Abstract
Insular gliomas represent 25% and 10% of low- and high-grade gliomas, respectively. Their resection proves challenging due to the intimate involvement of eloquent parenchyma and the lenticulostriate arteries (LSAs), limiting facility of achieving maximal safe resection. The majority of postoperative deficits following insular glioma resection is attributed to compromise of the LSAs. It is contemporaneously critical and challenging to preserve these vessels, given they are numerous and small, with an intraparenchymal course hidden from direct visualization during the operative intervention. A lesser degree of medially directed displacement of the LSAs predicts tumoral encasement of these vessels, which portends a decreased likelihood for achieving a gross total resection and increased probability of postoperative morbidity. Preservation of these vessels thus requires knowledge of their location during the entirety of the insular glioma resection and is facilitated by pre- and intra-operative imaging. Intraoperative real-time tracking, however, may prove rather challenging, especially with transcortical access. Conventional catheter digital subtraction angiography, computed tomographic angiography, magnetic resonance imaging and angiography, and three-dimensional ultrasound powered Doppler have proven effective modalities in assessing lenticulostriate position, and their use facilitates a greater extent of resection while minimizing the attendant morbidity consequent to LSA injury.
Keywords: Insular glioma, lenticulostriate artery, lenticulostriate, magnetic resonance angiography, operative, surgery, ultrasound
Introduction
The microsurgical resection of insular gliomas carries a significant risk of injury to the lenticulostriate arteries (LSAs) emanating from the precommunicating segment of the anterior cerebral artery and sphenoidal segment of the middle cerebral artery and supplying the deep white matter tracts and basal nuclei.[1,2,3,4,5,6] Iatrogenic injury of these fine vessels may precipitate the development of new onset hemiparesis[1,2,3,4,5] consequent to ischemic infarction of the highly eloquent pyramidal white matter tracts.[6] The LSAs may sustain damage as a consequence of suction aspirator trauma to the abluminal surface of the vessel wall during tumoral coagulation.[7,8] A plethora of examples of postoperative deficits sustained consequent to LSA injury or coagulation during insular glioma resection abound in the literature.[1,2,3,4,5,9,10] The preservation of the LSAs thus proves indispensable in order to ensure good neurological outcome.[2,4,9,11,12,13,14,15] Consequently, in order to mitigate these attendant risks accompanying insular glioma extirpation, the optimization of microsurgical resection technique is of utmost importance.[11,12,13,16,17,18]
Insular gliomas may medially displace or encase the lenticulostriate arteries.[8] An appreciation of lenticulostriate artery with respect to the tumor relative to the progression of the dissection proves critical intraoperatively, though frequently proves challenging.[7,11,12,13,18,19] While preoperative imaging represents an excellent modality in identifying the location of the LSAs relative to tumor and healthy parenchyma,[1,2,10,15,19,20] microsurgical dissection and tumor debulking generate intraoperative brain shifts[8,9] effectively changing the relationship between the tumor and the LSAs, invoking the necessity of innovation in real-time tracking imaging modalities.[1,5,8,9,10,15,21]
Lenticulostriate and Middle Cerebral Artery Perforator Anatomy
Emeritus Professor Dr. M. Gazi Yaşargil provides a beautiful description of the anatomy of the lenticulostriate arteries, as a series of fine branches varying from 5 to 24 vessels[22] arising principally from the inferomedial aspect of the M1 segment of the middle cerebral artery, typically as a single branch subsequently dividing into smaller branches [Figures 1 and 2]. The vessels may alternatively originate as two main stem arteries from the sphenoidal segment of the middle cerebral artery or the proximal aspect of the insular middle cerebral artery, subsequently dividing extensively, or as multiple small vessels directly emanating from the parent trunks of the middle cerebral artery (MCA).[23] Lenticulostriate origin deriving from the lateral fronto-orbital artery represents an anatomic variation characterized with an approximate prevalence of 3% of patients.[23] Following takeoff from the sphenoidal segment of the middle cerebral artery, the LSAs exhibit variable intracisternal course prior to penetrating the parenchyma of the central and lateral extent of the anterior perforated substance, after which these vessels supply the substantia innominata, caudate and lentiform nuclei, internal capsule, and corona radiata.[2,3,13,23,24,25,26,27] The medialmost aspect of the limen insulae may be found approximately 15–20 mm from the lateralmost lenticulostriate vessel.[26,27] Since parenchyma irrigated by the lenticulostriate arteries typically does not receive significant collateral flow, iatrogenic injury to a single lenticulostriate vessel may cause basal ganglionic or internal capsular infarction,[21] resulting in dense hemiplegia.[1,2,3,4,5]
Figure 1.
