Abstract
Runx2 is an essential transcription factor for skeletal development. It is expressed in multipotent mesenchymal cells, osteoblast-lineage cells, and chondrocytes. Runx2 plays a major role in chondrocyte maturation, and Runx3 is partly involved. Runx2 regulates chondrocyte proliferation by directly regulating Ihh expression. It also determines whether chondrocytes become those that form transient cartilage or permanent cartilage, and functions in the pathogenesis of osteoarthritis. Runx2 is essential for osteoblast differentiation and is required for the proliferation of osteoprogenitors. Ihh is required for Runx2 expression in osteoprogenitors, and hedgehog signaling and Runx2 induce the differentiation of osteoprogenitors to preosteoblasts in endochondral bone. Runx2 induces Sp7 expression, and Runx2, Sp7, and canonical Wnt signaling are required for the differentiation of preosteoblasts to immature osteoblasts. It also induces the proliferation of osteoprogenitors by directly regulating the expression of Fgfr2 and Fgfr3. Furthermore, Runx2 induces the proliferation of mesenchymal cells and their commitment into osteoblast-lineage cells through the induction of hedgehog (Gli1, Ptch1, Ihh), Fgf (Fgfr2, Fgfr3), Wnt (Tcf7, Wnt10b), and Pthlh (Pth1r) signaling pathway gene expression in calvaria, and more than a half-dosage of Runx2 is required for their expression. This is a major cause of cleidocranial dysplasia, which is caused by heterozygous mutation of RUNX2. Cbfb, which is a co-transcription factor that forms a heterodimer with Runx2, enhances DNA binding of Runx2 and stabilizes Runx2 protein by inhibiting its ubiquitination. Thus, Runx2/Cbfb regulates the proliferation and differentiation of chondrocytes and osteoblast-lineage cells by activating multiple signaling pathways and via their reciprocal regulation.
Keywords: Cbfb, fibroblast growth factor receptor, hedgehog, Runx2, Wnt
INTRODUCTION
Runx2 is a transcription factor that belongs to the Runx family composed of Runx1, Runx2, and Runx3. Runx2 is expressed in multipotent mesenchymal cells, osteoblast-lineage cells, and chondrocytes (Komori, 2018). It is also expressed in the thymus and mammary gland. Although Runx2 is not essential for T cell development, it is required for mammary gland development (Owens et al., 2014; Taniuchi et al., 2002). The transcription of the Runx2 gene is regulated by two promoters, P1 and P2. Both isoforms transcribed from P1 and P2 promoters are expressed in osteoblast-lineage cells and chondrocytes, but Runx2 expression in these lineages is regulated by the enhancers, most of which remain to be identified (Enomoto et al., 2000; Kawane et al., 2014). Runx2 forms a heterodimer with Cbfb, thereby acquiring increased DNA binding capacity, and binds the consensus sequence TGPyGGPyPy (Komori, 2018).
THE FUNCTIONS OF Runx2 IN CHONDROCYTES
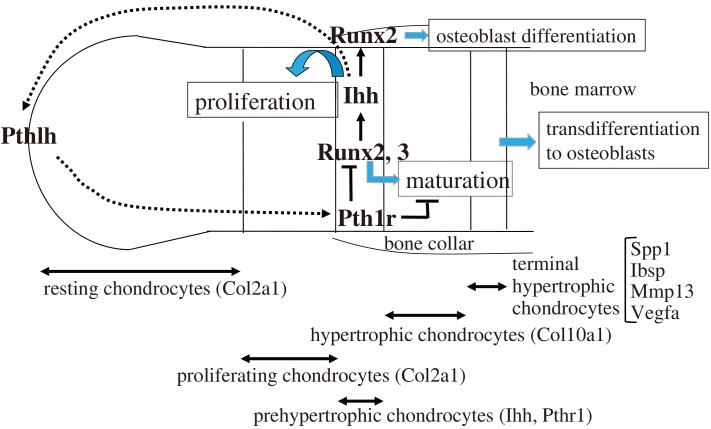
In skeletal development, Sox9 is required for a mesenchymal condensation, and Sox9, Sox5, and Sox6 are required for Col2a1 expression and cartilage formation (Lefebvre and Smits, 2005). In long bone, chondrocytes form the growth plate, which is composed of resting, proliferating, prehypertrophic, hypertrophic, and terminal hypertrophic chondrocyte layers (Fig. 1). Chondrocytes continuously differentiate (maturate) in this order. Runx2 is expressed in resting and proliferating chondrocytes weakly, and its expression is upregulated in prehypertrophic chondrocytes, mildly down-regulated in hypertrophic chondrocytes, and upregulated again in terminal hypertrophic chondrocytes (Inada et al., 1999). Runx2 is required for the differentiation (maturation) of prehypertrophic chondrocytes to hypertrophic chondrocytes (Enomoto et al., 2000; Takeda et al., 2001; Ueta et al., 2001). Runx2–/– mice lack hypertrophic chondrocytes in most of the skeleton except the tibia, fibula, radius, and ulna, in which hypertrophic and terminal hypertrophic chondrocytes are observed (Inada et al., 1999; Kim et al., 1999). Runx3 is also expressed in prehypertrophic chondrocytes, and Runx3–/– mice exhibit a mild delay in chondrocyte maturation at the embryonic stage. Double knockout mice of Runx2 and Runx3 lack hypertrophic chondrocytes in the entire skeleton (Yoshida et al., 2004). Thus, Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 plays a major role, whereas Runx3 plays a supplementary role in chondrocyte maturation. Runx2 induces Ihh expression in prehypertrophic chondrocytes, and Ihh increases chondrocyte proliferation in the proliferating chondrocyte layers. Therefore, Runx2 also regulates chondrocyte proliferation through the induction of Ihh expression (Yoshida et al., 2004). As Ihh induces Pthlh, which inhibits chondrocyte maturation at least partly through the suppression of Runx2, Runx2-Ihh-Pthlh forms a negative feedback loop for chondrocyte maturation (Iwamoto et al., 2003; Vortkamp et al., 1996) (Fig. 1).
