Abstract
Purpose of review:
This review synthesized the literature on predictors and mechanisms of post-bariatric alcohol problems, in order to guide future research on prevention and treatment targets.
Recent findings:
Consistent evidence suggests an elevated risk of developing problems with alcohol following bariatric surgery. While there is a paucity of empirical data on predictors of problematic alcohol use after bariatric surgery, being male, a younger age, smoking, regular alcohol consumption, pre-surgical alcohol use disorder, and a lower sense of belonging have predicted alcohol misuse post-operatively. This review synthesizes potential mechanisms including specific bariatric surgical procedures, peptides and reinforcement/reward pathways, pharmacokinetics, and genetic influences. Finally, potential misperceptions regarding mechanisms are explored.
Keywords: Obesity, Bariatric Surgery, Alcohol, Alcohol Use Disorder, Gastric Bypass, Sleeve Gastrectomy
Summary:
Certain bariatric procedures elevate the risk of alcohol misuse post-operatively. Future research should serve to elucidate the complexities of reward signaling, genetically-mediated mechanisms, and pharmacokinetics in relation to alcohol use across gender and developmental period by surgery type.
Introduction
There is now strong empirical evidence showing that individuals who undergo bariatric surgery are at an elevated risk of developing problems with alcohol, ranging from increased alcohol use to alcohol use disorder (AUD) [1,2∙∙,3∙], including overrepresentation of bariatric surgery patients in substance abuse treatment programs [4–6]. Of particular concern is that many of these cases are new-onset cases, developing at some time after surgery [1,7]. While there is now a robust body of empirical knowledge regarding the increased prevalence of post-bariatric alcohol problems, knowledge gaps remain with respect to the risk factors and etiology of these problems. This paper aims to synthesize the current knowledge regarding predictors and mechanisms of post-bariatric alcohol problems, in order to guide future research and clinical strategies for prevention and treatment.
Prevalence of Alcohol Misuse after Bariatric Surgery
It is difficult to synthesize this literature, as the studies vary significantly in terms of study design and methodology, sample size, duration of follow-up, and procedure type (see Table 1 for a chronological summary of existing studies). The variation among definitions used to operationalize alcohol problems has been particularly pronounced, including standardized, validated measures to assess quantity/frequency of alcohol use; non-standardized self-reported interviews or questionnaire items; validated questionnaires or interview items assessing various diagnostic criteria as a proxy for formal AUD; and diagnostic or billing codes or information on prescriptions for addiction medications from medical records or registries. However, taken together, the existing studies converge to demonstrate a number of important findings: 1) Rates of alcohol-related problems and/or AUDs increase in a subset of patients after bariatric surgery [1,2∙∙,4–11∙,12–15]; 2) This phenomenon is more likely to occur after Roux-en-Y gastric bypass (RYGB) than laparoscopic adjustable gastric band (LAGB) [1,2∙∙,13,15]; 3) In some reports, a decrease in alcohol use and/or an improvement or remission of alcohol problems has been observed after surgery [14]; and 4) Alcohol problems become increasingly likely as the patient becomes more temporally distal to the bariatric surgery procedure [1,2∙∙,9,14].
Table 1.
Descriptive summary of studies on alcohol use after bariatric surgery
| Author(s) | N | Surgery | F/U | % ETOH Misuse, Abuse, or Dependence |
|---|---|---|---|---|
| Mitchell et al. 2001 | 78 | RYGB | 13–15 Years | 7.8% |
| Buffington et al. 2007 | 318 | Variable | Variable | 28.4% |
| Ertelt et al. 2008 | 70 | RYGB | 6–10 Years | 10.0% |
| Welch et al. 2011 | 75 | RYGB | 2–3 Years | 1.3% |
| Suzuki et al. 2012 | 23/28 | RYGB/LAGB | 31–59 Month | 21.4%/0.0% |
| Svensson et al. 2013 | 164/135 | RYGB/Bands | 10/15 Years | % Not Indicated |
| Conason et al. 2013 | 100/55 | RYGB/Bands | 24 Months | % Not Indicated |
| Wee et al. 2014 | 328 | RYGB/LAGB/SG | 2 Years | 13% combined |
| Alfonsson et al. 2014 | 129 | RYGB | 1 Year | 2.3% |
| Ivezaj et al. 2014 | 143 | RYGB | Mean 2.7 Years | 19.6% |
| Burgos et al. 2015 | 277 | RYGB/LAGB | 2 Years | 9.4% combined |
| King et al. 2017 | 752/250 | RYGB/LAGB | 7 Years | 16.4%/---- |
| Spadola et al. 2017 | 69 | RYGB/LAGB/SG | 5–55 Months | 14.5% combined |
| Coluzzi et al. 2018 | 142 | LAGB | 1 Year | 2.1% |
| Ibrahim et al. 2019 | 5724 | RYGB/SG | 2 Year | 11.9%/14.4% |
The remainder of the review will discuss potential predictors and mechanisms, including certain bariatric procedures, peptides/reward pathways, pharmacokinetics, and genetics, as well as a concluding section on potential misperceptions regarding mechanisms.
Potential Predictors and Mechanisms of Alcohol Misuse Post-Bariatric Surgery
Longitudinal Predictors
There are a number of factors that have been shown to predict problematic alcohol use in the general population: sex and peer influence [16], other substance use disorders [17], depression [18], and socioeconomic status [19] to name a few. However, there is a general paucity of empirical data on predictors of problematic alcohol use in bariatric surgery patients. To our knowledge, only two studies have specifically addressed this topic among adults. King and colleagues [1] found that a number of variables predicted AUD: being male, younger age, smoking, regular alcohol consumption, pre-surgical AUD, and a lower sense of belonging. Data from the same study showed that relatively similar variables predicted AUD seven years after surgery: male, younger age, smoking, regular alcohol consumption, and lower social support [2∙∙]. Interestingly, evidence from a recent study [9] suggested that predictors might differ as a function of the type of surgical procedure. For example, in this cohort, greater income increased the odds of developing AUD two years following RYGB surgery, but not following sleeve gastrectomy (SG) surgery.
