Abstract
Purpose:
The use of intraoperative 5-aminolevulinic acid fluorescence has been shown to increase the extent of resection in high-grade glioma surgery. Sodium fluorescein is an alternate fluorescence agent with advantages of low cost, low adverse effect profile, and ability to visualize anatomical detail under the fluorescence filter. Sodium fluorescein-based fluorescence is not specific to tumor cells, and the significance of residual fluorescence at tumor margins has been questioned. In this article, the authors sought to correlate fluorescence intensity at tumor margins with the presence of residual contrast-enhancing tumor on magnetic resonance imaging (MRI).
Methods:
Eleven patients with a total of 12 lesions were enrolled in the study. Sodium fluorescein was administered at a dose of 5 mg/kg on induction of anesthesia. Relative intensity of fluorescence was extrapolated from intraoperative photographs through isolation of the green channel from the red/green/blue image, then graphically representing of pixel intensity through application of a thermal map. The correlation between areas of avid fluorescence at tumor cavity margins and the presence of residual contrast-enhancing tumor on postoperative MRI was evaluated.
Results:
All tumors demonstrated fluorescence. The presence of avid fluorescence at tumor cavity margins had a sensitivity of 66.7% and specificity of 75% for the presence of residual contrast-enhancing tumor on postoperative MRI. There were no adverse effects of fluorescein administration.
Conclusion:
Quantification of relative fluorescence intensity allows easy identification of areas that are high risk for residual contrast-enhancing tumor. Graphical representation of green pixel intensity requires validation through histopathological analysis but has the potential for real-time clinical application.
Keywords: Fluorescence brain tumor, fluorescence-guided surgery, high-grade glioma, sodium fluorescein
Introduction
Maximal safe resection has been shown to correlate with improvement in both progression free and overall survival in high-grade glioma.[1] Surgical adjuncts that have been shown to improve extent of resection include the use of fluorescence, intraoperative magnetic resonance imaging (MRI), and neuronavigation.[2] Fluorescence has the advantage of being readily available, as well as more time and cost-efficient than intraoperative MRI, and furthermore is not subject to the inaccuracies encountered with brain shift or registration error with neuronavigation.[3]
The primary fluorescence agent that has been studied in glioma is 5-aminolevulinic acid (5-ALA). This produces fluorescent porphyrins that accumulate in glioma cells, resulting in fluorescence under blue light.[4] The limitations of this agent include cost, the need to switch between blue light (for identification of fluorescent tissue) and white light (to delineate the anatomy of the nonfluorescent tissue and vessels for coagulation) frequently during surgery, as well as the side effects associated with photosensitivity of the 5-ALA compound.[3]
Sodium fluorescein has been suggested as an alternative to 5-ALA. Intravenous sodium fluorescein administration results in green fluorescence under yellow light in areas of blood–brain barrier impairment due to the accumulation of sodium fluorescein in the extracellular space.[3,5,6] The primary criticism regarding the use of sodium fluorescein pertains to the fact that fluorescence is not tumor cell specific – there is a potential for false-positive fluorescence garnered by accumulation in areas of perilesional edema and surgical tissue injury.[7,8]
At present, there are few reports regarding the efficacy of sodium fluorescein at tumor margins. Investigation of the specificity of sodium fluorescein in detecting residual tumor at resection cavity margins has been largely limited to biopsy-based histological studies in high-grade glioma patients.[3,5,9,10,11,12,13] In this study, we present our early experience with the use of sodium fluorescein in brain tumor surgery and describe a technique that may facilitate the correlation between marginal sodium fluorescein uptake and residual contrast-enhancing tumor.
Methods
Ethics approval was obtained through the Health District Human Research Ethics Committee, and a clinical trial notification was provided to the Therapeutic Goods Administration given the off-label use of the drug. Participants over the age of 18 years who were undergoing surgery for primary or secondary brain tumors at a single university-affiliated tertiary institution (Royal North Shore Hospital, Sydney, Australia) were invited to participate. Patients with extrinsic tumors, nonenhancing tumors, or with hypersensitivity to sodium fluorescein were excluded from the study. Written informed consent was obtained in all cases.
The dosing protocol of Acerbi et al. in the FLUOGLIO study was replicated.[3] A single intravenous dose of 5 mg/kg of sodium fluorescein (Retinofluor, Phebra Pty Ltd.) was administered immediately on induction of general anesthesia. Standard white light surgical resection of the tumor was performed with the aid of neuronavigation and the Zeiss KINEVO microscope (Carl Zeiss Meditec, Oberkochen, Germany). Fluorescence was not used to guide further surgical resection in this study.