Fig. 1. Regulation of the proliferation and differentiation of chondrocytes by Runx2.
The growth plate is composed of the resting and proliferating chondrocyte layers, which express Col2a1, prehypertrophic chondrocyte layer, which expresses Ihh and Pth1r, hypertrophic chondrocyte layer, which expresses Col10a1, and terminal hypertrophic chondrocyte layer, which expresses Spp1, Ibsp, Mmp13, and Vegfa. Runx2 expression is upregulated in the prehypertrophic chondrocyte layer and induces their maturation into hypertrophic chondrocytes. Runx3 is also involved in this process. Runx2 induces the expression of Ihh, which induces the proliferation of chondrocytes, and Ihh induces the expression of Pthlh, which inhibits Runx2 expression and chondrocyte maturation through Pth1r, forming a negative feedback loop. Runx2 also regulates the expression of Col10a1, Spp1, Ibsp, Mmp13, and Vegfa. Ihh induces Runx2 expression in the perichondrium for the differentiation of osteoblasts, which form the bone collar and primary spongiosa. Most terminal hypertrophic chondrocytes transdifferentiate into osteoblasts.
Overexpression of Runx2 in chondrocytes accelerates chondrocyte maturation in whole cartilage, including permanent cartilage, thereby impairing joint formation. Tenascin is expressed in permanent cartilage, but its expression is absent in the cartilaginous skeletons of chondrocyte-specific Runx2 transgenic mice. In chondrocyte-specific dominant-negative Runx2 transgenic mice, tenascin is expressed in the whole cartilaginous skeleton (Ueta et al., 2001). Thus, Runx2 determines whether chondrocytes become those in transient cartilage like the growth plate or those in permanent cartilage like articular cartilage (Komori, 2002). Runx2 induces the expression of Mmp13 and Adamts5, which disrupt the matrix of articular cartilage (Hess et al., 2001; Hirata et al., 2012; Jimenez et al., 1999; Selvamurugan et al., 2000; Takahashi et al., 2017; Tetsunaga et al., 2011; Thirunavukkarasu et al., 2007; Wang et al., 2004). Furthermore, Runx2+/– mice are resistant to osteoarthritis (OA) progression, chondrocyte-specific deletion of Runx2 decelerates OA progression, and tamoxifen-induced Runx2 expression in articular cartilage accelerates OA progression in an experimental OA mouse model (Catheline et al., 2019; Kamekura et al., 2006; Liao et al., 2017). In addition to Runx2 expression, the expression of Mmp13, Ihh, and Col10a1, which is regulated by Runx2, is increased in human OA cartilage (Cao et al., 2014). Chondrocyte maturation is an important aspect of OA, and Runx2 plays a key role in the pathogenesis of OA.
FUNCTIONS OF Runx2 IN OSTEOBLASTS
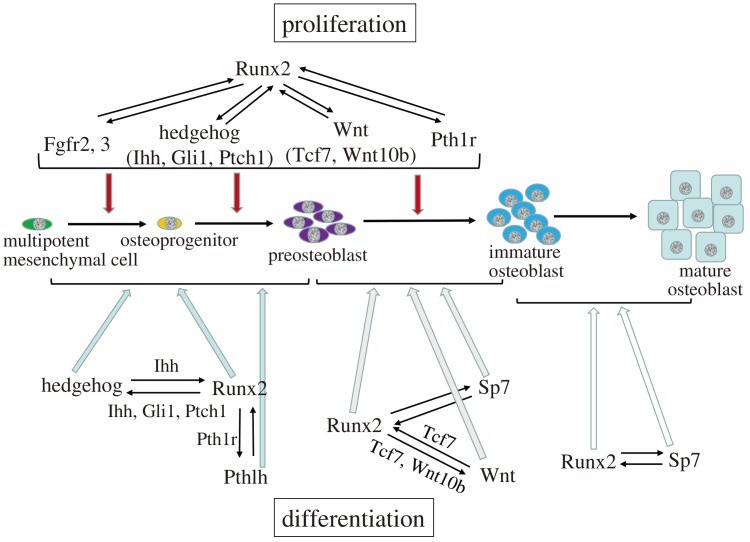
Ihh–/– mice lack osteoblasts in endochondral bones and Runx2 expression is absent in the perichondrium, suggesting that Ihh is required for Runx2 expression in osteoprogenitors in endochondral bones (St-Jacques et al., 1999). The binding of Ihh to the receptor Ptch relieves the repression of Smo by Ptch, and Smo ultimately regulates Gli (Simpson et al., 2009). In Smo conditional knockout mice using Col2a1 Cre, which directs Cre expression in chondrocytes and perichondrial cells that contain osteoblast progenitors, Runx2 expression is absent in the perichondrium but is detected in cells outside of the perichondrium (Long et al., 2004). However, deletion of Smo by Sp7 Cre, which directs Cre expression in preosteoblasts, does not affect osteoblast differentiation (Rodda and McMahon, 2006). Thus, the requirement of the activation of hedgehog signaling pathway for Runx2 expression and osteoblast differentiation is restricted to the stage of osteoprogenitors in endochondral bone development (Fig. 2).
Fig. 2. Regulation of the proliferation and differentiation of osteoblast-lineage cells by Runx2.
