Abstract
Atherosclerosis (AS), a major cause of cardiovascular disease, has developed into a serious challenge to the health system. The long non-coding RNA (lncRNA) metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is associated with the pathogenesis of AS. However, whether MALAT1 can affect cholesterol accumulation in macrophages during AS progression, and the potential molecular mechanism involved in this progression have not been elucidated. In the present study, the mRNA expression level of MALAT1 was measured using reverse transcription-quantitative PCR (RT-qPCR) and the protein expression level was detected via western blot analysis. Oil Red O staining was used for detecting lipid accumulation in macrophages. Bioinformatics, dual-luciferase reporter and RT-qPCR assays were used to investigate the relationship between MALAT1 and the microRNA (miR)-17-5p/ATP-binding cassette transporter A1 (ABCA1) axis. The present results suggested that the MALAT1 expression level was significantly decreased in patients with AS and in oxidized low-density lipoprotein (ox-LDL)-stimulated macrophages. Knockdown of MALAT1 increased ox-LDL uptake, lipid accumulation and the total cholesterol (T-CHO) level in ox-LDL-induced macrophages. In addition, MALAT1 inhibition significantly decreased the mRNA and protein expression levels of scavenger receptor (SR) class B member 1, apolipoprotein E (ApoE) and ABCA1. However, MALAT1 increased the expression level of SR class A. Subsequently, the present study investigated whether MALAT1 could target miR-17-5p to regulate the expression level of ABCA1, which is involved in cholesterol efflux from macrophages. The present results suggested that inhibition of miR-17-5p reversed the effects of MALAT1 knockdown on T-CHO content, and protein expression levels of ApoE and ABCA1 in ox-LDL-stimulated macrophages. In summary, knockdown of MALAT1 may promote cholesterol accumulation by regulating the miR-17-5p/ABCA1 axis in ox-LDL-induced THP-1 macrophages.
Keywords: lncRNA, MALAT1, microRNA-17-5p, ABCA1, cholesterol accumulation, macrophage
Introduction
Atherosclerosis (AS), a chronic inflammatory disease, is a major cause of cardiovascular disease and has developed to a serious challenge to the health system (1). AS is characterized by a series of pathological changes such as endothelial damage, lipid deposition, monocyte adhesion and immigration, the formation of foam cells, fatty streaks and atheromatous plaques (2,3). Macrophage migration and foam cell formation under the endothelium, and macrophage proliferation, aggregation and apoptosis in plaques contribute to the development of AS (4). Disruption of the homoeostasis of cholesterol intake and efflux in macrophages leads to lipid accumulation and formation of foam cells (5). However, there is a lack of therapeutics that can effectively inhibit these abnormal macrophage features during the progression of AS (6). Therefore, improved understanding of the potential biological mechanisms underlying the macrophage response in AS could facilitate the development of novel treatments for AS.
Long noncoding RNAs (lncRNAs), which are defined as being >200-nucleotide in length, can regulate gene expression in ischemic myocardial injury (for example, 2810403D21Rik/macrophage Ia-positive recruiting factor) (7), pathological cardiac hypertrophy [cardiac hypertrophy-associated regulator, (CHAR)] (8), acute myocardial infarction (CHAR, ZNFX1 antisense RNA 1) (9) and other cardiovascular diseases (10). Previous studies have indicated that lncRNAs are involved in AS. For example, Chen et al (11) demonstrated that knockdown of lncRNA growth arrest specific 5 suppresses atherogenesis by regulating the apoptosis of macrophages and endothelial cells via exosomes. In addition, lncRNA-FA2H-2 has been shown to alleviate the inflammatory response, which acts as an independent risk factor of atherogenesis induced by oxidized low-density lipoprotein (ox-LDL), via the induction of the autophagic flux (12). These previous studies showed that lncRNAs act as promoting or inhibiting factors in AS development. Mechanistically, lncRNAs exert ‘sponge-like’ effects on specific microRNAs (miRNAs/miRs) to affect miRNA binding to target genes (13).
Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), an 8.5-kB lncRNA located at 11q13, is recognized as a biomarker for various cancer types (14,15). Recent studies have shown that MALAT1 is lowly expressed in AS plaques (16) and that hematopoietic deficiency of MALAT1 promotes atherosclerotic lesion formation in mice via enhanced accumulation of hematopoietic cells (17). Therefore, MALAT1 may possess an important role in AS; however, the potential molecular mechanism of MALAT1 regulation of cholesterol accumulation in macrophages, which is a key event of AS progression (18), remains to be elucidated. In addition, our previous study has demonstrated that miR-17-5p was highly expressed in the peripheral blood of patients with AS, and the suppression of miR-17-5p could alleviate AS in mice (19). The present study hypothesized that MALAT1 may have a conserved miR-17-5p binding site, and ATP-binding cassette transporter A1 (ABCA1) could be a target of miR-17-5p. Moreover, ABCA1 has been reported to mediate cholesterol efflux from macrophages (20,21). In addition, the present study hypothesized that MALAT1 may regulate the miR-17-5p/ABCA1 axis to affect cholesterol accumulation in macrophages.
Materials and methods
Patient and control specimens
Peripheral blood samples were obtained from 30 patients with AS (age, 40–84 years; male patients, 13; female patients, 7) and 30 healthy controls (HC; age, 37–80 years; males, 9; females, 21) who had no coronary artery disease, diabetes or cardiac insufficiency. Peripheral venous whole-blood samples (volume, 2 ml) were collected from each subject after 12 h fasting and stored in EDTA anticoagulant vacutainers. The participants were consecutively recruited from The Second Affiliated Hospital of Shenyang Medical College between May 2018 and March 2019. The protocol was approved by The Ethics Committee of The Second Affiliated Hospital of Shenyang Medical College (approval no. 2019-002) and conformed to the recommendations in The Declaration of Helsinki. In addition, all patients signed an informed consent form. Characteristics of the patients are presented in Table SI.
Cell culture
Human monocytic THP-1 cells were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. and suspended in RPMI-1640 medium (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.) containing 10% FBS (GEHealthcare Life Sciences) maintained at 37°C in a humidified atmosphere containing 5% CO2. THP-1 cells were induced to differentiate into macrophages using the phorbol 12-myristate 13-acetate (PMA; MedChemExpress LLC; 100 nM) (22), for 48 h at 37°C prior to subsequent experimentation.
