Abstract
Context:
Venous thromboembolism (VTE) is a devastating complication of intracranial tumor surgery. The present study helps identify patients at the greatest risk of developing VTE.
Aims:
The aim of the study was to evaluate the incidence of and risk factors for VTE following craniotomy for intracranial tumors.
Setting and Designs:
This was a retrospective cohort study.
Methods:
Data from the institutional database (between January 2017 and December 2018) were reviewed. Consecutive patients with intracranial tumors who underwent craniotomy were included.
Statistical Analysis Used:
Patient characteristics were reported as descriptive data, and factors associated with VTE development were analyzed by the Cox regression model.
Results:
The study identified 177 patients. The incidence of VTE was 10.2% (deep-vein thrombosis [DVT], 8.5%; pulmonary embolism [PE] 1.7%; and simultaneous DVT and PE, 1.7%). In univariate analysis, VTE development was associated with diabetes mellitus (DM), operative duration of >420 min, blood transfusion, and new-onset postoperative motor deficits. DM and new-onset postoperative motor deficits were statistically significant factors in multivariable analysis, with hazard ratios of 4.52 (95% confidence interval [CI] = 1.38–14.82) and 3.46 (95% CI = 1.17–10.23), respectively.
Conclusions:
Postcraniotomy VTE was detected in 10.2% of patients with intracranial tumors. Risk factors for VTE included DM and new-onset postoperative motor deficits. Hence, intracranial tumor patients with these risk factors are the most likely to require VTE prophylaxis with an anticoagulant.
Keywords: Craniotomy, diabetes mellitus, intracranial tumor, motor deficit, venous thromboembolism
Introduction
Venous thromboembolism (VTE), including deep-vein thrombosis (DVT) and pulmonary embolism (PE), is a common postoperative complication.[1] It increases the economic burden[2] and leads to patient morbidity and mortality.[3,4] The pathophysiology of VTE – known as the Virchow's triad – involves immobilization, hypercoagulable state, and endothelial injury. Therefore, neurosurgery patients, particularly those with brain tumors, are at a high risk of developing VTE due to many reasons, such as muscle weakness, pro-coagulation factor secretion by tumor cells, and vascular injury following surgery.[5,6]
There has been extensive research on VTE in neurosurgery over the past two decades.[7] In a previous study, hospitalized patients with brain tumors, specifically those treated with surgery, showed a high incidence of VTE.[8] Recently, Rinaldo et al. reported a VTE incidence of 0.5%–42.6% from their literature review.[9] VTE development is associated with a number of factors related to patient characteristics, tumor characteristics, and treatment.[6] Therefore, recent VTE guidelines have classified brain tumor patients requiring anticoagulants for VTE prophylaxis into the high-risk group.[10,11,12] Although these strategies decrease VTE incidence, they increase the risk of intracranial bleeding complications.[13,14,15]
However, the studies referenced in the guidelines were limited to specific tumors, such as gliomas, used multiple screening tools, and lacked screening protocols for asymptomatic patients.[10,12] The real questions are “Are all patients undergoing brain tumor surgery at a high risk of developing VTE?” and “Do all such high-risk patients require VTE prophylaxis with an anticoagulant?” These questions warrant research, and their answers will help neurosurgeons to prescribe anticoagulants without hesitation.
To this end, we aimed to investigate the incidence of VTE in patients undergoing craniotomy for intracranial tumors and to identify the risk factors for VTE in our institution. Our data will help the surgeon to identify high-risk patients eligible to undergo pharmacological VTE prophylaxis.
Methods
Patients
This study was a retrospective review of the cohort database of patients who underwent elective craniotomy for intracranial tumors at Songklanagarind Hospital – a university hospital in Southern Thailand – between January 2017 and December 2018. We included all patients who were 15 years of age or older and presented a pathological diagnosis of intracranial tumors. Patients who underwent stereotactic tumor biopsy or trans-sphenoidal procedures were excluded from the study because they showed better postoperative recovery and earlier discharge than patients who underwent craniotomy.[9] Patients with history and preoperative VTE diagnosed were also excluded.
The database was extensively reviewed, and all patients' data were de-identified. The following parameters were recorded: sex, age, comorbidities, concurrent medications (steroids, antiepileptic drugs, or antiplatelet agents); body mass index (BMI), a history of tumor recurrence, previous radiotherapy or chemotherapy, type of neurosurgical procedure, operative time, estimated blood loss, and the use of blood products. Overweight was defined as BMI ≥23.0 kg/m2 according to the criteria for Asian patients.[16] We classified patients' ambulation on the basis of their ability to walk as independent when they were able to walk by themselves and dependent when they could not walk by themselves or required walking assistance devices. The overall performance status was evaluated by the Karnofsky Performance Scale (KPS). New-onset neurological deficits as motor deficits were recorded.