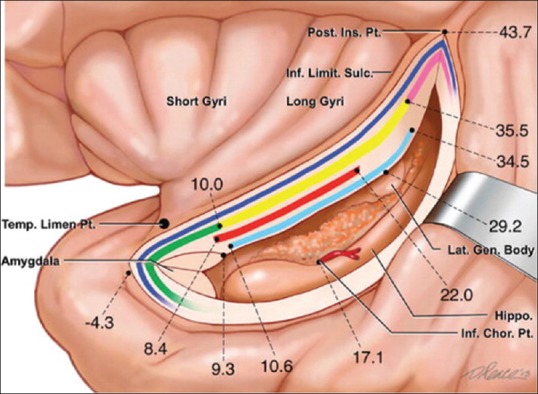
Anatomy of the insula. A schematic depiction of the insular region as would be viewed with retraction placed on the medial aspect of the superior temporal gyrus and incision through the inferior limiting sulcus of the insula. The insula is delimited anteriorly, superiorly, and inferiorly by the anterior, superior, and inferior limiting sulci of the insula, respectively. The insular cortex is arranged into anterior short and posterior long gyri. Fiber tracts coursing beneath the inferior limiting sulcus of the insula are represented. From superficial to deep: Extreme capsule (dark blue); uncinate fasciculus (green), inferior fronto-occipital fasciculus (yellow), and claustrocortical fibers (pink); anterior commissure (red); optic radiations (light blue). Distances of white matter pathways, the lateral geniculate body, and choroidal point from the limen insula are indicated. Modified with permission from Ribas et al., 2015
Figure 2.
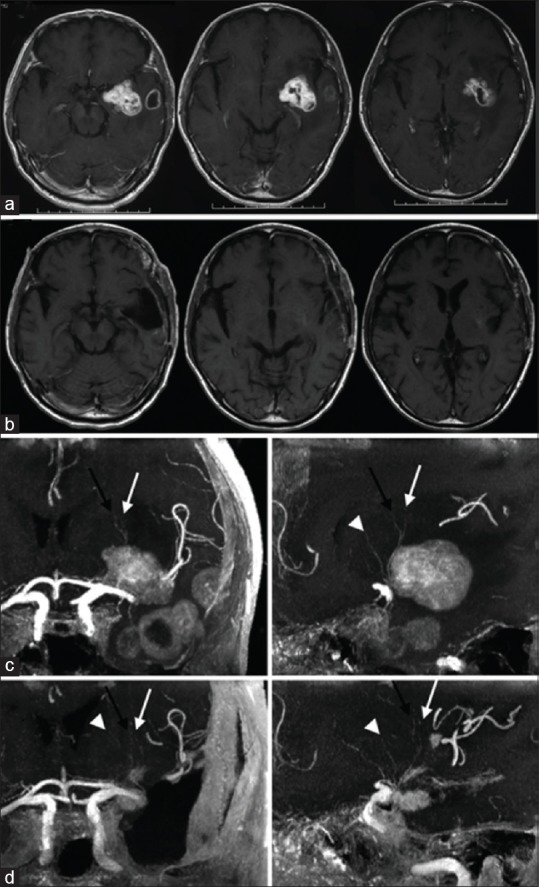
Lenticulostriate vessels and left operculoinsular glioblastoma demonstrated by contrast-enhanced magnetic resonance imaging. Preoperative (a and c) and postoperative (b and d) magnetic resonance imaging sequences. a: Axial T1-weighted magnetic resonance imaging sequences demonstrate a contrast enhancing left insuloopercular glioma with microcystic cavitation. b: Axial T1-weighted magnetic resonance imaging sequences demonstrate successful gross total resection of left operculoinsular glioblastoma. c: Coronal (left) and sagittal (right) views of three dimensional 3 Tesla time of flight magnetic resonace imaging sequences demonstrate the lenticulostriate vessels located at the anteromedial aspect of the tumor. d: Coronal (left) and sagittal (right) views of three dimensional 3 Tesla time of flight magnetic resonance imaging sequences demonstrate successful preservation of the lenticulostriate vessels following tumor resection. Black arrows = first perforators; white arrows = second perforators; white arrowheads = third perforators. Modified with permission from Saito et al., 2009
Preservation of middle cerebral artery branches during Sylvian dissection proves equivalently critical during the microsurgical extirpation of insular gliomas. Insular vessels deriving from the middle cerebral artery may be classified as short branches (85%–90%) supplying insular cortex and extreme capsule, intermediate (10%) branches irrigating claustrum and external capsule, or long (3%–5%) branches providing blood flow to the corona radiata.[10] Thus, the external capsule may be effectively conceptualized to represent a transition zone between insular cortex, extreme capsule, and claustrum supplied by branches of the insular segment of the middle cerebral artery and the basal ganglia and internal capsule supplied by the lenticulostriate arteries.[1,3,27,28] It is injury to either these groups of vessels (LSAs and long insular M2 branches) that presents the greatest risk of precipitating a dense hemiplegia.[1]
Identification of Lenticulostriate Arteries by Anatomic Landmarks