293T cells (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.) were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cell treatment and transfection
For ox-LDL induction, THP-1 macrophages were incubated with 50 mg/ml ox-LDL (Peking Union-Biology Co., Ltd.; http://www.unionbiol.com.cn/) for 48 h at room temperature. Negative control (NC) small interfering (si)RNA and MALAT1 siRNA were purchased from JTS Scientific (http://www.jtsbio.com/). The following siRNA sequences were used: MALAT1 siRNA forward, 5′-CCAGAGAACUUAAAGUCUUTT-3′, and reverse, 5′-AAGACUUUAAGUUCUCUGGTT-3′; and NC siRNA forward, 5′-UUCUCCGAACGUGUCACGUTT-3′, and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′. All transfections were performed using the Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions.
For MALAT1 knockdown, macrophages at ~70% confluence were transfected with 100 pmol NC siRNA (50 pmol/ml) or 100 pmol MALAT1 siRNA (50 pmol/ml) for 24 or 48 h at room temperature. Then, cells were stimulated with 50 mg/ml ox-LDL for 48 h at 37°C or directly collected for subsequent assays.
NC mimics forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′; miR-17-5p mimics forward, 5′-CAAAGUGCUUACAGUGCAGGUAG-3′ and reverse, 5′-ACCUGCACUGUAAGCACUUUGUU-3′; NC inhibitor 5′-UUGUACUACACAAAAGUACUG-3′; and miR-17-5p inhibitor 5′-CUACCUGCACUGUAAGCACUUUG-3′ were purchased from JTS Scientific. For knockdown or overexpression of miR-17-5p, NC mimics, miR-17-5p mimics, NC inhibitor or miR-17-5p inhibitor (100 pmol) were transfected into macrophages (~70% confluence) for 48 h at room temperature.
For co-transfection, macrophages were co-transfected with NC siRNA or MALAT1 siRNA (50 pmol), and NC inhibitor or miR-17-5p inhibitor (50 pmol). At 24 h after transfection, macrophages were stimulated with 50 mg/ml ox-LDL for 48 h at room temperature.
ox-LDL uptake assay
At 48 h post-transfection with NC siRNA or MALAT1 siRNA, macrophages were stimulated with 20 µg/ml Dil-ox-LDL (Peking Union-Biology Co., Ltd.) for 4 h at room temperature. After being washed twice with PBS, macrophages were fixed with 4% paraformaldehyde (0.5 ml) for 15 min at room temperature, and then stained with Hoechst staining solution (0.5 ml) for 5 min at room temperature. Dil-ox-LDL uptake was observed under a fluorescence microscope (magnification, ×400; Olympus Corporation).
Lipid accumulation and T-CHO content
Oil Red O staining was used for detecting lipid accumulation. After transfection with NC siRNA or MALAT1 siRNA for 24 h, and treatment with 50 mg/ml ox-LDL for 48 h, the macrophages (1×106/ml) were washed twice with PBS and then fixed in 4% paraformaldehyde for 20 min at room temperature. After being washed twice with PBS, macrophages were stained with 0.5% Oil Red O (Sangon Biotech Co., Ltd.) for 15 min at room temperature. Macrophages were then observed under a light microscope at ×400 magnification (Olympus Corporation).
T-CHO content in the macrophages was determined using a Total cholesterol test kit (cat. no. A111-1; Nanjing Jiancheng Bioengineering Institute Co., Ltd.) according to the manufacturer's instructions.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from human peripheral whole-blood or macrophages was extracted using TRIpure reagent (BioTeke Corporation) and cDNAs were synthesized using Super M-MLV reverse transcriptase (BioTeke Corporation) according to the manufacturer's instructions. For the RT of mRNA, the RT mixture contained 4 µl 5X RT buffer, 2 µl 2.5 mM dNTP, 0.5 µl RNAse inhibitor (BioTeke Corporation). After adding M-MLV reverse transcriptase, mixed liquid was incubated at 25°C for 10 min, at 42°C for 50 min and heated at 80°C for 10 min to terminate the reaction. RT-qPCR for mRNA was performed using SYBR Green reaction mix (Sigma-Aldrich; Merck KGaA) on an Exicycler 96 system (Bioneer Corporation) with the following thermocycling parameters: Initial denaturation at 94°C for 5 min, followed by 40 cycles of 94°C for 15 sec, 60°C for 20 sec and 72°C for 30 sec. For the RT of miRNA, the reverse transcription mixture contained 4 µl 5X RT buffer, 0.75 µl 2.5 mM dNTP and 0.25 µl RNAse inhibitor (BioTeke Corporation). After adding M-MLV reverse transcriptase, the mixed liquid was incubated at 37°C for 30 min, at 42°C for 30 min and heated at 70°C for 10 min to terminate the reaction. RT-qPCR for miRNA was performed using SYBR Green reaction mix (Sigma-Aldrich; Merck KGaA) on an Exicycler 96 system (Bioneer Corporation) with the following thermocycling parameters: Initial denaturation at 94°C for 2 min, followed by 40 cycles of 94°C for 10 sec, 60°C for 15 sec and 72°C for 15 sec. Gene expression levels were quantified via the 2−ΔΔCq method (23). Expression level of miRNA was normalized to U6 and expression level of mRNA was normalized to GAPDH. All primers for RT-qPCR were synthesized by GenScript Biotech Corporation and listed in Table I.
Table I.
Primer sequences used for reverse-transcription-quantitative PCR.
| Gene | Primer sequence (5′→3′) |
|---|---|
| MALAT1 | F: ATACCTAACCAGGCATAACA |
| R: AGTAGACCAACTAAGCGAAT | |
| miR-17-5p | F: CAAAGTGCTTACAGTGCAGGTAG |
| R: GCAGGGTCCGAGGTATTC | |
| U6 | F: GCTTCGGCAGCACATATACT |
| R: GCAGGGTCCGAGGTATTC | |
| SR-A | F: CACTGATTGCCCTTTACCTC |
| R: TTCCTCTTCGCTGTCATTTC | |
| SR-B1 | F: CGGCGGTGATGATGGAGAAT |
| R: AGAGCCCAGAGTCGGAGTTG | |
| ApoE | F: CAGCAGACCGAGTGGCAGAG |
| R: TGTTCCTCCAGTTCCGATTTGT | |
| ABCA1 | F: GGCATCGTGTATGAGAAGG |
| R: CTGTAGGGCAGCAGGTTT | |
| GAPDH | F: GACCTGACCTGCCGTCTAG |
| R: AGGAGTGGGTGTCGCTGT |
F, forward; R, reverse; SR-A, scavenger receptor class A; SR-B1, scavenger receptor class B member 1; ApoE, apolipoprotein E; ABCA1, ATP-binding cassette transporter A1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; miR, microRNA.