It is important to note that while male sex is a predictor of AUD in the general population and among bariatric surgery patients, women significantly outnumber men in bariatric surgery utilization. As such, a greater number of women than men may be struggling with post-bariatric AUD. For example, among bariatric patients seeking substance abuse treatment in an inpatient treatment facility, 70.4% (n=38) were women [4]. Moreover, cross-sectional analyses with adolescent bariatric patients suggest that female sex and age were associated significantly with alcohol use during the past year at the 24-month follow-up assessment after surgery [20∙∙]. Elucidating post-surgical AUD risk factors for women and adolescents is imperative, and particularly for adolescents, given that this developmental stage constitutes a high-risk period for onset of problematic drinking behaviors [20∙∙].
Bariatric Procedure Type
To generate hypotheses regarding surgery-specific predictors, it is important to understand the complex changes that occur as a function of bariatric surgeries. One area to examine is the differing impacts of the various bariatric procedures. Although the exact mechanisms are unknown, the effects of some bariatric procedures appear to be purely anatomical in nature and might induce significant weight-loss without significantly altering metabolic pathways. Other, more “metabolically-active” procedures alter the anatomy of the gastrointestinal tract in ways that alter certain physiological parameters, many of which interact with the brain [21]. This has led to a conceptualization of these procedures as metabolic bariatric surgeries (MBS). In general, with these procedures, one sees a decrease in orexigenic and increase in anorexigenic hormones, so physical hunger is dampened despite ongoing weight loss (the opposite of what is seen in non-surgically-induced weight loss [22]). Some of these hormones include ghrelin, leptin, glucagon-like peptide 1 (GLP-1), gastric inhibitory polypeptide (GIP) and peptide YY (PYY). Hormones involved in energy regulation typically have multiple functions. For instance, ghrelin stimulates appetite, leptin decreases appetite, GLP-1 increases satiety by slowing emptying of the stomach, GIP slows gastrointestinal motility and increases insulin secretion, and PYY both reduces appetite and slows gastric emptying [23]. However, these hormones also fulfill other functions, some of which may be related to post-bariatric alcohol problems. In addition to hormone changes, there are also alterations in the secretion of bile acids.
RYGB.
The RYGB has long been a “gold standard” intervention for patients with severe obesity. The RYGB has multiple mechanisms, including food restriction due to the small gastric pouch. More importantly, bypassing the proximal small intestine and passing food directly into the distal intestine causes a more rapid release of gut hormones that slow the passage of nutrients, decrease hunger, and increase satiety [24]. In the first year after RYGB, ghrelin decreases in most patients [25], decreasing hunger. At the same time, GLP-1 and PYY increase, causing enhanced and earlier satiety. In the longer term, ghrelin may increase [26], since the bypassed portion of the stomach remains intact, and this may lead to weight regain.
SG.
In the past several years, the SG has become the most commonly-performed MBS [27]. With SG, 70–80% of the outer portion of the stomach is removed from the body, leaving a narrow gastric tube. The SG does promote some restriction in intake, but also involves metabolic mechanisms of action, including increases in PYY and GLP-1, enhancing satiety. It also promotes a durable decrease in ghrelin levels due to resection of the cell mass responsible for its secretion [28].
The metabolic changes that result from the anatomical alterations and decreased food intake associated with RYGB and SG are also associated with changes in brain reward centers, possibly predisposing certain patients towards AUDs.
Peptides and Reinforcement/Reward Pathways
The gastrointestinal feeding peptide, ghrelin, targets the ghrelin-1a receptor (GHSR) in the central nervous system to stimulate alcohol intake and alcohol-reinforced behaviors. Preclinical studies indicate that RYGB regulates alcohol intake in laboratory rodents [29–32]. Pharmacologic studies suggest that surgically-induced alterations in ghrelin signaling may contribute to changes in alcohol intake after RYGB [29, 30,32]. Moreover, altered ghrelin signaling may be a key to the link between surgery-induced reductions of appetite, a key feature of the RYGB procedure [24], and new onset alcohol consumption.
Multiple reports have demonstrated that RYGB promotes new onset alcohol intake in rodents with low baseline intake prior to surgery [29–32∙∙]. Davis and colleagues [29] documented reduced plasma ghrelin levels in Long Evans RYGB rats that display elevated alcohol consumption after surgery. These data suggest that gastric ghrelin secretion does not likely regulate new onset alcohol intake, as plasma levels of ghrelin were reduced in RYGB rats that drank more after surgery. Data from the Hajnal group [33] reported that RYGB promoted increased alcohol intake in Sprague Dawley rats and that systemic treatment with a GHSR antagonist attenuated this effect [30]. This finding suggests that, independent of gastric ghrelin secretion, increases in GHSR activity may be involved in new onset alcohol intake after RYGB. More recent work by Jerlhag and colleagues [34] indicates that GHSR activity, but not gastric ghrelin secretion, regulates alcohol intake in rodents genetically predisposed to consume alcohol. These findings taken together suggest that GHSR activity may alter alcohol intake secondary to RYGB.