Lenticulostriate artery location may be estimated according to visualized anatomic landmarks intraoperatively or through the use of neuronavigation.[2] A vertical plane through the base of the periinsular sulci corresponds approximately to the location of the lateralmost lenticulostriate arteries, though typically only proves a reliable indicator in the setting of Yaşargil type 3A insular gliomas.[18,29] According to the eminent M.G. Yaşargil, in order to avoid injury to eloquent structures and the LSAs, the microsurgical resection of insular gliomas should be medially delimited by the white matter tracts overlying the putamen[30] However, the neurosurgeon may encounter the LSAs prior, since insular gliomas may not infrequently extend medially with respect to the LSAs and into the basal ganglia.[1] In Duffau et al.[9] series of 12 patients undergoing operative extirpation of insular glioma, dissection of the middle cerebral arterial tree allowed the origins of the lenticulostriate arteries to be successfully visualized in all patients, with the course and extent of the LSAs appreciated fully in 16.7% of patients. In their series, two patients were evaluated preoperatively with computed tomographic angiography (CTA) in order to determine the relationship of LSAs to insular tumor[9] one of whom developed postoperative hemiparesis consequent to iatrogenic microsurgical LSA injury.[9]
Identification of Lenticulostriate Arteries by Imaging
Overview
Preoperative identification of the lenticulostriate arteries may be effectively achieved through the use of conventional catheter transfemoral transaortic cranial digital subtraction angiography,[30] computed tomographic angiography (CTA),[9] and various magnetic resonance imaging (MRI).[2] The lenticulostriate arteries may be visualized on MRI as prominent flow voids[2] and their proximity to tumor evaluated. Superimposition of MRI and various angiographic modalities represents a useful strategy evaluate and elucidate tumor-LSA interface.[1] Three-dimensional time-of-flight magnetic resonance angiography (3D TOF MRA) effectively and directly evaluates the intracranial vasculature and has proven of benefit in identifying lenticulostriate artery position and course,[15,10] a knowledge of which significantly enhances the safely feasible extent of resection and reduces the attendant morbidity of operative expeditions upon tumors and lesions of the insula.[1,2,8,9,30] The insular tumoral-parenchymal interface may be most appropriately delineated by 3DT2 and 3DT2-fluid-attenuated inversion recovery (FLAIR) MRI.[10] Authors have also expounded upon the use of Micro-Doppler ultrasound (US) in the intraoperative real-time tracking of lenticulostriate artery position though rendering variably weighted opinions on utility.[8,21]
Magnetic Resonance Imaging and Angiography
Rao et al.[5] report on a series of 48 patients harboring insular gliomas contemporaneously undergoing 3D TOF MRI and 3D constructive interference in steady-state (CISS) MRI in order to evaluate the relationship of tumor interface with the lenticulostriate vessels. Insular gliomas were effectively visualized by the former in 29 of 48 cases and by the latter in all patients. Combining both modalities permitted exceptional visualization of tumor-lenticulostriate artery interface in 47 of 48 cases. Insular gliomas displacing the LSAs correlated with greater extents of resection compared with tumors encasing these vessels. The authors thus provided convincing evidence combining 3D TOF MRI and 3D CISS MRI effectively facilitates the identification and evaluation of tumor-LSA interface, contrasted with intermediate sensitivity for the same using the former alone. Among six patients developing postoperative new onset hemiparesis, two cases were attributable to iatrogenic microsurgical injury of the lenticulostriate arteries, underscoring the clinical significance and critical importance of identifying the location of these vessels relative to the progression of the dissection.