Ihh is required for Runx2 expression, and hedgehog signaling and Runx2 induce the differentiation of multipotent mesenchymal cells into preosteoblasts in endochondral bone. Runx2 activates the hedgehog signaling pathway through the induction of Ihh, Gli1, and Ptch1. Runx2, Sp7, and the canonical Wnt signaling pathway induce the differentiation of preosteoblasts into immature osteoblasts. Runx2 induces Sp7 expression at the preosteoblast stage, Runx2 activates the Wnt signaling pathway through the induction of Tcf7 and Wnt10b expression, and Sp7 and Tcf7 activate a Runx2 enhancer. Runx2 and Sp7 induce the differentiation of immature osteoblasts into mature osteoblasts. Runx2 induces the proliferation of multipotent mesenchymal cells, osteoprogenitors, and preosteoblasts through the regulation of Fgf, hedgehog, Wnt, and Pthlh signaling pathway genes. The mutual regulation between Runx2 and the signaling pathways, including hedgehog, Fgf, Wnt, and Pthlh, or Sp7 plays important roles in the proliferation and differentiation of osteoblast-lineage cells and their progenitors.
Conditional Ctnnb1 knockout mice using Twist2 Cre, which directs Cre expression in osteo-chondroprogenitors, Col2a1 Cre or Prrx1 Cre, which directs Cre expression in osteoprogenitors in calvaria and osteo-chondroprogenitors in limb skeletons, lack osteoblasts, but Runx2 is expressed in the perichondrium (Day et al., 2005; Hill et al., 2005; Hu et al., 2005; Rodda and McMahon, 2006). Thus, activation of the Wnt signaling pathway is essential for osteoblast differentiation, but not for Runx2 expression in osteoprogenitors (Fig. 2). Sp7 is another transcription factor essential for osteoblast differentiation. Sp7–/– mice lack osteoblasts, but Runx2 is expressed in the osteoprogenitors (Nakashima et al., 2002). Sp7 is expressed in preosteoblasts and osteoblasts, and Runx2 induces Sp7 expression (Yoshida et al., 2012). Therefore, it is an upstream transcription factor of Sp7. In conditional Ctnnb1 knockout mice and Sp7–/– mice, osteoprogenitors differentiate into chondrocytes. Therefore, osteoprogenitors that express Runx2 retain the ability to differentiate into chondrocytes, and Wnt signaling and Sp7 induce the differentiation of osteoprogenitors into osteoblasts, inhibiting their differentiation into chondrocytes (Fig. 2).
Osteoprogenitors and preosteoblasts weakly express type I collagen, which is a heterotrimeric protein composed of two α1(I) chains and one α2(I) chain encoded by Col1a1 and Col1a2, respectively. Immature osteoblasts upregulate Col1a1 and Col1a2 expression, and express Spp1 and Ibsp, and mature osteoblasts express osteocalcin encoded by Bglap2 and Bglap (Aubin and Triffitt, 2002; Maruyama et al., 2007). In vitro studies demonstrated that Runx2 upregulates the expression of these genes (Ducy et al., 1997; Harada et al., 1999). Indeed, Runx2–/– mice lack osteoblast-lineage cells expressing these genes (Komori et al., 1997). The expression of Spp1 and Bglap2 is reduced, but that of Col1a1 is increased, in the bone of type II Runx2-deficient mice, in which the P1 promoter and exon I of Runx2 are deleted (Xiao et al., 2004). Two groups reported conditional Runx2 knockout mice using 2.3 kb Col1a1 Cre, which directs Cre expression in osteoblasts. The deletion of the runt domain in osteoblasts resulted in no bone phenotype, whereas the deletion of exon 8, which creates cryptic Runx2 protein that retains DNA binding capacity but has lower transcriptional activation ability, resulted in reduced bone mass (Adhami et al., 2014; Takarada et al., 2013). As the cryptic Runx2 protein may interfere with the binding of Runx3, which is also involved in bone formation (Bauer et al., 2015), the regulation of bone matrix protein gene expression by Runx2 in vivo needs to be investigated further.
REGULATION OF THE PROLIFERATION OF OSTEOBLAST-LINEAGE CELLS BY Runx2
Overexpression of Runx2 using the Prrx1 promoter results in craniosynostosis and limb defects (Maeno et al., 2011). The severity of limb defects is dependent on the expression level of the transgene. Limbs develop through an epithelial-mesenchymal interaction loop formed by fibroblast growth factors (Fgfs) and Fgf receptors (Fgfrs) (Ohuchi et al., 1997; Xu et al., 1998). Fgf10, which is expressed in the mesenchyme, induces Fgf4 and Fgf8 expression in the epithelium through Fgfr2b with high affinity for Fgf10, which is expressed in the epithelium. Fgf4 and Fgf8 induce the proliferation of mesenchymal cells through Fgfr1c and Fgfr2c with high affinity for Fgf4 and Fgf8. In Runx2-overexpressing mice, Fgf4 and Fgf8 expression in the epithelium is impaired, and apical ectodermal ridge (AER) formation is interrupted. These phenotypes are caused by the upregulated expression of Fgfrs with a high affinity for Fgf10 in the mesenchyme. Runx2 induces the expression of Fgfr1, Fgfr2, and Fgfr3 via direct regulation of their promoters (Kawane et al., 2018).
