Abstract
Drosophila hematopoiesis is comparable to mammalian differentiation of myeloid lineages, and therefore, has been a useful model organism in illustrating the molecular and genetic basis for hematopoiesis. Multiple novel regulators and signals have been uncovered using the tools of Drosophila genetics. A Runt domain protein, lozenge, is one of the first players recognized and closely studied in the hematopoietic lineage specification. Here, we explore the role of lozenge in determination of prohemocytes into a special class of hemocyte, namely the crystal cell, and discuss molecules and signals controlling the lozenge function and its implication in immunity and stress response. Given the highly conserved nature of Runt domain in both invertebrates and vertebrates, studies in Drosophila will enlighten our perspectives on Runx-mediated development and pathologies.
Keywords: crystal cells, Drosophila melanogaster, hematopoiesis, lozenge, lymph gland, melanization, prophenoloxidase, RUNX
INTRODUCTION
The fruit fly is a holometabolous insect which undergoes four distinct phases in its development with each stage emphatically different in both anatomy and physiology (Fig. 1A) (Snodgrass, 1954). In brief, the adult female produces fertilized eggs after copulation, which then undergoes stages of internal rearrangements to form an embryo. Upon hatching, a larva goes through two more molts while consistently feeding before turning into a pupa. Active feeding at this stage ceases, allowing internal and external structures to be generated or reorganized within the pupal case (Ashburner and Novitski, 1976). A winged fly emerges after four to five days from the point of pupa formation, but the whole cycle takes about ten days under normal conditions. The emergent fly can fly and feed after a couple of hours and generally lives up to fifty days, within which the cycle is repeated several times (Linford et al., 2013; Robertson, 1936; Stocker and Gallant, 2008).
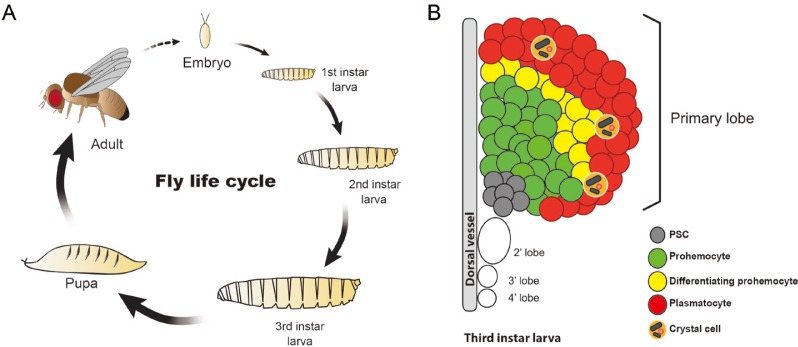
Fig. 1. Life cycle of Drosophila and constitution of the lymph gland.
(A) The fly undergoes four distinct phases of change that is commenced by the embryo which is formed after fertilization. The hatched embryo produces the first instar larva, which molts into second and then third instar larva, eventually forming a pupa. The pupa ecloses to the adult fly and the cycle repeats. (B) The lymph gland is the venue for definitive hematopoiesis. It comprises of four pairs of lobes and the primary lobe is divided into four regions: the posterior signaling center (PSC), the medullary zone (MZ), the intermediate zone (IZ), and the cortical zone (CZ). The prohemocytes of the MZ differentiate into plasmatocytes and crystal cells of the CZ. Lamellocytes are barely seen in healthy animals. The IZ contains differentiating prohemocytes that expresses both MZ and CZ markers.
HEMATOPOIESIS IN DROSOPHILA
Hematopoiesis in the fruit fly, though very multifaceted, is mainly classified into two waves: primitive and definitive hematopoiesis (Evans et al., 2003). In the first wave or primitive hematopoiesis, hemocytes are derived from the embryonic head mesoderm from which point they migrate to designated areas and facilitate organogenesis and immune responses (Holz et al., 2003; Moreira et al., 2010; Olofsson and Page, 2005; Tepass et al., 1994; Wood et al., 2006). Upon hatching, hemocytes are released either into circulation or to specialized sites including the hematopoietic pockets. During larval stages, these hemocytes perform diverse functions including phagocytosis of debris, immune responses, and metabolic regulation (Agaisse et al., 2003; Elrod-Erickson et al., 2000; Lanot et al., 2001; Lebestky et al., 2000; Makhijani et al., 2011; Márkus et al., 2009; Tepass et al., 1994).
