Abstract
Epithelial-mesenchymal transition (EMT) serves an important role in tumor migration and invasion. Astragalus polysaccharide (APS), which is the main component of the traditional Chinese medicine Astragalus membranaceus, has been identified to display an antitumor effect. However, the effects and mechanisms of APS during breast cancer migration and invasion are not completely understood. The present study investigated whether APS inhibited breast cancer migration and invasion by modulating the EMT pathway. An MTT assay and a Ki67 immunofluorescence staining assay demonstrated that APS inhibited the proliferation of breast cancer cells. The results of the wound healing and Transwell Matrigel invasion assays suggested that APS decreased the migration and invasion of breast cancer cells. The western blotting and immunofluorescence analyses further demonstrated that APS had a regulatory effect on EMT-related molecules. APS decreased the expression levels of Snail and vimentin, but increased E-cadherin expression. APS also downregulated Wnt1, β-catenin and downstream target expression. Additionally, the present results suggested that APS decreased the proliferation, and EMT-mediated migration and invasion of cells by inhibiting the Wnt/β-catenin signaling pathway. The present study suggested that APS may serve as a promising therapeutic agent for breast cancer.
Keywords: breast cancer, metastasis, epithelial-mesenchymal transition, Wnt/β-catenin pathway, Astragalus polysaccharide
Introduction
Breast cancer is prevalent among women worldwide (1) and the incidence is increasing rapidly with a large number of new cases being diagnosed each year, especially in areas where it was previously not prevalent, including a number of developing countries, such as China, Brazil and Mexico (2–4). The development of breast cancer is a complex process involving multiple factors and a variety of cytokines, such as IL-6 and TGF-β (5). Tumor migration and invasion contribute to cancer development, recurrence and mortality in patients with breast cancer, and 90% of patients with cancer suffer mortality due to metastasis (6). At present, except for chemotherapy, no effective drug treatment has been identified that can directly inhibit tumor migration and invasion in patients.
Metastasis is a programmed process that involves the following steps: Malignant tumor cells separate from the original location; invade the adjacent tissue via the surrounding extracellular matrix and stromal cell layers; survive in the circulatory system; translocate to distant tissues; re-initiate proliferative capabilities; and subsequently develop into a new tumor (7). Tumor metastasis displays a number of unique characteristics, including migration and invasion; however, the mechanisms of tumor migration and invasion have not been fully determined. Recently, it was identified that epithelial-mesenchymal transition (EMT), which is the transformation of epithelial cells into mesenchymal cells, can significantly enhance the motility of cancer cells (8).
EMT serves a vital role in tissue repair, embryonic developmental processes, including gastrulation, somite dissociation and neural crest development, and a number of diseases, for example, breast cancer (9). EMT is considered to occur prior to tumor migration and invasion (10). During EMT, epithelial cells lose cell adhesions and undergo cytoskeletal alterations (11). In breast cancer, EMT is associated with local invasion and migration, and is marked by the loss of epithelial properties and the acquisition of mesenchymal properties (12). Previous studies demonstrated that a variety of factors can induce EMT, including transforming growth factor β (TGF-β), Wnt, Notch and fibroblast growth factor (13–16). Among these inducers, the Wnt/β-catenin signaling pathway, which can be divided into classical and non-classical signaling pathways, has attracted increasing attention (17). Wnt has been identified as the promoter of the pathway (18) and, as an upstream factor, β-catenin affects the expression of downstream factors of the signaling pathway, including c-Myc and Cyclin D1 (19). Previous studies have suggested that the Wnt/β-catenin signaling pathway can influence developmental embryonic processes, determine cell polarity and regulate cell proliferation (20–23). Therefore, as the vital signaling pathway during EMT, dysfunctions in the Wnt/β-catenin signaling pathway are associated with a number of malignant tumors, including colorectal and ovarian cancer (24). Khramtsov et al (25) demonstrated that the Wnt signaling pathway enhanced migration and invasion during breast cancer. Lin et al (26) observed that suppressing the activity of the Wnt/β-catenin signaling pathway reduced the invasiveness of osteosarcoma cells by reversing EMT. Therefore, drugs that can interfere with the Wnt signaling pathway during EMT may have potential therapeutic effects on breast cancer.
Astragalus polysaccharide (APS) is one of the main bioactive components extracted from the root of Astragalus membranaceus, a traditional Chinese medicine that has been used for thousands of years (27). Previous studies demonstrated that APS displays immunoregulatory, antitumor, antioxidant and anti-inflammatory effects (28,29). Li et al (30) observed that APS stimulated the immune response and inhibited breast cancer cell growth. Lai et al (31) also demonstrated that APS displayed anticancer activities in hepatoma cells by inhibiting cell growth, and increasing the spleen and thymus indices. Additionally, Li et al (32) demonstrated that APS increased the sensitivity of ovarian cancer cells to cisplatin, potentially by activating the JNK signaling pathway. Zhou et al (33) also demonstrated that the combination of APS and a polysaccharopeptide had potent inhibitory effects on lung cancer. However, few studies have identified the anti-migratory and anti-invasion effects of APS on breast cancer. The aim of the present study was to investigate the effect of APS on the migration and invasion of breast cancer cells, and to identify the underlying molecular mechanisms.
Materials and methods
Cell lines and reagents
MCF-7 and MDA-MB-231 cells (Shanghai Cellular Research Institute) were maintained in DMEM (HyClone; GE Healthcare Life Sciences) supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences) and 1% penicillin/streptomycin. Cells were incubated at 37°C with 5% CO2. Both cell lines were used in all subsequent experiments. MCF-7 and MDA-MB-231 cells were cultured with APS (800 µg/ml) and LiCl (10 mM, Sigma-Aldrich; Merck KGaA) for 24 h for further investigation of the inhibitory effect of APS.
Cell proliferation assay
Cells were plated (5×103 cells/well) into 96-well plates and cultured overnight. According to previous literature (32,34,35), cells were treated with 0, 25, 50, 100, 200, 400, 800 or 1,600 µg/ml APS (purity, >98%; Tianjin Cinorch Pharmaceutical Co., Ltd.) at 37°C for 24 h. Subsequently, 10 µl MTT (Sigma-Aldrich; Merck KGaA) was added to each well for 4 h. Following the MTT incubation, the medium was removed and the formazan crystals were dissolved in 150 µl DMSO. Proliferation was analyzed at a wavelength of 568 nm using a plate reader.
Wound healing assay
Cells (5×105 cells/well) were plated in6-well plates and incubated in DMEM containing 10% FBS at 37°C. When the cells reached 100% confluency, a 10-µl pipette tip was used to make a single scratch through the center of the well and PBS was used to remove the dislodged cells. Following treatment with APS (0, 200, 400 or 800 µg/ml), cells were incubated in serum-free DMEM for 24 h. The wounds were observed at 0 and 24 h post-APS treatment using an inverted light microscope (magnification, ×100). The relative area compared with control group was measured.