Ctnnb1–/– mice and Sp7–/– mice exhibit similar phenotypes. Both lack osteoblasts, but have abundant osteoprogenitors in the presumptive bone regions (Day et al., 2005; Hill et al., 2005; Hu et al., 2005; Kawane et al., 2018; Nakashima et al., 2002). Although Runx2–/– mice also lack osteoblasts, osteoprogenitors are limited in the presumptive bone regions (Kawane et al., 2018; Komori et al., 1997). Osteoprogenitors in Sp7–/– mice express Runx2 and are actively proliferating. Of note, tibiae and fibulae in Sp7–/– mice are bent due to the accumulation of osteoprogenitors in the perichondrium (Kawane et al., 2018). These findings suggest that Runx2 is required for the expansion of osteoprogenitors. Indeed, Runx2 induced the proliferation of osteoprogenitors originating from wild-type and Sp7–/– mice, and increased Fgf2-induced proliferation. Fgfr2 and Fgfr3 are involved in proliferation, and Fgfr2 plays a major role because its expression level is much higher than that of Fgfr3 in calvaria. The amount of osteoprogenitors and their frequency of proliferation in Sp7–/–Runx2+/– mice are half of those in Sp7–/– mice, indicating that the proliferation of osteoprogenitors is dependent on the gene dosage of Runx2. Thus, Runx2 is required for the proliferation of osteoprogenitors, which is induced via the direct regulation of Fgfr2 and Fgfr3 (Kawane et al., 2018) (Fig. 2).
However, previous reports demonstrated that Runx2 inhibits the proliferation of osteoblast-lineage cells or mesenchymal stem cells in vitro (Galindo et al., 2005; Ghali et al., 2010; Lucero et al., 2013; Pratap et al., 2003; Thomas et al., 2004). Runx2–/– calvarial cells proliferate faster than wild-type calvarial cells in vitro (Kawane et al., 2018). Microarray analysis revealed that the expression of cell cycle-related genes is different between calvaria tissue and calvaria-derived cells in culture in Runx2–/– mice (Kawane et al., 2018). Although the reason why Runx2–/– calvarial cells acquire high proliferation activity in vitro is unclear, Runx2 increases the proliferation of osteoblast progenitors in vivo and in vitro.
MECHANISM OF THE PROLIFERATION OF MESENCHYMAL CELLS AND THEIR COMMITMENT TO OSTEOBLAST LINEAGE CELLS
Calvaria development is a good model to elucidate the mechanism of the differentiation of mesenchymal cells into osteoblasts. Runx2–/– mice have no calvarial bone and only a thin layer of mesenchymal cells (Komori et al., 1997). Runx2+/– mice develop calvarial bone, but the process is delayed and the sutures are not closed (Qin et al., 2019). Cleidocranial dysplasia, which is characterized by open fontanelle and sutures, dysplasia of clavicles, supernumerary teeth, and short stature, is caused by heterozygous mutation of the RUNX2 gene (Lee et al., 1997; Mundlos et al., 1997; Otto et al., 1997). Calvarial bone is formed through intramembranous ossification by osteoblasts, which differentiate from suture mesenchymal cells. Therefore, sutures close through the process of intramembranous ossification, but the posterior frontal suture is an exception. In the posterior frontal suture, mesenchymal condensation occurs at around P7 in mice, the mesenchymal cells differentiate into chondrocytes, the chondrocytes mature, and the cartilage is replaced by bone through endochondral ossification (Bradley et al., 1996; Qin et al., 2019; Sahar et al., 2005).
In Runx2+/– mice, chondrocytes never appear in the posterior frontal suture and the cell density is low in all of the sutures. The suture cells express both Sox9 and Runx2 in wild-type and Runx2+/– mice. The expression level of Sox9 is similar between wild-type and Runx2+/– mice. Indeed, the levels of Runx2 mRNA in suture cells and osteoblasts in Runx2+/– mice are approximately half of the respective level in wild-type mice. The expression level of Runx2 mRNA in suture cells is one-third to half of that in osteoblasts of calvarial bone. The proliferation of suture cells is markedly reduced in Runx2+/– mice compared with in wild-type mice. The expression of hedgehog, Fgf, Wnt, and Phhlh signaling pathway genes, including Gli1, Ptch1, Ihh, Fgfr2, Fgfr3, Tcf7, Wnt10b, and Pth1r, are reduced in the suture of Runx2+/– mice compared with in wild-type mice. Moreover, the expression of these genes is directly regulated by Runx2. However, their expression, as well as that of Sp7, Col1a1, and Bglap2, is not reduced in calvarial bone tissue of Runx2+/– mice compared with that of wild-type mice. This suggests that more than a half-dosage of Runx2 is required for the proliferation of suture mesenchymal cells and their commitment into osteoblast-lineage cells, and for the induction of hedgehog, Fgf, Wnt, and Phhlh signaling pathway genes; however this half-dosage of Runx2 is sufficient for the committed osteoblasts to induce the expression of these signaling pathway genes, Sp7, and bone matrix genes. Furthermore, hedgehog agonists, FGF2, Wnt3a, and Pthlh (1-34) increased calvarial bone formation, whereas antagonists reduced the bone formation and proliferation of suture mesenchymal cells. Therefore, Runx2 induces suture mesenchymal cell proliferation and their commitment into osteoblast-lineage cells by increasing the expression of hedgehog, Fgf, Wnt, and Pthlh signaling pathway genes (Qin et al., 2019) (Fig. 2). Among these signaling pathways, the Fgf signaling pathway likely plays the most important role in the proliferation of mesenchymal cells, osteoprogenitors, and preosteoblasts (Kawane et al., 2018; Qin et al., 2019).