Western blotting
Total proteins prepared from macrophages were lysed in RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd.) containing 1 mM PMSF (Beijing Solarbio Science & Technology Co., Ltd.). Protein concentrations were determined by a bicinchoninic acid kit (Beijing Solarbio Science & Technology Co., Ltd.). Equal quantities of protein (20 µg) were separated via 8–10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). After blocking with 5% (w/v) skim milk in TBS-0.1% Tween-20 buffer for 1 h at room temperature, the membranes were incubated overnight at 4°C with the following primary antibodies: Anti-scavenger receptor class A (SR-A; 1:3,000; cat. no. A14187; ABclonal Biotech Co., Ltd.); anti-SR-class B member 1 (SR-B1; 1:1,000; cat. no. A10799; ABclonal Biotech Co., Ltd.); anti-apolipoprotein E (ApoE; 1:500; cat. no. A16344; ABclonal Biotech Co., Ltd.); anti-ABCA1 (1:1,000; cat. no. ab7360; Abcam); and anti-GAPDH (1:10,000; cat. no. 60004-1-Ig; ProteinTech Group, Inc.). On the next day, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:3,000; cat. no. SE134; Beijing Solarbio Science & Technology Co., Ltd.) or goat anti-mouse (1:3,000; cat. no. SE131; Beijing Solarbio Science & Technology Co., Ltd.) immunoglobulin G secondary antibody for 1 h at 37°C. Then, ECL solution (Beijing Solarbio Science & Technology Co., Ltd.) was used to visualize these membranes, and a gel image processing system (Gel-Pro-Analyzer software; cat. no. WD-9413B; Beijing LIUYI Biotechnology Co., Ltd.) was used to analyze the relative intensities.
Dual-luciferase reporter assay
MALAT1 or the ABCA1 3′-untranslated region (3′UTR) containing the predicted potential miR-17-5p binding sites (StarBase prediction software; V3.0; http://starbase.sysu.edu.cn/index.php) (24) or corresponding mutant (mut) sequences were inserted into a pmirGLO vector constructed by GenScript Biotech Corporation. The plasmids were referred to as ‘wild-type (wt)-MALAT1’, ‘mut-MALAT1’, ‘wt-ABCA1-3′UTR’ or ‘mut-ABCA1-3′UTR’ reporter plasmid. 293T cells were co-transfected with these constructed reporter vectors, and NC mimics or miR-17-5p mimics (75 pmol) using Lipofectamine® 2000 reagent (9 µl; Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h after transfection, the relative luciferase activities were measured using a dual-luciferase reporter assay kit (cat. no. KGAF040; Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's protocols. The firefly luciferase expression was normalized to Renilla.
Statistical analysis
Data are presented as the mean ± SD of three independent replicates. Differences in sex, histories of hypertension, diabetes and smoking were compared using a χ2 test. Differences between two groups were analyzed with an unpaired Student's t-test and differences between multiple groups were analyzed using one-way ANOVA with Bonferroni post hoc test. All statistical analyses were performed using GraphPad Prism software (version 6.0; GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MALAT1 in patients with AS and ox-LDL-stimulated macrophages is decreased
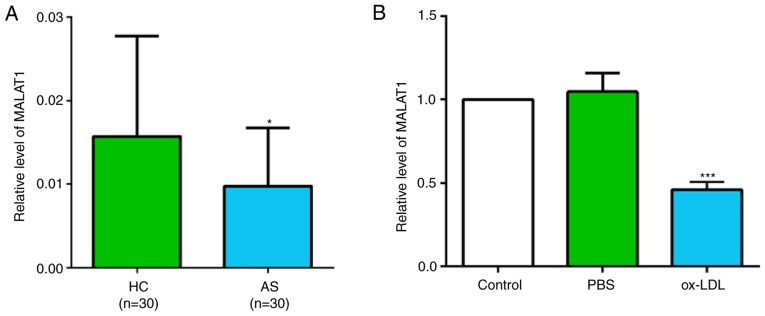
There were significant differences in age, hypertension incidence, diabetes incidence, T-CHO level and C-reactive protein level between patients with AS and healthy controls (Table SI). The relative expression level of MALAT1 was investigated in the peripheral blood of 30 patients with AS and 30 healthy volunteers. The results suggested that the MALAT1 expression level was significantly decreased in patients with AS (Fig. 1A). Similarly, the expression level of MALAT1 was significantly decreased in ox-LDL-stimulated THP-1 macrophages (Fig. 1B).
Figure 1.
Expression of MALAT1 is decreased in patients with AS and ox-LDL-induced THP-1 macrophages. (A) Expression level of MALAT1 in the peripheral blood of 30 patients with AS and 30 healthy volunteers. *P<0.05 vs. HC group. (B) Expression level of MALAT1 in macrophages after ox-LDL induction for 48 h. Data are presented as the mean ± SD of three independent experiments. ***P<0.001 vs. PBS group. HC, healthy controls; AS, atherosclerosis; MALAT1, metastasis associated lung adenocarcinoma transcript 1; ox-LDL, oxidized low-density lipoprotein.