Bykanov et al.[10] effectively used 3D TOF MRA to evaluate the relationship of the lenticulostriate arteries relative to insular gliomas. Non-contrast 3D TOF MRA was utilized in the evaluation of all patients of the series and six patients were additionally evaluated using contrast-enhanced sequences. Lateral and medial LSAs were identified in 18 of 20 and 19 of 20 patients, respectively. 3D TOF MRA proved excellent at visualizing the entire course of the lenticulostriate vessels, with use of contrast improving visualization of their distal extent. The authors of the study categorized three patterns of insular glioma growth relative to the lenticulostriate arteries as Type I: Encasement without displacement, occurring in 2 of 15 cases; Type II: Medial displacement without encasement, occurring in 11 of 15 cases; and Type III; encasement with displacement, occurring in 2 of 15 cases. In five patients, the tumor-LSA relationship could not be readily evaluated due to poor tumor visualization.
In the experience of Saito et al.[15], 3D TOF MRA proved quite effective in preoperatively identifying the LSAs, but not the long insular arteries emanating from the middle cerebral arterial tree. The latter vessels characteristically arise from the insular segment of the middle cerebral artery and course through the posterior insula to supply the corona radiata, with the arcuate fasciculus lying in close proximity. This typically renders the microsurgical resection of insular gliomas invading through the superior limiting sulcus dangerous, given the attendant risk of microsurgical compromise of the long insular M2 arteries.
Moshel et al.[1] report on a series of 38 patients harboring insular gliomas undergoing microsurgical tumor resection via a transylvanian approach. Superimposition of preoperative MRI tumor volume with preoperative stereotactic cerebral angiograms successfully allowed the classification of these tumors into two groups based on the relationship to the lenticulostriate arteries. Group I insular gliomas (n = 25) were accordingly designated to be those lesions located lateral to the LSAs, causing medial displacement of these vessels of 161%. Among 25 such gliomas, 20 were well-demarcated. Group II lesions (n = 13) were accordingly designated to be those lesions extending medially and around the LSAs and caused less displacement of these vessels (130%). The positive predictive value of an LSA shift >140% for a tumor being classified as a Group I lesion was 95.2% and that of an LSA shift <140% for a tumor being classified as a Group II lesion was 70.6%. Among 13 cases, 11 tumors exhibited a readily demonstrable tumoral-parenchymal interface, which was diffuse on T2 weighted MRI sequences. Worsening of pre-existent, or postoperative new onset, hemiparesis was attributable to LSA compromise in five patients. Among Group I and II insular gliomas, gross, near-total, and subtotal resection were successfully achieved in 68%, 16%, and 16% and 31%, 23%, and 46% of patients, respectively. Thus, insular glioma location lateral to the LSAs causing medial displacement of these vessels carried a positive predictive value for a well-demarcated tumor of 80% and was associated with increased probability of achieving gross or near-total resection, compared to insular gliomas which extended medially and around the LSAs (84 vs. 54%), as well as less morbidity.