Intraoperative photographs of tumors and perilesional tissues were taken under white light and with the Yellow 560 nm filter applied (Carl Zeiss Meditec, Oberkochen, Germany). Time from administration of fluorescein to early and final tumor cavity photographs was recorded. Tumor histology was recorded, and those patients with high-grade gliomas underwent a postoperative MRI within 48 h of surgery to quantify the extent of resection. A gross-total resection was defined as a >90% resection of contrast-enhancing tumor.
Residual marginal fluorescence following tumor removal was recorded through intraoperative photography with both white light and under the Yellow 560 nm filter. In order to distinguish areas of more avid fluorescence expression from general background fluorescence, an open-source biological-image analysis program (FIJI)[14] was then used to segment the original color Yellow 560 nm filtered photograph into red/green/blue monochrome channels. Following this, a thermal filter was applied to the green pixel substrate to aid in identifying the areas of relatively avid green fluorescence [Figure 1]. On these “thermal maps,” blue was interpreted as no fluorescence, green as mild-moderate, and red as avid fluorescence.
Figure 1.
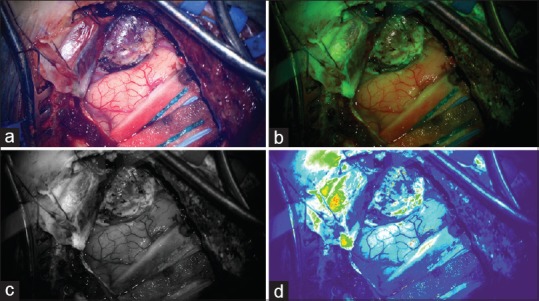
(a) White light photograph of a well-demarcated superficial lesion. (b) Heterogeneous fluorescence in lesion and dura demonstrated with Yellow 560 nm filter applied. (c) Green monochrome of fluorescence photograph. (d) Thermal filter applied to green monochrome to demonstrate moderate fluorescence intensity in the area of tumor adjacent to the cortex
This “fluorescence map” was then analyzed by the investigators to determine which surfaces of the final resection cavity featured ongoing high-intensity fluorescence. Those surfaces were then recorded and explored for the presence of residual contrast-enhancing disease on the postoperative MRI. Through this method, the sensitivity and specificity of sodium fluorescein in detecting residual contrast-enhancing disease at tumor margins was assessed.
The primary outcome was the specificity of marginal fluorescence in indicating the presence of residual contrast-enhancing tumor. Secondary outcomes were dynamic changes in intraoperative fluorescence and toxicity.
Results
Over a 3-month period (December 2018–February 2019), 11 patients with a total of 12 lesions were enrolled in the study. The mean age was 63.5 years (range: 36–74), with a male-to-female ratio of 1:1.2. The majority of the lesions were supratentorial (75%). All patients received a sodium fluorescein dose of 5 mg/kg without any adverse effect. The median time from injection to initial tumor exposure was 76 min and from injection to final cavity check was 138 min.
Histopathology demonstrated glioblastoma in seven patients (64%), metastasis in three patients (27%), and pilocytic astrocytoma in one patient. One patient had two metastatic lesions excised in the same operation. Three of the 11 patients were a recurrence of a previously excised lesion (27%). Demographic data are presented in Table 1.
Table 1.
Demographic characteristics
| No. | Age | Sex | Histopathology | Location | Recurrence | Post-Op MRI | GTR |
|---|---|---|---|---|---|---|---|
| 1 | 70 | M | GBM, IDH-WT | Right temporal | No | Y | Y |
| 2 | 69 | F | GBM, IDH-WT (Gliosarcoma) | Right temporo-parietal | Yes | Y | Y |
| 3 | 66 | F | Pilocytic Astrocytoma | Cerebellar | Yes | N | Y |
| 4 | 73 | M | Small cell neuroendocrine carcinoma | Cerebellar | No | N | Y |
| 5 | 74 | M | Sqaumous cell carcinoma | Left frontal | Yes | N | Y |
| 6 | 36 | M | GBM, IDH-WT | Right frontal | No | Y | Y |
| 7 | 61 | F | GBM, IDH-WT | Left temporal | No | Y | Y |
| 8 | 57 | F | GBM, IDH-WT | Left temporo-parietal | No | N | N |
| 9 | 67 | F | GBM, IDH-WT | Left temporal | No | Y | N |
| 10 | 72 | F | Melanoma | Left frontal | No | N | Y |
| 11 | 54 | M | GBM, IDH-WT | Right frontal | No | Y | Y |
GBM: Glioblastoma; IDH-WT: IDH wildtype, GTR: Gross Total Resection
Of the seven patients with glioblastoma, an early postoperative MRI was obtained in six patients. This demonstrated gross-total resection in four patients (67%). All four metastatic lesions were excised in an en bloc manner and deemed to be completely excised by the operating surgeon.