The second wave or definitive hematopoiesis occurs in a specific organ called the lymph gland (Rugendorff et al., 1994). The lymph gland originates from the cardiogenic mesoderm, distinctive from the embryonic lineage, which later differentiates into hemangioblast-like cells that give rise to the posterior signaling center (PSC) and pre-prohemocytes (Crozatier et al., 2004; Krzemień et al., 2007; Mandal et al., 2004; 2007; Rugendorff et al., 1994). Pre-prohemocytes turn into prohemocytes which produces three types of mature hemocytes: plasmatocytes, crystal cells, and lamellocytes (Jung et al., 2005; Krzemien et al., 2010; Lebestky et al., 2000; Shrestha and Gateff, 1982). The late-third-instar larval lymph gland is comprised of four pairs of lobes of which the biggest—the primary lobe—is further divided into four areas: the PSC, the medullary zone (MZ), the cortical zone (CZ), and the intermediate zone (IZ) (Fig. 1B) (Ferguson and Martinez-Agosto, 2014b; Krzemień et al., 2007; Krzemien et al., 2010; Mandal et al., 2007). The posterior lobes express similar markers as the primary lobe, yet, their detailed functions remain uncharacterized (Grigorian et al., 2011; Jung et al., 2005). The PSC, located at the medio-posterior region of the lymph gland, serves as a signaling center for the maintenance of prohemocytes (Crozatier et al., 2004; Krzemień et al., 2007; Lebestky et al., 2003; Mandal et al., 2004). Recent studies have suggested that the dorsal vessel plays additional signaling roles for the regulation of prohemocytes (Morin-Poulard et al., 2016). Closest to the PSC and the dorsal vessel is the MZ, possessing potentials to generate mature hemocytes of the lymph gland (Jung et al., 2005). The MZ is connected to the CZ via the IZ (Blanco-Obregon et al., 2019). The IZ is identified by reactive oxygen species (ROS), domeless, and Hemolectin as well as their transition states (Krzemien et al., 2010; Owusu-Ansah and Banerjee, 2009; Sinenko et al., 2009). The CZ which lies on the outermost region of the lymph gland, contains mature hemocytes (Blanco-Obregon et al., 2019; Crozatier et al., 2004; Jung et al., 2005).
During normal development, both circulation and the lymph gland maintain constant ratios of mature hemocytes. Plasmatocytes generally make up approximately 95% of the total hemocytes whereas crystal cells cover 5% (Bangs et al., 2000; De Gregorio et al., 2002; Holz et al., 2003; Lanot et al., 2001; Ramet et al., 2002; Rizki, 1957; Shrestha and Gateff, 1982; Tepass et al., 1994). Another group of hemocytes, which are seen under severe immune responses, is lamellocytes (Brehelin, 1982; Lanot et al., 2001; Rizki, 1957). Lamellocytes encapsulate eggs deposited by parasitic wasps into the larva and neutralize them as an active defense mechanism (Carton et al., 2008; Keebaugh and Schlenke, 2013; Russo et al., 1996).
RUNX IN DROSOPHILA DEVELOPMENT
Runt is a DNA-binding domain first identified in Drosophila runt and is highly conserved in invertebrates and vertebrates (Crute et al., 1996; Daga et al., 1996; Gergen and Wieschaus, 1986; Rennert et al., 2003). In mammals, acute myeloid leukemia 1 (AML1), also known as RUNX1, is involved in the regulation of hematopoiesis and its transposition causes myeloid leukemia (Lo Coco et al., 1997; Okuda et al., 1996; Rabbitts, 1994; Speck and Terryl, 1995). There are three paralogues of RUNX: RUNX1, RUNX2, and RUNX3, in mammals. Alternatively, there are two well-known Runt domain proteins in Drosophila, runt and lozenge (Rennert et al., 2003). Loss of runt or lozenge leads to comparable pleiotropic phenotypes during Drosophila development which include defects in: 1) neurogenesis (Dormand and Brand, 1998; Duffy et al., 1991), 2) eye development (Crew et al., 1997; Daga et al., 1996; Flores et al., 1998; Oliver, 1946), 3) segmentation (Ingham and Gergen, 1988), and 4) sex determination (Duffy and Gergen, 1991; Sánchez and Nöthiger, 1983). However, loss of lozenge causes two additional phenotypes: reproductive disorders and defects in hematopoiesis (Anderson, 1945; Milchanowski et al., 2004; Wang et al., 1996). Investigating the role of lozenge in Drosophila has provided mechanistic details on Runt domain protein functions in fly hematopoiesis and related signaling pathways (Canon and Banerjee, 2000; Milchanowski et al., 2004). Here on, we discuss the role of lozenge in Drosophila hematopoiesis and its genetic interactions.