Ihh is required for Runx2 expression (St-Jacques et al., 1999). Fgf2 and Fgf18 increase the capacity of Runx2 for transcriptional activation and stabilize Runx2 protein via phosphorylation though the MAPK pathway, and Runx2 is activated through the PI3K-Akt pathway (Fujita et al., 2004; Ge et al., 2009; Kawane et al., 2018; Park et al., 2010; Xiao et al., 2002). Wnt signaling and Sp7 activate the Runx2 enhancer (Kawane et al., 2014). Furthermore, parathyroid hormone (PTH), which has similar functions to Pthlh, increases Runx2 mRNA and increased its activity through protein kinase A in an osteosarcoma cell line, and anabolic functions of PTH in bone were induced in Runx2-dependent manner in metatarsal organ culture (Krishnan et al., 2003). Therefore, there is mutual regulation between Runx2 and these signaling pathways, including hedgehog, Fgf, Wnt, and Pthlh, or Sp7 (Fig. 2).
FUNCTIONS OF Cbfb IN Runx2-DEPENDENT BONE DEVELOPMENT
Runx1–/– mice and Cbfb–/– mice die at midgestation due to the absence of hematopoiesis in the fetal liver, demonstrating that Cbfb is required for Runx1-dependent hematopoiesis in the fetal liver (Okuda et al., 1996; Sasaki et al., 1996; Wang et al., 1996a; 1996b). To overcome the lethality due to the lack of hematopoiesis, it was partially rescued, confirming the requirement of Cbfb for skeletal development (Kundu et al., 2002; Miller et al., 2002; Yoshida et al., 2002). To more precisely evaluate the functions of Cbfb in skeletal development, several Cbfb conditional knockout mice have been generated using Twist2 Cre, Col2a1 Cre, Sp7 Cre, and Prrx1 Cre (Chen et al., 2014; Fei et al., 2014; Lim et al., 2015; Qin et al., 2015; Wu et al., 2014a; 2014b). These conditional knockout mice demonstrated that Cbfb is required for osteoblast differentiation, and chondrocyte proliferation and maturation. Furthermore, these mice revealed that Cbfb plays an important role in the stabilization of Runx2 protein by protecting it from degradation by ubiquitination (Lim et al., 2015; Qin et al., 2015). However, the capacity of Cbfb for protein stabilization differs among Runx family proteins (Qin et al., 2015). The protein levels of Runx1, Runx2, and Runx3 in cartilaginous skeleton in Cbfb conditional knockout mice using Twist2 Cre were 3%, 13%, and 8% of those in control mice, respectively. Those in calvariae were 7%, 55%, and 25%, respectively. Therefore, the degree of protein reduction is Runx1 > Runx3 > Runx2 in both the cartilaginous limb skeleton and calvaria. Moreover, the degree of reduction was more marked in cartilaginous limb skeleton than in calvaria. The development of calvaria and clavicle was affected more in Runx2+/– mice than in Cbfb conditional knockout mice, whereas the development of endochondral bone was affected more in Cbfb conditional knockout mice than in Runx2+/– mice (Qin et al., 2015). Calvaria and the lateral parts of clavicles are formed through intramembranous ossification (Huang et al., 1997). Therefore, intramembranous ossification is highly dependent on the gene dosage of Runx2, and the role of Cbfb is greater in endochondral ossification than in intramembranous ossification (Qin et al., 2015). This is explained by the lower stability of Runx family proteins in endochondral skeletons than in intramembranous skeletons in the absence of Cbfb, and by the significant roles of Runx1 and Runx3, which are more dependent on Cbfb than Runx2 for protein stability, in endochondral ossification (Qin et al., 2015). The abundance of proteins that can stabilize Runx proteins other than Cbfb may be different among tissues.
Cbfb has two functional isoforms, Cbfb1 and Cbfb2, which are formed by alternative splicing (Ogawa et al., 1993). Cbfb1–/– mice exhibit normal skeletal development, whereas Cbfb2–/– mice have impaired intramembranous and endochondral bone development (Jiang et al., 2016). Cbfb2 is upregulated in Cbfb1–/– mice, but Cbfb1 is not upregulated in Cbfb2–/– mice, resulting in markedly reduced Cbfb expression in Cbfb2–/– mice, but not in Cbfb1–/– mice. This is observed not only in cartilaginous skeletons and calvariae, but also in the liver, thymus, spleen, and heart. However, Cbfb1 has a greater capacity to induce the differentiation of chondrocytes and osteoblasts than Cbfb2. This is caused by the higher ability of Cbfb1 to increase DNA binding by Runx2. In wild-type mice, the expression level of Cbfb2 is three-times higher than that of Cbfb1 in cartilaginous skeletons, calvariae, liver, thymus, and brain. Thus, splicing of Cbfb1 is strictly regulated, and the more potent Cbfb1 and abundant Cbfb2 maintain Runx2 activity at an appropriate level during bone development (Jiang et al., 2016).
CONCLUSION
Hedgehog, Fgf, Wnt, and Pthlh signaling pathways induce Runx2 expression or activate Runx2. Therefore, the proliferation and differentiation of osteoblast-lineage cells are controlled by the reciprocal regulation of Runx2 and these signaling pathways, but not by their cascade (Fig. 2). Although the modification of Runx2 protein for activation is well studied, the transcriptional regulation of the Runx2 gene in chondrocytes and osteoblast-lineage cells remains to be clarified. Detailed elucidation of the interactions among Runx2, Sp7, and hedgehog, Wnt, Fgf, and Pthlh signaling pathways will reveal the general framework of bone development.