All tumors in our study demonstrated fluorescence under the Yellow 560 nm filter (100% sensitivity). As previously reported in the literature, the fluorescence characteristics of these tumors were heterogeneous with less avid enhancement noted in areas of central necrosis.[3,12] Where lesions presented to the cortical surface or were immediately subcortical, fluorescence borders correlated strongly with neuronavigation and white light assessment of abnormal tissue [Figure 2].
Figure 2.
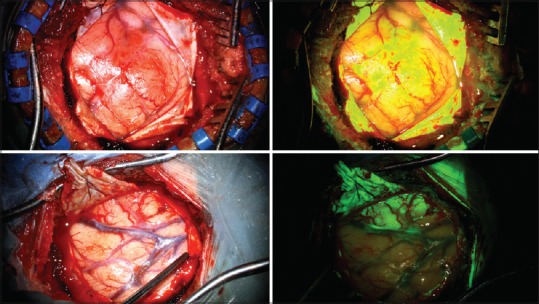
White light (left) and Yellow 560 nm (right) photographs demonstrating cortical and subcortical lesions with well-demarked superficial fluorescence boundaries
Some degree of perilesional fluorescence was seen in all cases. In particular, the three patients that were being treated for a recurrence of a previously excised lesion (pilocytic astrocytoma, glioblastoma, and metastasis) had marked fluorescence of the gliotic brain around the tumor cavity [Figure 3]. Interestingly, all three metastatic lesions also had notable homogenous tumor cavity fluorescence following en bloc resection. Intraoperatively, this was able to be distinguished from the more avid fluorescence of the lesion itself. No dynamic changes in the presence or intensity of marginal fluorescence were noted intraoperatively or on postoperative review of intraoperating images.
Figure 3.
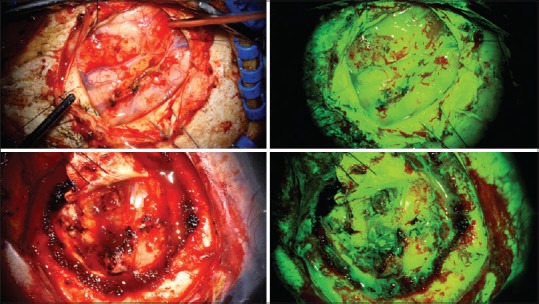
White light (left) and Yellow 560 nm (right) photographs of two patients with recurrent tumors demonstrating widespread sodium fluorescence uptake in tumor as well as the surrounding gliotic brain
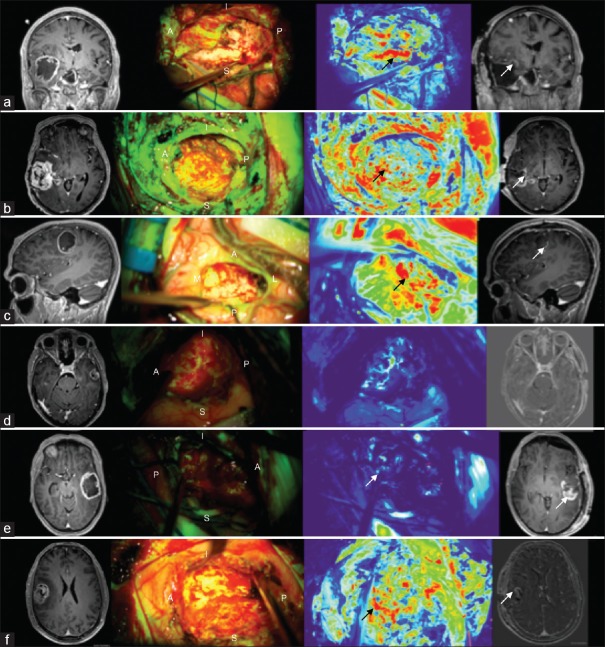
Peripheral fluorescence was more heterogeneous in high-grade glioma patients. In all six glioblastoma patients who had an early postoperative MRI, analysis of tumor cavity photographs taken with the Yellow 560 nm filter and aided by fluorescence intensity mapping demonstrated a strong correlation between areas of relative high fluorescence and the presence of residual contrast-enhancing tumor. Furthermore, areas of relative quiescence on fluorescence intensity-mapped photographs were associated with an absence of residual contrast-enhancing disease on MRI [Figure 4].
