Abstract
Single-cell RNA sequencing (scRNA-seq) of bone marrow or peripheral blood samples from patients with acute myeloid leukemia (AML) enables the characterization of heterogeneous malignant cells. A total of 87 cells from two patients with t(8;21) AML were analyzed using scRNA-seq. Clustering methods were used to separate leukemia cells into different sub-populations, and the expression patterns of specific marker genes were used to annotate these populations. Among the 31 differentially expressed genes in the cells of a patient who relapsed after hematopoietic stem cell transplantation, 13 genes were identified to be associated with leukemia. Furthermore, three genes, namely AT-rich interaction domain 2, lysine methyltransferase 2A and synaptotagmin binding cytoplasmic RNA interacting protein were validated as possible prognostic biomarkers using two bulk expression datasets. Taking advantage of scRNA-seq, the results of the present study may provide clinicians with several possible biomarkers to predict the prognostic outcomes of t(8;21) AML.
Keywords: acute myeloid leukemia, t(8;21), single-cell RNA-sequencing, prognosis
Introduction
As the most common type of adult leukemia, acute myeloid leukemia (AML) is characterized by the excessive expansion of immature myeloblasts from leukemic stem cells (LSCs) (1). LSC-based gene sets have previously been selected to predict the clinical outcomes of AML, particularly cytogenetically normal AML (CN-AML) (2,3). The t(8;21) chromosomal rearrangement is one of the most classic genetic abnormalities in AML, and results in a transcript encoding for the fusion protein acute myeloid leukemia 1 protein-protein ETO (AML1-ETO; also known as RUNX1-RUNX1T1) (4). Following conventional chemotherapy, patients with t(8;21) AML have a relatively favorable prognosis, and steady progress has been observed in the success of t(8;21) AML treatment (5). However, the relapse and long-term survival rate are less than optimal, and highlight the requirement for more accurate diagnostic and therapeutic strategies (6); elucidation of the molecular mechanisms of t(8;21) AML are fundamental to the development of more precise diagnostic and therapeutic methods.
Single-cell RNA sequencing (scRNA-seq) has been widely used in developmental biology and oncological research, primarily due to its ability to profile rare or heterogeneous populations of cells (7). In the present study, scRNA-seq analysis was performed on 87 cells from two patients with t(8;21) AML. Single cells were separated into subpopulations with specific gene marker expression patterns; 31 differentially expressed genes (DEGs) were identified from the cells of patient B, which were considered to be associated with poor patient outcome. Furthermore, three genes, namely AT-rich interaction domain 2 (ARID2), lysine methyltransferase 2A (MLL) and synaptotagmin binding cytoplasmic RNA interacting protein (SYNCRIP) were demonstrated to have prognostic significance in two bulk expression datasets of patients with t(8;21) AML (GSE37642 and GSE6891) (8,9). To conclude, the present study is, to the best of the authors' knowledge, the first to demonstrate the single-cell transcriptome profile of two patients with t(8;21) AML, and to suggest several possible prognostic biomarkers.
Materials and methods
Patients and specimens
Patient recruitment and sample collection took place at Chinese PLA General Hospital from January 2014 to December 2015. The present study was approved by The Institutional Review Board of Chinese PLA General Hospital, and written informed consent was obtained from both patients with AML. To classify the subtype and prognostic risk of the two patients, chromosome banding, immunophenotyping, flow cytometric analysis and real-time PCR for the fusion genes were conducted.
Targeted DNA sequencing
Targeted DNA sequencing of bone marrow samples was performed as previously described (10).
Single-cell isolation, cDNA amplification and RNA-sequencing
Single cells were isolated from the bone marrow (BM) and peripheral blood (PB) of the two patients. Single-cell loading, capture and cDNA amplification were carried out using the C1™ Single-Cell Auto Prep system (Fluidigm). A total of 87 single cells were loaded into a medium-sized C1 Single-Cell Auto Prep integrated fluidics circuit, as previously described (11). Afterwards, capture, reverse transcription and cDNA amplification were immediately performed according to the manufacturer's instructions (Fig. S1). RNA was extracted from samples and cDNA was reverse transcribed (Reverse Transcription System A3500; Promega Corporation) from RNA with TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The reaction conditions used were as follows: Pre-denaturation at 95°C for 15 min; then denaturation at 94°C for 30 sec, annealing at 53°C for 30 sec, and extension at 72°C for 30 sec, 28 cycles; final extension at 72°C for 8 min. Sequencing libraries were constructed using the Nextera XT DNA Sample Prep kit (Illumina, Inc.) and sequenced using the HiSeq2500 platform (Illumina, Inc.). Paired-end 100-bp reads were quality- and adapter-filtered using Trim Galore! software (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/; version 0.4.4).
Gene fusion prediction
For each cell, the clean reads were mapped to the human genome reference sequences (hg19 version) using the STAR aligner (v2.4.1) (12), and fusion gene detection was performed using STAR-Fusion (https://github.com/STAR-Fusion/STAR-Fusion; v1.3.2), which compared with other methods, was sufficient for fusion RNA prediction (13).
Quantitative-PCR (qPCR)
qPCR was performed with the iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Inc.) using cDNA from five cells from patient A (newly diagnosed) with potential AML1-ETO gene fusion. The following reaction conditions were used: Pre-denaturation at 95°C for 1 min; then denaturation at 95°C for 5 sec, and extension at 53°C for 20 sec, 40 cycles; final denaturation at 95°C for 1 min, 60°C for 1 min, 95°C for 30 sec. GAPDH was used as an internal reference gene, and the primer sequences of GAPDH were as follows: Forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTGATTTTGGAGGGATCTCG-3′; and the primer sequences of AML1-ETO were as follows: Forward, 5′-AACCACTCCACTGCCTTTAACC-3′ and reverse, 5′-TGGAGGAGTCAGCCTAGATTGC-3′. The 2−∆∆Cq method (14) was used to quantify the AML1-ETO gene fusions. Due to the shortages of cDNA left over after the construction of the sequencing libraries, AML1-ETO fusion in each cell was measured using both the Mx3005P qPCR System (Agilent Technologies, Inc.) and the ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Bioinformatics analysis
The high-quality reads were pseudo-aligned with the human genome reference sequence (Ensembl Release 72 of GRCh37) annotations using Kallisto (15), and quantified as transcript per million (TPM) using AltAnalyze (16). Expression levels were transformed as log2(TPM/10+1) as described in previous studies (17,18), and single cells were subjected to hierarchical clustering according to their expression levels. Principal component analysis (PCA) was then performed based on the results of hierarchical clustering. Furthermore, unsupervised clustering was performed with scRNA-seq data from patient B using the SC3 pipeline (version 1.12.0) (19). The functions of DGEs were determined through a literature review (20–36). Interactions between DEGs were analyzed using the Gene Multiple Association Network Integration Algorithm (GeneMANIA; http://www.genemania.org/; accessed July 24, 2019) (37). The Search Tool for the Retrieval of Interacting Genes (STRING; https://string-db.org/; accessed July 24, 2019) was used to investigate the protein-protein interactions between DEGs (38).