Intraoperative Ultrasound
Ascertaining medial tumor border permits intraoperative assessment of LSA location. While preoperative time of flight MRI may readily demonstrate the lenticulostriate arteries, the intraoperative shift of these vessels may render information provided by neuronavigation inaccurate.[1] Authors have accordingly proposed various modalities in order to thoroughly account for intraoperative brain and lenticulostriate artery shifts secondary to tumoral resection.[1,5,8,9,10,15,21] Emeritus Professor Dr. M. Gazi Yaşargil describes successfully using the acoustic signal from micro-Doppler in order to identify the position and course of the lenticulostriate arteries.[30] Three dimensional ultrasound powered Doppler represents a safe, fast, and reliable method effectively providing real-time identification of the LSAs intraoperatively, contemporaneously permitting visualization of residual glioma.[8] The use of intraoperative magnetic resonance imaging represents an alternative strategy achieving real-time intraoperative tracking, which provides excellent spatial resolution, though is costly and not as fast and facile as ultrasonography.[2,8]
Šteno et al.[8] demonstrated clinical utility of 3DUS powered Doppler in the intraoperative identification of the lenticulostriate arteries, though other authors have cited the need for technical improvements using this modality in order to improve sensitivity for detecting the lenticulostriate arteries.[31] This group reported on six patients undergoing microsurgical resection of insular gliomas evaluated preoperatively with structural and functional magnetic resonance imaging and diffusion tensor imaging with tractography, with two patients undergoing three dimensional time of flight magnetic resonance imaging to visualize the lenticulostriate arteries. Three patients each underwent single and two-staged resections. Kumabe et al., 1998 provide an outstanding description of two - staged insular glioma resection.[34] In the latter group, the first stage involved removal of the sphenoid bone and anterior temporal lobectomy, with patients undergoing removal of the remaining tumor during the second stage. Overall, five patients experienced subtotal resections, whereas one patient underwent a partial resection. Navigated 3D ultrasound powered Doppler successfully identified the lenticulostriate arteries intraoperatively in all patients and could provide an accurate estimate of tumor distance from these vessels. The relative location of the lenticulostriate arteries with respect to the tumor and the floor of the resection cavity was frequently periodically checked intraoperatively. In order to avoid injury to the lenticulostriate arteries, a small amount of gliomatous tissue was left unresected in a few patients.
Importantly, it may prove necessary to identify and preserve all lenticulostriate artery branches, as injury to even a single vessel could prove catastrophic.[21] While 3D ultrasound effectively identified lenticulostriate arteries in all cases of insular glioma resections, the proportion of lenticulostriate arteries identified using this modality is not known with precise certainty.[8] In an anatomical study, lenticulostriate arteries ranged from 1 to 15 (mean 7.75) per hemisphere, with an average diameter of 0.45 mm (range 0.1–1.5 mm) and with 73% of vessels <0.5 mm,[3] which could limit the sensitivity of 3D ultrasound in identifying all of the lenticulostriate vessels.