Venous thromboembolism screening, prophylaxis, and treatment
VTE is defined as DVT at lower limbs and/or PE.[11] All patients undergoing craniotomy for intracranial tumors were screened for DVT via compression ultrasonography (USG) of both the legs in the postoperative period. Experienced radiologists performed the screening. If the USG results were negative, subsequent USG was performed weekly until discharge. We do not have a screening protocol for PE, and screening was performed when PE was clinically suspected on the basis of unexplained hypoxemia or cardiac arrest. In such cases, chest computed tomography angiography (CTA) was performed to confirm the PE diagnosis. In the outpatient clinic, only those patients with clinically suspected VTE were evaluated via USG or CTA.
For VTE prophylaxis, all patients were weaned mechanical ventilators and were extubated early and mobilized as early as possible. A rehabilitation program was initiated if necessary. Mechanical prophylaxis via pneumatic calf compression, which is not routinely used, was applied at the attending neurosurgeon's discretion. No patient in this study received prophylaxis with anticoagulants due to the lack of experience of surgeons and concerns of intracerebral hemorrhage complications.
We consulted a vascular surgeon for further management of DVT patients and chest physicians or cardiothoracic surgeons for the management of PE patients. The VTE management depended on consulted physician's decisions. The choice of treatment for VTE patients was reviewed.
Statistical analysis
Patient characteristics were reported as descriptive statistics including frequencies, percentages, means with standard deviations (SDs) for parametric data, and medians with ranges for continuous nonparametric data. VTE-free possibilities were evaluated by survival curve analysis using the Kaplan–Meier (KM) method, and Cox proportional hazards regression analysis was used to identify univariable and multivariable factors associated with VTE. Variables associated with VTE with P ≤ 0.05 in univariable analysis were included in the multivariable analysis using the backward stepwise method. The desired power was set at 80%, and the alpha level for statistical significance using two-sided tests was set at 0.05. Statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria). The ethical committee of the institute approved this research (REC. 61-252-10-1).
Results
Patient characteristic, pathology, and surgical outcomes
We included 180 patients who met the study criteria; we excluded three patients with a history of prior admission VTE, but preoperative VTE symptomatic patient was not detected. We included the remaining 177 patients in the statistical analysis. Majority of the patients were females (54.2%), and the mean patient age was 50.4 years (SD, 13.8 years). Overweight was the most common comorbidity (58.2%), followed by hypertension (26.0%), dyslipidemia (18.1%), and diabetes mellitus (DM; 13.0%). Majority of the patients (75.7%) were of the physical status class 3 according to the American Society of Anesthesiologists classification. No moribund cases were operated in the elective setting during the study period. We encountered 13% of recurrent tumor patients with a history of irradiation (12.4%) and chemotherapy (7.9%). Majority of the patients were physically active with an independent status in the preoperative period (70.1%), and the median KPS was 60 (range, 30–80). However, 14.7% of the patients presented with altered consciousness. Perioperative steroids and prophylactic antiepileptic drugs were administered to 91.5% and 81.9% of the patients, respectively [Table 1].
Table 1.