Figure 4.
Cavity fluorescence in high-grade glioma patients. Orientation A – anterior, P – posterior, S – superior, I – inferior, M – medial, L – lateral. (a) Strong correlation between area of high relative fluorescence on thermal map (black arrow) and residual tumor on magnetic resonance imaging (white arrow). (b) Redo case with high relative fluorescence in surrounding gliotic cortex (under P) as well as medial avid fluorescence (black arrow) which correlates with residual disease on magnetic resonance imaging (white arrow). Residual tumor at posterior margin not obvious given more avid signal from other areas. (c) Posterior avid fluorescence (black arrow) with correlating with small area of posterior enhancement on magnetic resonance imaging (white arrow). (d) No avid enhancement on thermal imaging and no residual disease on magnetic resonance imaging. (e) Low-avidity signal from posterior cavity not reflecting of large posterior residual on magnetic resonance imaging (white arrow). (f) High relative fluorescence anteriorly (black arrow) correlating with small volume of residual enhancement on subtracted magnetic resonance imaging (white arrow)
Twenty margins were assessed for residual fluorescence in the six glioblastoma patients who had an early postoperative MRI. The presence of avid fluorescence (red on thermal maps) had a sensitivity of 66.7% and specificity of 75% for the presence of residual contrast-enhancing tumor on postoperative MRI. A false-negative result was recorded in patient B and patient E with lack of high-intensity fluorescence at the posterior cavity margin despite the presence of bulky residual disease on MRI. There was one false positive in patient F where appropriate avid tumor fluorescence was depicted anteriorly, but inappropriate avid fluorescence was present medially without residual contrast-enhancing disease here on MRI.
Discussion
The use of intraoperative fluorescence demonstrates great potential in facilitating maximal resection of tumors. 5-ALA has been shown in a Phase III study to significantly improve the extent of resection when compared to conventional white light microsurgery. This afforded a progression-free survival benefit in this group.[4] However, it has a number of limitations including cost, photosensitivity, loss of anatomical detail under blue light, and requirement for early dosing.
Sodium fluorescein has been proposed as an alternative fluorescence agent that has the advantages of significantly lower cost, ability to visualize anatomical detail under yellow light, relative simplicity of dosing, and a lower adverse effect profile. However, unlike 5-ALA which results in the accumulation of fluorescent porphyrins in malignant glioma cells, sodium fluorescein produces nonspecific fluorescence of areas where there is an impairment of the blood–brain barrier.[6]
Murray first reported on the sensitivity and specificity of sodium fluorescein in 1982.[15] In the past 5 years, six studies have reported on the sensitivity and specificity of sodium fluorescein in glioma surgery.[3,5,9,10,11,12,13] In the Phase II FlUOGLIO study, Acerbi et al. found sodium fluorescein to have a sensitivity of 80.8% and specificity of 79.1% through histopathological analysis of fifty biopsies of fluorescent and nonfluorescent tissue at tumor margins.[3] Other authors also document sensitivities and specificities in excess of 80% [Table 2].
Table 2.
Studies reporting sensitivity and specificity of sodium fluorescein
| Study | Patients | Dose (mg/kg) | Pathology | Fluorescent Biopsies | Non-fluorescent Biopsies | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Dios et al. (2014) | 6 | 3 | GBM | 15 | 11 | 79 | 100 |
| Diaz et al. (2015) | 12 | 3 | GBM | 39 | 28 | 82.8 | 90.9 |
| Acerbi et al. (2017) | 13 | 5 | IDH-wt GBM | 26 | 24 | 80.8 | 79.1 |
| Zhang et al. (2017) | 38 | 2-3 | Mixed glial | 54 | 35 | 94.4 | 88.6 |
| Neira et al. (2017) | 26 | 3 | GBM | 70 | 20 | 75.6 | 75 |
| Catapano et al. (2017) | 23 | 5 | High Grade Glioma | NS | NS | 84.6 | 95 |
NS: Not specified
However, these results must be interpreted with a degree of caution as some authors incorporate biopsies taken from fluorescent and nonfluorescent tissues within the tumor core into their specificity calculation.[10,12] These biopsies from known contrast-enhancing areas reflect the variable fluorescence seen with necrosis in the tumor core but fail to address the key clinical question of the significance of ongoing fluorescence in noncontrast-enhancing areas at tumor margins.