Footnotes
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Adhami M.D., Rashid H., Chen H., Javed A. Runx2 activity in committed osteoblasts is not essential for embryonic skeletogenesis. Connect. Tissue Res. 2014;55(Suppl 1):102–106. doi: 10.3109/03008207.2014.923873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J.E., Triffitt J.T. Mesenchymal stem cells and osteoblast differentiation. In: J.P. Bilezikian, L.G. Raisz, G.A. Rodan., editors. Principles of Bone Biology. Academic Press; Cambridge, MA: 2002. pp. 59–81. [DOI] [Google Scholar]
- Bauer O., Sharir A., Kimura A., Hantisteanu S., Takeda S., Groner Y. Loss of osteoblast Runx3 produces severe congenital osteopenia. Mol. Cell. Biol. 2015;35:1097–1109. doi: 10.1128/MCB.01106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J.P., Levine J.P., Roth D.A., McCarthy J.G., Longaker M.T. Studies in cranial suture biology: IV. temporal sequence of posterior frontal cranial suture fusion in the mouse. Plast. Reconstr. Surg. 1996;98:1039–1045. doi: 10.1097/00006534-199611000-00018. [DOI] [PubMed] [Google Scholar]
- Cao K., Wei L., Zhang Z., Guo L., Zhang C., Li Y., Sun C., Sun X., Wang S., Li P., et al. Decreased histone deacetylase 4 is associated with human osteoarthritis cartilage degeneration by releasing histone deacetylase 4 inhibition of runt-related transcription factor-2 and increasing osteoarthritis-related genes: a novel mechanism of human osteoarthritis cartilage degeneration. Arthritis Res. Ther. 2014;16:491. doi: 10.1186/s13075-014-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catheline S.E., Hoak D., Chang M., Ketz J.P., Hilton M.J., Zuscik M.J., Jonason J.H. Chondrocyte-specific RUNX2 overexpression accelerates post-traumatic osteoarthritis progression in adult mice. J. Bone Miner. Res. 2019;34:1676–1689. doi: 10.1002/jbmr.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Ma J., Zhu G., Jules J., Wu M., McConnell M., Tian F., Paulson C., Zhou X., Wang L., et al. Cbfbeta deletion in mice recapitulates cleidocranial dysplasia and reveals multiple functions of Cbfbeta required for skeletal development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:8482–848. doi: 10.1073/pnas.1310617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T.F., Guo X., Garrett-Beal L., Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A.L., Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Enomoto H., Enomoto-Iwamoto M., Iwamoto M., Nomura S., Himeno M., Kitamura Y., Kishimoto T., Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Fei T., Mengrui W., Lianfu D., Guochun Z., Junqing M., Bo G., Lin W., Yi-Ping L., Wei C. Core binding factor beta (Cbfβ) controls the balance of chondrocyte proliferation and differentiation by upregulating Indian hedgehog (Ihh) expression and inhibiting parathyroid hormone-related protein receptor (PPR) expression in postnatal cartilage and bone formation. J. Bone Miner. Res. 2014;29:1564–1574. doi: 10.1002/jbmr.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Azuma Y., Fukuyama R., Hattori Y., Yoshida C., Koida M., Ogita K., Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004;166:85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo M., Pratap J., Young D.W., Hovhannisyan H., Im H.J., Choi J.Y., Lian J.B., Stein J.L., Stein G.S., van Wijnen A.J. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J. Biol. Chem. 2005;280:20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C., Xiao G., Jiang D., Yang Q., Hatch N.E., Roca H., Franceschi R.T. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J. Biol. Chem. 2009;284:32533–32543. doi: 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali O., Chauveau C., Hardouin P., Broux O., Devedjian J.C. TNF-α's effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J. Bone Miner. Res. 2010;25:1616–1626. doi: 10.1002/jbmr.52. [DOI] [PubMed] [Google Scholar]
- Harada H., Tagashira S., Fujiwara M., Ogawa S., Katsumata T., Yamaguchi A., Komori T., Nakatsuka M. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J. Biol. Chem. 1999;274:6972–6978. doi: 10.1074/jbc.274.11.6972. [DOI] [PubMed] [Google Scholar]
- Hess J., Porte D., Munz C., Angel P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J. Biol. Chem. 2001;276:20029–20038. doi: 10.1074/jbc.M010601200. [DOI] [PubMed] [Google Scholar]
- Hill T.P., Spater D., Taketo M.M., Birchmeier W., Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kugimiya F., Fukai A., Saito T., Yano F., Ikeda T., Mabuchi A., Sapkota B.R., Akune T., Nishida N., et al. C/EBPbeta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2alpha as the inducer in chondrocytes. Hum. Mol. Genet. 2012;21:1111–1123. doi: 10.1093/hmg/ddr540. [DOI] [PubMed] [Google Scholar]
- Hu H., Hilton M.J., Tu X., Yu K., Ornitz D.M., Long F. Sequential roles of hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Huang L.F., Fukai N., Selby P.B., Olsen B.R., Mundlos S. Mouse clavicular development: analysis of wild-type and cleidocranial dysplasia mutant mice. Dev. Dyn. 1997;210:33–40. doi: 10.1002/(SICI)1097-0177(199709)210:1<33::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N., et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Kitagaki J., Tamamura Y., Gentili C., Koyama E., Enomoto H., Komori T., Pacifici M., Enomoto-Iwamoto M. Runx2 expression and action in chondrocytes are regulated by retinoid signaling and parathyroid hormone-related peptide (PTHrP) Osteoarthr. Cartil. 2003;11:6–15. doi: 10.1053/joca.2002.0860. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Qin X., Kawane T., Komori H., Matsuo Y., Taniuchi I., Ito K., Izumi S.I., Komori T. Cbfb2 isoform dominates more potent Cbfb1 and is required for skeletal development. J. Bone Miner. Res. 2016;31:1391–1404. doi: 10.1002/jbmr.2814. [DOI] [PubMed] [Google Scholar]