Statistical analysis
The heatmap of 31 DEGs were performed using the pheatmap (https://CRAN.R-project.org/package=pheatmap; version 1.0.12) R package. The expression levels of the four marker genes were analyzed using the ggpubr (https://CRAN.R-project.org/package=ggpubr; version 1.0.12) R package and Kruskal-Wallis test.
Survival analysis
The expression matrices of two GEO datasets GSE37642 (9) and GSE6891 (8) were downloaded from The National Center of Biotechnology Information using the GEOquery (version 2.52.0) R package (39). In total, 30 and 22 patients with t(8;21) AML from GSE37642 and GSE6891, respectively, were selected for survival analysis. For each gene, the expression value of a selected probe was used to represent the expression level of the gene. A median, tri-sectional quantile or quartile threshold of expression values was used to categorize patients into high- and low-expression groups. The survminer (https://CRAN.R-project.org/package=survminer; version 2.52.0) R package and Log-rank test were used for visualizing the Kaplan-Meier estimates of survival curves.
Results
scRNA-seq for two patients with t(8;21) AML
The two enrolled female patients represented the two stages of AML: Newly diagnosed (patient A) and relapse after hematopoietic stem cell transplantation (HSCT; patient B). The clinical information of these two patients is presented in Table I. The French American-British Cooperative Group Criteria (5), chromosomal karyotype analysis, flow cytometric analysis, reverse transcription and real-time fluorescent qPCR all suggested that both patients possessed the t(8;21) translocation, which classified them as AML type M2. A total of five cells from patient A were predicted to possess the AML1-ETO (RUNX1-RUNX1T1) gene fusion (Table SI), which was confirmed by qPCR (Fig. S2). These results based on scRNA-seq data demonstrated the existence of the AML1-ETO fusion in patient A. However, due to the low amount of data, none of the cells from patient B were predicted to harbor AML1-ETO fusions. Moreover, none of the known AML-associated somatic mutations were detected by targeted DNA-sequencing.
Table I.
Clinical information of two patients with acute myeloid leukemia.
| Name | Age, years | Sex | Tissue | Blast, %a | Stage at analysis | Karyotype |
|---|---|---|---|---|---|---|
| Patient A | 74 | Female | PB | 81 | New diagnosis | 46,XX,t(8;21) |
| Patient B | 29 | Female | BM | 94 | Relapse after HSCT | 46,XX,t(8;21) |
Percentage blast value was from PB samples of both patients. PB, peripheral blood; BM, bone marrow; HSCT, hematopoietic stem cell transplantation.
The treatment outcome for patient A was more favorable, as she achieved complete remission after a course of chemotherapy, and didn't relapse until death from another cause ~15 months later. On the contrary, the outcome for patient B was poor, due to relapse after the 15th course of chemotherapy and a second relapse 3 months after HSCT.
Single cells were isolated from the PB and BM of the two patients, and 87 cells qualified for the generation of RNA-seq data. With the exception of one of the cells (with a total read of 19 million), the total reads of the 87 cells ranged from 0.4 to 9 million (Table SI), which was sufficient for scRNA-seq analysis (10). The median reads of the patient B cells were relatively lower than those from patient A (Fig. S3), suggesting their abnormal transcriptional programs.
Separation of leukemia cell subpopulations
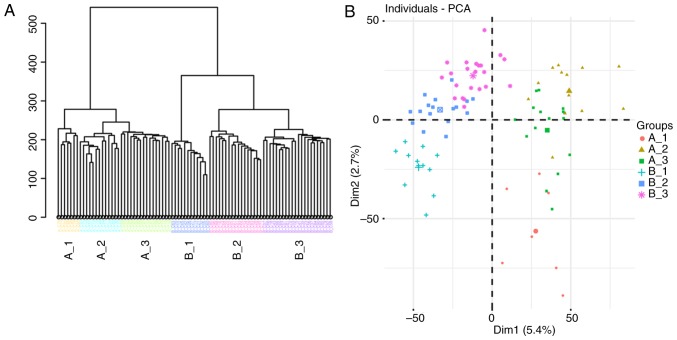
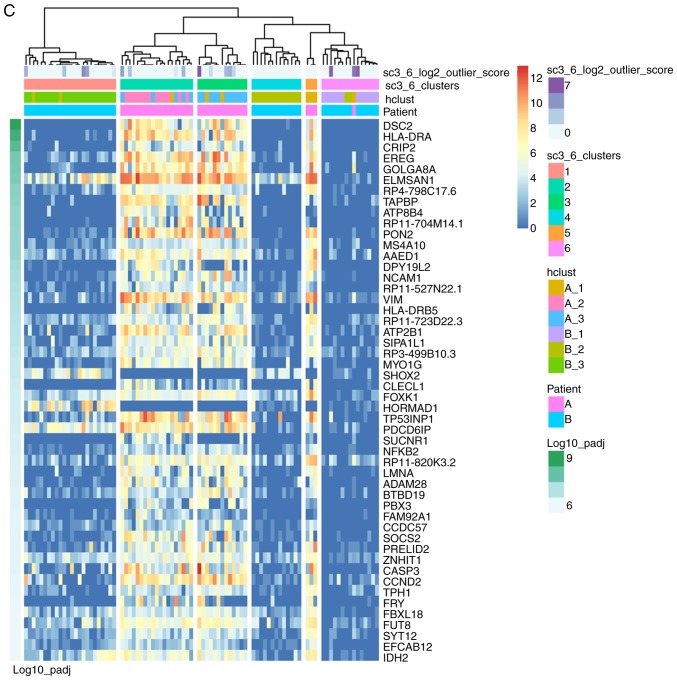
scRNA-seq data were pseudo-aligned and quantified as TPM using Kallisto, an alignment-free-based quantification method (13). TPM values were then log2 transformed (after dividing by 10 and adding 1) as in previous studies (17,18). Hierarchical clustering by ward. D2 linkage distance was used to separate the 87 cells into six groups (Fig. 1A): Cells from patient A were divided into three groups (A_1, A_2 and A_3), and cells from patient B were divided into another three groups (B_1, B_2 and B_3). As presented in Fig. 1B, the six groups were crudely separated by PCA, and the genes contributing to the separation of these subpopulations were examined using SC3 clustering (19). In total, 2,138 DEGs were detected (Table SII) and the top 50 DEGs are presented in Fig. 1C. Among them, immune-associated genes [major histocompatibility complex, class II, DR α (HLA-DRA), major histocompatibility complex, class II, DR β 1, major histocompatibility complex, class I, E and neural cell adhesion molecule 1] and a DNA methylation-associated gene [isocitrate dehydrogenase (NADP(+)) 2] were identified; 35 cells (40.2%) in clusters 2, 3 and 5 exhibited upregulation of these DEGs.