The most rigorous analysis of residual fluorescence at tumor margins has been performed by Neira et al.[9] In their study of 32 patients with glioblastoma, fluorescence always resulted in a histopathologically abnormal biopsy in contrast-enhancing regions of tumor. They reported an overall sensitivity of 75.6% and specificity of 75% across both contrast-enhancing and noncontrast-enhancing regions. However, when this was limited to assessment of residual fluorescence in the noncontrast-enhancing tumor margin, the sensitivity fell to 69.4% and specificity to 66.7%.
Through postoperative quantification of the degree of fluorescence, they were able to identify a “threshold value” of 0.1 normalized fluorescence intensity in only yielding biopsies specific for tumor or infiltrating tumor in noncontrast-enhancing areas at tumor margins.[9]
While Neira et al. report a valuable guide to identifying infiltrating tumor in nonenhancing regions, their method of quantification of fluorescence requires the selection of active and background regions of interest to determine specific quantitative fluorescence intensity. This may be difficult and time-consuming to do in real time in the intraoperative setting, rendering their technique somewhat impractical. Furthermore, their results also suggest that the correlation between subjective and objective classifications of fluorescence intensity is only strong when fluorescence is absent or high and prone to error when fluorescence intensity is medium or low.
Our method uses a similar approach to Neira et al. in isolating the green pixel monochrome but then utilizes a “thermal look-up table” to provide a visual display of relative fluorescence intensity without the need for manual selection of regions of interest. This allows quick identification of areas of intense fluorescence expression that may not be as obvious under Yellow 560 nm light given background fluorescence. In our study, areas of high relative fluorescence intensity correlated strongly with the presence of residual contrast-enhancing tumor on postoperative MRI [Figure 4]. It is feasible that the existing microscope software could be updated to include the ability to analyze relative fluorescence intensity in real time through overlay of a graphical representation of fluorescence intensity onto anatomical detail in intraoperative photographs.
The specificity of our technique (75%) matched that described by other authors who focused their assessment on fluorescence at tumor margins.[3,9] The sensitivity calculated by our technique (66.7%) was lower than that reported by groups in the literature with the exception of the only other group to apply quantitative analysis.[9] In our study, accurate calculation of sensitivity at tumor margins may have been confounded by the presence of more bulky residual disease in the two patients who recorded a false-negative margin – the lack of fluorescence may be attributable to the presence of ongoing necrotic tumor at this margin with an expected paucity of fluorescence expression. Although these figures are in keeping with those reported by other groups in the literature, they are not statistically robust due to the small sample size, lack of histopathological validation, and nonblinded assessment of postoperative imaging and thermal maps. If integrated into the microscope software in the future, this technique requires further validation with a combined postoperative imaging and intraoperative biopsy-based calculation of the sensitivity and specificity of areas of high relative fluorescence intensity for residual tumor.
A number of minor limitations regarding the use of sodium fluorescein were flagged in our early experience. Dural fluorescence was seen in all cases but did not interfere with assessment of fluorescence in the brain parenchyma. In some cases, we noted pooling of the fluorescence agent in blood at the surgical site and extradurally. There was no significant time difference between injection and assessment in these cases when compared to other cases in which this was not observed. The utility of fluorescein in detecting residual tumor at margins in redo surgical cases is limited by the avid fluorescence of the surrounding gliotic brain.
It is unclear why there is perilesional fluorescence in brain metastases. This was noted in all of our metastasis resections as well as by previous authors in a large study of patients with brain metastases.[16] Subjectively, residual marginal fluorescence was felt to be homogeneous and of lower intensity than that in the metastasis itself. Sequential intraoperative photographs in cerebral metastasis patients did not demonstrate dynamic changes in marginal fluorescence suggestive of fluorescence due to surgical tissue injury.
It is difficult to imagine that a histopathological study of fluorescence specificity in these patients will yield similar results to that in patients with infiltrating gliomas. A method of assessing relative fluorescence intraoperatively may be useful in differentiating small areas of residual tumor from background fluorescence in these cases. Our method of graphically depicting relative fluorescence intensity is one such way of doing this and further study with histopathological correlation would be the next stage in the validation of this technique.
