Abstract
Objective:
To evaluate the relationship between blood-brain-barrier (BBB) disruption and transient neurological deficits (TNDs) following neuroendovascular interventions (NEIs) using post-contrast T2/FLAIR (pcFLAIR) imaging.
Methods:
This is a prospective study of 41 consecutive patients undergoing flow diversion therapy for unruptured aneurysm treatment. Patients underwent post-procedural MR imaging within 24-hours of the procedure including DWI and pcFLAIR sequences. Regression analyses were performed to identify risk factors for developing TNDs.
Results:
13 patients (31.7%) developed neurologic complications ranging from visual field defects to dense hemiplegia. All deficits were transient, resolving spontaneously within 72 hours. 5 of 13 patients (38.5%) with TNDs had presence of DWI lesions while the remaining 8 patients (61.5%) did not. In contrast, all patients who developed TNDs had leptomeningeal enhancement (LME) on pcFLAIR imaging, and no patient with normal pcFLAIR imaging developed TNDs. Regression analysis revealed the extent of pcFLAIR enhancement is associated with development of post-procedure neurological deficits (p<0.0001). Video-EEG monitoring was performed in 4 symptomatic patients manifesting severe deficits. In all instances EEG demonstrates ipsilateral hemispheric slowing and eventual resolution corresponding to ensuing clinical improvement. Only one of these 4 patients presented with a lesion on DWI.
Conclusion:
This study challenges conventional dogma that TNDs are ischemic in etiology and suggests BBB impairment may be a potential alternative mechanism. These findings are applicable to stroke and other reversible neurological diseases.
Keywords: transient neurological deficits, blood-brain barrier, stroke, FLAIR imaging, Angiography, Stent, Cerebral Aneurysm, Cerebrovascular Procedures
Introduction
Neuroendovascular intervention (NEI) is an important modality for treatment of intracranial aneurysms. Neurologic complications following NEI are surprisingly common, occurring in up to 37.6% of patients1, 2. Most neurologic deficits are transient and conventionally attributed to ischemic events; however, diffusion weighted imaging (DWI) of symptomatic patients to evaluate suspected ischemic burden is often unrevealing1, 2. Contrast-induced encephalopathy is a rare yet widely reported entity that presents most commonly after angiography. Patients manifest clinically with transient neurological deficits (TNDs) ranging from cortical blindness to hemiplegia and aphasia experiencing complete resolution typically within 5 days without treatment3. Radiographic findings of contrast induced encephalopathy characteristically identifies leptomeningeal enhancement (LME) from contrast extravasation consistent with blood-brain-barrier (BBB) impairment3,4. This finding suggests that other non-ischemic mechanisms may contribute to post-procedural neurological deficits in some cases and exposes the need for a reliable imaging marker of cerebrovascular dysfunction following NEI. Post-contrast FLAIR (pcFLAIR) imaging has been established as an important radiographic marker of BBB impairment after ischemic stroke5. The presence of LME on pcFLAIR imaging increases the risk of reperfusion hemorrhage and indicates poorer neurological outcomes after ischemic stroke due to an incompetent BBB5. Therefore in this study, we utilize pcFLAIR imaging to evaluate the incidence of BBB disruption after NEI and investigate the correlation with development of reversible neurological complications post-procedure.
Methods:
This is a single-center prospective cohort study of 41 patients enrolled between July 2016 and November 2017 at the University of Wisconsin. All patients underwent post-NEI pcFLAIR MR-imaging within 24-hours of the procedure. This study was approved by the hospital’s institutional review board. Informed consent for treatment and post-operative management was obtained from the patient in all cases. The following data were collected for each patient: age, sex, aneurysm size, number of stents used, presence of hypertension, diabetes, smoking, presence of DWI, and extent of LME on pcFLAIR imaging.