In 2017 Sirohi et al., reported that Long Evans rats display increased preference for low concentration alcohol solutions following RYGB [32∙∙]. The group also reported that RYGB surgery facilitated the transition to alcohol dependence relative to sham controls in this strain of rodent. Furthermore, it was demonstrated that GHSR activity stimulated tonic dopamine firing in dopaminergic neurons in the ventral tegmental area (VTA). Importantly, antagonists that inhibit GHSR dopamine firing in control rats had no effect in RYGB rats behaviorally characterized by increased alcohol preference and facilitation to alcohol dependence. Moreover, RYGB rats that were behaviorally characterized for increased alcohol intake, alcohol preference and facilitation to alcohol dependence also displayed attenuated hedonic intake of palatable food. It was suggested that the reductions in gastric ghrelin secretion and subsequent malfunctions of GHSR signaling that are primarily involved in appetite reduction after RYGB may also indirectly stimulate new onset alcohol intake.
The finding that RYGB reduces GHSR control of tonic dopamine firing suggests that decreased plasma ghrelin levels after surgery may influence central GHSR activity in CNS reward regions. This finding becomes relevant when considering new evidence that indicates that ligand-independent GHSR activity (i.e., activity without ghrelin binding) regulates alcohol intake [34] and appetite [35]. For example, because alcohol directly activates CNS reward regions on its own, new onset alcohol intake may derive from a behavioral adaptation aimed at stimulating dopamine secretion once palatable food (i.e., GHSR signaling) is no longer performing this function. Alternatively, enhanced GHSR signaling, which has been demonstrated after RYGB [30], may also contribute to increased alcohol intake after surgery. More studies are needed to understand the complexities of GHSR signaling and adaptations to this process following surgeries that anatomically alter the gut.
Pharmacokinetics
Understanding of how different MBS procedures may affect the pharmacokinetics of alcohol is also important because it is well established that alterations in how quickly a substance of abuse reaches the brain can increase or decrease its addiction potential [36–37]. For instance, drugs that are smoked or delivered intravenously reach the brain faster and have a higher addiction potential than drugs delivered through routes such as oral or subcutaneous delivery, which take longer to reach the systemic circulation and central nervous system [36–37].
RYGB Pharmacokinetics.
In 2002, before the association between RYGB and increased risk of AUD was established, Klockoff and collaborators showed that blood alcohol concentrations (BAC) peaked faster (within five minutes after ingestion of the full alcohol dose), and about 28% higher in women who underwent RYGB than in a non-operated age- and sex- matched control group [38]. Consistent with these findings, more recent studies that estimated BAC from breath (BrAC) also show that despite drinking the same amount of alcohol, post-RYGB patients reach higher BrACs than non-operated control subjects [39] or than they themselves had before surgery [40]. Further, by measuring BAC at earlier time-points, Steffen and collaborators [41] showed that the effects of RYGB on peak BAC could be even more dramatic than previously thought. For example, one of the five participants they evaluated reached a peak BAC 2 minutes after ingesting the full dose of alcohol [41].
Notably, all above studies that measured BAC used venous blood samples; however, during the absorption phase, BAC is always higher in arteries than in veins [42–43], suggesting that the existing studies may be underestimating the full impact of RYGB on alcohol absorption. Pepino and collaborators (2015) measured BAC in arterialized blood samples and found that RYGB increased peak BAC by ~50% [44∙∙]. Taken together, the above findings suggest that the rate of delivery of ingested alcohol into the systemic circulation after RYGB resembles that of intravenous alcohol administration. Notably, such fast delivery of alcohol reduces gastric alcohol-first pass metabolism and increases alcohol bioavailability. Following RYGB, patients could be inadvertently engaging in binge drinking, a known risk factor for developing AUD, even when consuming just 1–2 drinks.
SG Pharmacokinetics.
In contrast to the findings described above for RYGB, findings on the effects of SG on alcohol pharmacokinetics have been less consistent. While two studies found SG did not change BAC [45–46], studies from two other laboratories found that SG, similar to RYGB, increased peak BAC after drinking alcohol [47,48∙∙]. A major difference between study methods is related to the fact that three of these studies, including the two that found no effects of SG on peak BAC, used a breathalyzer to estimate BAC [45–47], and only one study directly measured BAC [48∙∙]. The study of Acevedo and collaborators, which measured arterialized venous BAC, showed that, similar to that described above for RYGB, SG was associated with faster and higher peak BAC [48∙∙]. Importantly, by comparing BrAC and BAC simultaneously taken in the same participants, these authors found that the breathalyzer underestimated BAC by 27% and, because the breathalyzer cannot be used until 15 minutes after consuming the full alcohol dose to yield a valid breath sample, it missed peak BACs achieved after SG. These data highlight limitations of using a breathalyzer when examining pharmacokinetics after gastric surgery [48∙∙].
LAGB Pharmacokinetics.
We are aware of only one published study on the potential effects of LAGB on alcohol pharmacokinetics. While assessing the effects of SG, Changchien and collaborators also evaluated BrAC achieved in participants before, 3 months and 6 months after undergoing LAGB [45]. They found that LAGB did not change peak BrAC or time to reach peak BrAC at 3 or 6 months postoperatively. Although these findings are consistent with what one would expect based on the much smaller effect of LAGB on the anatomy of the gastrointestinal tract compared to RYGB or SG, it would be important to corroborate these findings with studies that directly measure BAC.
Genetics
There are substantial genetic influences on the development of both obesity and AUD, and increased risk of AUD after bariatric surgery suggests a common set of genetic and environmental factors that are common to AUD and obesity. In general, genetic association studies of alcohol use highlight the importance of genes related to alcohol or its intermediate metabolism (ADH, ADH1B, ADH1C, and ALDH2) and neurotransmission pathways thought to be involved in reward processing including dopaminergic, serotonergic, GABAergic and glutamatergic pathways [49]. Some of these genes may also be related in one way or another to obesity. For instance, Winnier and colleagues recently showed an inverse relationship between the expression of ADH1B, that encoded for a member of the alcohol dehydrogenase family, in adipocytes and various measures of obesity, suggesting its role in enhancing energy mobilization [50]. Decreased alcohol dehydrogenase activity in obesity may be associated with both decreased ethanol oxidation and reduced energy mobilization [50].