Figure 1.
Subpopulations of 87 acute myeloid leukemia single cells. (A) Hierarchical clustering separated single cells into six groups. (B) A total of six groups were separated with each other in PCA. (C) SC3 clustering of 87 single cells demonstrated the gene expression features of six groups. PCA, principal component analysis.
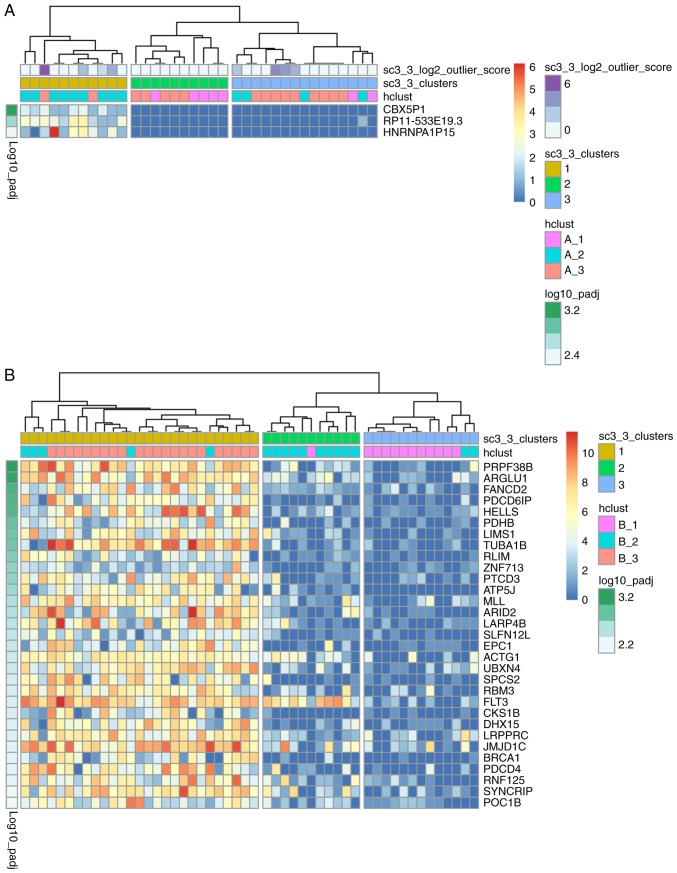
To examine the identity of cells based on the scRNA-seq data, single-cell consensus clustering (SC3) was performed (19) using raw read counts of the cells from both patients. As presented in Fig. 2A, 36 patient A cells were separated into three groups: The majority of cells in the A_2 group (9 in 13) were in cluster 1, the majority of the cells in the A_1 group (5 in 7) were in cluster 2, while the cells in the A_3 group were not evenly distributed in cluster 3 (n=9), cluster 2 (n=5) or cluster 1 (n=2). As shown in Fig. 2B, 51 cells from patient B were separated into 3 groups: The majority of cells in the B_1 group (11 in 12) were in cluster 3, cluster 2 comprised mainly of cells in the B_2 group, and all cells in the B_3 group were in cluster 1. The cells were previously separated into distinct subpopulations using hierarchical clustering (Fig. 1A), which was highly consistent with the results of PCA (Fig. 1B). The results of SC3 clustering (using read counts) were also concordant with those of hierarchical clustering using log2(TPM/10+1).
Figure 2.
Distributions of acute myeloid leukemia cell subpopulations in each patient. (A) SC3 clustering of 36 single cells from patient A demonstrated the gene expression features of three groups. (B) SC3 clustering of 51 single cells from patient B demonstrated the gene expression features of three groups.
Additionally, 3 and 31 DEGs with P<0.01, corrected for multiple testing using the ‘holm’ method (19), were identified in cells from patients A and B, respectively. The identified DEGs were upregulated in 11 of the patient A cells (30.6%) and 27 of the patient B cells (52.9%) in cluster 1 (Fig. 2A and B, respectively). The 3 DEGs in the patient A cells were not reported to be associated with AML, whereas the 31 DEGs in the patient B cells were primarily enriched in cancer-related functions from a literature review (Table I). There were differences between the cellular composition and transcription patterns of different tissues (including BM and PB), which has been previously described (40). The major subtypes and proportions of PB mononuclear cells from a healthy donor are >80% T cells, ~6% NK cells, ~6% B cells and ~7% myeloid cells; while the major subtypes of BM mononuclear cells (BMMCs) from a healthy donor are >50% T cells, ~20% B cells, ~10% monocytes and ~20% myeloid cells (40). Specifically, the level of blast cells and immature erythroids in the BMMCs of a healthy donor is ~15%; whereas, in patients with AML this could be 50–80% (40). The previous study also suggested that cells from BM could predict the status of patients with AML (40). Therefore, in the present study, only patient B cells from BM were used in the following analyses.