- Jimenez M.J., Balbin M., Lopez J.M., Alvarez J., Komori T., Lopez-Otin C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol. Cell. Biol. 1999;19:4431–4442. doi: 10.1128/MCB.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamekura S., Kawasaki Y., Hoshi K., Shimoaka T., Chikuda H., Maruyama Z., Komori T., Sato S., Takeda S., Karsenty G., et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- Kawane T., Komori H., Liu W., Moriishi T., Miyazaki T., Mori M., Matsuo Y., Takada Y., Izumi S., Jiang Q., et al. Dlx5 and mef2 regulate a novel Runx2 enhancer for osteoblast-specific expression. J. Bone Miner. Res. 2014;29:1960–1969. doi: 10.1002/jbmr.2240. [DOI] [PubMed] [Google Scholar]
- Kawane T., Qin X., Jiang Q., Miyazaki T., Komori H., Yoshida C.A., Matsuura-Kawata V., Sakane C., Matsuo Y., Nagai K., et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci. Rep. 2018;8:13551. doi: 10.1038/s41598-018-31853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I.S., Otto F., Zabel B., Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 1999;80:159–170. doi: 10.1016/S0925-4773(98)00210-X. [DOI] [PubMed] [Google Scholar]
- Komori T. Runx2, a multifunctional transcription factor in skeletal development. J. Cell. Biochem. 2002;87:1–8. doi: 10.1002/jcb.10276. [DOI] [PubMed] [Google Scholar]
- Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018;149:313–323. doi: 10.1007/s00418-018-1640-6. [DOI] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.H., Inada M., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Moore T.L., Ma Y.L., Helvering L.M., Frolik C.A., Valasek K.M., Ducy P., Geiser A.G. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol. Endocrinol. 2003;17:423–435. doi: 10.1210/me.2002-0225. [DOI] [PubMed] [Google Scholar]
- Kundu M., Javed A., Jeon J.P., Horner A., Shum L., Eckhaus M., Muenke M., Lian J.B., Yang Y., Nuckolls G.H., et al. Cbfbeta interacts with Runx2 and has a critical role in bone development. Nat. Genet. 2002;32:639–644. doi: 10.1038/ng1050. [DOI] [PubMed] [Google Scholar]
- Lee B., Thirunavukkarasu K., Zhou L., Pastore L., Baldini A., Hecht J., Geoffroy V., Ducy P., Karsenty G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat. Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- Lefebvre V.R., Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Liao L., Zhang S., Gu J., Takarada T., Yoneda Y., Huang J., Zhao L., Oh C.D., Li J., Wang B., et al. Deletion of Runx2 in articular chondrocytes decelerates the progression of DMM-induced osteoarthritis in adult mice. Sci. Rep. 2017;7:2371. doi: 10.1038/s41598-017-02490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.E., Park N.R., Che X., Han M.S., Jeong J.H., Kim S.Y., Park C.Y., Akiyama H., Kim J.E., Ryoo H.M., et al. Core binding factor beta of osteoblasts maintains cortical bone mass via stabilization of Runx2 in mice. J. Bone Miner. Res. 2015;30:715–722. doi: 10.1002/jbmr.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F., Chung U.I., Ohba S., McMahon J., Kronenberg H.M., McMahon A.P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- Lucero C.M., Vega O.A., Osorio M.M., Tapia J.C., Antonelli M., Stein G.S., Van Wijnen A.J., Galindo M.A. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J. Cell. Physiol. 2013;228:714–723. doi: 10.1002/jcp.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Moriishi T., Yoshida C.A., Komori H., Kanatani N., Izumi S., Takaoka K., Komori T. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone. 2011;49:673–682. doi: 10.1016/j.bone.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Maruyama Z., Yoshida C.A., Furuichi T., Amizuka N., Ito M., Fukuyama R., Miyazaki T., Kitaura H., Nakamura K., Fujita T., et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev. Dyn. 2007;236:1876–1890. doi: 10.1002/dvdy.21187. [DOI] [PubMed] [Google Scholar]
- Miller J., Horner A., Stacy T., Lowrey C., Lian J.B., Stein G., Nuckolls G.H., Speck N.A. The core-binding factor beta subunit is required for bone formation and hematopoietic maturation. Nat. Genet. 2002;32:645–649. doi: 10.1038/ng1049. [DOI] [PubMed] [Google Scholar]
- Mundlos S., Otto F., Mundlos C., Mulliken J.B., Aylsworth A.S., Albright S., Lindhout D., Cole W.G., Henn W., Knoll J.H., et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/S0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- Ohuchi H., Nakagawa T., Yamamoto A., Araga A., Ohata T., Ishimaru Y., Yoshioka H., Kuwana T., Nohno T., Yamasaki M., et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S.W., Grosveld G., Downing J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/S0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Otto F., Thornell A.P., Crompton T., Denzel A., Gilmour K.C., Rosewell I.R., Stamp G.W., Beddington R.S., Mundlos S., Olsen B.R., et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Owens T.W., Rogers R.L., Best S.A., Ledger A., Mooney A.M., Ferguson A., Shore P., Swarbrick A., Ormandy C.J., Simpson P.T., et al. Runx2 is a novel regulator of mammary epithelial cell fate in development and breast cancer. Cancer Res. 2014;74:5277–5286. doi: 10.1158/0008-5472.CAN-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park O.J., Kim H.J., Woo K.M., Baek J.H., Ryoo H.M. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J. Biol. Chem. 2010;285:3568–3574. doi: 10.1074/jbc.M109.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap J., Galindo M., Zaidi S.K., Vradii D., Bhat B.M., Robinson J.A., Choi J.Y., Komori T., Stein J.L., Lian J.B. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63:5357–5362. [PubMed] [Google Scholar]
- Qin X., Jiang Q., Matsuo Y., Kawane T., Komori H., Moriishi T., Taniuchi I., Ito K., Kawai Y., Rokutanda S., et al. Cbfb regulates bone development by stabilizing Runx family proteins. J. Bone Miner. Res. 2015;30:706–714. doi: 10.1002/jbmr.2379. [DOI] [PubMed] [Google Scholar]