In a study evaluating sensitivity of magnetic resonance imaging for the detection of the lenticulostriate arteries, 1.5 Tesla magnetic resonance imaging failed to display these vessels with sufficient clarity,[32] and at least 1 lenticulostriate artery per patient was missed by 3 Tesla magnetic resonance imaging.[32] Moreover, even three dimensional time of flight magnetic resonance angiography with a 7 Tesla magnetic field could not detect all lenticulostriate arteries, since perforators <0.25 mm are not adequately visualized.[20] While 3D ultrasound is believed to be relatively angle-independent, it may not visualize the entire course of the LSAs.[8] Complete angle independence of three dimensional ultrasound may be facilitated by the use of contrast agents, which would be expected to improve lenticulostriate artery visualization.[8]
Other Vascular Etiologies for Postoperative Morbidity Following Insular Glioma Resection
While injury to the lenticulostriate arteries accounts for the majority of postoperative hemiparesis following insular glioma resection, injury to the long insular branches of the M2 segments of the MCA is not infrequently implicated.[1,15] For example, Moshel et al.[1] describe one patient, a 31-year-old female, who suffered compromise of a long insular M2 branch during microsurgical resection of the posterosuperior aspect of the tumor, which led to an infarct in the corona radiata sans injury to the LSAs. The patient developed postoperative hemiparesis which resolved to a residual hand weakness. These authors also reported on a patient experiencing MCA vasospasm following resection of a Group I insular glioma and resulting in hemiparesis and aphasia.[1] Subsequent angioplasty precipitated intracerebral hemorrhage, requiring surgical evacuation, and the patient had persistent neurological deficits at 6 years' follow-up.[1] These vessels are readily visualized and protected with meticulous dissection of the middle cerebral artery tree.[33,34,35]
Conclusions
It is evident and clear from the foregoing discussion that preservation of the lenticulostriate arteries remains of utmost importance during microsurgical resection of insular gliomas.[36,37,38] Although this goal may prove challenging to achieve, a few strategies may be effectively employed in order to enhance the probability of avoiding iatrogenic injury to these vessels. Operatively, the middle cerebral arterial tree should be fully dissected out in order to identify the lenticulostriate artery vessels. Co-registration of parenchymal and angiographic imaging obtained preoperatively may help to precisely delineate the relationship of tumor with respect to the lenticulostriate arteriess, and the use of intraoperative ultrasound provides real-time tracking which may be used to update neuronavigation in order to account for brain shift. Further innovation in neuroimaging technology will greatly enhance the intraoperative identification and preservation of lenticulostriate vessels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts for interest.
References
- 1.Moshel YA, Marcus JD, Parker EC, Kelly PJ. Resection of insular gliomas: The importance of lenticulostriate artery position. J Neurosurg. 2008;109:825–34. doi: 10.3171/JNS/2008/109/11/0825. [DOI] [PubMed] [Google Scholar]
- 2.Lang FF, Olansen NE, DeMonte F, Gokaslan ZL, Holland EC, Kalhorn C, et al. Surgical resection of intrinsic insular tumors: Complication avoidance. J Neurosurg. 2001;95:638–50. doi: 10.3171/jns.2001.95.4.0638. [DOI] [PubMed] [Google Scholar]
- 3.Türe U, Yaşargil MG, Al-Mefty O, Yaşargil DC. Arteries of the insula. J Neurosurg. 2000;92:676–87. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- 4.Hentschel SJ, Lang FF. Surgical resection of intrinsic insular tumors. Neurosurgery. 2005;57:176–83. doi: 10.1227/01.neu.0000163603.70972.ab. [DOI] [PubMed] [Google Scholar]