Transwell Matrigel invasion assay
Transwell plates (24-well inserts; diameter, 6 mm; pore size, 8 µm; Corning Life Sciences) were used to assess the effect of APS on the invasion of breast cancer cells. Cells were resuspended and diluted to a concentration of 2×105 cells/ml in serum-free DMEM. Transwell membranes were precoated with Matrigel for 4 h at 37°C. Cells and different concentrations of APS (0, 200, 400 or 800 µg/ml) were added to the upper chambers of the Transwell plates. Complete medium (DMEM supplemented with 10% FPS and 1% penicillin/streptomycin) was plated in the lower chambers of the Transwell plates. Following incubation for 24 h, the Transwell membranes were removed and carefully cleaned using PBS. Subsequently, the invading cells were stained with crystal violet at room temperature for 20 min. Stained cells were counted using an inverted light microscope (magnification, ×200).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Cells were treated with different concentrations of APS (0, 200, 400 or 800 µg/ml) for 24 h. Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and the concentration of RNA was measured at wavelength of 260/280 nm using a spectrophotometer. RT-qPCR was performed using the reverse transcription kit and the SYBR Green Master mix (Vazyme, China) according to the manufacturer's protocol and the ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were: Initial denaturation at 95°C for 10 min, then 40 cycles of denaturation at 95°C for 30 sec and annealing and elongation at 60°C for 30 sec. The 2−ΔΔCq method was used for data analysis (36). The following primer pairs were used for qPCR: GAPDH forward, 5′-TCAAGAAGGTGGTGAAGCAGG-3′ and reverse, 5′-TCAAAGGTGGAGGAGTGGGT-3′; Wnt1 forward, 5′-CGCCTGTAACAGCTCGTCG-3′ and reverse, 5′-CGTGGCAGCACCAGTGGAAG-3′; β-catenin forward, 5′-CCAAGTGGGTGGTATAGAGG-3′ and reverse, 5′-AGTCCATAGTGAAGGCGAAC-3′; E-cadherin forward, 5′-CGTAGCAGTGACGAATGTGG-3′ and reverse, 5′-CTGGGCAGTGTAGGATGTGA-3′; Snail forward, 5′-ATGCACATCCGAAGCCACA-3′ and reverse, 5′-TGACATCTGAGTGGGTCTGG-3′; vimentin forward, 5′-TGAGTACCGGAGACAGGTGCAG-3′ and reverse, 5′-TAGCAGCTTCAACGGCAAAGTTC-3′; c-Myc forward, 5′-AACACACAACGTCTTGGAGC-3′ and reverse, 5′-GCACAAGAGTTCCGTAGCTG-3′; and Cyclin D1 forward, 5′-CGGACTACAGGGGAGTTTTG-3′ and reverse, 5′-AGGAGGTTGGCATCGGGGT-3′. mRNA levels were normalized to the internal reference gene GAPDH.
Western blot analysis
Cells were treated with 0, 200, 400 or 800 µg/ml APS for 24 h. The nuclear and cytoplasmic proteins were extracted from the cells using the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Institute of Biotechnology) according to the manufacturer's protocol. The BCA method was used to protein determination. Proteins (40 µg per lane) were separated by SDS-PAGE on 10% gels and transferred to PVDF membranes. Subsequently, the membranes were blocked with skimmed milk at room temperature for 2 h. Then the membranes were incubated at 4°C overnight with primary antibodies targeted against: Vimentin (Cell Signaling Technology, Inc., cat. no. 5741, dilution 1:1,000), Snail (Cell Signaling Technology, Inc., cat. no. 3879, dilution 1:1,000), E-cadherin (BD Biosciences, cat. no. 610812, dilution 1:1,000), β-catenin (Cell Signaling Technology, Inc., cat. no. 8480, dilution 1:1,000), Wnt1 (Santa Cruz Biotechnology, Inc., cat. no. SC-514531, dilution 1:1,000), c-Myc (Santa Cruz Biotechnology, Inc., cat. no. SC-40, dilution 1:1,000), Cyclin D1 (Santa Cruz Biotechnology, Inc., cat. no. SC-8396, dilution 1:1,000), Histone H3 (Bioworld Technology, Inc., cat. no. BS1405, dilution 1:1,000) and GAPDH (Santa Cruz Biotechnology, Inc., cat. no. SC-47724, dilution 1:1,000). Following primary incubation, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (OriGene Technologies, Inc., cat. nos. TA130024 and TA130005, dilution 1:5,000) for 2 h at room temperature. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL) kit (Applygen Technologies Inc.). Histone H3 and GAPDH were used as the loading controls. The photographic density was quantitated and analyzed using Glyko BandScan 5.0 software (Glyko).
Immunofluorescence staining
Cells at 60–80% confluence were seeded into 4-chamber dishes and incubated with different concentrations of APS (0, 200, 400 or 800 µg/ml) for 24 h. Subsequently, cells were fixed with 4% paraformaldehyde at room temperature for 15 min. Then cells were blocked with 5% normal goat serum (Boster Biological Technology.) at room temperature for 30 min. Subsequently, cells were incubated withanti-Ki67 (Abcam, ab15580, dilution 1:200) and anti-E-cadherin (BD Biosciences, cat. no. 610812, dilution 1:1,000) primary antibodies at 4°C overnight. Following primary incubation, cells were incubated with a fluorescence-labeled secondary antibody (Boster Biological Technology., cat. no. BA1032, dilution 1:100) at 37°C for 30 min. Then cells were counterstained with DAPI at 37°C for 5 min. Immunofluorescence staining was observed using a biological fluorescent microscope (magnification, ×400). The images were analyzed by Image-Pro Plus 6.0 (Media Cybernetics).
Statistical analysis
Data are presented as the mean ± standard deviation of ≥3 repeated experiments. Statistical analyses were performed using SPSS software (version 22.0; IBM Corp.). One-way ANOVA followed by Dunnett's or Tukey's post hoc test was used to analyze the data. P<0.05 was considered to indicate a statistically significant difference.
Results
APS inhibits breast cancer cell proliferation
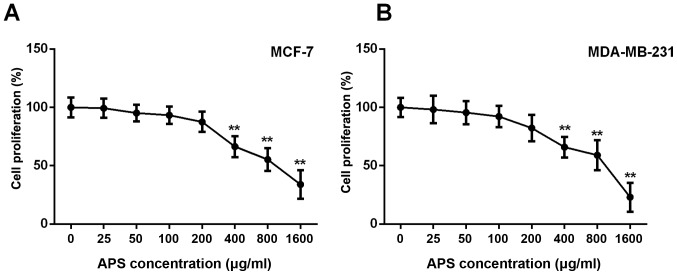
The MTT assay demonstrated that the proliferation of MCF-7 and MDA-MB-231 cells was not significantly different between cells treated with 0, 25, 50 and 100 µg/ml APS; however, the proliferation of MCF-7 and MDA-MB-231 cells was significantly inhibited by APS concentrations ≥400 µg/ml compared with the untreated group (Fig. 1). The proliferation rate of MCF-7 cells treated with 400, 800 and 1,600 µg/ml APS was significantly decreased by 33.6, 44.7 and 66.0%, respectively, compared with the untreated group (Fig. 1A). Similarly, the proliferation rate of MDA-MB-231 cells treated with 400, 800 and 1,600 µg/ml APS was significantly decreased by 34.0, 40.9 and 76.9%, respectively, compared with the untreated group (Fig. 1B). The IC50 value of APS at 24 h was 945 µg/ml for MCF-7 cells and 817 µg/ml for MDA-MB-231 cells.
Figure 1.