Several studies suggest the influence of disturbances in neurotransmission pathways within the reward processing on low brain dopamine activity predisposing subjects to both AUD and overeating, and subsequent obesity [51–53]. For example, data indicate that the dopamine receptor D2, encoded by the DRD2 shows reduced activity in persons with AUD and that the TaqA1 D2R variant, which is associated with reduced DRD2 activity, may be more prevalent among persons with obesity, especially in those with comorbid substance use disorder [54]. Another example concerns serotonergic pathway variations [55–56]. The 5-HTTLPR (serotonin-transporter-linked polymorphic region) is a degenerate repeat polymorphic region in SLC6A4, the gene that codes for the serotonin transporter. This variant is associated with the ability to control food intake and to modulate weight loss outcomes of behavioral/dietary treatment for obesity [49]. It has also been associated with AUD risk and differential treatment response. Additionally, a study using positron emission tomography suggests the role of epigenetic mechanisms, rather than 5-HTTLPR variation alone, on the in vivo serotonin availability in regions having a critical role in reward processing in individuals with obesity [57]. Data on glutamatergic pathways are more rare but significant [58–59].
Additionally, recent studies on alcohol exposure and subsequent changes in gene expression suggest the importance of epigenetic mechanisms in the neurobiology of AUDs. In particular, histone modifications and DNA methylation have emerged as important regulators of gene expression and associated phenotypes [60–61]. It is known that epigenetic signatures also influence both obesity phenotype and weight loss outcomes following bariatric surgery [62], which may provide clues to a connection with post-bariatic alcohol problems. In addition, changes in methylation may themselves contribute to metabolic improvements after bariatric surgery, by restoring methylation and gene expression levels in some genes that have been found to be altered in obesity [63∙∙,64–65]. However, it remains to be determined whether the post-surgical metabolic context may itself lead to changes in gene expression that favors AUDs through epigenetic mechanisms.
Potential Misperceptions Regarding Mechanisms
As reports of alcohol problems after MBS proliferate in both the empirical literature and the lay media, a number of hypotheses have been proposed to explain the etiology of these problems. We have discussed several potential causal models based on current empirical findings, but it is also informative to review some of the explanatory hypotheses that have been proposed but which are not supported by, or are even contradicted by, our empirical knowledge base.
In the lay media, and in some scientific writing as well, a frequently-posited idea is the “addiction transfer” or “cross addiction” hypothesis, which posits that the individuals developing problems with alcohol after bariatric surgery are people who, before surgery, had a pre-existing “addiction” to food, which was “transferred” to alcohol after surgery [66–67]. In addition to the fact that the construct of “food addiction” has yet to be fully scientifically validated [68], there are a number of other problems with this model. One clear argument against the “addiction transfer” hypothesis is failure of several studies to demonstrate a relationship between “food addiction” and/or binge eating before surgery and problems with alcohol after surgery [1,12,69].
Additionally, long-term studies of alcohol problems after MBS provide convergent evidence that these problems tend to develop after a relatively long latency following surgery – typically about one to two years after surgery [1,2∙∙ ], and some evidence suggests that the risk for onset of such problems continues to increase, rather than decrease, over many years following surgery [2∙∙,13]. If patients were experiencing a need to replace food and eating with some other type of substance or behavior, we would expect this need to be most acute within the early months after surgery, when patients are most limited in their intake capacity and their tolerance for highly palatable foods. In fact, by 1–2 years following MBS, most patients find that they are able to eat considerably larger quantities, and a wider variety, of foods [70] and a need to fulfill unmet eating-related needs would be less, rather than more, intense at this timepoint.
Findings that the risk for post-MBS alcohol problems vary by type of procedure (as noted above) also argue against the “addiction transfer” hypothesis. If the impetus behind “transferring” one’s addictive behaviors from eating to a substance like alcohol is that surgery impedes an individual’s ability to overeat, and/or because patients are purposefully changing their eating after surgery, then we would expect all MBS procedures to promote an equal risk for alcohol problems. Rather, the finding that risk varies by procedure strongly points to physiological/metabolic factors (as discussed above) as primary contributors to post-bariatric alcohol problems. It is notable that new-onset alcohol problems have also been observed in individuals who have undergone prophylactic total gastrectomy due to familial risk for gastric cancer [71–73]. This is not a population characterized as having obesity or engaging in overeating or “food addiction”, which weakens the “addiction transfer” model.
Further evidence contradicting the “addiction transfer” hypothesis may be found in the research demonstrating an increased proclivity to consume alcohol after RYGB in rodents – a phenomenon unlikely to be related to the concept of “addiction” as it is applied to humans. Further weakening the “addiction transfer” hypothesis, this increased proclivity to consume alcohol is seen in rodents who were not previously maintained on highly-palatable diets or fed in a “binge”-type paradigm (which would serve as a preclinical parallel to the construct of “food addiction” in humans).
A different explanatory model, which focuses on social/environmental etiological factors, posits that problems with alcohol develop after MBS because as individuals lose significant weight, become more mobile, and develop increased energy and social confidence, their frequency of socialization increases, increasing exposure to situations in which alcohol is consumed. However, evidence presented in the previous several paragraphs (increased risk of alcohol problems after prophylactic gastrectomy in patients who did not have obesity before surgery, differential risk for alcohol problems among different bariatric surgical procedures, evidence that MBS alters alcohol intake and reward in rodents), are all inconsistent with a model in which increased risk for alcohol problems after MBS is related to increased frequency of socialization in contexts where alcohol is available.