Baseline characteristics of brain tumor patients (n=177)
| Factor | n (%) |
|---|---|
| Gender | |
| Male | 81 (45.8) |
| Female | 96 (54.2) |
| Mean of age (year)±SD (range) | 50.4±13.8 (15-86) |
| Underlying disease | |
| Hypertension | 46 (26.0) |
| Dyslipidemia | 32 (18.1) |
| DM | 23 (13.0) |
| Pulmonary diseases | 5 (2.8) |
| Kidney diseases | 4 (2.3) |
| Cardiac diseases | 3 (1.7) |
| CVA | 1 (0.6) |
| Other cancer | 20 (11.3) |
| BMI (kg/m2) | |
| <18.5 | 17 (9.6) |
| 18.5-22.9 | 57 (32.2) |
| ≥23.0 | 103 (58.2) |
| ASA classification | |
| 2 | 36 (20.3) |
| 3 | 134 (75.7) |
| 4 | 7 (4.0) |
| Recurrence tumors | 23 (13.0) |
| Prior RT | 22 (12.4) |
| Prior CMT | 14 (7.9) |
| Antiplatelet use | 7 (4.0) |
| Steroid use | 162 (91.5) |
| Antiepileptic drug use | 145 (81.9) |
| Smoking | 49 (27.7) |
| Preoperative ambulation | |
| Independent | 124 (70.1) |
| Dependent | 53 (29.9) |
| Mean operative time (min)±SD (range) | 428.6±63.5 (145-1005) |
| Location of craniotomy | |
| Supratentorial | 145 (81.9) |
| Infratentorial | 32 (18.1) |
| Mean blood loss (ml)±SD (range) | 872.6±1290.3 (50-11,000) |
| PRC transfusion | 94 (53.1) |
| FFP transfusion | 87 (49.2) |
| Platelet transfusion | 25 (14.1) |
| New-onset postoperative motor deficits | 42 (23.7) |
| Postoperative ambulation | |
| Death | 6 (3.4) |
| Independent | 101 (57.1) |
| Dependent | 70 (39.5) |
SD – Standard deviation; CDV – Cardiovascular diseases; DM – Diabetes mellitus; BMI – Body mass index; ASA – American Society of Anesthesiologists; RT – Radiation therapy; CMT – Chemotherapy; PRC – Packed red blood cell; FFP – Fresh frozen plasma
Operative time was defined as the time from anesthesia induction to the completion of surgery. The mean operative time was 428.6 min (SD, 163.5 min). The mean estimated blood loss was 872.6 mL (SD, 1290.3 mL), and 63.3% of the patients required blood transfusion. The requirement of perioperative transfusion was determined by the attending anesthesiologist and neurosurgeon. The most common transfusion type was packed red blood cell (PRC) transfusion (53.1%), followed by plasma transfusion (49.2%), and only 14.1% of the patients required platelet transfusion. The median length of hospital stay was 12 days (range, 2–281 days). At discharge, 42 (23.7%) patients showed new-onset postoperative motor deficits. The mortality rate was 3.4%, and 101 (57.1%) patients showed independent ambulation. The postoperative and preoperative KPS was the same (median KPS, 60; range, 0–80).
All tumors were pathologically examined. The supratentorial region was the most common location (81.9%); however, the percentages of intra- and extra-axial tumors were comparable (49.7% and 50.3%, respectively). Meningioma was the most common pathology (36.7%), followed by glioblastoma (14.7%) and metastasis (13.0%). Two patients with pituitary adenomas underwent pterional craniotomy due to the large size of the tumor and its suprasellar extension [Table 2].
Table 2.
Intracranial tumors pathology in 177 patients undergoing craniotomy
| Type of pathology | n (%) |
|---|---|
| Intra-parenchymal lesions | 88 (49.7) |
| Glioblastoma | 26 (14.7) |
| Metastasis | 23 (13.0) |
| Astrocytoma | 19 (10.7) |
| Oligodendroglioma | 6 (3.4) |
| Lymphoma | 5 (2.8) |
| Gliosarcoma | 3 (1.7) |
| Ependymoma | 2 (1.1) |
| Hemangioblastoma | 2 (1.1) |
| Oligoastrocytoma | 1 (0.6) |
| Supratentorial neuroblastoma | 1 (0.6) |
| Extra-parenchymal lesions | 89 (50.3) |
| Meningioma | 65 (36.7) |
| Schwannoma | 15 (8.5) |
| Pituitary adenoma | 2 (1.1) |
| Craniopharyngioma | 2 (1.1) |
| Pineal germ cell tumor | 2 (1.1) |
| Central neurocytoma (lateral ventricle) | 1 (0.6) |
| Hemangiopericytoma | 1 (0.6) |
| Medulloblastoma | 1 (0.6) |
Incidence, prophylaxis, and management of venous thromboembolism
Leg USG for screening was performed in all patients. VTE occurred in 18 (10.2%) patients, including 12 (6.8%) with DVT, 3 (1.7%) with PE, and 3 (1.7%) with both DVT and PE. Mechanical prophylaxis with Intermittent pneumatic calf compression (IPC) was used postoperatively in 44 (24.9%) patients. The most common location of leg DVT is proximal leg veins, which found in ten patients (six patients in the common femoral vein and four patients in the superficial femoral vein). Distal leg DVT, popliteal vein, was detected in two patients in DVT alone patients and three patients with simultaneous DVT and PE patients. All PE patients were detected in both the lungs. The most common location of PE is the main pulmonary artery for four patients.