Marker-based classification of cell subpopulations from patient B
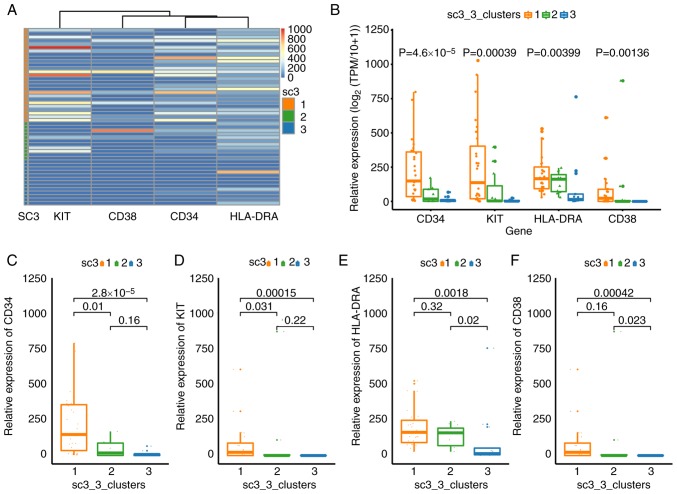
CD34, CD38, mast/stem cell growth factor receptor Kit (KIT or CD117) and HLA-DRA have been used to sort LSCs or hematopoietic stem/progenitor cells in different leukemia samples (41–44). Therefore, these four markers were selected to broadly classify the cell sub-populations from patient B. The expression levels of these four markers (Kruskal-Wallis; P=2.4×10−8), CD34 (Kruskal-Wallis; P=4.6×10−5), KIT (Kruskal-Wallis; P=0.00039), HLA-DRA (Kruskal-Wallis; P=0.00399) and CD38 (Kruskal-Wallis; P=0.00136) were significantly different between the cells of three clusters (Fig. 3B-F). The expression levels of these four genes were low in the cells of cluster 3, suggesting an inactive state (Fig. 3A and B). The expression levels of 31 DEGs in cluster 3 were also low as a result of SC3 clustering (Fig. 2B). Cells in cluster 1 expressed high levels of CD34 (Fig. 3C), KIT (Fig. 3D) and HLA-DRA (Fig. 3E), suggesting that these cells were ‘positive blasts’ (44). Significantly lower expression levels of CD34 and KIT were observed in cluster 2 compared with cluster 1 (Fig. 3C and D), suggesting that these were non-leukemic cells. These results not only demonstrate the functional identities of the cell sub-populations, but also confirmed the accuracy of SC3 clustering.
Figure 3.
Expression patterns of four markers in cell subpopulations from patient B. (A and B) Whole expression patterns of four markers in three cell subpopulations. Expression patterns of (C) CD34, (D) KIT (CD117), (E) HLA-DRA and (F) CD38 in three cell subpopulations. Expression values are presented in log2(TPM/10+1) scale, that is, TPM was log-transformed after dividing by 10 and adding 1. KIT, mast/stem cell growth factor receptor Kit; HLA-DRA, major histocompatibility complex, class II, DR α; TPM, transcript per million.
Functions and interactions of the 31 DEGs
Numerous genes among the 31 DEGs were associated with hematological malignancies (Table II). Internal tandem duplication of receptor-type tyrosine-protein kinase FLT3 (FLT3) and partial tandem duplication of lysine methyltransferase 2A (MLL) are the most common mutations in AML (with frequencies of 30–45% and 5–10%, respectively, in CN-AML), and are associated with poor therapeutic outcomes (20,21). Specifically, lymphoid-specific helicase and enhancer of polycomb homolog 1 were associated with epigenetic regulation in hematopoiesis (22), ring finger protein, LIM domain interacting (RLIM) was associated with the ubiquitylation of AML1-ETO and protein PML-retinoic acid receptor α (23), and programmed cell death 4 was associated with related signaling pathway (24) in myeloid leukemia. MLL (KMT2A) was associated with fusions and acute leukemia (25), and La ribonucleoprotein domain family member 4B (26), jumonji domain containing 1C (JMJD1C) (27) and SYNCRIP (28) were associated with LSC self-renewal. Additionally, FLT3 (29), DEAH-box helicase 15 (DHX15) (30) and JMJD1C (31) were with risk or survival in acute leukemia, FANCD2 was associated with drug resistance in leukemia (32), ARID2 was associated with hematopoietic stem cell (HSC) function (33), CDC28 protein kinase regulatory subunit 1B was associated with multiple myeloma (34), EPC1 was associated with the development of T-cell leukemia (35). Furthermore, the functions or variations of JMJD1C (31), RLIM (23) and DHX15 (36) were associated with t(8;21) AML.
Table II.
A total of 13 differentially expressed genes were associated with the progression of leukemia.
| Author, year | Gene symbol | Full name | Role in leukemia | (Refs.) |
|---|---|---|---|---|
| Prasad et al, 2014 | HELLS | Helicase, lymphoid specific | Specifically expressed in hematopoietic progenitor cells | (22) |
| Prasad et al, 2014; Nakahata et al, 2009 | EPC1 | Enhancer of polycomb homolog 1 | Lowly expressed in leukemia cells, involved in chromosomal translocation in ALL | (22,35) |
| Kramer et al, 2008 | RLIM | Ring finger protein, LIM domain interacting | A substrate of E3-ligase SIAH-1, contributing to the ubiquitin-dependent degradation of AML1-ETO and protein PML-retinoic acid receptor α fusion proteins | (23) |
| Espadinha et al, 2017 | PDCD4 | Programmed cell death 4 | A tumor suppressor, was repressed by phosphorylated STAT5 and microRNA-21 in chronic myeloid leukemia and AML models | (24) |
| Prasad et al, 2014; Meyer et al, 2018 | MLL (KMT2A) | Lysine methyltransferase 2A | Highly expressed in the lymphoid lineage, chromosomal rearrangements of MLL are associated with acute leukemias, and display a bad outcome | (22,25) |
| Zhang et al, 2015 | LARP4B | La ribonucleoprotein domain family member 4B | Involved in LSC maintenance, and may regulate the cell cycle of LSCs | (26) |
| Zhu et al, 2016; Chen et al, 2015 | JMJD1C | Jumonji domain containing 1C | A coactivator for RUNX1-RUNX1T1, mediates of MLL-AF9- and HOXA9-driven LSC function | (27,31) |
| Vu et al, 2017 | SYNCRIP | Synaptotagmin binding interacting cytoplasmic RNA protein | Interacts with MSI2 indirectly, controls the myeloid LSC program | (28) |
| Thiede et al, 2002; Cheng et al, 2018 | FLT3 | Fms related tyrosine kinase 3 | Internal tandem duplication of FLT3 results in the failure of leukemia treatment and contribute to a poor prognosis; significantly upregulated in AML and ALL, reduces survival rates | (20,29) |
| Pan et al, 2017; Christen et al, 2019 | DHX15 | DEAH-box helicase 15 | Regulates cell apoptosis through NF-κB signaling pathway, associated with poor prognosis in AML, with mutations in t(8;21) AML | (30,36) |
| Yao et al, 2015 | FANCD2 | FA complementation group D2 | May confer leukemia resistance to adriamycin via enhanced DNA interstrand crosslink repair | (32) |
| Liu et al, 2018 | ARID2 (BAF200) | AT-rich interaction domain 2 | Required for the maintenance of HSC homeostasis, ARID2 deficiency accelerates the progression of MLL-AF9-induced leukemia | (33) |
| Walker et al, 2019 | CKS1B | CDC28 protein kinase regulatory subunit 1B | Amplification (≥4 copies) of CKS1B was observed in high-risk multiple myeloma | (34) |
ALL, acute lymphoid leukemia; AML1-ETO, fusion protein acute myeloid leukemia 1 protein-protein ETO; AML, acute myeloid leukemia; LSC, leukemic stem cell; AF9, protein AF-9; HOXA9, homeobox protein Hox-A9; MSI2, musashi RNA binding protein 2; NF-κB, nuclear factor-κB; HSC, hematopoietic stem cell.