Conclusions and Future Directions
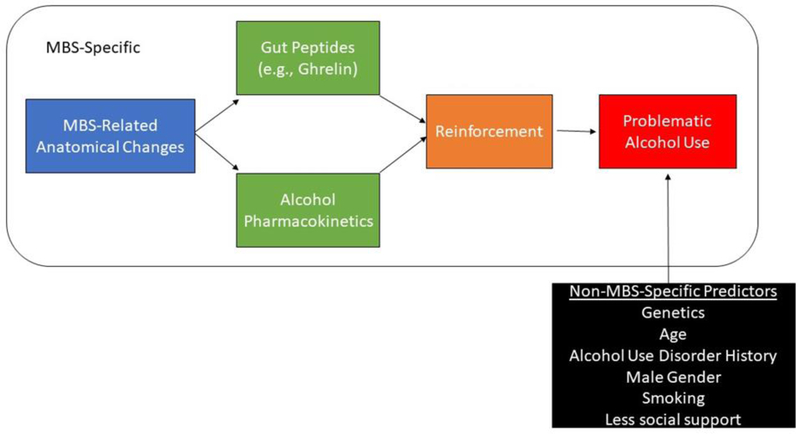
Taken together, there is a growing body of evidence that after certain types of MBS, patients can develop de novo alcohol or other substance use disorders or may relapse after a period of abstinence. Several mechanisms, including surgical- and non-surgical-specific factors, likely interact to increase the risk of AUD development following MBS. Based on the current review of the literature, we offer the following preliminary model of mechanisms related to AUD development following MBS (Figure 1).
Figure 1.
Preliminary model of potential mechanisms underlying AUD risk after MBS
Preliminary data suggest that male, younger age, smoking, regular alcohol consumption, pre-surgical AUD, and a lower sense of belonging predicted AUD following bariatric surgery. In addition, the decreased capacity for solid food intake, as well as the hormonal changes seen after metabolic operations, can likely explain some of the changes noted in the reward centers of the brain after surgery; however, there are a number of important, unanswered questions in this literature. First, the literature regarding SG is limited at this point [9]. Second, although the problem of alcohol misuse after bariatric surgery now seems well established, the process of the development of this complication remains largely unstudied, although the risk appears to remain substantial for many years after surgery. Third, of particular importance remains the need to identify patients at risk for such problems, ideally before surgery, and the need to identify whether patients with post-surgical AUDs have unique treatment needs. Fourth, we need to better understand potential differential predictors of AUD by surgical type, sex, developmental period, and new-onset versus continued use. Finally, more studies are needed to understand the complexities of GHSR signaling and adaptations to this process following surgeries that reconstruct the gut as well as genetically-mediated mechanisms, including the contribution of epigenetic regulation after bariatric surgery.
Notably, data suggest that both RYGB and SG, but not LAGB, dramatically affect alcohol pharmacokinetics. Therefore, patients who will undergo or have undergone RYGB or SG should be aware of these important changes in alcohol pharmacokinetics to avoid potential serious consequences of moderate alcohol consumption. It seems important to mention that most of the data have been collected in women. Although the effects of these surgeries on alcohol absorption most likely will apply to men, it would be important that future studies include men given some known sex-specific differences in alcohol pharmacokinetics. In addition, we are not aware of any published data on changes in alcohol pharmacokinetics of rodent models of metabolic surgeries; future research in this area is warranted.
Acknowledgement
The authors would like to acknowledge the Radcliffe Institute for Advanced Study at Harvard University for funding the meeting, which contributed to this review. All authors received travel reimbursement to attend the Radcliffe meeting, from which this manuscript resulted. Outside the submitted work, Dr. Pepino reports grants from the NIH and Dr. Steffen reports grants from Sanford Profile/NDSU, NIH, and Shire Pharmaceuticals.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.King W, Chen, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas A, Pories Walter J., and Yanovski SZ 2012. ‘Prevalence of alcohol use disorders before and after bariatric surgery’, JAMA, 307: 2516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King WC, Chen JY, Courcoulas AP, Dakin GF, Engel SG, Flum DR, Hinojosa MW, Kalarchian MA, Mattar SG, Mitchell JE, Pomp A, Pories WJ, Steffen KJ, White GE, Wolfe BM, and Yanovski SZ. 2017. ‘Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study’, Surg Obes Relat Dis, 13: 1392–402.Examined rates and predictors of alcohol use up to seven yeras following bariatric surgery.
- 3.∙Spadola Christine E., Wagner Eric F., Dillon Frank R., Trepka Mary Jo, De La Cruz-Munoz Nestor, and Messiah Sarah E. 2015. ‘Alcohol and Drug Use Among Postoperative Bariatric Patients: A Systematic Review of the Emerging Research and Its Implications’, Alcohol Clin Exp Res, 39: 1582–601.Provides a systematic review of the rates of alcohol and drug use following bariatric surgery.
- 4.Saules KK, Wiedemann Ashley A, Ivezaj V, Hopper JA, Foster-Hartsfield, and Schwarz D 2010. ‘Bariatric surgery history among substance abuse treatment patients: prevalence and associated features’, Surg Obes Relat Dis, 6: 615–21. [DOI] [PubMed] [Google Scholar]
- 5.Cuellar-Barboza AB, Frye MA, Grothe K, Prieto ML, Schneekloth TD, Loukianova LL, Hall-Flavin DK, Clark MM, Karpyak VM, Miller JD, and Abulseoud OA. 2015. ‘Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery’, Journal of Psychosomatic Research, 78: 199–204. [DOI] [PubMed] [Google Scholar]
- 6.Fogger SA, and McGuinness TM. 2012. ‘The relationship between addictions and bariatric surgery for nurses in recovery’, Perspect Psychiatr Care, 48: 10–5. [DOI] [PubMed] [Google Scholar]
- 7.Wiedemann AA, Saules KK, and Ivezaj V. 2013. ‘Emergence of New Onset substance use disorders among post-weight loss surgery patients’, Clin Obes, 3: 194–201. [DOI] [PubMed] [Google Scholar]
- 8.Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, and Marino JM. 2008. ‘Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set’, Surg Obes Relat Dis, 4: 647–50. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim N, Alameddine M, Brennan J, Sessine M, Holliday C, and Ghaferi AA New onset Alcohol use disorder after bariatric surgery. Surg Endoscopy, In Press. doi: 10.1007/s00464-018-6545-x [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JE, Lancaster KL, Burgard MA, Howell LM, Krahn DD, Crosby RD, Wonderlich SA, and Gosnell BA. 2001. ‘Long-term follow-up of patients’ status after gastric bypass’, Obesity Surgery, 11: 464–68. [DOI] [PubMed] [Google Scholar]
- 11.∙Spadola CE, Wagner EF, Accornero VH, Vidot DC, de la Cruz-Munoz N, and Messiah SE 2017. ‘Alcohol use patterns and alcohol use disorders among young adult, ethnically diverse bariatric surgery patients’, Substance Abuse, 38: 82–87.Examined alcohol use patterns and alcohol use disorder among a diverse group of young adults.