The clinical presentation of VTE depended on its location. DVT patients were more frequently asymptomatic than PE patients; only five DVT patients showed leg pain or edema. Only one patient with PE was asymptomatic [Table 3]. The median times of VTE, PE, and DVT diagnoses were postoperative days 13.5 (range, 2–52), 10 (range, 2–52), and 13 (range, 2–52), respectively. The mean time of diagnosis was not significantly different between asymptomatic and symptomatic VTE patients (18.3 vs. 23.9 days, P = 0.503).
Table 3.
Characteristics of venous thromboembolism patients (n=18)
| VTE characteristic | n (%) |
|---|---|
| Incidence of VTE | |
| DVT alone | 12 (6.8) |
| Asymptomatic | 7 |
| Symptomatic (leg edema or pain) | 5 |
| PE alone | 3 (1.7) |
| Asymptomatic | 0 |
| Symptomatic | |
| Dyspnea or deoxygenation | 3 |
| DVT + PE | 3 (1.7) |
| Asymptomatic | 1 |
| Symptomatic | |
| Dyspnea or deoxygenation | 1 |
| Cardiac arrest | 1 |
VTE – Venous thromboembolism; PE – Pulmonary embolism; DVT – Deep-vein thrombosis
Five DVT patients were treated with an inferior vena cava (IVC) filter and five received low molecular-weight heparin (LMWH); the symptoms were observed in the remaining patients. One patient with PE developed cardiac arrest but did not receive further treatment as per the relative's decision; two patients received LMWH. Three patients with simultaneous DVT and PE were treated with LMWH and IVC filters.
Risk factors for venous thromboembolism
Figure 1a shows the overall VTE-free probability as a KM curve; the calculated probability did not reach the median of overall probability. VTE-associated factors were DM and new-onset postoperative motor deficits. These two factors were presented in KM curves and log-rank tests, as shown in Figure 1b and c. The results of Cox proportional hazards regression analysis are presented in Table 4. In univariable analysis, DM (hazard ratio [HR] = 4.18, 95% CI = 1.39–12.57; P = 0.011), operative time of ≥420 min (HR = 2.74, 95% CI = 1.01–7.40; P = 0.047), PRC transfusion (HR = 3.46, 95% CI = 1.09–10.96; P = 0.035), fresh frozen plasma transfusion (HR = 2.99, 95% CI = 1.02–8.77; P = 0.047), and new-onset postoperative motor deficit (HR = 3.82, 95% CI = 1.40–10.38; P = 0.009) were associated with an increased risk of VTE development. After applying the Cox regression model with the backward stepwise method to establish significant factors in the univariable analysis, two significant factors were detected in multivariable analysis: DM (HR = 4.52, 95% CI = 1.38–14.82; P = 0.013) and new-onset postoperative motor deficit (HR = 3.46, 95% CI = 1.17–10.23; P = 0.025).
Figure 1.
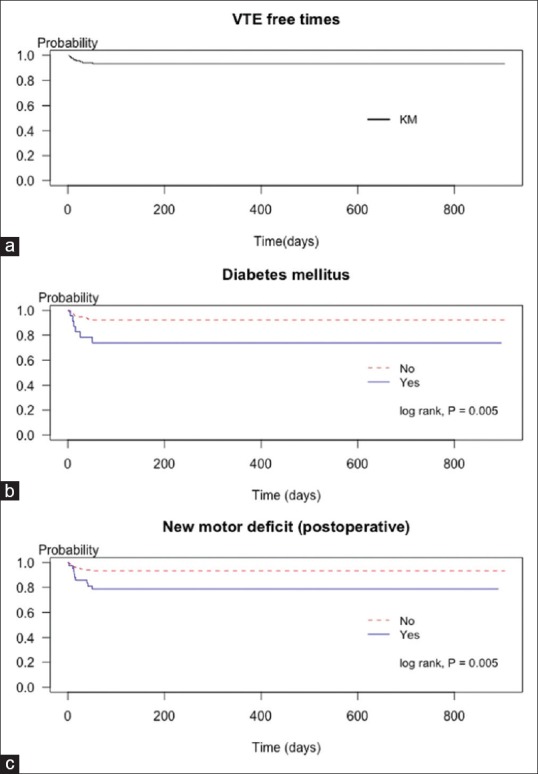
Venous thromboembolism-free probabilities presented by the Kaplan–Meier curve. (a) Kaplan–Meier curve for overall venous thromboembolism-free time. (b) Kaplan–Meier curve presented venous thromboembolism-free probabilities was lower in patients with diabetes mellitus (log-rank test, P = 0.005) and (c) in patients with new postoperative motor deficit (log-rank test, P = 0.005)
Table 4.