Knockdown of MALAT1 increases ox-LDL uptake, and causes changes in the expression levels of SR-A, SR-B1, ApoE and ABCA1
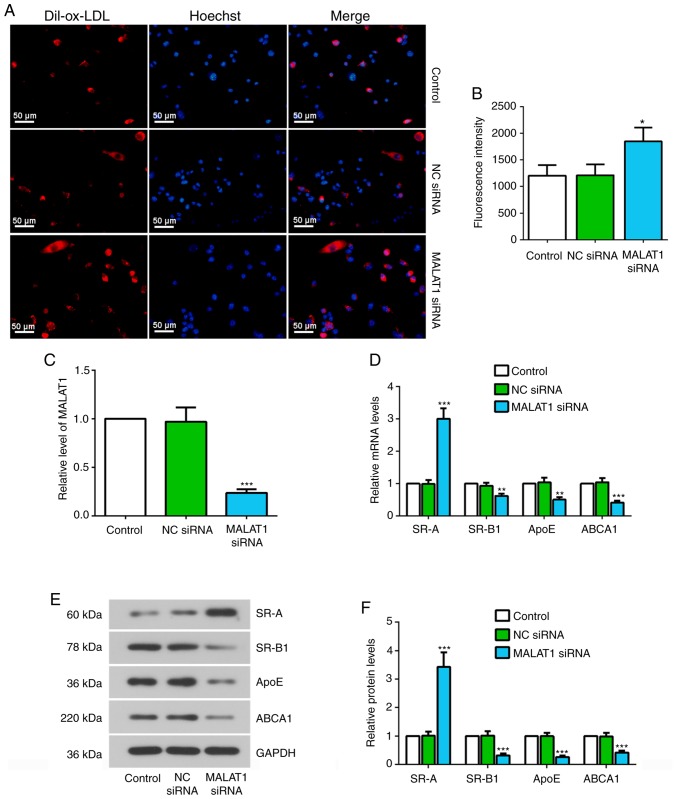
To investigate the effect of MALAT1 on ox-LDL uptake in macrophages, fluorescence staining with Dil-labeled ox-LDL, which stains red for intracellular lipid, and Hoechst staining solution, which stains nuclei blue, was performed. It was revealed that Dil-ox-LDL can be taken up by macrophages, and MALAT1 siRNA transfection significantly increased Dil-ox-LDL uptake (Fig. 2A and B). The efficiency of MALAT1 siRNA in suppressing MALAT1 expression was confirmed by RT-qPCR (Fig. 2C). At the molecular level, knockdown of MALAT1 resulted in changes in the expression levels of key genes implicated in the uptake and efflux of cholesterol in THP-1 macrophages (25). The mRNA and protein expression levels of SR-B1, ApoE and ABCA1 were significantly decreased, but the mRNA and protein expression levels of SR-A were significantly upregulated in MALAT1-inhibited macrophages (Fig. 2D-F).
Figure 2.
Knockdown of MALAT1 increases ox-LDL uptake, and causes changes in the expression levels of SR-A, SR-B1, ApoE and ABCA1. (A) At 24 h after transfection with NC siRNA or MALAT1 siRNA, macrophages were stimulated with 20 µg/ml Dil-ox-LDL for 4 h and the ox-LDL uptake was observed. Representative fluorescent images. Scale bar, 50 µm. (B) Quantification of mean fluorescence intensity for ox-LDL uptake assay. (C) Relative expression level of MALAT1 in macrophages transfected with NC siRNA or MALAT1 siRNA. (D) Relative mRNA expression levels of SR-A, SR-B1, ApoE and ABCA1 in macrophages transfected with NC siRNA or MALAT1 siRNA for 48 h. (E) Western blotting, and (F) quantification of the relative protein expression levels of SR-A, SR-B1, ApoE and ABCA1 in macrophages transfected with NC siRNA or MALAT1 siRNA for 48 h. Data are presented as the mean ± SD. *P<0.05, **P<0.01, ***P<0.001 vs. NC siRNA group. NC, negative control; siRNA, small interfering RNA; MALAT1, metastasis associated lung adenocarcinoma transcript 1; ox-LDL, oxidized low-density lipoprotein; SR-A, scavenger receptor class A; SR-B1, scavenger receptor class B member 1; ApoE, apolipoprotein E; ABCA1, ATP-binding cassette transporter A1.
Knockdown of MALAT1 increases lipid accumulation and T-CHO level in ox-LDL-induced macrophages
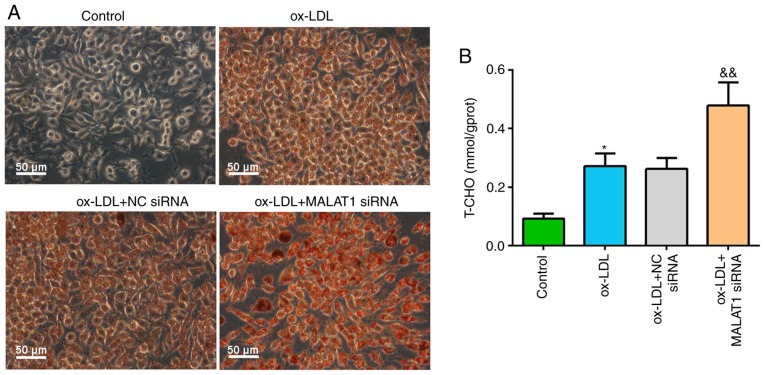
After transfection with NC siRNA or MALAT1 siRNA for 24 h, macrophages were stimulated with ox-LDL. It was demonstrated that ox-LDL stimulation induced foam cell formation, and MALAT1 siRNA cotreatment significantly increased lipid droplet accumulation in macrophage foam cells (Fig. 3A). Similar results were observed for T-CHO levels; ox-LDL stimulation significantly increased intracellular T-CHO level compared with control macrophages. In addition, ox-LDL + MALAT1 siRNA co-treatment induced increased T-CHO levels in macrophages compared with ox-LDL-induced macrophages with no co-transfection (Fig. 3B).
Figure 3.
Knockdown of MALAT1 increases lipid accumulation and the levels of T-CHO in ox-LDL-stimulated macrophages. Differentiated macrophages were transfected with NC siRNA or MALAT1 siRNA. At 24 h after transfection, cells were stimulated with 50 mg/ml ox-LDL for 48 h. (A) Representative images of Oil Red O staining. Scale bar, 50 µm. (B) Levels of T-CHO in macrophages. Data are presented as the mean ± SD. *P<0.05 vs. control group. &&P<0.01 vs. ox-LDL + NC siRNA group. NC, negative control; siRNA, small interfering RNA; ox-LDL, oxidized low-density lipoprotein; MALAT1, metastasis associated lung adenocarcinoma transcript 1; T-CHO, total cholesterol.