Effect of APS on the proliferation of breast cancer cells. Proliferation of (A) MCF-7 and (B) MDA-MB-231 cells was determined using an MTT assay. **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
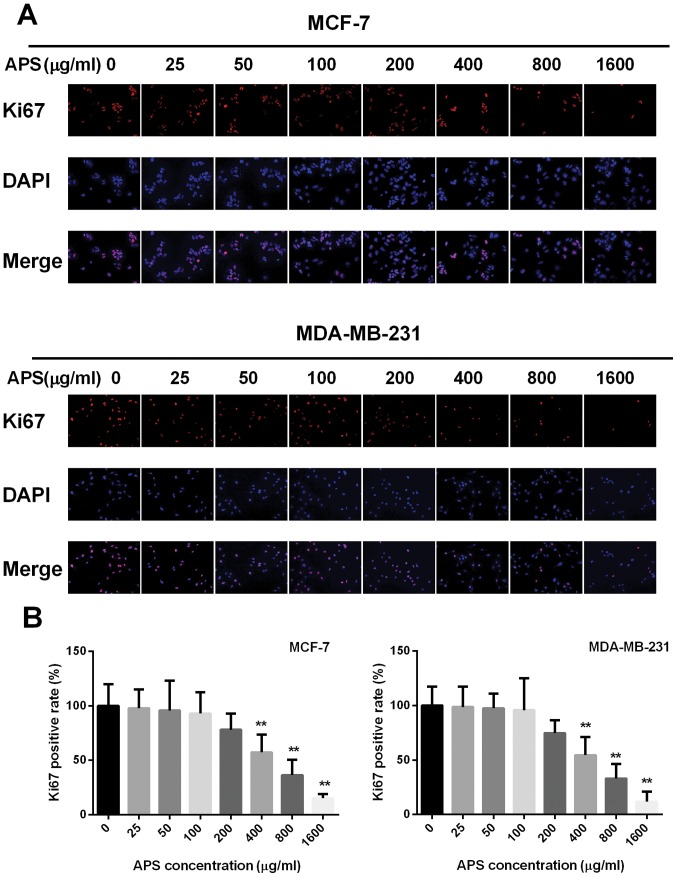
The effect of APS on cell proliferation was also assessed using a Ki67 immunofluorescence assay. The percentage of Ki67 positive cells decreased with increased APS concentration (Fig. 2). The highest APS concentration (1,600 µg/ml) demonstrated the most significant anti-proliferative effects on MCF-7 and MDA-MB-231 cells compared with the untreated group. Therefore, the lower APS concentrations (0, 200, 400 and 800 µg/ml) that decreased the proliferation of MCF-7 and MDA-MB-231 cells, compared with the untreated groups, were selected for subsequent experiments (Fig. 2).
Figure 2.
Immunofluorescence staining of Ki67. (A) Immunofluorescence staining was performed on MCF-7 and MDA-MB-231 cells using an anti-Ki67 antibody and (B) quantified. Magnification, ×400. **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
APS suppresses breast cancer cell migration
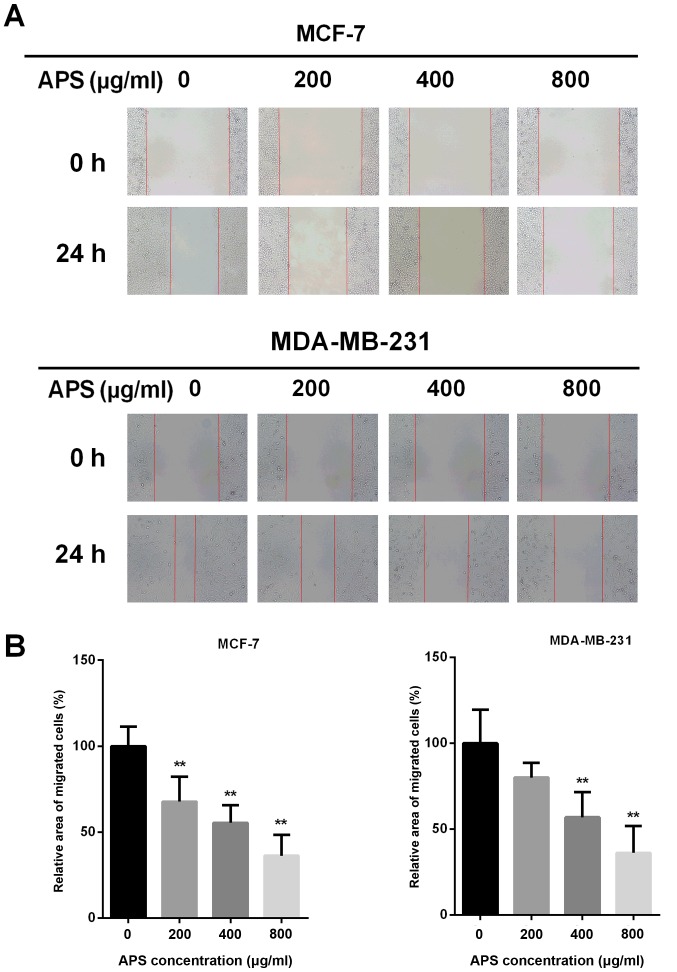
Due to EMT being closely associated with cancer cell migration, a wound healing assay was performed to assess the effect of APS on cell migration (Fig. 3A). The migratory potential of MCF-7 cells was decreased by 32.2, 44.6 and 63.7% after treatment with 200, 400 and 800 µg/ml APS for 24 h, respectively, compared with the control group (Fig. 3B). In MDA-MB-231 cells, the migratory potential was decreased by 19.9, 43.1 and 63.8% after treatment with 200, 400 and 800 µg/ml APS for 24 h, respectively, compared with the control group (Fig. 3B).
Figure 3.
APS inhibits the migration of breast cancer cells. (A) MCF-7 and MDA-MB-231 cells were scratched and subsequently incubated with different concentrations of APS. Images were acquired at 0 and 24 h following the addition of APS. Magnification, ×100. (B) Quantitative analysis of cell migration, expressed as a percentage of the control group (0 µg/ml). **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
APS inhibits breast cancer cell invasion
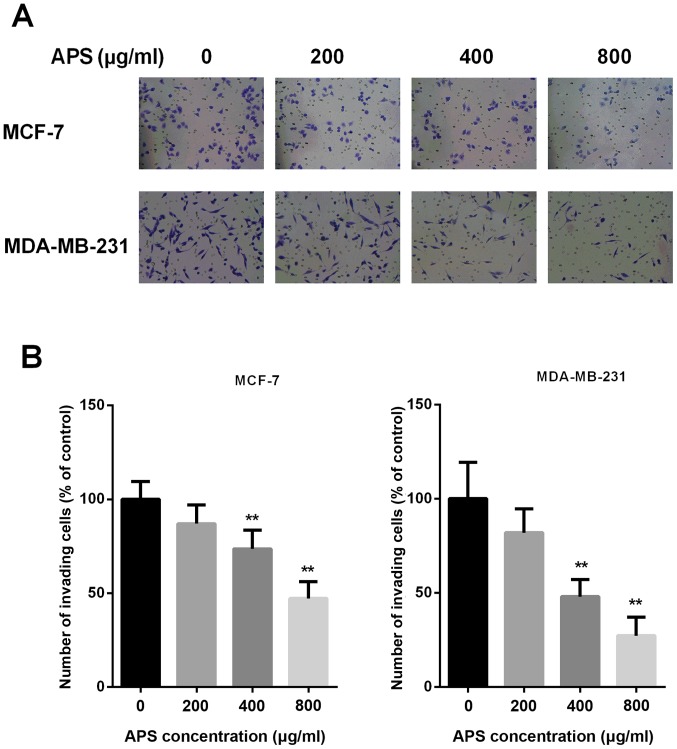
Invasion is an important characteristic that is closely associated with EMT; therefore, Transwell Matrigel assays were performed to assess whether APS inhibited the invasion of breast cancer cells (Fig. 4A). The results demonstrated that treatment with 200, 400 and 800 µg/ml APS decreased the number of invasive MCF-7 cells by 13.1, 26.4 and 52.9%, respectively, compared with the control group (Fig. 4B). The number of invasive MDA-MB-231 cells was also decreased by 18.0, 52.0 and 72.7% following treatment with 200, 400 and 800 µg/ml APS, respectively, compared with the control group (Fig. 4B). The results suggested that APS inhibited the invasion of cells in a dose-dependent manner.
Figure 4.