- 5.Rao AS, Thakar S, Sai Kiran NA, Aryan S, Mohan D, Hegde AS. Analogous three-dimensional constructive interference in steady state sequences enhance the utility of three-dimensional time of flight magnetic resonance angiography in delineating lenticulostriate arteries in insular gliomas: Evidence from a prospective clinicoradiologic analysis of 48 patients. World Neurosurg. 2018;109:e426–33. doi: 10.1016/j.wneu.2017.09.199. [DOI] [PubMed] [Google Scholar]
- 6.Ribas EC, Yagmurlu K, Wen HT, Rhoton AL., Jr Microsurgical anatomy of the inferior limiting insular sulcus and the temporal stem. J Neurosurg. 2015;122:1263–73. doi: 10.3171/2014.10.JNS141194. [DOI] [PubMed] [Google Scholar]
- 7.Rey-Dios R, Cohen-Gadol AA. Technical nuances for surgery of insular gliomas: Lessons learned. Neurosurg Focus. 2013;34:E6. doi: 10.3171/2012.12.FOCUS12342. [DOI] [PubMed] [Google Scholar]
- 8.Šteňo A, Jezberová M, Hollý V, Timárová G, Šteňo J. Visualization of lenticulostriate arteries during insular low-grade glioma surgeries by navigated 3D ultrasound power Doppler: Technical note. J Neurosurg. 2016;125:1016–23. doi: 10.3171/2015.10.JNS151907. [DOI] [PubMed] [Google Scholar]
- 9.Duffau H, Capelle L, Lopes M, Faillot T, Sichez JP, Fohanno D. The insular lobe: Physiopathological and surgical considerations. Neurosurgery. 2000;47:801–10. doi: 10.1097/00006123-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bykanov AE, Pitskhelauri DI, Pronin IN, Tonoyan AS, Kornienko VN, Zakharova NE, et al. 3D-TOF MR-angiography with high spatial resolution for surgical planning in insular lobe gliomas. Zh Vopr Neirokhir Im N N Burdenko. 2015;79:5–14. doi: 10.17116/neiro20157935-14. [DOI] [PubMed] [Google Scholar]
- 11.Vanaclocha V, Sáiz-Sapena N, García-Casasola C. Surgical treatment of insular gliomas. Acta Neurochir (Wien) 1997;139:1126–34. doi: 10.1007/BF01410972. [DOI] [PubMed] [Google Scholar]
- 12.Yaşargil MG. Microneurosurgery: Microneurosurgery of CNS Tumors. 4B. Stuttgart: Georg Thieme; 1996. pp. 263–8. [Google Scholar]
- 13.Yaşargil MG, Reeves JD. Tumours of the limbic and paralimbic system. Acta Neurochir (Wien) 1992;116:147–9. doi: 10.1007/BF01540867. [DOI] [PubMed] [Google Scholar]
- 14.Neuloh G, Pechstein U, Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg. 2007;106:582–92. doi: 10.3171/jns.2007.106.4.582. [DOI] [PubMed] [Google Scholar]
- 15.Saito R, Kumabe T, Inoue T, Takada S, Yamashita Y, Kanamori M, et al. Magnetic resonance imaging for preoperative identification of the lenticulostriate arteries in insular glioma surgery. Technical note. J Neurosurg. 2009;111:278–81. doi: 10.3171/2008.11.JNS08858. [DOI] [PubMed] [Google Scholar]
- 16.Zentner J, Meyer B, Stangl A, Schramm J. Intrinsic tumors of the insula: a prospective surgical study of 30 patients. J Neurosurg. 1996;85:263–71. doi: 10.3171/jns.1996.85.2.0263. [DOI] [PubMed] [Google Scholar]
- 17.Duffau H, Taillandier L, Gatignol P, Capelle L. The insular lobe and brain plasticity: Lessons from tumor surgery. Clin Neurol Neurosurg. 2006;108:543–8. doi: 10.1016/j.clineuro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ebeling U, Kothbauer K. Circumscribed low grade astrocytomas in the dominant opercular and insular region: A pilot study. Acta Neurochir (Wien) 1995;132:66–74. doi: 10.1007/BF01404850. [DOI] [PubMed] [Google Scholar]
- 19.Schätz CR, Kreth FW, Faist M, Warnke PC, Volk B, Ostertag CB. Interstitial 125-iodine radiosurgery of low-grade gliomas of the insula of Reil. Acta Neurochir (Wien) 1994;130:80–9. doi: 10.1007/BF01405506. [DOI] [PubMed] [Google Scholar]
- 20.Yaşargil MG, von Ammon K, Cavazos E, Doczi T, Reeves JD, Roth P. Tumours of the limbic and paralimbic systems. Acta Neurochir (Wien) 1992;118:40–52. doi: 10.1007/BF01400725. [DOI] [PubMed] [Google Scholar]