MALAT1 regulates the miR-17-5p/ABCA1 axis
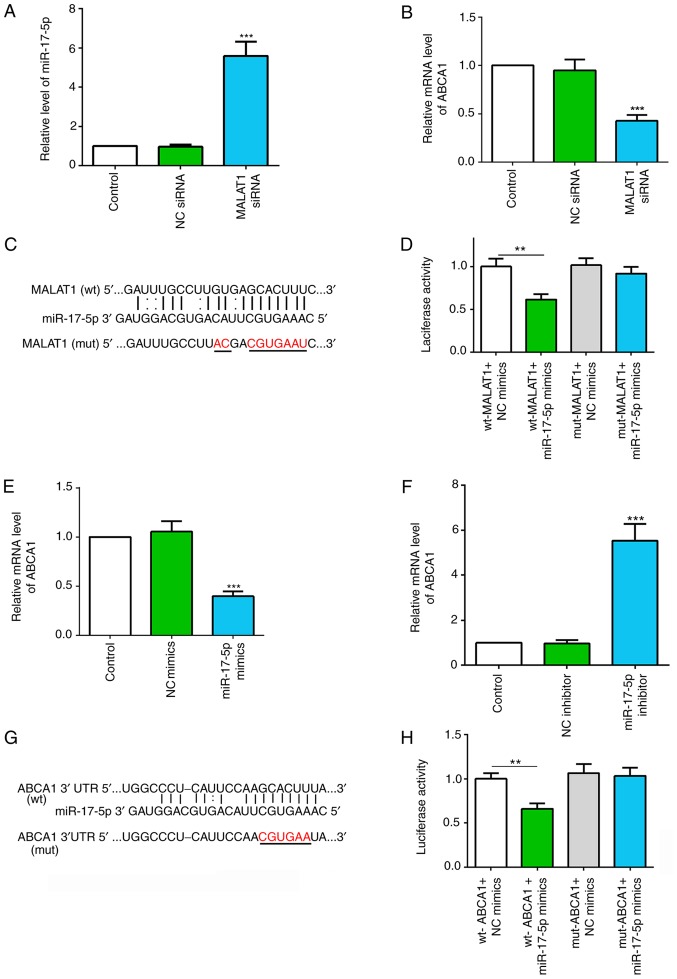
To investigate the functional mechanism of MALAT1 in macrophages, the present bioinformatic prediction analysis identified miR-17-5p as a potential target gene of MALAT1 and ABCA1. The present study investigated the effect of silencing MALAT1 on miR-17-5p and ABCA1 expression levels. The present results suggested that silencing MALAT1 significantly increased miR-17-5p expression and decreased ABCA1 mRNA expression in macrophages (Fig. 4A and B).
Figure 4.
MALAT1 regulates the miR-17-5p/ABCA1 axis. (A) Relative expression level of miR-17-5p in macrophages with NC or MALAT1 siRNA transfection. (B) Relative expression level of ABCA1 in macrophages with NC or MALAT1 siRNA transfection. (C) Putative binding sites between MALAT1 and miR-17-5p, and the mutant sites in the mut-MALAT1 reporter vector. (D) Luciferase activity analysis of 293T cells co-transfected with wt-MALAT1 or mut-MALAT1, and NC mimics or miR-17-5p mimics. (E) Relative mRNA expression level of ABCA1 in macrophages transfected with NC mimics or miR-17-5p mimics. (F) Relative mRNA expression level of ABCA1 in macrophages transfected with NC inhibitor or miR-17-5p inhibitor. (G) Putative binding sites between miR-17-5p and ABCA1 3′UTR, and the mutant sites in mut-ABCA1 3′UTR reporter vector. (H) Luciferase activity analysis in 293T cells co-transfected with wt-ABCA1 or mut-ABCA1, and NC mimics or miR-17-5p mimics. Data are presented as the mean ± SD. **P<0.01, ***P<0.001 vs. NC group [(A and B) NC siRNA; (D) wt-MALAT1 + NC mimics; (F) NC inhibitor; (H) wt-ABCA1 + NC mimics]. wt, wild-type; mut, mutant; NC, negative control; miR, microRNA; ABCA1, ATP-binding cassette transporter A1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; siRNA, small interfering RNA; 3′UTR, 3′-untranslated region.
Computational analysis revealed complementary sequences between MALAT1 and miR-17-5p (Fig. 4C). The dual-luciferase reporter assay revealed that co-transfection of the luciferase vector with the wt-MALAT1 and miR-17-5p mimics into 293T cells significantly decreased luciferase activity compared with co-transfection with NC mimics. However, there were no significant effects on the relative luciferase activity following co-transfection with mut-MALAT1 and miR-17-5p mimics (Fig. 4D). Therefore, the present results suggested that MALAT1 may target miR-17-5p.
The transfection efficiencies of miR-17-5p mimics and inhibitor were demonstrated via RT-qPCR (Fig. S1A and B). It was shown that miR-17-5p overexpression significantly decreased the mRNA expression levels of ABCA1, and miR-17-5p suppression using a miR-17-5p inhibitor significantly increased ABCA1 mRNA expression in macrophages (Fig. 4E and F). The putative miR-17-5p binding sites within the ABCA1 3′UTR are shown in Fig. 4G. Furthermore, dual-luciferase assay results revealed that miR-17-5p mimics and wt-ABCA1 3′UTR co-transfection significantly inhibited relative luciferase activity; however, luciferase activity was unchanged after mut-ABCA1 3′UTR and miR-17-5p mimic co-transfection (Fig. 4H). Therefore, the data suggested that miR-17-5p may directly target ABCA1. Collectively, the present results suggested that MALAT1 may regulate the miR-17-5p/ABCA1 axis.
Inhibition of miR-17-5p expression reverses the effect of MALAT1 in ox-LDL-stimulated macrophages
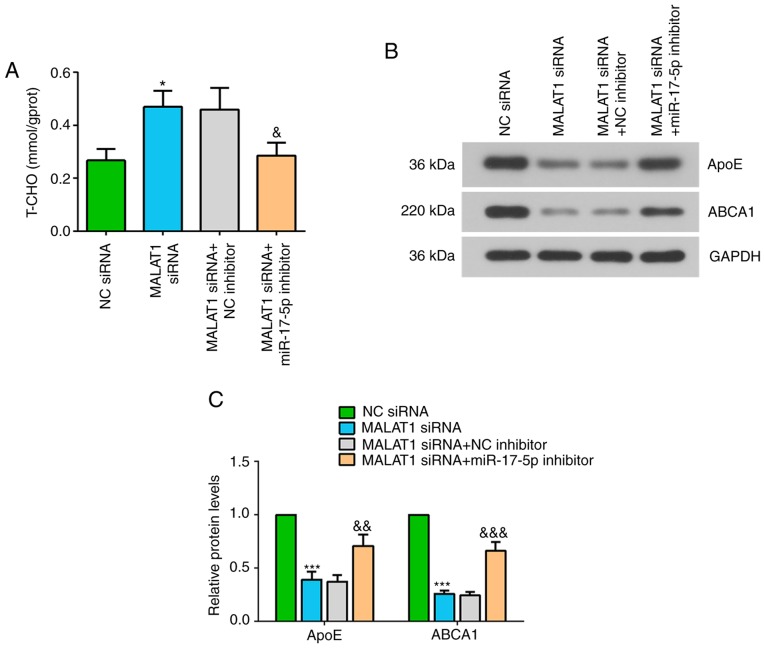
The present study investigated the effect of MALAT1 and miR-17-5p on T-CHO levels, and the protein expression levels of ApoE and ABCA1 in ox-LDL-stimulated THP-1 macrophages. It was revealed that inhibiting MALAT1 increased T-CHO level in ox-LDL-stimulated macrophages, but this effect was significantly suppressed after co-transfection with the miR-17-5p inhibitor (Fig. 5A). Furthermore, the protein expression levels of ApoE and ABCA1 were significantly decreased in ox-LDL stimulated macrophages transfected with MALAT1 siRNA, while addition of the miR-17-5p inhibitor attenuated the effect of MALAT1 knockdown on the expression level of these proteins (Fig. 5B and C). Collectively, the present results suggested that MALAT1 may regulate macrophage features by regulating the miR-17-5p/ABCA1 axis (Fig. 6).