APS inhibits the invasion of breast cancer cells. (A) MCF-7 and MDA-MB-231 cells were treated with different concentrations of APS for 24 h. Invasive ability was determined using a Transwell Matrigel invasion assay. Magnification, ×200. (B) Quantitative analysis of cell invasion, expressed as a percentage of the control group (0 µg/ml). **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
APS decreases the expression of EMT-associated molecules
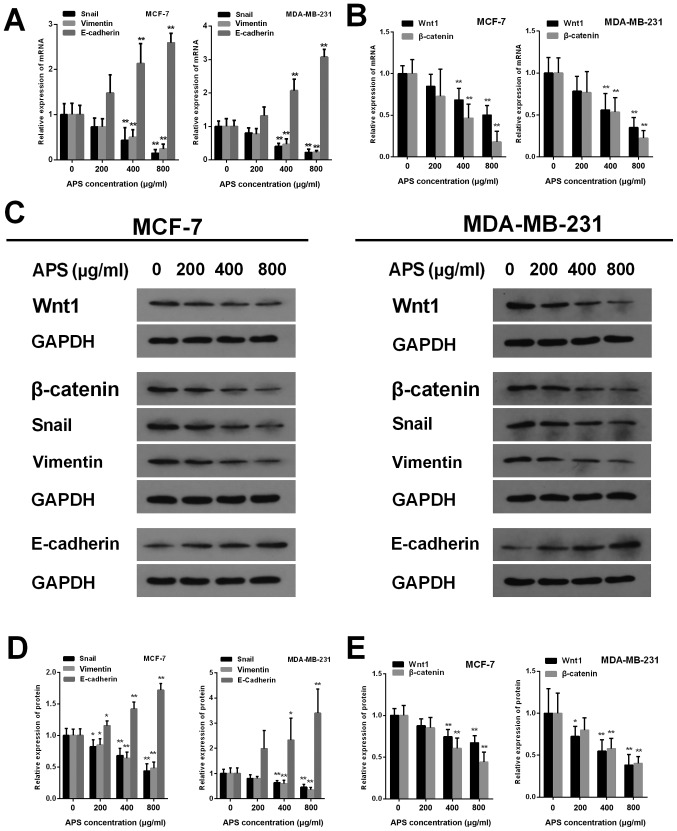
EMT is closely associated with tumor metastasis. To further investigate whether EMT was involved in the inhibitory effect of APS on breast cancer cell migration and invasion, the mRNA and protein expression levels of Snail, vimentin and E-cadherin were measured using RT-qPCR and western blot analysis, respectively (Fig. 5). The mRNA expression levels of Snail were significantly decreased by APS (400 and 800 µg/ml) in MCF-7 and MDA-MB-231 cells compared with the control group (Fig. 5A). Furthermore, MCF-7 cells treated with 200, 400 and 800 µg/ml APS demonstrated significantly decreased Snail protein expression compared with the control group (Fig. 5D). Similarly, Snail protein expression was decreased by 19.5, 36.6 and 54.2% in MDA-MB-231 cells treated with 200, 400 and 800 µg/ml APS, respectively, compared with the control group (Fig. 5D). The mRNA expression of vimentin was also significantly decreased by APS (400 and 800 µg/ml) in MCF-7 and MDA-MB-231 cells compared with the control group (Fig. 5A). In MCF-7 cells treated with 200, 400 and 800 µg/ml APS, vimentin protein expression was significantly decreased by 15.0, 36.0 and 51.8%, respectively, compared with the control group (Fig. 5D). MDA-MB-231 cells also demonstrated significantly decreased vimentin protein expression levels following treatment with 400 and 800 µg/ml APS compared with the control group (Fig. 5D).
Figure 5.
APS alters the expression of Wnt1, β-catenin and EMT-related molecules. mRNA expression levels of (A) Snail, vimentin, E-cadherin, (B) Wnt1 and β-catenin were assessed using reverse transcription-quantitative PCR. (C) Western blotting was used to determine the protein expression levels of Wnt, β-catenin and EMT-related molecules. The protein expression levels of (D) Snail, vimentin, E-cadherin, (E) Wnt1 and β-catenin were quantified. *P<0.05 and **P<0.01 vs. the respective control group. APS, Astragalus polysaccharide; EMT, epithelial-mesenchymal transition.
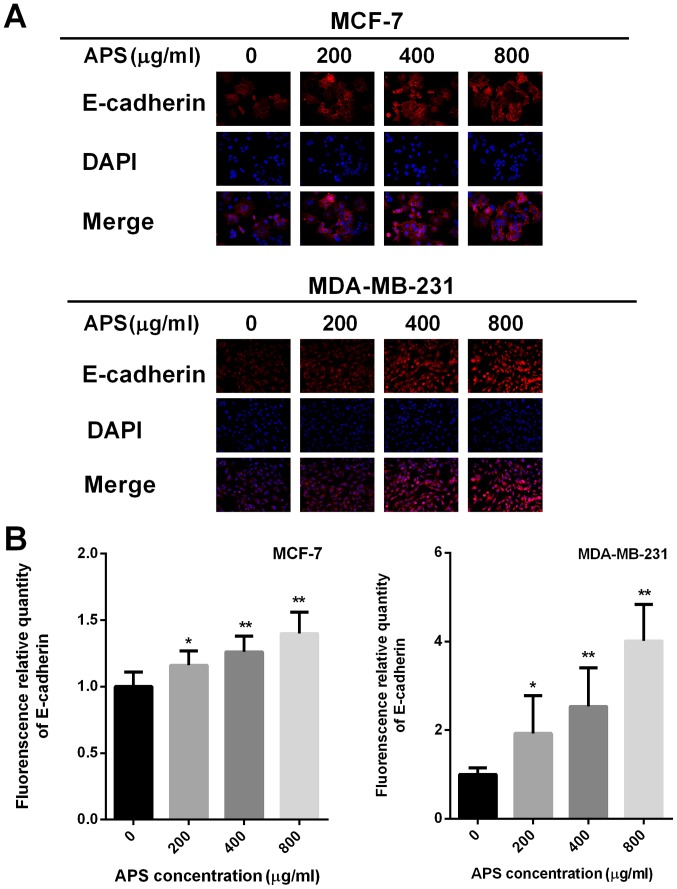
The protein level of E-cadherin increased in MCF-7 and MDA-MB-231 cells as APS concentration increased. The mRNA expression of E-cadherin was significantly increased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 5A). Compared with the control group, the protein level of E-cadherin inMCF-7 cells treated with 200, 400 and 800 µg/ml APS was significantly increased (Fig. 5D). Similarly, the protein level of E-cadherin was significantly increased by 2.33 and 3.40-fold in MDA-MB-231 cells treated with 400 and 800 µg/ml APS, respectively (Fig. 5D). Immunofluorescence staining was also used to assess E-cadherin expression (Fig. 6A). The relative fluorescence of E-cadherin staining was significantly increased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 6B).
Figure 6.
Immunofluorescence staining of E-cadherin. (A) Immunofluorescence staining was performed on MCF-7 and MDA-MB-231 cells using an anti-E-cadherin antibody and (B) quantified. Magnification, ×400.*P<0.05 and **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
APS inhibits EMT by suppressing the activity of the Wnt/β-catenin signaling pathway
The Wnt signaling pathway is an important classical pathway in EMT (17). The present study investigated whether APS exerted its antitumor effect by suppressing the activity of the Wnt/β-catenin signaling pathway. Wnt1 is one of the activators of the signaling pathway; therefore, the mRNA and protein expression levels of Wnt1 were assessed by RT-qPCR and western blotting, respectively. The mRNA expression levels of Wnt1 were significantly decreased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 5B). Wnt1 protein expression was also significantly decreased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 5E). β-catenin is a key factor that activates upstream and downstream factors of the Wnt/β-catenin signaling pathway (19). The mRNA and protein expression levels of β-catenin were also significantly decreased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 5B and E).