Factors associated with venous thromboembolism in brain tumor patients by Cox regression analysis
| Factors | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Patient related factors | ||||
| Female | 1.06 (0.39-2.83) | 0.906 | ||
| Age ≥50 years | 1.01 (0.38-2.69) | 0.986 | ||
| Hypertension | 1.49 (0.52-4.22) | 0.456 | ||
| DM | 4.18 (1.39-12.57) | 0.011 | 4.52 (1.38-14.82) | 0.013 |
| Dyslipidemia | 1.34 (0.41-4.37) | 0.631 | ||
| BMI ≥23.0 (kg/m2) | 1.50 (0.53-4.18) | 0.444 | ||
| ASA Class 4 | 1.41 (0.19-10.56) | 0.741 | ||
| Recurrent tumor | 0.82 (0.19-3.58) | 0.795 | ||
| Antiplatelet use | 1.50 (0.17-13.21) | 0.715 | ||
| Steroid use | 1.64 (0.20-13.27) | 0.642 | ||
| Anti-epileptic drug use | 1.86 (0.41-8.53) | 0.424 | ||
| Smoking | 1.35 (0.48-3.82) | 0.573 | ||
| Preoperative ambulatory dependent | 1.56 (0.49-4.98) | 0.453 | ||
| Treatment-related factors | ||||
| Operative time ≥420 mins | 2.74 (1.01-7.40) | 0.047 | ||
| Infratentorial surgery | 0.54 (0.12-2.46) | 0.424 | ||
| EBL ≥900 (mL) | 3.08 (1.14-8.30) | 0.026 | ||
| PRC transfusion | 3.46 (1.10-10.96) | 0.035 | ||
| FFP transfusion | 2.99 (1.02-8.77) | 0.047 | ||
| Platelet transfusion | 2.67 (0.86-8.30) | 0.089 | ||
| New-onset postoperative motor deficits | 3.82 (1.40-10.38) | 0.009 | 3.46 (1.17-10.23) | 0.025 |
| Postoperative ambulatory dependent | 2.27 (0.84-6.17) | 0.107 | ||
| IPC prophylaxis | 2.10 (0.76-5.80) | 0.153 | ||
| Tumor related factors | ||||
| Extra-axial tumor | 0.81 (0.30-2.16) | 0.674 | ||
| Glioblastoma | 1.39 (0.51-3.80) | 0.523 | ||
| Metastasis | 0.82 (0.18-3.83) | 0.802 | ||
| Meningioma | 1.18 (0.42-3.31) | 0.753 | ||
| Schwannoma | 1.64 (0.76-5.80) | 0.153 | ||
DM – Diabetes mellitus, BMI – Body mass index, ASA – American Society of Anesthesiologists, FFP – Fresh frozen plasma, EBL – Estimated blood loss, PRC – Packed red blood cell, IPC – Intermittent pneumatic compression, CI – Confidence interval
Discussion
In the present study, we investigated the incidence of and risk factors for VTE in patients undergoing craniotomy for intracranial tumors at our institution. In our cohort, all patients were screened with leg USG in the postoperative period. We detected a VTE rate of 10.2% (DVT, 6.8%; PE, 1.7%; and simultaneous DVT and PE, 1.7%).
We searched MEDLINE for studies assessing the incidence of and factors for postcraniotomy VTE. For review, we selected studies that included intracranial tumors, similar to our study. Details of nine studies, including the present study, are summarized in Table 5.[17,18,19,20,21,22,23,24] All studies were retrospective, and there were no previous prospective studies. The incidence of postcraniotomy VTE, DVT, and PE was 3%–23%, 1.9%–21.3%, and 0.8%–3.4%, respectively. The rate of simultaneous DVT and PE was 0%–1.7%; this rate was not reported in two studies.[20,21] A DVT screening protocol was used in three of the 9 (33.3%) studies. However, only the present study screened all patients, and two studies investigated only high-risk patients or those with high D-dimer values.[18,22] The mean rate of DVT was higher in studies that used a screening protocol[18,22] than in studies that did not (11.4% vs. 5.4%).[17,19,20,21,23,24]
Table 5.