Magnetic resonance (MR) examinations were performed within 24-hours of the procedure on 1.5T and 3T systems (GE Healthcare, Waukesha, WI) based on scanner availability. The imaging protocol consisted of a comprehensive MRI without and with contrast as well as time-of-flight MR angiogram of the head without contrast. This protocol included a post-contrast 3D sagittal T2-weighted FLAIR (TE 135 ms; TR 6000 ms; inversion time 1700 ms; slice thickness 1.6mm acquisition matrix 256 x 256). Post-contrast FLAIR (pcFLAIR) was obtained after the administration of (0.1 mmol/kg) of intravenous gadobenate dimeglumine contrast (Multihance; Bracco Diagnostics, Princeton, NJ). FLAIR sequences were read independently by one neuroradiologist and one neurological surgeon (both blinded) and the extent of pcFLAIR LME was scored as none, focal, lobar, or hemispheric to reflect the severity of BBB disruption.
Grading of LME was categorized as follows: none, focal (involving a few sulci but less than half of the ipsilateral lobule or cerebellar hemisphere), lobar (involving more than half of the ipsilateral lobule or cerebellar hemisphere), or hemispheric (involving more than half of at least two out of the four ipsilateral lobes or both cerebellar hemispheres). Further illustrations and examples are depicted in Figure 1.
Figure 1:
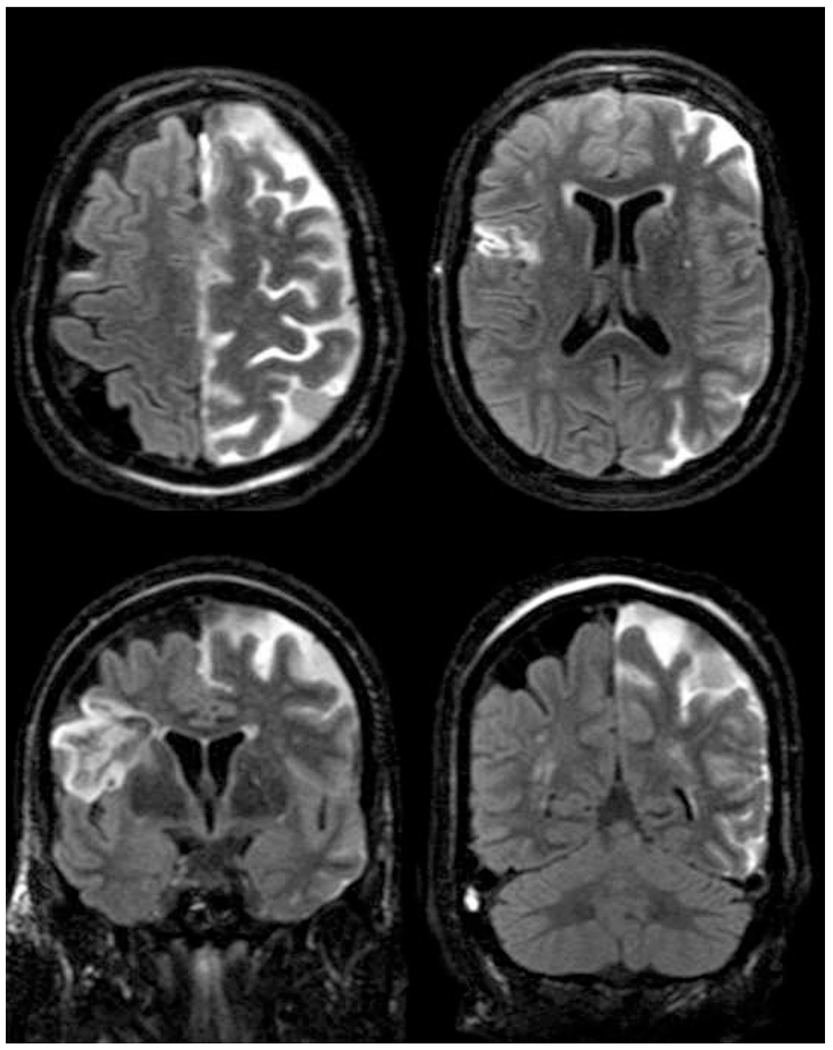
74 year old woman following Pipeline embolization of a dysplastic left ophthalmic segment internal carotid artery aneurysm. Axial (A,B) and coronal (C,D) pcFLAIR sequences demonstrate diffuse leptomeningeal T2/FLAIR signal hyper intensity throughout the left hemisphere. Note there is laminar necrosis in the right frontal lobe from a chronic prior infarct.