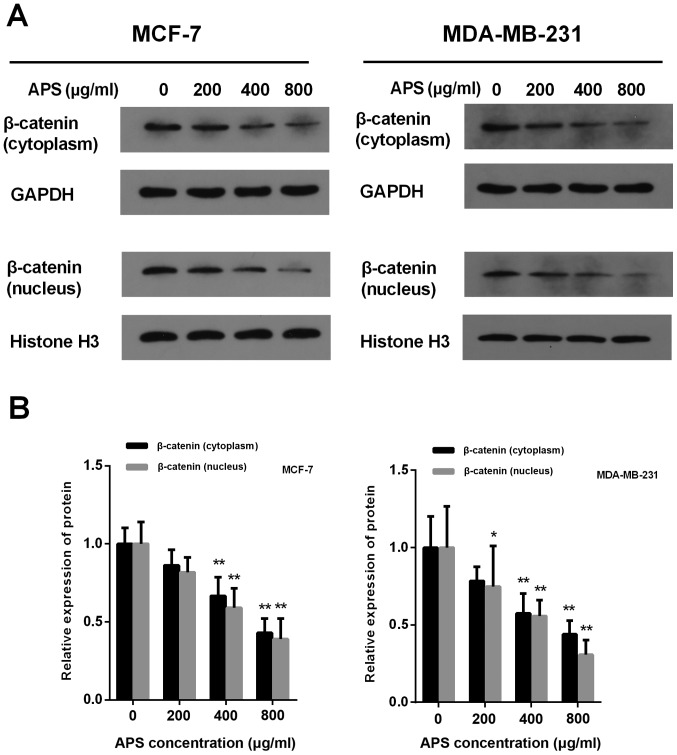
The accumulation of β-catenin in the cytoplasm and its translocation into the nucleus are critical for the activation of β-catenin target gene transcription (18). Treatment with APS (400 and 800 µg/ml) significantly decreased the protein expression level of cytoplasmic and nuclear β-catenin compared with the control group (Fig. 7). In MCF-7 cells treated with 200, 400 and 800 µg/ml APS, the expression of cytoplasmic β-catenin was decreased by 13.8, 33.4 and 57.1%, respectively, compared with the control group (Fig. 7B). In MDA-MB-231 cells treated with 200, 400 and 800 µg/ml APS, the expression of cytoplasmic β-catenin was decreased by 21.6, 42.5 and 56.1%, respectively, compared with the control group (Fig. 7B). In MCF-7 and MDA-MD-231 cells treated with 400 and 800 µg/ml APS, the expression of nuclear β-catenin was significantly decreased compared with the control group (Fig. 7B).
Figure 7.
APS decreases the expression of β-catenin in the cytoplasm and nucleus. Protein expression levels of cytoplasmic and nuclear β-catenin were (A) determined by western blotting and (B) quantified. *P<0.05 and **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
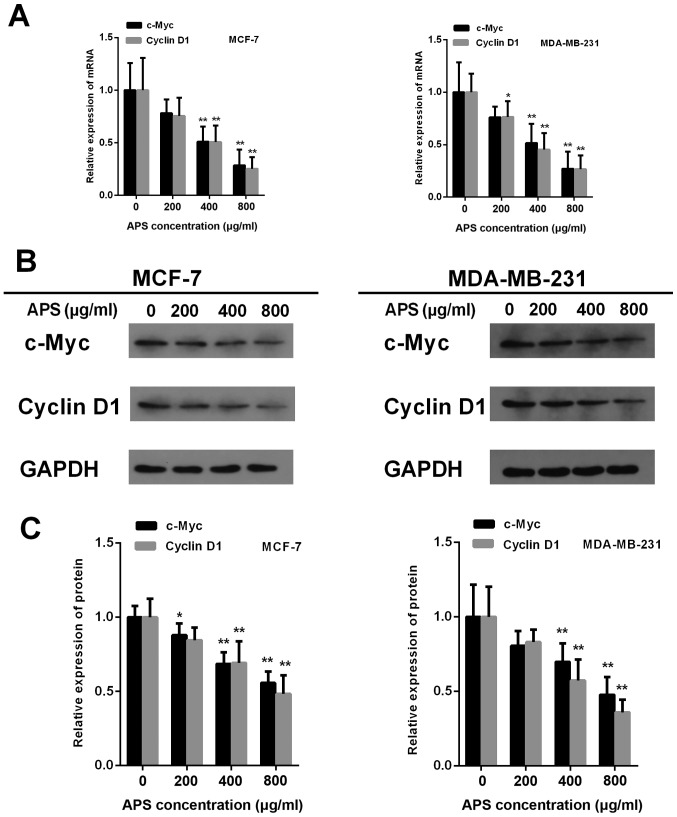
Cyclin D1and c-Myc are downstream factors of the Wnt/β-catenin signaling pathway (18). The mRNA expression of c-Myc was significantly decreased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS cells compared with the control group (Fig. 8A). The results also demonstrated that the protein expression of c-Myc was significantly decreased by 12.1, 31.4 and 44.3% in MCF-7 cells treated with 200, 400 and 800 µg/ml APS, respectively, compared with the control group (Fig. 8B and C). In MDA-MB-231 cells, the protein expression level of c-Myc was significantly decreased by 30.2 and 52.3% following treatment with 400 and 800 µg/ml APS, respectively, compared with the control group (Fig. 8B and C). Furthermore, the mRNA and protein expression levels of Cyclin D1 were significantly decreased in MCF-7 and MDA-MB-231 cells treated with 400 and 800 µg/ml APS compared with the control group (Fig. 8).
Figure 8.
APS decreases the expression of c-Myc and Cyclin D1. (A) mRNA levels of c-Myc and Cyclin D1 were assessed using reverse transcription-quantitative PCR. Protein expression levels of Cyclin D1 and c-Myc were (B) determined by western blotting and (C) quantified. *P<0.05 and **P<0.01 vs. the control group. APS, Astragalus polysaccharide.
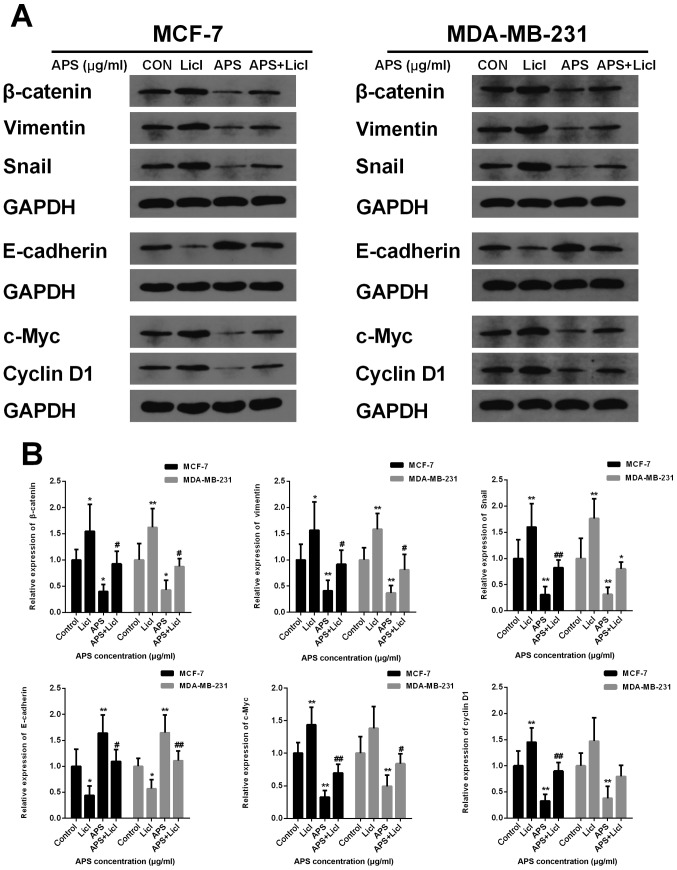
Lithium chloride (LiCl) reverses the inhibitory effect of APS on the Wnt/β-catenin signaling pathway
LiCl, an agonist of the Wnt/β-catenin signaling pathway, was used in the present study to further investigate the inhibitory effect of APS. MCF-7 and MDA-MB-231 cells were cultured with APS (800 µg/ml) and LiCl (10 mM) for 24 h. The protein expression levels of β-catenin, Snail, vimentin, c-Myc and Cyclin D1 were significantly decreased and the protein level of E-cadherin was significantly increased in the APS-treated group compared with the control group (Fig. 9). However, LiCl treatment reversed the APS-induced effects on protein expression (Fig. 9). The results further suggested that the mechanism underlying the inhibitory effects of APS involved the Wnt/β-catenin signaling pathway.