Summarized results of previous studies evaluating incidence and risk factors for postcraniotomy venous thromboembolism in brain tumor patients
| Authors and year | Country | Sample size | VTE | VTE detection time (POD) | Image screening protocol (criteria, modality) | Prophylaxis | VTE risk factors* | Remark | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (%) | DVT (%) | PE (%) | DVT + PE (%) | ||||||||
| Constantini et al., 1991 | Israel | 633 | 4.9 | 1.9 | 1.3 | 1.7 | DVT (mean) 9.7±5.0 days PE (mean) 10.0±5.4 days | No | NA | Supratentorial, Malignant glioma, Weakness | Only symptomatic patients |
| Aishima et al., 2013 | Japan | 419 | 5.5 | 2.9 | 1.0 | 1.6 | Median 5 days (range 0-47) | Yes (patient with high of abruptly increased D-dimer level, CT with contrast) | ECS + IPC | Malignant tumor, preoperative paresis | Included stereotactic biopsy (10.7%) |
| Chaichana et al., 2013 | USA | 4293 | 3.0 | 2.0 | 0.8 | 0.2 | NA | No | IPC + UFH | HGG, HTN, Motor deficit, Age >65, KPS ≤70 | Included stereotactic biopsy (7%) |
| Kimmel et al., 2014 | USA | 1741 | NA | 3.2 | 1.8 | NA | DVT (mean) 13.24 days PE (mean) 14.22 days | No | NA | Age >60, Op time >4 h, UTI, Septic shock | |
| Frisius et al., 2015 | Germany | 207 | NA | 7.2 | 1.9 | NA | NA | No | UFH or LMWH + IPC | Operative time >100 min | |
| Smith et al., 2015 | USA | 1148 | 17.1 | 13.7 | 3.4 | 0 | Mean 6.1±9.4 days | No | UFH or LMWH | Female, ICU LOS, High-grade tumors, Non-Caucasian, Prior VTE | |
| Nakano et al., 2018 | Japan | 61 | 23.0 | 21.3 | 1.7 | 0 | Median 8 days (range 1-64) | Yes (High risk or high D-dimer level, USG) | ECS + IPC | Postoperative infection | |
| Sender et al., 2018 | USA | 7376 | 3.5 | 2.0 | 0.9 | 0.6 | Inhospital (median) 6 days (IQR 3-8) Postdischarge (median) 13 days (IQR 6-19) | No | NA | Older age, Higher BMI | |
| Present study | Thailand | 177 | 10.2 | 6.8 | 1.7 | 1.7 | Median 13.5 days (range 2-52) | Yes (all cases, USG) | IPC (24.9%) | DM, New motor deficit | |
*The VTE risk factors included only statistically significant in multivariable analysis. DVT – Deep-vein thrombosis; ECS – Elastic compression stocking; ICU – Intensive care unit; IQR – Interuartile range; IPC – Intermittent pneumatic calf compression; KPS – Karnofsky performance status; LMWH – Low molecular-weight heparin; LOS – Length of stay; NA –Not available data; PE – Pulmonary embolism; POD – Postoperative day; UFH – Unfractionated heparin; UTI – Urinary tract infection; VTE – Venous thromboembolism; UGS – Ultrasonography; CT – Computed tomography
The timing of VTE development is a challenging issue. The exact time of VTE onset is not well established. Clinical studies reported that VTE diagnosis depends on several factors such as patients' symptoms, detection modality, and assessment timing. VTE is usually detected within the first 2 weeks after surgery, ranging from postoperative days 0–64. The reported VTE-associated risk factors include patient-associated factors (female sex,[23] old age,[19,20,24] non-Caucasian ethnicity,[23] prior VTE,[23] hypertension,[19] obesity,[24] KPS <70,[19] and motor deficits[17,18,19]), tumor-associated factors (supratentorial location[17] and malignant pathology[17,18,23]), and surgery-associated factors (prolonged operative time[20,21] and postoperative infection or sepsis[20,22]). The present study identified DM as a new independent risk factor for VTE.
Hyperglycemia has been evaluated as a factor associated with a high risk of thrombosis. Laboratory evidence has demonstrated that high serum glucose-induced oxidative stress leads to the malfunction of the endothelial layer, increase in coagulation factor levels, and impairment of fibrinolysis.[25] The results of our study contradict those of a recent population-based study[26] and another meta-analysis,[27] both of which reported a weak but positive or no association between DM and VTE risk. The meta-analysis indicated that the association of DM with VTE might be indirect, reflecting the effect of other VTE risk factors associated with DM, such as obesity.[27] Unfortunately, the association between perioperative blood glucose levels and VTE has not been extensively studied.