LOZENGE IN DROSOPHILA HEMATOPOIESIS
In Drosophila hematopoiesis, lozenge controls the lineage specification of prohemocytes into crystal cells (Fig. 2). This unique role of lozenge is observed in both waves of Drosophila hematopoiesis in the embryo and larval lymph gland (Holz et al., 2003; Lanot et al., 2001). In the embryo, a group of cells identified as crystal cell precursors (CCPs) express lozenge and are initially seen at the head mesoderm of stage 11 embryos (Lebestky et al., 2000). If CCPs lose lozenge expression, they give rise to plasmatocytes while CCPs with high lozenge become crystal cells (Lebestky et al., 2003). This implies that generation of crystal cells is dependent on lozenge expression in embryonic hemocytes. In addition to developmental studies, previous mutant screens of lozenge identified several lozenge alleles with variant phenotypes including loss of crystal cells resulting from lozengets and lozenger15 (Daga et al., 1996; Galko and Krasnow, 2004; Lebestky et al., 2000; Rizki et al., 1980). This reaffirms the assertion that early expression of lozenge is required for the formation of crystal cells in the embryo (Lebestky et al., 2000). Besides crystal cells generated from the embryonic head mesoderm, studies by Leitao and Sucena (2015) have demonstrated that lozenge-expressing primed cells can differentiate into crystal cells in a Notch-dependent manner at larval hematopoietic pockets.
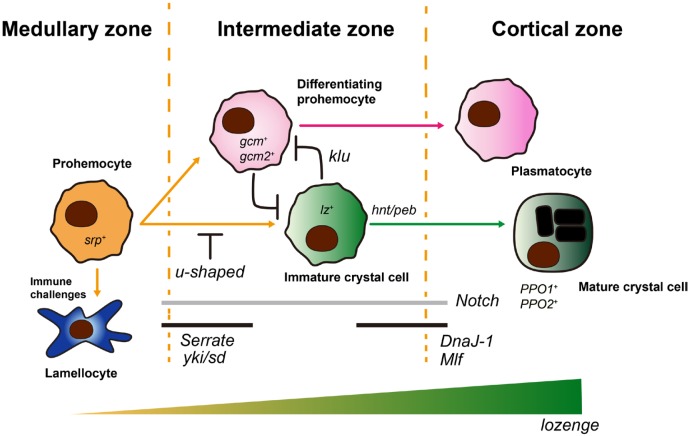
Fig. 2. lozenge in crystal cell formation.
serpent-positive (srp+) prohemocytes differentiate into gcm+/gcm2+ differentiating prohemocytes or lz+ immature crystal cells. Differentiation of prohemocytes into immature crystal cells is mediated by the Notch/Serrate interaction and moderated by u-shaped. Additionally, yki and sd control the crystal cell specification in a Notch/Serrate-dependent manner. High gcm/gcm2 expression reduces the number of crystal cells, however, fated crystal cells are inhibited by klu from becoming plasmatocytes. This high gcm/gcm2 cells become plasmatocytes. Increased lz in immature crystal cells coupled with hnt/peb, DnaJ-1, Mlf and Notch leads to formation of mature crystal cells which possess crystalline inclusions and express PPO1 and PPO2. The process of mature crystal cell formation is heavily dependent on lozenge expression from the onset to the late stage. The medullary, intermediate, and cortical zones demarcate three regions of the primary lymph gland lobe. Healthy animals do not actively generate lamellocytes. Though, prohemocyte tion is lamellocyte-biased upon immune challenges.
Similarly, in the second instar larval lymph gland, lozenge-positive cells emerge from differentiating prohemocytes in the IZ and divide into crystal cells (Ferguson and Martinez-Agosto, 2014a). Consequently, these lozenge-positive cells generate up to a hundred crystal cells per one lobe (Lebestky et al., 2000). lozenge, in determining the proportion of divided cells that become crystal cells, relies on an intricate circuit of interactions with other proteins. Over the years, though significant research is still required in this aspect, several transcription factors and related signaling proteins have been uncovered in Drosophila. First, the GATA-like transcriptional factor—serpent (srp), was identified as a marker for prohemocytes as well as an upstream regulator of lozenge that is crucial for the formation of crystal cells. Indeed, a small subset of srp-positive cells become lozenge-positive crystal cells and loss of srp turns off lozenge expression (Bataille et al., 2005; Lanot et al., 2001; Lebestky et al., 2000). Second, u-shaped (ush), a Friend of GATA homologue, was found to regulate the population size of crystal cells. Increasing or reducing u-shaped expression significantly diminishes or augments the number of crystal cells produced respectively (Fossett et al., 2001). Further investigation showed that a synergistic confluence of serpent and lozenge inhibits u-shaped to allow formation of crystal cells from its precursors (Fossett et al., 2003; Muratoglu et al., 2007; Waltzer et al., 2003). Third, glial cell missing (gcm)-positive cells later differentiate into plasmatocytes (Bernardoni et al., 1997; Lebestky et al., 2000). The absence of gcm in prohemocytes induces formation of lozenge positive cells which become crystal cells (Bataille et al., 2005; Lebestky et al., 2000). This process is identical in both embryonic and larval contexts.