Figure 9.
LiCl reverses the inhibitory effect of APS on the Wnt/β-catenin signaling pathway. MCF-7 and MDA-MB-231 cells were cultured with LiCl (10 mM) and 800 µg/ml APS. Protein expression levels of β-catenin, Snail, vimentin, E-cadherin, c-Myc and Cyclin D1 were (A) determined by western blotting and (B) quantified. *P<0.05 and **P<0.01 vs. the respective control group; #P<0.05 and ##P<0.01 vs. the APS group. LiCl, lithium chloride; APS, Astragalus polysaccharide; CON, control.
Discussion
Tumor migration and invasion are important causes of increased mortality in patients with breast cancer (6). EMT can significantly enhance the migratory ability of cancer cells, including breast cancer cells, which can lead to tumor migration and invasion (9); therefore, drugs targeting the EMT pathway may reduce mortality in patients with breast cancer (37). In recent years, a number of studies have reported that traditional Chinese medicine displays significant anticancer effects (38–40). AM, which is an important component of the qi-supplementing formula, is widely used in China (41) and the main extract of AM is APS. APS is primarily composed of α-1,4-(1,6)-glucan, rhamnus-galacturonic acid polysaccharide I, arabic-galactopolysaccharide and arabic-galactoprotein polysaccharide (42). APS has been demonstrated to display therapeutic effects in multiple diseases, including a variety of tumor diseases, such as hepatocellular carcinoma and lung carcinoma (28–33,43). However, there are only a few studies that have reported the effect of APS on breast cancer, and these studies primarily focused on the effects of APS on the proliferation and immune regulation of breast cancer cells (30,44). The antimigratory and anti-invasion effects, as well as the specific mechanisms of APS on breast cancer cells, are still unclear. The results of the present study suggested that APS significantly decreased the migration and invasion of breast cancer cells in vitro. Furthermore, the results suggested that the mechanism underlying the effects of APS was closely associated with EMT by downregulating the activity of the Wnt/β-catenin signaling pathway. Therefore, the present study may provide rationale for further investigation into the effects of the bioactive components of APS on breast cancer.
MCF-7 and MDA-MB-231 cells differ greatly in cell morphology, estrogen receptor expression, and migration and invasion potential (45). MDA-MB-231 cells proliferate rapidly and are prone to tumorigenesis (45), whereas MCF-7 cells are an estrogen receptor-positive cell line. The migration and invasion of MCF-7 cells is weaker compared with MDA-MB-231 cells; however, previous studies have demonstrated that the migratory and invasive abilities of MCF-7 cells are significantly enhanced under the effect of multiple molecules, including estrogen and Wnt/β-catenin signaling pathway-associated cytokines (46,47). A number of studies have used the two aforementioned cell lines to study the migration and invasion of breast cancer cells; therefore, the two cell lines were selected for the present study (48–50). Yang et al (51) demonstrated that APS inhibited the proliferation of hepatocellular carcinoma cells partly via immunomodulation. In the present study, an MTT assay and Ki67 immunostaining assay demonstrated that APS decreased the proliferation of breast cancer cells in a dose-dependent manner. Tumor migration and invasion involves the following steps: Tumor cells undergo a decrease in homogenous adhesion; detach from the original site of the tumor; adhere to the extracellular matrix (ECM); degrade the ECM; penetrate the ECM surrounding blood vessels; and enter circulation (52). In the present study, the number of migratory cells was significantly decreased in the APS-treated groups compared with the untreated group, as measured by a wound healing assay. The present results suggested that APS inhibited breast cancer cell migration. Additionally, the results of the Transwell Matrigel assay further suggested that the number of invading cells was decreased in the APS-treated groups compared with the untreated group. The present results suggested that APS may inhibit breast cancer cell migration and invasion.
EMT is a vital mechanism during cell growth, tissue repair and organ fibrosis (9); however, it is also closely associated with tumor invasion and migration (53). Previous studies have demonstrated that via the EMT process, cells gain invasive and anti-apoptotic abilities (10–12,54). The hallmark changes of EMT include a reduction in intercellular adhesion and a loss of cell polarity (55). It is well established that the Wnt signaling pathway can induce EMT, which subsequently leads to cell invasion and migration (56,57), and a number of previous studies demonstrated that the Wnt/β-catenin signaling pathway promotes breast cancer progression (58–60). The Wnt signaling pathway is composed of two different intracellular signaling pathways, the canonical and non-canonical pathway (61–63). The canonical pathway involves the activation of β-catenin as a result of triggering by Wnt1, Wnt3a and Wnt8. However, in the non-canonical pathway, Wnt proteins can trigger other effectors, including JNK (64–66). Wnt1 is one of the activators of the Wnt/β-catenin pathway (18). Following activation by Wnt1, β-catenin translocates to the nucleus to regulate the transcription of downstream genes, including Snail, vimentin and E-cadherin (57). Snail can induce EMT and increase cell motility to promote cell differentiation, and it has been reported that Snail expression is abnormal during tumor proliferation and metastasis (67). Vimentin is a downstream molecule of the tumor-related signaling pathway that is important for EMT during malignant transformation (68). Previous studies demonstrated that vimentin can regulate cell-cell adhesion to promote tumor migration and invasion (69,70). Furthermore, E-cadherin participates in the regulation of epithelium formation, maintaining homeostasis and forming adhesive connections. E-cadherin is also involved in the formation of polarized sheets of epithelial cells, and downregulation of E-cadherin can lead to decreased cell adhesion and aggregation (71). It was identified that E-cadherin displays a decreasing trend during EMT due to increased promoter methylation, which occurs during breast cancer (72,73). The present study assessed the expression of EMT-associated molecules, including Wnt1, β-catenin, Snail, vimentin and E-cadherin. Consistent with previous studies (72,73), the present results suggested that Wnt1, β-catenin, Snail and vimentin expression was increased, while E-cadherin expression was decreased in MCF-7 and MDA-MB-231 cells. However, APS treatment significantly downregulated Wnt1, vimentin, Snail and β-catenin expression, and upregulated E-cadherin expression in a dose-dependent manner. The intracellular localization of β-catenin is important for the activation of downstream target genes (57); therefore, cytoplasmic and nuclear β-catenin expression levels following APS treatment were determined. The present results demonstrated that APS reduced the expression levels of cytoplasmic and nuclear β-catenin in a dose-dependent manner. Additionally, Cyclin D1 and c-Myc are produced following β-catenin activation (19). c-Myc, a nuclear protein transcription factor, is associated with the activation of genes that are related to multiple cellular processes, such as proliferation, differentiation, and apoptosis. c-Myc is also important for cell cycle modulation and malignant transformation of cells (74). Cyclin D1, an oncogene, can regulate the function of the cell cycle function, and its abnormal expression can disrupt the cell cycle and promote tumorigenesis (75,76). The present results suggested that APS decreased the transcription of c-Myc and Cyclin D1 in a dose-dependent manner. However, further investigation into the effect of APS when protein degradation is inhibited is required.