Statistical measurements were calculated using SAS analytics software (SAS Institute, Cary, NC) with assistance from staff of the University of Wisconsin Biostatistics Department. Univariate analyses were performed using the Student’s t-test or Fisher’s exact test as appropriate. Logistic regression and Chi-Square test were used to assess the impact of multiple variables on the binary presence of post-operative deficits. Statistical significance was defined as a P-value of < 0.05.
Results:
Table-1 demonstrates patient data, demographics, and imaging findings. We included 41 patients (5 males) who underwent MRI within 24-hours of NEI. The mean patient age was 59 ± 14 years. The average aneurysm size was 6mm and ranged from 3-24mm in diameter. A total of 53 flow diverting stents (average 1.3 per patient) were used during NEI to achieve adequate embolization of 43 aneurysms.
Table 1:
Baseline patient characteristics.
| Parameter | Total | (−) neurologic deficit | (+) neurologic deficit |
|---|---|---|---|
| Sex | 5 male 36 female | 3 male 25 female | 2 male 11 female |
| Age | 58.9 ± 14.3 | 56.7 ± 14.0 | 63.5 ± 14.2 |
| Number of stents | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.4 ± 0.5 |
| Aneurysm size | 6.0 ± 3.8 | 6.1 ± 4.6 | 6.0 ± 1.7 |
| Hypertension | 17 (41.5%) | 10 (35.7%) | 7 (53.8%) |
| Diabetes | 5 (12.2%) | 3 (10.7%) | 2 (15.4%) |
| Smoking | 11 (26.8%) | 6 (21.4%) | 5 (38.5%) |
| Presence of FLAIR | 33 (80.5%) | 20 (71.4%) | 13 (100%) |
| Presence of DWI | 8 (19.5%) | 8 (28.6%) | 5 (38.5%) |
13 patients (31.7%) developed neurological deficits including: cortical blindness (1), hemianopia (4), aphasia and agitation (3), arm weakness (1), dense hemiplegia with or without aphasia (4). All neurological deficits were transient, resolving spontaneously within 72 hours. No patient had residual deficits on discharge between 1-6 days post-operatively.
Among patients who were symptomatic, 5 of 13 (38.4%) had presence of DWI lesions on MRI while the remaining 8 (61.5%) did not. No patient had evidence of cortical or subcortical infarct diameter greater than 0.5cm. 8 patients (19.5%) developed scattered punctate foci of lesions on DWI, none of which could fully account for clinical findings based on the extent or location.
34 patients (83%) developed ipsilateral pcFLAIR LME verified independently by one neuroradiologists and one neurological surgeon. The aggregate ratings of each reviewer is presented in Table 2. The inter-rater reliability of our proposed grading system was 90.2% (Table 3). All patients with TNDs developed LME and no patient with the absence of LME developed TNDs (Table 4). The diagnostic utility of pcFLAIR for predicting neurological deficits are presented in Table 3. On univariate analyses, extent of LME enhancement is associated with the development of post-procedure neurological deficits (p<0.05), whereas the presence of DWI lesions did not. The likelihood of developing neurological deficits with increasing extents of LME is 0% in the absence of LME and 16.7%, 36.4%, and 90% for focal, lobar, and hemispheric grades respectively (Table 2).