Figure 5.
Effects of MALAT1 inhibition on ox-LDL-stimulated macrophages are partly reversed by suppressing miR-17-5p expression. After co-transfection of NC siRNA or MALAT1 siRNA, and NC inhibitor or miR-17-5p inhibitor for 24 h, macrophages were stimulated with 50 mg/ml ox-LDL for 48 h. (A) Level of T-CHO in THP-1 macrophages. (B) Western blot analysis, and (C) quantification of the expression levels of ApoE and ABCA1. Data are presented as the mean ± SD. *P<0.05, ***P<0.001 vs. NC siRNA group. &P<0.05, &&P<0.01, &&&P<0.001 vs. MALAT1 siRNA + NC inhibitor. NC, negative control; miR, microRNA; ox-LDL, oxidized low-density lipoprotein; ABCA1, ATP-binding cassette transporter A1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; siRNA, small interfering RNA; ApoE apolipoprotein E; T-CHO, total cholesterol.
Figure 6.
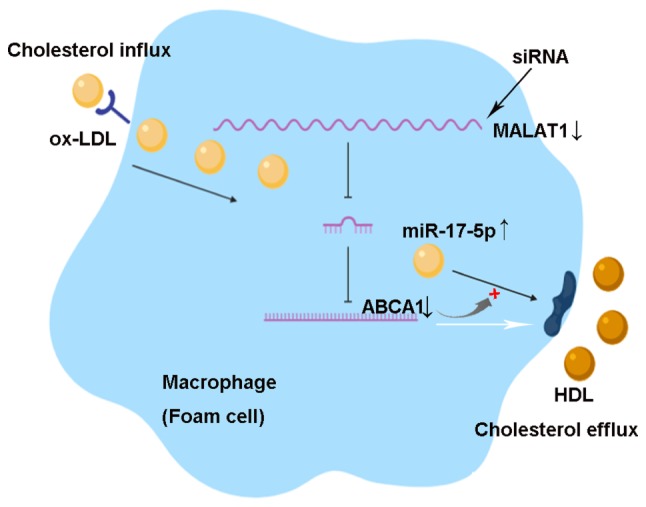
Knockdown of MALAT1 promotes cholesterol accumulation by regulating the miR-17-5p/ABCA1 axis in ox-LDL-induced THP-1 macrophages. ABCA1 is a cell membrane protein that mediates the transport of cholesterol, phospholipids and other metabolites from cells to form HDL apolipoproteins. Thus, ABCA1 promotes cholesterol efflux and inhibits macrophage foam cell formation. MALAT1 can sponge miR-17-5p, which targets the 3′-untranslated region of the ABCA1 mRNA, and facilitates its mRNA degradation or translational repression. Knockdown of MALAT1 by small interfering RNA increases ox-LDL uptake, lipid accumulation and total cholesterol levels via the miR-17-5p/ABCA1 axis in ox-LDL-induced macrophages. miR, microRNA; ABCA1, ATP-binding cassette transporter A1; MALAT1, metastasis associated lung adenocarcinoma transcript 1; HDL, high-density lipoprotein; ox-LDL, oxidized low-density lipoprotein.
Discussion
lncRNA MALAT1 has been identified as a transcript expressing conserved sequences across several species and demonstrated to be significantly associated with metastasis in human non-small cell lung cancer (26). MALAT1 has been reported to be upregulated in various types of cancer, such as bladder cancer, gastric cancer and osteosarcoma (27–29), but was reported to have reduced expression in AS plaques (16). It was revealed that MALAT1 expression was significantly downregulated in patients with AS and ox-LDL-induced macrophages. The present results suggested that knockdown of MALAT1 increased ox-LDL uptake, lipid accumulation and T-CHO levels in ox-LDL induced macrophages, therefore indicating a suppressive role for MALAT1 in AS, which was in line with previous results from Li et al (30).
AS is a progressive and chronic inflammatory disease associated with the involvement of lipid metabolism (31). One of the most important events of early-stage AS is the accumulation of lipid-laden foam cells derived from macrophages under the endothelium, which initiates the formation of fatty streaks (32). Macrophage-derived foam cell formation is caused by abnormal cholesterol deposition and transport (33). To investigate the effects of MALAT1 on cholesterol accumulation, the present study evaluated ox-LDL uptake by assessing the fluorescence intensity of Dil-ox-LDL, lipid accumulation and intracellular T-CHO in PMA-activated THP-1 monocytes after transfection with MALAT1 siRNA and ox-LDL stimulation. The incubation of macrophages with ox-LDL leads to cholesterol ester accumulation (34,35), and the present results suggested that ox-LDL may play a central role in AS plaque formation. The present results indicated the potential risk factor of MALAT1 inhibition in AS.
Macrophages regulate cholesterol homeostasis via several factors including scavenger receptors, cholesterol metabolism enzymes and cholesterol transporters (25,36). A series of scavenger receptors, including SR-A and SR-B1, mediate the binding of modified LDL (37). Smooth muscle cells within AS plaques, and smooth muscle cells co-incubated with macrophages or ox-LDL from macrophage-conditioned medium both express SR-A (38,39). The present findings indicated that MALAT1 silencing upregulated the expression of SR-A in ox-LDL-stimulated macrophages. SR-A-mediated uptake of modified LDL by macrophages leads to deposition of cholesterol and foam cell formation during atherogenesis (40). It was demonstrated that the mRNA and protein expression levels of SR-B1, ApoE and ABCA1 were significantly downregulated in macrophages co-treated with MALAT1 siRNA and ox-LDL. SR-B1 plays an antiatherogenic role, and is responsible for selective uptake of cholesterol esters from high-density lipoprotein (HDL) and LDL, and free cholesterol efflux to lipoprotein acceptors (41). ABCA1, a major protective factor against AS, is involved in directing cholesterol efflux from macrophages (42). ABCA1 can transfer excess free cholesterol to cholesterol acceptors such as ApoA-I or ApoE, thus promoting cholesterol efflux and inhibiting macrophage foam cell formation (42). The reduced SR-B1, ABCA1 and ApoE expression levels, and elevated SR-A expression were suggestive of cholesterol homeostasis disturbance at the molecular level in the MALAT1-silenced macrophages.