- 12.Suzuki Joji, Haimovici Florina, and Chang Grace. 2012. ‘Alcohol Use Disorders After Bariatric Surgery’, Obes Surg, 22: 201–07. [DOI] [PubMed] [Google Scholar]
- 13.Svensson PA, Anveden A, Romeo S, Peltonen M, Ahlin S, Burza MA, Carlsson B, Jacobson P, Lindroos AK, Lonroth H, Maglio C, Naslund I, Sjoholm K, Wedel H, Soderpalm B, Sjostrom L, and Carlsson LM. 2013. ‘Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study’, Obesity (Silver Spring), 21: 2444–51. [DOI] [PubMed] [Google Scholar]
- 14.Wee Christina C., Mukamal Kenneth J., Huskey Karen W., Davis Roger B., Colten Mary Ellen, Bolcic-Jankovic Dragana, Apovian Caroline M., Jones Daniel B., and Blackburn George L.. 2014. ‘High-risk alcohol use after weight loss surgery’, Surg Obes Relat Dis, 10: 508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, and Naslund E.. 2013. ‘Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery’, JAMA Surg, 148: 374–7. [DOI] [PubMed] [Google Scholar]
- 16.Nolen-Hoeksema S. 2004. ‘Gender differences in risk factors and consequences for alcohol use and problems’, Clinical Psychology Review, 24: 981–1010. [DOI] [PubMed] [Google Scholar]
- 17.Hasin DS, Stinson FS, Ogburn E, and Grant BF. 2007. ‘Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States - Results from the National Epidemiologic Survey on Alcohol and Related Conditions’, Archives of General Psychiatry, 64: 830–42. [DOI] [PubMed] [Google Scholar]
- 18.Conner KR, Pinquart M, and Gamble SA. 2009. ‘Meta-analysis of depression and substance use among individuals with alcohol use disorders’, Journal of Substance Abuse Treatment, 37: 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keyes KM, Hasin DS. Socioeconomic status and problem alcohol use: the positive relationship between income and the DSM-IV alcohol abuse diagnosis. Addiction. 2008;103:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.∙∙Zeller MH, Washington GA, Mitchell JE, Sarwer DB, Reiter-Purtill J, Jenkins TM, Courcoulas AP, Peugh JL, Michalsky MP, Inge TH, TEEN-LABS Consortium, and TeenView Study Grp. 2017. ‘Alcohol use risk in adolescents 2 years after bariatric surgery’, Surgery for Obesity and Related Diseases, 13: 85–94.Compared group differences in alcohol use between bariatric surgery and a matched control group of adolescents.
- 21.Ochner CN, Gibson C, Carnell S, Dambkowski C, Geliebter A. 2010. ‘The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity’, Physiol Behav, 100: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miras AD, le Roux CW 2013. ‘Mechanisms underlying weight loss after bariatric surgery’, Nature Reviews Gastroenterology & Hepatology, 10: 575–84. [DOI] [PubMed] [Google Scholar]
- 23.Dimitriadis GK, Randeva MS, and Miras AD. 2017. ‘Potential Hormone Mechanisms of Bariatric Surgery’, Curr Obes Rep, 6: 253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval D. 2011. ‘Bariatric surgeries: beyond restriction and malabsorption’, Int J Obes (Lond), 35 Suppl 3: S45–9. [DOI] [PubMed] [Google Scholar]
- 25.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, and Richart C.. 2004. ‘Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity’, Obes Res, 12: 962–71. [DOI] [PubMed] [Google Scholar]
- 26.Ochner CN, Gibson C, Shanik M, Goel V, and Geliebter A.. 2011. ‘Changes in neurohormonal gut peptides following bariatric surgery’, Int J Obes (Lond), 35: 153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozsoy Z, and Demir E.. 2018. ‘Which Bariatric Procedure Is the Most Popular in the World? A Bibliometric Comparison’, Obesity Surgery, 28: 2339–52. [DOI] [PubMed] [Google Scholar]
- 28.Akkary E, Duffy A, Bell R. 2008. ‘Deciphering the Sleeve: Technique, Indications, Efficacy, and Safety of Sleeve Gastrectomy’. Obes Surg, 18: 1323–9. [DOI] [PubMed] [Google Scholar]
- 29.Davis JF, Tracy AL, Schurdak JD, Magrisso IJ, Grayson BE, Seeley RJ, and Benoit SC. 2013. ‘Roux en Y Gastric Bypass Increases Ethanol Intake in the Rat’, Obesity Surgery, 23: 920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND, and Thanos PK. 2012. ‘Alcohol Reward Is Increased after Roux-en-Y Gastric Bypass in Dietary Obese Rats with Differential Effects following Ghrelin Antagonism’, Plos One, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, Volkow ND, and Hajnal A.. 2013. ‘Roux-en-Y Gastric Bypass Increases Intravenous Ethanol Self-Administration in Dietary Obese Rats’, Plos One, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.∙∙Sirohi S, Richardson BD, Lugo JM, Rossi DJ, and Davis JF 2017. ‘Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat’, Obesity (Silver Spring), 25: 1228–36.Discusses the importance of ghrelin signaling on appetite and alcohol using preclinical data.