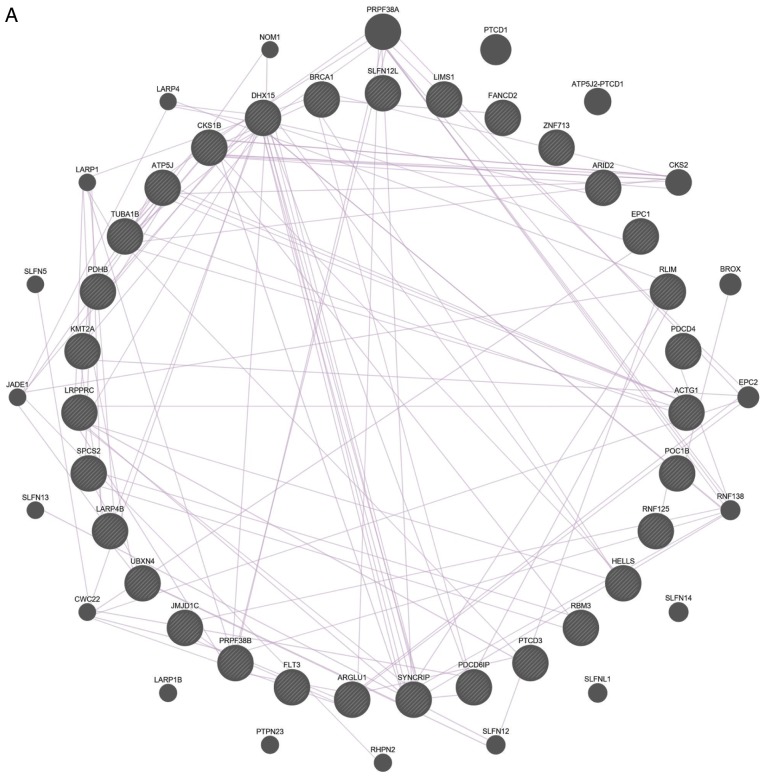
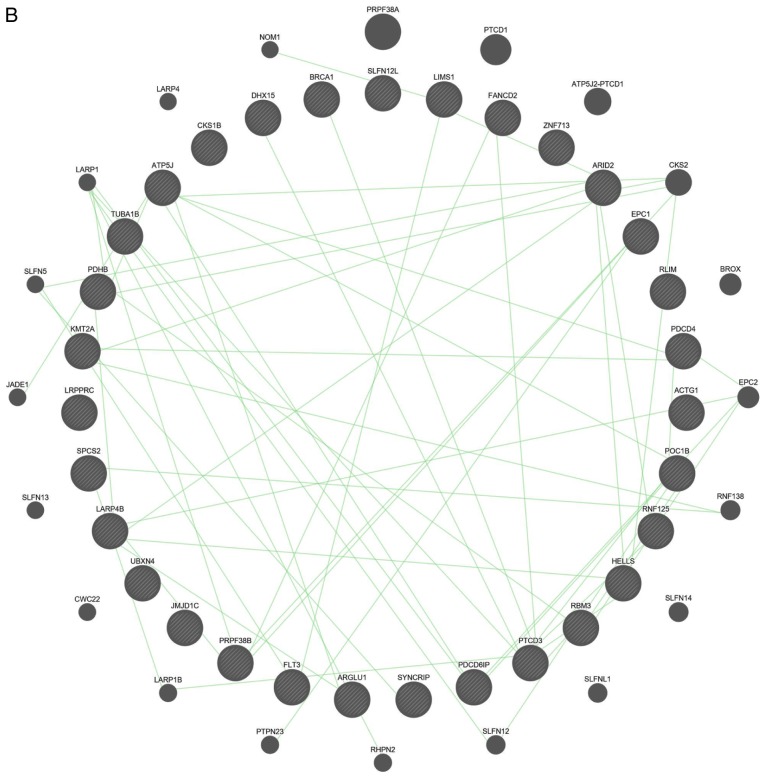
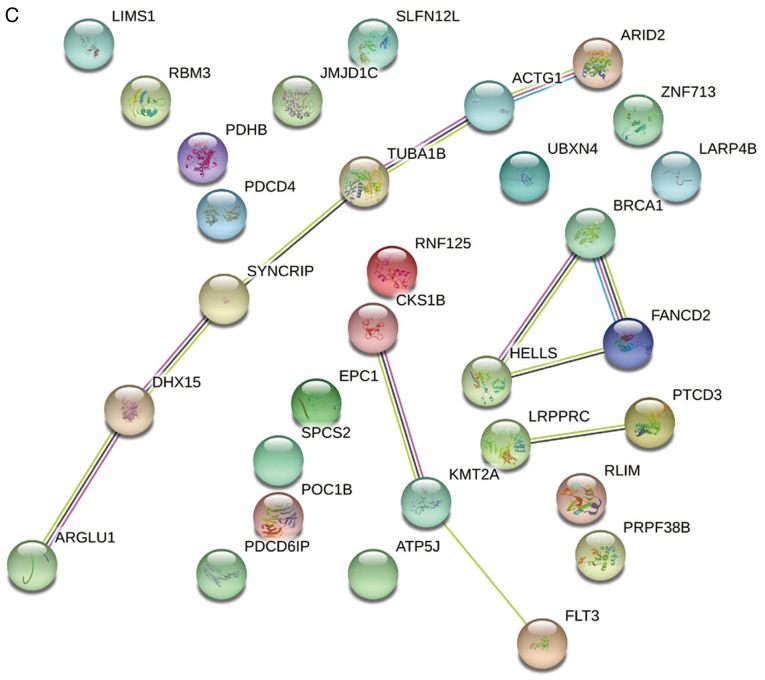
The interactions between these 31 DEGs were also examined (Fig. 4). The results of both gene-gene and protein-protein interaction analyses suggested that the DEGs are functionally related.