In addition to GATA and Zinc finger transcription factors, srp, ush and gcm and gcm2 respectively, one of the main signaling molecules that has been shown to impact the formation of crystal cells is Notch. The numbers of crystal cells or prohemocytes are shown to be reduced or absent in Notch-mutant clones of the lymph gland (Dey et al., 2016; Lebestky et al., 2003). In-depth studies in the lymph gland showed that a group of cells that are positive for Serrate, a Notch ligand, trigger the expression of lozenge and hence determine the crystal cell lineage (Duvic et al., 2002; Lebestky et al., 2003). Recently, endogenous expression of Serrate was revealed in the IZ, where Notch-mediated crystal cell differentiation takes place, emphasizing that Notch-Serrate activity in differentiating prohemocytes is critical for crystal cell specification (Cho et al., 2018). Of note, yorkie (Berson et al., 2019)- and scalloped (sd)- activation of lozenge is another important factor in Notch-dependent crystal cell determination. Loss of yki or sd reduces the crystal cell differentiation, and expression of yki does not rescue SerrateDN-mediated crystal cell depletion (Ferguson and Martinez-Agosto, 2014b). These results imply that yki/sd function requires Serrate-Notch signaling (Ferguson and Martinez-Agosto, 2014a). Lastly, pebbled (peb), myeloid leukemia factor (Mlf), DNA-J-like1 (DnaJ-1), klumpfuss (klu) have been implicated in Notch-lozenge dependent crystal cell formation. While DnaJ-1 and Mlf regulate Notch activation, peb regulates nuclear size of crystal cells. Klu, on the other hand, inhibits pre-destined crystal cells from becoming plasmatocytes (Miller et al., 2017; Terriente-Felix et al., 2013). The stabilization of lozenge by these proteins: klu, Mlf, and DnaJ-1, and their interaction with Notch supports proper differentiation of crystal cells (Miller et al., 2017; Terriente-Felix et al., 2013). It is worth noting that the Notch-RUNX collaboration is conserved in mammals and therefore, further investigations could elucidate their roles in mammalian hematopoiesis (Bras et al., 2012; Kulkarni et al., 2011; Miller et al., 2017; Terriente-Felix et al., 2013).
LOZENGE, CRYSTAL CELLS IN DROSOPHILA STRESS RESPONSES
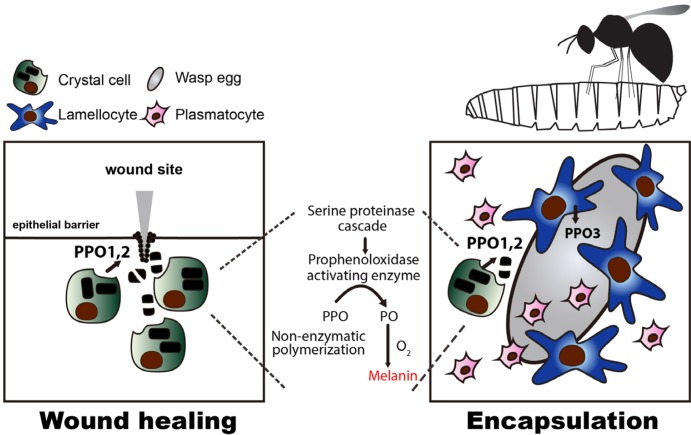
Crystal cells are responsible for a significant part of the Drosophila innate immunity and stress responses (Cho et al., 2018; Sorrentino et al., 2002). Though the population of crystal cells is sparse, upon invoking an immune reaction through wounding (Galko and Krasnow, 2004; Ramet et al., 2002; Rizki, 1960) or injection of wasp eggs into a larva, crystal cells rupture and melanization takes place (Fig. 3) (Dudzic et al., 2015; Tang et al., 2006). In melanization, crystal cells are mobilized via the c-Jun N-terminal Kinase (JNK), small GTPases, and Eiger (tumor necrosis factor, TNF) pathways, and they burst to activate the Serine protease cascade which is facilitated by phenoloxidases (Bidla et al., 2007; Peeples et al., 1968; 1969a; 1969b; Rizki, 1957). Within this cascade, proteolytic enzymes convert Prophenoloxidase 1 (PPO1) and Prophenoloxidase 2 (PPO2) released by crystal cells into active phenoloxidases. Phenoloxidases produce phenols and free radicals that eventually inactivate pathogens while building melanin via non-enzymatic polymerizations (Binggeli et al., 2014; Cerenius et al., 2008; Laifook, 1966; Ramet et al., 2002). Specifically, in wound healing, sterile wounding triggers calcium waves that in turn activates an NADPH oxidase, DUOX, required for the recruitment of hemocytes (Razzell et al., 2013). At this site, crystal cells rupture and induce melanization for the formation of a scab that blocks the punctured region. This precedes the crucial step of restoring epithelial integrity (Galko and Krasnow, 2004). Also, upon wasp infestation, lamellocytes inter-dependently contribute to melanization by secreting Prophenoloxidase 3 (PPO3) which augments crystal cells’ phenoloxidase activity, and mediate encapsulation (Dudzic et al., 2015; Gold and Brückner, 2015; Nam et al., 2008; Rizki, 1960; Rizki, 1957; Rizki and Rizki, 1974; 1980). Hence, in the absence of lozenge or PPOs in lozenger15, lozengets, PPO1∆ or PPO2∆, these immune reactions are abrogated (Binggeli et al., 2014; Dudzic et al., 2015; Ferguson and Martinez-Agosto, 2014a). Different from these phenomena, hypoxic stress activates crystal cell differentiation while keeping the cell intact (Mukherjee et al., 2011). While the innate immune functions of crystal cells and their PPO inclusions are well characterized, the function of crystal cells in hypoxia is still obscure. Considering that crystal cells are significantly induced by manipulation of CO2- or O2-sensing neurons, it is plausible that crystal cells may play a non-canonical role in this condition (Cho et al., 2018).