- 33.Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, Wang GJ, Volkow ND, and Hajnal A.. 2012. ‘Gastric bypass increases ethanol and water consumption in diet-induced obese rats’, Obes Surg, 22: 1884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerlhag E, Ivanoff L, Vater A, and Engel JA. 2014. ‘Peripherally Circulating Ghrelin Does NotMediate Alcohol- Induced Reward and Alcohol Intake in Rodents’, Alcoholism-Clinical and Experimental Research, 38: 959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holst B, and Schwartz TW. 2004. ‘Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation’, Trends in Pharmacological Sciences, 25: 113–17. [DOI] [PubMed] [Google Scholar]
- 36.Verebey K, and Gold MS. 1988. ‘From Coca Leaves to Crack - the Effects of Dose and Routes of Administration in Abuse Liability’, Psychiatric Annals, 18: 513–20. [Google Scholar]
- 37.Hatsukami DK, and Fischman MW. 1996. ‘Crack cocaine and cocaine hydrochloride. Are the differences myth or reality?’, JAMA, 276: 1580–8. [PubMed] [Google Scholar]
- 38.Klockhoff H, Naslund I, and Jones AW. 2002. ‘Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery’, Br J Clin Pharmacol, 54: 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagedorn JC, Encarnacion B, Brat GA, and Morton JM 2007. ‘Does gastric bypass alter alcohol metabolism?’, Surg Obes Relat Dis, 3: 543–48. [DOI] [PubMed] [Google Scholar]
- 40.Woodard Gavitt A., Downey John, Hernandez-Boussard Tina, and Morton John M. 2011. ‘Impaired Alcohol Metabolism after Gastric Bypass Surgery: A Case-Crossover Trial’, J Am Coll Surg, 212: 209–14. [DOI] [PubMed] [Google Scholar]
- 41.Steffen Kristine J., Engel Scott G., Pollert Garrett A., Li Cao, and Mitchell James E.. 2013. ‘Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass’, Surg Obes Relat Dis, 9: 470–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harger RN, Forney RB, and Baker RS. 1956. ‘Estimation of the Level of Blood-Alcohol from Analysis of Breath .2. Use of Rebreathed Air’, Quarterly Journal of Studies on Alcohol, 17: 1–18. [PubMed] [Google Scholar]
- 43.Jones AW, Lindberg L, and Olsson SG. 2004. ‘Magnitude and time-course of arterio-venous differences in blood-alcohol concentration in healthy men’, Clinical Pharmacokinetics, 43: 1157–66. [DOI] [PubMed] [Google Scholar]
- 44.∙∙Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, and Klein S. 2015. ‘Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4’, JAMA Surg.Examined the subjective effects of ingested alcohol using arterialized blood samples among individuals who underwent RYGB.
- 45.Changchien EM, Woodard GA, Hernandez-Boussard T, and Morton JM. 2012. ‘Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial’, J Am Coll Surg, 215: 475–9. [DOI] [PubMed] [Google Scholar]
- 46.Gallo AS, Berducci MA, Nijhawan S, Nino DF, Broderick RC, Harnsberger CR, Lazar S, Echon C, Fuchs HF, Alvarez F, Sandler BJ, Jacobsen G, and Horgan S.. 2015. ‘Alcohol metabolism is not affected by sleeve gastrectomy’, Surg Endosc, 29: 1088–93. [DOI] [PubMed] [Google Scholar]
- 47.Maluenda Fernando, Csendes Attila, Xabier De Aretxabala Jaime Poniachik, Salvo Karen, Delgado Iris, and Rodriguez Patricia. 2010. ‘Alcohol Absorption Modification After a Laparoscopic Sleeve Gastrectomy Due to Obesity’, Obes Surg, 20: 744–48. [DOI] [PubMed] [Google Scholar]
- 48.∙∙Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK, and Pepino MY 2018. ‘Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4’, Surgery for Obesity and Related Diseases, 14: 277–83.Examined pharmacologic and subjective effects of alcohol following SG and RYGB.