Figure 4.
Gene-gene and protein-protein interaction networks of 31 differentially expressed genes. (A) Co-expression network and (B) genetic network from Gene Multiple Association Network Integration Algorithm. Nodes indicate genes and lines indicate interactions. Gene-gene and protein-protein interaction networks of 31 differentially expressed genes. (C) Protein-protein interaction networks. Nodes indicate proteins and lines indicate interactions.
Possible biomarkers for rapid prediction of prognostic risk in t(8;21) AML
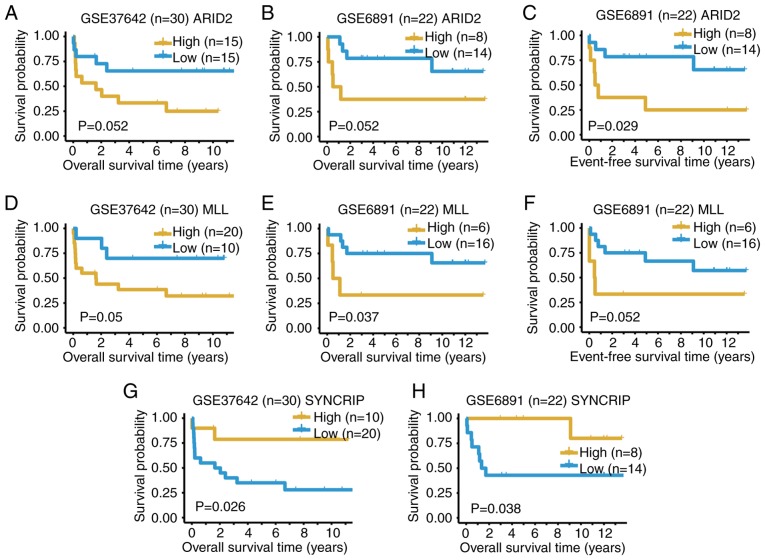
Potential prognostic biomarkers from the 31 DEGs of the patient B cells were investigated, which included the DEGs between ‘positive blasts’ and other cells. The dataset GSE6891 (8), containing both the overall survival (OS) and event-free survival information of 22 patients with t(8;21) AML, and the dataset GSE37642 (9) with the OS information of 30 patients with t(8;21) AML, were selected to determine the prognostic significance of the identified DEGs. The expression values of ARID2, MLL and SYNCRIP could predict the OS outcomes of patients with t(8;21) AML in both datasets with P≤0.052 (Fig. 5). High expression levels of ARID2 and MLL indicate a poor outcome, whilst high expression of SYNCRIP suggests a more favorable outcome (Fig. 5). The expression values of various genes in either dataset GSE37642 (Fig. S4) or GSE6891 (Fig. S5) could also predict the OS outcomes of t(8;21) AML. In summary, the present results from the two bulk expression datasets supported the conclusions from the scRNA-seq data.
Figure 5.
Kaplan-Meier survival curves of t(8;21) patients with acute myeloid leukemia from datasets GSE37642 (n=30) and GSE6891 (n=22) using genes ARID2, MLL and SYNCRIP. Overall survival curves using ARID2 for (A) GSE37642 and (B) GSE6891. (C) Event-free survival curve using ARID2 from the dataset GSE6891. Overall survival curves using MLL for (D) GSE37642 and (E) GSE6891. (F) Event-free survival curve using ARID2 from the dataset GSE6891. Overall survival curves using SYNCRIP for (G) GSE37642 and (H) GSE6891. ARID2, AT-rich interaction domain 2; MLL, lysine methyltransferase 2A; SYNCRIP, synaptotagmin binding cytoplasmic RNA interacting protein.
Discussion
scRNA-seq of AML undergoing allogeneic HSCT has been previously conducted (40); however, to the best of the authors' knowledge, investigations into the malignant development of t(8;21) AML are limited. In the present pilot study, the single-cell transcriptomes of two patients with t(8;21) AML were profiled, and the cells were separated into sub-populations with different gene expression patterns.
Among the 31 identified DEGs in cells from patient B (the treatment outcome for whom was poor), several genes were identified to be associated with leukemia; the prognostic significance of three of these genes, ARID2, MLL and SYNCRIP, was validated in two t(8;21) AML datasets. ARID2 is a tumor repressor that plays important roles in the maintenance of HSC homeostasis, and ARID2 deficiency accelerates the progression of MLL-protein AF-9-induced leukemia (33). Chromosomal rearrangements of MLL are associated with acute leukemia, and subsequently result in poor patient outcome (25). Together with musashi RNA binding protein 2 indirectly, SYNCRIP regulates the myeloid LSC program, and is required for the survival of leukemia cells (28). The prognostic significance of ARID2 and MLL determined in the present study are consistent with those presented in the literature (25,33), while that of SYNCRIP was the opposite, which may due to the tissue difference and requires further validation in the future. Furthermore, the functional and prognostic significance of the other various genes require future experimental clarification.
scRNA-seq is a powerful technology that is frequently used in cancer research, and the flow cytometric targeting of cell surface antigens has been used to isolate tumor cells in a number of previous studies (17,45). In the present study, the presence of ‘positive blasts’ was predicted using marker genes as indicated in a previous study (44). Different gene-based stemness scores have been developed to determine the risk of AML. The weighted sum of a subset of LSC-related genes has been used to determine the prognosis for AML in a number of previous studies, and a sufficient number of datasets and samples were used for training and validation. However, the LSC-related scores only perform well in CN-AML (2,3). In the present study, three prognostic biomarkers were identified in AML with an abnormal chromosomal karyotype. This differs from previous studies (2,3); the candidate genes were analyzed from the high-throughput sequencing data of single cells, rather than selected from microarray expression data of bulk cells, and the biomarkers in the present study are applicable to AML with a t(8;21) translocation.