- Qin X., Jiang Q., Miyazaki T., Komori T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum. Mol. Genet. 2019;28:896–911. doi: 10.1093/hmg/ddy386. [DOI] [PubMed] [Google Scholar]
- Rodda S.J., McMahon A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Sahar D.E., Longaker M.T., Quarto N. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev. Biol. 2005;280:344–361. doi: 10.1016/j.ydbio.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yagi H., Bronson R.T., Tominaga K., Matsunashi T., Deguchi K., Tani Y., Kishimoto T., Komori T. Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12359–12363. doi: 10.1073/pnas.93.22.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamurugan N., Pulumati M.R., Tyson D.R., Partridge N.C. Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor alpha1. J. Biol. Chem. 2000;275:5037–5042. doi: 10.1074/jbc.275.7.5037. [DOI] [PubMed] [Google Scholar]
- Simpson F., Kerr M.C., Wicking C. Trafficking, development and hedgehog. Mech. Dev. 2009;126:279–288. doi: 10.1016/j.mod.2009.01.007. [DOI] [PubMed] [Google Scholar]
- St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., de Andres M.C., Hashimoto K., Itoi E., Otero M., Goldring M.B., Oreffo R.O.C. DNA methylation of the RUNX2 P1 promoter mediates MMP13 transcription in chondrocytes. Sci. Rep. 2017;7:7771. doi: 10.1038/s41598-017-08418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada T., Hinoi E., Nakazato R., Ochi H., Xu C., Tsuchikane A., Takeda S., Karsenty G., Abe T., Kiyonari H., et al. An analysis of skeletal development in osteoblast-specific and chondrocyte-specific runt-related transcription factor-2 (Runx2) knockout mice. J. Bone Miner. Res. 2013;28:2064–2069. doi: 10.1002/jbmr.1945. [DOI] [PubMed] [Google Scholar]
- Takeda S., Bonnamy J.P., Owen M.J., Ducy P., Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi I., Osato M., Egawa T., Sunshine M.J., Bae S.C., Komori T., Ito Y., Littman D.R. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/S0092-8674(02)01111-X. [DOI] [PubMed] [Google Scholar]
- Tetsunaga T., Nishida K., Furumatsu T., Naruse K., Hirohata S., Yoshida A., Saito T., Ozaki T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr. Cartil. 2011;19:222–232. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu K., Pei Y., Wei T. Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol. Biol. Rep. 2007;34:225–231. doi: 10.1007/s11033-006-9037-3. [DOI] [PubMed] [Google Scholar]
- Thomas D.M., Johnson S.A., Sims N.A., Trivett M.K., Slavin J.L., Rubin B.P., Waring P., McArthur G.A., Walkley C.R., Holloway A.J. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J. Cell Biol. 2004;167:925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta C., Enomoto-Iwamoto M., Kanatani N., Yoshida C., Liu Y., Enomoto-Iwamoto M., Ohmori T., Enomoto H., Nakata K., Takada K., et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 2001;153:87–100. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A., Lee K., Lanske B., Segre G.V., Kronenberg H.M., Tabin C.J. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science (New York, NY) 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wang Q., Stacy T., Binder M., Marin-Padilla M., Sharpe A.H., Speck N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 1996a;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Stacy T., Miller J.D., Lewis A.F., Gu T.L., Huang X., Bushweller J.H., Bories J.C., Alt F.W., Ryan G., et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell. 1996b;87:697–708. doi: 10.1016/S0092-8674(00)81389-6. [DOI] [PubMed] [Google Scholar]
- Wang X., Manner P.A., Horner A., Shum L., Tuan R.S., Nuckolls G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wu M., Li C., Zhu G., Wang Y., Jules J., Lu Y., McConnell M., Wang Y.J., Shao J.Z., Li Y.P., et al. Deletion of core-binding factor beta (Cbfbeta) in mesenchymal progenitor cells provides new insights into Cbfbeta/Runxs complex function in cartilage and bone development. Bone. 2014a;65:49–59. doi: 10.1016/j.bone.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Li Y.P., Zhu G., Lu Y., Wang Y., Jules J., McConnell M., Serra R., Shao J.Z., Chen W. Chondrocyte-specific knockout of Cbfbeta reveals the indispensable function of Cbfbeta in chondrocyte maturation, growth plate development and trabecular bone formation in mice. Int. J. Biol. Sci. 2014b;10:861–872. doi: 10.7150/ijbs.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Jiang D., Gopalakrishnan R., Franceschi R.T. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J. Biol. Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- Xiao Z.S., Hjelmeland A.B., Quarles L.D. Selective deficiency of the "bone-related" Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J. Biol. Chem. 2004;279:20307–20313. doi: 10.1074/jbc.M401109200. [DOI] [PubMed] [Google Scholar]
- Xu X., Weinstein M., Li C., Naski M., Cohen R.I., Ornitz D.M., Leder P., Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yoshida C.A., Furuichi T., Fujita T., Fukuyama R., Kanatani N., Kobayashi S., Satake M., Takada K., Komori T. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat. Genet. 2002;32:633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- Yoshida C.A., Komori H., Maruyama Z., Miyazaki T., Kawasaki K., Furuichi T., Fukuyama R., Mori M., Yamana K., Nakamura K., et al. SP7 inhibits osteoblast differentiation at a late stage in mice. PLoS One. 2012;7:e32364. doi: 10.1371/journal.pone.0032364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C.A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K., Yamana K., Zanma A., Takada K., Ito Y., et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]