Table 2:
Extent of LME and relationship with developing neurologic deficits. P value of < 0.05 was considered significant.
| Extent of LME (2 raters) | (−) neurologic deficit (28) | (+) neurologic deficit (13) | Chance of neurologic deficit (%) | P value |
|---|---|---|---|---|
| None (0) | 15 | 0 | 0 | 0.0001 |
| Focal (1) | 20 | 4 | 16.7 | |
| Lobar (2) | 20 | 13 | 36.4 | |
| Hemispheric (3) | 1 | 9 | 90% | |
| Total | 56 | 26 | 82 |
Table 3 –
Inter-rater reliability
| Grade | Rater 1 | Rater 2 |
|---|---|---|
| 0 | 7 | 8 |
| 1 | 13 | 11 |
| 2 | 16 | 17 |
| 3 | 5 | 5 |
| Total | 41 | 41 |
| 90.20% | ||
| Inter-rater reliability | ||
Table 4:
pcFLAIR diagnostic utility. (PPV – Positive predictive value, NPV – Negative predictive value).
| (+) neurological deficits | (−) neurological deficits | Total | ||
|---|---|---|---|---|
| (+) pcFLAIR | 13 | 20 | 33 | 39% PPV |
| (−) pcFLAIR | 0 | 8 | 8 | 100% NPV |
| Total | 13 | 28 | 41 | |
| 100% sensitivity | 29% specificity |
Video EEG monitoring was performed in 4 of the 13 symptomatic patients whose presenting symptom was dense hemiparesis with or without aphasia due to clinical necessity and therefore data is not available for all patients. In all 4 cases, continuous EEG demonstrated temporary ipsilateral hemispheric slowing that resolved prior to ensuing clinical improvement. Only 1 of 4 of these patients presented with a lesion on DWI or MRI (Figure 2).
Figure 2:
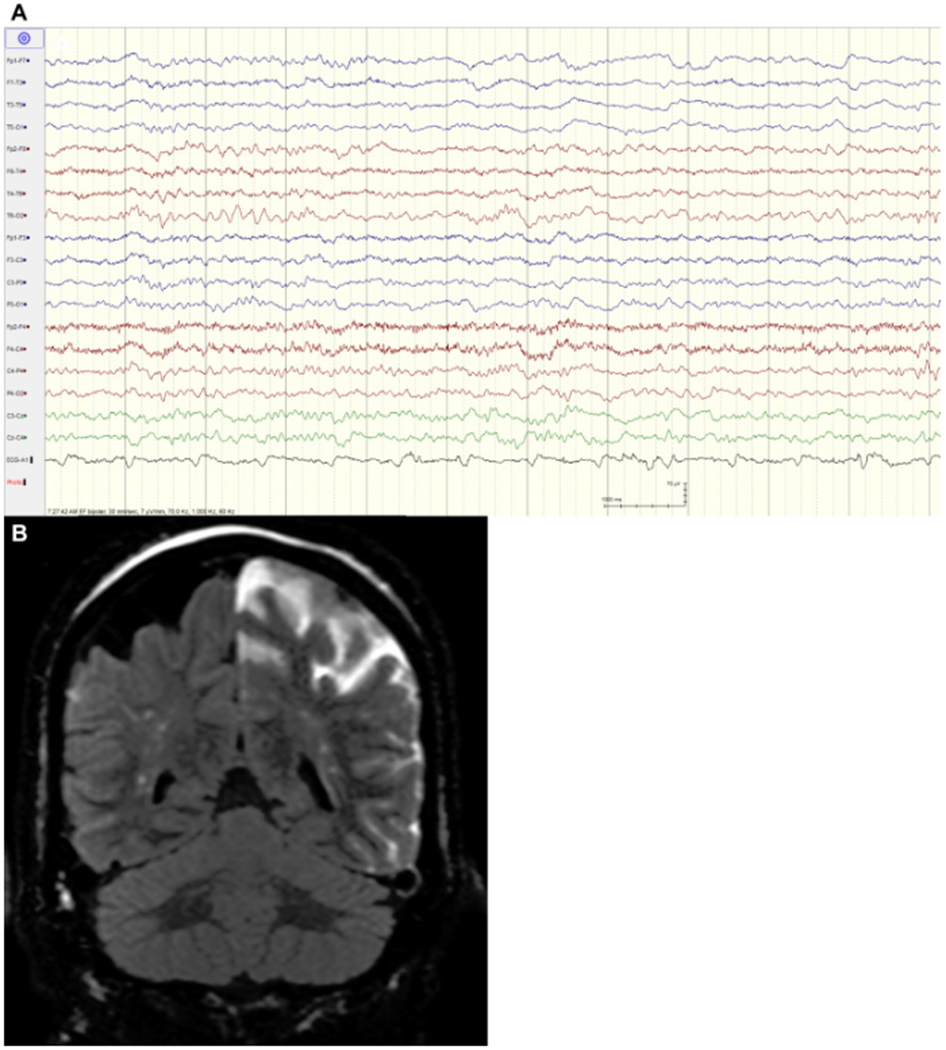
A 69-year-old woman who underwent Pipeline embolization of a left posterior communicating artery aneurysm presenting with confusion, aphasia, and right hemiparesis. Electroencephalography (A) demonstrates left hemispheric slowing. Postcontrast fluid-attenuated inversion recovery magnetic resonance imaging coronal (B) image demonstrates extensive hemispheric leptomeningeal enhancement consistent with significant blood–brain barrier impairment. There was no evidence of parent vessel thrombosis on angiography or stroke on diffusion-weighted imaging.
Discussion:
Neurological deficits following NEI are surprisingly common, yet the etiology of these events remains poorly understood. In this prospective study, we identified BBB impairment manifesting radiographically as LME on pcFLAIR imaging5 as an independent predictor for developing transient neurological deficits post-NEI (p<0.0001).
In contrast to our findings, only 5 of 13 (38.4%) patients who developed TNDs had lesions on DWI. Traditionally neurological complications were thought to be ischemic in nature. However, in our study DWI was not associated with development of neurologic complications on statistical analysis, consistent with prior studies4, 5. DWI is the gold standard for identifying ischemic stroke and therefore these findings suggest alternative mechanisms may explain transient neurological deficits seen after NEI.