lncRNAs play important regulatory roles in the expression and function of target mRNAs by adsorbing specific miRNAs (43). To detect the molecular mechanism of MALAT1 involved in the regulation of cholesterol transport, the present study predicted miR-17-5p binding to MALAT1 using bioinformatics prediction software. Our previous studies have shown that miR-17-5p was elevated in patients with AS and also macrophages of ApoE−/− mice with AS, and that inhibition of miR-17-5p reduced inflammation and lipid accumulation by interacting with ABCA1 in AS (19,44). Based on the present bioinformatics prediction and our previous results, the present study investigated the role of MALAT1 on the miR-17-5p/ABCA1 axis. Luciferase reporter assay results indicated that miR-17-5p was a potential target of MALAT1. Furthermore, reduced MALAT1 expression level by MALAT1 siRNA resulted in increased expression of miR-17-5p, which suggested a relationship between MALAT1 and miR-17-5p. In addition, the present results suggested that ABCA1 may be a target of miR-17-5p. Downregulation of miR-17-5p by interferon-stimulated gene 15 has been previously shown to increase cholesterol efflux from THP-1 macrophage-derived foam cells by targeting Beclin-1 (45). Tang et al (46) demonstrated that interleukin-8 affects ApoA-I-mediated ABCA1-dependent cholesterol efflux via the miR-183/ABCA1 axis in THP-1 macrophage-derived foam cells. These previous results suggest a risk factor role of miR-17-5p and a beneficial role of ABCA1, and that the miRNA/mRNA axis may play an intermediary role in cholesterol metabolism. The present study reported that MALAT1 silencing decreased T-CHO levels and the expression of proteins that regulate cholesterol efflux in ox-LDL-treated macrophages, whereas miR-17-5p inhibition reversed these effects. Hence, the present findings indicated that MALAT1 could regulate the miR-17-5p/ABCA1 axis to participate in cholesterol accumulation in macrophages.
In conclusion, the present results suggested that knockdown of MALAT1 may promote cholesterol accumulation by regulating the miR-17-5p/ABCA1 axis in ox-LDL-induced THP-1 macrophages. These findings provide insight into the potential molecular mechanism of MALAT1 in the progression of AS.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AS
atherosclerosis
- lncRNA
long non-coding RNA
- MALAT1
metastasis associated lung adenocarcinoma transcript 1
- RT-qPCR
reverse transcription-quantitative PCR
- ox-LDL
oxidized low-density lipoprotein
- T-CHO
total cholesterol
- SR-B1
scavenger receptor class B member 1
- ApoE
apolipoprotein E
- ABCA1
ATP-binding cassette transporter A1
- SR-A
scavenger receptor class A
Funding
This study was supported by grants from The Guide Project for Key Research and Development Project of Liaoning Province (grant nos. 2019010173-JH8/103 and 2017020258-201) and The Foundation of Shenyang Science and Technology Bureau (grant no. 18-014-4-68).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LL and LT were involved in the conception and design of the study, administrating the project, collecting the data and writing the manuscript. JY and LY performed the experiments and analyzed the data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The protocol was approved by The Ethics Committee of The Second Affiliated Hospital of Shenyang Medical College (approval no. 2019-002) and conformed to the Declaration of Helsinki. All patients signed an informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vieceli Dalla Sega F, Fortini F, Aquila G, Campo G, Vaccarezza M, Rizzo P. Notch signaling regulates immune responses in atherosclerosis. Front Immunol. 2019;10:1130. doi: 10.3389/fimmu.2019.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman JW, Calderon TM. The role of endothelial cell adhesion molecules in the development of atherosclerosis. Cardiovasc Pathol. 1992;1:17–28. doi: 10.1016/1054-8807(92)90005-9. [DOI] [PubMed] [Google Scholar]
- 3.Geng J, Liu H, Ge P, Hu T, Zhang Y, Zhang X, Xu B, Wang B, Xie J. PM2.5 promotes plaque vulnerability at different stages of atherosclerosis and the formation of foam cells via TLR4/MyD88/NFκB pathway. Ecotoxicol Environ Saf. 2019;176:76–84. doi: 10.1016/j.ecoenv.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 4.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. 2016;20:17–28. doi: 10.1111/jcmm.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn AV, Kramer MC, Vorpahl M, Kolodgie FD, Virmani R. Pharmacotherapy of coronary atherosclerosis. Expert Opin Pharmacother. 2009;10:1587–1603. doi: 10.1517/14656560902988494. [DOI] [PubMed] [Google Scholar]
- 7.Liang H, Su X, Wu Q, Shan H, Lv L, Yu T, Zhao X, Sun J, Yang R, Zhang L, et al. lncRNA 2810403D21Rik/Mirf promotes ischemic myocardial injury by regulating autophagy through targeting Mir26a. Autophagy. 2019;12:1–15. doi: 10.1080/15548627.2019.1687214. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Jiang Y, Guo X, Zhang B, Wu J, Sun J, Liang H, Shan H, Zhang Y, Liu J, et al. Long non-coding RNA cardiac hypertrophy-associated regulator governs cardiac hypertrophy via regulating miR-20b and the downstream PTEN/AKT pathway. J Cell Mol Med. 2019;23:7685–7698. doi: 10.1111/jcmm.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Wu D, Wu Q, Zou B, Huang X, Cheng X, Wu Y, Hong K, Li P, Yang R, et al. Knockdown of long non-coding RNA-ZFAS1 protects cardiomyocytes against acute myocardial infarction via anti-apoptosis by regulating miR-150/CRP. J Cell Biochem. 2017;118:3281–3289. doi: 10.1002/jcb.25979. [DOI] [PubMed] [Google Scholar]