- 49.Prom-Wormley EC, Ebejer J, Dick DM, and Bowers MS. 2017. ‘The genetic epidemiology of substance use disorder: A review’, Drug and Alcohol Dependence, 180: 241–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winnier DA, Fourcaudot M, Norton L, Abdul-Ghani MA, Hu SL, Farook VS, Coletta DK, Kumar S, Puppala S, Chittoor G, Dyer TD, Arya R, Carless M, Lehman DM, Curran JE, Cromack DT, Tripathy D, Blangero J, Duggirala R, Goring HHH, DeFronzo RA, and Jenkinson CP. 2015. ‘Transcriptomic Identification of ADH1B as a Novel Candidate Gene for Obesity and Insulin Resistance in Human Adipose Tissue in Mexican Americans from the Veterans Administration Genetic Epidemiology Study (VAGES)’, Plos One, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang GJ, Volkow ND, and Fowler JS. 2002. ‘The role of dopamine in motivation for food in humans: implications for obesity’, Expert Opin Ther Targets, 6: 601–9. [DOI] [PubMed] [Google Scholar]
- 52.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, and Fowler JS. 2001. ‘Brain dopamine and obesity’, Lancet, 357: 354–7. [DOI] [PubMed] [Google Scholar]
- 53.Romer AL, Kang MS, Nikolova YS, Gearhardt AN, and Hariri AR. 2019. ‘Dopamine genetic risk is related to food addiction and body mass through reduced reward-related ventral striatum activity’, Appetite, 133: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blum K, Braverman ER, Wood RC, Gill J, Li C, Chen TJ, Taub M, Montgomery AR, Sheridan PJ, and Cull JG. 1996. ‘Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report’, Pharmacogenetics, 6: 297–305. [DOI] [PubMed] [Google Scholar]
- 55.Thompson MD, Kenna GA Variation in the serotonin transporter gene and alcoholism : Risk and response to pharmacotherapy. Alcohol Alcohol. 2016. ;51 :164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Need AC, Ahmadi KR, Spector TD, Goldstein DB. Obesity is associated with genetic variants that alter dopamine availability. Ann Hum Genet. 2006;70:293–303. [DOI] [PubMed] [Google Scholar]
- 57.Drabe M, Rullmann M, Luthardt J, Boettcher Y, Regenthal R, Ploetz T, Becker GA, Patt M, Schinke C, Bergh FT, Zientek F, Hilbert A, Bresch A, Fenske W, Hankir MK, Sabri O, and Hesse S.. 2017. ‘Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity’, Translational Psychiatry, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Felix R, et al. LifeLines Cohort Study, ADIPOGen Consortium, AGEN-BMI Working Grp, CARDIOGRAMplusC4D Consortium, CKDGen Consortium, GLGC, ICBP, MAGIC Investigators, MuTHER Consortium, MIGen Consortium, PAGE Consortium, ReproGen Consortium, GENIE Consortium, and Int Endogene Consortium. 2015. ‘Genetic studies of body mass index yield new insights for obesity biology’, Nature, 518: 197–U401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serra-Juhe C, Martos-Moreno GA, de Pieri FB, Flores R, Gonzalez JR, Rodriguez-Santiago B, Argente J, and Perez-Jurado LA. 2017. ‘Novel genes involved in severe early-onset obesity revealed by rare copy number and sequence variants’, Plos Genetics, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahna D, Puri S, and Sharma S.. 2018. ‘DNA methylation signatures: Biomarkers of drug and alcohol abuse’, Mutation Research-Reviews in Mutation Research, 777: 19–28. [DOI] [PubMed] [Google Scholar]
- 61.Palmisano M, and Pandey SC. 2017. ‘Epigenetic mechanisms of alcoholism and stress-related disorders’, Alcohol, 60: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Still CD, Wood GC, Chu X, Erdman R, Manney CH, Benotti PN, Petrick AT, Strodel WE, Mirshahi UL, Mirshahi T, Carey DJ, and Gerhard GS. 2011. ‘High Allelic Burden of Four Obesity SNPs Is Associated With Poorer Weight Loss Outcomes Following Gastric Bypass Surgery’, Obesity, 19: 1676–83. [DOI] [PubMed] [Google Scholar]
- 63.∙Morcillo S, Macias-Gonzalez M, and Tinahones FJ 2017. ‘The Effect of Metabolic and Bariatric Surgery on DNA Methylation Patterns’, Current Atherosclerosis Reports, 19.Reviews the literature on DNA methylation patterns and metabolic improvement after bariatric surgery.
- 64.Nicoletti CF, Cortes-Oliveira C, Pinhel MAS, and Nonino CB. 2017. ‘Bariatric Surgery and Precision Nutrition’, Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day SE, Garcia LA, Coletta RL, Campbell LE, Benjamin TR, De Filippis EA, Madura JA 2nd, Mandarino LJ, Roust LR, and Coletta DK 2017. ‘Alterations of sorbin and SH3 domain containing 3 (SORBS3) in human skeletal muscle following Roux-en-Y gastric bypass surgery’, Clin Epigenetics, 9: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Testino G, and Fagoonee S.. 2018. ‘Alcohol Use Disorders and Bariatric Surgery’, Obesity Surgery, 28: 3304–05. [DOI] [PubMed] [Google Scholar]
- 67.McFadden KM 2010. ‘Cross-Addiction: From Morbid Obesity to Substance Abuse’, Bariatric Nursing and Surgical Patient Care, 5: 145–78. [Google Scholar]
- 68.Ziauddeen H, and Fletcher PC. 2013. ‘Is food addiction a valid and useful concept?’, Obesity Reviews, 14: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ivezaj V, Saules KK, and Schuh LM. 2014. ‘New-onset substance use disorder after gastric bypass surgery: rates and associated characteristics’, Obes Surg, 24: 1975–80. [DOI] [PubMed] [Google Scholar]
- 70.Lynch A. ‘“When the honeymoon is over, the real work begins:” 2016. Gastric bypass patients’ weight loss trajectories and dietary change experiences’, Soc Sci Med, 151: 241–9. [DOI] [PubMed] [Google Scholar]
- 71.Ito S, Izumi T, and Arakawa M.. 1999. ‘Gastrectomy is a risk factor for alcoholism’, Internal Medicine, 38: 751–51. [DOI] [PubMed] [Google Scholar]
- 72.Lundegardh G, Helmick C, Zack M, Adami HO. Mortality among patients with partial gastrectomy for benign ulcer disease. Dig Dis Sci. 1994;39:340–6. [DOI] [PubMed] [Google Scholar]
- 73.Yokoyama A, Takagi T, Ishii H, Wada N, Maruyama K, Takagi S, and Hayashida M.. 1995. ‘Gastrectomy Enhances Vulnerability to the Development of Alcoholism’, Alcohol, 12: 213–16. [DOI] [PubMed] [Google Scholar]
- 74.Burgos MG, Cabral PC, Maio R, et al. (2015). ‘Prevalence of alcohol abuse before and after bariatric surgery associated with nutritional and lifestyle factors’. Obes Surg, 25: 1716–1722. [DOI] [PubMed] [Google Scholar]