The results also demonstrated that LiCl, an agonist of the Wnt signaling pathway, partly reversed the inhibitory effects of APS on breast cancer cells. Therefore, the results of the present study suggested that APS may modulate EMT in breast cancer via the Wnt/β-catenin signaling pathway. Collectively, the present results suggested that the Wnt/β-catenin signaling pathway may serve as an effective target for the inhibition of breast cancer cell migration and invasion.
In conclusion, the present study suggested that APS significantly decreased the migration and invasion of breast cancer cells in vitro by inhibiting EMT and modulating the expression of components of the Wnt/β-catenin signaling pathway. The present study identified a novel mechanism of APS and demonstrated that APS may serve as a potential therapeutic agent for breast cancer. The effect of APS in other EMT-associated pathways, including the TGF-β signaling pathway, and which component of APS displays the antitumor effect requires further investigation.
Acknowledgements
The authors would like to thank Mrs. Qiyan Wang (School of Life Science, Beijng University of Chinese Medicine) for editing the manuscript.
Funding
The present study was supported by The Qingdao Post-Doctoral Research Project and The Natural Science Foundation of Shandong Province (grant no. ZR2017BH067).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SY and HW conceived and design the study. SY drafted the manuscript. SY and WX performed the experiments. SS, BY and GW performed the statistical analyses. HW and BY revised the manuscript. All authors read and approved the final manuscript to be published. All authors agreed to be accountable for the work in ensuring that questions related to the integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: Epidemiology and etiology. Cell Biochem Biophys. 2015;72:333–338. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990–2016: Evidence from Global Burden of Disease Study 2016. Breast Cancer. 2019;26:428–445. doi: 10.1007/s12282-018-00941-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhat V, Allan AL, Raouf A. Role of the microenvironment in regulating normal and cancer stem cell activity: Implications for breast cancer progression and therapy response. Cancers (Basel) 2019;11(pii):E1240. doi: 10.3390/cancers11091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;8:697–702. doi: 10.2217/fon.12.61. [DOI] [PubMed] [Google Scholar]
- 7.Weidle UH, Birzele F, Nopora A. MicroRNAs as potential targets for therapeutic intervention with metastasis of non-small cell lung cancer. Cancer Genomics Proteomics. 2019;16:99–119. doi: 10.21873/cgp.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan L, Xu F, Dai CL. Relationship between epithelial-to-mesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:203. doi: 10.1186/s13046-018-0887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai J, Kwok WC, Thiery JP. Traditional Chinese medicine and regulatory roles on epithelial-mesenchymal transitions. Chin Med. 2019;14:34. doi: 10.1186/s13020-019-0257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang BQ, Li ML, Quan HY, Hou PF, Li ZW, Chu SF, Zheng JN, Bai J. Functional roles of circular RNAs during epithelial-to-mesenchymal transition. Mol Cancer. 2019;18:138. doi: 10.1186/s12943-019-1071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjoberg E, Meyrath M, Milde L, Herrera M, Lovrot J, Hagerstrand D, Frings O, Bartish M, Rolny C, Sonnhammer E, et al. A novel ACKR2-dependent role of fibroblast-derived CXCL14 in Epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res. 2019;25:3702–3717. doi: 10.1158/1078-0432.CCR-18-1294. [DOI] [PubMed] [Google Scholar]
- 13.McCormack N, O'Dea S. Regulation of epithelial to mesenchymal transition by bone morphogenetic proteins. Cell Signal. 2013;25:2856–2862. doi: 10.1016/j.cellsig.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Farahmand L, Darvishi B, Majidzadeh AK, Madjid Ansari A. Naturally occurring compounds acting as potent anti-metastatic agents and their suppressing effects on Hedgehog and WNT/β-catenin singaling pathways. Cell Prolif. 2017:50. doi: 10.1111/cpr.12299. doi: 10.1111/cpr.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Gao XY, Yang SQ, Sun ZX, Dian LL, Qasim M, Phyo AT, Liang ZS, Sun YF. Jatrorrhizine inhibits colorectal carcinoma proliferation and metastasis through Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Drug Des Devel Ther. 2019;13:2235–2247. doi: 10.2147/DDDT.S207315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ram Makena M, Gatla H, Verlekar D, Sukhavasi S, K Pandey M, C Pramanik K. Wnt/β-catenin signaling: The culprit in pancreatic carcinogenesis and therapeutic resistance. Int J Mol Sci. 2019;20(pii):E4242. doi: 10.3390/ijms20174242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Li Q, Yang T, Li D, Ding F, Sun H, Bai G. Anti-cancer effect of Aquaporin 5 silencing in colorectal cancer cells in association with inhibition of Wnt/β-catenin pathway. Cytotechnology. 2018;70:615–624. doi: 10.1007/s10616-017-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 21.Liu XL, Meng J, Zhang XT, Liang XH, Zhang F, Zhao GR, Zhang T. ING5 inhibits lung cancer invasion and epithelial-mesenchymal transition by inhibiting the WNT/β-catenin pathway. Thorac Cancer. 2019;10:848–855. doi: 10.1111/1759-7714.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okura T, Ohkawara B, Takegami Y, Ito M, Masuda A, Seki T, Ishiguro N, Ohno K. Mianserin suppresses R-spondin 2-induced activation of Wnt/β-catenin signaling in chondrocytes and prevents cartilage degradation in a rat model of osteoarthritis. Sci Rep. 2019;9:2808. doi: 10.1038/s41598-019-39393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunt L, Scholpp S. The function of endocytosis in Wnt signaling. Cell Mol Life Sci. 2018;75:785–795. doi: 10.1007/s00018-017-2654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CH, Ji T, Chen CF, Hoang BH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. 2014;804:33–45. doi: 10.1007/978-3-319-04843-7_2. [DOI] [PubMed] [Google Scholar]
- 27.Bamodu OA, Kuo KT, Wang CH, Huang WC, Wu ATH, Tsai JT, Lee KY, Yeh CT, Wang LS. Astragalus polysaccharides (PG2) Enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients. 2019;11(pii):E2264. doi: 10.3390/nu11102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Xie JH, Jin ML, Morris GA, Zha XQ, Chen HQ, Yi Y, Li JE, Wang ZJ, Gao J, Nie SP, et al. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S60–S84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Hu X, Wang S, Wang H, Parungao R, Wang Y, Liu T, Song K. Detection and evaluation of Anti-cancer efficiency of astragalus polysaccharide via a tissue engineered tumor model. Macromol Biosci. 2018;18:e1800223. doi: 10.1002/mabi.201800223. [DOI] [PubMed] [Google Scholar]
- 31.Lai X, Xia W, Wei J, Ding X. Therapeutic effect of Astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose Response. 2017;15:1559325816685182. doi: 10.1177/1559325816685182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Hong L, Liu C, Min J, Hu M, Guo W. Astragalus polysaccharides increase the sensitivity of SKOV3 cells to cisplatin. Arch Gynecol Obstet. 2018;297:381–386. doi: 10.1007/s00404-017-4580-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Liu Z, Long T, Zhou L, Bao Y. Immunomodulatory effects of herbal formula of astragalus polysaccharide (APS) and polysaccharopeptide (PSP) in mice with lung cancer. Int J Biol Macromol. 2018;106:596–601. doi: 10.1016/j.ijbiomac.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Song K, Wang S, Zhang C, Zhuang M, Wang Y, Liu T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C Mater Biol Appl. 2019;98:685–695. doi: 10.1016/j.msec.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JX, Han YP, Bai C, Li Q. Notch1/3 and p53/p21 are a potential therapeutic target for APS-induced apoptosis in non-small cell lung carcinoma cell lines. Int J Clin Exp Med. 2015;8:12539–12547. [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Yang XG, Zhu LC, Wang YJ, Li YY, Wang D. Current advance of therapeutic agents in clinical trials potentially targeting tumor plasticity. Front Oncol. 2019;9:887. doi: 10.3389/fonc.2019.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Hua W, Li Y, Xian X, Zhao Z, Liu C, Zou J, Li J, Fang X, Zhu Y. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem Pharmacol. 2019;174:113776. doi: 10.1016/j.bcp.2019.113776. [DOI] [PubMed] [Google Scholar]