- 10.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J, Tong W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS One. 2017;12:e0185406. doi: 10.1371/journal.pone.0185406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo FX, Wu Q, Li P, Zheng L, Ye S, Dai XY, Kang CM, Lu JB, Xu BM, Xu YJ, et al. The role of the lncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ. 2019;26:1670–1687. doi: 10.1038/s41418-018-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayoumi AS, Sayed A, Broskova Z, Teoh JP, Wilson J, Su H, Tang YL, Kim IM. Crosstalk between long noncoding RNAs and MicroRNAs in health and disease. Int J Mol Sci. 2016;17:356. doi: 10.3390/ijms17030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NK, Lee JH, Ivan C, Ling H, Zhang X, Park CH, Calin GA, Lee SK. MALAT1 promoted invasiveness of gastric adenocarcinoma. BMC Cancer. 2017;17:46. doi: 10.1186/s12885-016-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y, Benyamini H, Elgavish S, Zong X, Prasanth KV, Karni R. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77:1155–1167. doi: 10.1158/0008-5472.CAN-16-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arslan S, Berkan Ö, Lalem T, Özbilüm N, Göksel S, Korkmaz Ö, Çetin N, Devaux Y, Cardiolinc™ network Long non-coding RNAs in the atherosclerotic plaque. Atherosclerosis. 2017;266:176–181. doi: 10.1016/j.atherosclerosis.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Cremer S, Michalik KM, Fischer A, Pfisterer L, Jaé N, Winter C, Boon RA, Muhly-Reinholz M, John D, Uchida S, et al. Hematopoietic deficiency of the long noncoding RNA MALAT1 promotes atherosclerosis and plaque inflammation. Circulation. 2019;139:1320–1334. doi: 10.1161/CIRCULATIONAHA.117.029015. [DOI] [PubMed] [Google Scholar]
- 18.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Meng L, Shi X, Yu B. Knockdown of microRNA-17-5p ameliorates atherosclerotic lesions in ApoE−/− mice and restores the expression of very low density lipoprotein receptor. Biotechnol Lett. 2017;39:967–976. doi: 10.1007/s10529-017-2337-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhao GJ, Mo ZC, Tang SL, Ouyang XP, He PP, Lv YC, Yao F, Tan YL, Xie W, Shi JF, et al. Chlamydia pneumoniae negatively regulates ABCA1 expression via TLR2-Nuclear factor-kappa B and miR-33 pathways in THP-1 macrophage-derived foam cells. Atherosclerosis. 2014;235:519–525. doi: 10.1016/j.atherosclerosis.2014.05.943. [DOI] [PubMed] [Google Scholar]
- 21.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Yuan Y, Shi J, Shi X, Gao X, Zhao Y, Ye J, Feng X. Metformin inhibits THP-1 macrophage-derived foam cell formation induced by lipopolysaccharide. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:168–172. (In Chinese) [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voloshyna I, Hai O, Littlefield MJ, Carsons S, Reiss AB. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARg and adenosine. Eur J Pharmacol. 2013;698:299–309. doi: 10.1016/j.ejphar.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 27.Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–2294. doi: 10.1039/c2mb25070e. [DOI] [PubMed] [Google Scholar]
- 28.Liu K, Huang J, Ni J, Song D, Ding M, Wang J, Huang X, Li W. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017;16:578–587. doi: 10.1080/15384101.2017.1288324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, Goel A. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Sun Y, Zhong L, Xiao Z, Yang M, Chen M, Wang C, Xie X, Chen X. The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs by long non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr Metab Cardiovasc Dis. 2018;28:1175–1187. doi: 10.1016/j.numecd.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology. 2013;218:1376–1384. doi: 10.1016/j.imbio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H, et al. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci. 2001;947:199–205. doi: 10.1111/j.1749-6632.2001.tb03941.x. discussion 205-196. [DOI] [PubMed] [Google Scholar]
- 33.Lin YW, Liu PS, Adhikari N, Hall JL, Wei LN. RIP140 contributes to foam cell formation and atherosclerosis by regulating cholesterol homeostasis in macrophages. J Mol Cell Cardiol. 2015;79:287–294. doi: 10.1016/j.yjmcc.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parthasarathy S, Quinn MT, Steinberg D. Is oxidized low density lipoprotein involved in the recruitment and retention of monocyte/macrophages in the artery wall during the initiation of atherosclerosis? Basic Life Sci. 1988;49:375–380. doi: 10.1007/978-1-4684-5568-7_58. [DOI] [PubMed] [Google Scholar]
- 36.Mangum LC, Hou X, Borazjani A, Lee JH, Ross MK, Crow JA. Silencing carboxylesterase 1 in human THP-1 macrophages perturbs genes regulated by PPARg/RXR and RAR/RXR: Down-regulation of CYP27A1-LXRα signaling. Biochem J. 2018;475:621–642. doi: 10.1042/BCJ20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Naito M, Suzuki H, Mori T, Matsumoto A, Kodama T, Takahashi K. Coexpression of type I and type II human macrophage scavenger receptors in macrophages of various organs and foam cells in atherosclerotic lesions. Am J Pathol. 1992;141:591–599. [PMC free article] [PubMed] [Google Scholar]
- 39.Mietus-Snyder M, Gowri MS, Pitas RE. Class A scavenger receptor up-regulation in smooth muscle cells by oxidized low density lipoprotein. Enhancement by calcium flux and concurrent cyclooxygenase-2 up-regulation. J Biol Chem. 2000;275:17661–17670. doi: 10.1074/jbc.275.23.17661. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 41.Rhainds D, Brissette L. The role of scavenger receptor class B type I (SR-BI) in lipid trafficking. Defining the rules for lipid traders. Int J Biochem Cell Biol. 2004;36:39–77. doi: 10.1016/S1357-2725(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Jiang T, Zhou HF, Liang Y, Zhao GJ. Apigenin retards atherogenesis by promoting ABCA1-mediated cholesterol efflux and suppressing inflammation. Cell Physiol Biochem. 2018;47:2170–2184. doi: 10.1159/000491528. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan L, Liu L, Jiang Z, Hao X. Inhibition of microRNA-17-5p reduces the inflammation and lipid accumulation, and up-regulates ATP-binding cassette transporterA1 in atherosclerosis. J Pharmacol Sci. 2019;139:280–288. doi: 10.1016/j.jphs.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Huang C, Yu XH, Zheng XL, Ou X, Tang CK. Interferon-stimulated gene 15 promotes cholesterol efflux by activating autophagy via the miR-17-5p/Beclin-1 pathway in THP-1 macrophage-derived foam cells. Eur J Pharmacol. 2018;827:13–21. doi: 10.1016/j.ejphar.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 46.Tang XE, Li H, Chen LY, Xia XD, Zhao ZW, Zheng XL, Zhao GJ, Tang CK. IL-8 negatively regulates ABCA1 expression and cholesterol efflux via upregulating miR-183 in THP-1 macrophage-derived foam cells. Cytokine. 2018;122:154385. doi: 10.1016/j.cyto.2018.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.