, et al. Attenuated poxviruses generate clinically relevant frequencies of CMV-specific T cells. Blood 2004; 104:847–56. [DOI] [PubMed] [Google Scholar]
- 16. Chiuppesi F, Nguyen J, Park S, et al. Multiantigenic modified vaccinia virus ankara vaccine vectors to elicit potent humoral and cellular immune reponses against human cytomegalovirus in mice. J Virol 2018; 92. doi: 10.1128/jvi.01012-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diamond DJ, La Rosa C, Chiuppesi F, et al. A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines 2018; 17:889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldoss I, Baden L, Ariza-Heredia E, et al. OS6-1 – multi-antigen vaccine (Triplex) based on attenuated poxvirus prevents cytomegalovirus viremia in a multi-center placebo-controlled, double-blinded, randomized phase 2 clinical trial in allogeneic HCT recipients [abstract]. 45 Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Frankfurt, March 22–27. 2019. [Google Scholar]
- 19. Aldoss I, La Rosa C, Baden LR, et al. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: a phase 2, randomized clinical trial [Epub ahead of print February 11, 2020]. Ann Intern Med 2020; doi:10.7326/M19-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 2002; 106:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. BenMohamed L, Krishnan R, Longmate J, et al. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(H) response. Hum Immunol 2000; 61:764–79. [DOI] [PubMed] [Google Scholar]
- 22. La Rosa C, Wang Z, Brewer JC, et al. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood 2002; 100:3681–9. [DOI] [PubMed] [Google Scholar]
- 23. Diamond DJ, York J, Sun JY, Wright CL, Forman SJ. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 1997; 90:1751–67. [PubMed] [Google Scholar]
- 24. Longmate J, York J, La Rosa C, et al. Population coverage by HLA class-I restricted cytotoxic T-lymphocyte epitopes. Immunogenetics 2001; 52:165–73. [DOI] [PubMed] [Google Scholar]
- 25. La Rosa C, Longmate J, Lacey SF, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis 2012; 205:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura R, La Rosa C, Longmate J, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 2016; 3:e87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. La Rosa C, Longmate J, Lingaraju CR, et al. Rapid acquisition of cytomegalovirus-specific T cells with a differentiated phenotype, in nonviremic hematopoietic stem transplant recipients vaccinated with CMVPepVax. Biol Blood Marrow Transplant 2019; 25:771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flatz L, Hegazy AN, Bergthaler A, et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med 2010; 16:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schleiss MR, Berka U, Watson E, et al. Additive protection against congenital cytomegalovirus conferred by combined glycoprotein B/pp65 vaccination using a lymphocytic choriomeningitis virus vector. Clin Vaccine Immunol 2017; 24. doi: 10.1128/cvi.00300-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith LR, Wloch MK, Chaplin JA, Gerber M, Rolland AP. Clinical development of a cytomegalovirus DNA vaccine: from product concept to pivotal phase 3 trial. Vaccines (Basel) 2013; 1:398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vincenti F, Budde K, Merville P, et al. A randomized, phase 2 study of ASP0113, a DNA-based vaccine, for the prevention of CMV in CMV-seronegative kidney transplant recipients receiving a kidney from a CMV-seropositive donor. Am J Transplant 2018; 18:2945–54. [DOI] [PubMed] [Google Scholar]
- 32. Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 2005; 102:18153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 2012; 86:7444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macagno A, Bernasconi NL, Vanzetta F, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 2010; 84:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lilleri D, Kabanova A, Revello MG, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 2013; 8:e59863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chandramouli S, Malito E, Nguyen T, et al. Structural basis for potent antibody-mediated neutralization of human cytomegalovirus. Sci Immunol 2017; 2. doi: 10.1126/sciimmunol.aan1457. [DOI] [PubMed] [Google Scholar]
- 37. John S, Yuzhakov O, Woods A, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 2018; 36:1689–99. [DOI] [PubMed] [Google Scholar]
- 38. Pötzsch S, Spindler N, Wiegers AK, et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog 2011; 7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke HG, Heldwein EE. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog 2015; 11:e1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishibashi K, Tokumoto T, Shirakawa H, et al. Lack of antibodies against the antigen domain 2 epitope of cytomegalovirus (CMV) glycoprotein B is associated with CMV disease after renal transplantation in recipients having the same glycoprotein H serotypes as their donors. Transpl Infect Dis 2011; 13:318–23. [DOI] [PubMed] [Google Scholar]
- 41. Baraniak I, Kropff B, McLean GR, et al. Epitope-specific humoral responses to human cytomegalovirus glycoprotein-B vaccine with MF59: anti-AD2 levels correlate with protection from viremia. J Infect Dis 2018; 217:1907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Axelsson F, Adler SP, Lamarre A, Ohlin M. Humoral immunity targeting site I of antigenic domain 2 of glycoprotein B upon immunization with different cytomegalovirus candidate vaccines. Vaccine 2007; 26:41–6. [DOI] [PubMed] [Google Scholar]
- 43. Ohlin M. A new look at a poorly immunogenic neutralization epitope on cytomegalovirus glycoprotein B. Is there cause for antigen redesign? Mol Immunol 2014; 60:95–102. [DOI] [PubMed] [Google Scholar]
- 44. Wen Y, Monroe J, Linton C, et al. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine 2014; 32:3796–804. [DOI] [PubMed] [Google Scholar]
- 45. Pepperl S, Münster J, Mach M, Harris JR, Plachter B. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J Virol 2000; 74:6132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Plachter B. Prospects of a vaccine for the prevention of congenital cytomegalovirus disease. Med Microbiol Immunol 2016; 205:537–47. [DOI] [PubMed] [Google Scholar]