Fig. 3. Crystal cell mediated stress responses.
When larva gets a sterile wound, crystal cells migrate towards the wound site, and rupture to initiate the melanization cascade (left). This promotes the formation of a scab at the wound site leading to a subsequent healing process. Upon wasp infestation, wasps lay eggs inside Drosophila larva that trigger innate immune responses including differentiation of lamellocytes. Lamellocytes encapsulate wasp eggs and activate PPO3 to neutralize them (right). During encapsulation, crystal cells co-opt PPO1 and PPO2 to facilitate melanization. In melanization, rupturing of crystal cells is coupled with the activation of Serine proteinase cascade which triggers the conversion of PPOs into POs initiating non-enzymatic melanin formation (middle).
PERSPECTIVES
Extensive and thorough studies on Drosophila hematopoiesis have unraveled novel insights into mechanisms underlying lozenge and its intricate molecular and genetic links. We have discussed a number of these factors, however, going forward additional analyses will be expedient in broadening our perspectives on lozenge/RUNX. Specifically, spatio-temporal interactions between lozenge and Notch/Serrate, srp, gcm, gcm2, ush, yki/sd, klu, peb, DnaJ-1, and Mlf in the embryo or the lymph gland contexts will require in-depth future investigations. Furthermore, explorations on additional functions of the crystal cell, other than its melanization effect, will be worth interrogating.
ACKNOWLEDGMENTS
This work was supported by National Research Foundation (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (NRF-2019R1A2C2006848) to J.S.
The authors thank members of the Shim lab for helpful discussions, and Bumsik Cho for Figure illustrations.
Footnotes
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Agaisse H., Petersen U.M., Boutros M., Mathey-Prevot B., Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 2003;5:441–450. doi: 10.1016/S1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Anderson R.C. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics. 1945;30:280–296. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Novitski E. Academic Press; London: 1976. Genetics and Biology of Drosophila. [Google Scholar]
- Bangs P., Franc N., White K. Molecular mechanisms of cell death and phagocytosis in Drosophila. Cell Death Differ. 2000;7:1027–103. doi: 10.1038/sj.cdd.4400754. [DOI] [PubMed] [Google Scholar]
- Bataille L., Auge B., Ferjoux G., Haenlin M., Waltzer L. Resolving embryonic blood cell fate choice in Drosophila: interplay of GCM and RUNX factors. Development (Cambridge, England) 2005;132:4635–4644. doi: 10.1242/dev.02034. [DOI] [PubMed] [Google Scholar]
- Bernardoni R., Vivancos V., Giangrande A. glide/gcm is expressed and required in the scavenger cell lineage. Dev. Biol. 1997;191:118–130. doi: 10.1006/dbio.1997.8702. [DOI] [PubMed] [Google Scholar]
- Berson A., Goodman L.D., Sartoris A.N., Otte C.G., Aykit J.A., Lee V.M., Trojanowski J.Q., Bonini N.M. Drosophila Ref1/ALYREF regulates transcription and toxicity associated with ALS/FTD disease etiologies. Acta Neuropathol. Commun. 2019;7:65. doi: 10.1186/s40478-019-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidla G., Dushay M.S., Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci. 2007;120:1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- Binggeli O., Neyen C., Poidevin M., Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014;10:e1004067. doi: 10.1371/journal.ppat.1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Obregon D.M., Katz M.J., Durrieu L., Gándara L., Wappner P. Context-specific functions of notch in Drosophila blood cell progenitors. bioRxiv. 2019:82658. doi: 10.1101/682658. [DOI] [PubMed] [Google Scholar]
- Bras S., Martin-Lanneree S., Gobert V., Auge B., Breig O., Sanial M., Yamaguchi M., Haenlin M., Plessis A., Waltzer L. Myeloid leukemia factor is a conserved regulator of RUNX transcription factor activity involved in hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4986–4991. doi: 10.1073/pnas.1117317109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehelin M. Comparative study of structure and function of blood cells from two Drosophila species. Cell Tissue Res. 1982;221:607–615. doi: 10.1007/BF00215704. [DOI] [PubMed] [Google Scholar]
- Canon J., Banerjee U. Runt and Lozenge function in Drosophila development. Semin. Cell Dev. Biol. 2000;11:327–336. doi: 10.1006/scdb.2000.0185. [DOI] [PubMed] [Google Scholar]
- Carton Y., Poirié M., Nappi A.J. Insect immune resistance to parasitoids. Insect Sci. 2008;15:67–87. doi: 10.1111/j.1744-7917.2008.00188.x. [DOI] [Google Scholar]