Conclusion
This is a pilot study of the authors' initial experience with sodium fluorescein and suffers a number of limitations including small population size, a nonhomogeneous patient population, lack of histopathological analysis, and nonblinded assessment of postoperative imaging and thermal maps. Although sodium fluorescein was felt to be useful in delineating residual disease, maximal fluorescence-guided resection was not pursued in this study due to our uncertainty regarding the sensitivity and specificity of sodium fluorescein at tumor margins and our awareness for the potential for false-positive fluorescence at tumor margins. The sensitivity of sodium fluorescein at tumor margins is likely to be greater than what was reported in our study given confounding by the presence of necrotic tumor layers on postoperative imaging.
These limitations preclude rigorous statistical analysis; however, we have been able to replicate the success of other authors in the safe use of sodium fluorescein at low doses and more significantly describe a potential method through which the specificity of sodium fluorescein in detecting residual disease at tumor margins may be amplified. The integration of this technique into the microscope software interface would permit real time use and facilitate histopathological validation in future studies.
Financial support and sponsorship
This study was financially supported by the Department of Neurosurgery, Royal North Shore Hospital.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130:269–82. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 2.Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, et al. The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res. 2000;22:354–60. doi: 10.1080/01616412.2000.11740684. [DOI] [PubMed] [Google Scholar]
- 3.Acerbi F, Broggi M, Schebesch KM, Höhne J, Cavallo C, De Laurentis C, et al. Fluorescein-Guided surgery for resection of high-grade gliomas: A multicentric prospective phase II study (FLUOGLIO) Clin Cancer Res. 2018;24:52–61. doi: 10.1158/1078-0432.CCR-17-1184. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 5.Diaz RJ, Dios RR, Hattab EM, Burrell K, Rakopoulos P, Sabha N, et al. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg. 2015;122:1360–9. doi: 10.3171/2015.2.JNS132507. [DOI] [PubMed] [Google Scholar]
- 6.Xiang Y, Zhu XP, Zhao JN, Huang GH, Tang JH, Chen HR, et al. Blood-brain barrier disruption, sodium fluorescein, and fluorescence-guided surgery of gliomas. Br J Neurosurg. 2018;32:141–8. doi: 10.1080/02688697.2018.1428731. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W. Poor man's fluorescence? Acta Neurochir (Wien) 2015;157:1379–81. doi: 10.1007/s00701-015-2471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stummer W. Fluorescein in brain metastasis and glioma surgery. Acta Neurochir (Wien) 2015;157:2199–200. doi: 10.1007/s00701-015-2576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neira JA, Ung TH, Sims JS, Malone HR, Chow DS, Samanamud JL, et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J Neurosurg. 2017;127:111–22. doi: 10.3171/2016.7.JNS16232. [DOI] [PubMed] [Google Scholar]
- 10.Rey-Dios R, Hattab EM, Cohen-Gadol AA. Use of intraoperative fluorescein sodium fluorescence to improve the accuracy of tissue diagnosis during stereotactic needle biopsy of high-grade gliomas. Acta Neurochir (Wien) 2014;156:1071–5. doi: 10.1007/s00701-014-2097-6. [DOI] [PubMed] [Google Scholar]
- 11.Catapano G, Sgulò FG, Seneca V, Lepore G, Columbano L, di Nuzzo G. Fluorescein-guided surgery for high-grade glioma resection: An intraoperative “contrast-enhancer”. World Neurosurg. 2017;104:239–47. doi: 10.1016/j.wneu.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Tian H, Huang D, Meng X, Guo W, Wang C, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant gliomas: Our first 38 cases experience. Biomed Res Int. 2017;2017:1–10. doi: 10.1155/2017/7865747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallo C, De Laurentis C, Vetrano IG, Falco J, Broggi M, Schiariti M, et al. The utilization of fluorescein in brain tumor surgery: A systematic review. J Neurosurg Sci. 2018;62:690–703. doi: 10.23736/S0390-5616.18.04480-6. [DOI] [PubMed] [Google Scholar]
- 14.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray KJ. Improved surgical resection of human brain tumors: Part I. A preliminary study. Surg Neurol. 1982;17:316–9. doi: 10.1016/0090-3019(82)90298-1. [DOI] [PubMed] [Google Scholar]
- 16.Höhne J, Hohenberger C, Proescholdt M, Riemenschneider MJ, Wendl C, Brawanski A, et al. Fluorescein sodium-guided resection of cerebral metastases-an update. Acta Neurochir (Wien) 2017;159:363–7. doi: 10.1007/s00701-016-3054-3. [DOI] [PubMed] [Google Scholar]