- 21.Duffau H. A personal consecutive series of surgically treated 51 cases of insular WHO grade II glioma: Advances and limitations. J Neurosurg. 2009;110:696–708. doi: 10.3171/2008.8.JNS08741. [DOI] [PubMed] [Google Scholar]
- 22.Liem MK, van der Grond J, Versluis MJ, Haan J, Webb AG, Ferrari MD, et al. Lenticulostriate arterial lumina are normal in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy: A high-field in vivo MRI study. Stroke. 2010;41:2812–6. doi: 10.1161/STROKEAHA.110.586883. [DOI] [PubMed] [Google Scholar]
- 23.Marinkovic S, Gibo H, Milisavljevic M, Cetkovic M. Anatomic and clinical correlations of the lenticulostriate arteries. Clin Anat. 2001;14:190–5. doi: 10.1002/ca.1032. [DOI] [PubMed] [Google Scholar]
- 24.Marinkovic SV, Milisavljevic MM, Kovacevic MS, Stevic ZD. Perforating branches of the middle cerebral artery. Microanatomy and clinical significance of their intracerebral segments. Stroke. 1985;16:1022–9. doi: 10.1161/01.str.16.6.1022. [DOI] [PubMed] [Google Scholar]
- 25.Yaşargil MG. Microneurosurgery: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain. Vol. 1. Stuttgart: Georg Thieme; 1984. pp. 77–83. [Google Scholar]
- 26.Rosner SS, Rhoton AL, Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468–85. doi: 10.3171/jns.1984.61.3.0468. [DOI] [PubMed] [Google Scholar]
- 27.Tanriover N, Kawashima M, Rhoton AL, Jr, Ulm AJ, Mericle RA. Microsurgical anatomy of the early branches of the middle cerebral artery: Morphometric analysis and classification with angiographic correlation. J Neurosurg. 2003;98:1277–90. doi: 10.3171/jns.2003.98.6.1277. [DOI] [PubMed] [Google Scholar]
- 28.Tanriover N, Rhoton AL, Jr, Kawashima M, Ulm AJ, Yasuda A. Microsurgical anatomy of the insula and the sylvian fissure. J Neurosurg. 2004;100:891–922. doi: 10.3171/jns.2004.100.5.0891. [DOI] [PubMed] [Google Scholar]
- 29.Türe U, Yaşargil DC, Al-Mefty O, Yaşargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–33. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- 30.Gibo H, Carver CC, Rhoton AL, Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–69. doi: 10.3171/jns.1981.54.2.0151. [DOI] [PubMed] [Google Scholar]
- 31.Yaşargil DC. Microneurosurgery. Vol. 4. Stuttgart: Thieme; 1994. [Google Scholar]
- 32.Yaşargil MG, Krisht AF, Türe U, Al-Mefty O, Yasargil DH. Microsurgery of insular gliomas: Part I. surgical anatomy of the sylvian cistern. Contemp Neurosurg. 2002;24:1–8. [Google Scholar]
- 33.Jakola AS, Berntsen EM, Christensen P, Gulati S, Unsgård G, Kvistad KA, et al. Surgically acquired deficits and diffusion weighted MRI changes after glioma resection – A matched case-control study with blinded neuroradiological assessment. PLoS One. 2014;9:e101805. doi: 10.1371/journal.pone.0101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumabe T, Nakasato N, Suzuki K, Sato K, Sonoda Y, Kawagishi J, Yoshimoto T. Two-staged resection of a left frontal astrocytoma involving the operculum and insula using intraoperative neurophysiological monitoring--case report. Neurol Med Chir (Tokyo) 1998;38:503–7. doi: 10.2176/nmc.38.503. [DOI] [PubMed] [Google Scholar]
- 35.Akashi T, Taoka T, Ochi T, Miyasaka T, Wada T, Sakamoto M, et al. Branching pattern of lenticulostriate arteries observed by MR angiography at 3.0 T. Jpn J Radiol. 2012;30:331–5. doi: 10.1007/s11604-012-0058-7. [DOI] [PubMed] [Google Scholar]
- 36.Heffez DS. Stereotactic transsylvian, transinsular approach for deep-seated lesions. Surg Neurol. 1997;48:113–24. doi: 10.1016/s0090-3019(96)00463-6. [DOI] [PubMed] [Google Scholar]
- 37.Sanai N, Polley MY, Berger MS. Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112:1–9. doi: 10.3171/2009.6.JNS0952. [DOI] [PubMed] [Google Scholar]
- 38.Yasargil MG, Abdulrauf S. Comment on: The insular lobe: Physiopathological and surgical considerations. Neurosurgery. 2000;47:801–10. doi: 10.1097/00006123-200010000-00001. [DOI] [PubMed] [Google Scholar]