There are some limitations to the present pilot study. Besides second relapse, the BM samples at other time points, such as new diagnosis, first relapse and after HSCT, were not collected from patient B and the patient has subsequently died. Therefore, it was not possible to track the clonal evolution of t(8;21) AML by taking advantage of scRNA-seq in the present study. A larger number of patients at different disease progression stages, and a larger number of collected cells may better illustrate the clonal evolution and development of t(8;21) AML. Additionally, due to the availability of resources, the dataset used for biomarker validation was not very large. Specific genomic variations, such as single nucleotide variants (40) and copy number variants (17), may be inferred in the assistance of genomic sequencing methods in future work. The present study provided evidence that scRNA-seq plays an important role in the study of t(8;21) AML and suggested that strategies promoting scRNA-seq may be valuable techniques for hematological malignancy therapy.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science Fund (grant. no. 81670162), The PLA General Hospital Science and Technology Project (grant. no. 18KMM01) and Beijing Natural Science Foundation (grant. no. 7204305).
Availability of data and materials
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Authors' contributions
LY, MQZ, YC, YZ, YHL, SH and NL designed the research. SH collected clinical samples and clinical information, and contributed to the acquisition of data. YC and LS performed single-cell RNA sequencing. QX, BLZ, LS and SHL analyzed the sequencing data. CL, YD, WWL and LLW performed the reverse transcription-PCR experiments. QX and SH drafted the manuscript. LY, BLZ, MQZ and YHL provided valuable advice and also critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Institutional Review Board of Chinese PLA General Hospital, and all patients provided signed informed consent for the collection of specimens and detailed analyses of the derived genetic material.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 2.Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA. 2010;304:2706–2715. doi: 10.1001/jama.2010.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, Arruda A, Popescu A, Gupta V, Schimmer AD, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–437. doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 4.Licht JD. AML1 and the AML1-ETO fusion protein in the pathogenesis of t(8;21) AML. Oncogene. 2001;20:5660–5679. doi: 10.1038/sj.onc.1204593. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Jiang MM, Chen GF, Qian K, Gao HH, Guan W, Shi JL, Liu AQ, Liu J, Wang BH, et al. Epigenetic silencing of eyes absent 4 gene by acute myeloid leukemia 1-eight-twenty-one oncoprotein contributes to leukemogenesis in t(8;21) acute myeloid leukemia. Chin Med J (Engl) 2016;129:1355–1362. doi: 10.4103/0366-6999.182838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reikvam H, Hatfield KJ, Kittang AO, Hovland R, Bruserud O. Acute myeloid leukemia with the t(8;21) translocation: Clinical consequences and biological implications. J Biomed Biotechnol. 2011;2011:104631. doi: 10.1155/2011/104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, Quake SR. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 2014;11:41–46. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaak RG, Wouters BJ, Erpelinck CA, Abbas S, Beverloo HB, Lugthart S, Lowenberg B, Delwel R, Valk PJ. Prediction of molecular subtypes in acute myeloid leukemia based on gene expression profiling. Haematologica. 2009;94:131–134. doi: 10.3324/haematol.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Herold T, He C, Valk PJ, Chen P, Jurinovic V, Mansmann U, Radmacher MD, Maharry KS, Sun M, et al. Identification of a 24-gene prognostic signature that improves the European LeukemiaNet risk classification of acute myeloid leukemia: An international collaborative study. J Clin Oncol. 2013;31:1172–1181. doi: 10.1200/JCO.2012.44.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Liu Y, Hou G, Wang L, Lv N, Xu Y, Xu Y, Wang X, Xuan Z, Jing Y, et al. Mutational spectrum and risk stratification of intermediate-risk acute myeloid leukemia patients based on next-generation sequencing. Oncotarget. 2016;7:32065–32078. doi: 10.18632/oncotarget.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollen AA, Nowakowski TJ, Shuga J, Wang X, Leyrat AA, Lui JH, Li N, Szpankowski L, Fowler B, Chen P, et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat Biotechnol. 2014;32:1053–1058. doi: 10.1038/nbt.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Vo AD, Qin F, Li H. Comparative assessment of methods for the fusion transcripts detection from RNA-Seq data. Sci Rep. 2016;6:21597. doi: 10.1038/srep21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–527. doi: 10.1038/nbt0816-888d. [DOI] [PubMed] [Google Scholar]
- 16.Emig D, Salomonis N, Baumbach J, Lengauer T, Conklin BR, Albrecht M. AltAnalyze and DomainGraph: Analyzing and visualizing exon expression data. Nucleic Acids Res. 2010;38((Web Server Issue)):W755–W762. doi: 10.1093/nar/gkq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171:1611–1624 e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C, Gao R, Sei E, Brandt R, Hartman J, Hatschek T, Crosetto N, Foukakis T, Navin NE. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell. 2018;173:879–893 e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiselev VY, Kirschner K, Schaub MT, Andrews T, Yiu A, Chandra T, Natarajan KN, Reik W, Barahona M, Green AR, Hemberg M. SC3: Consensus clustering of single-cell RNA-seq data. Nat Methods. 2017;14:483–486. doi: 10.1038/nmeth.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.V99.12.4326. [DOI] [PubMed] [Google Scholar]