- Cerenius L., Lee B.L., Soderhall K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immun. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Cho B., Spratford C.M., Yoon S., Cha N., Banerjee U., Shim J. Systemic control of immune cell development by integrated carbon dioxide and hypoxia chemosensation in Drosophila. Nat. Commun. 2018;9:2679. doi: 10.1038/s41467-018-04990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew J.R., Batterham P., Pollock J.A. Developing compound eye in lozenge mutants of Drosophila: lozenge expression in the R7 equivalence group. Dev. Genes Evol. 1997;206:481–493. doi: 10.1007/s004270050079. [DOI] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J.M., Vincent A., Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2:E196. doi: 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute B.E., Lewis A.F., Wu Z., Bushweller J.H., Speck N.A. Biochemical and biophysical properties of the core-binding factor alpha2 (AML1) DNA-binding domain. J. Biol. Chem. 1996;271:26251–26260. doi: 10.1074/jbc.271.42.26251. [DOI] [PubMed] [Google Scholar]
- Daga A., Karlovich C.A., Dumstrei K., Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- De Gregorio E., Han S.J., Lee W.J., Baek M.J., Osaki T., Kawabata S., Lee B.L., Iwanaga S., Lemaitre B., Brey P.T. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev. Cell. 2002;3:581–592. doi: 10.1016/S1534-5807(02)00267-8. [DOI] [PubMed] [Google Scholar]
- Dey N.S., Ramesh P., Chugh M., Mandal S., Mandal L. Dpp dependent hematopoietic stem cells give rise to Hh dependent blood progenitors in larval lymph gland of Drosophila. eLife. 2016;5:e18295. doi: 10.7554/eLife.18295.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormand E.L., Brand A.H. Runt determines cell fates in the Drosophila embryonic CNS. Development. 1998;125:1659–1667. doi: 10.1242/dev.125.9.1659. [DOI] [PubMed] [Google Scholar]
- Dudzic J.P., Kondo S., Ueda R., Bergman C.M., Lemaitre B. Drosophila innate immunity: regional and functional specialization of prophenoloxidases. BMC Biol. 2015;13:81. doi: 10.1186/s12915-015-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.B., Gergen J.P. The Drosophila segmentation gene runt acts as a position-specific numerator element necessary for the uniform expression of the sex-determining gene Sex-lethal. Genes Dev. 1991;5:2176–2187. doi: 10.1101/gad.5.12a.2176. [DOI] [PubMed] [Google Scholar]
- Duffy J.B., Kania M.A., Gergen J.P. Expression and function of the Drosophila gene runt in early stages of neural development. Development (Cambridge, England) 1991;113:1223. doi: 10.1242/dev.113.4.1223. [DOI] [PubMed] [Google Scholar]
- Duvic B., Hoffmann J.A., Meister M., Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr. Biol. 2002;12:1923–19. doi: 10.1016/S0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson M., Mishra S., Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol. 2000;10:781–784. doi: 10.1016/S0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- Evans C.J., Hartenstein V., Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 2003;5:673–690. doi: 10.1016/S1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Ferguson G.B., Martinez-Agosto J.A. Kicking it up a notch for the best in show: scalloped leads Yorkie into the haematopoietic arena. Fly (Austin) 2014a;8:206–217. doi: 10.1080/19336934.2015.1055427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G.B., Martinez-Agosto J.A. Yorkie and scalloped signaling regulates notch-dependent lineage specification during Drosophila hematopoiesis. Curr. Biol. 2014b;24:2665–2672. doi: 10.1016/j.cub.2014.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G.V., Daga A., Kalhor H.R., Banerjee U. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development. 1998;125:3681–3687. doi: 10.1242/dev.125.18.3681. [DOI] [PubMed] [Google Scholar]
- Fossett N., Hyman K., Gajewski K., Orkin S.H., Schulz R.A. Combinatorial interactions of Serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11451–11456. doi: 10.1073/pnas.1635050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N., Tevosian S.G., Gajewski K., Zhang Q., Orkin S.H., Schulz R.A. The friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko M.J., Krasnow M.A. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J.P., Wieschaus E. Dosage requirements for runt in the segmentation of Drosophila embryos. Cell. 1986;45:289–299. doi: 10.1016/0092-8674(86)90393-4. [DOI] [PubMed] [Google Scholar]
- Gold K.S., Brückner K. Macrophages and cellular immunity in Drosophila melanogaster. Semin. Immunol. 2015;27:357–368. doi: 10.1016/j.smim.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M., Mandal L., Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev. Genes Evol. 2011;221:121–131. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A., Bossinger B., Strasser T., Janning W., Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Ingham P., Gergen P. Interactions between the pair-rule genes runt, hairy, even-skipped and fushi tarazu and the establishment of periodic pattern in the Drosophila embryo. Development (Cambridge, England) 1988;104:51. [Google Scholar]