PcFLAIR imaging augments visualization of the leptomeninges and enables interrogation of BBB permeability. This technique has previously been shown to identify BBB breakdown following ischemic stroke4–6. In the post-NEI setting, the differential diagnosis of pcFLAIR LME includes subarachnoid hemorrhage, BBB disruption, and cerebral dysautoregulation7, 8. In our cohort, no patient who had both pre- and pcFLAIR imaging manifested LME on pre-contrast imaging, thereby supporting BBB disruption as the most likely etiology.
Previous neuroimaging research has identified findings consistent with BBB disruption following NEI. For instance, transient cortical hyper-attenuation on CT scans was seen in 49% of patients immediately after uncomplicated NEI, which the authors hypothesized was due to extravasation of contrast used during the NEI procedure secondary to disruption of the BBB8. What leads to BBB hyperpermeability to gadolinium following NEI? We examined the correlation between many variables that could be reasonably thought to predispose to BBB breakdown, including aneurysm size, hypertension, diabetes, smoking, and age. However, we did not identify significant associations between these variables with the development of pcFLAIR LME. This may be due to the small sample size of this study. Additional research using larger patient cohorts could elucidate predisposing or protective factors for development of BBB disruption.
The BBB is essential in preventing entry of toxic substances into the cerebral cortex. Disruption of the BBB increases vascular permeability to a wide range of potentially neurotoxic drugs and metabolites into the central nervous system, potentially leading to cortical dysfunction and may explain the radiographic and clinical findings observed after intra-arterial contrast administration seen during contrast-induced encephalopathy. Additionally, we found that four patients who had EEG testing for workup of severe neurological symptoms had diffuse ipsilateral slowing that eventually resolved prior to symptomatic improvement. This finding would be extremely atypical in the absence of large vessel occlusion or large territorial ischemic stroke. We hypothesize that BBB disruption is reversible and ultimately regains its ability to auto-regulate the permeability of neurotoxic metabolites thereby leading to eventual clinical recovery.