- 21.Basecke J, Whelan JT, Griesinger F, Bertrand FE. The MLL partial tandem duplication in acute myeloid leukaemia. Br J Haematol. 2006;135:438–449. doi: 10.1111/j.1365-2141.2006.06301.x. [DOI] [PubMed] [Google Scholar]
- 22.Prasad P, Rönnerblad M, Arner E, Itoh M, Kawaji H, Lassmann T, Daub CO, Forrest AR, Lennartsson A, Ekwall K, FANTOM consortium High-throughput transcription profiling identifies putative epigenetic regulators of hematopoiesis. Blood. 2014;123:e46–e57. doi: 10.1182/blood-2013-02-483537. [DOI] [PubMed] [Google Scholar]
- 23.Kramer OH, Muller S, Buchwald M, Reichardt S, Heinzel T. Mechanism for ubiquitylation of the leukemia fusion proteins AML1-ETO and PML-RARalpha. FASEB J. 2008;22:1369–1379. doi: 10.1096/fj.06-8050com. [DOI] [PubMed] [Google Scholar]
- 24.Espadinha AS, Prouzet-Mauleon V, Claverol S, Lagarde V, Bonneu M, Mahon FX, Cardinaud B. A tyrosine kinase-STAT5-miR21-PDCD4 regulatory axis in chronic and acute myeloid leukemia cells. Oncotarget. 2017;8:76174–76188. doi: 10.18632/oncotarget.19192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer C, Burmeister T, Groger D, Tsaur G, Fechina L, Renneville A, Sutton R, Venn NC, Emerenciano M, Pombo-de-Oliveira MS, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Peng L, Hu T, Wan Y, Ren Y, Zhang J, Wang X, Zhou Y, Yuan W, Wang Q, et al. La-related protein 4B maintains murine MLL-AF9 leukemia stem cell self-renewal by regulating cell cycle progression. Exp Hematol. 2015;43:309–318 e2. doi: 10.1016/j.exphem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhu N, Chen M, Eng R, DeJong J, Sinha AU, Rahnamay NF, Koche R, Al-Shahrour F, Minehart JC, Chen CW, et al. MLL-AF9- and HOXA9-mediated acute myeloid leukemia stem cell self-renewal requires JMJD1C. J Clin Invest. 2016;126:997–1011. doi: 10.1172/JCI82978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu LP, Prieto C, Amin EM, Chhangawala S, Krivtsov A, Calvo-Vidal MN, Chou T, Chow A, Minuesa G, Park SM, et al. Functional screen of MSI2 interactors identifies an essential role for SYNCRIP in myeloid leukemia stem cells. Nat Genet. 2017;49:866–875. doi: 10.1038/ng.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng J, Qu L, Wang J, Cheng L, Wang Y. High expression of FLT3 is a risk factor in leukemia. Mol Med Rep. 2018;17:2885–2892. doi: 10.3892/mmr.2017.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan L, Li Y, Zhang HY, Zheng Y, Liu XL, Hu Z, Wang Y, Wang J, Cai YH, Liu Q, et al. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget. 2017;8:89643–89654. doi: 10.18632/oncotarget.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Zhu N, Liu X, Laurent B, Tang Z, Eng R, Shi Y, Armstrong SA, Roeder RG. JMJD1C is required for the survival of acute myeloid leukemia by functioning as a coactivator for key transcription factors. Genes Dev. 2015;29:2123–2139. doi: 10.1101/gad.267278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao C, Du W, Chen H, Xiao S, Huang L, Chen FP. Involvement of Fanconi anemia genes FANCD2 and FANCF in the molecular basis of drug resistance in leukemia. Mol Med Rep. 2015;11:4605–4610. doi: 10.3892/mmr.2015.3288. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Wan X, Zhou P, Zhou X, Zhang W, Hui X, Yuan X, Ding X, Zhu R, Meng G, et al. The chromatin remodeling subunit Baf200 promotes normal hematopoiesis and inhibits leukemogenesis. J Hematol Oncol. 2018;11:27. doi: 10.1186/s13045-018-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker BA, Mavrommatis K, Wardell CP, Ashby TC, Bauer M, Davies F, Rosenthal A, Wang H, Qu P, Hoering A, et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia. 2019;33:159–170. doi: 10.1038/s41375-018-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakahata S, Saito Y, Hamasaki M, Hidaka T, Arai Y, Taki T, Taniwaki M, Morishita K. Alteration of enhancer of polycomb 1 at 10p11.2 is one of the genetic events leading to development of adult T-cell leukemia/lymphoma. Genes Chromosomes Cancer. 2009;48:768–776. doi: 10.1002/gcc.20681. [DOI] [PubMed] [Google Scholar]
- 36.Christen F, Hoyer K, Yoshida K, Hou HA, Waldhueter N, Heuser M, Hills RK, Chan W, Hablesreiter R, Blau O, et al. Genomic landscape and clonal evolution of acute myeloid leukemia with t(8;21): An international study on 331 patients. Blood. 2019;133:1140–1151. doi: 10.1182/blood-2018-05-852822. [DOI] [PubMed] [Google Scholar]
- 37.Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008;9(Suppl 1):S4. doi: 10.1186/gb-2008-9-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis S, Meltzer PS. GEOquery: A bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 40.Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8:14049. doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao X, Gao S, Wu Z, Kajigaya S, Feng X, Liu Q, Townsley DM, Cooper J, Chen J, Keyvanfar K, et al. Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells. Blood. 2017;130:2762–2773. doi: 10.1182/blood-2017-08-803353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Bie J, Demeyer S, Alberti-Servera L, Geerdens E, Segers H, Broux M, De Keersmaecker K, Michaux L, Vandenberghe P, Voet T, et al. Single-cell sequencing reveals the origin and the order of mutation acquisition in T-cell acute lymphoblastic leukemia. Leukemia. 2018;32:1358–1369. doi: 10.1038/s41375-018-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giustacchini A, Thongjuea S, Barkas N, Woll PS, Povinelli BJ, Booth CAG, Sopp P, Norfo R, Rodriguez-Meira A, Ashley N, et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat Med. 2017;23:692–702. doi: 10.1038/nm.4336. [DOI] [PubMed] [Google Scholar]
- 44.Yan B, Hu Y, Ban KHK, Tiang Z, Ng C, Lee J, Tan W, Chiu L, Tan TW, Seah E, et al. Single-cell genomic profiling of acute myeloid leukemia for clinical use: A pilot study. Oncol Lett. 2017;13:1625–1630. doi: 10.3892/ol.2017.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. Landscape of infiltrating t cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169:1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.