- 39.Song M, Wang X, Luo Y, Liu Z, Tan W, Ye P, Fu Z, Lu F, Xiang W, Tang L, et al. Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem Biol Interact. 2020;317:108939. doi: 10.1016/j.cbi.2020.108939. [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Lin J, Tang D, Zhang M, Wen F, Xue D, Zhang H. Paris saponin H suppresses human hepatocellular carcinoma (HCC) by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Int J Clin Exp Pathol. 2019;12:2875–2886. [PMC free article] [PubMed] [Google Scholar]
- 41.Shan H, Zheng X, Li M. The effects of Astragalus membranaceus active extracts on autophagy-related diseases. Int J Mol Sci. 2019;20(pii):E1904. doi: 10.3390/ijms20081904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun S, Yang S, An N, Wang G, Xu Q, Liu J, Mao Y. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J Ethnopharmacol. 2019;238:111857. doi: 10.1016/j.jep.2019.111857. [DOI] [PubMed] [Google Scholar]
- 43.Zhao M, Zhang ZF, Ding Y, Wang JB, Li Y. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-κB in C2C12 myotubes. Molecules. 2012;17:7083–7092. doi: 10.3390/molecules17067083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou X, Bao Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. 2017;7:44822. doi: 10.1038/srep44822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karamanou K, Franchi M, Vynios D, Brezillon S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin Cancer Biol. 2019 Aug 8; doi: 10.1016/j.semcancer.2019.08.003. (Epub ahead of print). doi: 10.1016/j.semcancer.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Song C, Sun P, He Q, Liu LL, Cui J, Sun LM. Long non-coding RNA LINC01287 promotes breast cancer cells proliferation and metastasis by activating Wnt/β-catenin signaling. Eur Rev Med Pharmacol Sci. 2019;23:4234–4242. doi: 10.26355/eurrev_201905_17928. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Fang J, Zhao H, Yu Y, Cao X, Zhang B. Downregulation of microRNA-1469 promotes the development of breast cancer via targeting HOXA1 and activating PTEN/PI3K/AKT and Wnt/β-catenin pathways. J Cell Biochem. 2019;120:5097–5107. doi: 10.1002/jcp.27313. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Yan F, Wei W, Deng J, Li L, Liu L, Sun J. Litchi seed aqueous extracts play a role in suppression of epithelial-mesenchymal transition, invasion and migration in breast cancer cells. Cell Cycle. 2020:1–9. doi: 10.1080/15384101.2019.1710912. doi: 10.1080/15384101.2019.1710912 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Duan X, Guo G, Pei X, Wang X, Li L, Xiong Y, Qiu X. Baicalin inhibits cell viability, migration and invasion in breast cancer by regulating miR-338-3p and MORC4. Onco Targets Ther. 2019;12:11183–11193. doi: 10.2147/OTT.S217101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei T, Xiaojun X, Peilong C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed Pharmacother. 2020;121:109139. doi: 10.1016/j.biopha.2019.109139. [DOI] [PubMed] [Google Scholar]
- 51.Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287–290. doi: 10.1016/j.ijbiomac.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsubakihara Y, Moustakas A. Epithelial-mesenchymal transition and metastasis under the control of transforming growth factor β. Int J Mol Sci. 2018;19(pii):E3672. doi: 10.3390/ijms19113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijay GV, Zhao N, Den Hollander P, Toneff MJ, Joseph R, Pietila M, Taube JH, Sarkar TR, Ramirez-Pena E, Werden SJ, et al. GSK3β regulates epithelial-mesenchymal transition and cancer stem cellproperties in triple-negative breast cancer. Breast Cancer Res. 2019;21:37. doi: 10.1186/s13058-019-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Zhou BP. Epithelial-mesenchymal Transition-A Hallmark of breast cancer metastasis. Cancer Hallm. 2013;1:38–49. doi: 10.1166/ch.2013.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Y, Zheng S, An N, Athanasopoulos T, Popplewell L, Liang A, Li K, Hu C, Zhu Y. β-catenin as a potential key target for tumor suppression. Int J Cancer. 2011;129:1541–1551. doi: 10.1002/ijc.26102. [DOI] [PubMed] [Google Scholar]
- 57.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rahmani F, Amerizadeh F, Hassanian SM, Hashemzehi M, Nasiri SN, Fiuji H, Ferns GA, Khazaei M, Avan A. PNU-74654 enhances the antiproliferative effects of 5-FU in breast cancer and antagonizes thrombin-induced cell growth via the Wnt pathway. J Cell Physiol. 2019;234:14123–14132. doi: 10.1002/jcp.28104. [DOI] [PubMed] [Google Scholar]
- 59.Hseu YC, Lin YC, Rajendran P, Thigarajan V, Mathew DC, Lin KY, Way TD, Liao JW, Yang HL. Antrodia salmonea suppresses invasion and metastasis in triple-negative breast cancer cells by reversing EMT through the NF-κB and Wnt/β-catenin signaling pathway. Food Chem Toxicol. 2018;124:219–230. doi: 10.1016/j.fct.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhang K, Liu P, Tang H, Xie X, Kong Y, Song C, Qiu X, Xiao X. AFAP1-AS1 promotes epithelial-mesenchymal transition and tumorigenesis through Wnt/β-catenin signaling pathway in Triple-negative breast cancer. Front Pharmacol. 2018;9:1248. doi: 10.3389/fphar.2018.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamieson C, Sharma M, Henderson BR. Wnt signaling from membrane to nucleus: β-catenin caught in a loop. Int J Biochem Cell Biol. 2012;44:847–850. doi: 10.1016/j.biocel.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: A prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 63.Carayol N, Wang CY. IKKalpha stabilizes cytosolic beta-catenin by inhibiting both canonical and non-canonical degradation pathways. Cell Signal. 2006;18:1941–1946. doi: 10.1016/j.cellsig.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 64.De A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y. Wnt/Planar cell polarity signaling: A new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 66.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 67.Kang Y, Massague J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Danielsson F, Peterson MK, Caldeira Araujo H, Lautenschläger F, Gad AKB. Vimentin diversity in health and disease. Cells. 2018;7(pii):E147. doi: 10.3390/cells7100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977–4984. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- 70.Maskarinec G, Ju D, Fong J, Horio D, Chan O, Loo LWM, Hernandez BY. Mammographic density and breast tissue expression of inflammatory markers, growth factors and vimentin. BMC Cancer. 2018;18:1191. doi: 10.1186/s12885-018-5088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aristizabal-Pachon AF, Takahashi CS. Effect of genetics, epigenetics and variations in the transcriptional expression of cadherin-E in breast cancer susceptibility. Biomedica. 2016;36:593–602. doi: 10.7705/biomedica.v36i4.3135. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 73.Wang Q, Gun M, Hong XY. Induced Tamoxifen resistance is mediated by increased methylation of E-cadherin in estrogen receptor-expressing breast cancer cells. Sci Rep. 2019;9:14140. doi: 10.1038/s41598-019-50749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miliani de Marval PL, Macias E, Rounbehler R, Sicinski P, Kiyokawa H, Johnson DG, Conti CJ, Rodriguez-Puebla ML. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538–7547. doi: 10.1128/MCB.24.17.7538-7547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubin SM. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem Sci. 2013;38:12–19. doi: 10.1016/j.tibs.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.