- Jung S.H., Evans C.J., Uemura C., Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development (Cambridge, England) 2005;132:2521. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Keebaugh E., Schlenke T. Insights from natural host-parasite interactions: the Drosophila model. Dev. Comp. Immunol. 2013;42:111–123. doi: 10.1016/j.dci.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemień J., Dubois L., Makki R., Meister M., Vincent A., Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Krzemien J., Oyallon J., Crozatier M., Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev. Biol. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Kulkarni V., Khadilkar R.J., Magadi S.S., Inamdar M.S. Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS One. 2011;6:e27667. doi: 10.1371/journal.pone.0027667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laifook J. The repair of wounds in the integument of insects. Phys. Ther. 1966;46:195–226. doi: 10.1016/0022-1910(66)90136-3. [DOI] [PubMed] [Google Scholar]
- Lanot R., Zachary D., Holder F., Meister M. Postembryonic hematopoiesis in Drosophila. Dev. Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lebestky T., Chang T., Hartenstein V., Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lebestky T., Jung S.H., Banerjee U. A serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao A.B., Sucena E. Drosophila sessile hemocyte clusters are true hematopoietic tissues that regulate larval blood cell differentiation. eLife. 2015;4:e06166. doi: 10.7554/eLife.06166.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford N.J., Bilgir C., Ro J., Pletcher S.D. Measurement of lifespan in Drosophila melanogaster. J. Vis. Exp. 2013;71:50068. doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Coco F., Pisegna S., Diverio D. The AML1 gene: a transcription factor involved in the pathogenesis of myeloid and lymphoid leukemias. Haematologica. 1997;82:364–370. [PubMed] [Google Scholar]
- Makhijani K., Alexander B., Tanaka T., Rulifson E., Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development (Cambridge, England) 2011;138:5379. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L., Banerjee U., Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J.A., Evans C.J., Hartenstein V., Banerjee U. A hedgehog- and antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márkus R., Laurinyecz B., Kurucz É., Honti V., Bajusz I., Sipos B., Somogyi K., Kronhamn J., Hultmark D., Andó I. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4805–4809. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milchanowski A.B., Henkenius A.L., Narayanan M., Hartenstein V., Banerjee U. Identification and characterization of genes involved in embryonic crystal cell formation during Drosophila hematopoiesis. Genetics. 2004;168:325–339. doi: 10.1534/genetics.104.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Chen A., Gobert V., Auge B., Beau M., Burlet-Schiltz O., Haenlin M., Waltzer L. Control of RUNX-induced repression of Notch signaling by MLF and its partner DnaJ-1 during Drosophila hematopoiesis. PLoS Genet. 2017;13:e1006932. doi: 10.1371/journal.pgen.1006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S., Stramer B., Evans I., Wood W., Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Morin-Poulard I., Sharma A., Louradour I., Vanzo N., Vincent A., Crozatier M. Vascular control of the Drosophila haematopoietic microenvironment by Slit/Robo signalling. Nat. Commun. 2016;7:11634. doi: 10.1038/ncomms11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T., Kim W.S., Mandal L., Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332:1210–1213. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratoglu S., Hough B., Mon S.T., Fossett N. The GATA factor Serpent cross-regulates lozenge and u-shaped expression during Drosophila blood cell development. Dev. Biol. 2007;311:636–649. doi: 10.1016/j.ydbio.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H.J., Jang I.H., Asano T., Lee W.J. Involvement of pro-phenoloxidase 3 in lamellocyte-mediated spontaneous melanization in Drosophila. Mol. Cells. 2008;26:606–610. [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S.W., Grosveld G., Downing J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/S0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Oliver C.P. A study of the relationship between facet irregularities and eye color in lozenge alleles of Drosophila melanogaster. Anat. Rec. 1946;94:416. [PubMed] [Google Scholar]
- Olofsson B., Page D.T. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev. Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E., Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples E.E., Barnett D.R., Oliver C.P. Phenol oxidases of a lozenge mutant of Drosophila. Science (New York, NY) 1968;159:548–552. doi: 10.1126/science.159.3814.548. [DOI] [PubMed] [Google Scholar]