VTE preventive strategies can be implemented using many approaches, from encouraging patients to engage in physical activity as early as possible to mechanical and/or pharmacological prophylaxis.[7,28,29] In this study, we mainly used the motivation of patients using both active and passive mobilizations by physical therapists.[30] However, the use of mechanical prophylaxis in our institution is only one-fourth, mainly due to the limited number of devices and lack of funds to purchase compression stockings; these are commonly faced issues in hospitals with limited resources[31] and warrant systematic and policy-based resolution.
The strength of this study is that we screened all patients, achieving the most realistic incidence. However, there are many limitations, such as the retrospective study bias, inconsistent screening periods, and inconsistent IPC usage in patients with or without VTE. Finally, the lack of preoperative screening data made us unable to know the exact time of VTE occurrence.
Conclusions
Patients who underwent craniotomy for brain tumors were examined. Such patients, specifically those with DM and new-onset motor deficits after surgery, are at a high risk of developing VTE. Therefore, such high-risk patients should be prioritized for receiving VTE prophylaxis.
Financial support and sponsorship
The authors would like to thank the Faculty of Medicine, Prince of Songkla University, Thailand.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–73. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 2.Kourlaba G, Relakis J, Mylonas C, Kapaki V, Kontodimas S, Holm MV, et al. The humanistic and economic burden of venous thromboembolism in cancer patients: A systematic review. Blood Coagul Fibrinolysis. 2015;26:13–31. doi: 10.1097/MBC.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 3.Cote LP, Greenberg S, Caprini JA, Stone J, Arcelus JI, López-Jiménez L, et al. Outcomes in neurosurgical patients who develop venous thromboembolism: A review of the RIETE registry. Clin Appl Thromb Hemost. 2014;20:772–8. doi: 10.1177/1076029614532008. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto J, Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, et al. Cancer-associated venous thromboembolism in the real world – From the command VTE registry. Circ J. 2019;83:2271–81. doi: 10.1253/circj.CJ-19-0515. [DOI] [PubMed] [Google Scholar]
- 5.Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost. 2014;40:325–31. doi: 10.1055/s-0034-1370791. [DOI] [PubMed] [Google Scholar]
- 6.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117:219–30. doi: 10.1160/TH16-08-0615. [DOI] [PubMed] [Google Scholar]
- 7.Ganau M, Prisco L, Cebula H, Todeschi J, Abid H, Ligarotti G, et al. Risk of deep vein thrombosis in neurosurgery: State of the art on prophylaxis protocols and best clinical practices. J Clin Neurosci. 2017;45:60–6. doi: 10.1016/j.jocn.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer-associated venous thromboembolism: An update. Semin Thromb Hemost. 2019;45:321–5. doi: 10.1055/s-0039-1688494. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldo L, Brown DA, Bhargav AG, Rusheen AE, Naylor RM, Gilder HE, et al. Venous thromboembolic events in patients undergoing craniotomy for tumor resection: Incidence, predictors, and review of literature. J Neurosurg. 2020;132:10–21. doi: 10.3171/2018.7.JNS181175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraoni D, Comes RF, Geerts W, Wiles MD ESA VTE Guidelines Task Force. European guidelines on perioperative venous thromboembolism prophylaxis: Neurosurgery. Eur J Anaesthesiol. 2018;35:90–5. doi: 10.1097/EJA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 11.Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–81. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 12.Nyquist P, Bautista C, Jichici D, Burns J, Chhangani S, DeFilippis M, et al. Prophylaxis of venous thrombosis in neurocritical care patients: An evidence-based guideline: A statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016;24:47–60. doi: 10.1007/s12028-015-0221-y. [DOI] [PubMed] [Google Scholar]
- 13.Algattas H, Damania D, DeAndrea-Lazarus I, Kimmell KT, Marko NF, Walter KA, et al. Systematic review of safety and cost-effectiveness of venous thromboembolism prophylaxis strategies in patients undergoing craniotomy for brain tumor. Neurosurgery. 2018;82:142–54. doi: 10.1093/neuros/nyx156. [DOI] [PubMed] [Google Scholar]