Our reported findings are of immediate clinical utility in the management neurological complications post-NEI. We confirm pcFLAIR as an important imaging biomarker of BBB disruption which may serve as an indicator of potential transient nature of any neurological deficits experienced post-procedure. Additionally, the implication of a link between TNDs and BBB impairment suggests agents targeting the BBB may be protective of neurological complications from NEI.
Future animal and large clinical studies are needed to elucidate the causative factors of BBB breakdown following NEI, which may help explain the pathophysiology behind TNDs seen after ischemic stroke, NEIs, hyper-perfusion syndromes, drug induced encephalopathy, and other inflammatory cerebral dysautoregulation syndromes such as posterior reversible encephalopathy syndrome.
Conclusion:
This study challenges conventional dogma that TNDs are ischemic in etiology and suggests BBB impairment as a potential alternative mechanism in some cases. These findings are applicable to stroke and other reversible neurological diseases and provides an avenue for future research.
Highlights:
Diffusion Weighted Imaging does not fully explain the present or severity of neurological deficits after cerebral angiographic procedures
Leptomeningeal enhancement seen on post-contrast FLAIR imaging is consistent with impairment of the blood-brain-barrier
Blood-brain-barrier dysfunction may facilitate the development of neurological complications after cerebral angiography procedures
Acknowledgment:
The authors would like to thank the staff of the University of Wisconsin Carbone Cancer Center (UWCCC) Biostatistics Shared Resource for their valuable contributions to this research. Shared research services at the UWCCC are supported by Cancer Center Support Grant P30 CA014520.
Funding: No sources of funding were provided for this study.
Abbreviations:
- TND
Transient neurological deficits
- IA
intracranial aneurysm
- SAH
subarachnoid hemorrhage
- BBB
blood brain barrier
- DWI
diffusion weighted imaging
- NEI
neuro endovascular intervention
- LME
leptomeningeal enhancement
Footnotes
Disclosure: The authors have no conflict of interest to disclose.
References:
- 1.Pierot L, Spelle L, Vitry F, Investigators A. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39:2497–2504. [DOI] [PubMed] [Google Scholar]
- 2.Soeda A, Sakai N, Murao K, et al. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol 2003;24:2035–2038. [PMC free article] [PubMed] [Google Scholar]
- 3.Dangas G, Monsein LH, Laureno R, et al. Transient contrast encephalopathy after carotid artery stenting. J Endovasc Ther. 2001;8:111–113. [DOI] [PubMed] [Google Scholar]
- 4.Ozturk A, Saatci I, Pamuk AG, et al. Focal increased cortical density in immediate postembolization CT scans of patients with intracranial aneurysms. AJNR Am J Neuroradiol 2006;27:1866–1875. [PMC free article] [PubMed] [Google Scholar]
- 5.Suthiphosuwan S, Hsu CC, Bharatha A, HARMless: Transient Cortical and Sulcal Hyperintensity on Gadolinium-Enhanced FLAIR after Elective Endovascular Coiling of Intracranial Aneurysms, AJNR Am J Neuroradiol (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latour LL, Kang D-W, Ezzeddine MA, Chalela JA, Warach S. Early Blood-Brain Barrier Disruption in Human Focal Brain Ischemia. Annals of Neurology 2004;56:468–477. [DOI] [PubMed] [Google Scholar]
- 7.Warach S, Latour LL. Evidence of Reperfusion Injury, Exacerbated by Thrombolytic Therapy, in Human Focal Brain Ischemia Using a Novel Imaging Marker of Early Blood-Brain Barrier Disruption. Stroke 2004;35:2659–2661. [DOI] [PubMed] [Google Scholar]
- 8.Berge J, Tourdias T, Moreau JF, Barreau X, Dousset V. Perianeurysmal brain inflammation after flow-diversion treatment. AJNR Am J Neuroradiol 2011;32:1930–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]