- Peeples E.E., Geisler A., Whitcraft C.J., Oliver C.P. Activity of phenol oxidases at the puparium formation stage in development of nineteen lozenge mutants of Drosophila melanogaster. Biochem. Genet. 1969a;3:563–5. doi: 10.1007/BF00485477. [DOI] [PubMed] [Google Scholar]
- Peeples E.E., Geisler A., Whitcraft C.J., Oliver C.P. Comparative studies of phenol oxidase activity during pupal development of three lozenge mutants (lz8,lz,lzk) of Drosophila melanogaster. Genetics. 1969b;62:161–170. doi: 10.1093/genetics/62.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T.H. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Ramet M., Lanot R., Zachary D., Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 2002;241:145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- Razzell W., Evans I.R., Martin P., Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert J., Coffman J.A., Mushegian A.R., Robertson A.J. The evolution of Runx genes I. A comparative study of sequences from phylogenetically diverse model organisms. BMC Evol. Biol. 2003;3:4. doi: 10.1186/1471-2148-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki M.T. Melanotic tumor formation in Drosophila. J. Morphol. 1960;106:147–157. doi: 10.1002/jmor.1051060203. [DOI] [PubMed] [Google Scholar]
- Rizki M.T.M. Alterations in the haemocyte population of Drosophila melanogaster. J. Morphol. 1957;100:437–458. doi: 10.1002/jmor.1051000303. [DOI] [Google Scholar]
- Rizki R.M., Rizki T.M. Basement membrane abnormalities in melanotic tumor formation of Drosophila. Experientia. 1974;30:543–546. doi: 10.1007/BF01926343. [DOI] [PubMed] [Google Scholar]
- Rizki R.M., Rizki T.M. Hemocyte responses to implanted tissues in Drosophila melanogaster larvae. Wilehm Roux Arch. Dev. Biol. 1980;189:207–213. doi: 10.1007/BF00868679. [DOI] [PubMed] [Google Scholar]
- Rizki T.M., Rizki R.M., Grell E.H. A mutant affecting the crystal cells in Drosophila melanogaster. Wilehm Roux Arch. Dev. Biol. 1980;188:91–99. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- Robertson C.W. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 1936;59:351–399. doi: 10.1002/jmor.1050590207. [DOI] [Google Scholar]
- Rugendorff A., Younossi-Hartenstein A., Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux Arch. Dev. Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- Russo J., Dupas S., Frey F., Carton Y., Brehelin M. Insect immunity: early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology. 1996;112:135–142. doi: 10.1017/S0031182000065173. [DOI] [PubMed] [Google Scholar]
- Sánchez L., Nöthiger R. Sex determination and dosage compensation in Drosophila melanogaster: production of male clones in XX females. EMBO J. 1983;2:485–491. doi: 10.1002/j.1460-2075.1983.tb01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R., Gateff E. Ultrastructure and cytochemistry of the cell-types in the tumorous hematopoietic organs and the hemolymph of the mutant lethal (1) malignant blood neoplasm (l(1)mbn) of Drosophila melanogaster (Drosophila/mutant blood cells/ultrastructure/cytochemistry) Dev. Growth Differ. 1982;24:83–98. doi: 10.1111/j.1440-169X.1982.00083.x. [DOI] [PubMed] [Google Scholar]
- Sinenko S.A., Mandal L., Martinez-Agosto J.A., Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass R.E. Smithsonian Institution; Washington D.C.: 1954. Insect Metamorphosis. [Google Scholar]
- Sorrentino R.P., Carton Y., Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Speck N.A., Terryl S. A new transcription factor family associated with human leukemias. Crit. Rev. Eukaryot. Gene Expr. 1995;5:337–364. doi: 10.1615/CritRevEukarGeneExpr.v5.i3-4.60. [DOI] [PubMed] [Google Scholar]
- Stocker H., Gallant P. Getting started. In: C. Dahmann., editor. Drosophila: Methods and Protocols. Humana Press; Totowa, USA: 2008. pp. 27–44. [DOI] [Google Scholar]
- Tang H., Kambris Z., Lemaitre B., Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J. Biol. Chem. 2006;281:28097–28104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- Tepass U., Fessler L.I., Aziz A., Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development (Cambridge, England) 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Terriente-Felix A., Li J., Collins S., Mulligan A., Reekie I., Bernard F., Krejci A., Bray S. Notch cooperates with Lozenge/Runx to lock haemocytes into a differentiation programme. Development. 2013;140:926–937. doi: 10.1242/dev.086785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltzer L., Ferjoux G., Bataille L., Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 2003;22:6516–6525. doi: 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Stacy T., Binder M., Marin-Padilla M., Sharpe A.H., Speck N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3444. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., Faria C., Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]