- 14.Alshehri N, Cote DJ, Hulou MM, Alghamdi A, Alshahrani A, Mekary RA, et al. Venous thromboembolism prophylaxis in brain tumor patients undergoing craniotomy: A meta-analysis. J Neurooncol. 2016;130:561–70. doi: 10.1007/s11060-016-2259-x. [DOI] [PubMed] [Google Scholar]
- 15.Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: An updated systematic review and meta-analysis. J Neurosurg. 2018;129:906–15. doi: 10.3171/2017.2.JNS162040. [DOI] [PubMed] [Google Scholar]
- 16.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-pacific recommendations. Asia Pac J Clin Nutr. 2008;17:370–4. [PubMed] [Google Scholar]
- 17.Constantini S, Kornowski R, Pomeranz S, Rappaport ZH. Thromboembolic phenomena in neurosurgical patients operated upon for primary and metastatic brain tumors. Acta Neurochir (Wien) 1991;109:93–7. doi: 10.1007/BF01403001. [DOI] [PubMed] [Google Scholar]
- 18.Aishima K, Yoshimoto Y. Screening strategy using sequential serum D-dimer assay for the detection and prevention of venous thromboembolism after elective brain tumor surgery. Br J Neurosurg. 2013;27:348–54. doi: 10.3109/02688697.2012.737958. [DOI] [PubMed] [Google Scholar]
- 19.Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, et al. Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res. 2013;35:206–11. doi: 10.1179/1743132812Y.0000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimmell KT, Walter KA. Risk factors for venous thromboembolism in patients undergoing craniotomy for neoplastic disease. J Neurooncol. 2014;120:567–73. doi: 10.1007/s11060-014-1587-y. [DOI] [PubMed] [Google Scholar]
- 21.Frisius J, Ebeling M, Karst M, Fahlbusch R, Schedel I, Gerganov V, et al. Prevention of venous thromboembolic complications with and without intermittent pneumatic compression in neurosurgical cranial procedures using intraoperative magnetic resonance imaging. A retrospective analysis. Clin Neurol Neurosurg. 2015;133:46–54. doi: 10.1016/j.clineuro.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Nakano F, Matsubara T, Ishigaki T, Hatazaki S, Mouri G, Nakatsuka Y, et al. Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: A Japanese single-center, retrospective study. Thromb Res. 2018;165:95–100. doi: 10.1016/j.thromres.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Smith TR, Nanney AD, 3rd, Lall RR, Graham RB, McClendon J, Jr, Lall RR, et al. Development of venous thromboembolism (VTE) in patients undergoing surgery for brain tumors: Results from a single center over a 10 year period. J Clin Neurosci. 2015;22:519–25. doi: 10.1016/j.jocn.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Senders JT, Goldhaber NH, Cote DJ, Muskens IS, Dawood HY, de Vos FY, et al. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: A national surgical quality improvement program analysis. J Neurooncol. 2018;136:135–45. doi: 10.1007/s11060-017-2631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB. Hyperglycemia: A prothrombotic factor? J Thromb Haemost. 2010;8:1663–9. doi: 10.1111/j.1538-7836.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4:163–73. doi: 10.1001/jamacardio.2018.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell EJ, Folsom AR, Lutsey PL, Selvin E, Zakai NA, Cushman M, et al. Diabetes mellitus and venous thromboembolism: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;111:10–8. doi: 10.1016/j.diabres.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell S, Bashar K, Broderick BJ, Sheehan J, Quondamatteo F, Walsh SR, et al. The use of intermittent pneumatic compression in orthopedic and neurosurgical postoperative patients: A systematic review and meta-analysis. Ann Surg. 2016;263:888–9. doi: 10.1097/SLA.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 29.Salmaggi A, Simonetti G, Trevisan E, Beecher D, Carapella CM, DiMeco F, et al. Perioperative thromboprophylaxis in patients with craniotomy for brain tumours: A systematic review. J Neurooncol. 2013;113:293–303. doi: 10.1007/s11060-013-1115-5. [DOI] [PubMed] [Google Scholar]
- 30.Hillegass E, Puthoff M, Frese EM, Thigpen M, Sobush DC, Auten B, et al. Role of physical therapists in the management of individuals at risk for or diagnosed with venous thromboembolism: Evidence-based clinical practice guideline. Phys Ther. 2016;96:143–66. doi: 10.2522/ptj.20150264. [DOI] [PubMed] [Google Scholar]
- 31.Akaraborworn O, Chittawatanarat K, Chatmongkolchart S, Kitsiripant C. Modalities in venous thromboembolism prophylaxis and symptomatic venous thromboembolism occurrence in critically Ill surgical patients (THAI-SICU Study) J Med Assoc Thai. 2016;99(Suppl 6):S112–7. [PubMed] [Google Scholar]


